Abstract
Marine-derived small molecules and peptides have played a central role in elaborating pharmacological specificities and neuronal functions of mammalian ionotropic glutamate receptors (iGluRs), the primary mediators of excitatory synaptic transmission in the central nervous system (CNS). As well, the pathological sequelae elicited by one class of compounds (the kainoids) constitute a widely-used animal model for human mesial temporal lobe epilepsy (mTLE). New and existing molecules could prove useful as lead compounds for the development of therapeutics for neuropathologies that have aberrant glutamatergic signaling as a central component. In this chapter we discuss natural source origins and pharmacological activities of those marine compounds that target ionotropic glutamate receptors.
1 Introduction
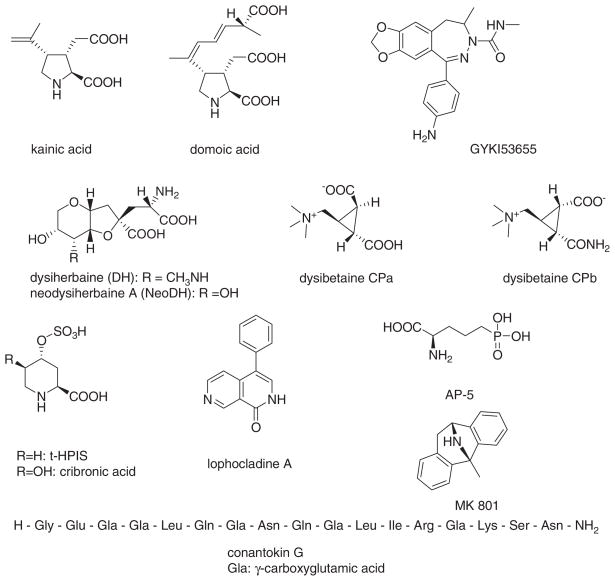
Marine organisms have provided some of the most well-known and widely used ligands for mammalian iGluRs (Fig. 1). Indeed, one family of iGluRs, the kainate receptors, was named for a molecule derived from a common variety of seaweed that was used extensively in Japanese native medicine as an anthelmintic to treat ascariasis. Despite the prominence in neuroscience research of a limited set of marine natural products that target iGluRs, in sheer numbers far more iGluR-active secondary metabolites, particularly amino acid derivatives, have been derived from terrestrial sources (Moloney 1998, 1999, 2002). Notable examples include quisqualic acid, from the fruit of the Rangoon creeper Quisqualis indica (Takemoto et al. 1975; Takemoto 1978), and willardiine, from the pea seedling of Acacia willardinia (Gmelin 1959; Ashworth et al. 1972), both of which have played important roles in the pharmacological characterization of iGluRs. Wasp toxins also are sources of metabolites with affinity for iGluRs. Philanthotoxins, which are polyamine-containing toxins from the digger wasp Philanthus triangulum, and Joro spider toxins (JSTX) from the Joro spider Nephila clavata are open-channel blockers for a subset of glutamate receptors (Clark et al. 1982; Bruce et al. 1990; Blagbrough et al. 1994; Usherwood 2000; Estrada et al. 2007). Numerous other examples of molecules derived from terrestrial organisms exist whose activity on iGluRs have been characterized to varying degrees (e.g., Takemoto et al. 1964; Evans and Usherwood 1985; Konno et al. 1988; Shin-ya et al. 1997a, b; McCormick et al. 1999; Watanabe and Kitahara 2007). This rich abundance of iGluR ligands from terrestrial sources perhaps explains why comparatively few new molecules have been isolated from marine organisms, which are often more difficult to collect and exhibit more limited diversity at the species level. Nevertheless, molecules isolated from marine sources are structurally novel in many cases and for that reason could serve both as important tools in neurobiological research and as templates for development of clinically relevant drugs.
Fig. 1.
Chemical structures of representative iGluR ligands discussed in this chapter
In this chapter we will discuss several broad families of marine-derived iGluR ligands, including peptides isolated from Conus snails that target N-methyl-Daspartate (NMDA) receptors and rigid analogs of the excitatory amino acid L-glutamate that predominantly activate non-NMDA receptors, which consist of (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptor families. Molecules that target non-NMDA receptors include kainic acid (KA) and domoic acid (DOM), which are algal products collectively referred to as kainoids because they share a 2,3,4-trisubstituted pyrrolidine core structure (Laycock et al. 1989). The terrestrial excitotoxin acromelic acid, from the Japanese mushroom Clitocybe acromelalga, also falls into this structural class. More recent studies discovered two new natural iGluR ligands, dysiherbaine and neodysiherbaine A (Sakai et al. 1997, 2001a), underscoring the potential utility of screening marine benthic organisms for neuroactive molecules. These molecules are structurally distinct from the kainoids and thus constitute a third family of marine-derived ligands for ionotropic glutamate receptors. As a necessary introduction to the molecules themselves, we first briefly review the genetics, pharmacology, and neurophysiology of the three primary members of the iGluR family of subunits, the AMPA, kainate and NMDA receptors.
2 Ionotropic Glutamate Receptors
Ionotropic glutamate receptors are essential to the appropriate function of the mammalian CNS. They mediate chemical synaptic transmission at the vast majority of excitatory synapses, underlie well-characterized cellular models of learning and memory, modulate excitability of neuronal networks, and are required for maturation of synaptic connections during early development (reviewed in Mayer et al. 1992; Hollmann and Heinemann 1994; Aamodt and Constantine-Paton 1999; Dingledine et al. 1999; Huettner 2003; Lerma 2006; Paoletti and Neyton 2007). Modulation of the strength of excitatory synaptic transmission by enhancing or inhibiting glutamate receptor function is under active investigation for therapeutic benefits in a number of neuropathologies, including mild to moderate cognitive impairment and chronic pain (Bleakman et al. 2006; Lynch and Gall 2006; Planells-Cases et al. 2006).
Ionotropic glutamate receptors have been highly conserved during evolution of marine and terrestrial organisms. They subserve similar functional roles in neurotransmission in higher and lower vertebrates, including fish (Nawy and Copenhagen 1987; Kung et al. 1996), and structurally related homologues have been identified from invertebrates such as Drosophila melanogaster (Schuster et al. 1991) and Caenorhabditis elegans (Hart et al. 1995; Maricq et al. 1995). Their central role in excitatory neurotransmission in a wide variety of organisms in part accounts for the occurrence of natural iGluR ligands used aggressively for prey immobilization (e.g., conantokins). In the following section we confine our brief discussion to the structure, physiology and pharmacology of mammalian iGluRs, because the vast majority of such research has been focused on these molecules. The general principles of receptor function, however, are likely applicable to the true targets of marine excitotoxins in fish and other predatory marine organisms. Reviews with significantly greater detail on the structure, function and significance of each type of mammalian iGluR are available in the recently published book The Glutamate Receptors (Gereau and Swanson 2008).
2.1 iGluR Gene Families and Structure
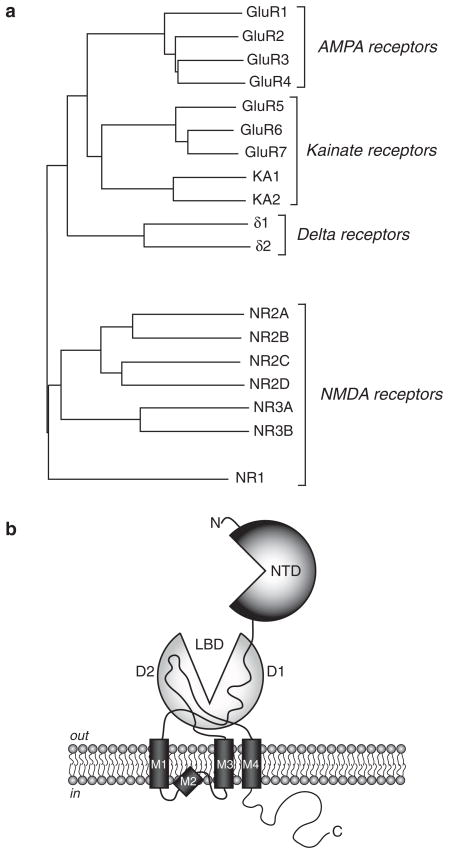
Ionotropic glutamate receptors are ligand-gated cation channels formed from subunit proteins of the AMPA, kainate, NMDA or delta receptor gene families. In mammalians, these gene families are known by the acronyms GRIA, GRIK, GRIN, and GRID, for Glutamate Receptor, Ionotropic, AMPA (Kainate, NMDA, or Delta) (Fig. 2a). While these subunits have shared structural features and similar primary amino acid sequences, assembly of functional tetrameric receptors is strictly controlled so that each subunit only oligomerizes with partners within its gene family. This restriction ensures, for example, that functional AMPA receptors in the mammalian CNS only contain AMPA receptor subunits; similar stoichiometric restrictions exist for other iGluRs.
Fig. 2.
a The mammalian ionotropic glutamate receptor gene families represented in a dendrogram, with the distance of the connecting lines proportional to primary sequence identity. b The structure of a representative iGluR subunit. The N-terminal domain (NTD) is involved in assembly of tetrameric receptors. The ligand-binding domain (LBD) is extracellular and a bi-lobate structure composed of two distinct domains (D1 and D2). Four membrane domains are noted (M1–M4), with the M2 domain constituting a P-loop. The c-terminal domain contains trafficking, targeting and modulatory determinants.
A functional iGluR is composed of four subunit proteins, which can be identical (homomeric receptors) or heterogeneous (heteromeric receptors). The secondary structure of a single iGluR subunit has a generally modular design, with different domains in the proteins playing distinct roles in receptor biogenesis, trafficking, or function (Madden 2002; Greger and Esteban 2007) (Fig. 2b). All iGluR subunits have three transmembrane domains (M1, M3 and M4) and one re-entrant P-loop (M2) similar to that found in voltage-gated channels. The M2 domains form the pore of the channel, whereas other membrane domains are intimately involved in channel gating processes. The large extracellular amino-terminal domain (NTD) is critical for appropriately restricted oligomerization during early steps in receptor assembly and contains allosteric modulatory sites in NMDA receptor subunits (Paoletti and Neyton 2007). The extracellular ligand-binding domain (LBD) is formed from two discontiguous segments of the protein, with the first (S1) located immediately before M1 and the second (S2) between M3 and M4 (Stern-Bach et al. 1994). Each subunit protein contains one binding site for its primary ligand, which in most cases is the excitatory neurotransmitter L-glutamate. While several subunits bind ligands other than glutamate, the tertiary structures of the ligand binding domains of each iGluR subunits are remarkably conserved (e.g., Armstrong et al. 1998; Furukawa et al. 2005; Mayer 2005; Naur et al. 2007), suggesting the fundamental mechanisms for ligand binding and channel gating are common to the different receptor families. Finally, the intracellular carboxy-terminal domains of receptor subunits interact with signaling systems and other protein complexes to modulate function and control trafficking and targeting to synaptic and non-synaptic sites in neurons (Perez-Otaño and Ehlers 2004; Jaskolski et al. 2005; Greger and Esteban 2007).
2.2 AMPA Receptors
AMPA receptors are the “workhorses” of mammalian excitatory synapses. Glutamate release from presynaptic vesicles binds to closely apposed receptors, resulting in channel gating and a brief, localized depolarization of the postsynaptic neuron. Typically, AMPA receptor-mediated excitatory postsynaptic potentials (EPSPs) have a half-time of less than 10 ms, ensuring that receptors are available for re-activation during periods of relatively high frequency input. Summation and propagation of these depolarizing signals to the cell soma can result in initiation of action potentials. Generalized inhibition of AMPA receptors results in cessation of excitatory neurotransmission and, essentially, brain activity.
AMPA receptors are formed from a combination of four individual gene products known as GluR1–4 (with the corresponding genes named GRIA1–4) (Hollmann and Heinemann 1994). All AMPA receptor subunits contain a binding site for glutamate and form channels that are permeable primarily to monovalent cations. Notably, receptors lacking the GluR2 subunit are also weakly permeable to calcium (Hollmann et al. 1991). Incorporation of a GluR2 subunit restricts divalent cation permeability as a result of a single amino acid difference from GluR1, GluR3, and GluR4 subunits in the critical pore-forming M2 domain (Dingledine et al. 1992). This amino acid difference (an arginine instead of a glutamine) arises not from a difference in the gene sequence, but rather from a post-transcriptional enzymatic alteration (RNA editing) of an adenosine within the glutamine codon in the mRNA, resulting in its translation as an arginine (Sommer et al. 1991). Functional diversity between receptor subunits is further introduced by an additional RNA editing event, as well as alternative splicing in both extracellular and intracellular domains (Sommer et al. 1990; Lomeli et al. 1994).
Neurons in the mammalian brain appear to use AMPA receptors with distinct stoichiometric combinations of subunits. For example, AMPA receptor excitatory postsynaptic currents (EPSCs) at hippocampal Schaffer collateral–CA1 pyramidal neuron synapses are thought to arise primarily from GluR2/GluR3 receptors. This is altered during periods of strong synaptic stimulation, which induce insertion of GluR1/GluR2 or GluR2/GluR4 AMPA receptors (Hayashi et al. 2000; Zhu et al. 2000; Shi et al. 2001). In contrast to pyramidal neurons, hippocampal interneurons tend to express AMPA receptors that lack the GluR2 subunit and thus have calcium-permeable populations of receptors (Geiger et al. 1995), which make them sensitive to open-channel blockage by natural polyamine toxins such as philanthotoxin and Joro spider toxin (Iino et al. 1996; Washburn and Dingledine 1996). In principle, this diversity suggests that specific populations of receptors could be targeted pharmacologically, but in actuality there are few identified ligands that exhibit high degrees of selectivity between AMPA receptor subunits.
As will be discussed in subsequent sections, a number of marine-derived agonists that activate kainate receptors with high affinity also act upon AMPA receptors with lower affinity. Kainic acid itself is the best-known example of an agonist with overlapping but divergent affinities for AMPA and kainate receptors. It is clear now, however, that the potent excitant activity elicited by kainoids and dysiherbaines arise largely (though perhaps not exclusively) from their affinity for and activation of kainate rather than AMPA receptors (Mulle et al. 1998; Sakai et al. 2001b).
2.3 Kainate Receptors
Kainate receptors play a variety of roles in the mammalian CNS (Lerma 2006). They modulate excitatory and inhibitory synaptic transmission (Rodriguez-Moreno et al. 1997; Contractor et al. 2000; Kamiya and Ozawa 2000; Schmitz et al. 2000; Frerking et al. 2001; Jiang et al. 2001), modulate some forms of synaptic plasticity (Bortolotto et al. 1999; Contractor et al. 2001; Lauri et al. 2001; Schmitz et al. 2003), control neuronal excitability through inhibitory actions on intrinsic conductances (Melyan et al. 2002, 2004; Fisahn et al. 2005), and can contribute to temporal summation of postsynaptic depolarization in response to bursts of action potentials (Castillo et al. 1997; Vignes and Collingridge 1997; Frerking and Ohliger-Frerking 2002; Jin et al. 2006). Despite these widespread actions in neuronal function, inhibition of kainate receptors (or genetic ablation of one or more subunits) does not have the profound impact on brain activity observed upon inhibition of AMPA receptors (Mulle et al. 1998, 2000; Simmons et al. 1998; Smolders et al. 2002; Contractor et al. 2003; Alt et al. 2007; Pinheiro et al. 2007). This has led to the hypothesis that the roles subserved by kainate receptors are largely modulatory, fine-tuning the balance between excitation and inhibition in the CNS, rather than being obligatory constituents of central synaptic transmission.
Kainate receptors are formed from a combination of five individual gene products, which are further subdivided into two groups based on primary sequence identity and pharmacological specificity. GluR5, GluR6, and GluR7 comprise the first sub-family to be isolated (with the corresponding genes GRIK1–3) (Hollmann and Heinemann 1994). These three subunits are collectively referred to as “low-affinity” kainate receptor subunits, because they have a lower affinity for the eponymous ligand, kainic acid, than do the two members of the second sub-family, KA1 and KA2 (GRIK4 and GRIK5), which consequently have been referred to as the “high-affinity” kainate receptor subunits. There are important differences in the physiological function of these receptor subunits as well: low-affinity subunits can assemble into functional homo-oligomeric receptors, whereas high-affinity subunits KA1 and KA2 must combine with GluR5, GluR6 or GluR7 to form functional hetero-oligomeric receptors (Egebjerg et al. 1991; Herb et al. 1992; Sommer et al. 1992; Schiffer et al. 1997; Ren et al. 2003). For this reason, KA1 and KA2 are also denoted “auxiliary” subunits to the “principal” GluR5, GluR6 and GluR7 subunits. This concept is somewhat misleading, however, because the majority of neuronal kainate receptors are likely composed of one or more members of both sub-families of subunits as heteromeric receptors (Wisden and Seeburg 1993; Bahn et al. 1994), and thus both “principal” and “auxiliary” subunits are obligatory to the appropriate functioning of these receptors in the brain.
While inhibition of kainate receptors appears to be well-tolerated by mammalian nervous systems, activation of neuronal kainate receptors with potent and high-affinity agonists, such as kainic or domoic acid, elicits characteristic stereotyped behaviors, tonic-clonic seizures, or even death at high concentrations (Nadler 1979; Ben-Ari 1985). Long-term pathological consequences of sub-lethal exposure to kainate receptor agonists include deterioration of hippocampal pyramidal neurons and lesions similar to that observed in human patients with mTLE (Nadler 1981; Ben-Ari 1985). A similar neuropathology can arise from ingestion of marine organisms (fish or shellfish) containing highly concentrated domoic acid, as is discussed in more detail in the subsequent section.
2.4 NMDA Receptors
NMDA receptors are essential mediators of many forms of learning and memory, and NMDA receptor activation is a requisite early step in most models of long-term synaptic plasticity of excitatory neurotransmission in the mammalian CNS (Dingledine et al. 1999). These receptors have a number of unusual functional features central to their important roles at excitatory synapses. For example, they are the only type of glutamate receptor that requires binding of two distinct agonists, glutamate and glycine, for channel gating (Johnson and Ascher 1987).
As well, NMDA receptors are occluded at physiological membrane potentials by the presence of a Mg2+ ion bound to a high-affinity site within the channel pore (Nowak et al. 1984). Voltage-dependent channel block by Mg2+ is relieved upon strong depolarization of the postsynaptic membrane, which can occur as a result of robust AMPA or kainate receptor activation or from back-propagating action potentials in the dendritic arbor (Bliss and Collingridge 1993; Spruston et al. 1995; Magee and Johnston 1997). Thus, NMDA receptors function as “coincidence detectors”: in order to gate current, they must receive both a presynaptic signal (glutamate released from the synaptic vesicle) and a postsynaptic signal (depolarization). If these conditions are met, NMDA receptors will open and allow permeation of both monovalent and divalent cations. Relative to AMPA and kainate receptors, NMDA receptors are highly Ca2+ permeable (MacDermott et al. 1986; Mayer and Westbrook 1987; Burnashev et al. 1995), and this Ca2+ entry through the channel plays a unique role in mediating downstream signals that lead to alterations in synaptic strength. Calcium- and calmodulin-dependent kinase II (CaMKII) and the Ca2+ dependent phosphatase calcineurin are perhaps the best-characterized signaling proteins that play central roles in synaptic plasticity downstream of Ca2+ entry through NMDA receptors. In addition, Ca2+ entry stimulates gene expression to effect protein synthesis-dependent stabilization of alterations in synaptic strength (Nicoll and Malenka 1999; Xia and Storm 2005).
NMDA receptors are formed from heteromeric combinations of three subfamilies of gene products: the NR1, NR2, and NR3 subunits. A single NR1 gene exists in mammals (named GRIN1). NR2 subunits are encoded by four distinct genes (GRIN2A–GRIN2D). NR3 subunits, the most recent sub-family to be cloned and characterized, are produced by two distinct genes (GRIN3A and −3B). Further diversity is introduced into the NR1 family of subunits by alternate splicing events in both the amino-and carboxy-terminal domains, such that a total of eight unique transcripts are utilized in mammalian brains. Functional NMDA receptors are composed of NR1 in combination with either NR2 or NR3 subunits (or, potentially, both types of subunits) (Dingledine et al. 1999; Chatterton et al. 2002; Awobuluyi et al. 2007; Smothers and Woodward 2007). As with all ionotropic glutamate receptor subunits, each NMDA receptor subunit contains a single binding site for a neurotransmitter, but the identity of the endogenous agonist molecule differs between NMDA receptor subunits. Glycine is the native ligand for NR1 and NR3 subunits (Kuryatov et al. 1994; Chatterton et al. 2002), whereas glutamate binds selectively to NR2 subunits (Laube et al. 1997). Thus, NR1/NR2 NMDA receptors (likely the predominant form found in the brain) have glutamate and glycine as obligate coagonists and are the primary contributors to plasticity in the CNS. NR1/NR3 “NMDA” receptors, in contrast, can be gated by glycine alone (Chatterton et al. 2002); the importance of these unusual receptors in excitatory neurotransmission is not well-characterized.
2.5 Delta Receptors
Delta receptors are a fourth family of receptors that are classified as iGluRs based on structural, rather than functional, similarity (Araki et al. 1993; Lomeli et al. 1993). Glutamate does not bind to or activate either delta-1 (δ1) or -2 (δ2) receptors to produce a current. Indeed, the endogenous ligand remains unknown for these receptors, which were classified for many years after their cloning as “orphans.” Recent data suggests that glycine or D-serine could represent physiological ligands (Naur et al. 2007), based on binding studies and resolved crystal structures, but neither elicit a detectable current from the receptors when applied in voltage-clamp experiments. Thus, their mechanism of action remains a mystery. No marine-derived molecules have been identified that target delta receptors, and for that reason we limit our discussion here of these interesting molecules. Several in-depth reviews discuss their potential function and role in development and neuropathology (Yuzaki 2004; Hirano 2006).
3 AMPA/Kainate Receptor Ligands: Kainoids
3.1 Natural Sources and Synthetic Analogs of Kainoids
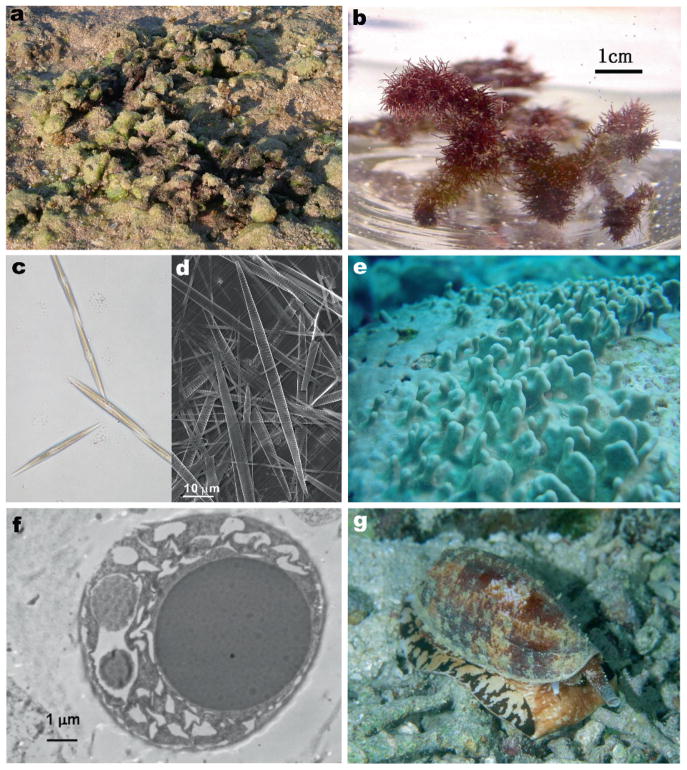
Kainic acid (KA), the original member of the kainoid family of molecules, was first found from marine red alga Digenea simplex (Ceramiales, Rhodomelaceae) (Fig. 3a, b). Aqueous extracts of D. simplex were used as anthelmintics in traditional Chinese and Japanese medicine for many centuries (Nitta et al. 1958; Pei-Gen and Shan-Lin 1986), but the isolation and structural determination of KA as the vermicidal principle was first achieved by Takemoto in 1953 (Murakami et al. 1953; Takemoto and Daigo 1958). KA has also been isolated from other species of Ceramiales red algae such as Alsidium helmithochorton (Calaf et al. 1989), Caloglossa leprieurii (Pei-Gen and Shan-Lin 1986), Centroceras clavulatum (Impellizzeri et al. 1975), and certain variety of non-Ceramiales red algae Palmaria palmata (Laycock et al. 1989). Tank cultures of a naturally occurring “dwarf” mutant of P. palmata provided a source for isolation of KA and other excitatory amino acids such as D-homocysteic acid and glutamate (Laycock et al. 1989). A survey of 46 marine red and green algae found KA or the structural analog domoic acid (DOM) in four and five Rhodomelaceae species, respectively. Interestingly, D. simplex itself contained a small amount of DOM (100-fold less than KA), which had not been noted previously (Sato et al. 1996).
Fig. 3.
Marine organisms that contain iGluR compounds. a Digenea simplex (Iriomote, Okinawa). The algae are often covered by sand. b Digenea simplex in culture. c Light micrograph of Pseudonitzschia multiseries. d Scanning electron micrograph of P. multiseries. e Lendenfeldia chondrodes (Yap, Micronesia). f Transparent electron micrograph of a symbiotic Synecocystis sp. in mesohyl of L. chondrodes. g Conus geographus in Palau. Images acquired by G. T. Swanson (A), R. Sakai (B, E, F) Y. Kotaki (C), K. Koike (D), and K. Nomura (G)
In addition to use as a veterinary anthelmintic, KA is well-established as a standard probe in neurological research to elicit currents from neuronal or recombinant kainate receptors and to induce seizure-related behaviors and pathology in animal models of epilepsy. Production of KA for these purposes has relied on both total synthesis and isolation of natural product from algae. The latter process provided a stable and economical resource until the last decade, when isolation from D. simplex for commercial purposes ceased, leading to a widespread shortage of KA (Tremblay 2000). Recently, cultivation of P. palmata by Ocean Produce International Inc. and synthetic production by other commercial agents re-established a stable production of KA.
Two natural congeners of KA have been isolated from marine organisms. Allokainic acid is the C-4 epimer of KA and was isolated from D. simplex. 1′-hydroxykainic acid was found from a KA-producing mutant of P. palmata (Ramsey et al. 1994). In addition, a “kainic-peptide” was isolated from a red alga A. helminthocorton; this molecule appears to be a naturally occurring peptide that contains kainic acid as two of its 37 amino acids residues (Calaf et al. 1989), but no further structural information, beyond amino acid analysis, or biological activities have been described.
Recently Sakai and co-workers determined the cellular and subcellular localization of KA in D. simplex using immunohistochemical and immunocytochemical techniques (Sakai et al. 2005). A KA-specific antibody localized immunoreactivity within the outmost layer cells in the algal thallus. In subcellular observations using transmission electron microscopy, KA immunoreactivity was found in electron dense cytosolic granule bodies, nuclei, and pit plugs of the cells. No immunoreactivity was observed in epibionts, including bacteria attached to the outer surface of the thallus. Localization of KA in nuclei is of particular interest because the accumulation of secondary metabolites in this structure had not previously been reported. The function of KA in the nucleus, however, remains unknown. The pit plugs are cell-to-cell connective apparatus unique in Rhodophyceae; their physiological role(s) have been elusive but could involve transport of nutritive materials based on morphological observations. Localization of KA immunoreactivity in the pit plug constitutes the first evidence for translocation of cellular material through this structure.
The distribution and occurrence of another marine-derived kainoid, domoic acid (DOM), bears significant importance in the realms of public health and food hygiene. This kainoid is produced by algae that enter the food chain of marine mammals and seabirds, and potentially humans, through accumulation in marine primary producers and higher filter feeders, such as anchovies and certain shellfish (Olney 1994; Watters 1995; Clark et al. 1999; Lefebvre et al. 1999; Mos 2001). DOM was first isolated from red alga Chondria armata (Takemoto 1978), and several related compounds – isodomoic acids A to D, isodomoic acid G and H, and domoilactones A and B – were later identified from the same source (Takemoto 1978; Maeda et al. 1986, 1987; Zaman et al. 1997). To date, ten stereo- and regio-isomers or congeners of DOM have been detected (Clayden et al. 2005).
The ecological and toxicological threat posed by DOM has become evident over the last two decades. DOM-containing algae were also used as vermifugal agents in Japanese folk medicine, similar to D. simplex, because DOM is a potent neuronal excitant in both vertebrates and invertebrates arising from its high affinity for kainate receptors (indeed, significantly greater affinity than that of KA itself) (Debonnel et al. 1989; Lomeli et al. 1992). The use of DOM-containing algae for medicinal purposes in humans did not result in any reported incidences of severe toxicity. However, an outbreak of food poisoning resulting from ingestion of DOM-containing blue mussels on Prince Edward Island Canada in 1987 demonstrated its potential for toxicity in humans and was the proximal cause of three deaths (Bates et al. 1989; Wright et al. 1989; Perl et al. 1990b). The clinical manifestation of DOM toxicity included moderate to severe gastrointestinal disorders and neurological symptoms that included disorientation, seizure and memory deficits in a subset of individuals (Perl et al. 1990b; Teitelbaum et al. 1990). One elderly individual who survived the initial intoxication later developed complex partial seizures and exhibited hippocampal neuronal loss and sclerosis similar to that observed in animals following KA-induced toxicity (Cendes et al. 1995). As a result of the striking degree of acute and, in some cases, long-term anterograde amnesia, the term “Amnesiac Shellfish Poisoning” (ASP) was used to describe the clinical consequences of DOM intoxication resulting from consumption of contaminated shellfish (Perl et al. 1990a; Jeffery et al. 2004).
Pennate diatoms belonging to Pseudo-nitzschia multiseries species were identified initially as the source organisms that led to ASP after consumption of blue mussels in Canada (Bates et al. 1989). The toxic events localized to the western coast of North America are attributed to Pseudo-nitzschia australis (Fritz et al. 1992; Lefebvre et al. 1999; Scholin et al. 2000). Additionally, DOM has been detected in diatoms in Japan (P. multiseries) (Fig. 3c, d) (Kotaki et al. 1999), the United Kingdom (P. australis Frenguelli, P. seriata f. seriata) (Cusack et al. 2002; Fehling et al. 2004), and Vietnam (Nitzschia navis-varingica) (Kotaki et al. 2000; Lundholm and Moestrup 2000; Kotaki et al. 2004), demonstrating that DOM-producing diatoms can occur throughout many marine ecologies (Bates 2000). Accumulation and depuration of DOM following ingestion of Pseudo-nitzschia algae occur at variable rates and in distinct tissues in marine organisms, with the highest concentrations found in anchovies, razor clams and blue mussels following algal blooms on the Pacific coast of North America (Wekell et al. 1994; Lefebvre et al. 2002a, b, 2007).
Mass mortalities of sea mammals and coastal birds of California and Baja California, including the sea lions Zalophus californianus (Lefebvre et al. 1999; Scholin et al. 2000), brown pelicans Pelecanus occidentalis (Fritz et al. 1992; Work et al. 1993; Sierra Beltran et al. 1997), and Brant’s cormorants Phalacrocorax penicillatus (Fritz et al. 1992; Work et al. 1993), were attributed to consumption of DOM-containing anchovies Engraulis mordax. A decade long monitoring study suggested that increasing numbers of California sea lions with neurological dysfunction and neuroanatomical damage (hippocampal atrophy and sclerosis) was attributable to chronic sub-lethal exposure to DOM (Goldstein et al. 2008). As well, krill (Euphasia pacifica) have been identified as a potential source of DOM toxicity that pose a risk to planktivorous organisms (Bargu et al. 2002; Lefebvre et al. 2002a). Governmental and fisheries organizations now routinely screen marine food sources for DOM levels. The US Food and Drug Safety sets a critical limit of 20 ppm DOM in the “edible portion of raw shellfish” (Guide for the Control of Molluscan Shellfish, 2005, available at http://www.cfsan.fda.gov), which is well below the levels toxic to humans (Iverson and Truelove 1994). Detection of supra-threshold DOM accumulation in marine organisms has resulted in several instances of temporary bans on fishing of particular species or within affected geographical areas (Trainer et al. 1998; Lefebvre et al. 2002a; Bill et al. 2006).
The physiological function of unusual secondary metabolites such as the kainoids within their marine ecosystem remains a matter of speculation. The most obvious possibility is that their potent and excitotoxic activity on vertebrate and invertebrate iGluRs serves in a defensive capacity to discourage attack.
Intraperitoneal or intracoelomic injection reproduces neurotoxicity observed in vertebrates, but oral gavage or ingestion of DOM is not neurotoxic in fish; (Hardy et al. 1995; Lefebvre et al. 2001, 2007). Furthermore, DOM accumulates without apparent lethality in a variety of benthic organisms (Lefebvre et al. 2002a, b, 2007), although more subtle neurological effects have been observed following exposure during development (Tiedeken et al. 2005). DOM also does not appear to subserve an allelopathic role to discourage competition between algal species (Lundholm et al. 2005). More recently, it was suggested that the excitotoxin might serve as a physiological defense mechanism against krill, which consume the diatoms (Bargu et al. 2006). In this intriguing study, the authors found that DOM effectively reduced krill grazing behaviors and thereby could serve to perpetuate algal blooms.
3.2 Biological Activities of Kainoids
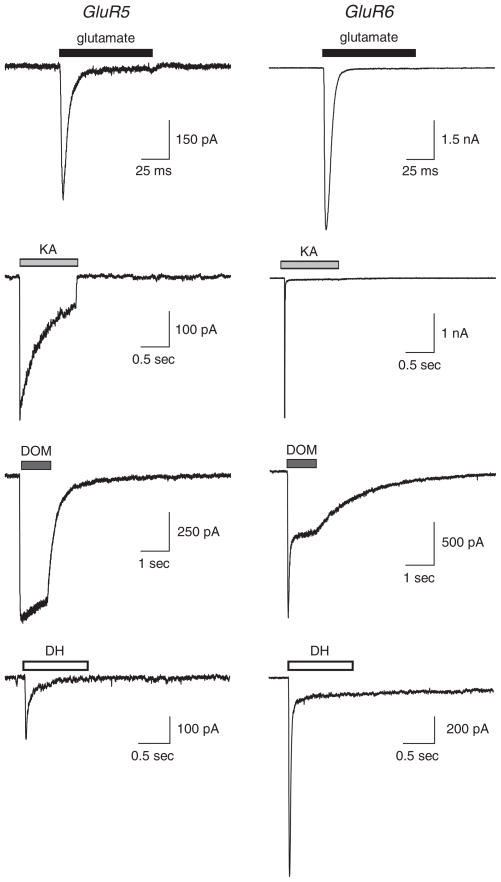
All kainoids elicit currents from both kainate and AMPA receptors. In general, they exhibit higher affinity and potency for kainate receptors, depending on structure and stereochemistry of the side chain functional groups. KA itself exhibits >10-fold higher binding affinity for kainate receptors as compared to AMPA receptors (reviewed in Hollmann and Heinemann 1994). It is a full (or nearly so) agonist that elicits desensitizing currents from most combinations of kainate receptors (see examples in Fig. 4), but only weakly activates AMPA receptors to produce steady-state currents with a very small desensitizing component. Within the kainate receptor subunit family, KA1 and KA2 kainate receptor subunits have a higher binding affinity for kainate (KD values of 5–15 nM) than do GluR5, GluR6, and GluR7 (KD values of 13–95 nM) (Bettler et al. 1990, 1992; Werner et al. 1991; Herb et al. 1992; Lomeli et al. 1992; Swanson et al. 1997). As mentioned previously, KA1 and KA2 have been referred to as “high-affinity” kainate receptor subunits and GluR5–7 as “low-affinity” subunits; it is important to keep in mind that this nomenclature, while useful for categorizing the two sub-families of subunits, is not relevant to their sensitivities to the endogenous neurotransmitter, glutamate.
Fig. 4.
Representative currents evoked by natural products from kainate receptors. Homomeric GluR5–2a (left column) or GluR6a (right column) receptors were expressed in HEK 293 cells. L-glutamate (10 mM), kainate (KA, 1 mM), domoate (DOM, 30 μM), or dysiherbaine (DH, 100 μM) were rapidly applied to cells in whole-cell voltage clamp recordings
Application of KA to almost all types of neurons in the mammalian brain causes marked depolarization through the activation of kainate and AMPA receptors, in a concentration- and receptor composition-dependent manner. Thus, KA is only a moderately selective agonist and, in general, currents evoked by high concentrations of kainate (>100 μM) will arise largely from AMPA receptors, because these receptors tend to be present at much higher density in neuronal membranes compared to kainate receptors. The respective contributions of AMPA and kainate receptors to KA-evoked currents can be differentiated more effectively using a selective AMPA receptor antagonist, such as GYKI 53655, to isolate kainate receptor currents (Paternain et al. 1995, 1996; Wilding and Huettner 1997). Currents evoked by low concentrations of KA (<5 μM) are largely carried by kainate receptors because of their significantly higher affinity for the marine toxin. This approach has been used, for example, to characterize the modulatory action of presynaptic kainate receptors on AMPA receptor-mediated synaptic currents in the hippocampus (e.g., Kamiya and Ozawa 1998; Contractor et al. 2000). KA played an important role in the early pharmacological and structural differentiation of iGluRs. It was central to definitively establishing its cognate receptor family as a distinct pharmacological entity from AMPA receptors (or Quisqualate receptors, as they initially were denoted) in seminal studies from dorsal root ganglion sensory neurons (Agrawal and Evans 1986; Huettner 1990), which constitute a relatively unique population of neurons that predominantly express kainate receptors as their sole type of iGluR. KA was a critical tool in the iGluR pharmacologist’s armamentarium for nearly a decade because of its unparalleled selectivity, commercial availability and low cost.
A variety of synthetic analogs of KA have been generated, but in large part these molecules have not been characterized as extensively on defined combinations of recombinant kainate or AMPA receptors or on neuronal iGluRs. Structure-activity relationship studies with the KA template indicated that the configurations at C2 and C4 and the composition of the C4 side-chain are particularly critical determinants of receptor selectivity. For example, dihydrokainate, which has a fully saturated C4 isopropenyl group, is a potent competitive substrate for electro-genic glutamate transporters rather than a high-affinity kainate receptor agonist (Johnston et al. 1979; Shinozaki 1988), whereas trans-2-carboxy-3-pyrrolidineacetic acid, which lacks the C4 group entirely, exhibits agonist activity on NMDA receptors (Tsai et al. 1988). Also, high affinity binding to and agonist activity on human recombinant GluR6 receptors was maintained in a variety of analogs with aryl substitutions of the C4 side-chain, but stereochemical reversal of the C4 position greatly reduced affinity for the receptor subunit (Cantrell et al. 1996). The natural terrestrial toxin acromelic acid is a C4 aryl-substituted KA analog (Konno et al. 1988) and thus may show a similar high affinity for a subset of kainate receptor subunits, although this has not been demonstrated formally.
The pharmacological actions of DOM are similar to that of KA; it is a high-affinity kainate receptor agonist and somewhat lower affinity AMPA receptor agonist. It binds, activates and desensitizes all homomeric and heteromeric kainate receptors, albeit to differing degrees and with distinct potencies. GluR5–2a receptors, which exhibit a particularly high affinity for domoate, gate a slowly desensitizing current upon activation by the marine toxin, whereas GluR6a receptors rapidly desensitize to a stable equilibrium current (Fig. 4) (Herb et al. 1992; Swanson et al. 1997). DOM is more potent and has a higher binding affinity for kainate receptors than KA (Ki values of ~2–60 nM) (reviewed in Hollmann and Heinemann 1994). Like KA, it is a partial agonist for AMPA receptors that elicits steady-state currents with a minimal desensitizing component. The actions of KA and DOM on non-NMDA receptors have been compared in a detailed review (Hampson and Manalo 1998).
Those natural analogs of DOM (the isodomoic acids) that have been examined generally display a lower potency and affinity for kainate and AMPA receptors. The radioligand binding affinities of isodomoic acid A and C for the GluR6 subunit are ~40- and ~240-fold lower than that that of DOM, respectively (Holland et al. 2005; Sawant et al. 2007); consistent with this observation, isodomoic acids A and C were less effective than DOM in reducing hippocampal population spike amplitudes (an effect known to be mediated predominantly by neuronal kainate receptors) (Sawant et al. 2007). Isodomoic acids D, E, and F have 5–280-fold lower affinities for high-affinity KA binding sites in the rat brain, which (at low radioligand concentrations) primarily arise from kainate receptors (Hampson et al. 1992).
In addition to its central importance to iGluR pharmacology research, KA has been widely used as an excitotoxic agent in behavioral and neuropathological studies (Nadler 1979, 1981; Ben-Ari 1985; Ben-Ari and Cossart 2000). Acute administration of kainoids induces characteristic acute behavioral changes in rodents, including stereotyped movement such as scratching behavior, head bobbing, and frequent grooming after ~10 min (Sperk 1994). The symptoms progress into more frequent and violent behaviors and, dependent upon the concentration of the toxins, animals display clonic whole body convulsions. At high doses, animals die after severe seizure episodes similar to those observed in humans. Repeated administration of KA will induce a permanent hyperexcitable state in animals marked by recurring convulsions, thought to mimic status epilepticus in humans (Ben-Ari and Cossart 2000). The seizurogenic action of DOM is more potent than that of KA, and it can induce long-lasting status epilepticus persisting for hours in mice (Chiamulera et al. 1992; Sakai et al. 2001b).
The neuroanatomical alterations and damage to the limbic regions produced by kainoid injection into rodents or ingestion of environmental DOM by some marine organisms partially reproduces that observed in patients with mTLE (Ben-Ari 1985). The rodent kainate-induced neuropathology model continues to be used as one diagnostic assay in screening potential anti-epileptic drugs (Loscher 2002). A discussion of the extensive literature on this model is beyond the scope of this chapter, but it has been the subject of a number of excellent reviews and book chapters (Sperk 1994; Dudek et al. 2006; Ratte and Lacaille 2006).
4 AMPA/Kainate Receptor Ligands: Dysiherbaines
4.1 Natural Sources and Synthetic Analogs of Dysiherbaines
Dysiherbaine (DH) is the first member of a new structural class of marine-derived iGluR agonists with a high degree of specificity for kainate receptors. In the course of screening for new excitatory amino acids from marine benthic organisms, Sakai and co-workers found that an aqueous extract of a sponge initially identified as Dysidea herbacea, which later analysis revealed was instead Lendenfeldia chodrodes (Fig. 3e), exhibited potent convulsant activity when injected into mice. The active principal isolated from the sponge extract was unprecedented and was comprised of a functionalized perhydro furanopyrane skeleton furnished with a 2-ami-nopropanoic acid side-chain (Sakai et al. 1997, 2006). Similar to kainoids, the structure of L-glutamate was embedded in DH and thus the toxin can be considered a C di-substituted analogue of L-glutamate. Subsequent searches for related compounds from the same sponge species resulted in an isolation of neodysiherbaine A (NDH A), an analog of DH with similar convulsant activity in mice (Sakai et al. 2001a). In addition to DH and NDH A, several structurally novel betaines, denoted dysibetaine PP, CPa and CPb, were isolated from the same sponge (Sakai et al. 2004). Weak affinity for NMDA and kainate receptors was observed in ligand-binding assays, but the pharmacological activity of the dysibetaines is not well-characterized beyond these preliminary results. The production of this array of structurally unusual molecules in the marine sponge underscores its diverse biosyn-thetic machinery and suggests that additional bioactive molecules await discovery (Sakai et al. 2006).
Recently Sakai and co-workers examined the localization of DH within the sponge tissue using immunohisto- and immunocytochemical techniques. Molecular analysis of the ribosomal DNA sequence resulted revealed that the taxonomy of the sponge was in fact Lendenfeldia chodrodes rather than D. herbacea. Moreover, localization of DH using a selective antibody found the toxin exclusively in the cells of endosymbiotic cyanobacteria, of Synechosystis sp. (Fig. 3f), suggesting that DH is in fact a metabolite of the cyanobacteria rather than the sponge itself (Sakai et al. 2008).
Because of its intriguing structural and biological features, intense efforts were undertaken towards the synthesis of DH. The first total synthesis by Hatakeyama and co-workers confirmed the proposed structure and absolute stereochemistry of DH (Masaki et al. 2000). To date, four total and one formal syntheses of DH, four total syntheses of NDH A, and structure-activity relationship (SAR) studies of NDH A have been reported (Masaki et al. 2000; Sasaki et al. 2000, 2007; Snider and Hawryluk 2000; Phillips and Chamberlin 2002; Lygo et al. 2005; Takahashi et al. 2006). A variety of DH analogues have been described, although only DH and NDH A are natural products derived from the sponge (Sasaki et al. 1999, 2006; Cohen et al. 2006; Shoji et al. 2006). Extensive molecular pharmacological and electro-physiological characterizations, as well as in vivo pharmacology, demonstrate that DH and its structural analogues are a new generation of excitatory amino acids with distinct receptor selectivity and agonist actions as described in the next section.
4.2 Biological Activities of Dysiherbaines
DH and neoDH are extraordinarily potent convulsants with high-affinity agonist activity on mammalian kainate receptors, a lesser potency for AMPA receptors, and (in the case of DH) an extremely weak activity on mGlu5 metabotropic glutamate receptors (Sakai et al. 2001b). Their pharmacological specificity for different KAR subunits diverges significantly from kainoids, which likely underlies their marked convulsant activity. Whereas KA exhibits highest affinity for the KA1 and KA2 subunits, DH and neoDH instead bind with very high affinity to GluR5, GluR6 and GluR7 KAR subunits (Ki values of ~0.5–1.5 nM) (Sakai et al. 2001b) but only have very weak interaction with KA2 subunits (Ki value of 4.3 μM, comparable to their affinity for AMPA receptor subunits) (Swanson et al. 2002). The action of DH on homomeric GluR5 and GluR6 receptors is unusually long-lived because the marine toxin effectively promotes a stable, desensitized conformation of the receptor, which can prevent unbinding of the agonist and subsequent re-activation by agonists (Swanson et al. 2002). While the nanomolar binding affinities exhibited by these molecules for the “primary” KAR subunits are indeed quite high, they are not so high as to suggest that the ligand-receptor interaction would be effectively irreversible (as is the case of DH and homomeric GluR5 receptors, for example). Receptors composed of both high- and low-affinity subunits, and in particular GluR5/KA2 receptors, exhibit a further twist in their biophysical response to DH. Application of DH to GluR5/KA2 receptors elicits a slowly desensitizing current, as is typical for many agonists, but upon removal of the agonist from the bathing solution a slowly developing, steady-state current emerges that arises from the stable association of DH with GluR5 subunits, resulting in a tonic partial activation of the heteromeric GluR5/KA2 receptors (Swanson et al. 2002). Thus, the pharmacological activity of the DH molecules on KARs is critically determined by the subunit composition of the receptors, which can be complex and which is not well understood at the molecular level. A more detailed review of this topic can be found in Sakai et al. (2006). Kainate receptors have diverse compositions in the mammalian brain, and therefore DH will impact neuronal function dependent upon a variety of factors, including toxin concentration and neuronal site of action (Sakai et al. 2001b).
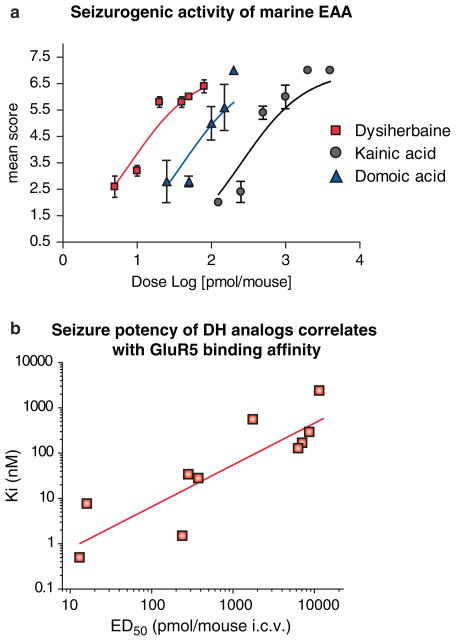
DH has been shown to be the most potent seizurogenic excitatory amino acid isolated from natural sources (Fig. 5a) (Sakai et al. 2001b). The convulsant activity of DH was found to be approximately six-fold more potent than that of DOM (Table 1). Seizure behaviors induced by injection of DH in mice were chiefly distinguished from those elicited by kainoids in the duration of the status epilepticus. Mice receiving DH (40 pmol/mouse, i.c.v. or 1.6 mg/kg, i.p) experienced severe whole body convulsions for more than 3 h, which then stabilized into periodic recurrent seizures. This state, which was not replicated by KA or DOM, lasted for more than 24 h. It is possible that this unique behavior arises from stable binding of the toxin with a subset of kainate receptors similar to that observed with recombinant receptor subunits. Indeed, a recent study showed that the seizurogenic potency of a diverse panel of DH-related molecules was strongly correlated with their affinity for the GluR5 KAR subunit (Fig. 5b), suggesting that activation of receptors comprised of this subunit primarily underlies toxin convulsant activity (Lash et al. 2007). It remains unknown whether the longer-term neuropathological consequences of seizure induction with DH closely resemble the well-characterized pattern of limbic structural reorganization, neuronal loss, and sclerosis produced in the kainate model of mTLE.
Fig. 5.
Seizure activity of marine excitatory amino acids and synthetic analogs of neodysiherbaine A. (a) Seizure behaviors induced by i.c.v. injection of dysiherbaine, kainic acid, and domoic acid in mice were graded using a seven-point scale (Sakai et al. 2001b). Values, the mean scores ±S.E.M., were fit on a sigmoidal curve using Prism™ software. (b) Binding affinity at GluR5–2a subunits correlates with seizure potency (r = 0.86; p < 0.01). Linear correlation graph is plotted as Ki (nanomolar) versus ED50 (picomoles per mouse) after i.c.v. injection of the following compounds: DH, neoDH, MSVII19, 8-deoxy-neoDH, 9-deoxy-neoDH, 8-epi-neoDH, 9-epi-neoDH, 9-F-8-epineoDH, 2,4-epi-neoDH, and 4-epi-neoDH (Lash et al. 2007)
Table 1.
Convulsant activities of marine excitatory amino acids
The structural determinants that underlie DH and neoDH affinity for kainate receptors have been explored in studies with synthetic analogs (Shoji et al. 2006). The C8 and C9 functional groups, in particular, confer specificity for the GluR5 and GluR6 KAR subunits. Elimination or epimerization of the C9 hydroxyl essentially eliminates binding to GluR6 subunits, as does similar alterations to the C8 group; in contrast, binding to GluR5 subunits is more tolerant to modification at C9 and is unaffected by elimination of the C8 moiety (Lash et al. 2007). This likely arises from the spatially larger binding pocket and the presence of favorable hydrophobic and polar interactions in the GluR5 binding domain as compared to GluR6 (Mayer 2005; Naur et al. 2005; Sanders et al. 2006). Interestingly, removal of both functional groups to produce dideoxy-neoDH (also known as MSVIII-19) fundamentally altered pharmacological activity; this molecule was an antagonist for homomeric GluR5 receptors, rather than an agonist (Sanders et al. 2005). Molecules with similar pharmacological profiles are under active examination for efficacy in a variety of animal models of neuropathologies, including epilepsy and chronic pain. MSVIII-19 is weak convulsant that additionally promotes an reversible unconscious state when injected intracerebroventricularly (i.c.v) in mice (Sasaki et al. 1999) but has relatively modest effects on motor function when introduced via intrathecal or intraperitoneal injection. Finally, epimerization of neoDH at the C4 position, which disrupts the glutamate congener in the molecule, reduced but did not eliminate affinity for GluR5 and GluR6 subunits (Lash et al. 2007). Given these precedents, further modifications to the DH template structure may yet produce molecules with distinct pharmacological profiles or activities on kainate receptor subunits.
5 NMDA Receptor Ligands: Amino Acids
5.1 Natural Sources of Amino Acid Ligands Acting on NMDA Receptors
Several compounds with selectivity for NMDA receptors have been identified from marine organisms. NMDA itself was detected from foot muscle of blood shell, Scapharca broughtonii (Sato et al. 1987). More recently, endogenous NMDA was discovered in the tunicate Ciona intestinalis (D’Aniello et al. 2003), where it is was biosynthesized from precursor D-aspartate; in the tunicate gonads, NMDA induced synthesis of gonadotropin-releasing hormone (GnRH), which in turn led to production of sex steroid hormones. A survey in marine algae for N-methyl aspartic acid (with an unspecified stereochemical configuration) found that eight out of 42 algae collected contained the compound (Sato et al. 1996); the physiological function of these amino acids in the algae is unknown.
Two 4,5-substituted analogs of pipecolic acid with activity on NMDA receptors have been isolated: cribronic acid [(2S,4R,5R)-5-hydroxy-4-sulfooxy-piperidine2-carboxylic acid], from the Palauan sponge Cribrochalina olemda, and (2S,4S) 4-sulfooxy-piperidine-2-carboxylic acid (trans-4-hydoroxypipecolic acid sulfate, t-HPIS) from the Micronesian sponges Axynella carteri and Stylotella aurantium (Sakai et al. 2003). Cribronic acid was a new compound while t-HPIS had been isolated previously from the legume Peltophorum africanum and characterized as NMDA agonist (Evans et al. 1985; Moroni et al. 1995).
Lophocladines are alkaloids with 2,7-naphthyridine skeletons isolated from red algae Lophocladia sp. collected in the Fijian Islands. One of the isolates, Lophocladine A, was shown to have affinity for the MK-801 binding site in the channel pore of NMDA receptors, suggesting that this compound might represent a novel class of naturally occurring small-molecule NMDA receptor antagonists. Validation of this possibility awaits physiological studies. A closely related analog, Lophocladine B, did not show equivalent affinity for NMDA receptors; rather, it inhibited microtubule formation and was cytotoxic (Gross et al. 2006).
5.2 Biological Activities of Natural NMDA Receptor Agonists
Both cribronic acid and t-HPIS were potent convulsants when injected i.c.v. in mice, producing dose-dependent behaviors, from running, jumping, and tonic extension to lethal convulsions, with ED50 values of ~20–30 pmol/mouse (Sakai et al. 2003). t-HPIS and cribronic acid displaced CGP 39653, a ligand for the glutamate binding site on NMDA receptors, from rat cerebrocortical membrane preparations with IC50 values of 214 nM and 83 nM, respectively. Neither compound displaced radiola-beled ligands from AMPA and kainate receptors. The agonist activity of t-HPIS on NMDA receptors was confirmed earlier in mouse cortical wedge preparations, in which the molecule caused dose-dependent depolarizations that were reduced by AP-5, an NMDA-receptor antagonist (Moroni et al. 1995). The relative depolarization potency of t-HPIS was about 5 times that of NMDA in the cortical preparation. Nothing is known regarding the NMDA receptor selectivity of these compounds. Interestingly, structurally related three- and four-substituted pipecolic acid analogs act as potent NMDA receptor antagonists and have been modified and studied extensively in pursuit of neurotherapeutic drugs (e.g., cis-4-phosphonomethyl-2-piperidine carboxylic acid, CGS 19755, Selfotel) (Lehmann et al. 1988). Thus far, however, this pharmacological approach has not proven beneficial in clinical trials for stroke mediation, and instead have tended to exacerbate neurotoxicity associated with ischemia (Davis et al. 2000).
6 NMDA Receptor Ligands: Conantokins
6.1 Natural Sources of Conantokins
Venoms from fish hunting snails, a genus of Conus, are a rich source of diverse neuroactive peptides targeting various voltage-gated ion channels, neurotransmitter receptors and transporters (Terlau and Olivera 2004). Conus peptides are categorized into a variety of families based on pharmacological targets and structural characteristics. Until very recently, the conantokin family of cone snail peptides contained four members, conantokin-G, -L, -R and –T (con-G, con-L, con-R, and con-T), which were shown to act as NMDA receptor antagonists (reviewed in Prorok and Castellino 2007). Conantokins are relatively unusual because they lack the disulfide bridges critical for structural integrity in most conopeptides and because the conantokins contain four to five γ-carboxyglutamates, a modified amino acid, in their structure. These first four conantokins range in size from 17 (Con-G) to 27 amino acids (Con-R). Con-G, from Conus geographus (Fig. 3g), was the first member of the family discovered and was isolated on the basis of its unusual bioactivity in mice: it produced a sleep-like state (McIntosh et al. 1984). This “sleeper peptide” was proposed initially to target NMDA receptors based on indirect biochemical assays (Mena et al. 1990) and later confirmed using physiological recordings from NMDA receptor channels (Hammerland et al. 1992). Con-T was isolated from Conus tulipa based on similar behavioral effects (“sleep” induction) in mice and found to have structural similarities to con-G (Haack et al. 1990); namely the initial glycine-glutamate-γ-carboxyglutamate-γ-carboxyglutamate residues were conserved in the two peptides. This sequence is also present in con-R, from Conus radiatus (White et al. 2000), and con-L, from Conus lynceus (Jimenez et al. 2002). A new, closely related series of conopeptides targeting NMDA receptors, conantokin-Pr1 to -Pr3 (con-Pr1 to -Pr3), was recently discovered from Conus parius (Teichert et al. 2007). Notably, this species of cone snail is the first whose venom contains multiple conantokin peptides. They diverge structurally from the four original conantokins, primarily in that the con-Pr toxins have only three γ-carboxyglutamate residues and two of the members (con-Pr2 and con-Pr3) contain another modified amino acid, 4-trans-hydroxyproline. Con-Pr peptides also induce a sleep-like state in mice (Teichert et al. 2007).
6.2 Biological Activities of Conantokins
Conantokins are peptide antagonists selective for NMDA receptors (Haack et al. 1990; Mena et al. 1990; Hammerland et al. 1992; Jimenez et al. 2002; Prorok and Castellino 2007). Con-G, the most extensively characterized conantokin, has appeared to have both competitive and noncompetitive antagonist activity in different assays for NMDA receptor function (Prorok and Castellino 2007). Competitive antagonism occurs at the glutamate binding site on the NR2 subunit (Hammerland et al. 1992; Donevan and McCabe 2000; Wittekindt et al. 2001), with an IC50 of ~0.5–1 μM for inhibition of NMDA-evoked currents in cultured mouse cortical neurons or for inhibition of synaptic NMDA-EPSCs in CA1 pyramidal neurons in rat hippocampal slice preparations (Donevan and McCabe 2000; Barton et al. 2004). The molecular binding site of con-G could be heterotopic, because the inhibitory activity is enhanced by polyamines such as spermine (Donevan and McCabe 2000), which binds to an extracellular allosteric modulatory site. Con-G also exhibits a high degree of subunit selectivity; inhibition of NR1/NR2B NMDA receptors is potent, whereas NR1/NR2A receptors (or those containing NR2C or NR2D) are relatively unaffected by the peptide (Donevan and McCabe 2000; Klein et al. 2001). This degree of selectivity does not extend to all conantokins, however, as con-R and con-T inhibit both NR2A- and NR2B-containing receptors (White et al. 2000; Klein et al. 2001). Conantokins, and in particular con-G, have attracted significant attention for their potential as therapeutics in a variety of neuropathologies (Layer et al. 2004). Con-G and con-T are effective antinociceptive agents in models of chronic pain (Malmberg et al. 2003), although their peptide structures restrict potential routes of administration. Con-G and con-R, but not con-L, also show anticonvulsant efficacy in a number of mouse seizures models (White et al. 2000; Jimenez et al. 2002; Barton et al. 2003). Con-G, which in its pre-clinical form is known as CGX-1007, also has neuroprotective effects in stroke models (Williams et al. 2000, 2003). While CGX-1007 was found to be safe in Phase I clinical trials, further clinical trials have not been disclosed (Olivera 2006).
Con-Pr peptides also induced the characteristic sleep-like state after injection observed decades earlier with con-G (McIntosh et al. 1984) and inhibited recombinant NR1/NR2 NMDA receptors expressed in Xenopus oocytes, with varying degrees of selectivity for NR2B-containing receptors (Teichert et al. 2007). Their pharmacological profiles were distinct from those of the earlier conantokins and consequently will provide additional tools and clues for understanding how the structure of these unusual peptides determines their functional activity and subunit specificity.
7 Unpurified Bioactive Extracts
Several studies found crude extracts with bioactivity that appeared to target iono-tropic glutamate receptors. For example, extracts of cultured bacteria associated with the marine sponge Halichondria panacea activate rat cortical NMDA receptors; the active principle(s) were not further isolated, however (Perovic et al. 1998). Garateix and colleagues carried out an ecologically-inspired search for iGluR lig-ands from marine organisms that prey on crustaceans, leading to the discovery of bioactive peptide-containing fractions from a sea anemone, Phyllactis flosculifera (Garateix et al. 1996). Peptide fractions of extracts from the animal diminished both the excitatory and the inhibitory responses to glutamate agonists in neurons of the land snail Zachrysia guanensisin. Similarly, a crude extract from the sea anemone Bunodosoma caissarum induced convulsions following intracerebro-ventricular (i.c.v.) injection in mice. The convulsion was suppressed by chlorokynurenic acid, an antagonist of the glycine site on the NMDA receptor (Gondran et al. 2002). Isolation and structures for these sea anemone products have not been reported to date.
8 Conclusion
Marine-derived compounds have played key roles in iGluR research. The recent discovery of the dysiherbaines and the con-Pr peptides, and the variety of bioactive extracts with unknown active principles, suggest that additional and novel molecules await the attention of neuroscience researchers. One of the central challenges for finding new molecules lies in the very early steps of characterizing bioactivity. While stereotypic seizure behavior and convulsions or sleep-inducing activity are obvious behavioral responses that lend insight into potential biological activity, extracts containing bioactive molecules that elicit less dramatic responses, but which have novel pharmacological profiles, could potentially be overlooked. It is clear, however, that synthetic modification of natural analogs can dramatically alter their pharmacological action (see, for example the DH analog MSVIII-19), and thus it is worthwhile to pursue even those active principles lacking obvious clinical application (such as convulsants). A revived appreciation of the potential utility of drugs from the sea and other natural sources is evident in the form of new initiatives, from funding bodies such as the US National Institutes of Health, which hope to spur development of higher-throughput identification and isolation of natural source compounds that could impact human health. It is likely that these efforts will produce molecules with new structures and specificities for iGluRs to supplement those described in this chapter.
Acknowledgments
We thank Professor Y. Kotaki (Kitasato University, Japan), Professor K. Koike (Hiroshima University, Japan), and Mr. K. Nomura for images of natural organisms and Dr. Anis Contractor (Northwestern University School of Medicine, US) for helpful comments on the text. G. T. Swanson is supported by a grant from the National Institutes for Health (R01 NS44322). R. Sakai was financially supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (15580183 and 17380125).
Contributor Information
Geoffrey T. Swanson, Email: gtswanson@northwestern.edu, Department of Molecular Pharmacology and Biological Chemistry, Northwestern University, Feinberg School of Medicine, 303 E. Chicago Ave., Chicago, IL, 60611
Ryuichi Sakai, Faculty of Fisheries Sciences, Hokkaido University, Hakodate 041-8611, Japan.
References
- Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–144. [PubMed] [Google Scholar]
- Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br J Pharmacol. 1986;87:345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt A, Weiss B, Ornstein PL, Gleason SD, Bleakman D, Stratford RE, Jr, Witkin JM. Anxiolytic-like effects through a GLU(K5) kainate receptor mechanism. Neuropharmacology. 2007;52:1482–1487. doi: 10.1016/j.neuropharm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel delta 2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- Ashworth TS, Brown EG, Roberts FM. Biosynthesis of willardiine and isowillardiine in germinating pea seeds and seedlings. Biochem J. 1972;129:897–905. doi: 10.1042/bj1290897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awobuluyi M, Yang J, Ye Y, Chatterton JE, Godzik A, Lipton SA, Zhang D. Subunit-specific roles of glycine-binding domains in activation of NR1/NR3 N-methyl-D-aspartate receptors. Mol Pharmacol. 2007;71:112–122. doi: 10.1124/mol.106.030700. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargu S, Powell CL, Coale SL, Busman M, Doucette GJ, Silver MW. Krill: a potential vector for domoic acid in marine food webs. Mar Ecol Prog Ser. 2002;237:209–216. [Google Scholar]
- Bargu S, Lefebvre K, Silver MW. Effect of dissolved domoic acid on the grazing rate of krill Euphausia pacifica. Mar Ecol Prog Ser. 2006;312:169–175. [Google Scholar]
- Barton ME, Peters SC, Shannon HE. Comparison of the effect of glutamate receptor modulators in the 6 Hz and maximal electroshock seizure models. Epilepsy Res. 2003;56:17–26. doi: 10.1016/j.eplepsyres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Barton ME, White HS, Wilcox KS. The effect of CGX-1007 and CI-1041, novel NMDA receptor antagonists, on NMDA receptor-mediated EPSCs. Epilepsy Res. 2004;59:13–24. doi: 10.1016/j.eplepsyres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Bates SS. Domoic-acid-producing diatoms: another genus added! J Phycol. 2000;36:978–983. [Google Scholar]
- Bates SS, Bird CJ, Defreitas ASW, Foxall R, Gilgan M, Hanic LA, Johnson GR, McCulloch AW, Odense P, Pocklington R, Quilliam MA, Sim PG, Smith JC, Rao DVS, Todd ECD, Walter JA, Wright JLC. Pennate diatom Nitzschia-Pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can J Fish Aquat Sci. 1989;46:1203–1215. [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, O’Shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bettler B, Egebjerg J, Sharma G, Pecht G, Hermans-Borgmeyer I, Moll C, Stevens CF, Heinemann S. Cloning of a putative glutamate receptor: a low affinity kainate-binding subunit. Neuron. 1992;8:257–265. doi: 10.1016/0896-6273(92)90292-l. [DOI] [PubMed] [Google Scholar]
- Bill BD, Cox FH, Horner RA, Borchert JA, Trainer VL. The first closure of shellfish harvesting due to domoic acid in Puget Sound, Washington, USA. Afr J Mar Sci. 2006;28:435–440. [Google Scholar]
- Blagbrough IS, Moya E, Taylor S. Polyamines and polyamine amides from wasps and spiders. Biochem Soc Trans. 1994;22:888–893. doi: 10.1042/bst0220888. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Bruce M, Bukownik R, Eldefrawi AT, Eldefrawi ME, Goodnow R, Jr, Kallimopoulos T, Konno K, Nakanishi K, Niwa M, Usherwood PN. Structure-activity relationships of analogues of the wasp toxin philanthotoxin: non-competitive antagonists of quisqualate receptors. Toxicon. 1990;28:1333–1346. doi: 10.1016/0041-0101(90)90098-r. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol (Lond) 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaf R, Barlatier A, Garçon D, Balansard G, Pellegrini M, Reynaud J. Isolation of an unknown kainic peptide from the red alga Alsidium helminthocorton. J Appl Phycol. 1989;1:257–266. [Google Scholar]
- Cantrell BE, Zimmerman DM, Monn JA, Kamboj RK, Hoo KH, Tizzano JP, Pullar IA, Farrell LN, Bleakman D. Synthesis of a series of aryl kainic acid analogs and evaluation in cells stably expressing the kainate receptor humGluR6. J Med Chem. 1996;39:3617–3624. doi: 10.1021/jm960155a. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Carpenter S, Zatorre RJ, Cashman NR. Temporal lobe epilepsy caused by domoic acid intoxication: evidence for glutamate receptor-mediated excitotoxicity in humans. Ann Neurol. 1995;37:123–126. doi: 10.1002/ana.410370125. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Costa S, Valerio E, Reggiani A. Domoic acid toxicity in rats and mice after intracerebroventricular administration: comparison with excitatory amino acid agonists. Pharmacol Toxicol. 1992;70:115–120. doi: 10.1111/j.1600-0773.1992.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Clark RB, Donaldson PL, Gration KA, Lambert JJ, Piek T, Ramsey R, Spanjer W, Usherwood PN. Block of locust muscle glutamate receptors by delta-philanthotoxin occurs after receptor activations. Brain Res. 1982;241:105–114. doi: 10.1016/0006-8993(82)91233-1. [DOI] [PubMed] [Google Scholar]
- Clark RF, Williams SR, Nordt SP, Manoguerra AS. A review of selected seafood poisonings. Undersea Hyperb Med. 1999;26:175–184. [PubMed] [Google Scholar]
- Clayden J, Read B, Hebditch KR. Chemistry of domoic acid, isodomoic acids, and their analogues. Tetrahedron. 2005;61:5713–5724. [Google Scholar]
- Cohen JL, Limon A, Miledi R, Chamberlin AR. Design, synthesis, and biological evaluation of a scaffold for iGluR ligands based on the structure of (−)-dysiherbaine. Bioorg Med Chem Lett. 2006;16:2189–2194. doi: 10.1016/j.bmcl.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O’Gorman S, Heinemann SF. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Heinemann SF. Kainate receptors are involved in short and long term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack CK, Bates SS, Quilliam MA, Patching JW, Raine R. Confirmation of domoic acid production by Pseudo-nitzschia australis (bacillariophyceae) isolated from Irish waters. J Phycol. 2002;38:1106–1112. [Google Scholar]
- D’Aniello A, Spinelli P, De Simone A, D’Aniello S, Branno M, Aniello F, Fisher GH, Di Fiore MM, Rastogi RK. Occurrence and neuroendocrine role of D-aspartic acid and N-methyl-D-aspartic acid in Ciona intestinalis. FEBS Lett. 2003;552:193–198. doi: 10.1016/s0014-5793(03)00921-9. [DOI] [PubMed] [Google Scholar]
- Davis SM, Lees KR, Albers GW, Diener HC, Markabi S, Karlsson G, Norris J. Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke. 2000;31:347–354. doi: 10.1161/01.str.31.2.347. [DOI] [PubMed] [Google Scholar]
- Debonnel G, Beauchesne L, de Montigny C. Domoic acid, the alleged “mussel toxin,” might produce its neurotoxic effect through kainate receptor activation: an electrophysiological study in the dorsal hippocampus. Can J Physiol Pharmacol. 1989;67:29–33. doi: 10.1139/y89-005. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Hume RI, Heinemann SF. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci. 1992;12:4080–4087. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–62. [PubMed] [Google Scholar]
- Donevan SD, McCabe RT. Conantokin G is an NR2B-selective competitive antagonist of N-methyl-D-aspartate receptors. Mol Pharmacol. 2000;58:614–623. doi: 10.1124/mol.58.3.614. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Clark S, Williams PA, Grabenstatter HL. Kainate-induced Status Epilepticus: a chronic model of acquired epilepsy. In: Pitkänen A, Schwartzkroin PA, Moshé SL, editors. Models of Seizures and Epilepsy. Burlington, MA: Elsevier Academic Press; 2006. [Google Scholar]
- Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991;351:745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Estrada G, Villegas E, Corzo G. Spider venoms: a rich source of acylpolyamines and peptides as new leads for CNS drugs. Nat Prod Rep. 2007;24:145–161. doi: 10.1039/b603083c. [DOI] [PubMed] [Google Scholar]
- Evans ML, Usherwood PN. The effect of lectins on desensitisation of locust muscle glutamate receptors. Brain Res. 1985;358:34–39. doi: 10.1016/0006-8993(85)90945-x. [DOI] [PubMed] [Google Scholar]
- Evans SV, Shing TKM, Aplin RT, Fellows LE, Fleet GWJ. Sulphate ester of trans-4-hydroxypipecolic acid in seeds of Peltophorum. Phytochemistry. 1985;24:2593–2596. [Google Scholar]
- Fehling J, Green DH, Davidson K, Bolch CJ, Bates SS. Domoic acid production by Pseudounitzschia seriata (bacillariophyceae) in Scottish waters. J Phycol. 2004;40:622–630. [Google Scholar]
- Fisahn A, Heinemann SF, McBain CJ. The kainate receptor subunit GluR6 mediates metabotropic regulation of the slow and medium AHP currents in mouse hippocampal neurones. J Physiol. 2005;562:199–203. doi: 10.1113/jphysiol.2004.077412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Ohliger-Frerking P. AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci. 2002;22:7434–7443. doi: 10.1523/JNEUROSCI.22-17-07434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA. Kainate receptors depress excitatory synaptic transmission at CA3- > CA1 synapses in the hippocampus via a direct presynaptic action. J Neurosci. 2001;21:2958–2966. doi: 10.1523/JNEUROSCI.21-09-02958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz L, Quilliam MA, Wright JLC, Beale AM, Work TM. An outbreak of domoic acid and poisoning attributed to the pennate diatom Pseudonitzschia australius. J Phycol. 1992;28:438–442. [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Garateix A, Flores A, Garcia-Andrade JM, Palmero A, Aneiros A, Vega R, Soto E. Antagonism of glutamate receptors by a chromatographic fraction from the exudate of the sea anemone Phyllactis flosculifera. Toxicon. 1996;34:443–450. doi: 10.1016/0041-0101(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gereau RW, Swanson GT, editors. The Glutamate Receptors. Totawa, NJ: Humana Press; 2008. [Google Scholar]
- Gmelin R. The free amino acids in the seeds of Acacia willardiana (Mimosaceae). Isolation of willardiin, a new plant amino acid which is probably L-beta-(3-uracil)-alpha-aminopropionic acid. Hoppe Seylers Z Physiol Chem. 1959;316:164–169. doi: 10.1515/bchm2.1959.316.1.164. [DOI] [PubMed] [Google Scholar]
- Goldstein T, Mazet JA, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FM. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc Biol Sci. 2008;275(1632):267–276. doi: 10.1098/rspb.2007.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondran M, Eckeli AL, Migues PV, Gabilan NH, Rodrigues AL. The crude extract from the sea anemone, Bunodosoma caissarum elicits convulsions in mice: possible involvement of the glutamatergic system. Toxicon. 2002;40:1667–1674. doi: 10.1016/s0041-0101(02)00181-2. [DOI] [PubMed] [Google Scholar]
- Greger IH, Esteban JA. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol. 2007;17:289–297. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Gross H, Goeger DE, Hills P, Mooberry SL, Ballantine DL, Murray TF, Valeriote FA, Gerwick WH. Lophocladines, bioactive alkaloids from the red alga Lophocladia sp. J Nat Prod. 2006;69:640–644. doi: 10.1021/np050519e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack JA, Rivier J, Parks TN, Mena EE, Cruz LJ, Olivera BM. Conantokin-T. A gamma-carboxyglutamate containing peptide with N-methyl-D-aspartate antagonist activity. J Biol Chem. 1990;265:6025–6029. [PubMed] [Google Scholar]
- Hammerland LG, Olivera BM, Yoshikami D. Conantokin-G selectively inhibits N-methyl-D-aspartate-induced currents in Xenopus oocytes injected with mouse brain mRNA. Eur J Pharmacol. 1992;226:239–244. doi: 10.1016/0922-4106(92)90067-6. [DOI] [PubMed] [Google Scholar]
- Hampson DR, Manalo JL. The activation of glutamate receptors by kainic acid and domoic acid. Nat Toxins. 1998;6:153–158. doi: 10.1002/(sici)1522-7189(199805/08)6:3/4<153::aid-nt16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Hampson DR, Huang XP, Wells JW, Walter JA, Wright JL. Interaction of domoic acid and several derivatives with kainic acid and AMPA binding sites in rat brain. Eur J Pharmacol. 1992;218:1–8. doi: 10.1016/0014-2999(92)90140-y. [DOI] [PubMed] [Google Scholar]
- Hardy RW, Scott TM, Hatfield CL, Barnett HJ, Gauglitz EJ, Wekell JC, Eklund MW. Domoic acid in rainbow trout (Oncorhynchus mykiss) feeds. Aquaculture. 1995;131:253–260. [Google Scholar]
- Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Hirano T. Cerebellar regulation mechanisms learned from studies on GluRdelta2. Mol Neurobiol. 2006;33:1–16. doi: 10.1385/MN:33:1:001. [DOI] [PubMed] [Google Scholar]
- Holland PT, Selwood AI, Mountfort DO, Wilkins AL, McNabb P, Rhodes LL, Doucette GJ, Mikulski CM, King KL. Isodomoic acid C, an unusual amnesic shellfish poisoning toxin from Pseudo-nitzschia australis. Chem Res Toxicol. 2005;18:814–816. doi: 10.1021/tx0496845. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Iino M, Koike M, Isa T, Ozawa S. Voltage-dependent blockage of Ca(2+) permeable AMPA receptors by joro spider toxin in cultured rat hippocampal neurones. J Physiol. 1996;496:431–437. doi: 10.1113/jphysiol.1996.sp021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impellizzeri G, Mangiafico S, Oriente G, Piattelli M, Sciuto S, Fattorusso E, Magno S, Santacroce C, Sica D. Amino acids and low-molecularweight carbohydrates of some marine red algae. Phytochemistry. 1975;14:1549–1557. [Google Scholar]
- Iverson F, Truelove J. Toxicology and seafood toxins: domoic acid. Nat Toxins. 1994;2:334–339. doi: 10.1002/nt.2620020514. [DOI] [PubMed] [Google Scholar]
- Jaskolski F, Coussen F, Mulle C. Subcellular localization and trafficking of kainate receptors. Trends Pharmacol Sci. 2005;26:20–26. doi: 10.1016/j.tips.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Jeffery B, Barlow T, Moizer K, Paul S, Boyle C. Amnesic shellfish poison. Food Chem Toxicol. 2004;42:545–557. doi: 10.1016/j.fct.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Jiang L, Xu J, Nedergaard M, Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron. 2001;30:503–513. doi: 10.1016/s0896-6273(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Jimenez EC, Donevan S, Walker C, Zhou LM, Nielsen J, Cruz LJ, Armstrong H, White HS, Olivera BM. Conantokin-L, a new NMDA receptor antagonist: determinants for anticonvulsant potency. Epilepsy Res. 2002;51:73–80. doi: 10.1016/s0920-1211(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Jin XT, Pare JF, Raju DV, Smith Y. Localization and function of pre- and postsynaptic kainate receptors in the rat globus pallidus. Eur J Neurosci. 2006;23:374–386. doi: 10.1111/j.1460-9568.2005.04574.x. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Johnston GA, Kennedy SM, Twitchin B. Action of the neurotoxin kainic acid on high affinity uptake of L-glutamic acid in rat brain slices. J Neurochem. 1979;32:121–127. doi: 10.1111/j.1471-4159.1979.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol (Lond) 1998;509:833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J Physiol (Lond) 2000;523:653–665. doi: 10.1111/j.1469-7793.2000.t01-1-00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Prorok M, Galdzicki Z, Castellino FJ. The amino acid residue at sequence position 5 in the conantokin peptides partially governs subunit-selective antagonism of recombinant N-methyl-D-aspartate receptors. J Biol Chem. 2001;276:26860–26867. doi: 10.1074/jbc.M102428200. [DOI] [PubMed] [Google Scholar]
- Konno K, Hashimoto K, Ohfune Y, Shirahama H, Matsumoto T. Acromelic acids A and B. Potent neuroexcitatory amino acids isolated from Clitocybe acromelalga. J Am Chem Soc. 1988;110:4807–4815. [Google Scholar]
- Kotaki Y, Koike K, Sato S, Ogata T, Fukuyo Y, Kodama M. Confirmation of domoic acid production of Pseudo-nitzschia multiseries isolated from Ofunato Bay, Japan. Toxicon. 1999;37:677–682. doi: 10.1016/s0041-0101(98)00210-4. [DOI] [PubMed] [Google Scholar]
- Kotaki Y, Koike K, Yoshida M, Van Thuoc C, Huyen NTM, Hoi NC, Fukuyo Y, Kodama M. Domoic acid production in Nitzschia sp (Bacillariophyceae) isolated from a shrimp-culture pond in Do Son, Vietnam. J Phycol. 2000;36:1057–1060. [Google Scholar]
- Kotaki Y, Lundholm N, Onodera H, Kobayashi K, Bajarias FFA, Furio EF, Iwataki M, Fukuyo Y, Kodama M. Wide distribution of Nitzschia navis-varingica, a new domoic acid-producing benthic diatom found in Vietnam. Fish Sci. 2004;70:28–32. [Google Scholar]
- Kung SS, Wu YM, Chow WY. Characterization of two fish glutamate receptor cDNA molecules: absence of RNA editing at the Q/R site. Brain Res Mol Brain Res. 1996;35:119–130. doi: 10.1016/0169-328x(95)00193-v. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Laube B, Betz H, Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron. 1994;12:1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Lash LL, Sanders JM, Akiyama N, Shoji M, Postila P, Pentikainen OT, Sasaki M, Sakai R, Swanson GT. Novel analogs and stereoisomers of the marine toxin neodysiherbaine with specificity for kainate receptors. J Pharmacol Exp Ther. 2007;324:484–496. doi: 10.1124/jpet.107.129890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- Laycock MV, de Freitas ASW, Wright JLC. Glutamate agonists from marine algae. J Appl Phycol. 1989;1:113–122. [Google Scholar]
- Layer RT, Wagstaff JD, White HS. Conantokins: peptide antagonists of NMDA receptors. Curr Med Chem. 2004;11:3073–3084. doi: 10.2174/0929867043363901. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Powell CL, Busman M, Doucette GJ, Moeller PD, Silver JB, Miller PE, Hughes MP, Singaram S, Silver MW, Tjeerdema RS. Detection of domoic acid in northern anchovies and California sea lions associated with an unusual mortality event. Nat Toxins. 1999;7:85–92. doi: 10.1002/(sici)1522-7189(199905/06)7:3<85::aid-nt39>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Dovel SL, Silver MW. Tissue distribution and neurotoxic effects of domoic acid in a prominent vector species, the northern anchovy Engraulis mordax. Marine Biology. 2001;138:693–700. [Google Scholar]
- Lefebvre KA, Bargu S, Kieckhefer T, Silver MW. From sanddabs to blue whales: the pervasiveness of domoic acid. Toxicon. 2002a;40:971–977. doi: 10.1016/s0041-0101(02)00093-4. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Silver MW, Coale SL, Tjeerdema RS. Domoic acid in planktivorous fish in relation to toxic Pseudo-nitzschia cell densities. Marine Biology. 2002b;140:625–631. [Google Scholar]
- Lefebvre KA, Noren DP, Schultz IR, Bogard SM, Wilson J, Eberhart BT. Uptake, tissue distribution and excretion of domoic acid after oral exposure in coho salmon (Oncorhynchus kisutch) Aquat Toxicol. 2007;81:266–274. doi: 10.1016/j.aquatox.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Hutchison AJ, McPherson SE, Mondadori C, Schmutz M, Sinton CM, Tsai C, Murphy DE, Steel DJ, Williams M, et al. CGS 19755, a selective and competitive N-methyl-D-aspartate-type excitatory amino acid receptor antagonist. J Pharmacol Exp Ther. 1988;246:65–75. [PubMed] [Google Scholar]
- Lerma J. Kainate receptor physiology. Curr Opin Pharmacol. 2006;6:89–97. doi: 10.1016/j.coph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Wisden W, Köhler M, Keinänen K, Sommer B, Seeburg PH. High-affinity kainate and domoate receptors in rat brain. FEBS Lett. 1992;307:139–143. doi: 10.1016/0014-5793(92)80753-4. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Laurie DJ, Kohr G, Herb A, Seeburg PH, Wisden W. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Lundholm N, Moestrup Ø. Morphology of the marine diatom Nitzschia navis-varingica, sp. Nov. (bacillariophyceae), another producer of the neurotoxin domoic acid. J Phycol. 2000;36:1162–1174. [Google Scholar]
- Lundholm N, Hansen PJ, Kotaki Y. Lack of allelopathic effects of the domoic acid-producing marine diatom Pseudo-nitzschia multiseries. Mar Ecol Prog Ser. 2005;288:21–33. [Google Scholar]
- Lygo B, Slack D, Wilson C. Synthesis of neodysiherbaine. Tetrahedron Lett. 2005;46:6629–6632. [Google Scholar]
- Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554–562. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Madden DR. The structure and function of glutamate receptor ion channels. Nat Rev Neurosci. 2002;3:91–101. doi: 10.1038/nrn725. [DOI] [PubMed] [Google Scholar]
- Maeda M, Kodama T, Tanaka T, Yoshizumi H, Takemoto T, Nomoto K, Fujita T. Structures Of isodomoic acid-A, acid-B And acid-C, novel insecticidal amino-acids from the red alga Chondria armata. Chem Pharm Bull. 1986;34:4892–4895. [Google Scholar]
- Maeda M, Kodama T, Tanaka T, Yoshizumi H, Takemoto T, Nomoto K, Fujita T. Structures of domoilactone A and B, novel amino acids from the red alga. Tetrahedron Lett. 1987;28:633–636. [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Gilbert H, McCabe RT, Basbaum AI. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain. 2003;101:109–116. doi: 10.1016/s0304-3959(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peckol E, Driscoll M, Bargmann CI. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- Masaki H, Maeyama J, Kamada K, Esumi T, Iwabuchi Y, Hatakeyama S. Total synthesis of (−)-dysiherbaine. J Am Chem Soc. 2000;122:5216–5217. [Google Scholar]
- Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying Kainate receptor selectivity. Neuron. 2005;45:539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Benveniste M, Patneau DK, Vyklicky L., Jr Pharmacologic properties of NMDA receptors. Ann N Y Acad Sci. 1992;648:194–204. doi: 10.1111/j.1749-6632.1992.tb24538.x. [DOI] [PubMed] [Google Scholar]
- McCormick J, Li Y, McCormick K, Duynstee HI, van Engen AK, van der Marel GA, Ganem B, van Boom JH, Meinwald J. Structure and total synthesis of HF-7, a neuroactive glyconucleoside disulfate from the funnel-web spider Hololena curta. J Am Chem Soc. 1999;121:5661–5665. [Google Scholar]
- McIntosh JM, Olivera BM, Cruz LJ, Gray WR. Gamma-carboxyglutamate in a neuroactive toxin. J Biol Chem. 1984;259:14343–14346. [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of Isahp and excitability in pyramidal cells. Neuron. 2002;34:107–114. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Lancaster B, Wheal HV. Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J Neurosci. 2004;24:4530–4534. doi: 10.1523/JNEUROSCI.5356-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena EE, Gullak MF, Pagnozzi MJ, Richter KE, Rivier J, Cruz LJ, Olivera BM. Conantokin-G: a novel peptide antagonist to the N-methyl-Daspartic acid (NMDA) receptor. Neurosci Lett. 1990;118:241–244. doi: 10.1016/0304-3940(90)90637-o. [DOI] [PubMed] [Google Scholar]
- Moloney MG. Excitatory amino acids. Nat Prod Rep. 1998;15:205–219. doi: 10.1039/a815205y. [DOI] [PubMed] [Google Scholar]
- Moloney MG. Excitatory amino acids. Nat Prod Rep. 1999;16:485–498. doi: 10.1039/a800247i. [DOI] [PubMed] [Google Scholar]
- Moloney MG. Excitatory amino acids. Nat Prod Rep. 2002;19:597–616. doi: 10.1039/b103777n. [DOI] [PubMed] [Google Scholar]
- Moroni F, Galli A, Mannaioni G, Carla V, Cozzi A, Mori F, Marinozzi M, Pellicciari R. NMDA receptor heterogeneity in mammalian tissues: focus on two agonists, (2S,3R,4S) cyclo-propylglutamate and the sulfate ester of 4-hydroxy-(S)-pipecolic acid. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:371–376. doi: 10.1007/BF00169077. [DOI] [PubMed] [Google Scholar]
- Mos L. Domoic acid: a fascinating marine toxin. Environ Toxicol Pharmacol. 2001;9:79–85. doi: 10.1016/s1382-6689(00)00065-x. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Pérez-Otaño I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Swanson GT, Brana C, O’Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron. 2000;28:475–484. doi: 10.1016/s0896-6273(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Murakami S, Takemoto T, Shimizu Z. Studies on the effective principles of Digenea simplex Aq. I. Separation of the effective fraction by liquid chromatography. J Pharm Soc Jpn. 1953;73:1026–1028. [Google Scholar]
- Nadler JV. Kainic acid: neurophysiological and neurotoxic actions. Life Sci. 1979;24:289–299. doi: 10.1016/0024-3205(79)90325-4. [DOI] [PubMed] [Google Scholar]
- Nadler JV. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- Naur P, Vestergaard B, Skov LK, Egebjerg J, Gajhede M, Kastrup JS. Crystal structure of the kainate receptor GluR5 ligand-binding core in complex with (S)-glutamate. FEBS Lett. 2005;579:1154–1160. doi: 10.1016/j.febslet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, Olsen L, Vestergaard B, Egebjerg J, Gajhede M, Traynelis SF, Kastrup JS. Ionotropic glutamate-like receptor delta2 binds D-serine and glycine. Proc Natl Acad Sci USA. 2007;104:14116–14121. doi: 10.1073/pnas.0703718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Copenhagen DR. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987;325:56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Nitta I, Watase H, Tomiie Y. Structure of kainic acid and its isomer, allokainic acid. Nature. 1958;181:761–762. doi: 10.1038/181761b0. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olivera BM. Conus peptides: biodiversity-based discovery and exogenomics. J Biol Chem. 2006;281:31173–31177. doi: 10.1074/jbc.R600020200. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitotoxins in foods. Neurotoxicology. 1994;15:535–544. [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Vicente A, Nielsen EO, Lerma J. Comparative antagonism of kainate-activated kainate and AMPA receptors in hippocampal neurons. Eur J Neurosci. 1996;8:2129–2136. doi: 10.1111/j.1460-9568.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- Pei-Gen X, Shan-Lin F. Traditional antiparasitic drugs in China. Parasitol Today. 1986;2:353–355. doi: 10.1016/0169-4758(86)90057-8. [DOI] [PubMed] [Google Scholar]
- Perez-Otaño I, Ehlers MD. Learning from NMDA receptor trafficking: clues to the development and maturation of glutamatergic synapses. Neurosignals. 2004;13:175–189. doi: 10.1159/000077524. [DOI] [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, McNutt LA, Remis RS. Amnesic shellfish poisoning: a new clinical syndrome due to domoic acid. Can Dis Wkly Rep. 1990a;16 (Suppl 1E):7–8. [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990b;322:1775–1780. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- Perovic S, Wickles A, Schutt C, Gerdts G, Pahler S, Steffen R, Muller WEG. Neuroactive compounds produced by bacteria from the marine sponge Halichondria panicea: activation of the neuronal NMDA receptor. Environ Toxicol Pharmacol. 1998;6:125–133. doi: 10.1016/s1382-6689(98)00028-3. [DOI] [PubMed] [Google Scholar]
- Phillips D, Chamberlin AR. Total synthesis of dysiherbaine. J Org Chem. 2002;67:3194–3201. doi: 10.1021/jo0107610. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells-Cases R, Lerma J, Ferrer-Montiel A. Pharmacological intervention at ionotropic glutamate receptor complexes. Curr Pharm Des. 2006;12:3583–3596. doi: 10.2174/138161206778522092. [DOI] [PubMed] [Google Scholar]
- Prorok M, Castellino FJ. The molecular basis of conantokin antagonism of NMDA receptor function. Curr Drug Targets. 2007;8:633–642. doi: 10.2174/138945007780618481. [DOI] [PubMed] [Google Scholar]
- Ramsey UP, Bird CJ, Shacklock PF, Laycock MV, Wright JLC. Kainic acid and 1′-hydroxykainic acid from Palmariales. Nat Toxins. 1994;2:286–292. doi: 10.1002/nt.2620020507. [DOI] [PubMed] [Google Scholar]
- Ratte S, Lacaille JC. Selective degeneration and synaptic reorganization of hippocampal interneurons in a chronic model of temporal lobe epilepsy. Adv Neurol. 2006;97:69–76. [PubMed] [Google Scholar]
- Ren Z, Riley NJ, Garcia EP, Sanders JM, Swanson GT, Marshall J. Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J Neurosci. 2003;23:6608–6616. doi: 10.1523/JNEUROSCI.23-16-06608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Sakai R, Kamiya H, Murata M, Shimamoto K. Dysiherbaine: a new neurotoxic amino acid from the Micronesian marine sponge Dysidea herbacea. J Am Chem Soc. 1997;119:4112–4116. [Google Scholar]
- Sakai R, Koike T, Sasaki M, Shimamoto K, Oiwa C, Yano A, Suzuki K, Tachibana K, Kamiya H. Isolation, structure determination, and synthesis of neodysiherbaine A, a new excitatory amino acid from a marine sponge. Org Lett. 2001a;3:1479–1482. doi: 10.1021/ol015798l. [DOI] [PubMed] [Google Scholar]
- Sakai R, Swanson GT, Shimamoto K, Green T, Contractor A, Ghetti A, Tamura-Horikawa Y, Oiwa C, Kamiya H. Pharmacological properties of the potent epileptogenic amino acid dysiherbaine, a novel glutamate receptor agonist isolated from the marine sponge Dysidea herbacea. J Pharmacol Exp Ther. 2001b;296:650–658. [PubMed] [Google Scholar]
- Sakai R, Matsubara H, Shimamoto K, Jimbo M, Kamiya H, Namikoshi M. Isolations of N-methyl-D-aspartic acid-type glutamate receptor ligands from Micronesian sponges. J Nat Prod. 2003;66:784–787. doi: 10.1021/np020590+. [DOI] [PubMed] [Google Scholar]
- Sakai R, Suzuki K, Shimamoto K, Kamiya H. Novel betaines from a Micronesian sponge Dysidea herbacea. J Org Chem. 2004;69:1180–1185. doi: 10.1021/jo0355045. [DOI] [PubMed] [Google Scholar]
- Sakai R, Minato S, Koike K, Koike K, Jimbo M, Kamiya H. Cellular and subcellular localization of kainic acid in the marine red alga Digenea simplex. Cell Tissue Res. 2005;322:491–502. doi: 10.1007/s00441-005-0035-x. [DOI] [PubMed] [Google Scholar]
- Sakai R, Swanson GT, Sasaki M, Shimamoto K, Kamiya H. Dysiherbaine: a new generation of excitatory amino acids of marine origin. CNS Agents Med Chem. 2006;6:83–108. [Google Scholar]
- Sakai R, Yoshida K, Kimura A, Koike K, Jimbo M, Koike K, Kobiyama A, Kamiya H. Cellular origin of dysiherbaine, a marine sponge-derived excitatory amino acid. ChemBioChem. 2008;9(4):543–551. doi: 10.1002/cbic.200700498. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Ito K, Settimo L, Pentikainen OT, Shoji M, Sasaki M, Johnson MS, Sakai R, Swanson GT. Divergent pharmacological activity of novel marine-derived excitatory amino acids on glutamate receptors. J Pharmacol Exp Ther. 2005;314:1068–1078. doi: 10.1124/jpet.105.086389. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Pentikainen OT, Settimo L, Pentikainen U, Shoji M, Sasaki M, Sakai R, Johnson MS, Swanson GT. Determination of binding site residues responsible for the subunit selectivity of novel marine-derived compounds on kainate receptors. Mol Pharmacol. 2006;69:1849–1860. doi: 10.1124/mol.106.022772. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Maruyama T, Sakai R, Tachibana K. Synthesis and biological activity of dysiherbaine model compound. Tetrahedron Lett. 1999;40:3195–3198. [Google Scholar]
- Sasaki M, Koike T, Sakai R, Tachibana K. Total synthesis of (−)-dysiherbaine, a novel neuroexcitotoxic amino acid. Tetrahedron Lett. 2000;41:3923–3926. [Google Scholar]
- Sasaki M, Tsubone K, Shoji M, Oikawa M, Shimamoto K, Sakai R. Design, total synthesis, and biological evaluation of neodysiherbaine A derivative as potential probes. Bioorg Med Chem Lett. 2006;16:5784–5787. doi: 10.1016/j.bmcl.2006.08.082. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Akiyama N, Tsubone K, Shoji M, Oikawa M, Sakai R. Total synthesis of dysiherbaine. Tetrahedron Lett. 2007;48:5697–5700. [Google Scholar]
- Sato M, Inoue F, Kanno N, Sato Y. The occurrence of N-methyl-Daspartic acid in muscle extracts of the blood shell, Scapharca broughtonii. Biochem J. 1987;241:309–311. doi: 10.1042/bj2410309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Nakano T, Takeuchi M, Kanno N, Nagahisa E, Sato Y. Distribution of neuroexcitatory amino acids in marine algae. Phytochemistry. 1996;42:1595–1597. [Google Scholar]
- Sawant PM, Weare BA, Holland PT, Selwood AI, King KL, Mikulski CM, Doucette GJ, Mountfort DO, Kerr DS. Isodomoic acids A and C exhibit low KA receptor affinity and reduced in vitro potency relative to domoic acid in region CA1 of rat hippocampus. Toxicon. 2007;50:627–638. doi: 10.1016/j.toxicon.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxyterminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Breustedt J, Nicoll RA. Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nat Neurosci. 2003;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R, III, Miller PE, McLellan WA, Moeller PDR, Powell CL, Rowles T, Silvagni P, Silver M, Spraker T, Trainer V, Van Dolah FM. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403:80–84. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Ultsch A, Schloss P, Cox JA, Schmitt B, Betz H. Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle. Science. 1991;254:112–114. doi: 10.1126/science.1681587. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shin-ya K, Kim J-S, Furihata K, Hayakawa Y, Seto H. Structure of kaitocephalin, a novel glutamate receptor antagonist produced by Eupenicillium shearii. Tetrahedron Lett. 1997a;38:7079–7082. [Google Scholar]
- Shin-ya K, Kim JS, Hayakawa Y, Seto H. Protective effect of a novel AMPA and NMDA antagonist kaitocephalin against glutamate neurotoxicity. J Neurochem. 1997b;73:S190. [Google Scholar]
- Shinozaki H. Pharmacology of the glutamate receptor. Prog Neurobiol. 1988;30:399–435. doi: 10.1016/0301-0082(88)90009-3. [DOI] [PubMed] [Google Scholar]
- Shoji M, Akiyama N, Tsubone K, Lash LL, Sanders JM, Swanson GT, Sakai R, Shimamoto K, Oikawa M, Sasaki M. Total synthesis and biological evaluation of neodysiherbaine A and analogues. J Org Chem. 2006;71:5208–5220. doi: 10.1021/jo0605593. [DOI] [PubMed] [Google Scholar]
- Sierra Beltran A, Palafox-Uribe M, Grajales-Montiel J, Cruz-Villacorta A, Ochoa JL. Sea bird mortality at Cabo San Lucas, Mexico: evidence that toxic diatom blooms are spreading. Toxicon. 1997;35:447–453. doi: 10.1016/s0041-0101(96)00140-7. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Li DL, Hoo KH, Deverill M, Ornstein PL, Iyengar S. Kainate GluR5 receptor subtype mediates the nociceptive response to formalin in the rat. Neuropharmacology. 1998;37:25–36. doi: 10.1016/s0028-3908(97)00188-3. [DOI] [PubMed] [Google Scholar]
- Smolders I, Bortolotto ZA, Clarke VR, Warre R, Khan GM, O’Neill MJ, Ornstein PL, Bleakman D, Ogden A, Weiss B, Stables JP, Ho KH, Ebinger G, Collingridge GL, Lodge D, Michotte Y. Antagonists of GLU(K5) containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat Neurosci. 2002;5:796–804. doi: 10.1038/nn880. [DOI] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-Daspartate NR1 and NR3 subunits. J Pharmacol Exp Ther. 2007;322:739–748. doi: 10.1124/jpet.107.123836. [DOI] [PubMed] [Google Scholar]
- Snider BB, Hawryluk NA. Synthesis of (−)-dysiherbaine. Org Lett. 2000;2:635–638. doi: 10.1021/ol991393d. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Sommer B, Köhler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinänen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G. Kainic acid seizures in the rat. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y, Bettler B, Hartley M, Sheppard PO, O’Hara PJ, Heinemann SF. Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron. 1994;13:1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Gereau RW, IV, Green T, Heinemann SF. Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron. 1997;19:913–926. doi: 10.1016/s0896-6273(00)80972-1. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Green T, Sakai R, Contractor A, Che W, Kamiya H, Heinemann SF. Differential activation of individual subunits in heteromeric kainate receptors. Neuron. 2002;34:589–598. doi: 10.1016/s0896-6273(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Matsumura T, Corbin GRM, Ishihara J, Hatakeyama S. A highly stereocon-trolled total synthesis of (−)-neodysiherbaine A. J Org Chem. 2006;71:4227–4231. doi: 10.1021/jo060410r. [DOI] [PubMed] [Google Scholar]
- Takemoto T. Isolation and structural identification of naturally occurring excitatory amino acids. In: McGreer EG, editor. Kainic Acid as a Tool in Neurobiology. New York: Raven Press; 1978. pp. 1–15. [Google Scholar]
- Takemoto T, Daigo K. Constituents of Chondria armata. Chem Pharm Bull. 1958;6:578–580. doi: 10.1002/ardp.19602930608. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Nakajima T, Sakuma R. Isolation of a flycidal constituent “Ibotenic Acid” from Amanita muscaria and A. pantherina. Yakugaku Zasshi. 1964;84:1233–1234. [PubMed] [Google Scholar]
- Takemoto T, Nakajima T, Arihara S, Koike K. Studies on the constituents of Quisqualis Fructus. II. Structure of quisqualic acid. Yakugaku Zasshi. 1975;95:326–332. doi: 10.1248/yakushi1947.95.3_326. [DOI] [PubMed] [Google Scholar]
- Teichert RW, Jimenez EC, Twede V, Watkins M, Hollmann M, Bulaj G, Olivera BM. Novel conantokins from conus parius venom are specific antagonists of NMDA receptors. J Biol Chem. 2007;282(51):36905–36913. doi: 10.1074/jbc.M706611200. [DOI] [PubMed] [Google Scholar]
- Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Engl J Med. 1990;322:1781–1787. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS, Ramsdell AF. Developmental toxicity of domoic acid in zebrafish (Danio rerio) Neurotoxicol Teratol. 2005;27:711–717. doi: 10.1016/j.ntt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Trainer VL, Adams NG, Bill BD, Anulacion BF, Wekell JC. Concentration and dispersal of a Pseudo-nitzschia bloom in Penn Cove, Washington, USA. Nat Toxins. 1998;6:113–126. doi: 10.1002/(sici)1522-7189(199805/08)6:3/4<113::aid-nt14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Tremblay J-F. Shortage of kainic acid hampers neuroscience research. Chem Eng News. 2000;78:14–15. [Google Scholar]
- Tsai C, Schneider JA, Lehmann J. Trans-2-carboxy-3-pyrrolidineacetic acid (CPAA), a novel agonist at NMDA-type receptors. Neurosci Lett. 1988;92:298–302. doi: 10.1016/0304-3940(88)90606-4. [DOI] [PubMed] [Google Scholar]
- Usherwood PN. Natural and synthetic polyamines: modulators of signalling proteins. Farmaco. 2000;55:202–205. doi: 10.1016/s0014-827x(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Dingledine R. Block of alpha-amino-3-hydroxy-5-methyl4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther. 1996;278:669–678. [PubMed] [Google Scholar]
- Watanabe H, Kitahara T. Revision of the stereochemistries of natural products through the synthetic study: Synthesis of fudecalone and kaitocephalin. J Synth Org Chem Jpn. 2007;65:511–519. [Google Scholar]
- Watters MR. Organic neurotoxins in seafoods. Clin Neurol Neurosurg. 1995;97:119–124. doi: 10.1016/0303-8467(95)00015-c. [DOI] [PubMed] [Google Scholar]
- Wekell JC, Gauglitz EJ, Jr, Barnett HJ, Hatfield CL, Simons D, Ayres D. Occurrence of domoic acid in Washington State razor clams (Siliqua patula) during 1991–1993. Nat Toxins. 1994;2:197–205. doi: 10.1002/nt.2620020408. [DOI] [PubMed] [Google Scholar]
- Werner P, Voigt M, Keinänen K, Wisden W, Seeburg PH. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991;351:742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- White HS, McCabe RT, Armstrong H, Donevan SD, Cruz LJ, Abogadie FC, Torres J, Rivier JE, Paarmann I, Hollmann M, Olivera BM. In vitro and in vivo characterization of conan-tokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiatus. J Pharmacol Exp Ther. 2000;292:425–432. [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Dave JR, Phillips JB, Lin Y, McCabe RT, Tortella FC. Neuroprotective efficacy and therapeutic window of the high-affinity N-methyl-D-aspartate antagonist conantokin-G: in vitro (primary cerebellar neurons) and in vivo (rat model of transient focal brain ischemia) studies. J Pharmacol Exp Ther. 2000;294:378–386. [PubMed] [Google Scholar]
- Williams AJ, Ling G, Berti R, Moffett JR, Yao C, Lu XM, Dave JR, Tortella FC. Treatment with the snail peptide CGX-1007 reduces DNA damage and alters gene expression of c-fos and bcl-2 following focal ischemic brain injury in rats. Exp Brain Res. 2003;153:16–26. doi: 10.1007/s00221-003-1566-6. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekindt B, Malany S, Schemm R, Otvos L, Maccecchini ML, Laube B, Betz H. Point mutations identify the glutamate binding pocket of the N-methyl-D-aspartate receptor as major site of conantokin-G inhibition. Neuropharmacology. 2001;41:753–761. doi: 10.1016/s0028-3908(01)00112-5. [DOI] [PubMed] [Google Scholar]
- Work TM, Barr BB, Beale AM, Fritz L, Quilliam MA, Wright JLC. Epidemiology of domoic acid poisoning in brown pelicans (Pelicanus occidentalis) and Brandt’s cormorants (Phalacrocorax pencillatus) in California. J Zool Wildlife Med. 1993;24:54–62. [Google Scholar]
- Wright JLC, Boyd RK, Defreitas ASW, Falk M, Foxall RA, Jamieson WD, Laycock MV, McCulloch AW, McInnes AG, Odense P, Pathak V, Quilliam MA, Ragan M, Sim PG, Thibault P, Walter JA, Gilgan M, Richard DJA, Dewar D. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can J Chem. 1989;67:481–490. [Google Scholar]
- Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- Yuzaki M. The delta2 glutamate receptor: a key molecule controlling synaptic plasticity and structure in Purkinje cells. Cerebellum. 2004;3:89–93. doi: 10.1080/14734220410028921. [DOI] [PubMed] [Google Scholar]
- Zaman L, Arakawa O, Shimosu A, Onoue Y, Nishio S, Shida Y, Noguchi T. Two new isomers of domoic acid from a red alga, Chondria armata. Toxicon. 1997;35:205–212. doi: 10.1016/s0041-0101(96)00123-7. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]