Abstract
Background
Studies of visual backward masking have frequently revealed an elevated masking threshold in schizophrenia. This finding has frequently been interpreted as indicating a low-level visual deficit. However, more recent models suggest that masking may also involve late and higher-level integrative processes, while leaving intact early “bottom-up” visual processing.
Objectives
We tested the hypothesis that the backward masking deficit in schizophrenia corresponds to a deficit in the late stages of conscious perception, whereas the subliminal processing of masked stimuli is fully preserved.
Method
28 patients with schizophrenia and 28 normal controls performed two backward-masking experiments. We used Arabic digits as stimuli and varied quasi-continuously the interval with a subsequent mask, thus allowing us to progressively “unmask” the stimuli. We finely quantified their degree of visibility using both objective and subjective measures to evaluate the threshold duration for access to consciousness. We also studied the priming effect caused by the variably masked numbers on a comparison task performed on a subsequently presented and highly visible target number.
Results
The threshold delay between digit and mask necessary for the conscious perception of the masked stimulus was longer in patients compared to control subjects. This higher consciousness threshold in patients was confirmed by an objective and a subjective measure, and both measures were highly correlated for patients as well as for controls. However, subliminal priming of masked numbers was effective and identical in patients compared to controls.
Conclusions
Access to conscious report of masked stimuli is impaired in schizophrenia, while fast bottom-up processing of the same stimuli, as assessed by subliminal priming, is preserved. These findings suggest a high-level origin of the masking deficit in schizophrenia, although they leave open for further research its exact relation to previously identified bottom-up visual processing abnormalities.
Keywords: Adolescent, Adult, Comorbidity, Consciousness Disorders, diagnosis, epidemiology, psychology, Female, Form Perception, physiology, Humans, Male, Middle Aged, Neuropsychological Tests, statistics & numerical data, Perceptual Masking, physiology, Psychomotor Performance, physiology, Schizophrenia, diagnosis, epidemiology, Schizophrenic Psychology, Sensory Thresholds, physiology, Subliminal Stimulation
Introduction
A functional breakdown of large-scale cortical integrative processes, caused by abnormal cortico-cortical and cortico-subcortical long-range connectivity is postulated in schizophrenia [1–14]. A crucial issue concerns whether, in addition to this deficit at the level of cognitive integration, patients with schizophrenia also suffer from other, possibly unrelated deficits of a lower and more modular nature [15–22]. Indeed, some experimental results arising from studies of visual backward masking have suggested a low-level visual deficit. In visual backward masking, the visibility of a briefly presented stimulus is reduced by a mask presented very shortly after the stimulus [23, 24]. Patients with schizophrenia consistently show a deficit in the perception of backward-masked stimuli: compared with healthy controls, they require a longer interval between stimulus and mask in order to identify the stimulus. [25–27]
Breitmeyer and Ganz have proposed a model where masking depends on the interactions between transient (magnocellular) and sustained (parvocellular) channels within the early visual pathways. In that model, backward masking would occur when the transient channels of the mask interfere with the sustained channels of the stimulus and therefore interrupt the formation of the percept. [23, 24]. Abnormal masking in schizophrenia would be linked to deficits in transient magnocellular channels [25, 28–32]. An additional deficit in sustained channels (causing abnormal gamma range activity) has also been proposed [33, 34].
More recently, however, new models of masking have appeared, according to which this phenomenon may also involve late and higher-level integrative processes [35–43]. Enns and Di Lollo, for instance, suggest that some forms of masking may be caused by a disruption in the integration between bottom-up inputs and a top-down attentional signal [44, 45]. Similarly, the global neuronal workspace model of conscious perception [39–41, 46] postulates that conscious access is associated with recurrent interactions between distant brain areas. Top-down feedback from prefrontal and parietal areas to lower-level sensory regions would establish a self-amplified reverberant neuronal assembly associated with conscious reportability. During masking, a stimulus would fail to reach consciousness if the mask replaces the stimulus before this recurrent activity has become stable. Simulations show that this process is stochastic and may fail due to random fluctuations in prestimulus spontaneous activity. The model therefore predicts an all-or-none, bimodal distribution of subjective visibility scores and of event-related brain activation measures, which was recently observed experimentally during the attentional blink. [40, 47, 48]
The global workspace model, like most current models of masking, suggests that the initial feedforward processing of masked stimuli can be largely intact, in spite of their reduced subjective visibility. This hypothesis is largely confirmed by studies of subliminal priming. In those studies, a masked shape called the “prime” is shown to influence the processing of a subsequent target stimulus. Behavioral and neuroimaging studies of subliminal priming suggest that processing of stimuli made subjectively invisible by masking is extensive and can include visual recognition, but also lexical and even semantic levels. [49–52] [53]. In particular, Dehaene et al demonstrated a non-conscious motor conflict induced by masked primes during a number comparison task. [54] Most relevant to present purposes, they showed that patients with schizophrenia showed a normal masked priming effect, but became disproportionately slower and showed an absence of conflict, relative to controls, when the primes were unmasked [55]. These data, which suggested intact low-level visual processes and abnormal higher-level executive control in schizophrenia, were related to a major hypoactivation of prefrontal and anterior cingulate cortices. Dehaene et al. tentatively suggested that the lack of top-down amplification from these areas might jointly explain both the higher-level cognitive deficits and the change in visual masking threshold in patients with schizophrenia.
The main purpose of the present work is to provide a further test of the hypothesis that the backward masking deficit in schizophrenia corresponds to a deficit in the late stages of conscious perception. Our aim was to evaluated whether the bottom-up subliminal processing of masked stimuli is preserved by studying whether normal subliminal priming could be observed in schizophrenia. We used a new form of masking, with a component of spatial attention, inspired by Vorberg et al. [56] and Di Lollo et al. [44, 45], where the stimulus and mask shapes are not overlapping. Using Arabic digits as stimuli, we varied quasi-continuously the interval between a digit and the subsequent mask, thus allowing us to progressively “unmask” the stimulus. This manipulation allowed us to study both the subliminal priming effect caused by these variably masked numbers, as well as their degree of visibility, and to precisely quantify the threshold duration for access to consciousness. Based on the global neuronal workspace model of conscious access, the following predictions were made. 1) Masking should affect conscious visibility in an “all or none” manner: either the stimulus is fully consciously perceived or it is not perceived at all. 2) The threshold for conscious access should be higher in patients than in controls, i.e. a longer interval between stimulus and mask should be necessary for them to identify the stimulus. 3) The subliminal processing of non-consciously perceived stimuli, measured by their priming effect, should be preserved and identical in the two groups. Thus, we expected response times to be faster when the prime provided some valid information about the target, either about its exact identity (repetition priming) or about the nature of the forthcoming response (response priming). The preservation of both priming effects would constitute strong evidence in favour of preserved fast bottom-up perceptual processing in schizophrenia.
Method
Participants
Twenty eight patients with schizophrenia (mean age, 34yr, range 18–53, 9 women and 19 men) participated in the study. All were native French speakers. Patients met DSM-IV criteria for schizophrenia and were recruited from the psychiatric department of Creteil University Hospital (Assistance Publique, Hôpitaux de Paris). They had a chronic course and were stable at the time of the experiment. 22 patients were medicated by atypical antipsychotics and 6 with typical antipsychotics. This treatment had been unchanged for at least three weeks. The comparison group consisted of twenty eight subjects (mean age, 32yr, range 18–55, 18 men and 10 women). Comparison subjects were excluded for history of any psychotic disorder, bipolar disorder, schizotypal or paranoid personality disorder, recurrent depression. Patients and controls with a history of brain injury, epilepsy, alcohol or substance abuse, or other neurological or ophthalmologic disorders were excluded. Patients and controls did not differ significantly in sex, age and level of education.
All experiments were approved by the French regional ethical committee for biomedical research (Hôpital de la Pitié Salpétrière), and subjects gave written informed consent.
Stimuli
In our material, a first number, that we will call the “prime”, was masked by a shape containing in its structure a second number, the “target” (see figure 1). We varied quasi-continuously the interval between the prime and the subsequent mask, thus allowing us to progressively unmask the stimulus digit. The delay between the onset of the prime and the onset of the mask could take one out of eight values (0, 16, 33, 50, 66, 83, 100 and 150 ms). For the delay of 0 ms, the prime and the mask had the same onset, but the mask persisted after the prime had disappeared.
Figure 1. Experiment design.
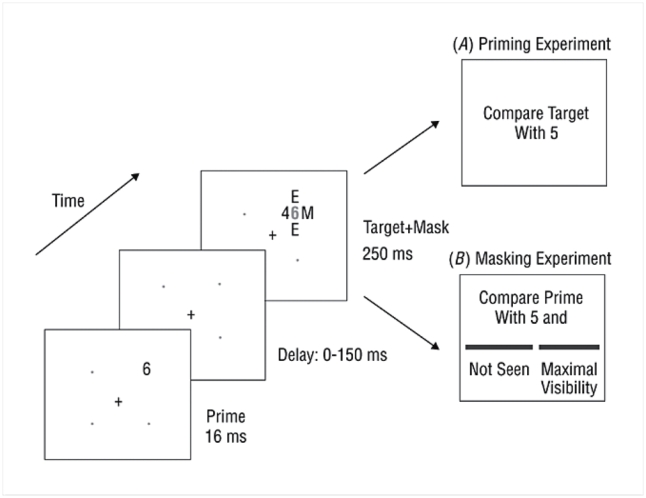
The prime was presented for 16 ms at one of four positions (1.4 degrees above or below and 1.4 degrees right or left to the fixation cross). The mask (duration of presentation 250ms) was composed of three letters (M, M, E) and the target number (1 ° from the fixation cross). Those four symbols surrounded the prime number without touching it.
In the first experiment, referred to as the “priming experiment”, subjects were asked to compare each target number with 5, pressing the right-hand key as fast as possible for numbers larger than 5 and the left hand key for numbers smaller than 5.
The second experiment aimed at measuring the consciousness threshold in two different ways. We measured an objective visibility threshold by examining subjects’ ability to perform the number comparison task on the prime. We also measured a subjective threshold by collecting introspective ratings of prime visibility, on a subjective continuous scale.
The stimulus set consisted of 16 pairs of prime and target numbers, consisting in all pairs of the numbers 1, 4, 6 and 9 written in Arabic format. As a consequence, the following factors could be analyzed: target distance (close or far from 5), target size (larger or smaller than 5), response congruity (whether or not the prime and target fell on the same side of 5), and repetition (within the congruent trials, whether or not the prime and target were the same number).
Procedure
One part of the experiment was dedicated to a measurement of both objective and subjective thresholds. We measured an objective visibility threshold by examining subjects’ ability to perform a number comparison task on the prime. We also measured a subjective threshold by collecting introspective ratings of prime visibility, on a subjective continuous scale, identical to the one used in previous studies of the attentional blink (see refs. [40, 47, 57] for detailed instructions). The experiment consisted in 20 training trials followed by 20 trials for each delay, for a total 180 randomly presented trials.
Another part evaluated subliminal and supraliminal priming. Subjects were asked to compare each target number with 5, pressing the right-hand key as fast as possible for numbers larger than 5 and the left hand key for numbers smaller than 5. This experiment consisted in 20 training trials followed by 320 experimental trials (8 blocks of 40 trials, one block for each delay). The different delays were presented in random order but in different blocks in order to facilitate the subject’s task. It was felt that, if the delays had been randomly mixed, it would have been too difficult for patients to avoid responding to the prime on conscious trials. Blocking helped them to learn to focus on the target and neglect the prime, regardless of its visibility. For similar reasons, the priming experiment, which was the most difficult, was always run prior to threshold measurement.
Results
Measuring the threshold for access to consciousness
Figure 2 shows the distributions of visibility scores in each group and for each delay on prime-present trials. In both groups, we observed a bimodal repartition of scores, with a first set of responses close to maximal visibility (scale score > 75%) and a second set of responses peaking at zero visibility (score < 25%). Responses between 25% and 75% were rare (< 10%). Thus, in both groups, increasing delays did not lead to a progressive increase in subjective experience of prime visibility, but to a shift in the probability of reporting one of two discrete subjective states (“seen” or “not seen”).
Figure 2. Distribution of subjective visibility ratings.
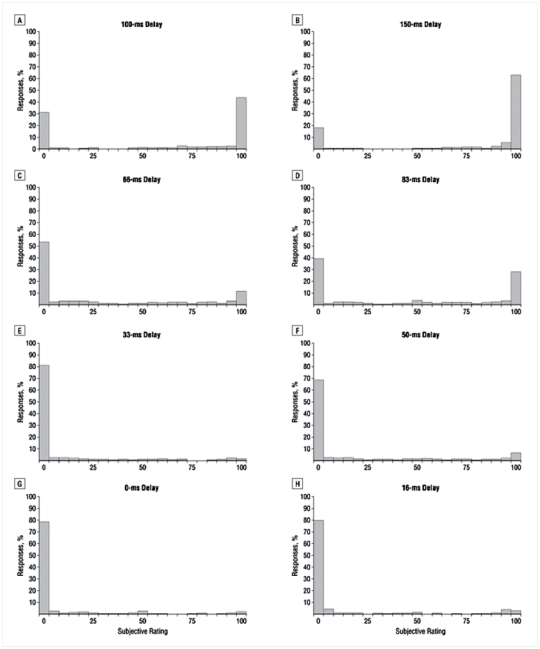
In both groups, we observed a bimodal repartition of scores, with a first set of responses close to maximal visibility (scale score > 16) and a second set of responses peaking at zero visibility (score < 6). Responses between 6 and 16 were rare (< 10%). More not seen responses were observed in patients than in controls, particularly at short delays.
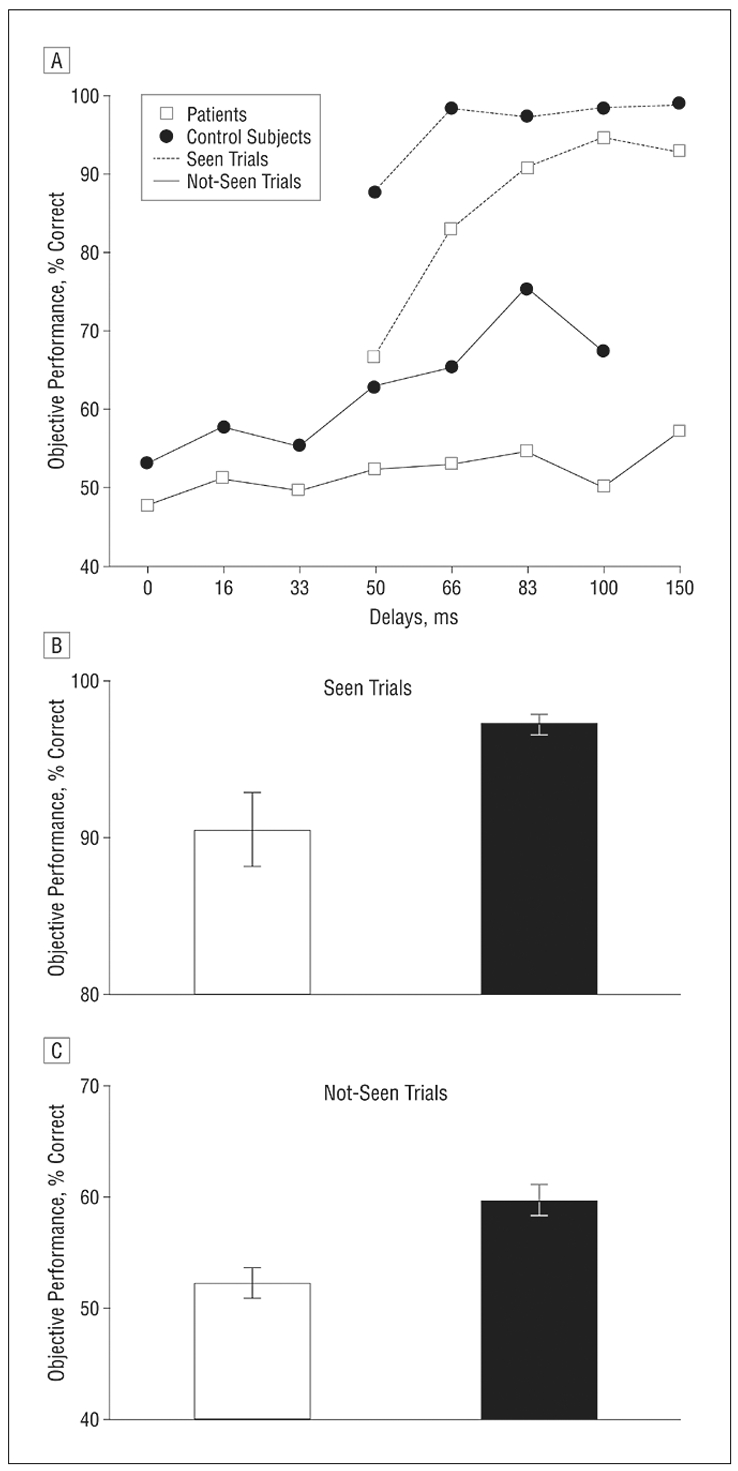
Based on this bimodal distribution, we arbitrarily defined “seen” trials as those whose visibility score was above the middle of the scale (>50%). Figure 3b shows the proportion of “seen” trials as a function of delay. The proportion increased steadily with delay, but at a slower rate in patients than in the controls. This pattern was evaluated with an ANOVA on the proportion of “seen trials”, with factors of group and delay. The proportion of “seen” trials was significantly higher in controls than in patients (F(1,54)=12.90; p<0.001). We also found a significant delay effect (F(7,378)=270.64; p<0.001) and a group by delay interaction (F(7,378)=8.17; p<0.001). At all delays above 33 ms, the proportion of “seen” trials was significantly lower in patients (all p<0.05).
Figure 3. Objective and subjective measures of access to consciousness.
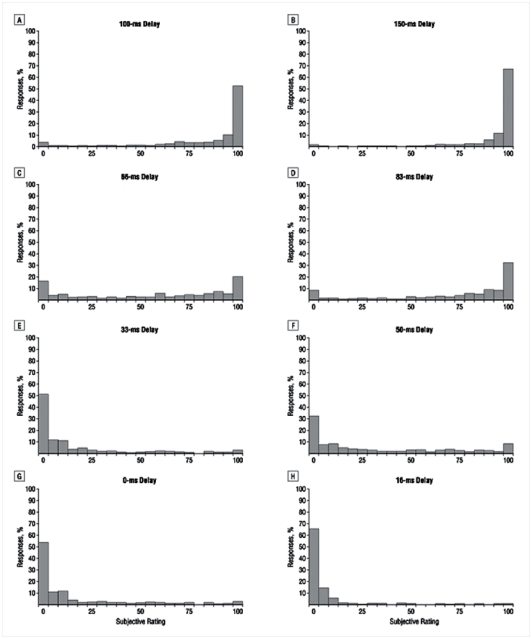
a) Percentage of correct responses in prime comparison to 5 as a function of delay. At each delay, the controls outperformed the patients. In controls, performance was significantly superior to chance at all the delays whereas in patients, performance only became superior to chance for delays of 50 ms and above.
b) Proportion of trials subjectively rated as “seen” as a function of delay. At all delays above 33 ms, the proportion of “seen” trials was significantly lower in patients.
In both graphs, the sigmoid curve fitting the data is represented as a continuous line. The mean objective θo and subjective θs thresholds were defined in each group as the delay for which the sigmoid curve reached its inflexion point.
Error bars represent the standard error.
A similar analysis was applied to the objective measure of prime processing. For each subject and each delay, we calculated the proportion of correct responses in the prime comparison task (Figure 3a). Again, this measure increased non-linearly with delay, at a slower rate for patients than for controls. An ANOVA indicated that performance was significantly higher in controls than in patients (78.2% against 59.6% correct; F(1,54)=87.53; p<0.001). There was a main effect of delay (F(7,378)=100.63; p<0.001) and a group by delay interaction (F(7,378)=5.02; p<0.001). At each delay, the controls outperformed the patients (all p<0.05). In the controls, performance was significantly superior to chance at all the delays. However, in the patients, performance only became superior to chance for delays of 50 ms and above.
In summary, both objective and subjective measures of prime conscious perception indicated lower performance in the patients. In order to characterize, for each subject, a subjective and an objective threshold for access to consciousness, we used non-linear regression to fit the curves in figure 3 with a sigmoid defined as , where the αi are free parameters. The threshold was defined as the delay for which the sigmoid curve reached its inflexion point, i.e. parameter α4. In all subjects, the fit provided an excellent account of the data (mean r2 =0.948, range=0.871–0.996). The mean objective threshold was 59 ms (SD: 13.76; range: 43–91.5) for controls and 90 ms (SD: 32.62; range: 47–163.5) for patients. This threshold was significantly higher for patients than for controls. (Wilcoxon test: W = 539, p<0.001). The mean subjective threshold was 62 ms (SD: 14.68 range: 41–96) for controls and 93 ms (SD: 36.11 range: 47–182) for patients. Again, this threshold was significantly higher for patients than for controls. (Wilcoxon test: W = 523, p<0.001).
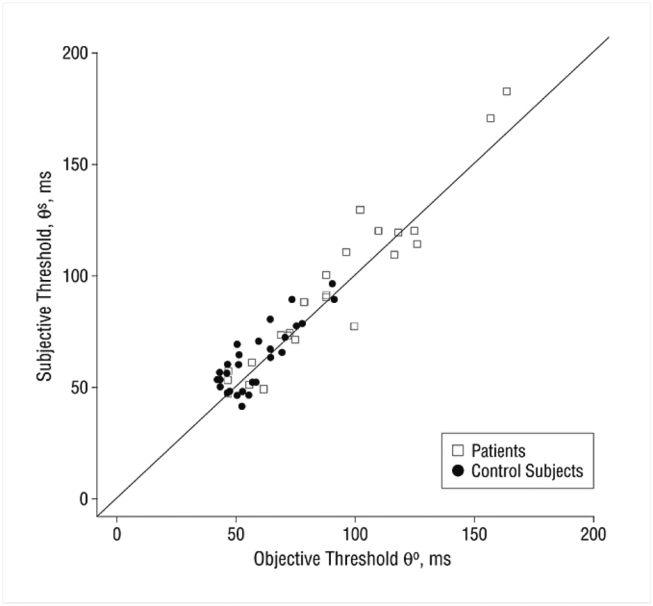
Figure 4 shows the relation between the individual objective and subjective thresholds. Linear regression showed that the two values were highly correlated, both as a whole (r2 = 0.951, p<0.001), within the controls (r2 = 0.834, p<0.001) and within the patients (r2 = 0.955 p<0.001). In all cases, the slope did not differ from 1, and the intercept was not significant. Thus, the objective and subjective tasks appeared to provide essential identical measures of the threshold for access to consciousness. Those results indicate that our paradigm quantifies this threshold with high cross-task reliability. In the following, we arbitrarily use the objective measure as our index of the conscious access threshold.
Figure 4. Positive correlation between objective and subjective consciousness thresholds across subjects.
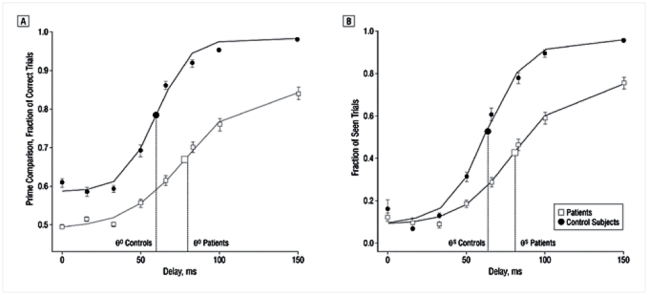
The two values were highly correlated, both as a whole (r2 = 0.951, p<0.001), within the controls (r2 = 0.834, p<0.001) and within the patients (r2 = 0.955 p<0.001). In all cases, the slope did not differ from 1, and the intercept was not significant.
Given the large variability in the observed threshold in the patient group, we further studied whether this threshold correlated with the patients’ clinical symptoms. To this aim, we used the French translation of the Signs and Symptoms of Psychotic Illness (SSPI) scale. [58, 59] The consciousness threshold was positively correlated with psychomotor poverty (Pearson’s r2 = 0.551, p<0.001), depression (r2 = 0.473, p=0.017), and disorganization (r2 = 0.459, p<0.01). We also found a significant correlation with reality distortion (r2 = 0.459, p=0.021) and inside this cluster of symptoms, a positive correlation with hallucinations (r2 = 0.629, p<0.001) and delusion (r2 = 0.4, p=0.047).
Occasional mismatch between subjective and objective measures
We further analyzed the rare trials in which subjective and objective measures did not match. One potential cause for such a mismatch is a capacity for subliminal performance in the objective number comparison task, which is known to be partly feasible under subliminal conditions [51, 54, 60]. As previously shown in figure 3, for short delays (inferior to 50 ms), subjective visibility was around zero and did not differ between groups, but objective performance was above chance for controls only, thus creating a significant difference between the two groups. To study whether this phenomenon was imputable to subliminal processing in the control subjects, we analyzed objective performance while restricting ourselves solely to subjectively defined “not seen” trials, the latter being defined using a conservative criterion (subjective score smaller or equal to 25%).
Figure 5c shows, across delays, the objective performance in each group for “not seen trials” and Figure 5a shows this performance for each delay. Averaged across delays, objective performance on “not seen” trials was significantly above chance (50%) in controls (objective performance= 0.6; p<0.001), indicating some capacity for subliminal number comparison, but not in patients (objective performance= 0.52; p=0.099). An ANOVA indicated that this performance was significantly superior in controls than in patients (F(1,44)=19.69, p<0.001). There was a main effect of delay (F(6,230)=2.695; p=0.015), indicating that performance increased with delay, without delay by group interaction (F(6,230)=1.551; p=0.163).
Figure 5.
Objective performance in prime comparison in each group respectively for “seen trials” (conservatively defined as a subjective score greater than to 16) and “not seen” trials (subjective score smaller or equal to 5). a) Performance at each delay b) and c) performance averaged across delays.
The results demonstrate, in normal subjects a capacity for objective prime processing even on trials subjectively rated as “not seen” (subliminal perception); and, conversely, in patients, an invalability of objective information on some trials judged as “seen” (hallucinations).
Error bars represent the standard error.
Another potential cause for mismatch between objective and subjective measures might be a propensity for illusory perception. To objectify the impression that patients had more such illusory perceptions than controls, we analyzed prime comparison performance during subjectively defined “seen” trials (conservatively defined as a subjective score greater than to 75%). The objective performance was 97.12 % correct in controls and 90.5 % correct in patients (Figure 5b), indicating the presence of comparison errors in both groups, as is usual in response time experiments. However, using an ANOVA on these percentages, we found that objective performance was significantly higher for controls than for patients (F(1,42)=13.61, p<0.001). There was a main effect of delay (F(6,192)= 16.191; p<0.001) without delay by group interaction (F(6,192)= 1.06; p= 0.388) indicating that erroneous objective performance was particularly frequent in the controls and in the patients at short delays of 50, 66 and 83 ms.
It seems likely that, at those delays, several schizophrenic patients experienced illusory perception of the primes, which led them to respond erroneously in the comparison task (see discussion). Indeed, performance within “seen” trials averaged across delays was only negatively correlated with hallucinations (r2 = −0.402, p=0.034), indicating that clinical hallucinations likely were accompanied by visual illusions of seeing the masked prime.
Subliminal and conscious priming effects
We now turn to the priming experiment, in which we measured the ability of the subliminal number prime to influence the processing of the subsequent conscious target. We performed an analysis of variance (ANOVA) on error rate and on mean RT from all trials with RTs below 1000 ms (representing 1,5%, SD = 1.7, of all the trials for the controls and 3.6%, SD = 4.7, for the patients), with factors of group, prime-target relation (congruent repeated, congruent non-repeated, and incongruent), distance of the target number to five, and delay.
First, we tested that patients and controls did not differ on number comparison processes: first, they were affected by a similar distance effect: they answered faster for large than for small distance (37 ms for patients: (1,25)=130.63 p<0.001, and 46 ms for controls: F(1,25)=144.36 p<0.001) without significant distance*group interaction; F(1,49)=2.191 p=0.145). Furthermore, the error rate in target comparison to 5 was non-significantly different between patients and controls (5.017% versus 4.91% errors, p=0.905). We found a significant effect of delay (F(7,364)= 4.463 p<0.001) indicating that subjects made more errors for longer delays, without significant difference between groups (F(7,364)= 0.705; p=0.668).
Figure 6a shows the mean RT for each group, for each condition of prime-target relation and for each delay. Overall, patients were slower than controls (618 ms versus 513 ms, F(1,52)=21.461, p<0.001). The main effect of delay was globally significant (F(7,364)=20.589; p<0.001): RT increased with delay, particularly at the longer delays where the prime becomes conscious. The main effect of prime-target relation was significant overall (F(2,104)=91.059, p<0.001), and a significant delay by relation interaction (F(14,728)=5.332; p<0.001) indicated that the priming effect tended to increase with delay. Crucially, none of these effects interacted with group. There was no interaction of delay and group (F(7,364)=1.105; p=0.359), of prime-target relation and group (F(2,104)=0.017; p=0.983), nor any triple interaction (F(14,728)=1.139; p=0.319). At each delay, we found no significant difference between groups for the prime-target relation effect. Furthermore, the overall prime-target relation effect was significant in both groups (all p<0.001).
Figure 6. Measures of priming during the target number comparison task.
a) Mean RT for each group, for each condition of prime-target relation and for each delay. Response priming was defined as the difference in reaction time between incongruent (InCong) and congruent non-repeated (CongNonRep) trials, and repetition priming as the difference between congruent non-repeated and congruent repeated (CongRep) trials. Both effects were significant across and within each group without significant difference between groups.
b) and c) Delays were sorted into two categories according whether they fell below and above the previously measured consciousness thresholds.
Error bars represent the standard error.
The effect of prime-target relation on RT could be decomposed into two distinct effects: response priming and repetition priming. Both effects were significant across and within each group (all p<0.05) without significant difference between groups. We then separated the delays into those above and those below the consciousness threshold (60ms in controls and 90ms in patients). In both cases, however, priming was not significantly different between groups, neither for repetition priming nor for response priming (Figure 6c).
Discussion
Overall, our results demonstrate the non-linear character of conscious access, as postulated in the global workspace theory. In both patients and controls, the bimodal distribution of subjective visibility ratings suggests that conscious access corresponds to a discontinuous” all-or-none” process (with a higher threshold in patients). Note that all subjects were instructed to report even minimal changes in visibility, clarity or brightness. Indeed, previous research established that normal subjects could do so with remarkable sensitivity, and with continuous changes in their ratings, in a pattern masking paradigm where prime duration was manipulated [47]. A contrario, the bimodal distribution observed here does not seem attributable to a failure to comply with the instructions, but may correspond to a genuine discontinuity in perception during the present object substitution paradigm, as previously observed in an attentional blink paradigm [47, 48]. In both cases, the lack of conscious access may be imputable to the all-or-none availability of central top-down attention.
Our experiment allowed us to measure both an objective and a subjective threshold within each subject, and we found a good correlation of these two measures within each subject and in both groups. Therefore, the variability found in the masking thresholds was not noise but could be considered as a genuine inter-individual difference between subjects. Furthermore, this threshold captured some of the clinical variability in schizophrenia, as we found a positive correlation between conscious threshold and psychomotor poverty dimension, depression, hallucinations, and disorganization. The tight correlation between objective and subjective measures indicates that patients possessed an excellent ability for introspection or metacognition about their vision.
Our results were similar to those of previous studies which also found backward masking performance deficit in patients. However, we also demonstrate that subliminal priming effect is identical between patients and controls. Our results corroborate findings of normal or even enhanced repetition and semantic priming effects in schizophrenia [61–63]. In particular, they replicate previous results where intact subliminal priming was found for numbers written both in word and in Arabic formats [55]. The preservation of non-conscious repetition and response priming in schizophrenia suggests that the fast feedforward processing stages that are thought to support these priming effects are largely intact, including early visual analysis, but also numerical comparison and automatic response programing. A contrario, the deficit of the patients in perceiving backward masked stimuli would then be due to a dysfunction in the late stages of conscious perception. Compatible with this interpretation, an auditory masking deficit involving central processes and contrasting with preserved “low level” masking has been reported in schizophrenia and was attributed to a deficient central cross-modal stage. [64]
Our finding that even subtle measures of automatic visual processing can be preserved in schizophrenia has to be discussed in the context of the many studies which support a low-level visual dysfunction of the magnocellular (M) pathway in schizophrenia [20, 65, 66]. Our experiment studied the subliminal processing of stimuli relying principally on the parvocellular (P) pathway (high contrast digits and letters). It did not assess of the integrity of the magnocellular response to the stimuli and/or to the mask. Therefore, we cannot exclude that an abnormal “bottom up” magnocellular response to the mask contributes to the stronger masking effect in patients, as previously postulated.[20, 28–32, 67, 68] Such a deficit would not affect the processing in the P pathway, known to project predominantly to ventral visual areas, whereas it might affect the speed or strength of activity in dorsal stream areas where the M pathway is known to project predominantly. In turn, such a weaker dorsal input may lead to a slower orientation of spatial attention, thus leading to a prolonged period of susceptibility to substitution masking. [44, 45] Foxe et al [69–71] have reported a decrease in a dorsal parietal subcomponent of the visual evoked potential P1, contrasting with preserved processing in the visual ventral stream. Whether this early magnocellular visual impairment and the higher-level conscious access deficit are linked or independent remains uncertain. According to Foxe et al, the magnocellular impairment would lead to a deficit in later “effortful conceptual processing requiring intact dorsal stream input”. Alternatively, the deficit observed with low-contrast visual stimuli, and imputed to a magnocellular impairment, could be an indirect consequence of impaired parieto-frontal networks for top-down effortful processing in schizophrenia, which would induce a deficient top-down amplification of lower-level processing crucial for such tasks. Those two possibilities are not necessarily incompatible and remain open for further research.
The hypothesis of a perturbed central stage associated with conscious cognitive control [55, 72–77] may explain, not only the elevated threshold for perception of masked stimuli, but also the observed difference in the ability to exploit subliminal information below that threshold. Normal subjects performed the objective prime comparison task better than chance, even on trials where they reported not seeing the prime; patients, however, remained at chance level on those trials (see figure 3). We speculate that, during this experiment, normal subjects are able to concentrate on the masked stimulus even though there are many trials in which this stimulus cannot be seen. This considerable effort of top-down attention may enable them to subliminally extract some information about the stimulus. Indeed, other experiments have shown that attention can modulate and enhance subliminal processing [78]. This ability to maintain a strong top-down task set in the absence of any visible stimulus, a function which depends particularly on prefrontal resources, may be impaired in schizophrenia [79, 80].
Some patients (about thirteen) occasionally seemed to experience an erroneous perception of the masked stimuli. On a few trials, they reported to have seen a number but were wrong when comparing this number to 5, thus suggesting that their introspection did not correspond to reality. Given the design of the experiment, we cannot exclude that some of these responses were merely errors in the comparison task. However, for several reasons, we believe that the phenomenon goes beyond this interpretation. First, during the experiment, although their task only required comparing the prime with 5, some patients verbally reported the prime identity and occasionally gave a verbal response that did not correspond to the digit that was actually presented. Furthermore, such errors on “seen” trials decreased with delay (figure 5a), and were frequent only at the intermediate delays of 50 and 66 ms. This pattern would not be expected if the errors resulted merely from a failure in comparison of a consciously seen digit. Third, across subjects, the occurrence of such errors correlated with a high hallucination score and with an elevated consciousness threshold. What could be the mechanism for such erroneous perceptions? In recent simulations of the global neuronal workspace model, spontaneous activity of neural populations was shown to compete with the entry of external inputs. [81] Thus, for some trials and obviously only for some patients, it is possible that local, spontaneously activated internal representations competed with the concurrent visual input for access to consciousness. Elevated spontaneous activity could simultaneously explain both the elevated threshold and the intrusion of hallucinated digits.
Our previous study [35] had revealed impaired conscious priming in patients with schizophrenia: when the prime was made visible by removing the masks, response priming was reduced whereas repetition priming remained unchanged. Here, however, there was no difference between patients and controls at any of the delays, including delays that were supposedly above the consciousness thresholds. A possible explanation is that, in the present study, even at the longest delays, the mask is always present. This might have helped patients focus their attention on the mask and neglect the prime. Because of this neglect of the prime in the priming task, we think that there was hardly ever for most of the patients a conscious conflict between prime and target even for the primes presented at the longest delay.
In conclusion, our results suggest that the process of access to conscious report of masked stimuli is impaired in schizophrenia, while some fast bottom-up processes of visual perception and number comparison are fully preserved. This deficit could be due to anomalies in the recurrent interactions between visual and higher level associative areas necessary for conscious access. Indeed, a functional disconnection hypothesis of schizophrenia has been proposed, based on abnormal patterns of long-distance correlation in functional neuroimaging studies [4, 82, 83] and on structural anomalies observed with diffusion tensor imaging in the long-distance white matter bundles, particularly in the fronto-cingular cortices, the uncinate fasciculus area and corpus callosum. [84–92]. Although these functional and anatomical anomalies can be plausibly related to an impaired “global workspace” associated with conscious processing, further experiments are needed to clarify the exact relation between this high-level masking deficit and the lower-level visual abnormalities previously described in schizophrenia..
Acknowledgments
This work was supported by grants from the Assistance Publique-Hôpitaux de Paris (PHRC AOM98152), the Fondation pour la Recherche Médicale and the Institut de la Santé et de la Recherche Médicale (INSERM) and a centennial fellowship from the McDonnell donation to S.D. We are grateful to the following individuals for their help and useful comments: Bertrand Audoin, Laurent Cohen, Raphaël Gaillard, Caroline Huron, Alexandre Meary, Lionel Naccache, Christophe Pallier, Jean-Philippe Ranjeva, Françoise Reuter, Jerome Sackur, Franck Schürhoff, Claire Sergent and Andrei Szocke.
References
- 1.Meyer-Lindenberg A, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158(11):1809–17. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher P, et al. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9(3):337–42. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 3.Haraldsson HM, et al. Transcranial Magnetic Stimulation in the investigation and treatment of schizophrenia: a review. Schizophr Res. 2004;71(1):1–16. doi: 10.1016/j.schres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Lawrie SM, et al. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51(12):1008–11. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 5.Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Res Brain Res Rev. 2000;31(2–3):391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 6.Fuster JM. Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr Scand Suppl. 1999;395:51–7. doi: 10.1111/j.1600-0447.1999.tb05983.x. [DOI] [PubMed] [Google Scholar]
- 7.Friston K. Disconnection and cognitive dysmetria in schizophrenia. Am J Psychiatry. 2005;162(3):429–32. doi: 10.1176/appi.ajp.162.3.429. [DOI] [PubMed] [Google Scholar]
- 8.Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30(2):115–25. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 9.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 10.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- 11.Spencer KM, et al. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23(19):7407–11. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer KM, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101(49):17288–93. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreasen NC, et al. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46(7):908–20. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- 14.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–18. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 15.Judd LL, et al. Sensory gating deficits in schizophrenia: new results. Am J Psychiatry. 1992;149(4):488–93. doi: 10.1176/ajp.149.4.488. [DOI] [PubMed] [Google Scholar]
- 16.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47(2):181–8. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 17.Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49(3):206–15. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 18.Braff DL, Swerdlow NR, Geyer MA. Gating and habituation deficits in the schizophrenia disorders. Clin Neurosci. 1995;3(2):131–9. [PubMed] [Google Scholar]
- 19.Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berl) 2004;174(1):75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- 20.Butler PD, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62(5):495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meincke U, et al. Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatry Res. 2004;126(1):51–61. doi: 10.1016/j.psychres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Lindstrom L, et al. Abnormal auditory brain-stem responses in hallucinating schizophrenic patients. Br J Psychiatry. 1987;151:9–14. doi: 10.1192/bjp.151.1.9. [DOI] [PubMed] [Google Scholar]
- 23.Breitmeyer BG. Visual Masking: An Integrative Approach. Oxford University Press; 1984. [Google Scholar]
- 24.Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: a comparison, review, and update. Percept Psychophys. 2000;62(8):1572–95. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- 25.Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry. 1998;43(2):132–8. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- 26.Green M, Walker E. Symptom correlates of vulnerability to backward masking in schizophrenia. Am J Psychiatry. 1986;143(2):181–6. doi: 10.1176/ajp.143.2.181. [DOI] [PubMed] [Google Scholar]
- 27.Rund BR. Backward-masking performance in chronic and nonchronic schizophrenics, affectively disturbed patients, and normal control subjects. J Abnorm Psychol. 1993;102(1):74–81. doi: 10.1037//0021-843x.102.1.74. [DOI] [PubMed] [Google Scholar]
- 28.Butler PD, et al. Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res. 2003;59(2–3):199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 29.Schechter I, et al. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64(2–3):91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- 30.Bedwell JS, Brown JM, Miller LS. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophr Res. 2003;63(3):273–84. doi: 10.1016/s0920-9964(02)00356-0. [DOI] [PubMed] [Google Scholar]
- 31.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51(12):945–51. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- 32.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch Gen Psychiatry. 1994;51(12):939–44. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 33.Green MF, et al. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003;53(12):1113–9. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- 34.Green MF, et al. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. Am J Psychiatry. 1999;156(9):1367–73. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- 35.Shelley-Tremblay J, Mack A. Metacontrast Masking and Attention. Psychological Science. 1999;10(6):508–515. [Google Scholar]
- 36.Williams MC, Weisstein N. Spatial frequency response and perceived depth in the time-course of object superiority. Vision Res. 1981;21(5):631–46. doi: 10.1016/0042-6989(81)90071-7. [DOI] [PubMed] [Google Scholar]
- 37.Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23(11):571–9. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- 38.Lamme VA, Zipser K, Spekreijse H. Masking interrupts figure-ground signals in V1. J Cogn Neurosci. 2002;14(7):1044–53. doi: 10.1162/089892902320474490. [DOI] [PubMed] [Google Scholar]
- 39.Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 40.Dehaene S, Sergent C, Changeux JP. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci U S A. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dehaene S, Changeux JP. Neural mechanisms for access to consciousness. In: Gazzaniga M, editor. The cognitive neurosciences. 3. Norton; New York: 2004. pp. 1145–1157. [Google Scholar]
- 42.Ramachandran VS, Cobb S. Visual attention modulates metacontrast masking. Nature. 1995;373(6509):66–8. doi: 10.1038/373066a0. [DOI] [PubMed] [Google Scholar]
- 43.King DL, Mose JF, Nixon NS. One line decreases the visibility of a simultaneous identical distant second line. Percept Psychophys. 1995;57(3):393–401. doi: 10.3758/bf03213063. [DOI] [PubMed] [Google Scholar]
- 44.Di Lollo V, Enns JT, Rensink RA. Competition for consciousness among visual events: the psychophysics of reentrant visual processes. J Exp Psychol Gen. 2000;129(4):481–507. doi: 10.1037//0096-3445.129.4.481. [DOI] [PubMed] [Google Scholar]
- 45.Enns JT, Di Lollo V. What’s new in visual masking? Trends Cogn Sci. 2000;4(9):345–352. doi: 10.1016/s1364-6613(00)01520-5. [DOI] [PubMed] [Google Scholar]
- 46.Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci U S A. 1998;95(24):14529–34. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sergent C, Dehaene S. Is consciousness a gradual phenomenon? Evidence for an all-or-none bifurcation during the attentional blink. Psychol Sci. 2004;15(11):720–8. doi: 10.1111/j.0956-7976.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- 48.Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci. 2005;8(10):1391–400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 49.Dehaene S. The neural bases of subliminal priming. In: Kanwisher N, Duncan J, editors. Functional Neuroimaging of Visual Cognition (Attention and Performance Series, 20) Oxford University Press; New York: 2004. [Google Scholar]
- 50.Dehaene S, et al. Letter binding and invariant recognition of masked words: behavioral and neuroimaging evidence. Psychol Sci. 2004;15(5):307–13. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- 51.Naccache L, Dehaene S. Unconscious semantic priming extends to novel unseen stimuli. Cognition. 2001;80(3):215–29. doi: 10.1016/s0010-0277(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 52.Greenwald AG, Draine SC, Abrams RL. Three cognitive markers of unconscious semantic activation. Science. 1996;273(5282):1699–702. doi: 10.1126/science.273.5282.1699. [DOI] [PubMed] [Google Scholar]
- 53.Dehaene S, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4(7):752–8. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 54.Dehaene S, et al. Imaging unconscious semantic priming. Nature. 1998;395:597–600. doi: 10.1038/26967. [DOI] [PubMed] [Google Scholar]
- 55.Dehaene S, et al. Conscious and subliminal conflicts in normal subjects and patients with schizophrenia: the role of the anterior cingulate. Proc Natl Acad Sci U S A. 2003;100(23):13722–7. doi: 10.1073/pnas.2235214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vorberg D, et al. Different time courses for visual perception and action priming. Proc Natl Acad Sci U S A. 2003;100(10):6275–80. doi: 10.1073/pnas.0931489100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nature Neuroscience. 2005 doi: 10.1038/nn1549. (In Press) [DOI] [PubMed] [Google Scholar]
- 58.Liddle PF. The symptoms of chronic schizophrenia: a re-examination of the positive-negative dichotomy. British Journal of Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
- 59.Liddle PF, et al. Signs and Symptoms of Psychotic Illness (SSPI): a rating scale. Br J Psychiatry. 2002;180:45–50. doi: 10.1192/bjp.180.1.45. [DOI] [PubMed] [Google Scholar]
- 60.Koechlin E, et al. Primed numbers: Exploring the modularity of numerical representations with masked and unmasked semantic priming. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1882–1905. [Google Scholar]
- 61.Minzenberg MJ, Ober BA, Vinogradov S. Semantic priming in schizophrenia: a review and synthesis. J Int Neuropsychol Soc. 2002;8(5):699–720. doi: 10.1017/s1355617702801357. [DOI] [PubMed] [Google Scholar]
- 62.Condray R, et al. Automatic activation of the semantic network in schizophrenia: evidence from event-related brain potentials. Biol Psychiatry. 2003;54(11):1134–48. doi: 10.1016/s0006-3223(03)00699-1. [DOI] [PubMed] [Google Scholar]
- 63.Baving L, et al. Increased semantic and repetition priming in schizophrenic patients. J Abnorm Psychol. 2001;110(1):67–75. doi: 10.1037//0021-843x.110.1.67. [DOI] [PubMed] [Google Scholar]
- 64.Kallstrand J, et al. Auditory masking experiments in schizophrenia. Psychiatry Res. 2002;113(1–2):115–25. doi: 10.1016/s0165-1781(02)00248-2. [DOI] [PubMed] [Google Scholar]
- 65.Keri S, et al. Visual-perceptual dysfunctions are possible endophenotypes of schizophrenia: evidence from the psychophysical investigation of magnocellular and parvocellular pathways. Neuropsychology. 2005;19(5):649–56. doi: 10.1037/0894-4105.19.5.649. [DOI] [PubMed] [Google Scholar]
- 66.Kim D, et al. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2005 doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients. Evidence for a vulnerability indicator. Arch Gen Psychiatry. 1997;54(5):465–72. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- 68.Butler PD, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158(7):1126–33. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 69.Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12(17):3815–20. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- 70.Doniger GM, et al. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59(11):1011–20. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 71.Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: a high-density electrical mapping and source-analysis investigation of illusory contour processing. Cereb Cortex. 2005;15(12):1914–27. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- 72.Carter CS, et al. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154(12):1670–5. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 73.Cohen JD, Braver TS, O’Reilly RC. A computational approach to prefrontal cortex, cognitive control and schizophrenia: recent developments and current challenges. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1515–27. doi: 10.1098/rstb.1996.0138. [DOI] [PubMed] [Google Scholar]
- 74.Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Brain Res Rev. 2000;31(2–3):357–63. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 75.Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. 1996;5(1–2):175–81. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- 76.Frith C. The role of the prefrontal cortex in self-consciousness: the case of auditory hallucinations. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1505–12. doi: 10.1098/rstb.1996.0136. [DOI] [PubMed] [Google Scholar]
- 77.Frith CD. Consciousness, information processing and schizophrenia. Br J Psychiatry. 1979;134:225–35. doi: 10.1192/bjp.134.3.225. [DOI] [PubMed] [Google Scholar]
- 78.Naccache L, Blandin E, Dehaene S. Unconscious masked priming depends on temporal attention. Psychological Science. 2002;13:416–424. doi: 10.1111/1467-9280.00474. [DOI] [PubMed] [Google Scholar]
- 79.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46(5):650–61. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 80.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6(4):348–57. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 81.Dehaene S, Changeux JP. Ongoing spontaneous activity controls access to consciousness: a neuronal model for inattentional blindness. PLoS Biol. 2005;3(5):e141. doi: 10.1371/journal.pbio.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breakspear M, et al. A disturbance of nonlinear interdependence in scalp EEG of subjects with first episode schizophrenia. Neuroimage. 2003;20(1):466–78. doi: 10.1016/s1053-8119(03)00332-x. [DOI] [PubMed] [Google Scholar]
- 83.Lee KH, et al. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41(1):57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 84.Lim KO, et al. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56(4):367–74. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 85.Burns J, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–43. [PubMed] [Google Scholar]
- 86.Wolkin A, et al. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003;160(3):572–4. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- 87.Wang F, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161(3):573–5. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- 88.Kubicki M, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2005 doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kubicki M, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26(4):1109–18. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kubicki M, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54(11):1171–80. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kubicki M, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159(5):813–20. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szeszko PR, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162(3):602–5. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]


