Abstract
Background
Little is known about detection of genital human papillomavirus (HPV) types in women’s fingertips. The study objectives were to determine the presence of genital HPV types in fingertip samples and agreement between fingertip and genital samples for detecting HPV.
Methods
At tri-annual visits, genital and fingertip samples were collected from female university students and tested for 37 HPV genotypes by PCR-based assay. Type-specific concordance between paired fingertip and genital samples was evaluated using a kappa statistic for percent positive agreement (“kappa +”). Paired samples with type-specific concordant fingertip and genital results were selected for variant characterization.
Results
A total of 357 fingertip samples were collected from 128 women. HPV prevalence in fingertip samples was 14.3%. Although percent positive agreement between fingertips and genitals for detecting type-specific HPV was low (17.8%; kappa+=0.17, 95%CI:0.10–0.25), 60.4% of type-specific HPV detected in the fingertips was detected in a concurrent genital sample. All but one of 28 paired concordant samples were positive for the same type-specific variant in the fingertip and genital sample. Re-detection of HPV types at the subsequent visit was more common in genital samples (73.3%) than in fingertip samples (14.5%) (p<.001).
Conclusions
Detection of genital HPV types in the fingertips was not uncommon. While impossible to distinguish between deposition of DNA from the genitals to the fingertips and true fingertip infection, the rarity of repeat detection in the fingertips suggests that deposition is more common.
Impact
Finger-genital transmission is plausible, but unlikely to be a significant source of genital HPV infection.
Keywords: human papillomavirus, fingertip, genital, women, epidemiology
INTRODUCTION
While sexual intercourse is the primary route of genital human papillomavirus (HPV) transmission, transmission via non-penetrative sexual contact (including finger-genital contact) is plausible (1). Genital HPV types have been detected in palm or fingertip samples from patients with genital warts (2) and asymptomatic men and women (3, 4). In newly sexually active female university students, our objectives were to evaluate presence of genital HPV types in fingertip samples and agreement between fingertip/genital samples for detecting HPV.
METHODS
Between December 2000–March 2007, 18–22 year old female university students were recruited into a longitudinal study of HPV infections. Recruitment procedures and data collection were described previously (5). Women were eligible if they had never had vaginal intercourse with a male partner or first had intercourse with one partner within the previous three months. Informed consent was obtained, and the human-experimentation guidelines of the University of Washington Institutional Review Board were followed.
Women were followed with tri-annual gynecological examinations. At each visit, genital swab samples were collected for HPV testing. Starting in February 2006, fingertip samples were collected for HPV testing prior to collection of genital swab samples. Women were instructed to rub each fingertip and the underside of each fingernail tip on both hands with a CytoSoft cytology brush (MPC). The brushes were placed into tubes containing 1 ml of specimen-transport medium. Prior to pelvic examination, women were instructed to self-collect a vaginal sample (6). During the pelvic examination, the nurse practitioner collected separate cervical and vulvar/vaginal samples (5).
Samples were tested for HPV using polymerase chain reaction-based methods. Of each sample, 200 µl was used to extract DNA. As described previously, samples testing positive by generic probe were typed using a reverse line-blot assay (Roche Molecular Systems) for 37 HPV types (5) . Paired samples with type-specific concordant fingertip/genital results were selected for variant characterization; sequence analysis of the E6 gene was performed using type-specific primers. Given previous findings that individual pairs of cervical and vulvar/vaginal samples were positive for identical HPV-16 variants (Long Fu Xi, personal communication), sequence analysis was performed on only one genital sample (the cervical sample was selected unless it was negative, in which case the vulvar/vaginal or self-collected sample was selected).
A genital sample was considered positive if HPV was detected in either the clinician-collected cervical, clinician-collected vulvar/vaginal, or self-collected sample. In analyses referring to incident type-specific genital HPV infections, incident infection was defined as the first positive type-specific result for that type (in either clinician-collected sample or the self-collected sample) following negative type-specific baseline results in all genital samples.
The proportion positive agreement (PPA) between corresponding fingertip/genital samples was calculated by dividing the number of samples positive for HPV in both samples by the number of samples positive in either sample. To determine the PPA beyond that expected by chance, a modified unweighted Kappa statistic (“Kappa+”) was calculated by dividing the difference between the observed positive agreement and expected positive agreement by one minus the expected positive agreement. Expected positive agreement was computed under the assumption of independence of the assay results. To account for correlation within subjects, 95% confidence intervals (CI) were computed using percentile bootstrap methods with 1,000 repetitions.
Logistic regression was used to evaluate whether site of type-specific genital HPV detection (cervix and vulva/vagina versus one site only [using the results from the clinician-collected samples only]) or HPV risk type (high- or probable high-risk [types 16/18/26/31/33/35/39/45/51/52/53/56/58/59/66/68/73/82/IS39 (7)] versus low- or undetermined risk [types 6/11/40/42/54/55/57/61/62/64/67/69/70/71/72/81/83/84/CP6108 (7)]) was associated with the likelihood of detection in the corresponding fingertip sample. Robust variance estimates were used to account for correlation within subjects.
The Kaplan-Meier method was used to calculate the cumulative prevalence of type-specific HPV detection in the fingertips at a visit concurrent with or subsequent to incident detection in the genitals.
RESULTS
Three-hundred and fifty-seven fingertip samples were collected from 128 women over a mean of 8.7 months (SD, 5.4 months) follow-up. Twenty-two (6.2%) samples were insufficient for testing, leaving 335 samples from 127 women for analysis (mean 2.6 [SD, 1.2, range 1–5] samples per woman). The mean age at sampling was 18.9 years (SD, 0.8 years). Fourteen samples (4.2%) were collected from women reporting no history of vaginal intercourse.
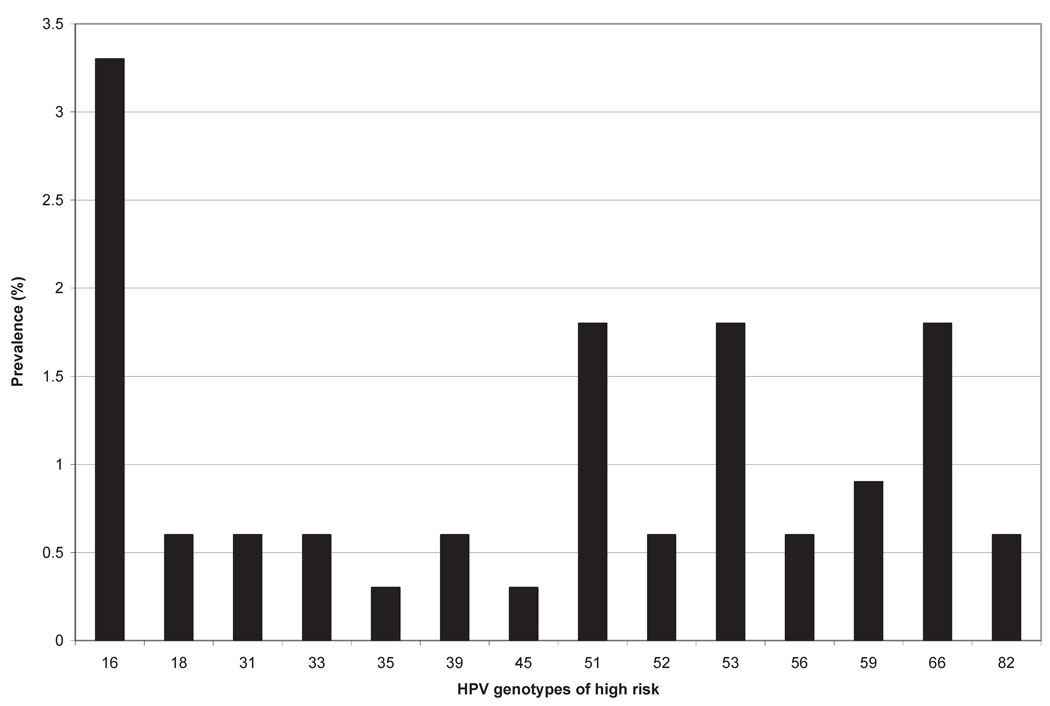
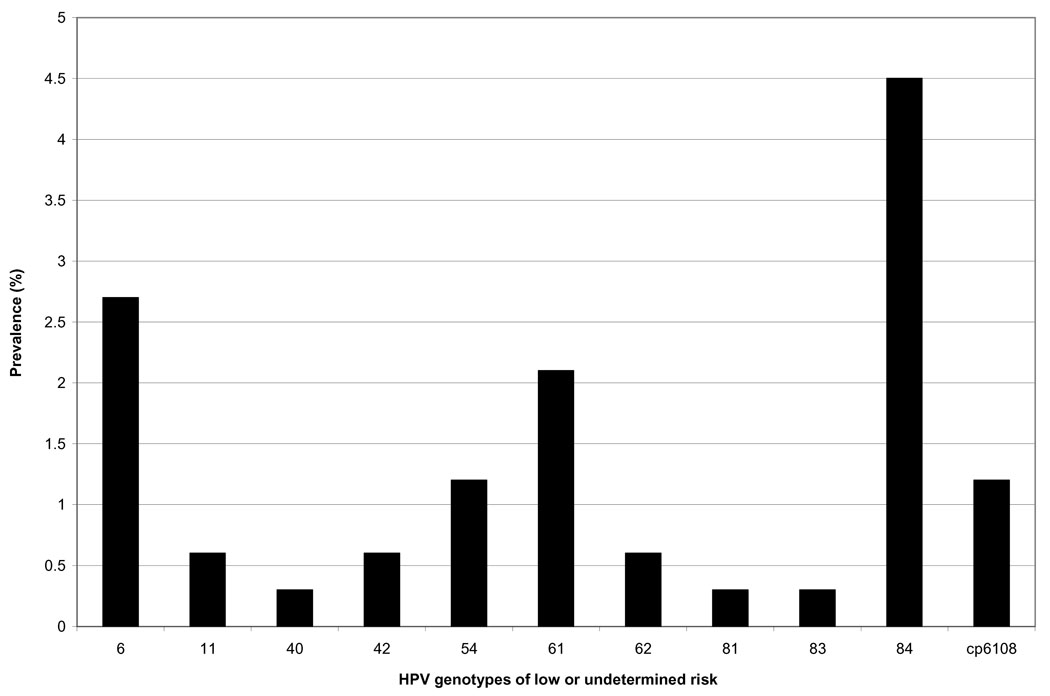
All 14 samples collected from virgin women tested negative for HPV. Forty-six of 321 fingertip samples (14.3%) collected from sexually active women tested positive for HPV (any type). The mean number of types detected in samples testing positive was 2.1 (SD, 1.6, range 1–7). The most prevalent types detected in fingertip samples were HPV-84 (4.5% positivity) and HPV-16 (3.3% positivity) (Figures 1 and 2).
Figure 1.
High-risk type-specific HPV DNA prevalence in 335 fingertip samples collected from 127 women. Types 58, 68, 73, and is39 were not detected in fingertip samples. HPV-68 was detected in 0.6% of concurrently tested genital samples and HPV-73 was detected in 0.3% of concurrently tested genital samples.
Figure 2.
Low- or undermined-risk type-specific HPV DNA prevalence in 335 fingertip samples collected from 127 women. Types 55, 57, 64, 67, 69, 70, 71, and 72 were not detected in fingertip samples. HPV-55 was detected in 1.2% of concurrently tested genital samples, HPV-67 was detected in 2.4% of concurrently tested genital samples, and HPV-70 was detected in 0.6% of concurrently tested genital samples.
HPV (any type) was detected in 38.5% (129/335) of genital samples collected at the same visit as a fingertip sample. 20.1% of type-specific HPV detected in the genitals (58/288 types) was detected in the concurrent fingertip sample. Conversely, 60.4% of type-specific HPV detected in the fingertips (58/96 types) was detected in the concurrent genital sample. Pooling across HPV types, the type-specific PPA for detecting HPV between fingertip/genital samples was 17.8% (kappa+=0.17, 95% CI:0.10–0.25).
Forty-one paired type-specific concordant fingertip/genital samples were selected for variant characterization, of which 13 tested negative by PCR-based DNA sequencing in either the fingertip sample (10) or both samples (3). In the remaining 28 pairs, all but one displayed the same variant(s) in each pair (including one pair with two different HPV-16 variants in both samples). The exception had HPV-84 prototype found in the fingertip sample and the variant (with changes of C-to-T at position 19, C-to-T at positive 66, C-to-T at position 171, and T-to-C at position 346) in the genital sample.
If a given HPV type was detected simultaneously in both clinician-collected samples, that type was more likely to be detected in the concurrent fingertip sample than if it were detected in only the cervical or only the vulvar/vaginal sample (OR=6.21, 95%CI:2.32–16.58). HPV types of low or undetermined risk detected in the genitals were borderline statistically significantly more likely to be detected in the concurrent fingertip sample than HPV types of high- or probable high-risk (OR=1.85, 95%CI:0.95–3.60).
Of 109 incident type-specific genital HPV infections with corresponding fingertip samples, 20 (18.3%) were concurrently positive for the same type in the fingertip sample. Of 89 infections with corresponding negative fingertip results, 53 were followed by ≥1 more visit with fingertip sampling (range 1–3 more visits). The same HPV type was detected in a subsequent fingertip sample in 13 cases (24.5%), and all had a corresponding genital sample that was still positive for the same type. The one-year cumulative prevalence of detecting the same HPV type in a concurrent or subsequent fingertip sample was 46.1% (95%CI:33.6–60.7).
On a per-woman level, 29.1% of women (37/127) had HPV detected in ≥1 samples collected from the fingertips. Fifty-five (of 82) type-specific positives were first detected before the last follow-up visit. Of these, eight (14.5%) were re-detected at the next follow-up visit. Thirty of the 55 type-specific positives had a corresponding genital positive. Re-detection was more common in corresponding genital samples; 73.3% (22/30) of types detected in ≥1 genital sites were re-detected at ≥1 genital sites at the next follow-up visit (p<.001). (The likelihood of re-detection did not differ by genital site [73.3% in the vulva/vagina versus 71.4% in the cervix].) When type-specific HPV was detected in both the fingertips and genitals, there were no cases where type-specific HPV was re-detected in the fingertip sample but not in a corresponding genital sample.
DISCUSSION
In this cohort of newly sexually active female university students, detection of genital HPV types in the fingertips was not uncommon; close to 30% of participants had at least one 4-monthly HPV-positive fingertip sample during an average of 9 months of study follow-up. Sixty percent of type-specific positives were concurrently detected in the genitals (with the same variant(s) detected in all but one of 28 pairs), suggesting that a majority of HPV in the fingertips represents either DNA deposition from the genitals, autoinoculation between the genitals and the fingertips, or an additional site of infection from a single source (e.g. a recent sex partner). (Of note, the two most common genital HPV types detected in the fingertips [HPV-84 and HPV-16] were also the two most common types detected in the genitals in the same cohort (5).) While impossible to distinguish between these three possibilities, it is likely that all three occur. However, given that repeated detection of HPV in the fingertip samples was uncommon relative to repeated detection in genital samples, we suspect that a majority is due to deposition (rather than true infection).
Considering all HPV-positive samples, the PPA between genital and fingertip samples was low. However, given that the prevalence of HPV in fingertip samples was less than half that in genitals samples, the low positive agreement is not surprising. Even between genital sites, HPV discordance occurs; we previously reported that perfect type-specific agreement was only 50% between cervical and vulvar/vaginal samples collected from these same women (6). In the present analysis, type-specific HPV was more likely to be concurrently detected in the fingertips if both genital sites (versus only one) were positive. Multi-site genital HPV infections may reflect infections with higher viral loads (and increased likelihood of either genital-finger transmission or deposition of DNA from the genitals to the fingertips). Alternatively, multi-site genital HPV infections may simply present a greater number of potential sites of exposure for the fingertips.
When HPV was detected in the genitals, the same type was more likely to be detected in the concurrent fingertip sample if it was a low-/undetermined-risk type than if it was a high-/probable high-risk type. While of borderline statistical significance, this may signify that the lower genital tract/external genitalia is a likely source of HPV in the fingertips (based on our previous report that HPV types of low-/undetermined-risk were more likely than high-risk types to be detected in the vulva/vagina than the cervix (8)). It is also worth noting that all 18 fingertip samples collected at visits with a type-specific cervical positive and vulvar/vaginal negative were negative. Another possible interpretation is that low-risk HPV types display tropism for fingertip tip epithelium.
While transmission of HPV from fingertips to genital or oral sites is plausible, the lower detection rates in fingertips (compared to genitals) and the predominantly transient nature of detectable HPV in fingertips suggest that fingertips are unlikely to be a significant source of HPV transmission. Nonetheless, given that genital HPV infections are detected (albeit rarely) in virgins, and that non-penetrative sexual contact (including finger-vulvar, penile-vulvar, and oral-penile) has been shown to be associated with an increased risk of genital HPV infection in virgin women (9), the potential for transmission from fingertips to other sites cannot be ruled out. In a small longitudinal study of HPV transmission in 25 heterosexual couples, sequential detection of type-specific HPV DNA at different anatomic sites (both within individuals and between partners) was observed, suggesting possible autoinoculation or between-partner transmission from hands to genitals, or vice versa (4). Furthermore, given that cutaneous HPV types can be transmitted indirectly via fomites (e.g., plantar warts [caused by HPV types 1/2/4/63] can be transmitted to the feet via contaminated showers or swimming pools) (10), it is possible that the fingertips could be a vector for HPV transmission to genital or oral sites in the absence of true fingertip infection.
A limitation of our study is that we did not capture information on non-penetrative sexual contact. Therefore, we could not evaluate whether finger-genital contact was associated with an increased likelihood of detecting HPV in fingertips. Also, because we did not start collecting fingertip samples until the 6th year of the study, it was not possible to evaluate and compare the incidence of fingertip HPV to the incidence of genital HPV; in a study of young men attending the same university (who were followed with both fingertip/genital sampling throughout the entire study period), the two-year cumulative incidence of detecting HPV in the fingertips (32%) was approximately half the two-year cumulative incidence of detecting HPV in a genital site (62%) (3).
In conclusion, while detection of HPV in fingertips of newly sexually active university women was not uncommon, prevalence was significantly lower than in genitals. While DNA deposition could not be distinguished from true infection, the predominantly transient nature of detecting fingertip HPV suggests that deposition is more common than infection. Finally, while transmission of HPV from fingertips to genital or oral sites is plausible, it seems unlikely that fingertips are a significant source of HPV transmission.
Acknowledgments
Financial support was provided by grants from the National Institute of Allergy and Infectious Diseases (R01-A138383 and T32AI007140-24).
Footnotes
Dr. Koutsky has received commercial research grant support from Merck. None of the other authors have commercial or other associations that might pose a conflict of interest.
REFERENCES
- 1.Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006 Aug 21;24(Suppl 3):S52–S61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Sonnex C, Strauss S, Gray JJ. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex Transm Infect. 1999;75(5):317–319. doi: 10.1136/sti.75.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007 Oct 15;196(8):1128–1136. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008 Jun;14(6):888–894. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006 Jun 22;354(25):2645–2654. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 6.Winer RL, Feng Q, Hughes JP, et al. Concordance of self-collected and clinician-collected swab samples for detecting human papillomavirus DNA in women 18 to 32 years of age. Sex Transm Dis. 2007 Jun;34(6):371–377. doi: 10.1097/01.olq.0000240315.19652.59. [DOI] [PubMed] [Google Scholar]
- 7.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006 Aug 21;24S:S1–S10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 8.Winer RL, Hughes JP, Feng Q, O'Reilly S, Kiviat NB, Koutsky LA. Comparison of incident cervical and vulvar/vaginal human papillomavirus infections in newly sexually active young women. J Infect Dis. 2009 Mar 15;199(6):815–818. doi: 10.1086/597118. [DOI] [PubMed] [Google Scholar]
- 9.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Incident infection with genital human papillomavirus: rates and risk factors among a cohort of female university students. Am J Epidemiol. 2003;157(3):218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 10.Laurent R, Kienzler JL. Epidemiology of HPV infections. Clin Dermatol. 1985 Oct–Dec;3(4):64–70. doi: 10.1016/0738-081x(85)90050-1. [DOI] [PubMed] [Google Scholar]