Abstract
Objective
Cognitive benefit of postmenopausal hormone use is controversial; however, timing treatment close to menopause may increase the likelihood of preserving cognitive function. We examined effects of early-initiation hormone use on visual working memory, hypothesizing that long-term hormone use is associated with greater brain activation during visual working memory.
Methods
This is a cross-sectional comparison of long-term early hormone users – current (n=13) and past (n=24, 2.1±1.0 years off hormones) – to never-users (n=18), using a visual memory task and functional MRI. We evaluated 55 women over age 60 at the University of Michigan’s General Clinical Research Center. Hormone users had completed at least ten continuous years of conjugated equine estrogens with or without medroxyprogesterone acetate, began within two years of menopause. Women were excluded for illness, medication, intermittent estrogen use, phytoestrogen use, recent smoking, and MRI contraindications. The primary outcome was functional MRI-detected brain activity during the visual memory task.
Results
Compared to never-users, both hormone-user groups had increased activation in the frontal and parietal cortices, insula, hippocampus, and cingulate; combined hormone-users also had increased activation in the putamen and raphe (corrected p<0.05 or uncorrected p<0.001 with a priori hypothesis). Across the entire sample, medial temporal cortex (p<0.000 right; p<0.018 left) and right hippocampus (p<0.000) positively correlated with task performance.
Conclusions
Hormone use was associated with increased brain activation during the visual memory task, in regions used for visual working memory. A positive correlation between activation and task performance suggests that early-initiated long-term postmenopausal hormone use may benefit visual working memory.
Keywords: Estrogen, Hormone Therapy, Postmenopause, Functional Magnetic Resonance Imaging, Memory, Cognition
INTRODUCTION
Estrogens are present throughout the central nervous system, contributing to neuronal integrity and cognitive function 1–4. As women approach menopause, declining estrogen levels may negatively affect learning and memory 5, 6. Early observations suggested that estrogen treatment preserves cognitive ability, however clinical trials have not uniformly supported this conclusion 7–11. Increasing evidence suggests that beginning treatment within a ‘critical window’ is necessary to achieve a cognitive benefit 8, 11–16.
Contrary to previous observational studies, the Women’s Health Initiative Memory Study (WHIMS) found an increased risk of dementia and mild cognitive impairment in women, all over 65 years old, randomized to estrogen or combined estrogen and progestin therapies 17–22. Paradoxically, a sub-study of WHIMS, the Women’s Health Initiative Study of Cognitive Aging (WHISCA), found a trend towards a benefit of hormone use in nonverbal cognitive domains 23. These studies demonstrate the limitations of hormone use initiated well past menopause, but it is unclear whether the conclusions can be extrapolated to earlier hormone initiation.
Reviews of randomized studies conclude that earlier treatment yields cognitive benefits in the majority of studies, while later hormone use is not associated with such benefit 8, 9. Similarly, a follow-up study of early postmenopausal women randomized to hormones or placebo detected less cognitive impairment in those who received the active hormone use 24. Likewise, in the REMEMBER study, early hormone initiators performed better than late initiators on tests of attention, concentration, and mental status 25.
Functional magnetic resonance imaging (fMRI), which indirectly measures synaptic activity in the brain, can provide additional insight into the effects of postmenopausal hormone use on memory and cognition. Our group recently used fMRI to compare brain activity in women after 4 weeks each of placebo and combined estrogen plus progestin treatment while they performed a visual memory task 26. Regional brain activity differed between treatment arms, suggesting the hormones influenced how visual information was processed.
The present study is a cross-sectional fMRI-based evaluation of visual working memory in early-initiating long-term estrogen users, combined estrogen and progestin users, and never-users of hormones. We compared hormone-treated to never-treated women, and evaluated the effects of estrogen only (ET) versus combined estrogen plus progestin therapy (EPT). We hypothesize that ET and EPT are both associated with increased activity in the frontal cortex, parietal cortex, hippocampus, and parahippocampal gyrus, brain regions known to be involved in visual cognition and working memory.
METHODS
Subjects
Three groups of healthy right-handed postmenopausal women, 60 years or older, were recruited by advertisement or through the University of Michigan Women’s Health Registry 27, 28; those who took hormones without interruption for at least 10 years (two groups: ET and EPT), and those who had never received treatment. All hormone-treated subjects began use within two years of menopause onset (defined as the absence of menstrual periods for one year, onset of severe symptoms after hysterectomy, or the time of hysterectomy with bilateral oophorectomy). Users took the same dose and preparation of estrogen, conjugated equine estrogens 0.625 mg/day (Premarin, Wyeth Pharmaceuticals, Philadelphia, PA), with or without cyclic or continuous medroxyprogesterone acetate (Provera, Pfizer, New York, NY or Prempro, Wyeth Pharmaceuticals, Philadelphia, PA). Fifty-five women were included in the study: 17 ET users, 20 EPT users, and 18 never-users.
Hormone treatment (HT) groups included current and past long-term users. Of the treated women, 13 of 37 women were currently taking hormones (7 of 17 women in the estrogen only group and 6 of 20 women in the EPT group). For those no longer taking hormones, mean time since treatment ended was 2.1 ± 1 years. All women in the estrogen only group had undergone hysterectomy, including 10 of 17 women (64.7%) with bilateral oophorectomy.
Initial phone screening was followed by medical, psychiatric and neurologic histories, and physical exam. Screening laboratory tests included electrolytes, glucose, complete blood count, TSH and estradiol. Exclusion criteria included diabetes, heart disease, cancer, stroke, other acute or uncorrected medical illnesses, use of centrally acting medications, intermittent estrogen usage, use of phytoestrogen supplements, smoking within the last 5 years, inability to tolerate the scanning procedures, and contraindications to MRI. After a full description of the study, written informed consent was obtained. All procedures were approved by the University of Michigan’s Institutional Review Board.
Study protocol
This was a cross-sectional study of post-menopausal long-term hormone users versus never-users. A neuropsychological battery of tests was given to exclude the presence of dementia or specific deficits in visual spatial skills, including: 1) Mini-mental State Examination 29, a brief screening measure of dementia; 2) Shipley Institute of Living Scale 30, a short estimate of intellectual power; 3) Geriatric Depression Rating Scale 31, to exclude the presence of depression, and 4) Benton Visual Retention Test, revised 32, 33, a measure of visual memory ability.
fMRI visual delayed matching to sample task
The fMRI paradigm to evaluate visual working memory used a validated Visual Delayed Matching to Sample task 34, 35. The visual stimuli consisted of 9 × 9 grids, with 40 squares darkened to form a random pattern, presented under three conditions: 1) matching to sample, 2) 1-second delayed matching to sample, or 3) 4-second delayed matching to sample. In the matching condition, a target stimulus was presented with two additional test items simultaneously underneath the target. The women selected which of the test items matched the target via a response pad. Stimuli were presented for 3 seconds, followed by a 7-second fixation cross before presentation of the next item. The delay conditions assessed visual working memory: the target stimulus was presented alone for 1.5 seconds and was followed by a 1-second or 4-second delay. After the delay, test items were presented for 3 seconds, during which time women indicated the match. The 4-second delay condition engaged working memory to a greater extent as it required the visual information to be remembered during the delay. Four blocked trials from each condition were counterbalanced over three 6-minute runs, for 180 total scans with a 2-second interscan interval. E-Prime software (Psychology Software Tools Inc., Pittsburgh, PA) controlled the stimulus presentation timing. To minimize performance differences, participants practiced the task until they achieved at least 70% accuracy.
fMRI acquisition and processing
Scans were acquired using a 3T whole-body MRI scanner (General Electric, Milwaukee, WI) equipped with a standard head coil. Anatomical MRI scans were acquired axially with a spoiled gradient recalled (SPGR) three dimensional volumetric acquisition [repetition time (TR) = 9.6, echo time (TE) = 3.3, inversion recovery preparation (IR PREP) = 200 millisecond, flip angle (FA) = 17°, bandwidth = 15.63, 24-cm field of view (FOV), 1.5-mm slice thickness, 106–110 slices, 256 × 256 matrix, 2 excitations]. fMRI acquisition was sensitized for the blood oxygen level-dependent (BOLD) effect using a T2* weighted single-shot spiral pulse sequence with 32 oblique-axial slices prescribed to be approximately parallel to the anterior commissure – posterior commissure (AC-PC) line (spiral gradient echo (GRE), TE = 25, TR = 2000, FA = 60°, 4-mm-thick contiguous slices, 24-cm FOV, 64 × 64 image matrix). Image reconstruction included processing steps to remove distortions caused by magnetic field inhomogeneity and other sources of misalignment to the structural data 36. Data were sinc-interpolated in time, slice-by-slice, to correct for the staggered sequence of slice acquisition 37. The first four functional volumes of each run were discarded to remove magnetic saturation effects, and the remaining images were realigned to the fifth volume to eliminate movement artifacts using SPM2-based algorithms 38. Realignment parameters for each subject were examined to ensure that head movement did not exceed 2 mm. Anatomical and functional images were coregistered to each other through rigid body affine transformation using a mutual information algorithm, as previously described 39. The subject’s MRI was spatially normalized into standard stereotactic space via linear and nonlinear warping, to a minimum deformation (MDT2) template derived from T1-weighted MR images from 25 normal older women, created by the Imaging of Dementia and Aging laboratory (IdeALab) at the University of California, Davis 40. The transformation matrix was applied to all functional images, and a three-dimensional Gaussian smoothing kernel set at 8 mm full width half maximum was applied to accommodate for residual anatomical variability and improve signal-to-noise ratios.
fMRI data analysis
fMRI data analyses were conducted using the general linear model in SPM2 (Wellcome Department of Cognitive Neurology, London, UK). For the first-level analyses, contrast images were generated for each subject to assess activation differences between visual task conditions. These initial contrast images (4-second delay - matching and 1-second delay - matching) were subtracted from each other to isolate the visual working memory component. We assessed effects of hormone use using this working memory component. Regions included in our analysis were significant at false discovery rate (FDR)-corrected p< 0.05 using a 1 sample t test to evaluate effect of task in our study population, as well as regions deemed significant by a priori hypothesis at uncorrected p< 0.001. Regions meeting this criterion were included in further evaluations. An initial effect-of-treatment analysis was performed using ANOVA with treatment group as the independent variable and 4-second delay – 1-second delay fMRI BOLD signal as the dependent variable. Subsequent post-hoc analyses were performed between treatment groups using 2 sample t tests and general linear models. A correlation between task performance and brain activation during the visual task was determined using multiple linear regression in SPM2, and confirmed with partial correlation analyses (to describe the linear relationship between performance and activation while holding age and education constant) in SPSS (SPSS Inc, Chicago, IL), using beta coefficients extracted from the regions found to be correlated in SPM2. All comparisons made between two HT groups controlled for age and education level, and comparisons made between the hormone-using groups or between past and current hormone users additionally controlled for duration of treatment and age at treatment initiation.
RESULTS
Study Demographics
The demographic and baseline information for study participants is described in Table 1. Neuropsychological testing documents normal IQ, spatial perception, and memory, and absence of dementia and depression. The average age of participants was 66.2 ± 5.5 years. In the three-group comparison, there were no demographic differences between the groups. Post-hoc two-group comparisons show that age, education, age at treatment initiation, and duration of hormone use differ between the estrogen only and the combined estrogen and progestin treatment groups. Current and past hormone users were similar to each other in most respects, except that current hormone users had fewer years of education than past users.
Table 1.
demographic and baseline information
| n=55 | n=17 | n=20 | n=28 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Subjects | ET | EPT | T Test* | Never-Treated ANOVA† | ||||||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | p | Mean | ± | SD | p | |
| Age (yr) | 66.2 | ± | 5.5 | 68.5 | ± | 6.1 | 64.4 | ± | 4.8 | 0.05 | 66.1 | ± | 5.2 | 0.12 |
| Education | 16.4 | ± | 2.8 | 15.0 | ± | 2.6 | 17.3 | ± | 2.6 | 0.01 | 16.6 | ± | 3.0 | 0.04 |
| Age began HT | 48.0 | ± | 4.1 | 46.2 | ± | 4.2 | 49.4 | ± | 3.3 | 0.00 | 0.02 | |||
| Years on HT | 16.4 | ± | 6.2 | 20.2 | ± | 6.3 | 13.3 | ± | 4.1 | 0.00 | 0.00 | |||
| Benton Visual Retention Test | 6.5 | ± | 1.8 | 6.2 | ± | 2.3 | 6.3 | ± | 1.5 | 0.79 | 7.1 | ± | 1.5 | 0.11 |
| Mini-Mental State Examination | 28.6 | ± | 1.5 | 28.3 | ± | 1.5 | 28.7 | ± | 0.3 | 0.42 | 28.8 | ± | 1.8 | 0.65 |
| Shipley-Estimated IQ | 113.0 | ± | 9.9 | 110.0 | ± | 9.4 | 113.0 | ± | 8.5 | 0.34 | 116.0 | ± | 11.4 | 0.20 |
| Geriatric Depression rating Scale | 0.6 | ± | 0.9 | 0.9 | ± | 1.2 | 0.5 | ± | 0.8 | 0.19 | 0.6 | ± | 0.8 | 0.29 |
| n=13 | n=24 | |||||||||||||
| Current Users | Past Users | T Test‡ | ||||||||||||
| Mean | ± | SD | Mean | ± | SD | p | ||||||||
| Age (yr) | 67.3 | ± | 7.3 | 65.9 | ± | 4.8 | 0.49 | |||||||
| Education | 14.9 | ± | 2.9 | 17.1 | ± | 2.5 | 0.02 | |||||||
| Age began HT | 47.6 | ± | 4.7 | 48.1 | ± | 3.6 | 0.74 | |||||||
| Years on HT | 18.2 | ± | 7.4 | 15.7 | ± | 5.4 | 0.26 | |||||||
| Benton Visual Retention Test | 6.7 | ± | 2.1 | 6.1 | ± | 1.8 | 0.35 | |||||||
| Mini-Mental State Examination | 28.3 | ± | 1.6 | 28.7 | ± | 1.2 | 0.40 | |||||||
| Shipley-Estimated IQ | 109.6 | ± | 9.6 | 113.0 | ± | 8.5 | 0.28 | |||||||
| Geriatric Depression rating Scale | 0.6 | ± | 1.0 | 0.3 | ± | 1.0 | 0.66 | |||||||
| Time since stopped HT | 2.1 | ± | 1.0 | |||||||||||
t test comparison between ET and EPT groups
ANOVA comparison between all three treatment groups
t test comparison between current and past hormone users
Neuroimaging Task Performance
There were no significant differences between treatment groups on the visual memory task. Women in the estrogen only group scored 82 ± 11 and 80 ± 10 percent correct, those in the EPT group scored 85 ± 10 and 82 ± 11 percent correct, and the never-treated group scored 88 ± 9 and 86 ± 8 percent correct for the 1- and 4-second delay tasks, respectively (two-tailed unpaired t tests, p> 0.05). Current and past hormone users did not score significantly differently from each other on the visual memory task: current users had a mean accuracy of 78.6 ± 9.9% and past users had a mean accuracy of 82.8 ± 11.1%.
Regional Activity during Visual Working Memory Task
For all subjects, regions activated by the visual working memory task are detailed in Table 2, including the right inferior frontal cortex, anterior cingulate, bilateral anterior insula, bilateral superior parietal cortex, left hippocampus and bilateral parahippocampal gyrus, and the midbrain raphe region. After introducing age and education as covariates, activation patterns remained similar, except that the right parahippocampal gyrus and the raphe no longer achieved statistical significance. Further activations became significant in the left prefrontal cortex (z = 3.11), anterior cingulate (z = 3.96), right hippocampus (z = 2.96), and posterior cingulate (z = 3.95).
Table 2.
areas activated in all subjects during the visual working memory task
| Region | Coordinates | Z† | Cluster Size |
|---|---|---|---|
| (x,y,z)* | (mm3) | ||
| Right inferior frontal lobe | 50,32,2 | 4.27 | 3448 |
| Anterior cingulate | 6, 24, 42 | 3.17 | 1672 |
| Right anterior insula | 54, 20, 8 | 4.05 | 7696 |
| Left anterior insula | −40, 14,6 | 4.44 | 3208 |
| Left superior parietal lobe | −26, −54, 46 | 4.26 | 10584 |
| Right superior parietal lobe | 20, −58, 52 | 3.56 | 2104 |
| Right superior parietal lobe | 40, −40, 38 | 3.14 | 2016 |
| Left hippocampus | −20, −30,−16 | 4.16 | 448 |
| Left parahippocampal gyrus | −44, −56, −6 | 5.15 | 11984 |
| Right parahippocampal gyrus | 38, −62, 8 | 3.34 | 4472 |
| Raphe | 4, −28, 0 | 3.18 | 304 |
ICBM coordinates where regions were centered
All regions significant at p < 0.05, corrected for multiple comparisons (false detection rate method)
Task performance correlations to regional activation
We used linear regression and partial correlation analyses to assess correlations between regional activation and performance on the 1- and 4-second delay conditions of the visual task, controlling for age and education, across the entire sample. Performance accuracy for the 1-second delay condition was not positively or negatively correlated to increased activation anywhere in the brain. For the 4-second delay condition, increased activation was positively correlated with better performance in the right hippocampus (z = 3.45, p< 0.000 regression; R = 0.41, p = 0.003 partial correlation), and bilaterally in the medial temporal lobe (right: z = 3.46, p< 0.000 regression; R = 0.19, p = 0.17 partial correlation; left: z = 3.12, p = 0.001 regression; R = 0.33, p = 0.019 partial correlation).
Effect of Hormone Use on Visual Working Memory
Significant activation differences were observed between treatment groups during the visual working memory task (Table 3, Figure 1). Main effects of treatment occurred in the right inferior frontal lobe, anterior cingulate, left anterior insula, right parietal and left superior parietal cortices, left hippocampus and bilateral hippocampal gyrus, posterior cingulate, and the raphe. Compared to never-treated women, both HT groups had more regional activation (Table 4). ET women had more activation than never-treated women bilaterally in the superior frontal lobe, left anterior and bilateral posterior insula, right superior and bilateral inferior frontal lobe, left hippocampus, and posterior cingulate. EPT women had more activation than never-treated women in the right inferior and left superior frontal lobe, anterior cingulate, bilateral insula, right superior parietal lobe, bilateral hippocampus, left parahippocampal gyrus, and raphe. Never-treated women did not have more activation in any region than women in either of the HT groups.
Table 3.
differences between treatment groups activation patterns during visual working memory task
| Region | Coordinates | F† | Cluster Size |
|---|---|---|---|
| (x,y,z)* | (mm3) | ||
| Right inferior frontal lobe | 54,20,6 | 9.2 | 2912 |
| Anterior cingulate | 8,60,0 | 7.52 | 2000 |
| Anterior cingulate | 12,30,36 | 6.06 | 432 |
| Left anterior insula | −40,14,12 | 8.77 | 6288 |
| Right parietal lobe | 58,−62,28 | 6.95 | 1168 |
| Left superior parietal lobe | −26, −60, 52 | 7.91 | 3024 |
| Left hippocampus | −18,−30,−14 | 7.53 | 1976 |
| Right parahippocampal gyrus | 38, −62, 8 | 5.87 | 1128 |
| Left parahippocampal gyrus | −44, −56, −4 | 14.56 | 20112 |
| Posterior cingulate | 2,−36,32 | 7.41 | 9096 |
| Raphe | 2,−28,−2 | 6.3 | 392 |
ICBM coordinates where regions were centered
All regions significant at p < 0.05, corrected for multiple comparisons (false detection rate method), or by a priori hypothesis at p < 0.001
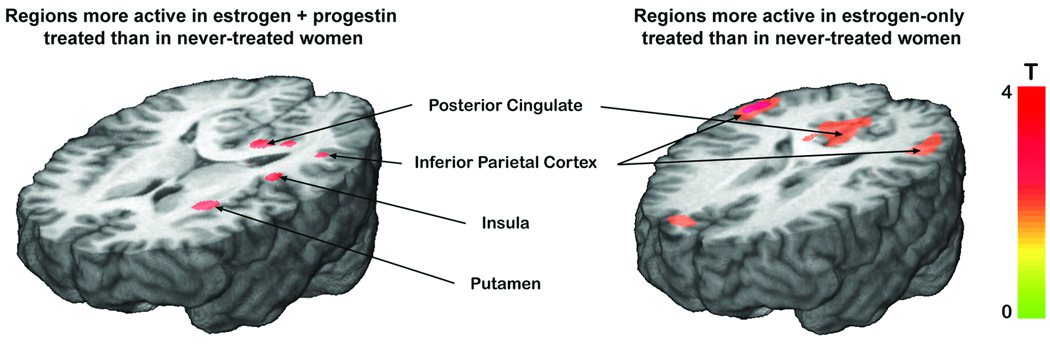
Figure 1.
neural activity during visual working memory is greater in women treated with combined estrogen and progestin (left) or estrogen only (right) than in never-treated controls. During the working memory component of the delayed matching-to-sample task (4-second delay - 1-second delay), regions that generated greater fMRI-BOLD signal in combined EPT treated women than in never-treated women (left) include the posterior cingulate, inferior parietal cortex, insula, and putamen. Regions with greater fMRI-BOLD signal in estrogen-only treated women than in never-treated controls (right) include the posterior cingulate and inferior parietal cortex. Color scale depicts T score of significant regions.
Table 4.
regional activaty during visual working memory in long-term hormone users compared to never-users, controlling age & education
| Region | Coordinates | Z† | Cluster Size |
|---|---|---|---|
| (x,y,z)* | (mm3) | ||
| ET > Never-Users | |||
| Right superior frontal lobe | 24,26,48 | 3.49 | 1704 |
| Left superior frontal lobe | −30,20,42 | 3.32 | 4088 |
| Left anterior insula | −44,4,−16 | 3.38 | 1880 |
| Right posterior insula | 46,−16,−2 | 3.18 | 352 |
| Left posterior insula | −44,−28,12 | 3.41 | 1272 |
| Right superior parietal lobe | 24,26,48 | 3.9 | 1704 |
| Right inferior parietal lobe | 60,−62,28 | 3.66 | 4456 |
| Left inferior parietal lobe | −50,−72,18 | 3.13 | 1480 |
| Left hippocampus | −18,−22,−8 | 3.16 | 936 |
| Posterior cingulate | 2,−20,44 | 2.47 | 184 |
| EPT > Never-Users | |||
| Right prefrontal cortex | 18,30,38 | 3.46 | 1296 |
| Right inferior frontal lobe | −70,−22,0 | 3.01 | 192 |
| Left superior frontal lobe | −12,14,62 | 3.07 | 2224 |
| Left putamen | −28,8,10 | 3.6 | 2232 |
| Right insula | 56,18,4 | 2.92 | 720 |
| Left interior insula | −44,6,−16 | 3.1 | 272 |
| Left posterior insula | −42,−30,16 | 3.58 | 9416 |
| Right superior parietal lobe | 60,−54,38 | 3.91 | 9272 |
| Right hippocampus | 18,−30,−14 | 2.77 | 288 |
| Left hippocampus | −14,−30,−16 | 3.04 | 304 |
| Left parahippocampal gyrus | −10,−50,12 | 3.22 | 2768 |
| Posterior cingulate | −20,−42,4 | 3.36 | 9416 |
| Raphe | 0,−28,−4 | 3.04 | 1312 |
ICBM coordinates where regions were centered
All regions significant at p < 0.05, corrected for multiple comparisons (false detection rate method), or by a priori hypothesis at p < 0.001
Table 5 presents a comparison of regional activation between the HT groups. Compared to ET women, the EPT group had greater activation than the estrogen only group in the left superior parietal cortex and bilateral parahippocampal gyrus. ET women had greater regional activity in the right superior frontal cortex, prefrontal cortex, and superior parietal cortex.
Table 5.
comparison of ET and EPT on visual working memory, controlling age, education, age at hormone initiation, and duration of treatment
| Region | Coordinates | Z† | Cluster Size |
|---|---|---|---|
| (x,y,z)* | (mm3) | ||
| EPT > ET | |||
| Left superior parietal cortex | −22, −36, 54 | 3.21 | 248 |
| Right parahippocampal gyrus | 22,−50,10 | 2.53 | 24 |
| Left parahippocampal gyrus | −22,−60,14 | 2.66 | 208 |
| ET > EPT | |||
| Right superior frontal lobe | 54,−8,52 | 2.75 | 648 |
| Right prefrontal cortex | 48,8,36 | 2.96 | 504 |
| Right superior parietal cortex | 36, −34, 62 | 2.78 | 552 |
ICBM coordinates where regions were centered
All regions significant at p < 0.05, corrected for multiple comparisons (false detection rate method), or by a priori hypothesis at p < 0.001
Because both current and past hormone users were included in the study, we compared activation patterns from current and past users of both hormone treatments, controlling for age, education, duration of hormone treatment, and age at treatment initiation. Past users had more activation than current users in a 544 mm3 cluster within the right prefrontal cortex (Z = 2.98), while current users did not have any regions significantly more activated than past users.
DISCUSSION
Historically, cognitive benefits of postmenopausal hormone therapy have not been consistently demonstrated. The timing of treatment in relation to menopause is a likely source of differences in results between studies. Evidence suggests that treatment began soon after menopause is most likely to impart a cognitive benefit 9, 13, 15, 41–44. Accordingly, continued studies focusing on cognitive outcomes in those women who began hormone use near the time of menopause are warranted. In this study, we addressed the cognitive effects of hormones in women who began long-term treatment close to the time of menopause, and considered the type of hormone used. Our results indicate that early-initiated long-term hormone use is associated with increased regional brain activation during a visual working memory task, with women in both hormone-treatment groups exhibiting a more robust neural response than never-treated women. In contrast to previous studies that have found a differential effect of progestins on cognitive performance measures, we did not identify a benefit to estrogen only compared to combined estrogen plus progestin therapy 7, 16, 23. Our results support the hypothesis that long-term hormone therapy, when initiated close to menopause, maintains a positive effect on visual memory processes over a decade later.
Prior neuroimaging studies have found differences in neural activity between postmenopausal hormone and non-hormone users. The blood oxygen level-dependent fMRI (fMRI-BOLD) method has measured increased regional activity in estrogen users versus non-users during working memory tasks in the frontal and parietal cortices, regions involved in working memory 45. fMRI-BOLD has also demonstrated increased frontal activity during both verbal and spatial working memory tasks in perimenopausal and postmenopausal estrogen users versus non-users 44. Our group previously used fMRI-BOLD to compare brain activation patterns in women in their fifties after four weeks each of placebo or hormone therapy (5 µg ethinyl estradiol and 1 mg norethindrone acetate) while the subjects performed this same visual memory task 26. The short-term hormone use was associated with increased bilateral activation of the prefrontal cortex. The results of this previous study of acute estrogen effects in younger women are similar to those of the current study of chronic long-term hormone use, except the current study also identified greater activation in hormone users in brain regions associated with learning and memory, including the hippocampus, parahippocampal gyrus, and raphe.
In the present study, increased fMRI-BOLD signal in hormone-treated women appears to reflect a benefit of hormone use. Increased brain activation can indicate less effective cognitive processing or inefficient compensatory mechanisms during early mild cognitive impairment (MCI), and may predict future cognitive decline 46, 47. Other studies, however, have found decreased neural activity indicative of cognitive decline, with increased activation in normal older adults 48. In the current study, greater regional activity occurred in regions expected to be used during the visual working memory task, reflecting neural activation associated with processing visual information as required by the task. These results agree with increased neural activity observed in previous studies, including our own study of mid-age women after four weeks of hormone treatment, a timeframe unlikely to result in compensatory mechanisms associated with MCI, but rather upregulated synaptic activity reflecting greater estrogen availability 26, 49. We also detected increased posterior cingulate activation in hormone users, a region where decreased activity can be predictive of Alzheimer’s disease 50, 51. Finally, fMRI-BOLD signal in the right hippocampus, a region central to memory processing, positively correlated to task accuracy, implying a cognitive benefit to the increased activity measured during the visual memory task. Increased brain activation was correlated only to performance on the longer 4-second delay condition, and not the short 1-second delay condition, suggesting that the increased regional activation is specific to visual working memory processes.
Synthetic progestins, including the medroxyprogesterone acetate used by the women in this study, have potentially deleterious effects on cognitive performance 9, 16, 52. However, we found that women in both HT groups had different activation patterns than never-treated women during the visual memory task, and these patterns did not differ greatly between the hormone-treated groups. Women in the EPT group had slightly more regional activation than those in the ET group, most notably bilaterally in the parahippocampal gyrus, even after accounting for differences between the groups.
Many of the regions differentially activated between treatment groups are dense in estrogen receptors, progesterone receptors, or both. Estrogen receptor subtypes are present in limbic areas associated with emotional and cognitive processing, and in regions connecting to cognitive association areas, including the amygdala, hippocampus, thalamus, and cortex 53–56. Progesterone receptors are found in many of the same regions as estrogen receptors, partly because they are induced by estrogens 57, 58. Women in both HT groups showed greater activation in many of these regions during the visual memory task compared to never-treated women. Estrogen and progesterone receptors regionally decrease with age, and are sensitive to exogenous hormones 57, 59, 60. As both estrogens and progesterone provide neuroprotection and help prevent degenerative effects of aging 2, 58, 61, 62, the increased activation in hormone treated women may reflect preservation of cognitive association areas used during the visual task. This idea is supported by prior studies demonstrating increased parietal, occipital, cerebellar, and hippocampal volumes in postmenopausal women currently taking estrogen 63, 64, although these findings are in contradiction to that of the WHIMS-MRI study showing increased hippocampal atrophy and size reduction in hormone users, albeit in older women 65.
The goal of this study was to examine differences in visual working memory after chronic hormone use compared to no hormone use, so women who recently quit long-term hormones were included along with current hormone users. Apart from a statistically significant difference in mean years of education, these two groups were demographically similar (although the smaller sample size used to compare current and past users may increase the likelihood of a type II error). Current and past hormone users had a similar pattern of neural activation during the visual memory task. After accounting for variations in age, education, duration of hormone use, and age at hormone initiation, current users did not have more activation than past users in any part of the brain. Past users showed slightly more activation than current users in only one small region within the prefrontal cortex. While it is difficult to make conclusions based on one differing region, these results may be consistent with others that have indicated a persisting cognitive benefit to past hormone users 24, 64. Other than this region, the activation patterns between these two HT groups were sufficiently similar to justify including them in the same groups when comparing hormone ever-users to never-users.
Strengths of this study include the comparison of consistent hormone users to never-users, with separate analyses of ET and EPT women, all on identical dose and formulations. This assessment of long-term use required a cross-sectional design, as randomization to long-term hormone use was unfeasible. Our study examined effects of chronic, rather than acute, hormone use, so allowed inclusion of both current and past hormone users; women falling into these groups were demographically similar, had similar behavioral outcomes, and had only minor differences in brain activation during the visual task, suggesting a persisting chronic effect of long-term hormone use in these women. Demographic and hormone-use characteristics were kept as similar as possible between groups, and any factors that differed between groups were controlled for in the analyses. We did not include a comparison group of women who began hormone use well past menopause, but limiting our study to women who began hormone use early allowed us to only include women who used hormones long-term. A limitation of this design is the inability to separate effects of early initiation from those of long-term use of hormones. Onset of menopause was self-defined, although whenever possible we confirmed dates with healthcare records.
CONCLUSION
This study of early-initiation, long-term hormone use suggests postmenopausal hormone use is beneficial to visual working memory. During our test of visual working memory, we observed more regional brain activation in hormone-treated women than in never-treated women, specifically in regions important to visual working memory. Controlling for differences in age and education, this increased regional activation was associated with better performance on the visual memory task. We found similarly positive effects in both estrogen-only and combined estrogen-progestin treated women. While long-term hormone therapy may not be beneficial in terms of chronic disease prevention 66, 67, our results suggest that if begun close to the onset of menopause, hormone use may benefit visual memory processes in postmenopausal women.
ACKNOWLEDGEMENTS
We thank the Michigan Clinical Research Unit (formerly General Clinical Research Center), and the fMRI laboratory (Eve Gochis, Keith Newnham, Luis Hernandez, Ph.D., and Douglas Noll, Ph.D.) at the University of Michigan for their assistance. We especially thank the participants of our study.
Financial Support
This work was supported by the National Center for Research Resources (K23 RR17043 and UL1RR024896), and for investigator support, by the National Institute for Child Health and Human Development (5T32HD007048), National Institute on Aging and the Office for Research on Women’s Health (RO1AG027675), and the University of Michigan’s Postdoctoral Translational Scholars Program award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest & Disclosures
The authors have nothing to disclose
REFERENCES
- 1.Jensen EJH. Basic guides to the mechanism of estrogen action. Recent Progress Hormone Research. 1962;18:387–408. [Google Scholar]
- 2.McEwen B. Estrogen actions throughout the brain. Recent progress in hormone research. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine reviews. 1999 Jun;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 4.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007 May;72(5):381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH. Possible acceleration of age effects on cognition following menopause. Journal of psychiatric research. 1995 May–Jun;29(3):153–163. doi: 10.1016/0022-3956(95)00005-p. [DOI] [PubMed] [Google Scholar]
- 6.Markou A, Duka T, Prelevic GM. Estrogens and brain function. Hormones (Athens) 2005 Jan–Mar;4(1):9–17. doi: 10.14310/horm.2002.11138. [DOI] [PubMed] [Google Scholar]
- 7.Maki PM, Sundermann E. Hormone therapy and cognitive function. Hum Reprod Update. 2009 May;25 doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Hormones and behavior. 2005 Mar;47(3):371–375. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Annals of the New York Academy of Sciences. 2005 Jun;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 10.Henderson VW. Cognition and cognitive aging. Climacteric. 2007 Oct;10 Suppl 2:88–91. doi: 10.1080/13697130701537363. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. Journal of neuroscience research. 2003 Dec 1;74(5):637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- 12.Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? Journal of neuroendocrinology. 2007 Feb;19(2):77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 13.Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138(3):1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Maki PM. The timing of estrogen therapy after ovariectomy--implications for neurocognitive function. Nature clinical practice. 2008 Sep;4(9):494–495. doi: 10.1038/ncpendmet0901. [DOI] [PubMed] [Google Scholar]
- 15.Maki PM. Potential importance of early initiation of hormone therapy for cognitive benefit. Menopause. 2006 Jan–Feb;13(1):6–7. doi: 10.1097/01.gme.0000194822.76774.30. [DOI] [PubMed] [Google Scholar]
- 16.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Frontiers in neuroendocrinology. 2008 Jan;29(1):88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. Jama. 2004 Jun 23;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 18.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. Jama. 2003 May 28;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 19.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997 Jun;48(6):1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 20.Tang MX, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996 Aug 17;348(9025):429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 21.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. Jama. 2002 Nov 6;288(17):2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 22.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. Jama. 2004 Jun 23;291(24):2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 23.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. The Journal of clinical endocrinology and metabolism. 2006 May;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 24.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005 Jan–Feb;12(1):12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan AH, Henderson VW, Paine BJ, et al. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006 Jan–Feb;13(1):28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- 26.Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. The Journal of clinical endocrinology and metabolism. 2006 Nov;91(11):4476–4481. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith YR, Johnson AM, Newman LA, Greene A, Johnson TR, Rogers JL. Perceptions of clinical research participation among African American women. Journal of women's health (2002) 2007 Apr;16(3):423–428. doi: 10.1089/jwh.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers JL, Johnson TR, Brown MB, Lantz PM, Greene A, Smith YR. Recruitment of women research participants: the Women's Health Registry at the University of Michigan. Journal of women's health (2002) 2007 Jun;16(5):721–728. doi: 10.1089/jwh.2006.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Shipley WC. Institute of Living Scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]
- 31.Yesavage JA, Rose TL, D L. Validity of the geriatric depression scale in subjects with senile dementia. Palo Alto: Clinical Diagnostic and Rehabilitation Unit, Veterans Administrative Medical Center; 1981. [Google Scholar]
- 32.Benton AL. Revised visual retention test. New York: The Psychological Corporation; 1974. [Google Scholar]
- 33.Benton AL, Hamsher K, Varney N, Spreen O. Contributions to neuropsychological assessment. New York: Oxford University Press; 1983. [Google Scholar]
- 34.Lencz T, Bilder RM, Turkel E, et al. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Archives of general psychiatry. 2003 Mar;60(3):238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- 35.Phillips W. On the distinction between sensory storage and short-term visual memory. Perception and Psychophysics. 1974;16:283–290. [Google Scholar]
- 36.Noll D, Stenger V, Vasquez A, Peltier S. Spiral scanning in functional MRI. Heidelberg: Springer-Verlag; 1999. [Google Scholar]
- 37.Acquirre C, D'Esposito M. Experimental design for brain fMRI. Heidelberg: Springer-Verlag; 1999. [Google Scholar]
- 38.Friston K, Ashburner J, Frith C, Poline J-B, Heather J, Frackowiak R. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- 39.Meyer CR, Boes JL, Kim B, et al. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Medical image analysis. 1997 Apr;1(3):195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- 40.Sun FT, Schriber RA, Greenia JM, He J, Gitcho A, Jagust WJ. Automated template-based PET region of interest analyses in the aging brain. NeuroImage. 2007 Jan 15;34(2):608–617. doi: 10.1016/j.neuroimage.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith YR, Giordani B, Lajiness-O'Neill R, Zubieta JK. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertility and sterility. 2001 Dec;76(6):1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- 42.Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997 Dec;49(6):1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- 43.Kimura D. Estrogen replacement therapy may protect against intellectual decline in postmenopausal women. Hormones and behavior. 1995 Sep;29(3):312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- 44.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006 May–Jun;13(3):411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 45.Shaywitz SE, Shaywitz BA, Pugh KR, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. Jama. 1999 Apr 7;281(13):1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 46.Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer's disease: insights from functional MRI studies. Neuropsychologia. 2008;46(6):1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. Journal of neurology, neurosurgery, and psychiatry. 2008 Jun;79(6):630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology. 2003 Aug 26;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maki PM, Dumas J. Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Seminars in reproductive medicine. 2009 May;27(3):250–259. doi: 10.1055/s-0029-1216278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Annals of neurology. 1997 Jul;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 51.Rasgon NL, Silverman D, Siddarth P, et al. Estrogen use and brain metabolic change in postmenopausal women. Neurobiology of aging. 2005 Feb;26(2):229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002 Jan;143(1):205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 53.Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95(2):333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- 54.Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. The Journal of clinical endocrinology and metabolism. 2000 Oct;85(10):3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- 55.Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 2001 Jun;64(3):251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 56.Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003 Dec;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 57.Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Experimental biology and medicine (Maywood, N.J. 2004 Nov;229(10):977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- 58.Brinton RD, Thompson RF, Foy MR, et al. Progesterone receptors: form and function in brain. Frontiers in neuroendocrinology. 2008 May;29(2):313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shima N, Yamaguchi Y, Yuri K. Distribution of estrogen receptor beta mRNA-containing cells in ovariectomized and estrogen-treated female rat brain. Anat Sci Int. 2003 Jun;78(2):85–97. doi: 10.1046/j.0022-7722.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain research. 2007 Jun 25;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annual review of pharmacology and toxicology. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- 62.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in neuroendocrinology. 2008 May;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiology of aging. 2008 Jan;29(1):95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Ghidoni R, Boccardi M, Benussi L, et al. Effects of estrogens on cognition and brain morphology: involvement of the cerebellum. Maturitas. 2006 Jun 20;54(3):222–228. doi: 10.1016/j.maturitas.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Resnick SM, Espeland MA, Jaramillo SA, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009 Jan 13;72(2):135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 67.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. Jama. 2004 Apr 14;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]