Abstract
A primary focus in autoimmunity is the breakdown of central and peripheral tolerance resulting in the survival and eventual activation of autoreactive T cells. As CD4+ T cells are key contributors to the underlying pathogenic mechanisms responsible for the onset and progression of most autoimmune diseases, they are a logical target for therapeutic strategies. One method for restoring self-tolerance is to exploit the endogenous regulatory mechanisms that govern CD4+ T cell activation. In this review, we discuss tolerance strategies with the common goal of inducing antigen (Ag)-specific tolerance. Emphasis is given to the use of peptide-specific tolerance strategies, focusing on ethylene carbodiimide (ECDI)-peptide-coupled cells (Ag-SP) and non-mitogenic anti-CD3, which specifically target the T-cell receptor (TCR) in the absence of costimulatory signals. These approaches induce a TCR signal of insufficient strength to cause CD4+ T cell activation and instead lead to functional T-cell anergy/deletion and activation of Ag-specific induced regulatory T cells (iTregs) while avoiding generalized long-term immunosuppression.
1. Title: Prospects for Antigen-Specific Tolerance Based Therapies for the Treatment of Multiple Sclerosis
1.1 Introduction
This chapter will focus on tolerance mechanisms in multiple sclerosis (MS) and it’s mouse model, experimental autoimmune encephalomyelitis (EAE). An important goal of current research is to develop new therapies for autoimmune diseases by specifically inhibiting and/or tolerizing autoreactive CD4+ T cells. Although this chapter focuses on tolerance strategies that directly target autoreactive T cells in MS and EAE, similar approaches are ongoing in other autoimmune diseases, as well as in tissue transplantation.
MS is an immune-mediated disease of the central nervous system (CNS) characterized by perivascular CD4+ and CD8+ T cell and mononuclear cell infiltration with subsequent primary demyelination of axonal tracks leading to progressive paralysis (Wekerle, 1991). MS is generally understood to be an autoimmune disease characterized by T cell responses to myelin basic protein (MBP), proteolipid protein (PLP), and/or myelin-oligodendrocyte glycoprotein (MOG) (Bernard and de Rosbo, 1991; de Rosbo et al., 1997; Ota et al., 1990), however a straightforward cause-effect relationship between myelin reactivity and disease pathology has not been demonstrated.
Characteristically there are four courses of clinical disease in MS: 1) relapsing-remitting, 2) secondary-progressive, 3) primary-progressive, and 4) progressive-relapsing. Correspondingly, there are relapsing-remitting and chronic mouse models of MS, i.e. EAE. Relapsing-remitting EAE (R-EAE) is characterized by transient ascending hind limb paralysis, perivascular mononuclear-cell infiltration, and fibrin deposition in the brain and spinal cord with adjacent areas of acute and chronic demyelination (Paterson and Swanborg, 1988). Given that the etiology of MS is unknown, the inducing antigen (Ag) has yet to be identified and the probability that CD4+ T cell respond to multiple epitopes contained within several myelin proteins are responsible for chronic disease progression, the use of Ag-specific tolerance-based immunotherapy targeting a single protein is challenging. Furthermore, a pathological role for epitope spreading is difficult to verify in human MS because the initiating Ag is not known. In contrast, animal models, such as EAE, have the advantage that the initiating Ag is known. For example, in the SJL model of disease in which mice are primed with PLP139–151 in complete Freund’s adjuvant (CFA), PLP139–151-specific CD4+ T cell reactivity in secondary lymphoid organs is maintained throughout the disease course. Beside the activation of CD4+ T cells specific for the initiating antigen, PLP178–191-specific CD4+ T cell reactivity arises (intramolecular epitope spreading) during the first disease relapse, and CD4+ T cells specific for a myelin basic protein epitope, MBP84–104, arise (intermolecular epitope spreading) during the second disease relapse (McRae et al., 1995b; Vanderlugt et al., 2000). While Ag-specific tolerance can be induced in this experimental model as the self peptides are well characterized, this is not true for humans with MS.
Although the etiology of MS is unknown, both genetic (Ebers et al., 1995) and environmental factors appear to play a role in susceptibility and initiation of disease. Epidemiological studies provide strong circumstantial evidence for an environmental trigger, most likely viral, in the induction of MS (Kurtzke, 1993; Olson et al., 2001b; Waksman, 1995). CNS pathology may therefore result from bystander myelin damage mediated via T cells targeting a CNS-persisting virus; and/or from activation of autoreactive T cells secondary to an encounter with a pathogen directly by molecular mimicry (Fujinami and Oldstone, 1985; Olson et al., 2001a; Wucherpfennig and Strominger, 1995), or indirectly by epitope spreading resulting from the release of sequestered antigens secondary to virus-specific T cell-initiated myelin damage (McRae et al., 1995a; Miller and Karpus, 1994; Miller et al., 1997). It is believed that the combination of persistent CNS inflammation and resulting myelin/nerve damage produces the clinical symptoms of MS. In addition to the EAE model of MS, another commonly used and virally induced model of MS is Theiler’s murine encephalomyelitis virus (TMEV) induced demyelinating disease (TMEV-IDD) (Dal Canto et al., 1997). TMEV is a naturally occurring mouse pathogen and disease is induced in recipient mice by intracerebral injection of the positive strand RNA virus that leads to a primary progressive demyelinating disease (Miller et al., 1997).
As integral members of the adaptive immune system, CD4+ T cells are key mediators in multiple phases of the protective immune response by recognizing foreign Ags via their antigen-specific T cell receptor (TCR) complex during cognate interactions with antigen presenting cells (APCs) displaying peptide/major histocompatibility complex (MHC) class II complexes. Thus, an essential characteristic of intrathymic T cell development is the generation of TCR diversity enabling T cells to respond to an unlimited number of foreign antigens (Ags). However, one inevitable consequence of TCR diversity is the generation of self-reactive TCRs creating the potential for autoimmune disease. To balance this, the immune system has developed regulatory checkpoints that govern lymphocyte development which includes the biphasic processes of central tolerance which only permits the generation of T cells with a functional TCR while deleting populations of T cells which express TCRs strongly reactive to self-peptides (Hogquist et al., 2005). Additionally, the immune system has created peripheral tolerance mechanisms to safe guard against autoreactive T cells that escape thymic deletion. Mechanisms of peripheral tolerance include T cell intrinsic mechanisms (ignorance, anergy, phenotypic skewing/immune deviation and deletion/apoptosis) as well as T cell extrinsic mechanisms (induction of tolerogenic dendritic cells (DCs) and/or regulatory T cells (Treg) (Walker and Abbas, 2002). Thus, when functioning properly, the process of central and peripheral tolerance ensures the selective generation and regulation of functional, non-self-reactive T cells.
The breakdown of immune tolerance resulting in the persistence and eventual activation of autoreactive T cells (Christen and von Herrath, 2004) is a fundamental theme in autoimmunity. Since CD4+ T cells are key contributors to the underlying pathogenic mechanisms responsible for the onset and progression of most autoimmune diseases, they are also a logical target for therapeutic intervention. However, as discussed above, these cells are critical to the induction of adaptive immunity, thus creating a complex functional dichotomy that underscores the necessity for active regulatory mechanisms, such as the two-signal hypothesis, and therapeutic interventions that both promote immunity against foreign Ags, while inhibiting self-directed responses.
One technique for restoring self-tolerance is to exploit the endogenous regulatory mechanisms that govern CD4+ T cell activation. Typically, endogenous ligation of the TCR by peptide/MHC class II alone produces a signal of insufficient strength to activate a CD4+ T cell and can instead induce functional anergy or deletion. As a consequence, additional APC-derived costimulatory signals (e.g. CD80/86 engagement of CD28) are required to lower the threshold required for successful T cell activation. This “two-signal” hypothesis predicts that TCR stimulation in the absence of costimulatory signals leads to CD4+ T cell anergy, tolerance, and/or depletion (Sharpe and Freeman, 2002). Therefore, either TCR ligation in the absence of costimulatory signals or exogenous targeting of the costimulatory pathway would appear to be a logical target of therapeutic strategies to down-regulate the pathologic functions of autoreactive CD4+ T cells. In light of this, various therapeutic approaches have been designed to block autoreactive CD4+ T cell function during autoimmune disease, including the administration of blocking antibodies directed against a variety of epitopes including CD3, CD4, CD28, CD40, CD80, CD86, CD154, ICOS, OX40, and 4-1BB, as well as CTLA4-Ig (Karandikar et al., 1998; Zhang et al., 2003). However, these treatment strategies, if administered over a long time period, often result in either non-specific immune suppression or other undesirable side effects. In this chapter techniques with the common purpose of inducing Ag-specific tolerance by specifically targeting the TCR to avoid detrimental influences on non-specific/bystander immune processes will be discussed.
1.2 Monoclonal Antibody induced tolerance
In an attempt to further test the two-signal hypothesis, several groups have investigated the therapeutic potential of anti-CD3 mAb treatment in the absence of costimulatory signals for the treatment of various autoimmune diseases. However, treatment with an unaltered anti-CD3 mAb is potentially a double-edged sword. While treatment may modify the activity of pathogenic autoreactive CD4+ T cells, thereby ameliorating autoimmune disease progression, this therapy may also induce non-specific side effects through the activation of bystander T cells. For example, the induction of general immunosuppression which increases the patient’s susceptibility to opportunistic infection and the common occurrence of high-dose syndrome in which treatment recipients suffer severe side-effects due to the non-specific production of inflammatory cytokines including TNF-α. Furthermore, cross-linking of CD3 may in some cases initiate a signal of sufficient strength that eliminates the need for a costimulatory molecule-induced reduction in the signal threshold required for T cell activation. Due to the aforementioned complications associated with the use of mitogenic anti-CD3 mAb, structural alterations have been made to the Fc binding domain so that the deleterious side effects may be avoided by lowering the level of non-specific T cell signaling. Non-mitogenic anti-CD3 mAb treatments induce lower levels of TCR-mediated signaling (Herold et al., 2003), and it is believed that lower levels of TCR-mediated signaling favor immune deviation from a Th1/17 to a Th2 phenotype and the activation of Tregs. In this scenario, the T cell-mediated immune response is changed from a Th1/17-like (disease-promoting) response, to a Th2-like (disease-regulating) response.
The therapeutic efficacy of bypassing costimulatory signals has been demonstrated using non-mitogenic anti-CD3 mAb therapy for the treatment of both EAE and the non-obese diabetic (NOD) mouse model of type 1 diabetes (TID) (Chatenoud, 2003; Chatenoud et al., 1994; Kohm et al., 2005). While anti-CD3 mAb treatment causes the functional cross-linking and activation of the TCR in an Ag-non-specific manner, a humanized form of anti-CD3 mAb, mutated to prevent binding to Fc receptors (OKT3 Ala-Ala), has had some success in phase I/II clinical trials for delaying onset of type I diabetes and treating psoriatic arthritis (Herold et al., 2002; Keymeulen et al., 2005; Pozzilli et al., 2000; Utset et al., 2002). This non-mitogenic anti-CD3 mAb induces a suboptimal level of TCR-mediated signalling (Herold et al., 2003) and the success of the therapy is thought to be due to multiple mechanisms including anergy induction, immune deviation and activation of Tregs (Belghith et al., 2003; Kohm et al., 2005). Administration of the humanized form of non-mitogenic anti-CD3 mAb in patients was however associated with side effects including a moderate cytokine release syndrome as well as symptoms of Epstein-Barr viral mononucleosis (Herold et al., 2005). Thus, broad-based TCR-directed therapies may have other undesired long-term effects on the immune system.
Other studies using monoclonal antibody therapies directed against molecules involved in lymphocyte/monocyte recruitment and activation include the use of antibodies directed against α4β1-integrin (VLA4; Tysabri; natalizumab) (Miller et al., 2003; Yednock et al., 1992), chemokines such as CC-chemokine ligand 3 (CCL3) (Karpus et al., 1995), or pro-inflammatory cytokines such as lymphotoxin (Ruddle et al., 1990). These and other immunosuppressive strategies promote the physical deletion or inactivation of entire subsets of T cells or cause non-specific inhibition of Ag presentation, pro-inflammatory cytokine production or T-cell trafficking. All of these strategies can compromise the ability of the host to combat opportunistic pathogens and/or increase the risk of neoplasia. This is illustrated by the recent deaths from progressive multifocal leukoencephalopathy (PML), an infection of the CNS by JC virus, which destroys myelin-producing oligodendrocytes, of several participants in an MS clinical trial following treatment with Tysabri (Khalili et al., 2007). Thus, Ag-specific tolerance strategies have the best therapeutic potential, but their success requires a more precise understanding of the autoantigen(s) and epitope(s) involved in the ongoing pathogenesis of a particular autoimmune disease.
1.3 Antigen specific induced tolerance induction
The direct targeting of autoreactive T cells is the ideal treatment strategy for autoimmune disease, resulting in Ag-specific unresponsiveness without global immunosuppression. There are currently four different protocols employed for inducing peptide-specific immune tolerance: altered peptide ligand (APL)-induced tolerance, mucosal (oral–nasal)-induced tolerance, soluble-peptide-induced tolerance, and ECDI-coupled-cell-induced tolerance. Each of these methods of peptide-specific tolerance induction is briefly discussed in the following section, as well as their putative mechanisms of action.
1.3.1. Altered peptide ligand (APL) induced tolerance
APLs are peptide analogues that bind to the same TCR, but elicit different functional responses as compared to the native autoepitope (Anderton and Wraith, 2002). APLs compete with the naïve peptide for TCR binding, altering the cascade of signalling events necessary for full T cell activation. APLs contain one or more amino-acid substitutions, typically binding with lower affinity to the TCR than the native peptide and function as either antagonists or partial agonists. Antagonistic APLs induce T cell anergy, while partial-agonist APLs induce incomplete activation of T cells. This partial activation can induce cytokine production in the absence of proliferation, thereby inducing immune deviation from Th1- and Th17-cell dependent responses to Th2- and Th3-cell dependent responses, or bystander suppression through the induction of Tregs (Nicholson et al., 1997; Young et al., 2000). A number of groups have tested the therapeutic efficacy of APLs of various myelin epitopes in treating established CNS autoimmune disease. In vivo administration of these myelin APLs were reported to prevent or reverse clinical disease progression in EAE, and were effective regardless of the route of administration, including subcutaneous (s.c.), intraperitoneal (i.p.) in incomplete Freund’s adjuvant, intranasal (i.n.), or intravenous (i.v.) routes (Nicholson and Kuchroo, 1997; Samson and Smilek, 1995; Wraith et al., 1989). Disease prevention with APLs of PLP139–151 is associated with the induction of Th2-cell differentiation. In support of immune deviation as the mechanistic basis of APL treatment, therapeutic clones responsive to PLP139–151-derived APLs have been shown to produce IL-4, IL-10, IL-13, and TGF-β, all of which are believed to suppress EAE, and antibodies directed against each of these cytokines attenuate the protective influence of APL-mediated treatment in EAE (Nicholson et al., 1995).
APLs with substitutions at amino-acid positions necessary for TCR engagement, that compete with the natural ligand and interfere with T cell activation, have also been tested in clinical trials. As discussed previously, APL therapy successfully inhibits EAE, however two separate MS Phase II clinical trials testing an APL of MBP83–99, the immunodominant HLA-DR2-restricted MBP T cell epitope, were halted due to safety concerns (Bielekova et al., 2000; Kappos et al., 2000). The number of CNS lesions of patients undergoing therapy were assessed by MRI and incidence of clinical relapse and hypersensitivity reactions were monitored. Participants in the first study, which included only eight patients tested at a 50 mg dose, demonstrated a higher incidence of MS exacerbations, as determined by both MRI and clinical criteria, and the APL cross-stimulated self-antigen reactive Th1 cells (Bielekova et al., 2000). A second double-blind, placebo-controlled study included 142 patients receiving various APL doses (Kappos et al., 2000) and was halted because 9% of patients (mostly in the group receiving the highest dose) developed hypersensitivity reactions. A potential problem with this approach is that a particular APL may be antagonistic for certain T cell clones, but at the same time serve as an agonist or super-agonist for peptide-specific clones expressing different TCRs. These studies aptly demonstrate the difficulty in moving from animal model to human patient.
In order to induce peptide-specific tolerance and for the effective prevention and treatment of disease, it is hypothesized that either the peptide(s) responsible for disease induction or the dominant peptide(s) driving ongoing autoimmunity must be identified. However, the development of glatiramer acetate (GA, Teva Pharmaceuticals), a random mixture of glutamine, lysine, alanine, and tyrosine peptides of various lengths, is thought to act as an APL for the treatment of patients with MS by simulating MBP reactive T cells (Bornstein et al., 1987; Duda et al., 2000; Neuhaus et al., 2000). Recent studies suggest that GA induces immune deviation from a Th1/Th17 cell-type response to a Th2 cell-type response and does not induce anergy or the deletion of the autoreactive T cells (Aharoni et al., 1999). In contrast to the aforementioned trials using the MBP APL, GA is the only approved semi-Ag-specific approved drug for the treatment of MS. Although GA appears to be well tolerated, 10% of patients experience a transient systemic post-injection reaction characterized by flushing, chest tightness, palpitations, dyspnea, and anxiety (Korczyn and Nisipeanu, 1996). As with all approved MS therapies, treatment requires daily s.c. injections and is beneficial to only a minority of MS patients (Johnson et al., 1995).
1.3.2 Mucosal tolerance
Tolerance induced by the mucosal (oral/nasal) route is biologically relevant as individual foreign dietary Ags are normally tolerated by the host, except in the case of food allergy. T cells found within the gastrointestinal (GI) tract are exposed regularly to ingested foreign Ags, yet they remain largely unresponsive to these non-self Ags. GI surfaces are constantly exposed to exogenous foreign Ags and allow for protective tolerance against some (primarily food) Ags while at the same time serving as an immunological defence against other harmful (pathogenic) Ags. For this reason, the induction of tolerance using the mucosal route for the administration of soluble Ags is appealing as it is antigen-specific, is a relatively easy method of administration, and it carries decreased risk of toxicity when compared with the parenteral injection of soluble Ag.
The efficiency of oral tolerance is dependent on various factors including the animal model employed, type of Ag, and the whether a high or low treatment dose is used (Faria and Weiner, 1999; Mayer and Shao, 2004; Mowat et al., 1982). High-dose oral tolerance results in the induction of anergy or deletion of peripheral Ag-specific T cells (Bitar and Whitacre, 1988; Whitacre et al., 1991). At high doses, Ag can diffuse through the GI wall and into the systemic circulation, where it can induce T cell unresponsiveness via anergy and/or deletion. By contrast, low doses of oral Ag act by bystander suppression via activation of regulatory-cell-driven tolerance within the target organ (Chen et al., 1994; Khoury et al., 1992). Low-dose antigen is taken up by mucosa-associated APCs that activate Tregs to secrete suppressive cytokines, such as TGF-β, IL-4 and IL-10 (Miller et al., 1992a). Thus, the induction of tolerance using high doses of Ag is believed to act by mechanisms that directly influence CD4+ T cells, whereas the use of low doses is believed to induce tolerance by indirect bystander suppression.
Oral Ag administration has been shown to suppress the initiation of disease in multiple animal models of autoimmune disease, including EAE, uveitis, and colitis, as well as asthma (Faria and Weiner, 2006; Mowat et al., 2004; Weiner, 2004). Multiple studies in EAE have shown that pre-administration of soluble myelin peptides by the oral or nasal routes protects against disease induction (Metzler and Wraith, 1993), however attempts to treat EAE following onset of clinical symptoms with oral tolerance have been less successful (Bai et al., 1998; Bai et al., 1997; Benson et al., 1999; Karpus et al., 1996; Kennedy et al., 1997; Meyer et al., 1996) without the addition of other compounds such as soluble IL-10 delivered either orally or nasally (Slavin et al., 2001). In addition, treatment with orally administered bovine MBP in MS clinical trials proved unsuccessful as a therapy (Barnett et al., 1998; Faria and Weiner, 2006; Weiner, 2004; Weiner et al., 1993). Thus, oral tolerance appears to be effective at preventing the induction of EAE, but it is significantly less effective in treating pre-established EAE and MS. While the use of mucosal tolerance remains an attractive possibility for the induction of tolerance, this therapy is currently limited in its ability to induce tolerance in ongoing disease, which restricts its potential for treating human autoimmune disease.
1.3.4 Soluble peptide tolerance
Injection of high doses of soluble peptides leads to a state of anergy by blocking T cell proliferation and/or IL-2 cytokine production upon re-stimulation with the cognate peptide (Burstein et al., 1992; Critchfield et al., 1994; Gaur et al., 1992). Upon encountering a high-dose of Ag, T cells undergo an initial burst of proliferation and on repeated encounter are rendered anergic or deleted due to activation induced cell death (AICD) (Critchfield et al., 1994; Racke et al., 1996). For this reason, it has been hypothesized that tolerance induced by soluble peptides may be useful for Ag-specific immunotherapy for human autoimmune diseases. For example, the induction of tolerance to MBP was examined in a Phase I clinical trial in patients with primary-progressive MS using a peptide that is immunodominant for MBP-specific T cells and B cells. The induction of tolerance was monitored by quantification of MBP-specific autoantibodies in cerebrospinal fluid (CSF). Following a single i.v. injection of MBP85–96 peptide, autoantibodies were undetectable for 3–4 months in the CSF of these patients and tolerance was more prolonged following a second injection (Warren et al., 1997).
A cautionary note for the use of i.v. injected soluble-myelin peptide monomers or oligomers as a therapy in mice with pre-established adjuvant-induced EAE, is that this treatment regimen was found to induce a fatal anaphylactic response in various mouse strains (Smith et al., 2005). Furthermore, i.v. administration of soluble MOG in a primate model of EAE was shown to exacerbate disease (Genain et al., 1996). Due to the highly variable outcome of treatment soluble-peptide-induced tolerance, there is currently a significant level of uncertainty regarding its safety. Anaphylactic responses to i.v. soluble peptide administration appear to occur in an Ag-specific manner, such that the same Ag must be administered during both the initial sensitization phase and the re-challenge phase to induce the effect. In light of the fact that recurrent tolerogenic treatments may be required to ameliorate disease progression, soluble-peptide-induced anaphylaxis is a significant safety concern. Contributing to the complications of i.v. administered soluble-peptide-induced anaphylaxis is the observation that not all autoantigens induce anaphylaxis. Moreover, the capacity of specific Ags to induce an anaphylactic response has been reported to directly correlate with the thymic expression of each Ag, such that if the self-peptide is expressed in the thymus and therefore is subject to central tolerance, anaphylaxis does not occur (Pedotti et al., 2001). This hypothesis is however not supported by findings from our laboratory that showed that equivalent levels of anaphylaxis were induced in mice with pre-established EAE regardless of whether or not the peptide was expressed in the thymus. For example, MBPAc1–11 and MBP84–104 are expressed in the thymus, whereas PLP139–151 and MOG35–55 are not, yet all four peptides equally induced anaphylaxis when the soluble peptide was administered to mice with actively-induced EAE (Smith et al., 2005). In addition, the capacity of a specific self-Ag to elicit an anaphylactic response failed to correlate with its ability to induce an antibody (Ab) response; PLP178–191, which is a B cell epitope and induces IgG production, failed to promote anaphylactic shock during autoimmune disease treatment (Smith et al., 2005). Although i.v. administration of soluble peptides has been shown to ameliorate EAE in an Ag-specific manner, the technique has significant efficacy and safety concerns.
1.3.5 ECDI-peptide-coupled cell induced tolerance
One of the more promising modes of tolerance induction for prevention and treatment of autoimmune diseases, as well as in preventing transplant rejection, is the i.v. treatment with Ag-coupled, ethylene carbodiimide (ECDI)-fixed splenocytes (referred to as Ag-coupled cells, Ag-SP). Treatment with Ag-SP is a powerful method to induce anergy in vitro and peripheral tolerance in vivo (Miller et al., 1995a; Miller et al., 1979), as i.v. injection of myelin-Ag-coupled cells induces rapid and long-lived Ag-specific tolerance in mice with EAE. The fixation of donor cells with ECDI in the presence of Ag results in the formation of peptide bonds between free amino and carboxyl groups, which binds the peptide to the cells. Specific peptides as well as intact proteins can be used to induce tolerance with this method.
Experimentally, this tolerogenic method not only prevents the onset of EAE in mice, but is also an effective treatment for ameliorating the progression of established disease in both the active and adoptive transfer models of EAE by tolerizing host CD4+ T cells that are specific for spread epitopes (Kennedy et al., 1990a; Kennedy et al., 1990b; Su and Sriram, 1991; Vandenbark et al., 1996; Vanderlugt et al., 2000). The induction of tolerance by Ag-SP treatment has also been shown to be an effective therapy in other disease models, including experimental autoimmune thyroiditis (Braley-Mullen et al., 1980), uveitis (Dua et al., 1992), and neuritis (Gregorian et al., 1993) and in the NOD model of diabetes (Fife et al., 2006). Ag-SP therapy appears to be non-toxic and well tolerated at all stages of disease progression. Unlike soluble-peptide therapy, in which the tolerizing Ag can induce an anaphylactic response resulting in the death of treated mice, Ag-SP therapy does not induce an anaphylactic response regardless of the Ag used (Pedotti et al., 2001; Smith et al., 2005).
I.v. administration of myelin antigen- or peptide-coupled splenocytes is a highly efficacious way to regulate EAE in mice and rats. Pre-tolerization of mice with the initiating myelin protein or epitope inhibits the induction of disease in various active R-EAE models. In addition, Ag-SP inhibit the expression of R-EAE when administered shortly after the adoptive transfer of pre-activated neuroantigen-peptide-specific T cells or during disease remission (Karpus et al., 1994; Miller and Karpus, 1994; Miller et al., 1995a; Miller et al., 1992b; Tan et al., 1991; Vanderlugt et al., 2000). The induction of peripheral tolerance with Ag-SP has also been useful for defining immunodominant myelin proteins within the myelin sheath and immunodominant epitopes within myelin proteins. For example, MBP84–104-specific tolerance significantly inhibits relapsing-remitting EAE initiated by MBP-primed lymph-node-derived T cells and PLP139–151-specific tolerance significantly inhibits active R-EAE induced by either mouse spinal cord homogenate (MSCH) or intact PLP. This indicates that the MBP84–104 and PLP139–151 peptides are immunodominant in their respective proteins, but also that other epitopes also contribute to disease induced by the intact proteins (McRae et al., 1995a; Miller and Karpus, 1994; Miller et al., 1995a; Miller et al., 1992b; Miller et al., 1995b; Tan et al., 1992). Ag-SP tolerance has also helped define the specificity and pathological contribution of epitope spreading to endogenous myelin epitopes in clinical relapses. Tolerance studies showed a major pathological contribution of PLP139–151-specific T cells to the relapses in MBP84–104-induced R-EAE and of PLP178–191-specific T cells to relapses in the PLP139–151-induced EAE and that responses to the initiating epitope do not play a major role in the chronic disease phase (McRae et al., 1995a; Miller et al., 1995a; Tan et al., 1991; Vanderlugt et al., 2000; Vanderlugt and Miller, 1996).
In a virally induced model of MS, TMEV, pre-tolerance with TMEV-coupled cells was found to induce ‘split-tolerance’. This split-tolerance is due to the tolerization of virus-specific Th1-cells, concomitant with activation of virus-specific Th2-cell responses (Karpus et al., 1994; Peterson et al., 1993). Split-tolerance was characterized by a decrease in virus-specific delayed-type hypersensitivity (DTH) and IgG2a Ab responses, but a normal to elevated IgG1 Ab response compared to sham tolerized control mice (Peterson et al., 1993). Likewise, the level of T cell-dependent IL-2 and IFNγ produced were decreased upon re-challenge with viral epitopes, but no change was observed in Th2-cell-derived (IL-4) cytokine levels. ECDI-fixed peripheral-blood lymphocytes (PBLs) coupled with MBP peptides also selectively induced anergy in vitro in human Th1-cell but not Th2-cell clones (Vandenbark et al., 2000). While oral administration of soluble PLP139–151 to SJL mice efficiently prevented acute and relapsing EAE induced by either PLP139–151 or intact PLP (Karpus et al., 1996), tolerance induced with PLP139–151-coupled cells significantly down-regulated ongoing adoptive R-EAE when administered at either disease onset or the peak of acute disease, whereas oral tolerance is not effective at these time points (Kennedy et al., 1997).
The mechanism(s) underlying Ag-SP-induced tolerance has yet to be fully elucidated, however the route of administration, dosage, levels of costimulation (the two-signal hypothesis), Th cell polarization and Treg cell induction are all likely factors that contribute to the efficacy of treatment. A likely mechanism for Ag-SP tolerance is by suboptimal T cell activation through the engagement of the TCR in the absence of costimulation. In vitro studies revealed that ECDI-treated splenocytes pulsed with soluble peptide Ag are unable to deliver critical costimulatory signals for activation of Th1 clones leading to anergy (Jenkins and Schwartz, 1987). Data from our laboratory demonstrates that the effectiveness of Ag-SP tolerance in PLP139–151-induced EAE in the SJL mouse is dependent on having a low level of CD80 and CD86 expression on the fixed APCs (Eagar et al., 2002). This is further supported by the observation that blocking CTLA-4:CD80/CD86 interaction at the time of secondary antigen encounter reverses the tolerized state (Eagar et al., 2004). Programmed cell death ligand-1 (PDL-1)/PD1 engagement has also been demonstrated to be important for the maintenance of insulin-coupled-cell-induced tolerance in the NOD diabetes model (Fife et al., 2006) and in islet graft transplant survival with allogeneic-coupled-cell-tolerance (X. Lou and S. D. Miller, submitted). The efficiency of this therapy is also critically dependent upon i.v. administration of antigen-coupled cells, whereas neither i.p. nor s.c. injection are effective at inducing tolerance, with the latter actually enhancing immune responses to the target Ag (Tan et al., 1992). Furthermore, ECDI fixation of the cells is absolutely necessary for the induction of tolerance, however, de novo Ag processing by the donor cells is not a contributing factor as the inclusion of Ag-processing inhibitors in the coupling reaction does not reverse the tolerance phenotype (Pope et al., 1992).
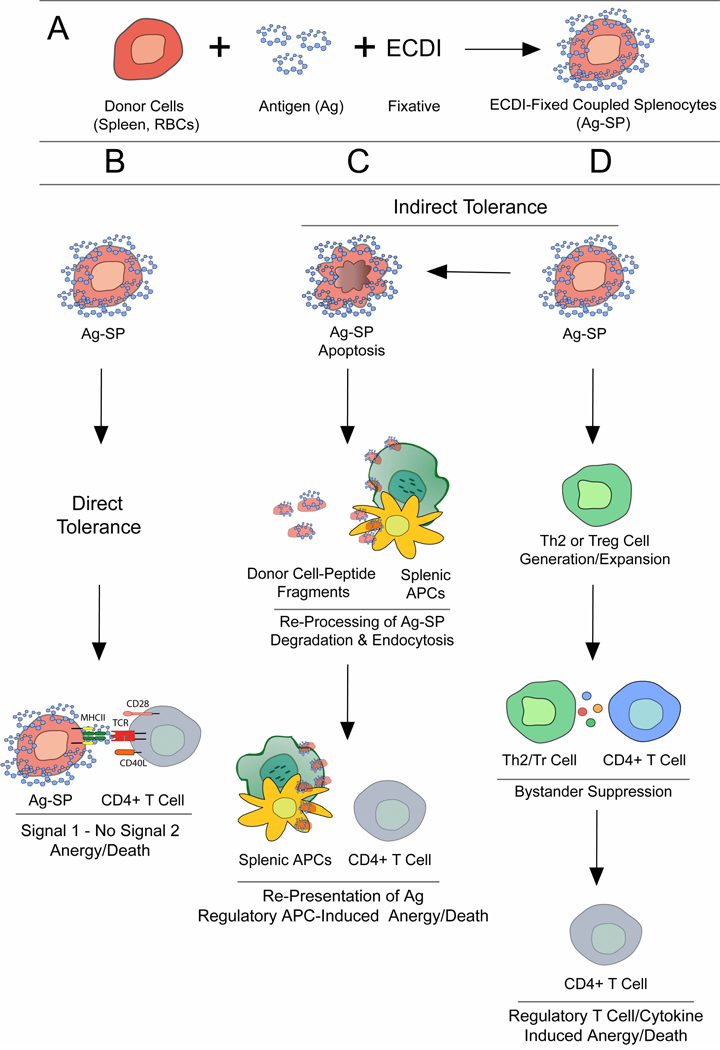
The mechanism of Ag-SP tolerance is believed to be two-fold through both direct and indirect interaction of the Ag-SP and recipient T cells (Fig. 1). We have hypothesized that direct induction of tolerance occurs via interaction of host autoreactive CD4+ T cells with the donor Ag-SP cells and is strongly dependent on costimulation. Alternatively indirect tolerance can occur through re-presentation of the bound Ag by host APCs such as splenic macrophages, B cells and immature dendritic cells (DCs,) that phagocytize the donor Ag-SP and re-process and represent the bound antigen to host T cells in a tolerogenic fashion in a costimulatory-deficient manner (Turley and Miller, 2007). Ag-SP tolerance induction occurs even if the donor cells are coupled with intact proteins or if they are derived from donors that are deficient in MHC class I and/or MHC class II molecules, however twice the number of donor MHC deficient donor cells are required to induce levels of protection equivalent to that of syngeneic MHC-expressing donor cells. Additionally, ECDI fixation actively induces apoptosis of the donor cells (Turley and Miller, 2007) likely assisting in the ability of the peptide-coupled allogeneic derived donor cells to tolerize recipient T cells. In addition to these findings, tolerance is inhibited in splenectomized recipients (D. M. Turley and S. D. Miller, unpublished observations) suggesting the requirement for a recipient splenic APC population for re-presentation of Ag in a tolerance-inducing manner. Tolerizing Ag can also be coupled to donor red blood cells (RBCs) to induce tolerance capable of both prevent disease induction and treat ongoing EAE (D. M. Turley and S. D. Miller, unpublished observations). The use of RBCs as donor carrier cells greatly increases to the clinical efficacy of Ag-SP as a therapy for MS as RBCs are more readily available than purified APCs. Collectively this data suggests that ECDI non-specifically cross-links Ag to the cell surface while inducing apoptosis, which allows for the donor cells to be perceived by the host in a non-immunogenic fashion in the spleen. This would aid in the ability of immature/tolerogenic host splenic APCs to re-process and re-present the coupled Ag in a tolerance-inducing manner.
Fig. 1. Potential Mechanisms of ECDI-fixed Ag-coupled cell (Ag-SP) Tolerance.
(A) Schematic representation of Ag-SP formation. Donor cells [bulk splenocytes or red blood cells (RBCs)] are fixed with ethylene carbodiimide (ECDI) in the presence of peptide for 1 hour at 4°C to generate Ag-SP. (B–D) Possible mechanisms of Ag-SP-induced tolerance: Ag-SP can induce tolerance by direct (B) or indirect mechanisms (C, D). (B) Donor Ag-SP can directly interact with host antigen-specific T cells delivering signal 1 (MHC/peptide:TCR) without signal 2 (co-stimulation; B7-1,-2/CD28; CD40/CD40L), rendering the cells anergic. (C) Alternatively Ag-SP can induce indirect or cross-tolerance as the donor Ag-SP undergo apoptosis, leading to phagocytic uptake by host splenic antigen presenting cells (APCs), which then can present peptide fragments to host T cells inducing anergy. (D) Another possible mechanism for Ag-SP tolerance is the indirect generation/expansion of a Th2 or regulatory T cell (Treg) population, which through bystander suppression can also render antigen-specific CD4+ T cells tolerant.
Another interesting aspect of Ag-SP tolerance is the finding that multiple peptides can be coupled to a donor cell, allowing for the simultaneous targeting of multiple T cell specificities. This may be critical for directed tolerance therapy of chronic autoimmune diseases, in which responses to multiple tissue Ags, activated by epitope spreading, are likely important in sustaining the destruction of self-tissue (Smith and Miller, 2006). T cells removed from the CNS of mice with ongoing EAE that were tolerized at the peak of the acute clinical stage of disease with Ag-SP also make increased amounts of the anti-inflammatory cytokines IL-10 and TGFβ, compared with control mice, although the levels of these cytokines in the periphery remain roughly equivalent to control mice (Smith and Miller, 2006). This pattern of increased regulatory cytokine production is suggestive of an increase in natural Treg function in ongoing EAE. Tregs appear to have a role in Ag-SP tolerance and are required for the long-term maintenance of tolerance, but are not necessary for tolerance induction (E. Feeny, S. D. Miller, unpublished observations). However the opposite was found in the allogeneic-coupled-cell tolerance in islet transplant survival, Tregs were found to be required for tolerance induction (X. Lou, et al., submitted). Additionally, the necessity of Treg cells in adoptively transferred tolerance also suggests that a regulatory population of cells is either induced, activated and/or expanded by the administration of Ag-SP (C. Smith and S. D. Miller, unpublished observations) and necessary for the transfer of tolerance. Based on the current success of Ag-SP cell tolerance in treating EAE, this therapy is in the final stages of toxicity testing for an initial Phase I/IIa clinical trial designed to test the safety and efficacy of this therapy in treating new-onset RRMS patients. It is proposed to intravenously re-infuse autologous peripheral blood leukocytes (PBLs), collected from patients using leukocytapharesis, ECDI-coupled with a cocktail of 5–7 previously identified immunodominant myelin peptides including MBP13–32, MBP111–129, MBP83–99, MBP146–170, MOG1–20, MOG35–55 and PLP139–154 which cause T cell expansion in peripheral blood T cells of MS patients (Bielekova et al., 2004). The goal is to induce long-term tolerance in effector autoreactive T cells as well as to prevent future relapses by tolerizing naïve T cells specific for potential endogenously released myelin epitopes without compromising immune responses to foreign pathogens. Our data demonstrating the use of RBCs as efficient donor ECDI-fixed carrier cells to induce tolerance lends support to the clinical efficacy of Ag-SP tolerance induction for the treatment of human autoimmune disease.
1.4 Conclusions
When moving research studies from the lab to the clinic, it is necessary to keep in mind that a contributing factor to the variable results gained from Ag-specific tolerance approaches which are initially used for treatment of disease models in inbred mice and those observed to date in humans in the clinical setting is likely the effect of the diverse nature of human MHC polymorphisms. Continued research to better understand the underlying molecular mechanisms of tolerance and to enhance the specificity and efficacy of each of these treatment strategies, perhaps using combinatorial approaches, is thus necessary to deal with the complexity of the human immune system.
The development/identification of therapeutics that either inhibit signalling intermediates necessary for T cell activation or activate T cell anergy-associated signalling intermediates, when used in combination with therapies such as Ag-SP presents a possible combinatorial strategy that may increase therapeutic efficacy. Therefore, continued research to enhance specificity and efficacy of treatment in Ag-SP as well as the other aforementioned antigen-specific strategies is necessary in both animal models as well as in the clinic with advanced patient screening using modern genomic and pharmacogenomic techniques. The use of these tolerogenic approaches in combination with non-Ag-specific therapies also has the potential to one day provide ‘tailored therapy’ to deal with the complexity of the human immune system.
References
- Aharoni R, Teitelbaum D, Arnon R, Sela M. Copolymer 1 acts against the immunodominant epitope 82–100 of myelin basic protein by T cell receptor antagonism in addition to major histocompatibility complex blocking. Proc Natl Acad Sci USA. 1999;96:634–639. doi: 10.1073/pnas.96.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton SM, Wraith DC. Selection and fine-tuning of the autoimmune T-cell repertoire. Nat Rev Immunol. 2002;2:487–498. doi: 10.1038/nri842. [DOI] [PubMed] [Google Scholar]
- Bai XF, Li HL, Shi FD, Liu JQ, Xiao BG, van der Meide PH, Link H. Complexities of applying nasal tolerance induction as a therapy for ongoing relapsing experimental autoimmune encephalomyelitis (EAE) in DA rats. ClinExpImmunol. 1998;111:205–210. doi: 10.1046/j.1365-2249.1998.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai XF, Shi FD, Xiao BG, Li HL, van der Meide PH, Link H. Nasal administration of myelin basic protein prevents relapsing experimental autoimmune encephalomyelitis in DA rats by activating regulatory cells expressing IL-4 and TGF-beta mRNA. J Neuroimmunol. 1997;80:65–75. doi: 10.1016/s0165-5728(97)00133-1. [DOI] [PubMed] [Google Scholar]
- Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, Fletcher MJ, Chasan-Taber S, Finger E, Morales A, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41:290–297. doi: 10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- Benson JM, Stuckman SS, Cox KL, Wardrop RM, Gienapp IE, Cross AH, Trotter JL, Whitacre CC. Oral administration of myelin basic protein is superior to myelin in suppressing established relapsing experimental autoimmune encephalomyelitis. Journal of Immunology. 1999;162:6247–6254. [PubMed] [Google Scholar]
- Bernard CC, de Rosbo NK. Immunopathological recognition of autoantigens in multiple sclerosis. Acta Neurol(Napoli) 1991;13:171–178. [PubMed] [Google Scholar]
- Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Sung MH, Kadom N, Simon R, McFarland H, Martin R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. Journal of Immunology. 2004;172:3893–3904. doi: 10.4049/jimmunol.172.6.3893. [DOI] [PubMed] [Google Scholar]
- Bitar DM, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Cellular Immunology. 1988;112:364–370. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- Bornstein MB, Miller A, Slagle S, Weitzman M, Crystal H, Drexler E, Keilson M, Merriam A, Wassertheilsmoller S, Spada V, et al. A Pilot Trial of Cop-1 in Exacerbating Remitting Multiple-Sclerosis. N Engl J Med. 1987;317:408–414. doi: 10.1056/NEJM198708133170703. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H, Tompson JG, Sharp GC, Kyriakos M. Suppression of experimental autoimmune thyroiditis in guinea pigs by pretreatment with thyroglobulin-coupled spleen cells. Cellular Immunology. 1980;51:408–413. doi: 10.1016/0008-8749(80)90272-5. [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Shea CM, Abbas AK. Aqueous antigens induce in vivo tolerance selectively in IL-2- and IFN-gamma-producing (Th1) cells. Journal of Immunology. 1992;148:3687–3691. [PubMed] [Google Scholar]
- Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol. 2003;3:123–132. doi: 10.1038/nri1000. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Christen U, von Herrath MG. Initiation of autoimmunity. Curr Opin Immunol. 2004;16:759–767. doi: 10.1016/j.coi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Critchfield JM, Racke MK, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- Dal Canto MC, Kim BS, Miller SD, Melvold RW. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelination: A model for human multiple sclerosis. Neuroprotocols. 1997;10:453–461. doi: 10.1006/meth.1996.0123. [DOI] [PubMed] [Google Scholar]
- de Rosbo NK, Hoffman M, Mendel I, Yust I, Kaye J, Bakimer R, Flechter S, Abramsky O, Milo R, Karni A, Ben-Nun A. Predominance of the autoimmune response to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis: reactivity to the extracellular domain of MOG is directed against three main regions. Eur J Immunol. 1997;27:3059–3069. doi: 10.1002/eji.1830271144. [DOI] [PubMed] [Google Scholar]
- Dua HS, Gregerson DS, Donoso LA. Inhibition of experimental autoimmune uveitis by retinal photoreceptor antigens coupled to spleen cells. Cellular Immunology. 1992;139:292–305. doi: 10.1016/0008-8749(92)90072-w. [DOI] [PubMed] [Google Scholar]
- Duda PW, Schmied MC, Cook SL, Krieger JI, Hafler DA. Glatiramer acetate (Copaxone (R)) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. JClinInvest. 2000;105:967–976. doi: 10.1172/JCI8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagar TN, Karandikar NJ, Bluestone J, Miller SD. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. EurJImmnol. 2002;32:972–981. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Eagar TN, Turley DM, Padilla J, Karandikar NJ, Tan LJ, Bluestone JA, Miller SD. CTLA-4 regulates expansion and differentiation of Th1 cells following induction of peripheral T cell tolerance. Journal of Immunology. 2004;172:7442–7450. doi: 10.4049/jimmunol.172.12.7442. [DOI] [PubMed] [Google Scholar]
- Ebers GC, Sadovnick AD, Risch NJ. A genetic basis for familial aggregation in multiple sclerosis. Nature. 1995;377:150–151. doi: 10.1038/377150a0. [DOI] [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami RS, Oldstone MB. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Gaur A, Wiers B, Liu A, Rothbard J, Fathman CG. Amelioration of autoimmune encephalomyelitis by myelin basic protein synthetic peptide-induced anergy. Science. 1992;258:1491–1494. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- Genain CP, Abel K, Belmar N, Villinger F, Rosenberg DP, Linington C, Raine CS, Hauser SL. Late complications of immune deviation therapy in a nonhuman primate. Science. 1996;274:2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- Gregorian SK, Clark L, Heber-Katz E, Amento EP, Rostami A. Induction of peripheral tolerance with peptide-specific anergy in experimental autoimmune neuritis. Cellular Immunology. 1993;150:298–310. doi: 10.1006/cimm.1993.1198. [DOI] [PubMed] [Google Scholar]
- Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala) JClinInvest. 2003;111:409–418. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB The Copolymer 1 Multiple Sclerosis Study Group. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L The Altered Peptide Ligand in Relapsing MS Study Group. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med. 2000;6:1176–1182. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- Karandikar NJ, Vanderlugt CL, Bluestone JA, Miller SD. Targeting the B7/CD28:CTLA-4 costimulatory system in CNS autoimmune disease. J Neuroimmunol. 1998;89:10–18. doi: 10.1016/s0165-5728(98)00058-7. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Kennedy KJ, Smith WS, Miller SD. Inhibition of relapsing experimental autoimmune encephalomyelitis in SJL mice by feeding the immunodominant PLP139-151 molecule. J Neurosci Res. 1996;45:410–423. doi: 10.1002/(SICI)1097-4547(19960815)45:4<410::AID-JNR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Karpusx WJ, Lukacs NW, McRae BL, Streiter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. Journal of Immunology. 1995;155:5003–5010. [PubMed] [Google Scholar]
- Karpus WJ, Peterson JD, Miller SD. Anergy in vivo : Down-regulation of antigen-specific CD4+ Th1 but not Th2 cytokine responses. IntImmunol. 1994;6:721–730. doi: 10.1093/intimm/6.5.721. [DOI] [PubMed] [Google Scholar]
- Kennedy KJ, Smith WS, Miller SD, Karpus WJ. Induction of antigen-specific tolerance for the treatment of ongoing, relapsing autoimmune encephalomyelitis - A comparison between oral and peripheral tolerance. Journal of Immunology. 1997;159:1036–1044. [PubMed] [Google Scholar]
- Kennedy MK, Tan LJ, Dal Canto MC, Miller SD. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. Journal of Immunology. 1990a;145:117–126. [PubMed] [Google Scholar]
- Kennedy MK, Tan LJ, Dal Canto MC, Tuohy VK, Lu ZJ, Trotter JL, Miller SD. Inhibition of murine relapsing experimental autoimmune encephalomyelitis by immune tolerance to proteolipid protein and its encephalitogenic peptides. Journal of Immunology. 1990b;144:909–915. [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- Khalili K, White MK, Lublin F, Ferrante P, Berger JR. Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology. 2007;68:985–990. doi: 10.1212/01.wnl.0000257832.38943.2b. [DOI] [PubMed] [Google Scholar]
- Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, Bluestone JA, Miller SD. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. Journal of Immunology. 2005;174:4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- Korczyn AD, Nisipeanu P. Safety profile of copolymer 1: Analysis of cumulative experience in the United States and Israel. JNeruol. 1996;243:S23–S26. doi: 10.1007/BF00873698. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4:407–419. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995a;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995b;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int Immunol. 1993;5:1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- Meyer AL, Benson JM, Gienapp IE, Cox KL, Whitacre CC. Suppression of murine chronic relapsing experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Journal of Immunology. 1996;157:4230–4238. [PubMed] [Google Scholar]
- Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor · after antigen-specific triggering. Proceedings of the National Academy of Sciences. 1992a;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GPA, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O'Connor PW. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- Miller SD, Karpus WJ. The immunopathogenesis and regulation of T-cell mediated demyelinating diseases. Immunol Today. 1994;15:356–361. doi: 10.1016/0167-5699(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Miller SD, McRae BL, Vanderlugt CL, Nikcevich KM, Pope JG, Pope L, Karpus WJ. Evolution of the T cell repertoire during the course of experimental autoimmune encephalomyelitis. Immunol Rev. 1995a;144:225–244. doi: 10.1111/j.1600-065x.1995.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Miller SD, Tan LJ, Pope L, McRae BL, Karpus WJ. Antigen-specific tolerance as a therapy for experimental autoimmune encephalomyelitis. Int Rev Immunol. 1992b;9:203–222. doi: 10.3109/08830189209061791. [DOI] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Lenschow DJ, Pope JG, Karandikar NJ, Dal Canto MC, Bluestone JA. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995b;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM, Parker LA, Beacock-Sharp H, Millington OR, Chirdo F. Oral tolerance: overview and historical perspectives. Annals NY Acad Sci. 2004;1029:1–8. doi: 10.1196/annals.1309.001. [DOI] [PubMed] [Google Scholar]
- Mowat AM, Strobel S, Drummond HE, Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982;45:105–113. [PMC free article] [PubMed] [Google Scholar]
- Neuhaus O, Farina C, Yassouridis A, Wiendl H, Bergh FT, Dose T, Wekerle H, Hohlfeld R. Multiple sclerosis: Comparison of copolymer-1-reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7452–7457. doi: 10.1073/pnas.97.13.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson LB, Greer JM, Sobel RA, Lees MB, Kuchroo VK. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Nicholson LB, Kuchroo VK. T cell recognition of self and altered self antigens. Crit RevImmunol. 1997;17:449–462. [PubMed] [Google Scholar]
- Nicholson LB, Murtaza A, Hafler BP, Sette A, Kuchroo VK. A T cell receptor antagonist peptide induces T cells that mediate bystander suppression and prevent autoimmune encephalomyelitis induced with multiple myelin antigens. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9279–9284. doi: 10.1073/pnas.94.17.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Croxford JL, Calenoff M, Dal Canto MC, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. JClinInvest. 2001a;108:311–318. doi: 10.1172/JCI13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Croxford JL, Miller SD. Virus-induced autoimmunity: Potential role of viruses in initiation, perpetuation, and progression of T cell-mediated autoimmune diseases. Viral Immunol. 2001b;14:227–250. doi: 10.1089/088282401753266756. [DOI] [PubMed] [Google Scholar]
- Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Paterson PY, Swanborg RH. Demyelinating diseases of the central and peripheral nervous systems. In: Sampter M, Talmage DW, Frank MM, Austen KF, Claman HN, editors. Immunological Diseases. Boston, Little: Brown and Co; 1988. pp. 1877–1916. [Google Scholar]
- Pedotti R, Mitchell D, Wedemeyer J, Karpuj M, Chabas D, Hattab EM, Tsai M, Galli SJ, Steinman L. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. NatImmunol. 2001;2:216–222. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Karpus WJ, Clatch RJ, Miller SD. Split tolerance of Th1 and Th2 cells in tolerance to Theiler's murine encephalomyelitis virus. Eur J Immunol. 1993;23:46–55. doi: 10.1002/eji.1830230109. [DOI] [PubMed] [Google Scholar]
- Pope L, Paterson PY, Miller SD. Antigen-specific inhibition of the adoptive transfer of experimental autoimmune encephalomyelitis in Lewis rats. J Neuroimmunol. 1992;37:177–190. doi: 10.1016/0165-5728(92)90002-3. [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, Spera S, Suraci C, Multari G, Cervoni M, et al. IMDIAB Group. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII) Diabetologia. 2000;43:1000–1004. doi: 10.1007/s001250051482. [DOI] [PubMed] [Google Scholar]
- Racke MK, Critchfield JM, Quigley L, Cannella B, Raine CS, McFarland Hf, Lenardo MJ. Intravenous antigen administration as a therapy for autoimmune demyelinating disease. Ann Neurol. 1996;39:46–56. doi: 10.1002/ana.410390108. [DOI] [PubMed] [Google Scholar]
- Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson MF, Smilek DE. Reversal of acute experimental autoimmune encephalomyelitis and prevention of relapses by treatment with a myelin basic protein peptide analogue modified to form long-lived peptide-MHC complexes. Journal of Immunology. 1995;155:2737–2746. [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Slavin AJ, Maron R, Weiner HL. Mucosal administration of IL-10 enhances oral tolerance in autoimmune encephalomyelitis and diabetes. Int Immunol. 2001;13:825–833. doi: 10.1093/intimm/13.6.825. [DOI] [PubMed] [Google Scholar]
- Smith CE, Eagar TN, Strominger JL, Miller SD. Differential induction of IgE-mediated anaphylaxis after soluble vs. cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc NatlAcadSciUSA. 2005;102:9595–9600. doi: 10.1073/pnas.0504131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Miller SD. Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities. J Autoimmunity. 2006;27:218–231. doi: 10.1016/j.jaut.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su XM, Sriram S. Treatment of chronic relapsing experimental allergic encephalomyelitis with the intravenous administration of splenocytes coupled to encephalitogenic peptide 91–103 of myelin basic protein. J Neuroimmunol. 1991;34:181–190. doi: 10.1016/0165-5728(91)90128-t. [DOI] [PubMed] [Google Scholar]
- Tan LJ, Kennedy MK, Dal Canto MC, Miller SD. Successful treatment of paralytic relapses in adoptive experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance. Journal of Immunology. 1991;147:1797–1802. [PubMed] [Google Scholar]
- Tan LJ, Kennedy MK, Miller SD. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. II. Fine specificity of effector T cell inhibition. Journal of Immunology. 1992;148:2748–2755. [PubMed] [Google Scholar]
- Turley DM, Miller SD. Peripheral tolerance Induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- Utset TO, Auger JA, Peace D, Zivin RA, Xu D, Jolliffe L, Alegre ML, Bluestone JA, Clark MR. Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol. 2002;29:1907–1913. [PubMed] [Google Scholar]
- Vandenbark AA, Barnes D, Finn T, Bourdette DN, Whitham R, Robey I, Kaleeba J, Bebo BF, Jr., Miller SD, Offner H, Chou YK. Differential susceptibility of human T(h)1 versus T (h) 2 cells to induction of anergy and apoptosis by ECDI/antigen-coupled antigen- presenting cells. IntImmunol. 2000;12:57–66. doi: 10.1093/intimm/12.1.57. [DOI] [PubMed] [Google Scholar]
- Vandenbark AA, Vainiene M, Ariail K, Miller SD, Offner H. Prevention and treatment of relapsing autoimmune encephalomyelitis with myelin peptide-coupled splenocytes. J Neurosci Res. 1996;45:430–438. doi: 10.1002/(SICI)1097-4547(19960815)45:4<430::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Eagar TN, Neville KL, Nikcevich KM, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. Journal of Immunology. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8:831–836. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman BH. Multiple sclerosis: More genes versus environment. Nature. 1995;377:105–106. doi: 10.1038/377105a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- Warren KG, Catz I, Wucherpfennig KW. Tolerance induction to myelin basic protein by intravenous synthetic peptides containing epitope P85 VVHFFKNIVTP96 in chronic progressive multiple sclerosis. J Neurol Sci. 1997;152:31–38. doi: 10.1016/s0022-510x(97)00130-5. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Current issues in the treatment of human diseases by mucosal tolerance. Annals NY Acad Sci. 2004;1029:211–224. doi: 10.1196/annals.1309.053. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Mackin GA, Matsui M, Orav EJ, Khoury SJ, Dawson DM, Hafler DA. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- Wekerle H. Immunopathogenesis of multiple sclerosis. Acta Neurol(Napoli) 1991;13:197–204. [PubMed] [Google Scholar]
- Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis: III. Evidence for clonal anergy. Journal of Immunology. 1991;147:2155–2163. [PubMed] [Google Scholar]
- Wraith DC, Smilek DE, Mitchell DJ, Steinman L, McDevitt HO. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide-mediated immunotherapy. Cell. 1989;59:247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Young DA, Lowe LD, Booth SS, Whitters MJ, Nicholson L, Kuchroo VK, Collins M. IL-4, IL-10, IL-13, and TGF-beta from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. Journal of Immunology. 2000;164:3563–3572. doi: 10.4049/jimmunol.164.7.3563. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hupperts R, De Baets M. Monoclonal antibody therapy in experimental allergic encephalomyelitis and multiple sclerosis. ImmunolRes. 2003;28:61–78. doi: 10.1385/IR:28:1:61. [DOI] [PubMed] [Google Scholar]