Abstract
Objectives
To determine the relationships among dietary protein source, cardiovascular risk, reproductive hormones and ovarian aging.
Methods
Adult female cynomolgus monkeys (Macaca fascicularis) were assigned randomly to one of two diets containing saturated fat and cholesterol, differing only by protein source: 1) casein-lactalbumin (n = 29) or 2) soy protein with isoflavones (n = 32). Cardiovascular risk markers and reproductive hormones were measured at baseline and after 32 months of treatment, at which time the ovaries were removed, serially sectioned, and ovarian follicles counted in every 100th section.
Results
Casein-lactalbumin-fed monkeys had fewer primordial, primary, and secondary follicles (all p's <0.05) than did their soy-fed counterparts. AMH was significantly correlated with all follicle types (r's≥0.66, p's< 0.001) for casein-fed monkeys, and was significantly correlated with primary (rsoy=0.47, p=0.005) and secondary (rsoy=0.45, p=0.007) follicles in soy-fed monkeys. No significant associations were seen between any of the other reproductive hormones measured and follicle counts. Casein-lactalbumin-fed monkeys had a more atherogenic lipoprotein profile and increased atherosclerosis extent (p< 0.05), but despite these differences in cardiovascular risk between casein-lactalbumin- and soy-fed monkeys, none of the individual cardiovascular risk markers measured in this study explained the relationship between dietary protein source and follicle counts (linear regression, all p's>0.05).
Conclusions
Diet influences the rate of follicular depletion in cynomolgus macaques; however, the mechanism for this effect remains undetermined.
Keywords: ovary, monkey, menopause, cardiovascular disease, diet
Introduction
During the menopausal transition and post menopause, risk factors for cardiovascular disease (e.g., elevated plasma lipids, carotid artery intimal thickness, markers of inflammation, metabolic syndrome) have been reported to increase1-6 and an increase in cardiovascular disease is observed postmenopausally.7 Recently it has been observed that early follicle decline, in younger women, is associated with elevated cardiovascular disease risk (e.g., elevated total and low density lipoprotein [LDL] cholesterol).8 Determination of mediators that either reflect early ovarian aging or hasten the onset of menopause, could facilitate the identification of premenopausal women at risk for postmenopausal cardiovascular disease. Unfortunately, factors influencing the tempo of ovarian aging (i.e., the rate of follicle loss) within an individual (and thus leading to the inter-individual variation observed in the age at menopause) are not well understood currently. While it is generally accepted that the loss of ovarian hormones and some environmental conditions (e.g., an imprudent diet) are associated with and precede cardiovascular disease in women, the exact nature of these relationships is relatively unexplored.
It has been reported that ∼50% of inter-individual variability in age at menopause can be attributed to genetic effects,9 with the remaining variability reflecting various environmental factors. Research on lifestyle characteristics that might predict the age of menopause10-12 has produced inconsistent results beyond showing that cultural and ethnic variables may play a role13 and that smoking decreases the age at menopause.14,15 With the exception of a well described hereditary disorder in galactose metabolism resulting in premature ovarian failure,16 relatively few studies have explored directly the effect of dietary components (such as protein source, effect of isoflavones, cholesterol content, etc.) on ovarian aging in women.17-19
It is well known that diet has a profound impact on cardiovascular health, and it was recently reported that women in the Framingham Heart Study who had an increased cardiovascular risk profile (e.g., elevated cholesterol, high blood pressure, being overweight, etc.) had a younger age at menopause.15 The authors suggested that, since ovaries are highly vascularized, diet-induced atherosclerotic blockage in the arteries supplying blood to the ovaries may increase the speed of ovarian aging and thus decrease the age at menopause.
In the study presented here, we report the effect of diet (either plant [soy with isoflavones] - or animal-based [casein-lactalbumin]) on ovarian aging (follicle counts) in monkeys. In addition, several potential mediators of the dietary effect (lipids, markers of inflammation, and hormones) were also evaluated. The data presented here are from a retrospective study of female monkeys used in an investigation of the effects of soy, life stage (premenopausal vs. postmenopausal), and stress on atherosclerosis progression.20-23 As such, the diets were specially formulated to model “typical” American consumption and to allow the development of atherosclerosis in this monkey model. In addition, the use of this diet allows the investigation of ovarian aging in monkeys in a dietary context similar to that experienced by many American women.
Materials and Methods
Animal Subjects/Diet
The data presented here were from a retrospective study of 61 female monkeys that were used in a larger (n=95) investigation of the effects of soy, life stage (premenopausal vs. postmenopausal), and stress on atherosclerosis progression.20-23 Briefly, subjects were cynomolgus macaques (Macaca fascicularis) originally imported as adults from Indonesia (Institute Pertanian Bogor). Adult status was confirmed by dentition and evidence of epiphyseal closure and the average age was estimated at 9-15 years, approximately equivalent to 27-45 human years.21 For eight months following quarantine, the monkeys were fed an isoflavone-free semi-purified diet containing casein and lactalbumin (C/L) and wheat flour as sources of protein, as well as added cholesterol. Social groups of monkeys were then randomized to one of two treatment groups, stratified for body weight and plasma lipids (total plasma cholesterol [TPC] and high density lipoprotein cholesterol [HDL-C]) as follows: 1) Diet containing C/L as the major protein source (C/L, n=29); or 2) Diet containing soy with isoflavones as the major protein source (Soy, n=32).
All diets were prepared in the Wake Forest University Primate Center diet laboratory and were formulated to be equivalent for cholesterol (0.28mg/Cal for the first year and then 0.20mg/Cal for the next 2.5 years) and macronutrient content (protein, fat, carbohydrate; Figure 1). DL-methionine was added to the soy-containing diets to make the content of sulfur–containing amino acids comparable to that in the C/L diet. The monkeys were fed 120 Cal of diet/kg body weight (BW) daily. To adjust for differences in the metabolic rate between monkeys and women, a women's equivalent dose of soy isoflavones (IF) was calculated based on the average woman consuming approximately 1800 calories per day. Therefore, the monkeys in the Soy group were given 139.5 mg IF/1800 calories of diet per day which equates to 9.3 mg IF/Kg BW/day (female monkeys consume on average ∼360 calories/day or 1/5th that of the average woman). All procedures were done in compliance with state and federal laws, standards of the US DHHS, and regulations and guidelines established by the Wake Forest University Animal Care and Use Committee. Wake Forest University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Figure 1.
Composition of both diets used in the study.
Ovarian Follicle Number
After 32 months of consuming the experimental diets, both ovaries were removed from all monkeys via laparotomy. The ovaries were trimmed of fat and fixed in Bouin's solution (75mL picric acid solution [1.3%], 25mL of formaldehyde [37%], and 5mL glacial acetic acid) for 24 hours and then transferred to 70% ethanol. One ovary from each monkey was transferred to the University of Arizona for follicle counting (PBH). The ovaries were sectioned serially (every 4 to 5μm) and every 50th section was saved, placed in order of sectioning on glass slides and stained with hematoxylin and eosin. Although the total number of sections per ovary varied from 2-10 depending on individual differences in ovarian size, the number of sections collected for both Soy and C/L groups was equivalent (median number of sections = 7, p=0.44), indicating that variability in ovarian size was similar in both treatment conditions. As published previously,24,25 follicles were classified and counted in every 100th section and only follicles with an oocyte nucleus were counted. Data are reported as total follicles counted per ovary (i.e., the sum of all follicles in each section counted per ovary). Follicles were classified as primordial (oocytes surrounded by a single layer of flattened granulosa cells), primary (oocyte surrounded by a single layer of cuboidal granulosa cells), and secondary (oocytes surrounded by two or more layers of cuboidal granulosa cells).26 In support of counting follicles in every 100th section of ovary are data from a previous study24 in which we found that there was no change in the rank order of monkeys on the basis of their primordial follicle count whether every 100th section or twice that number (every 50th section) was counted. In addition, the relative effect of treatment with a compound known to destroy primordial follicles is identical whether follicles are counted every 50th or 100th section. The same relationships were true for primary and secondary follicles.
Reproductive Hormones
All samples for hormone measurement were collected between 0900 and 1200 hours, following an overnight fast. Blood samples were stored at −20°C or below until assayed (Biomarkers Core Lab, Yerkes National Primate Research Center of Emory University, MEW). Antimüllerian hormone (AMH) determinations were done at baseline (∼6 months) and at the time of ovariectomy (32 months) (ELISA, Diagnostics Systems Laboratories, Inc., [DSL] Webster, TX). Consistent with previous findings that human AMH ELISA recognizes cynomolgus macaque AMH,27 no problems with the cross reactivity between DSL's ultrasensitive assay and the AMH of cynomolgus macaques were encountered in the study presented here. The coefficients of variation (CV) were: intra-assay CV: 4.53% at 13.62ng/mL; inter-assay CV: 19.75% at 0.23ng/mL, 11.42% at 9.61ng/mL, and 11.27% at1.89ng/mL. Estradiol was measured on the day of ovariectomy by a modification28 of a commercially available radioimmunoassay (RIA) from Diagnostic Products Corporation (Los Angeles, CA). Intra-assay and inter-assay CVs were <4% and 10%, respectively.
Androstenedione was quantified by RIA with commercially available kits (DSL, Webster, TX) using antibody coated tubes (Coat-A-Count, Diagnostics Products Corporation, Los Angeles, CA). Inter-assay CVs for androstenedione were 12.06% at 1.07ng/mL and 13.34% at 5.48ng/mL. The intra-assay CV was 11.21% at 1.01ng/mL. Testosterone concentrations (T) were measured using a commercially available kit (DSL, Webster, TX). The inter-assay CVs for testosterone were 5.95% at 0.68ng/mL and 4.14% at 5.67ng/mL. The intra-assay CV was 6.3%.
Cardiovascular Risk Markers
a) Plasma Lipids and Lipoproteins
Plasma lipids and lipoproteins were measured at baseline, 12 months and at the time of ovariectomy (32 months). Total plasma cholesterol (TPC) and high density lipoprotein cholesterol (HDL-C) concentrations were measured at the Wake Forest University Primate Center using enzymatic methods on the Ace Alera analyzer (Alfa Wassermann, West Caldwell, NJ), with protocols and reagents supplied by Alfa Wassermann (intra-assay CV was 3% and inter-assay CV was 5%). Non-HDL cholesterol (LDL + VLDL [low density + very low density lipoprotein]) concentrations were calculated using the above data.
b) Iliac Artery Atherosclerosis
To determine change in atherosclerosis extent during the 32-month experimental period, a paired artery, the common iliac artery, was collected from each monkey at the time of ovariectomy. The common iliac artery has been shown previously to have essentially the same plaque sizes in the left and right arteries (r=0.97) and to be highly associated with coronary artery plaque extent (r=0.71).23,29 The methods for collection and atherosclerosis determination, as well as the differences between diet groups, have been published previously.30 Briefly, based on a considerable amount of archival data indicating that monkeys have undetectable atherosclerosis prior to the initiation of an atherogenic diet, all animals will were assigned an initial (baseline) atherosclerosis value of 0. The contribution of diet to atherosclserosis extent was determined by measuring each monkey's plaque area in the left common iliac at the time of ovariectomy. The extent of atherosclerosis was measured as the cross-sectional area of plaque in one section of the artery as described previously.30
c) Markers of Inflammation
Monocyte chemoattractant protein-1 (MCP-1), C-reactive protein (CRP), and interleukin-6 (IL-6) were assessed from serum collected at baseline and at ovariectomy (i.e., after 32 months of treatment). MCP-1 and IL-6 were analyzed with a commercially available ELISA (R&D Systems, Minneapolis, MN).31 CRP was measured using a commercially available ELISA (Alpco, Salem, NH).31 For these measurements, intra-assay and inter-assay CVs were 10%.
Serum Isoflavone Measurements
Serum was collected from all monkeys 4 hours after feeding at two time points (7 and 19 months following the beginning of feeding experimental diets), and the mean total serum isoflavones was determined. Serum isoflavone and isoflavone metabolite concentrations were determined by liquid chromatography-mass spectrometry (AAF);32 details including detection limits and inter- and intra-assay CVs for isoflavone measurements have been described previously.30
Data Analysis
The main outcome variable in this study was follicle counts. The distribution of the three types of follicle counts is highly skewed. Thus, square root transformation was used throughout the analyses to achieve normality, an assumption required for linear regression analysis. For each follicle type, a linear regression model was fit to assess the effect of diet, social status (a demonstrated modifier of atherosclerosis in socially-housed monkeys33), and potential interactions between diet and social status. The interaction effect and the main effect of social status were examined using F-tests and were not statistically significant. Therefore social status was removed from subsequent analyses of follicle counts.
AMH is an indirect marker of follicle numbers in cynomolgus monkeys25 and was measured at both baseline and at the end of the experiment. Using a linear regression model which included the main effect of diet, AMH, and their interactions, we found that significant AMH by diet interactions were present in the primordial and the secondary follicle counts but not in the primary follicle counts. To illustrate this interaction effect, the correlations between AMH and follicle counts were reported separately for the C/L diet and the soy diet groups.
Finally, we used two sets of models to examine the effect of various factors on the association between diet and the follicle counts. These factors included iliac artery atherosclerosis, plasma lipids, reproductive hormones, and markers of inflammation. The first model contained only the main effect of diet and yielded an estimated total effect of diet on follicle counts. The second model contained the main effect of both diet and a factor of interest (e.g., iliac artery atherosclerosis, plasma lipids, etc.) and yielded an estimated direct effect of diet on follicle counts. The difference between the total effect and the direct effect is indicative of the indirect, or mediational, effect that a factor of interest has on the association between diet and the follicle counts.34 Since iliac artery atherosclerosis extent was associated with diet in this study and in previous cynomolgus monkey studies,30,35,36 we investigated further its potential to explain the differences in follicle counts observed between the two diet groups. A bootstrapping method was used to estimate the indirect effect of atherosclerosis as a potential mediator. Confidence intervals were set at 95% and calculated to examine if the indirect effect was significantly different from 0.
All analyses were done using SAS v9.1.3 (SAS Institute, Cary, NC). Significance was defined as a p-value < 0.05.
Results
Ovarian Follicle Number / AMH
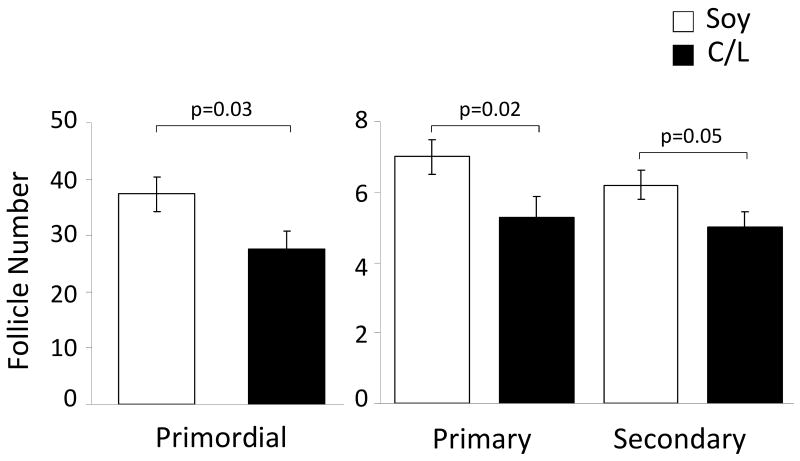
After 32 months of consuming the diets, monkeys fed the C/L diet had fewer primordial (755, CI's 440-1156 vs. 1397, CI's 983-1882, p=0.03), primary (28, CI's 18-40 vs. 49, CI's 37-63, p=0.02) and secondary follicles (25, CI's 17-35 vs. 39, CI's 29-50, p= 0.05) than did their soy-fed counterparts (Figure 2). Because an ovary was not collected at baseline, pre-experimental follicle counts could not be determined and consequently, a surrogate marker of follicle number, AMH, was used to determine whether follicle counts differed at baseline. We have reported previously that AMH is highly associated with all follicle classes (i.e., primordial, primary, secondary) (r>0.60, p <0.001 for all) in cynomolgus macaques.25 At baseline there was no significant difference in AMH concentration between diet groups (C/L=2.8±0.36 ng/mL; Soy=3.0±0.36 ng/mL, p=0.77), indicating that follicle numbers were similar in both groups. At the end of the experimental period, mean AMH was slightly lower in casein-fed monkeys but the difference was not statistically significant (C/L=5.2±0.6 ng/mL, Soy=6.3±0.6 ng/mL; p=0.18) (Figure 3).
Figure 2.
Mean number of primordial, primary, and secondary follicles counted in each diet group after 32 months of treatment. Numbers are expressed as the square root transformation ± SE. CL group, n=29; soy group, n=32.
Figure 3.
Mean (± SE) AMH concentrations for each diet group at baseline and after 32 months of treatment (i.e., at the time of ovariectomy / iliac artery atherosclerosis measurement). CL group, n=29; soy group, n=32.
AMH was highly correlated with all follicle classes among C/L-fed monkeys (primordial rC/L = 0.78, primary rC/L=0.66, secondary rC/L=0.79, p<0.001 for all). However, among soy fed monkeys the correlations were numerically lower and in fact were clearly significant for primary (rsoy=0.47, p=0.005) and secondary (rsoy=0.45, p=0.007) but not for primordial follicles (rsoy=0.31, p=0.07). To more accurately assess the difference in correlations of AMH with follicle counts between the diets, a linear regression including the main effect of diet, AMH, and their interactions was done. Significant AMH by diet interactions were present in the primordial and the secondary follicle counts (indicating differences between the two diets) but not in the primary follicle counts.
Reproductive Hormones
At the time of ovariectomy there was no significant difference in estradiol concentration between soy and casein-fed monkeys (C/L= 18.4±2.7ng/L, Soy= 32.0±11.8ng/L, p=0.64). No significant associations between estradiol or androgens (testosterone and androstenedione) and any of the follicle classes were observed, regardless of whether the data were analyzed for all monkeys or by diet group (p's>0.05).
Cardiovascular Risk Markers
a) Plasma Lipids, Lipoproteins and Iliac Artery Atherosclerosis
Mean plasma lipid/lipoprotein concentrations and iliac artery atherosclerosis extent for all monkeys in the larger study (n=95) have been published previously.30 The mean values for animals in this study (n=61) were similar and are shown in Table 1. Briefly, C/L-fed monkeys had a more atherogenic lipid/lipoprotein profile (higher TPC, higher non-HDL-C and lower HDL-C, p<0.01 for all). Similarly, iliac artery atherosclerosis extent (artery plaque size/intimal area) was greater in C/L-fed monkeys than in soy-fed monkeys (p=0.01). The mean intimal area was 0.48mm2 (95% CI: 0.35-0.64) and 0.25mm2 (95% CI: 0.17-0.36) for the C/L group and the Soy group, respectively.
Table 1. Effect of Diet on Cardiovascular Disease Risk Factors.
| Baseline | Post Treatment (∼32 month) | |||||
|---|---|---|---|---|---|---|
| Variable | C/L (n=29) | Soy (n=32) | p | C/L (n=29) | Soy (n=32) | p |
| TPC (ng/dL) | 279.6 ± 11.6 | 278.8 ± 11.6 | 0.96 | 321.43 ± 18.0 | 242.12 ± 16.3 | 0.002 |
| Non-HDL-C (ng/dL) | 239.4 ± 12.4 | 239.4 ± 12.8 | 0.79 | 265.29 ± 20.69 | 154 ± 18.7 | 0.0002 |
| HDL-C (ng/dL) | 40.6 ± 2.7 | 39.4 ± 2.7 | 0.99 | 56.14 ± 5.2 | 88.1 ± 4.8 | < 0.0001 |
| Iliac athero (mm2) | NA | NA | NA | 0.48 ± 0.07 | 0.25 ± 0.04 | < 0.05 |
| MCP-1(pg/ml) | 322 ± 15.3 | 353.2 ± 20.8 | 0.54 | 380.9 ± 22.2 | 337.26 ± 17.2 | 0.13 |
| CRP (ng/ml) | 425.9 ± 54.2 | 423.4 ± 51.4 | 0.91 | 413.8 ± 48.1 | 473.7 ± 75.5 | 0.50 |
| IL-6 (pg/ml) | 1.48 ± 0.4 | 1.15 ± 0.3 | 0.63 | 2.6 ± 0.30 | 2.41 ± 0.3 | 0.68 |
The effect of diet on cardiovascular endpoints. NA= not applicable (baseline atherosclerosis assumed to be 0, but not measured directly). TPC = total plasma cholesterol; Non-HDL-C = non-high density lipoprotein cholesterol; HDL-C = high density lipoprotein cholesterol; MCP-1 = monocyte chemoattract protein-1; CRP = C-reactive protein; IL-6 = interleukin-6.
b) Markers of Inflammation
For the most part, circulating inflammation markers (CRP, IL-6, MCP-1) were not different between diet groups (Table 1). C/L-fed monkeys experienced a 15.3% increase from baseline MCP-1 compared to a 6.7% increase from baseline in the soy group (p=0.2). No significant associations were found among any follicle types with CRP, MCP-1 or IL-6 (p's >0.05 for all).
Serum Isoflavones
As previously described,30 total serum isoflavone concentrations were significantly elevated in soy-fed monkeys compared to the C/L-fed group (Soy= 713±61 nM vs. C/L= 25±8 nM, p<0.0001). Mean values for isoflavone concentrations (nM) in the Soy group were as follows: daidzein 114 ± 16 and genistein 115 ± 17. Among those fed soy protein, there was no association between total serum isoflavone concentration and any of the follicle types (p >0.77 for all).
Relationships between Follicle Counts and Potential Explanatory Variables
We used the approach described above (“Data analysis” in the Materials and Methods section) to identify potential mediators that might explain the observed differences in follicle counts between diet groups. After fitting a regression model with only diet groups to obtain an estimated total effect of diet on follicle counts, the following variables were added separately to the above regression model to obtain the estimated direct effect of diet on follicle counts: iliac artery atherosclerosis, all lipids, CRP, IL-6, MCP-1, androgens and estrogen. There was a suggestion that iliac artery atherosclerosis may have contributed to the effect of dietary protein source on follicle counts. However, further tests of the indirect effects did not reach statistical significance. For example, the estimated indirect effect of atherosclerosis as a potential mediator on the square root of primordial counts was 0.41 (95% bootstrap CI -2.10, 3.79).
Discussion
The present study provides evidence that the source of dietary protein, or isoflavones, affects ovarian reserve (follicle number) in cynomolgus monkeys. Monkeys fed a casein-lactalbumin (C/L)-based diet had fewer primordial, primary, and secondary follicles than did their soy-fed counterparts after 32 months of consuming the diets. AMH concentrations did not differ between the groups at baseline, indicating that follicle number did not differ at baseline. Thus, it appears that either the C/L diet increased the tempo of ovarian follicle decline (e.g., via reduced blood flow to the ovary as a result of atherosclerosis or direct effects of diet-induced inflammation or oxidation), or that the soy diet slowed the ovarian aging process (e.g., via antioxidant or anti-inflammatory properties). With respect to cardiovascular disease risk and inflammation markers measured in this study, C/L-fed monkeys did have a more atherogenic lipoprotein profile, increased iliac artery plaque size and slight increases in MCP-1. However, none of these risk markers (considered individually) were significant mediators of the effect of dietary protein source on follicle count in this study. These results highlight the importance of nutrition with respect to ovarian aging, although the mechanism underlying this relationship remains to be determined.
Although iliac artery atherosclerosis extent did not explain the effect of diet on follicle counts in this study, we cannot rule out the effect of other cardiovascular disease risk markers (i.e. ovarian atherosclerosis or other CVD risk markers not measured here) on follicle counts and ovarian aging. The hypothesis that CVD risk could influence ovarian aging, rather than the reverse, was proposed by Kok and colleagues (2006) in a report of women in the Framingham Risk Study.15 Data were collected over a 12-year period from women (n=695) aged 34-55 and cardiovascular risk markers (TPC, body weight, Framingham risk score, change in lipids, blood pressure) were measured at least twice per woman during the premenopausal phase. Among women in the study, 42% reported smoking during the time period proceeding menopause, and those women reached menopause approximately 1.6 years earlier than non-smokers. After adjusting for smoking, however, a subgroup of women with increasing TPC during the perimenopausal transition reached menopause approximately 2.6 years earlier for every 20mg/dL increase in TPC. Conversely, among women with decreasing TPC during perimenopause, each 20mg/dL decrease in individual TPC was associated with a 4.6-year delay in menopause. The authors suggested that increased atherosclerosis in arteries supplying the ovary, leading to decreased perfusion and ischemia, might be responsible for this finding. However, blood pressure appeared to be the largest determinant of menopausal age in this population. Adjusted for smoking, women experienced a 7.38-year earlier menopause for each 10-mm Hg increase in diastolic pressure, while menopause was experienced 2.48 years later for each 10-mm Hg decrease in diastolic pressure. Unfortunately, blood pressure was not one of the cardiovascular risk factors measured in the current study, so it is unclear if blood pressure differences might have been correlated with ovarian follicle number.
There are many potential confounders in the above mentioned study, and they are outlined in an editorial suggesting that because risk measurements were taken after the hormonal changes characterizing the perimenopause had already begun, it was impossible to distinguish between cause and effect.37 The risk-factor trends calculated in that study were based on an average of 4.9 measurements before menopause, corresponding to an average of 10 years of observation before the final menstrual period. These risk-factor trends were thus measured in the late reproductive phase (i.e., at a time when endocrine changes in the reproductive system may have already been in progress). Therefore it cannot be known whether endocrine changes and risk-factor changes simply occurred concurrently, whether risk-factor changes influenced the endocrine changes in progress and thus changed menopausal age as the authors hypothesize, or whether endocrine changes caused both a change in risk-factor levels over time and determined menopausal age. Despite the controversial nature of these findings, these data suggest that it is possible that changes in cardiovascular risk factors could influence ovarian aging.
While a casein-rich diet could have hastened follicular depletion through an elevation in CVD risk factors, it is also possible that soy (protein or isoflavones or both) has beneficial effects on the ovary, thus slowing the rate at which primordial follicles are depleted. There are some data consistent with this hypothesis. For example, monkeys consuming diets that derive their primary protein from isoflavone-rich soy meal have plasma concentrations of the soy metabolites (genistein, daidzein and the non-steroidal estrogen equol) several orders of magnitude greater than endogenous estrogen concentrations.38,39 These isoflavone metabolites all bind to one or both of the α and β estrogen receptors and have both receptor and non-receptor-mediated biological effects.40 These marked elevations in plasma isoflavones and isoflavone metabolites may be responsible, at least in part, for inhibiting ovarian aging. For example, isoflavones inhibit oxidative stress, which has been shown to affect mid-luteal phase corpus luteum and its steroidogenic capacity of ovaries both in vitro and in vivo.41 In addition, soy isoflavones have been shown to reduce certain markers of inflammation both in cynomolgus monkeys31 and in human beings.42 One set of studies assessing the effect of oral antioxidants on oocytes from aged mice is of particular interest.43 Here, mice were supplemented with vitamins C and E either starting on the first day of weaning or from age 32 weeks until necropsy (40-62 wks). With both early and late administration of oral antioxidants, there was an increased percentage of normal oocytes and a decrease in apoptotic oocytes, indicating that dietary intervention counteracted some of the effects of ovarian aging. Nonetheless, despite intriguing findings in women15 and experimental data from animals, the mechanisms underlying nutritional effects on ovarian aging are unknown. Hence, in the current study it remains unclear whether casein/lactalbumin hastened follicular depletion, soy impeded it, or both.
The study presented here is not without limitations. Because an ovary could not be collected at baseline to determine pre-treatment follicle counts, an indirect measure of follicle number (AMH) was used. While AMH has been shown to be highly correlated with all follicle types in cynomolgus monkeys25 and women44-50 and should have provided reasonable evidence that the treatment groups were balanced at baseline, a direct measure of follicle counts would have been more reliable. Nonetheless, animals were assigned to dietary conditions using a structured randomization protocol that should have minimized pre-existing differences. We note that we also observed an increase from baseline AMH in both treatment groups, which may have been due to travel-induced stress and follicle suppression just after arrival from Indonesia, or possibly to degradation of AMH protein over time during storage. A second potential limitation relates to the method used to estimate follicle counts. Ovaries were serially sectioned and follicles were counted in every 100th section. This method provides an estimate of follicle numbers from which relative differences between treatment conditions can be determined, but does not provide true total follicle counts per ovary. A third limitation of the study relates to the need to use monkeys whose age has been estimated. Since ovarian follicle counts decline with age in both monkeys51 and women,52-54 it would have been preferable to use monkeys in which the exact age was known. However, adult status of each monkey was confirmed by dentition and evidence of epiphyseal closure, and the average age of monkeys at arrival was estimated to be greater than 9-10 years, although equivalent for both treatment groups. Finally, because samples collected for estradiol measurement were not timed for a specific day of the menstrual cycle, associations between E2 and follicle numbers may have been missed. A major strength of this study is the use of a controlled diet with complete adherence and the approximately 3-year treatment period (comparable to 9 years of a woman's experience).
Conclusions
The study presented here provides evidence that diet influences ovarian aging in cynomolgus monkeys. Monkeys consuming the animal protein-based C/L diet had significantly fewer follicles than those consuming the soy protein-based (with isoflavones) diet. Although the C/L-fed monkeys were at increased risk for cardiovascular disease compared to their soy-fed counterparts (e.g., they had elevated plasma lipids, atherosclerosis, metabolic measures and markers of inflammation), these differences in CVD risk did not account statistically for the difference in follicle numbers. Similarly, no associations among isoflavone concentration (in monkeys fed soy), androgens or estradiol with follicle numbers were seen, and the mechanism of the dietary effect on follicle counts remains undetermined.
Acknowledgments
The authors would like to acknowledge the following for their technical contributions to this work: Melissa Ayers, Dewayne Cairnes, Patty Christian, Laurie Custer, Debbie Golden, Andrea Grantham, Chrissy May, and Maryanne Post.
This work was supported by grant R01 HL079421 from the National Heart, Lung, and Blood Institute (JRK); grant R24 RR022191 (JRK) and S10 RR020890 (AAF) from the National Center for Research Resources; grant R01 AG027847 from the National Institute on Aging (JRK); grant R01 AG021948 from the National Institute of Aging (PBH); and Center Grant ES06694 (PBH).
Footnotes
The authors have no disclaimers to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Susan E. Appt, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine
Haiying Chen, Department of Biostatistical Sciences, Wake Forest University School of Medicine
Amanda K. Goode, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine
Patricia B. Hoyer, Department of Physiology, the University of Arizona
Thomas B. Clarkson, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine
Michael R. Adams, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine
Mark E. Wilson, Yerkes National Primate Research Center and the Division of Psychobiology, Emory University
Adrian A. Franke, The Cancer Research Center of Hawaii
Jay R. Kaplan, Wake Forest University Primate Center and the Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine
References
- 1.Derby CA, FitzGerald G, Lasser NL, Pasternak RC. Application of national screening criteria for blood pressure and cholesterol to perimenopausal women: prevalence of hypertension and hypercholesterolemia in the Study of Women's Health Across the Nation. Prev Cardiol. 2006;9(3):150–9. doi: 10.1111/j.1520-037x.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- 2.Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol. 2009;169(11):1352–61. doi: 10.1093/aje/kwp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women's Health across the Nation. Arch Intern Med. 2008;168:1568–75. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews KA, Santoro N, Lasley B, et al. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab. 2006;91:1789–95. doi: 10.1210/jc.2005-1057. [DOI] [PubMed] [Google Scholar]
- 5.Sowers MR, Matthews KA, Jannausch M, et al. Hemostatic factors and estrogen during the menopausal transition. J Clin Endocrinol Metab. 2005;90(11):5942–8. doi: 10.1210/jc.2005-0591. [DOI] [PubMed] [Google Scholar]
- 6.Wildman RP, Colvin AB, Powell LH, et al. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women's Health Across the Nation (SWAN) Menopause. 2008;15(3):414–21. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosano GMC, Vitale C, Marazzi G, Volteranni M. Menopause and cardiovascular disease: the evidence. Climacteric. 2007;10(Suppl 1):19–24. doi: 10.1080/13697130601114917. [DOI] [PubMed] [Google Scholar]
- 8.Chu MC, Rath KM, Huie J, Taylor HS. Elevated basal FSH in normally cycling women is associated with unfavorable lipid levels and increased cardiovascular risk. Hum Reprod. 2003;18(8):1570–3. doi: 10.1093/humrep/deg330. [DOI] [PubMed] [Google Scholar]
- 9.Murabito JM, Yang Q, Fox C, Wilson PW, Cupples LA. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90(6):3427–30. doi: 10.1210/jc.2005-0181. [DOI] [PubMed] [Google Scholar]
- 10.Castelo-Branco C, Blümel JE, Chedraui P, et al. Age at menopause in Latin America. Menopause. 2006;13(4):706–12. doi: 10.1097/01.gme.0000227338.73738.2d. [DOI] [PubMed] [Google Scholar]
- 11.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–74. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 12.Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol. 1994;139(1):64–76. doi: 10.1093/oxfordjournals.aje.a116936. [DOI] [PubMed] [Google Scholar]
- 13.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol. 2008;167(11):1287–94. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen A. Environment, human reproduction, menopause, and andropause. Environ Health Perspect. 1993;101(Suppl 2):91–100. doi: 10.1289/ehp.93101s291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kok HS, van Asselt KM, van der Schuow YT, et al. Heart disease determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47(10):1976–83. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 16.Forges T, Monnier-Barbarino P, Leheup B, Jouvet P. Pathophysiology of impaired ovarian function in galactosaemia. Hum Reprod Update. 2006;12(5):573–84. doi: 10.1093/humupd/dml031. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - a clinician's perspective. Reprod Biomed Online. 2005;11(5):641–50. doi: 10.1016/s1472-6483(10)61174-1. [DOI] [PubMed] [Google Scholar]
- 18.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198(2):210.e1–210.e7. doi: 10.1016/j.ajog.2007.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorjgochoo T, Kallianpur A, Gao YT, et al. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause. 2008;15(5):924–33. doi: 10.1097/gme.0b013e3181786adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratzke C, Jarajapu YP, Christ GJ, et al. Effects of long-term dietary soy treatment on female urethral morphology and function in ovariectomized nonhuman primates. J Urol. 2008;180(5):2247–53. doi: 10.1016/j.juro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Lees CJ, Kaplan JR, Chen H, Jerome CP, Register TC, Franke AA. Bone mass and soy isoflavones in socially housed, premenopausal macaques. Am J Clin Nutr. 2007;86:245–50. doi: 10.1093/ajcn/86.1.245. [DOI] [PubMed] [Google Scholar]
- 22.Riddick NV, Czoty PW, Gage HD, et al. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158(4):1257–65. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker SE, Adams MR, Franke AA, Register TC. Effects of dietary soy protein on iliac and carotid artery atherosclerosis and gene expression in male monkeys. Atherosclerosis. 2008;196:106–13. doi: 10.1016/j.atherosclerosis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appt SE, Kaplan JR, Clarkson TB, Cline JM, Christian PJ, Hoyer PB. Destruction of primordial ovarian follicles in adult cynomolgus macaques after exposure to 4-vinylcyclohexene diepoxide: a nonhuman primate model of the menopausal transition. Fertil Steril. 2006;86(4 Suppl 1):1210–6. doi: 10.1016/j.fertnstert.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Appt SE, Clarkson TB, Chen H, et al. Serum antimüllerian hormone predicts ovarian reserve in a monkey model. Menopause. 2009;16(3):597–601. doi: 10.1097/gme.0b013e3181906fb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pederson T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–7. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 27.Lee MM, Gustafson ML, Ukiyama E, et al. Developmental changes in müllerian inhibiting substance in the cynomolgus monkey, Macaca fascicularis. J Clin Endocrinol Metab. 1994;78:615–21. doi: 10.1210/jcem.78.3.8126132. [DOI] [PubMed] [Google Scholar]
- 28.Pazol K, Wilson ME, Wallen K. Medroxyprogesterone acetate antagonizes the effects of estrogen treatment on social and sexual behavior in female macaques. J Clin Endocrinol Metab. 2004;89:2998–3006. doi: 10.1210/jc.2003-032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995;15(12):2094–100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- 30.Walker SE, Register TC, Appt SE, et al. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008;15(5):950–7. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Register TC, Cann JA, Kaplan JR, et al. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab. 2005;90:1734–40. doi: 10.1210/jc.2004-0939. [DOI] [PubMed] [Google Scholar]
- 32.Franke AA, Custer LJ, Wilkens LR, et al. Liquid chromatographic analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777(1-2):45–59. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan JR, Manuck SB, Chen H. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am J Primatol. 2009;71(9):732–41. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 35.Anthony MS, Clarkson TB, Williams JK. Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am J Clin Nutr. 1998;68:1390S–3S. doi: 10.1093/ajcn/68.6.1390S. [DOI] [PubMed] [Google Scholar]
- 36.Register TC. Primate models in women's health: inflammation and atherogenesis in female cynomolgus macaques (Macaca fascicularis) Am J Primatol. 2009;71(9):766–75. doi: 10.1002/ajp.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bittner V. Menopause and cardiovascular risk: cause or consequence? J Am Coll Cardiol. 2006;47(10):1984–6. doi: 10.1016/j.jacc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Honoré EK, Williams JK, Anthony MS, Clarkson TB. Soy isoflavones enhance coronary vascular reactivity in atherosclerotic female macaques. Fertil Steril. 1997;67(1):148–54. doi: 10.1016/s0015-0282(97)81872-9. [DOI] [PubMed] [Google Scholar]
- 39.Stroud FC, Appt SE, Wilson ME, Franke AA, Adams MR, Kaplan JR. Concentrations of isoflavones in macaques consuming standard laboratory monkey diet. J Am Assoc Lab Anim Sci. 2006;45(4):20–3. [PubMed] [Google Scholar]
- 40.Muthyala RS, Ju YH, Sheng S, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12(6):1559–67. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 2006;18(3):325–32. doi: 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins DJ, Kendall CW, Connelly PW, et al. Effects of high- and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism. 2002;51(7):919–24. doi: 10.1053/meta.2002.33352. [DOI] [PubMed] [Google Scholar]
- 43.Tarín JJ, Pérez-Albalá S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev. 2002;61(3):385–97. doi: 10.1002/mrd.10041. [DOI] [PubMed] [Google Scholar]
- 44.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women's Midlife Health Project. Hum Reprod Update. 2007;13(6):559–65. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 45.de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–62. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 46.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92:3060–7. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 47.La Marca A, de Leo V, Giulini S, et al. Anti-mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Investig. 2005;12:545–8. doi: 10.1016/j.jsgi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Robertson DM, Hale GE, Fraser IS, Hughes CL, Burger HG. A proposed classification system for menstrual cycles in the menopause transition based on changes in serum hormone profiles. Menopause. 2008;15(6):1139–44. doi: 10.1097/gme.0b013e3181735687. [DOI] [PubMed] [Google Scholar]
- 49.Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-müllerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–83. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rooij IAJ, den Tonkelaar I, Broekmans FJM, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6):601–6. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 51.Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum Reprod. 2005;20:79–83. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65(6):1231–7. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 53.Richardson SJ, Nelson JF. Follicular depletion during the menopausal transition. Ann N Y Acad Sci. 1990;592:13–20. doi: 10.1111/j.1749-6632.1990.tb30312.x. [DOI] [PubMed] [Google Scholar]
- 54.Richardson SJ. The biological basis of the menopause. Baillieres Clin Endocrinol Metab. 1993;7(1):1–16. doi: 10.1016/s0950-351x(05)80267-8. [DOI] [PubMed] [Google Scholar]