Abstract
Improved detection and isolation of rickettsial agents from naturally infected dogs would facilitate understanding the epidemiologic roles of these hosts. In this study, current methods were refined for rapid screening of carrier blood and ticks for Ehrlichia canis, and the feasibility of blood culture after PCR screening was addressed.
Keywords: Ehrlichia canis, Rhipicephalus sanguineus, blood culture, PCR assay
Several members of the rickettsial family Anaplasmataceae that naturally infect dogs have emerged as tick-borne zoonotic pathogens over recent decades. Isolation of canine strains of these pathogens is needed for experimental studies to elucidate roles of dogs as sentinels, reservoirs and models for tick-borne zoonoses. This is challenging due to time constraints associated with detection and cultivation of these pathogens. For example, screening and culture of blood submitted to diagnostic laboratories requires a rapid, sensitive PCR assay, but until recently our most sensitive procedure required time-consuming phenol-chloroform extraction and ethanol precipitation (P-E) [1-3]. The objectives of this study were to determine (1) if a more rapid template isolation procedure is applicable for detection of Ehrlichia canis, a monocytotropic agent of canine ehrlichiosis (CME) [4], in carrier blood and ticks, and (2) if E. canis can be cultured from blood refrigerated during the time required for this process.
Canine blood was collected in the presence of heparin or EDTA from purpose-bred Beagles cared for in accordance with a protocol on file with the University of Missouri ACUC, and divided into 1 ml or 200 μl aliquots for methods that required buffy coats (BC) or whole blood (WB), respectively. Rhipicephalus sanguineus nymphs were purchased from Oklahoma State University and fed on dogs BMN and BIW during the chronic phase of CME as previously described [1-3]. For P-E, BC and ticks were prepared as described elsewhere with nonionic detergents [1] or SDS [3]. For the silica adsorption (SA) method, ticks, WB and BC were processed with the High Pure Viral Nucleic Acid Kit (Roche, USA) with polyA according to the manufacturer’s instructions. P-E and SA methods were compared with a p30-based PCR assay of blood from an E. canis (Ebony strain) carrier that was infected by tick transmission [1]. Cell culture methods were performed as described elsewhere [5].
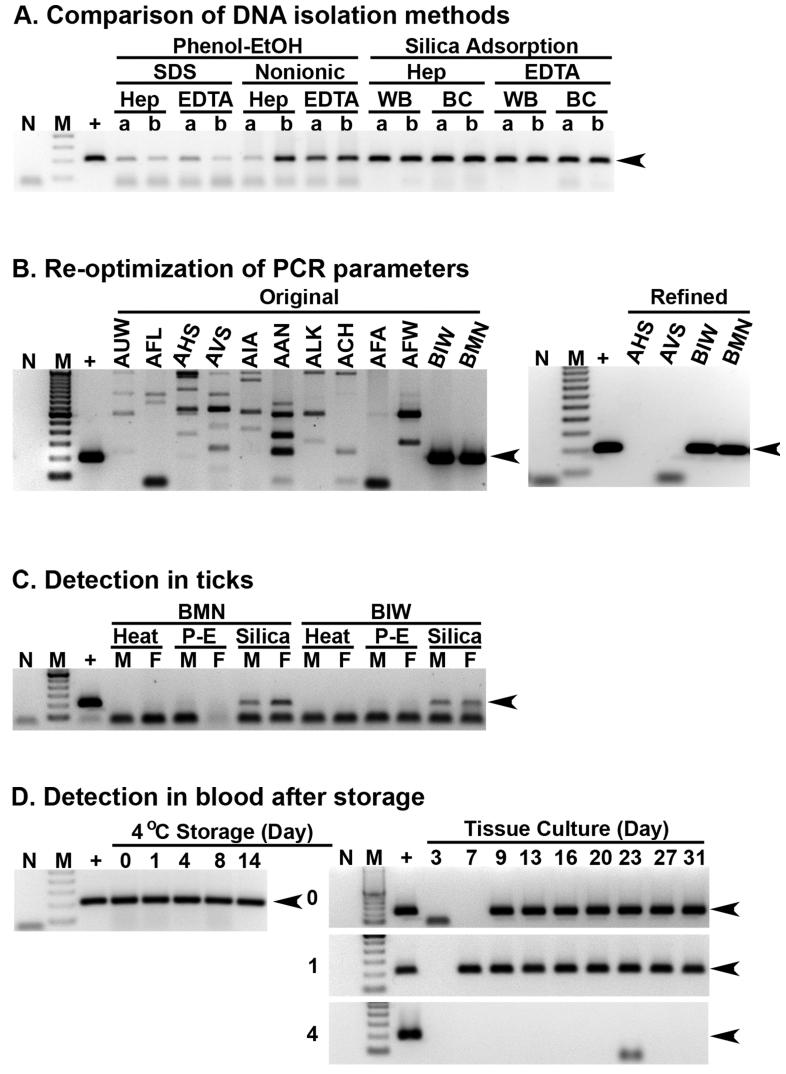
All of the samples tested PCR-positive for E. canis, but notable differences were observed with the different procedures. For P-E, brighter amplicons were observed from templates digested in the presence of nonionic detergents than with SDS (Fig 1A). Notably, protease digestion of BC was also more rapid with nonionic detergents. Interestingly, the most robust and consistent bands were observed with SA, without any effect attributable to the anticoagulant used. No differences were observed among WB versus BC prepared with SA in this experiment, but in a subsequent experiment E. canis was detected sooner from BC than WB simultaneously collected from dogs after experimental inoculation (data not shown); thus BC were optimal for SA.
Figure 1. Refined methods for detection of E. canis.
Each panel represents a minimum of two trials. No template (N) and infected DH82 cells (+) were controls. Molecular standards (M) were 100 bp ladders, with 200 bp target amplicons indicated by arrowheads. Template isolation methods (Panel A) were compared with E. canis carrier whole blood (WB) or buffy coats (BC), with heparin or EDTA, digested with SDS or nonionic detergents and extracted with phenol and ethanol (P-E) or silica adsorption methods in replicate (a and b). Original and refined assays were compared (Panel B) with silica adsorption from normal (dogs AUW, AFL, AHS, AVS, AIA, AAN, ALK, ACH, AFA and AFW) and infected (dogs BIW and BMN) buffy coat samples. For E. canis detection in ticks (Panel C), silica adsorption was compared to protease with nonionic detergents followed by heat denaturation or P-E of male (M) and female (F) ticks exposed as nymphs on BIW or BMN. Refrigerated carrier blood (Panel D) was stored for 0, 1, 4, 8 or 14 days before PCR assay and cultures that were also assayed semiweekly.
Unspecific amplification of uninfected BC DNA was observed when SA was used to assay unexposed dogs (Fig 1B). Therefore PCR parameters were re-optimized with infected and uninfected BC prepared with SA, and amplicon intensity from infected BC combined with background amplification from uninfected BC were used to define optimal parameters. The refined PCR assay resulted in specific amplification of target without artifact bands observed from uninfected BC (Fig 1B). Optimized PCR parameters for SA were 2.0 mM MgCl2, 0.3 μM each of primers ECA30-384S (ATAAACACGCTGACTTTACTGTTCC) and ECA30-583A (GTGATGAGATAGAGCGCAGTACC), 0.03 U/μl of Platinum Taq polymerase (Invitrogen, USA) and 2% DMSO; which were incubated at 94 °C for 2 min followed by 75 cycles at 94, 65 and 72 °C for 30 sec each, and a final extension for 7 min at 72 °C.
Superior results with carrier blood prompted a similar comparison of these methods with ticks exposed to E. canis. Adult R. sanguineus, fed as nymphs on experimentally infected dogs, were bisected and halves of each tick pooled into groups of 5 (2.5 tick equivalents per pool). Tick halves from one pool were digested with protease k and nonionic detergents, which was divided in half and subjected to heat denaturation or P-E. The remaining pool was subjected to SA. Faint bands were observed from ticks subjected to P-E, and more robust amplicons were observed with SA (Fig 1C).
Rapid screening of blood will make pathogen isolation more feasible. However, cultivation of monocytotropic Ehrlichia from refrigerated blood would provide additional logistic advantages. Thus heparinised E. canis carrier blood was refrigerated and tested via PCR and culture after 0 to 14 days (Figure 1D). E. canis was detected throughout this period by PCR, but could not be cultivated after four days of refrigeration in two of three trials. Interestingly, cultures started after one day became positive sooner than the non-refrigerated controls in all three trials.
In conclusion, these refined methods are expected to facilitate investigations of monocytotropic Ehrlichia and related agents in both vertebrate and invertebrate hosts. This SA procedure was more rapid, robust and reliable for detection of E. canis in blood and ticks than P-E, and this refined process is expected to facilitate culture of such organisms from blood samples within 24 hr.
Acknowledgments
This work was supported by NIH grant R01AI47932.
REFERENCES
- 1.Stich RW, Rikihisa Y, Ewing SA, Needham GR, Grover DL, Jittapalapong S. Detection of Ehrlichiacanis in canine carrier blood and in individual experimentally infected ticks with a p30-based PCR assay. J Clin Microbiol. 2002;40:540–546. doi: 10.1128/JCM.40.2.540-546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bremer WG, Schaefer JJ, Wagner ER, Ewing SA, Rikihisa Y, Needham GR, Jittapalapong S, Moore DL, Stich RW. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet Parasitol. 2005;131:95–105. doi: 10.1016/j.vetpar.2005.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer JJ, Needham GR, Bremer WG, Rikihisa Y, Ewing SA, Stich RW. Tick acquisition of Ehrlichia canis from dogs treated with doxycycline hyclate. Antimicrob Agents Chemother. 2007;51:3394–3396. doi: 10.1128/AAC.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewing SA. Canine ehrlichiosis. Adv Vet Sci Comp Med. 1969;13:331–353. [PubMed] [Google Scholar]
- 5.Zhang XF, Zhang JZ, Long SW, Ruble RP, Yu XJ. Experimental Ehrlichia chaffeensis infection in Beagles. J Med Microbiol. 2003;52:1021–1026. doi: 10.1099/jmm.0.05234-0. [DOI] [PubMed] [Google Scholar]