Abstract
Background
Based on empirical evidence, a personal history of non-melanoma skin cancer (NMSC) has been hypothesized to be a risk factor for other cancers. Others hypothesize that NMSC may be a marker of high cutaneous vitamin D synthesis and therefore inversely associated with risk of other malignancies. To reconcile these divergent views, we carried out a systematic review to determine the association between NMSC and subsequent risk of other cancers.
Methods
Bibliographic databases were searched through March 2009. Studies were included if sufficient information was presented to estimate the risk of developing other cancers following NMSC. Studies were reviewed and data abstracted independently in duplicate with disagreements resolved by consensus.
Results
Of the 21 included studies, 15 reported the association between NMSC and risk of all other cancers combined. NMSC was significantly associated with increased risk of another malignancy among cohort studies based on cancer registries (summary random-effects RR (SRR) 1.12, 95% confidence interval (CI) 1.07-1.17, n=12 studies) and those with individual-level data (SRR 1.49, 95% CI 1.12 – 1.98, n=3). In stratified analyses of registry studies, this association held true for both squamous (SRR 1.17, 95% CI 1.12-1.23, n=7) and basal cell carcinoma (SRR 1.09, 95% CI 1.01-1.17, n=7), and both men (SRR 1.14, 95% CI 1.09-1.20, n=12) and women (SRR 1.10, 95% CI 1.04-1.15, n=12).
Conclusions
Strong, consistent evidence indicates that a personal history of NMSC is associated with increased risk of developing other malignancies.
Impact
For unknown reasons, NMSC may be a risk factor for other cancers.
Keywords: non-melanoma skin cancer, second primary cancer, meta-analysis
INTRODUCTION
Non-melanoma skin cancer (NMSC), comprised mainly of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), is the most common malignancy in the United States, with more than two million new cases annually (1, 2). Disturbingly, NMSC incidence continues to rise (3). Although NMSC is frequently curable, its high prevalence and expense of treatment place NMSC as a major public health problem and among the costliest cancers in the United States (4). Accurate population-based data on the occurrence of NMSC have been difficult to obtain, as NMSC is not routinely included in most cancer registries and is often not rigorously followed up in prospective cohort studies. Some of the issues complicating population-based ascertainment of NMSC include treatment based on clinical diagnosis without biopsy, and deferment of biopsy due to misclassification as pre-malignant or non-malignant lesions.
The major cause of NMSC is exposure to solar ultraviolet radiation. Skin characteristics, such as ability to tan and pigmentation, largely determine host susceptibility to ultraviolet radiation in causing NMSC. Individuals with NMSC are known to be at increased risk of developing subsequent NMSCs, as well as malignant melanoma, likely through the shared risk factor of ultraviolet radiation (1, 5).
Individuals with a personal history of NMSC may also be at an increased risk for second primary cancers other than just NMSC and melanoma (6-29). Despite accumulating evidence that appears to support this association, some have hypothesized that individuals with prior NMSC may in fact have a decreased risk of other cancers (28, 30-32). The hypothesis of an inverse association is based on the supposition that the extensive exposure to sunlight among those who develop NMSC serves as a proxy for elevated levels of vitamin D due to the cutaneous synthesis that occurs upon exposure to sunlight. In turn, vitamin D is hypothesized to have anti-cancer properties (33).
Clarifying this issue has important scientific, clinical, and public health implications. To help reconcile these divergent views of the relationship between a personal history of NMSC and risk of subsequent malignancies, we carried out a systematic review of the evidence on this topic.
METHODS
Data Sources and Searches
With the assistance of a research librarian, we conducted searches of the Ovid/MEDLINE and PubMed databases using the following Medical Subject Heading terms: Skin neoplasms; Neoplasms, second primary; and Risk. This search identified 107 potentially relevant papers as of March 3, 2009. Searching the CINAHL Plus and CRISP databases did not identify any current studies. The electronic searches were supplemented with hand-searches based on references in relevant papers and the related articles identified in PubMed citations. Authors were not contacted in the case of missing data.
Study Selection
Studies eligible for inclusion in this systematic review met the following criteria: 1) were prospective studies in which those with and without NMSC were followed-up over time for the occurrence of other cancers; 2) provided an estimate of relative risk (RR) for the association of NMSC and second primary cancers, plus 95% CI or standard error; and 3) were reported in English. When study populations were overlapping, we used only the most recent data, or the report presenting the greatest levels of stratification. Potential studies were sequentially screened in duplicate for relevance by title, abstract, and then full-text. Discrepancies in judgments of relevance to the topic were discussed and resolved by consensus.
One report presented the combined data from thirteen national cancer registries (28). Due to overlap with published reports from many of these countries, and the resultant loss of ability to perform stratified analyses, this study was not included in the primary analysis. To assess the impact of this report on the body of evidence, results from this large study were included in ancillary analyses. Results from these ancillary analyses are presented in instances when they differed from those of the primary analyses.
Data Extraction and Quality Assessment
From each included study, relative risks (RR) for the association between NMSC and subsequent risk of all other malignancies combined were abstracted in duplicate. If more than one RR was presented, the most fully adjusted estimate was used. To obtain a study-specific estimate of the overall association between NMSC and risk of another malignancy in studies that did not present this information, we calculated it from published results by combining stratum-specific estimates (usually males and females). Rather than conduct formal quality scoring of the included studies, we conducted stratified analyses by study design as described below, in accordance with the recommendation of the Meta-Analysis of Observational Studies in Epidemiology group (34).
Data Synthesis and Analysis
The evidence identified by the search was generated from two types of prospective study designs: 1) cancer registry-based studies that entailed linking nationwide databases, and 2) cohort studies in which individual-level information was collected from study participants who were followed over time for the occurrence of cancer. As the methods used to analyze these two designs have different underlying assumptions limiting the comparability of their respective risk estimates, all analyses were stratified by study design (registry-based studies vs cohort studies with individual-level data). To measure the association between NMSC and the risk of second primary cancers, we combined the study-specific results. Summary relative risks (SRR) and 95% confidence intervals (CI) were calculated using Dersimonian and Laird random effects models (35). Stratified analyses were performed by sex and histologic type (BCC, SCC). SRRs were calculated for specific anatomic cancers sites in instances when the association between NMSC and that cancer site was reported on in at least 5 studies. Consequently, stratified analyses were not conducted for cohort studies with individual-level data (n=3), but rather were limited to registry-based studies only (n=12). I2 was used to assess the magnitude of heterogeneity among studies (36). Funnel plots (1/SE) were constructed to assess publication bias, augmented by trim-and-fill plots. The results of these analyses did not provide evidence of publication bias.
Standard errors were estimated to weight each study in calculating the summary RR measure. For those studies reporting a 95% CI only and not a standard error (SE), we calculated the SE using the equation: SE = [ln(upper limit) – ln(lower limit)] / 3.92. Where the published lower limit was zero, we estimated the SE using the equation: SE = [ln(upper limit) – ln(RR)]/1.96. For registry-based studies that presented only results stratified by gender or NMSC histology, we combined strata to obtain measures for total NMSC by adding the stratum-specific observed and expected numbers of cases, then dividing the total number of observed cases by the total number of expected cases to calculate the association for the total unstratified population. If the expected number of cases was not reported, we estimated it using the equation: expected = observed / SIR. 95% confidence intervals were calculated assuming a Poisson distribution for the number of observed cancers following NMSC using exact limits (37).
To assess factors contributing to heterogeneity, a meta-regression was conducted for the overall association between NMSC and risk of subsequent cancer. Covariates that were considered in the regression models were histology, year of study, and latitude of study location.
Mix software, version 1.7 was used for all primary analyses (38, 39) except the meta-regression was conducted using SAS 9.1 (SAS Institute, Cary, NC) (40). Two-sided p-values of less than 0.05 were considered statistically significant.
RESULTS
The initial search yielded 107 studies. Of these, 81 did not measure the incidence of second cancers after NMSC, three studies were duplications, one study presented a point estimate outside of its confidence interval, and one study did not present gender strata that were obtainable from earlier studies. The hand searches did not identify any additional studies. This left twenty-one articles that met the inclusion criteria (Table 1). Of these, 18 were registry-based studies, and three were cohort studies that collected individual-level data (9, 11, 12). These represented 13 populations from 10 countries in Europe (Denmark, England, Finland, Ireland, Italy, the Netherlands, Sweden, Switzerland) and North America (Canada, United States). Six of the registry-based studies presented data on only specific cancer sites and not risk of cancer overall (10, 16, 17, 22, 30, 32).
Table 1.
Included studies on the risk of subsequent cancers in individuals with a personal history of NMSC compared to those with no prior history of NMSC
| First Author | Year | Location | Study Type | Study Years |
# NMSC cases |
# subsequent cancers |
Mean follow up for cases (years) |
Adjustments |
|---|---|---|---|---|---|---|---|---|
| Bower | 2000 | United Kingdom |
Registry-based (South and West) |
1981-1995 | 13961 | 789 | 8.45 | Cancer site, age |
| Cantwell | 2009 | Ireland | Registry-based (Northern Ireland) |
1993-2002 | 20843 | 1377 | not reported | age, 5-year period, sex |
| Chen | 2008 | Maryland | Cohort with individual-level data (CLUE II) |
1989-2005 | 769 | 181 | 8.02 | age, sex, BMI, smoking, education |
| Crocetti | 2001 | Italy | Registry-based | 1976-1995 | 198303 | 6974 | 2.57 | Cancer site, age, sex, registry, 5-year period |
| de Vries | 2007 | Netherlands | Registry-based (Eindhoven) |
1972-2002 | 12078 | 253 (prostate) | 5.0 SCC, 5.6 BCC | age, 5-year period |
| Efird | 2002 | California | Cohort with individual-level data (Kaiser Permanente) |
1974-1995 | 822 | 144 | 7.80 | education, BMI (alcohol, smoking, occupational exposure, marital status did not change model) |
| Friedman | 2000 | California | Cohort with individual-level data (Kaiser Permanente) |
1974-1997 | 3164 | 556 | 11.30 | matched on sex, zip code, skin color, age ± 2, date joined. Multivariate: age at dx, parent hx cancer, marital status, education, smoking, alcohol, occupational exposure, BMI, parity, age at menarche and menopause |
| Frisch | 1995 | Denmark | Registry-based | 1978-1989 | 5132 | 815 | 4.47 | Cancer site, age, sex |
| Frisch | 1996 | Denmark | Registry-based | 1978-1991 | 37674 | 3663 | 5.07 | Cancer site, age, sex, calendar period |
| Hemminki | 2000 | Sweden | Registry-based (Sweden) |
1958-1996 | 17673 | 3624 | not reported | Age, sex, calendar period |
| Hemminki | 2001 | Sweden | Registry-based (Sweden) |
1958-1996 | 17280 | 192 (HPV- related cancers) |
not reported | Age, sex, calendar period |
| Hemminki | 2003 | Sweden | Registry-based (Sweden) |
1958-1998 | Not reported | 120 (non- Hodgkin) |
not reported | Age, sex, calendar period |
| Jaeger | 1999 | Denmark | Registry-based | 1978-1993 | 1147 | 201 | 5.63 | sex, age, period, cancer site |
| Levi | 1997 | Switzerland | Registry-based (Vaud, Neuchatel) |
1974-1994 | 4639 | 729 | 4.99 | Cancer site, age, calendar period |
| Levi | 1998 | Switzerland | Registry-based (Vaud, Neuchatel) |
1974-1994 | 11878 | 1543 | 6.44 | Cancer site, sex, age, calendar period |
| Levi | 2008 | Switzerland | Registry-based (Vaud, Neuchatel) |
1974-2005 | 28031 | 1517 (prostate, breast, colorectal) |
not reported | Cancer site, sex, age, calendar period |
| Lindelof | 1991 | Sweden | Registry-based (Sweden) |
1971-1983 | 1973 | 236 | 6.5 | sex, cancer site |
| Maitra | 2005 | England | Registry-based (Thames) |
1961-2000 | 25731 | 3359 | not reported | Cancer site, age, sex |
| Milan | 2000 | Finland | Registry-based (Finland) |
1953-1995 | 71924 | 11042 | 8.69 | Cancer site, age ,sex, period |
| Nugent | 2005 | Manitoba | Registry-based (Manitoba) |
1956-2000 | 36789 | 5935 | 9.36 | Cancer site, age ,sex |
| Soerjomataram | 2008 | Netherlands | Registry-based (Eindhoven) |
1972-2002 | 23408 | 463 (breast and colorectal) |
5.65 | Cancer site, age, sex, stage, duration of follow up |
Classification of NMSC status is a key study quality criterion. Not all studies registered BCC, but all of the studies except one reported using pathologic confirmation of registered NMSCs (24). Even for the study that did not specify using pathologic confirmation, a report from the registry used in the study indicated that in the final year of the study’s data collection, greater than 96% of registered cases of SCC had pathologic confirmation, and completeness was estimated to be 98% (41). Greater than 90% completeness was reported for the studies in Switzerland (20-22), while the remaining studies based on national cancer registries were assumed by the authors to have completeness near 100%. These studies relied on mandated reporting and national health systems with universal coverage to provide high detection and confirmation rates. The two cohorts similarly had access to pathology reports for confirmation of lesions.
Among registry-based studies, compared to those with no NMSC diagnosis, those with a personal history of NMSC had a statistically significant 12% increased risk of developing subsequent malignancies other than NMSC (SRR 1.12, 95% CI 1.07 - 1.17, n=12, I2 = 92%), whereas among cohort studies with individual-level data, a much stronger association was observed (SRR 1.49, 95% CI 1.12 – 1.98, n=3, I2 = 92%)(Figure 1). As previously described, the following stratified analyses were conducted in the 12 registry-based studies only.
Figure 1.
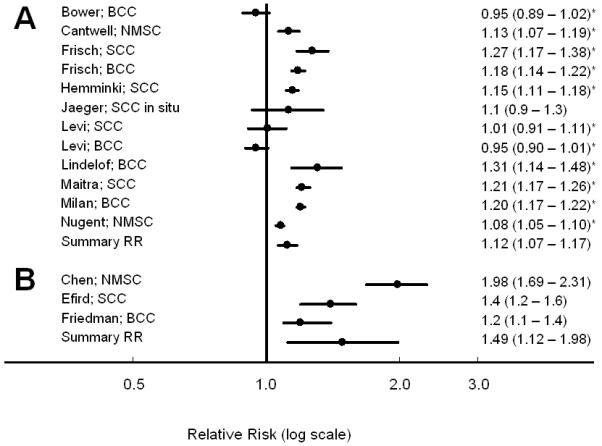
Risk of subsequent cancer following a diagnosis of nonmelanoma skin cancer, compared to those with no prior history of skin cancer among (A) registry-based studies, and (B) cohort studies. * Calculated from data presented to include all gender and subtype strata, as well as melanoma.
The association between NMSC and increased risk of subsequent cancer persisted in both males (RR 1.14, 95% CI 1.09 - 1.20; n= 12; I2 = 89%) and females (RR 1.10, 95% CI 1.04 - 1.15; n= 12; I2 = 79%). The significantly increased risk was present following both BCC (RR 1.09, 95% CI 1.01 - 1.17; n = 7; I2 = 95%) and SCC (RR 1.17, 95% CI 1.12 - 1.23; n = 7; I2 = 78%)(Table 2).
Table 2.
Risk of total cancer following NMSC, compared to those with no prior NMSC
| Non-Melanoma Skin Cancer | SRR (95% CI) * | I2 |
|---|---|---|
| Individual-level cohort studies (n=3) | 1.49 (1.12 – 1.98) | 92% |
| Registry-based studies (n=12) | 1.12 (1.07 - 1.17) | 93% |
| Males (n=12) | 1.14 (1.09 - 1.20) | 89% |
| Females (n=12) | 1.10 (1.04 - 1.15) | 79% |
| Basal Cell Carcinoma (n=7) | 1.09 (1.01 - 1.17) | 95% |
| Males (n=7) | 1.09 (1.00 - 1.18) † | 93% |
| Females (n=7) | 1.09 (1.02 - 1.16) | 82% |
| Squamous Cell Carcinoma (n=7) | 1.17 (1.12 - 1.23) | 78% |
| Males (n=7) | 1.20 (1.15 - 1.26) | 61% |
| Females (n=7) | 1.09 (1.00 - 1.19) | 75% |
Summary relative risk and 95% confidence interval
CI excludes 1.00
When we examined the association between NMSC and risk of specific types of cancer, 13 of 15 associations were in the direction of increased risk (Wilcoxon Signed-Rank p = 0.003) (Table 3). However, not all of these associations were statistically significant and the magnitude of the associations varied considerably across cancer sites (Figure 2). Cervical and pancreatic cancers were the only malignancies with a SRR<1.0. For breast, prostate, colorectal, bladder, and esophageal cancers, the SRRs were weak (<1.08) and not statistically significant. The strongest associations were observed for cancers of the salivary glands (SRR 4.57, 95% CI 2.92 - 7.15), melanoma (SRR 2.74, 95% CI 2.49 - 3.02), lip (SRR 2.38, 95% CI 2.03 - 2.79), mouth and pharynx (SRR 1.69, 95% CI 1.31 - 2.18), and for non-Hodgkin lymphoma (SRR 1.58, 95% CI 1.37 - 1.81).
Table 3.
Risk of site-specific cancers following BCC, SCC, NMSC, and males and females among registry-based studies
| Site of second primary cancer |
NMSC SRR (95% CI) * |
NMSC: Males SRR (95% CI) * |
NMSC: Females SRR (95% CI) * |
BCC SRR (95% CI) * |
SCC SRR (95% CI) * |
|---|---|---|---|---|---|
| Lung | 1.23 (1.13 – 1.33) | 1.27 (1.16 – 1.39) | 1.19 (1.06 – 1.33) | 1.13 (1.01 – 1.27) | 1.34 (1.22 – 1.47) |
| Breast | 1.04 (0.96 – 1.14) | -- † | 1.04 (0.96 – 1.14) | 1.08 (0.99 – 1.19) | 0.97 (0.88 – 1.08) |
| Colorectal | 1.05 (0.97 – 1.13) | 1.05 (0.95 – 1.16) | 1.03 (0.94 – 1.13) | 1.02 (0.93 – 1.13) | 1.03 (0.93 – 1.15) |
| Melanoma | 2.74 (2.49 – 3.02) | 2.73 (2.52 – 2.96) | 2.61 (2.24 – 3.05) | 2.75 (2.39 – 3.16) | 2.84 (2.45 – 3.29) |
| Pancreas | 0.94 (0.82 – 1.07) | 1.07 (0.97 – 1.18) | 0.91 (0.78 – 1.06) | -- ‡ | -- ‡ |
| Leukemia | 1.26 (1.04 – 1.52) | 1.45 (1.17 – 1.79) | 1.24 (0.99 – 1.55) | 1.12 (0.93 – 1.35) | 1.45 (0.93 – 2.11) |
| Cervix | 0.93 (0.62 – 1.41) | -- † | 0.93 (0.62 – 1.41) | 0.84 (0.50 – 1.41) | -- ‡ |
| Non-Hodgkin lymphoma | 1.58 (1.37 – 1.81) | 1.56 (1.33 – 1.84) | 1.58 (1.37 – 1.82) | 1.39 (1.22 – 1.58) | 2.00 (1.80 – 2.22) |
| Myeloma | 1.18 (1.06 – 1.32) | -- ‡ | -- ‡ | -- ‡ | -- ‡ |
| Salivary Gland | 4.57 (2.92 – 7.15) | 5.22 (3.32 – 8.22) | 3.65 (1.91 – 6.95) | -- ‡ | 7.15 (4.09 – 12.52) |
| Thyroid | 1.28 (0.95 – 1.71) | 1.72 (0.55 – 5.33) | 1.23 (0.99 – 1.53) | 1.24 (0.92 – 1.67) | -- ‡ |
| Prostate | 1.04 (0.98 – 1.12) | 1.04 (0.98 – 1.12) | -- † | 1.07 (0.97 – 1.17) | 1.00 (0.94 – 1.07) |
| Lip | 2.38 (2.03 – 2.79) | 2.09 (1.88 – 2.31) | 4.45 (2.76 – 7.19) | -- ‡ | 3.79 (3.15 – 4.57) |
| Mouth and pharynx | 1.69 (1.31 – 2.18) | 1.94 (1.50 – 2.49) | 1.87 (1.26 – 2.78) | 1.32 (1.13 – 1.55) | 2.05 (1.33 – 3.19) |
| Esophagus | 1.06 (0.85 – 1.32) | 1.15 (0.92 – 1.45) | 1.11 (0.90 – 1.36) | 0.90 (0.67 – 1.21) | 1.21 (0.83 – 1.74) |
Summary relative risk and 95% confidence interval
Not applicable
Relative risk estimate presented in fewer than 5 studies
Figure 2.
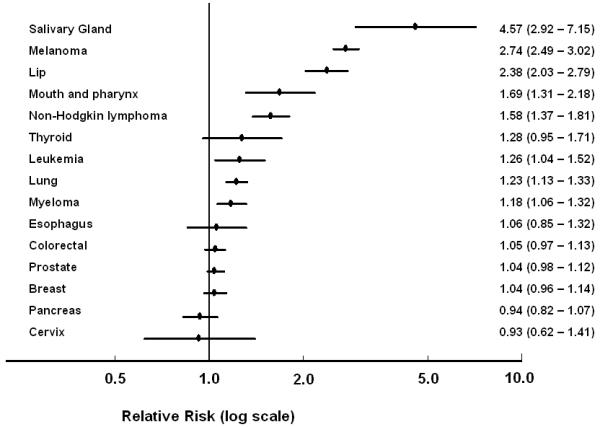
Relative risk and 95% CI of developing specific cancers following NMSC, compared to those with no prior NMSC.
The increase in total cancer risk remained upon inclusion of the study by Tuohimaa et al. and excluding the studies with overlapping data, although this result was no longer statistically significant (SRR 1.10, 95% CI 0.96 - 1.26, n=6) (28). The only two instances where inclusion of the study of Tuohimaa et al. yielded a different conclusion were with thyroid cancer following NMSC, and esophageal cancer after specifically SCC, both of which became statistically significant upon inclusion (thyroid: SRR 1.41, 95% CI 1.20 – 1.66 vs. SRR 1.28, 95% CI 0.95 – 1.71; esophagus: SRR 1.26, 95% CI 1.02 – 1.56, vs. SRR 1.06, 95% CI 0.85 – 1.32).
In the results described above, considerable heterogeneity was present. In meta-regression to investigate potential sources of heterogeneity among the registry-based studies, differences in latitude accounted for 55% of the variation, while histology (10%) and year of publication (2%) were much weaker individual contributors. The association between NMSC and risk of other cancers was stronger the further from the equator (p < 0.01).
DISCUSSION
Differing views have been expressed concerning whether a personal history of NMSC is associated with increased (6-29) or decreased (28, 30-32) risk of subsequent noncutaneous malignancy. Some have suggested that NMSC may be inversely associated with subsequent cancer risk based on the hypothesis that the sunlight exposure that causes NMSC also increases cutaneous synthesis of vitamin D, which in turn may protect against cancer. To help resolve this issue, we carried out a systematic review to objectively summarize the available evidence on this topic. The synthesis was based on a substantial body of evidence: 21 reports, mostly based on national cancer registry data, from a total of 13 different populations in 10 different countries. We did not find evidence of a significant protective association overall or for any individual cancer site in any of our analyses.
In fact, the data synthesis showed that compared to those with no prior NMSC, among registry-based studies those with a personal history of NMSC had a significant 12% increased risk of subsequent cancers other than NMSC. The association between NMSC and other cancers not only persisted, but was consistent with a much stronger 49% increased risk in cohort studies with individual-level data (n=3) that adjusted for potential confounders such as smoking status (Figure 1) (9, 11, 12). The registry-based studies were unable to control for individual-level confounders other than age and sex, which limits the strength of the inferences; however, the large study populations tended to offer more precise risk estimates, as well as risk estimates for rarer cancers not observed in the cohort studies with smaller sample sizes but more detailed data collection. There did not appear to be meaningful differences between the two types of study designs with respect to confirmation of NMSC, as all studies had access to pathology reports. Each study also linked to national or regional cancer registries for data on confirmed second primary cancers. The completeness of the ascertainment of NMSC may be relevant to the differing results by study design, but the information to assess this issue in greater detail is not readily available. The fact that cohort studies with the ability to adjust for individual-level risk factors have thus far observed stronger associations suggests that the registry-based studies may be biased toward the null, but additional cohort studies are needed to definitively determine if this is the case.
Despite consistent evidence of a positive association, two registry-based studies found a non-significant inverse association (7, 20). Both of these studies examined second cancers after BCC. The RR following SCC in these countries was greater than one, as was consistently seen in every instance where both estimates were presented. There were no obvious differences in study design or methodology that would explain the lack of perfect consistency in the direction of the associations, so they may have been due to chance.
The systematic review approach is of particular value for summing up the data across the subgroups where strata from single studies may be lacking in statistical precision. A contribution of this systematic review is clarifying that the increased cancer risk is equally relevant for both sexes and major histologic types of NMSC, and seems to be relevant to numerous anatomic cancer sites, as the association between a personal history of NMSC and risk of other malignancies was in the direction of increased risk for 13 of 15 cancer sites with sufficient data (Figure 2). This systematic review also highlights the difference in the risk estimates based on two major study designs.
For many reasons, the association between NMSC and risk of other cancers is likely to represent a true etiologic association. First, we limited the evidence synthesis to studies reporting follow-up after NMSC, unequivocally establishing that the NMSC diagnosis preceded the occurrence of other malignancies. Second, the large number of studies was remarkably consistent: almost all studies showed a significantly increased risk for all other cancers. Third, the association between NMSC and other cancers not only persisted, but actually increased in strength among studies adjusting for potential confounders such as smoking status (9, 11, 12). This raises the possibility that lack of control for confounding may be generating a bias toward the null in the registry-based studies. There are also several plausible biological mechanisms that could explain the association between NMSC and risk of other cancers, including immunosuppression, chronic inflammation, and variation in DNA repair efficiency, all of which act systemically and play a role in both cutaneous and internal carcinogenesis.
Non-melanoma skin cancer also has several unique features that make it particularly well-suited for study as a risk factor for other cancers. Most NMSCs are non-fatal, allowing for follow-up sufficiently long to detect second primary cancers. The majority of NMSCs will also be cured by surgical excision, eliminating the need for systemic chemotherapy, radiation, and their concomitant side effects. When studying the risk of second cancers, primary cancers that are more lethal run the risk of introducing a survival bias whereby individuals with aggressive cancers do not live long enough to develop second primary cancers, while the treatments themselves may also be carcinogenic. If there were a common risk factor for both cutaneous and internal malignancies, one would expect to see increased risk of non-melanoma skin cancer after other cancers as well. For reasons just mentioned, this association would be very difficult to isolate, though several unadjusted studies have indeed observed an increased risk of NMSC after other cancers, especially following cancers of hematopoietic origin (6, 10, 17, 42-45).
The data synthesis we report in this systematic review is subject to the limitations of the published studies. One possible explanation that has yet to be adequately addressed is surveillance bias, whereby individuals with a prior NMSC may be followed more closely than others, and as a result may have a greater likelihood of having subsequent cancers detected. If this were true, the association between NMSC and risk of other cancers would be expected to dissipate with long-term follow-up. Although there were no data specifically on frequency of surveillance, studies that have examined the length of follow-up have found that the risk of a subsequent primary cancer remains elevated as many as 15 years after NMSC (14, 15, 22, 25), suggesting surveillance bias may not explain the association between NMSC and subsequent cancers. On the other hand, other studies have observed the risk diminish over time, leaving this an open question (18-20, 30, 32). The only study we are aware of to directly account for this issue had information about having a health care provider, which did not differ according to NMSC status (27), but this was only a crude measure of potential surveillance.
Not all studies that have reported on information relevant to this topic were included in the formal quantitative synthesis of the evidence. These included cross-sectional studies (27), cohort studies comprised entirely of those with a personal history of all NMSC (19), and studies with mortality as their endpoint (46). Results from the Women’s Health Initiative, a cross-sectional study of more than 93,000 women aged 50-79, showed a significant association between self-reported NMSC and other self-reported cancers (OR 2.25, 95% CI 2.11 – 2.39) (27). In the American Cancer Society Cancer Prevention Study II (n = 1,184,569), compared to those with no prior NMSC, there was an increased risk of cancer mortality in individuals with previous NMSC (men: RR 1.30, 95% CI 1.23 – 1.36; women: RR 1.26, 95% CI 1.17 – 1.35) (46), even after adjustment for age, race, education, smoking, obesity, exercise, alcohol, vegetable and fat intake, diabetes, aspirin use, menopausal status, oral contraceptive use, parity, and estrogen replacement therapy. Not all excluded studies found a significant association, though. One study, which was excluded due to the point estimate being outside its reported confidence interval, found no overall association with cancers following NMSC, but a significantly increased risk after SCC (47). Another study was excluded because it collected cases from a single hospital and controls from the general population in Sweden, with known differences in BCC incidence between the two populations; this study found no increased risk following BCC (48). Other studies in Sweden consistently reported an increased risk following SCC (15-17), but there has not been another study for BCC in Sweden. It is thus reassuring that the results of the higher quality studies that were not included in the quantitative synthesis generally corroborate an association between NMSC and other cancers.
In contrast to our results, a previous meta-analysis on this topic concluded that solar UV exposure, as indicated by a personal history of NMSC, was associated with an overall decreased risk of cancer that was attributed to vitamin D (31). The previous result was obtained using most of the studies included in this systematic review, none of which showed a significantly decreased risk of second cancers. In addition to applying a less rigorous systematic review protocol, the author of this earlier meta-analysis attempted to control for cigarette smoking by using the RR for lung cancer as an internal reference value for each study against which all other cancer risks were compared. That is, RRs for total and site-specific cancers were divided by the stratum-specific RR for lung cancer. These differences in systematic review protocol clearly make a major difference in the results and interpretation of the data. That the three cohort studies with individual-level data that adjusted for smoking actually resulted in stronger associations than registry-based studies suggests it is important to rely on the observed data than attempt artificial corrections.
The results of the quantitative synthesis of the data were quite consistent, but substantial heterogeneity was present. We conducted a meta-regression to investigate contributing factors. Among registry-based studies, a great deal of variation was explained by differences in latitude. The majority of studies included were based on national cancer registries and thus were unable to control for confounders such as cigarette smoking. As mentioned, the few studies with individual-level data on potential confounders actually observed a substantially stronger association (1.49 versus 1.12). Additional cohort studies with the ability to adjust for individual-level risk factors are needed to determine if this stronger association persists (49, 50).
In summary, this systematic review revealed strong evidence that NMSC is associated with approximately 10% increased risk of subsequent primary cancer in registry-based studies and that this association may be substantially larger (50% increased risk) based on cohort studies with more detailed individual-level data. This association applies to both NMSC subtypes, and both men and women. The increased risk associated with NMSC appears to apply to a broad spectrum of malignancies.
ACKNOWLEDGEMENTS
The authors would like to thank Terri Lyn Herbert, MLIS, MS for her help in the development of the search strategy.
Supported by NIH/NCI grant R01CA105069 (Alberg) and Lee Wheless was supported by grant number T32RR023258 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Conflicts of interest: None
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2009. American Cancer Society; Atlanta: 2009. [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.de Vries E, van de Poll-Franse LV, Louwman WJ, de Gruijl FR, Coebergh JW. Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol. 2005;152:481–8. doi: 10.1111/j.1365-2133.2005.06386.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen JG, Fleischer AB, Jr, Smith ED, et al. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg. 2001;27:1035–8. doi: 10.1046/j.1524-4725.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 5.Marcil I, Stern R. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer. Arch Dermatol. 2000;136:1524–30. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 6.Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin’s lymphoma and skin cancer. BMJ. 1995;310:1491–5. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bower CPR, Lear J, Bygrave S, Etherington D, Harvey I, Archer CB. Basal cell carcinoma and risk of subsequent malignancies: a cancer registry-based study in southwest England. J Am Acad Dermatol. 2000;42:988–91. [PubMed] [Google Scholar]
- 8.Cantwell MM, Murray LJ, Catney D, et al. Second primary cancers in patients with skin cancer: a population-based study in Northern Ireland. Br J Cancer. 2009;100:174–7. doi: 10.1038/sj.bjc.6604842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Ruczinski I, Jorgensen TJ, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst. 2008;100:1215–22. doi: 10.1093/jnci/djn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crocetti E, Buiatti E, Falini P, the Italian Multiple Primary Cancer Working Group Multiple primary cancer incidence in Italy. Eur J Cancer. 2001;37:2449–56. doi: 10.1016/s0959-8049(01)00314-8. [DOI] [PubMed] [Google Scholar]
- 11.Efird JT, Friedman GD, Habel L, Tekawa IS, Nelson LM. Risk of subsequent cancer following invasive or in situ squamous cell skin cancer. Ann Epidemiol. 2002;12:469–75. doi: 10.1016/s1047-2797(01)00276-9. [DOI] [PubMed] [Google Scholar]
- 12.Friedman GD, Tekawa IS. Association of basal cell skin cancers with other cancers (United States) Cancer Causes Control. 2000;11:891–7. doi: 10.1023/a:1026591016153. [DOI] [PubMed] [Google Scholar]
- 13.Frisch M, Hjalgrim H, Olsen JH, Melbye M. Risk for subsequent cancer after diagnosis of basal-cell carcinoma. Ann Intern Med. 1996;125:815–21. doi: 10.7326/0003-4819-125-10-199611150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Frisch M, Melbye M. New primary cancers after squamous cell skin cancer. Am J Epidemiol. 1995;141:916–22. doi: 10.1093/oxfordjournals.aje.a117358. [DOI] [PubMed] [Google Scholar]
- 15.Hemminki K, Dong C. Subsequent cancers after in situ and invasive squamous cell carcinoma of the skin. Arch Dermatol. 2000;136:647–51. doi: 10.1001/archderm.136.5.647. [DOI] [PubMed] [Google Scholar]
- 16.Hemminki K, Jiang Y, Dong C. Second primary cancers after anogenital, skin, oral, esophageal, and rectal cancers: etiological links? Int J Cancer. 2001;93:294–8. doi: 10.1002/ijc.1319. [DOI] [PubMed] [Google Scholar]
- 17.Hemminki K, Jiang Y, Steineck G. Skin cancer and non-Hodgkin’s lymphoma as second malignancies: markers of impaired immune function? Eur J Cancer. 2003;39:223–9. doi: 10.1016/s0959-8049(02)00595-6. [DOI] [PubMed] [Google Scholar]
- 18.Jaeger AB, Gramkow A, Hjalgrim H, Melbye M, Frisch M. Bowen disease and risk of subsequent malignant neoplasms. Arch Dermatol. 1999;135:790–3. doi: 10.1001/archderm.135.7.790. [DOI] [PubMed] [Google Scholar]
- 19.Karagas MR, Greenberg ER, Mott LA, Baron JA, Ernster VL. Occurrence of other cancers among patients with prior basal cell and squamous cell skin cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:157–61. [PubMed] [Google Scholar]
- 20.Levi F, La Vecchia C, Te VC, Randimbison L, Erler G. Incidence of invasive cancers following basal cell skin cancer. Am J Epidemiol. 1998;147:722–726. doi: 10.1093/oxfordjournals.aje.a009516. [DOI] [PubMed] [Google Scholar]
- 21.Levi F, Randimbison L, La Vecchia C, Erler G, Te VC. Incidence of invasive cancers following squamous cell skin cancer. Am J Epidemiol. 1997;146:734–739. doi: 10.1093/oxfordjournals.aje.a009349. [DOI] [PubMed] [Google Scholar]
- 22.Levi F, Randimbison L, Te VC, Conconi MM, La Vecchia C. Risk of prostate, breast, and colorectal cancer after skin cancer diagnosis. Int J Cancer. 2008;123:2899–901. doi: 10.1002/ijc.23816. [DOI] [PubMed] [Google Scholar]
- 23.Lindelof B, Sigurgeirsson B, Wallberg P, Eklund G. Occurrence of other malignancies in 1973 patients with basal cell carcinoma. J Am Acad Dermatol. 1991;25:245–8. doi: 10.1016/0190-9622(91)70189-9. [DOI] [PubMed] [Google Scholar]
- 24.Maitra SK, Gallo H, Rowland-Payne C, Robinson D, Moller H. Second primary cancers in patients with squamous cell carcinoma of the skin. Br J Cancer. 2005;92:570–1. doi: 10.1038/sj.bjc.6602306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milán T, Pukkala E, Verkasalo PK, et al. Subsequent primary cancers after basal-cell carcinoma: a nationwide study in Finland from 1953 to 1995. Int J Cancer. 2000;87:283–8. [PubMed] [Google Scholar]
- 26.Nugent Z, Demers A, Wiseman MC, Mihalcioiu C, Kliewer EV. Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2584–90. doi: 10.1158/1055-9965.EPI-05-0379. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg CA, Greenland P, Khandekar J, Loar A, Ascensoa J, Lopez AM. Association of nonmelanoma skin cancer with second malignancy. Cancer. 2004;100:130–8. doi: 10.1002/cncr.11874. [DOI] [PubMed] [Google Scholar]
- 28.Tuohimaa P, Pukkala E, Scélo G, et al. Does solar exposure, as indicated by the nonmelanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer. 2007;43:1701–12. doi: 10.1016/j.ejca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Wassberg C, Thörn M, Yuen J, Ringborg U, Hakulinen T. Second primary cancers in patients with squamous cell carcinoma of the skin: a population-based study in Sweden. Int J Cancer. 1999;80:511–5. doi: 10.1002/(sici)1097-0215(19990209)80:4<511::aid-ijc5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.de Vries E, Soerjomataram I, Houterman S, Louwman MWJ, Coebergh JWW. Decreased risk of prostate cancer after skin cancer diagnosis: a protective role of ultraviolet radiation? Am J Epidemiol. 2007;165:966–72. doi: 10.1093/aje/kwk084. [DOI] [PubMed] [Google Scholar]
- 31.Grant WB. A meta-analysis of second cancers after a diagnosis of nonmelanoma skin cancer: additional evidence that solar ultraviolet-B irradiance reduces the risk of internal cancers. J Steroid Biochem Mol Biol. 2007;103:668–74. doi: 10.1016/j.jsbmb.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Soerjomataram I, Louwman W, Lemmens WEPP, Coebergh JWW, de Vries E. Are patients with skin cancer at lower risk of developing colorectal or breast cancer? Am J Epidemiol. 2008;167:1421–9. doi: 10.1093/aje/kwn077. [DOI] [PubMed] [Google Scholar]
- 33.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000 Apr 19;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 35.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JPT, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. International Agency for Research on Cancer; Lyon, France: 1987. pp. 65–71. [PubMed] [Google Scholar]
- 38.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Medical Research Methodology. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2007;7:40. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houwelingen HC, Arends LR, Stinjen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 41.Thames Cancer registry . Cancer in South East England 2000. King’s College; London: 2003. [Google Scholar]
- 42.Bjørge T, Hennig EM, Skare GB, Søreide O, Thoresen SO. Second primary cancers in patients with carcinoma in situ of the uterine cervix. The Norwegian experience 1970-1992. Int J Cancer. 1995 Jul 4;62:29–33. doi: 10.1002/ijc.2910620108. [DOI] [PubMed] [Google Scholar]
- 43.Chute CG, Chuang TY, Bergstralh EJ, Su WP. The subsequent risk of internal cancer with Bowen’s disease. A population-based study. JAMA. 1991 Aug 14;266:816–9. [PubMed] [Google Scholar]
- 44.Hartmann JT, Nichols CR, Droz JP, et al. The relative risk of second nongerminal malignancies in patients with extragonadal germ cell tumors. Cancer. 2000 Jun 1;88:2629–35. [PubMed] [Google Scholar]
- 45.Olsen JH, Garwicz S, Hertz H, et al. Nordic Society of Paediatric Haematology and Oncology Association of the Nordic Cancer Registries Second malignant neoplasms after cancer in childhood or adolescence. BMJ. 1993 Oct 23;307:1030–6. doi: 10.1136/bmj.307.6911.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahn H, Tatham L, Patel AV, Thun MJ, Heath CW. Increased cancer mortality following a history of nonmelanoma skin cancer. JAMA. 1998;280:910–2. doi: 10.1001/jama.280.10.910. [DOI] [PubMed] [Google Scholar]
- 47.Troyanova P, Danon S, Ivanova T. Nonmelanoma skin cancer and risk of subsequent malignancies: a cancer registry-based study in Bulgaria. Neoplasma. 2002;49:81–5. [PubMed] [Google Scholar]
- 48.Sandstrom A, Larsson LG, Damber L. Occurrence of other malignancies in patients treated for basal cell carcinoma of the skin. Acta Radiol Oncol. 1984;23:227–30. doi: 10.3109/02841868409136016. [DOI] [PubMed] [Google Scholar]
- 49.Frisch M. Re: Are patients with skin cancer at lower risk of developing colorectal or breast cancer? [letter] Am J Epidemiol. 2009;169:918–918. doi: 10.1093/aje/kwp046. [DOI] [PubMed] [Google Scholar]
- 50.Grimsrud TK, Andersen A. Protective effect from solar exposure, risk of an ecological fallacy. Eur J Cancer. 2008;44:16–18. doi: 10.1016/j.ejca.2007.10.019. [DOI] [PubMed] [Google Scholar]


