Abstract
Exposure of developing fish to polycyclic aromatic hydrocarbons (PAHs) and halogenated aromatic hydrocarbons (HAHs) results in a suite of defects including cardiac malformation, pericardial and yolk sac edema, craniofacial defects, and hemorrhaging. Several populations of Atlantic killifish or mummichog (Fundulus heteroclitus) on the Atlantic coast of the United States are resistant to the developmental and acute toxicity caused by PAHs and HAHs; this has made Fundulus a valuable model for studying aryl hydrocarbon sensitivity and adaptation. In order to further increase the utility of Fundulus, better understanding of the components of the molecular pathways governing aryl hydrocarbon response in Fundulus is required. The aryl hydrocarbon receptor (AHR) is known to mediate many of the toxic responses to PAHs and HAHs. A single AHR has been identified in mammals, but Fundulus has two AHRs and their relative roles are not clear. In the current study, translation-blocking and splice-junction morpholino gene knockdown was used to determine the roles of AHR1 and AHR2 in mediating cardiac teratogenesis induced by β-naphthoflavone (BNF), benzo[k]fluoranthene (BkF), and 3, 3′, 4, 4′, 5-pentachlorobiphenyl (PCB-126). Here we report that AHR2 and not AHR1 knockdown resulted in rescue of teratogenicity induced by BNF, BkF, and PCB-126. These data demonstrate that AHR2 is the primary mediator of cardiac teratogenesis caused by multiple aryl hydrocarbons in Fundulus and suggest that suppression of the AHR pathway through modulation of AHR2 is a plausible mechanism for PAH resistance in adapted fish. Additionally, this is the first reported use of splice-junction morpholinos in Fundulus.
Keywords: aryl hydrocarbon receptor, polycyclic aromatic hydrocarbon, PCB-126, morpholino, Fundulus heteroclitus, adaptation
1. Introduction
The Atlantic killifish or mummichog (Fundulus heteroclitus), found in estuaries along the Atlantic coast of North America from Newfoundland to Florida (Shute, 1980), has a variety of attributes that make it an important field and laboratory toxicology model (Burnett et al., 2007). Fundulus are tolerant of significant variation in environmental conditions, including salinity, temperature, oxygen, and pH (Dunson et al., 1993, Wood and Marshall, 1994, Smith and Able, 2003, Stierhoff et al., 2003, Nordlie, 2006). Because of their wide distribution and abundance, Fundulus are important components of estuarine ecosystems (Meredith and Lotrich, 1979, Kneib, 1986). However, despite wide distribution, individual Fundulus have relatively small home ranges (Lotrich, 1975) and are ideal for studying the impacts of local contamination and other stressors (Burnett et al., 2007). Fundulus have been used in studies of a wide variety of contaminants including toxicity of polycyclic aromatic hydrocarbons (PAHs) (e.g. Wassenberg and Di Giulio, 2004), polychlorinated biphenyls (PCBs) (e.g. Nacci et al., 1999, Jonsson et al., 2007), metals (e.g. Roling et al., 2006), and pesticides (e.g. Fortin et al., 2008); Fundulus have also been used to study processes such as endocrine disruption (e.g. Kelly and Di Giulio, 2000), environmental carcinogenesis (e.g. Vogelbein et al., 1990, Wills et al., accepted), and evolutionary adaptation (e.g. Schulte et al., 2000). Additionally, individual Fundulus populations have been identified that are resistant to a number of anthropogenic stressors, including mercury (Weis and Weis, 1984), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Prince and Cooper, 1995b), PCBs (Nacci et al., 1999, Meyer and Di Giulio, 2002), and PAHs (Meyer et al., 2002, Ownby et al., 2002).
Of the Fundulus populations adapted to environmental contaminants, most are resistant to the acute toxicity and teratogenic effects caused by PCBs and PAHS, which are known to interact with the aryl hydrocarbon receptor (AHR) pathway. PCBs and dioxin have long been known to cause a suite of deformities collectively known as “blue sac disease” (reviewed in Peterson et al., 1993) and some PAHs and PAH mixtures also induce similar cardiac deformities (Billiard et al., 1999, Wassenberg and Di Giulio, 2004). Furthermore, alteration of the AHR pathway appears to be an important component of adaptation to these contaminants. Notably, these populations exhibit a marked recalcitrance to induction of cytochrome P4501A (CYP1A) in response to exposure to a variety of AHR agonists (Prince and Cooper, 1995a, Van Veld and Westbrook, 1995, Elskus et al., 1999, Nacci et al., 1999, Bello et al., 2001, Arzuaga and Elskus, 2002, Meyer et al., 2002, Wills et al., 2010).
The AHR mediates responses to halogenated hydrocarbons and PAHs, such as CYP1A induction, by recognizing and binding these ligands and driving gene expression (Schmidt and Bradfield, 1996). Following activation by ligand binding, the AHR dimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT); this complex associates with specific DNA sequences known as xenobiotic response elements (XREs) or AHR response elements (AHREs) to induce transcription of a number of genes, including components of Phase I and II metabolism (i.e. CYP1A, 1B1, 1C1; some glutathione S-transferases (GSTs); NADP(H):oxidoreductase; UDP-glucuronosyltransferase (UGT) (Nebert et al., 2000). The AHR complex also induces transcription of a factor called the aryl hydrocarbon receptor repressor (AHRR), which down-regulates the pathway by competing with the AHR-ARNT complex for XREs (Karchner et al., 2002).
In order to further increase the utility of Fundulus for the study of aryl hydrocarbon toxicity and adaptation, better understanding of the components of the molecular pathways governing PAH response in Fundulus is crucial. The AHR pathway appears to be well-conserved among mammals and lower vertebrates (Hahn, 2002, Hahn et al., 2006) and a variety of genes in the AHR pathway have been characterized in Fundulus. Due to gene and genome duplication events, multiple AHRs have arisen and been identified in fish, including two in Fundulus, AHR1 and AHR2 (Karchner et al., 1999, Tanguay et al., 1999, Andreasen et al., 2002). ARNT and the AHRR have also been identified in Fundulus and other fish (Powell et al., 1999, Andreasen et al., 2002, Karchner et al., 2002, Evans et al., 2005). In zebrafish, AHR2 mediates teratogenesis induced by PAH mixtures (Billiard et al., 2006), 3,3′,4,4′,5-pentachlorobiphenyl (PCB-126) (Jonsson et al., 2007), and TCDD (Prasch et al., 2003, Teraoka et al., 2003). Currently, it is unclear whether AHR1, AHR2, or both perform this role in Fundulus. In vitro, both AHR1 and AHR2 have been demonstrated to bind TCDD and the model PAH β–naphthoflavone (BNF) and to activate a luciferase reporter upon TCDD binding (Karchner et al., 1999, Karchner et al., 2002).
In the current study, we used a morpholino gene knockdown approach to investigate the roles of AHR1 and AHR2 in mediating cardiac teratogenesis induced by PAHs and PCB-126 in Fundulus heteroclitus. We confirmed knockdown of AHR1 and AHR2 protein by Western blot analysis. Our findings lead us to conclude that AHR2 and not AHR1 is the primary mediator of this response in Fundulus. We also verified the lack of effect of AHR1 knockdown using a second morpholino directed at a splice junction, which allowed confirmation of knockdown via RT-PCR.
2. Materials and methods
2.1. Fish
Adult killifish were collected with minnow traps on King’s Creek, a relatively-uncontaminated tributary of the Severn River which feeds into the lower Chesapeake Bay in southeastern Virginia (37° 17′52.4″N, 76° 25′31.4″W). In the lab, fish were maintained at 23-25 °C, in 20‰ artificial sea water (ASW; Instant Ocean, Foster & Smith, Rhinelander, WI, USA), with a 14:10 light:dark cycle, and were fed pelleted fish feed (Aquamax ® Fingerling Starter 300, PMI Nutritional International, LLC, Brentwood, MO, USA). Eggs were collected by manual spawning of females and fertilized in vitro by expressing sperm from males directly into a beaker containing eggs in ASW. Eggs were set aside for approximately one hour for fertilization, then washed briefly with 0.3% hydrogen peroxide in ASW. Eggs were then rolled on durable wet paper towels to remove mucous and debris from the chorion (Matson et al., 2008). Adult care and reproductive techniques were non-invasive and approved by the Duke University Institutional Animal Care & Use Committee (A234-07-08).
2.2. Morpholino and microinjection
Morpholino antisense oligos were designed and manufactured by Gene Tools (Philomath, OR, USA). Translation blocking morpholinos were designed against F. heteroclitus AHR1 (GenBank, AF024591) and AHR2 (GenBank, U29679) (Karchner et al., 1999); the AHR1 MO sequence was 5′-TTCTCCTCTTGCGTCCAGCATACAT-3′ and the AHR2 MO sequence was 5′-GGTTCACAGACATCTTGCCGCTCGG-3′. Additionally, a splice-junction morpholino was used to further demonstrate the efficient knockdown of AHR1. The morpholino targeted the exon 8-intron 8 boundary (S. Karchner, personal communication) and began at nucleotide 1004 (counting started with ATG) and continued into the intron (AHR1-spl MO, 5′-GACCTCCATTTAATATGCACTTACT-3′). Splice-junction morpholinos cause aberrant splicing of pre-mRNA; unlike translation blocking morpholinos, knockdown via splice-junction morpholinos can be quantified using PCR. This morpholino targeted the ligand binding domain of AHR1, which should result in a non-functional receptor. Finally, Gene Tools’ standard control morpholino (control-MO, 5′-CCTCTTACCTCAGTTACAATTTATA-3′) was used as a control for effects of morpholino injection. All morpholinos were tagged with fluorescein to visualize incorporation. Individual morpholinos were prepared as 250 μM injection stocks in RNase-free water and maintained at 4 °C. When injected in combination, the concentration of each individual morpholino was halved so that the total concentration of morpholino was maintained at 250 μM. Injection stocks were briefly vortexed and centrifuged for two minutes prior to use. Injection volume was approximately 4 nL, yielding morpholino doses of 1 pmol/embryo (250 μM) or 0.5 pmol/embryo (125 μM).
Microinjection was performed as described previously (Matson et al., 2008) using a system consisting of a Nikon SMZ-1500 zoom stereomicroscope (Nikon Instruments, Inc, Lewisville, TX, USA), MDI PM 1000 Cell Microinjector (MicroData Instrument, Inc., S. Plainfield, NJ, USA), course manipulator (Narishige International USA, Inc., New York, NY, USA), and a three-axis joysick oil hydraulic micromanipulator (Narishige). Borosilicate glass tubing (1.0 mm o.d., 0.5 mm i.d.) was pulled into microinjection needles on a PC-10 Puller (Narishige) with heater output set at 53. All injections were into a cell during the 1-4 cell stages. Embryos were maintained overnight in an incubator at 27 °C; confirmation of morpholino incorporation and proper early development was performed at 22-24 hours post fertilization (hpf) using a Leica MZ FLIII fluorescence zoom stereomicroscope (Leica Microsystems Inc., Bannockburn, IL, USA) with GFP/FITC filter. Only normally developing embryos with strong, uniform morpholino incorporation were used.
2.3. Chemicals and dosing
β-naphthoflavone (BNF) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Benzo[k]fluoranthene (BkF) and benzo[a]pyrene (BaP) were purchased from Sigma-Aldrich (St. Louis, MO, USA) or from Absolute Standards, Inc. (Hamden, CT, USA) and 3,3′,4,4′,5-pentachlorobiphenyl (PCB-126) was purchased from AccuStandard (New Haven, CT, USA). Stocks were prepared by dissolving BNF, BkF, BaP or PCB-126 in DMSO. All chemicals used in buffers for Western blotting or for gel electrophoresis were purchased from Sigma-Aldrich.
Following manual spawning and microinjection as described previously, morpholino-injected and non-injected embryos were dosed individually in 20-mL glass scintillation vials (VWR, West Chester, PA) beginning at 24 hpf. Exposures were conducted in 10 mL of dosing solution with final concentrations of 10 μg/L BNF, 300 μg/L BkF, 1 μg/L PCB-126, and 100 μg/L BaP. All dosing solutions were made in 20 ppt ASW. Control embryos were exposed to DMSO at a concentration (v/v) equal to that in the dosed group; DMSO concentrations were held at <0.03% across all treatments. Embryos were maintained in dosing solution in the incubator at 27 °C from 24 hpf until the appropriate time for the given analysis. All exposures consisted of at least three experimental replicates with n≥10 embryos per treatment group unless otherwise noted.
2.3.1. Dosing for deformities
To determine the role of each AHR in the cardiac deformities caused by several AHR agonists, morpholino-injected (control MO, AHR1 MO, AHR2 MO, or combination of AHR1&2 MO) and non-injected embryos were exposed as described previously with deformity assessment occurring at 144 hpf. In a second experiment, non-injected embryos and embryos injected with an AHR1 splice morpholino (AHR1-spl MO) or AHR2 MO were dosed only with DMSO, 10 μg/L BNF, or 300 μg/L BkF and assessed in the same manner.
2.3.2. Dosing for Western blot analysis
Morpholino-injected (AHR1 MO, AHR2 MO, and combination of AHR1&2 MO) and non-injected embryos were exposed to 10 μg/L BNF at 24 hpf and flash frozen in groups of ten at 144 hpf. Because exposure at 24 hpf may have resulted in induction of AHR protein expression and subsequent degradation prior to protein analysis at 144 hpf (see review by Pollenz, 2002), a second experiment was conducted with dosing beginning later; morpholino-injected (AHR1 MO, AHR2 MO, and control MO) and non-injected embryos were exposed to 100 μg/L BaP at 96 hpf and flash frozen in groups of ten at 144 hpf. Embryos were stored at −80 °C until analysis.
2.3.3. Dosing for reverse transcription PCR
To further demonstrate efficient knockdown of AHR1 and confirm the results observed with the translation-blocking morpholino, AHR1-spl MO-injected, AHR2 MO-injected, and non-injected embryos (n≥12 embryos per treatment group) were dosed individually with 300 μg/L BkF at 24 hpf. At 48 hpf, embryos were paired, placed in 50 μL RNAlater (Qiagen, Valencia, CA, USA), and flash frozen for reverse transcription PCR (RT-PCR) analysis. Embryos were stored at −80 °C until analysis.
2.4. Deformity assessment
Following morpholino injection and dosing, cardiac teratogenesis was scored blind at 144 hpf under light microscopy. Deformity severity was scored as a 0 (normal), 1 (moderate deformity), or 2 (severe deformity) as described previously (Matson et al., 2008). Observed heart abnormalities consisted primarily of heart elongation, improper atrial-ventricular alignment, and pericardial edema. Embryos scored as a 1 on the deformity scale have a range of heart abnormalities from moderate changes in atrial-ventricular alignment to misalignment of the chambers accompanied by elongation of the atrium and sinus venosus (the inflow vessel of the heart). All embryos scored as a 1 maintained some blood flow as observed by light microscopy. Embryos scored as a 2 on the deformity scale exhibited severe misalignment and elongation of the atrium and ventricle or complete alteration of the heart to a single, elongated tube. Many of the individuals scored as a 2 did not have any apparent blood flow, but some exhibited severely reduced blood flow. Representative images (144 hpf) of each deformity class are shown in Figure 1.
Figure 1.
Representative images of embryos at 144 hpf exhibiting each score on the deformity scale. A score of 0 corresponds to a normal heart, 1 to a moderately deformed heart, and 2 to a severely deformed heart. Clearly visible in the normal heart are the ventricle (V), atrium (A), and sinus venosus (Sv). Pericardial edema (Pe, margin marked by dashed line) surrounds elongated hearts in embryos scored as 1 and 2.
2.5. Confirmation of morpholino efficacy by Western blot analysis
After thawing on ice, samples were homogenized in homogenization buffer (300 mM sucrose, 50 mM KCL, 50 mM NaCl, 8 mM EGTA, 30 mM HEPES, pH 7.5) with 1 mM phenylmethanesulfonyl fluoride, 1 μg/mL leupeptin, and 1 μg/mL aproponin with a hand-held tissue homogenizer. The homogenate was centrifuged at 500 g for 15 min at 4 °C to remove the chorion and non-homogenized tissues. Supernatant was moved to a new tube and spun at 18,000 g for 15 min at 4 °C to remove the nuclei and mitochondria. The supernatant was aliquoted and stored at − 80 °C till further analyses. SDS-Page and Western blot analysis were performed as described by Jung and Di Giulio (2010) for CYP1A, with the following differences for AHR analysis. AhR1 (1:50 dilution) and AhR2 (1:500 dilution) polyclonal antibodies raised against the killifish (Merson et al., 2006) were used as primary antibody, and goat anti-rabbit IgG horseradish peroxidase antibody (1:10,000 dilution, Jackson laboratory, Bar Harbor, ME, USA) was used as secondary antibody. Blots were visualized on X-ray film by enhanced chemiluminescence (SuperSignal® West Pico Chemiluminescence Substrate, Thermo Scientific, Rockford, IL, USA).
2.6. Confirmation of AHR1 splice-MO efficacy by reverse transcription PCR analysis
Paired embryos were thawed on ice, homogenized with RNA-Bee for 30 seconds, and mRNA was extracted by modified phenol-chloroform extraction according to the RNA-Bee protocol (Tel-Test, Inc., Friendswood, Texas, USA). RNA quantity and quality was analyzed using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). Using the Omniscript cDNA synthesis kit for Reverse Transcription (Qiagen), cDNA was prepared according to manufacturer’s instructions using 500 ng of RNA, random hexamers, and RNAse inhibitor, and carried out in a Biometra T1 thermocycler (Göttingen, Germany) for 1 h at 37°C. RT-PCR was performed in a 25 μL reaction containing 62.5 ng template, 12.5 μL AmpliTaq Gold PCR master mix (Applied Biosystems, Foster City, CA, USA), 9 μL distilled H2O, and 0.5 μL each of 10 μM primer. Thermocycler conditions were 5 min at 95°C, 35 cycles of 15 s at 95°C, 15 s at 58°C, and 4 min at 72°C, followed by 4 min at 72°C. Two fragments were amplified from each sample. To amplify the region targeted by the splice morpholino, the forward primer was located in exon 7 (5′-AACATCCAGGGCAGGCTCAAGTTT-3′) and the reverse in exon 9 (5′-CATTTCCAGCGATTCTCTTTAGTGAG-3′). A reference fragment from a region untargeted by the morpholino was amplified for comparison with the forward primer located in exon 2 (5′-ACCAAGTCCAATCCGTCCAAACGA-3′) and the reverse in exon 3 (5′-GCTGCTCCATTCCCACTGTTCA-3′). All reactions were performed in triplicate. Gel electrophoresis of 10 μL of PCR product was performed in 2% agarose gels stained with ethidium bromide. Gel images were captured with a Bio-Rad Molecular Imager Gel Doc (Bio-Rad, Hercules, CA, USA).
2.7. Statistical analysis
All analyses were performed using JMP 8.0 (SAS Institute Inc, Cary, NC, USA). All data were analyzed by non-parametric analysis of variance (ANOVA) controlling for morpholino and dose, followed by least square means (LSMeans) procedures. As stated previously, experiments were replicated a minimum of three times; no differences between experimental replicates were observed for any test. Tukey-adjusted pairwise comparisons were conducted to determine differences between groups. Statistical significance was accepted at p≤0.05 for all tests.
3. Results
3.1. Deformity assessment
Targeted morpholino knockdown of AHR1, AHR2, or the combination was used to determine the role of each of the Fundulus AHRs in mediating the cardiac deformities induced by PAHs and PCB-126. Morpholino-injected and non-injected embryos were exposed to BNF, BkF, and PCB-126 as described previously and the effect of gene knockdown on response was assessed.
We have previously demonstrated that 10 μg/L of the model PAH BNF induces cardiac deformities of mild to intermediate severity in naïve Fundulus (Matson et al., 2008) and this was confirmed in this study. BNF-exposed embryos generally exhibited a heart phenotype of misalignment of the atrium and ventricle; this was accompanied by some overall lengthening of the heart, reduction of the apparent size of the chambers, and pericardial edema (see representative images Fig 1). The mean deformity scores of BNF-exposed embryos are shown in Figure 2A. As expected, non-injected embryos exposed to BNF displayed a mean deformity score of 0.59±0.04. Embryos injected with AHR1 MO and dosed with BNF had a mean deformity score (0.71±0.06) that was not statistically different from that of non-injected, BNF-dosed embryos (p=0.0962). In contrast, AHR2 knockdown resulted in a mean deformity score (0.15±0.04) in BNF-dosed embryos that was approximately 21% of that for non-injected, BNF-dosed embryos. Additionally, the deformity score for AHR2 MO-injected, BNF-dosed individuals was not statistically different from that of the DMSO-dosed non-injected control group (p=0.1089). Embryos with both AHR1 and AHR2 knocked down had a mean deformity score of 0.37±0.10, which was intermediate between the non-injected BNF treatment and the AHR2 knockdown BNF treatment, but was not statistically different from the control morpholino BNF-dosed group (p=0.2155). DMSO-dosed morpholino treatments were not statistically different from the non-injected DMSO treatment (0.06±0.03); these groups are not shown in the figure for simplicity. Additionally, the severity of cardiac deformities in BNF-dosed individuals injected with control MO was no different from non-injected, BNF-dosed embryos (p=0.4620).
Figure 2.
Effect of 250 μM AHR1 MO, 250 μM AHR2 MO, a combination of 125 μM AHR1 MO and 125 μM AHR2 MO, or 250 μM control MO on mean deformity score (± SEM) of Fundulus embryos exposed to DMSO or 10 μg/L BNF (A), 300 μg/L BkF (B), or 1 μg/L PCB-126 (C) compared to non-injected (NI) controls. Embryos were exposed at 24 hpf and scored at 144 hpf. None of the MO-injected, DMSO control groups differed from the non-injected, DMSO controls in any experiment; for figure clarity only the non-injected, DMSO controls are shown. Bars not marked by the same letter are statistically different at p≤0.05 (ANOVA, Tukey adjusted LSMeans).
To determine the effect of AHR knockdown on response to a PAH found in the environment, embryos were exposed to BkF at 300 μg/L. At this concentration, BkF induces a much more severe deformity phenotype in naïve embryos than does 10 μg/L BNF. Typically, there was significant misalignment of the atrium and ventricle, accompanied by lengthening of the heart and pericardial edema; individuals ranged in severity from a 1 to 2 on the deformity scale (see Figure 1). The results of BkF exposure are shown in Figure 2B. Non-injected embryos exposed to BkF had a mean deformity score of 1.23±0.08. Control MO-injected embryos exposed to BkF had a slightly lower mean deformity score (1.08±0.14), but were not statistically different from non-injected individuals (p=0.9964). AHR1 MO-injected embryos exhibited a mean deformity score (0.86±0.09) that was approximately 70% of that of the non-injected BkF dosed control group. However, the mean deformity score of AHR1 MO-injected embryos was not statistically different from that of the non-injected or control MO-injected individuals dosed with BkF (p=0.0782 or p=0.9481, respectively). In contrast, AHR2 knockdown resulted in a BkF-induced mean deformity score of 0.33±0.07, which was significantly lower than that of both the non-injected and control MO-injected Bkf-dosed groups (p<0.0001 and p=0.0002, respectively). The BkF-induced deformity score for AHR2 MO-injected embryos was approximately 27% of the non-injected group and 31% of the control MO-injected group. BkF-dosed embryos with both AHR1 and AHR2 knocked down had a mean deformity score of 0.68±0.09, which was statistically lower than the deformity score for non-injected embryos dosed with BkF (p=0.0002), but not those with injected with control MO (p=0.3076). Additionally, this reduction was not as great as that for the individuals with only AHR2 knocked down. Again, DMSO-dosed embryos injected with any morpholino were not statistically different (p>0.05) from the non-injected DMSO treatment (0.07±0.05); these groups are not shown in Figure 2B for simplicity. Additionally, injection of control MO did not affect the severity of cardiac deformities compared to non-injected embryos in BkF-dosed treatments (p=0.9964).
To investigate the role of the Fundulus AHRs in mediating teratogenesis caused by a halogenated aromatic hydrocarbon, embryos were exposed to 1 μg/L PCB-126. At this concentration, greater than 70% of dosed, non-injected embryos are scored as a 2 on the deformity scale. Typically, the hearts are greatly elongated tubes with no recognizable chambers. This is accompanied by little to no observable blood flow and a large volume of pericardial edema (see Figure 1). The results of PCB-126 exposure are shown in Figure 2C. Both non-injected and AHR1 knockdown embryos dosed with PCB-126 exhibited severe deformities (1.80±0.12 and 1.76±0.11, respectively), which did not differ statistically (p=0.9999). AHR2 knockdown resulted in a statistically reduced (p<0.0001) mean deformity score of 0.96±0.11 after PCB-126 exposure, which was about 53% of that for the non-injected individuals. Knockdown of both AHR1 and AHR2 resulted in a mean deformity score of 1.00±0.16 in PCB-126 dosed embryos, which was a statistically significant reduction compared to the non-injected, PCB-126-dosed group (p=0.0027). However, knockdown of AHR1 and AHR2 was not different from AHR2 knockdown alone in protecting from PCB-126 exposure (p=0.9999).
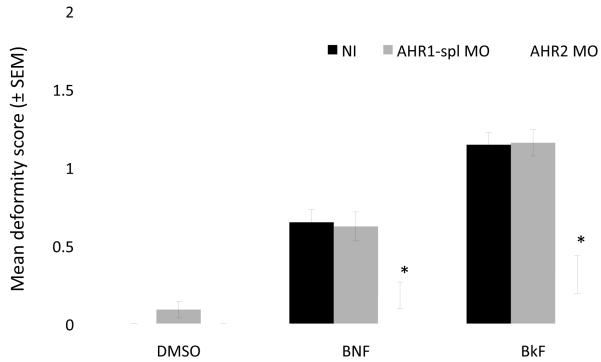
Because AHR1 knockdown did not appear to result in deformity rescue and because Western blots of AHR1 protein knockdown were somewhat inconclusive, a second morpholino targeting AHR1 was utilized to confirm that AHR1 knockdown was achieved. The use of a splice-junction morpholino allows confirmation of knockdown by PCR. The results of this experiment, shown in Figure 3, were similar to the BNF and BkF exposures with translation-blocking morpholino described previously. Non-injected and AHR1-spl knockdown embryos dosed with BNF had mean deformity scores that were not statistically different (p=0.9999; 0.64±0.08 and 0.62±0.09, respectively). AHR2 knockdown again resulted in a statistically significant protection from BNF compared to non-injected embryos (p=0.0100; mean deformity score 0.18±0.10). When dosed with BkF, non-injected and AHR1-spl knockdown embryos exhibited deformity scores that were not statistically different (p=0.9999; 1.14±0.07 and 1.16±0.06, respectively), whereas AHR2 knockdown was again protective from BkF (p<0.0001; mean deformity score 0.31±0.12).
Figure 3.
Effect of 250 μM AHR1-spl MO and 250 μM AHR2 MO on mean deformity score (± SEM) of Fundulus embryos exposed to DMSO, 10 μg/L BNF, or 300 μg/L BkF compared to non-injected (NI) controls. Embryos were exposed at 24 hpf and scored at 144 hpf. Bars marked by * are statistically different from the respective non-injected dosed group p≤0.05 (ANOVA, Tukey adjusted LSMeans).
3.2. Confirmation of morpholino efficacy by Western blot analysis
To confirm morpholino knockdown, AHR1 and AHR2 protein was measured in two exposure experiments. In the first, morpholino-injected and non-injected embryos were dosed individually with 10 μg/L BNF or DMSO at 24 hpf and flash frozen in pools of ten at 144 hpf. In the second, embryos were dosed individually with 100 μg/L BaP at 96 hpf and flash frozen in pools of ten at 144 hpf.
As shown in the representative gel image (Figure 4) for BNF exposure, the greatest expression of AHR1 and AHR2 protein was measured in DMSO control treatments and appeared to decrease in BNF treatments. Compared to the non-injected DMSO-dosed group, AHR1 MO injection resulted in a reduction of AHR1 protein; this reduction was less apparent for comparison of the BNF-dosed groups due to the lower overall expression of AHR protein. Overall, visualization of changes in AHR1 protein was not as good as visualization of AHR2. In BNF-dosed treatment groups, there was a reduction of AHR2 protein in the AHR2 MO-injected group and the group injected with both AHR1 and AHR2 morpholinos. Additionally, induction of CYP1A protein expression by BNF was observed in the non-injected and AHR1 MO-injected groups, but was prevented in the groups injected with AHR2 MO or both AHR1 MO and AHR2 MO.
Figure 4.
Effect of 250 μM AHR1 MO, 250 μM AHR2 MO, and a combination of 125 μM AHR1 MO and 125 μM AHR2 MO on expression of AHR1, AHR2, and CYP1A protein in Fundulus embryos exposed individually to DMSO or 10 μg/L BNF at 24 hpf. Embryos were pooled in groups of ten for Western blot analysis at 144 hpf.
Because of the reduced AHR protein levels in individuals dosed with BNF at 24 hpf and analyzed at 144 hpf, a second experiment was conducted with BaP wherein embryos were dosed later (96 hpf) and flash frozen only 48 hours later (144 hpf). As shown in the representative gel image (Figure 5), this resulted in similar levels of AHR protein in both DMSO control and BaP-dosed treatment groups. Compared to the other treatments, AHR1 protein was reduced in the two AHR1 MO groups. Additionally, AHR2 protein was greatly reduced in the two AHR2 MO groups compared to the other treatments. However, visualization of AHR1 protein knockdown was again not as clear as for AHR2 protein.
Figure 5.
Effect of 250 μM AHR1 MO, 250 μM AHR2 MO, or 250 μM control MO on expression of AHR1 and AHR2 protein in Fundulus embryos exposed individually to DMSO or 300 μg/L BkF at 96 hpf. Embryos were pooled in groups of ten for Western blot analysis at 144 hpf.
3.3. Confirmation of AHR1 splice-MO efficacy by reverse transcription PCR analysis
To further confirm AHR1 knockdown, the effect of an AHR1 splice morpholino was measured with RT-PCR of AHR1 cDNA (Figure 6). The image is representative of a minimum of five pairs of embryos per group. Knockdown was demonstrated by comparing amplification of a cDNA fragment in the region targeted by the morpholino (exon 7 to exon 9) to amplification of a fragment in exons 2 and 3 that is unaffected by the splice morpholino. In both DMSO and BkF dosed groups, non-injected embryos exhibited strong amplification of a band in both regions of the gene. In contrast, AHR1-spl MO injection resulted in a loss of the band in the morpholino targeted region, but no loss of the band in exon 2.
Figure 6.
Loss of mRNA fragment targeted by AHR1-spl MO in embryos exposed to DMSO or 300 μg/L BkF at 24 hpf. Embryos were sacrificed at 48 hpf and paired for mRNA extraction and analysis by RT-PCR. AHR1 knockdown is demonstrated by comparing amplification of a cDNA fragment in the region targeted by the splice morpholino (exon 7 to exon 9, 374 nucleotide expected band size) to amplification of a fragment in exons 2 and 3 (191 nucleotide expected band size) that is unaffected by the morpholino. Representative image shows both RT-PCR amplifications for 2 of ≥6 pairs of embryos per treatment group. Ld = 1 kb DNA ladder.
4. Discussion
Fundulus are an important and highly-studied model in environmental toxicology, in part due to identification of multiple populations adapted to contamination from the AHR agonists PAHs, PCBs, and dioxin. Because populations adapted to these contaminants demonstrate a recalcitrant AHR pathway, it is logical to suspect that alteration of the AHR pathway plays a crucial role in circumventing toxicity. However, it was previously unclear what role AHR1 and AHR2 played in mediating the toxic effects of these contaminants in Fundulus. Karchner et al. (1999) identified two AHRs in Fundulus; both AHR1 and AHR2 were able to bind TCDD with high affinity fashion and were able to bind DNA in the presence of ligand. Subsequently, they showed that both AHR1 and AHR2 also bound BNF (Karchner et al., 2002). They further demonstrated that both AHR1 and AHR2 could mediate TCDD-induced expression of luciferase in vitro. Collectively, these results indicated that both AHR1 and AHR2 had the potential to mediate effects of AHR agonists in Fundulus. It has been suggested that the presence of two AHRs in fish could contribute to their high degree of sensitivity to AHR agonists (Karchner et al., 1999). However, results from the current study indicate that AHR2 is the primary mediator of cardiac teratogenesis due to a variety of AHR agonists.
4.1. The role of AHR1 and AHR2 in cardiac teratogenesis
In these experiments, only AHR2 knockdown was protective from the effects of BNF and PCB-126. Furthermore, the combination of AHR1 and AHR2 knockdown was no different than AHR2 knockdown alone in protection from PCB-126. AHR2 knockdown was also protective from teratogenesis caused by BkF, but the results of the experiments using translation-blocking morpholinos yielded some ambiguity regarding the effect of AHR1 knockdown. The mean deformity score in AHR1 MO injected individuals was statistically lower than that of the non-injected individuals dosed with BkF. However, it was not lower than the control MO-injected individuals dosed with BkF. This may indicate a role for both AHR1 and AHR2 in mediating BkF toxicity, but injection with a combination of both morpholinos did not improve protection when compared to knockdown of AHR2 alone. An intermediate level of protection in embryos with concurrent AHR1 and AHR2 knockdown also occurred in BNF-dosed embryos. It is likely that the lower dose of morpholino used for the combination knockdowns is responsible for the reduction in rescue compared to embryos with AHR2 knockdown alone. It is also possible that the lower dose of morpholino masked a combined protective effect of AHR1 and AHR2 knockdown. However, this seems unlikely considering the results of the second set of experiments utilizing a splice junction morpholino. Individuals injected with AHR1-spl MO and dosed with BNF or BkF exhibited mean deformity scores no different from non-injected controls, while the AHR2 morpholino was again protective from BNF and BkF. In view of the weight of evidence from these experiments, it seems likely that the difference in mean deformity scores between AHR1 MO-injected individuals and non-injected individuals dosed with BkF in the first experiment is artifactual.
Previous studies provide support that AHR2 is the primary mediator of the effects of AHR agonists in Fundulus. While Karchner et al. (2002) showed that both receptors bound agonists, they also demonstrated that AHR2 was somewhat better able to drive luciferase expression in vitro. In adult killifish, AHR2 transcripts were similarly abundant in multiple tissues, whereas AHR1 transcripts were found primarily in brain, heart, ovary, and testis; the authors hypothesized that this suggests a more specialized role for AHR1 in certain tissues (Karchner et al., 1999, Powell et al., 2000). It is possible that there is a tissue-specific role for AHR1 in mediating response to AHR agonists that could not be addressed due to the approach of the current study. It is also possible that AHR1 and AHR2 mediate toxicity in a compound-specific fashion. In the current study we utilized a variety of AHR agonists (BNF, BaP, BkF, and PCB-126) and found no evidence to support this conclusion. However, there is great diversity of structure and activity among potential AHR agonists and it is possible that compounds not investigated here act through AHR1 or both AHR2 and AHR1.
Our results are significantly informed by comparison of Fundulus AHRs to those of zebrafish (Danio rerio), which have been used extensively to investigate the mechanisms of induction of cardiac toxicity by PAHs, PCB-126, and TCDD. Zebrafish AHR2 is an ortholog of Fundulus AHR2; unlike Fundulus, they also have two identified AHR1s. In zebrafish, morpholino knockdown of AHR2 was protective from cardiac toxicity caused by TCDD (Prasch et al., 2003, Teraoka et al., 2003), PCB-126 (Jonsson et al., 2007), the PAHs pyrene and benz[a]anthracene (Incardona et al., 2005, Incardona et al., 2006), and PAH mixtures (Billiard et al., 2006). In zebrafish exposed to high doses of the tricyclic PAHs dibenzothiophene and phenanthrene, cardiac toxicity was not altered by either AHR1a or AHR2 knockdown (Incardona et al., 2005). Subsequently, that research group showed that embryos with AHR1a knockdown also exhibited pyrene resistance, but that it did not affect vascular CYP1A induction (Incardona et al., 2006). Currently, the role of AHR1b in cardiac toxicity in zebrafish has not been demonstrated, although in vitro it binds TCDD with high affinity and activates a luciferase reporter (Karchner et al., 2005). Collectively, findings in zebrafish further support the conclusion that Fundulus AHR2 is the primary mediator of similar effects. Phylogenetically, Fundulus AHR2 is most closely related to zebrafish AHR2 and Fundulus AHR1 is most closely related to zebrafish AHR1b; zebrafish AHR1a is isolated from the other identified zebrafish and Fundulus AHRs (Karchner et al., 2005, Hahn et al., 2006).
4.2. Consequence of AHR1 and AHR2 knockdown for normal development
Beyond its adaptive functions, the AHR serves poorly understood physiological roles. AHR homologs have been identified in invertebrates, but they lack the ability to bind TCDD and other aryl hydrocarbons, indicating that they serve a purpose other than xenobiotic response (Hahn, 2002, Hahn et al., 2006). Studies in AHR null mice and mice with constitutively active AHR have show that the mouse AHR plays a role in normal development and function of the liver, heart, immune system, vasculature, and reproductive system (reviewed in Nguyen and Bradfield, 2008). In the current study, no developmental effects of knockdown of either AHR1 or AHR2 were observed. Each embryo was observed carefully, but assessment was focused on cardiac teratogenesis. It is possible that the effects of AHR knockdown were not apparent in this gross assessment. However, it is important to note that morpholinos result in gene knockdown and not gene knockout. It is therefore possible that sufficient AHR remained to carry out its physiological role(s) in morpholino-injected embryos.
4.3. Assessment of morpholino efficacy
In this study use of several AHR agonists resulted in a range of severity of cardiac malformation. Although AHR2 knockdown was protective in all cases, it only reduced the effects of PCB-126 by about half and did not rescue the BkF-induced effects to control levels. This is probably due to the severity of the effects and the fact that morpholino knockdown is not complete. Similar incomplete rescue has been observed in studies utilizing morpholinos to investigate aryl hydrocarbon teratogenesis in zebrafish (Billiard et al., 2006, Jonsson et al., 2007). However, it was important in the current study to assess the efficacy of the morpholino knockdown process, particularly due to the relative lack of use of morpholino knockdown in Fundulus. With Western blots we demonstrated that both translation blocking morpholinos reduced expression of their respective targeted proteins. However, it was difficult to measure AHR protein levels early in development, at least in part due to interference by the relative excess of vitellogenin. As a result, we analyzed protein from pooled 144 hpf embryos, which allowed good measurement of AHR. When embryos were dosed at 24 hpf, we observed lower AHR protein levels in BNF-dosed fish compared to controls. The rapid degradation of AHR protein following binding of a ligand has been described previously (reviewed in Pollenz, 2002) and is not unexpected given the amount of time between dosing and protein assessment. While dosing at 24 hpf still allowed assessment of morpholino efficacy, it made comparison of the morpholino effects on protein levels under dosed conditions difficult. When individuals were dosed at 96 hpf and protein measured at 144 hpf in a second experiment, morpholino efficacy was more apparent under both control and dosed conditions. However, visualization of protein knockdown for AHR1 was somewhat inconclusive, so an AHR1-spl MO was also used to allow better confirmation of AHR1 knockdown.
In addition to providing a second method (RT-PCR) for confirmation of AHR knockdown, use of the AHR1-spl MO provided allowed us to demonstrate knockdown much earlier than was possible with Western blot analysis. Although the splice morpholino-injected individuals clearly lost the targeted region of AHR1, we were unable to amplify an altered sequence created by the action of the splice morpholino. This may be due to a large increase in target size generated by inclusion of the intron, which is one of the possible outcomes reported by GeneTools. This is the first report of the use of a splice morpholino in Fundulus, further demonstrating the application of molecular tools in this environmental model.
4.4. The role of AHR1 and AHR2 in aryl hydrocarbon-resistant Fundulus populations
The pivotal role of the AHR in the toxicity of both dioxin-like compounds (DLCs) and PAHs makes it a likely target for development of resistance to these contaminants in field populations. Recalcitrance to CYP1A induction, indicative of depression of the AHR pathway, is shared by aryl hydrocarbon-resistant Fundulus populations from the Elizabeth River, VA (Van Veld and Westbrook, 1995, Meyer et al., 2002), New Bedford Harbor, MA (Nacci et al., 1999, Bello et al., 2001), and Newark Bay, NJ (Prince and Cooper, 1995a). However, there has been limited work investigating the specific role of the AHRs in these resistant populations.
In one study, Fundulus from Newark Bay exhibited lower hepatic expression of AHRs than reference fish, possibly contributing to resistance (Arzuaga and Elskus, 2002). However, the use of photoaffinity labeling to measure AHR protein did not distinguish between AHR1 and AHR2. Previous work in our lab analyzed transcriptional expression of AHR pathway genes in liver of BNF-treated adult Fundulus from the PAH-adapted Elizabeth River population and a reference population (Meyer et al., 2003). AHR2 expression was induced in reference fish, but not in the Elizabeth River population. Overall, expression of AHR1 was low and the populations did not differ in their level of AHR1 expression. Importantly, there were no differences in basal mRNA expression of AHR pathway genes, so this was not the source of resistance. It is notable that AHR2 was moderately inducible in reference Fundulus. One would then expect that Fundulus directly from the Elizabeth River might have elevated basal levels due to exposure to high levels of PAHs in situ. In keeping with results from the current study demonstrating the importance of AHR2 in mediating PAH toxicity, the lack of induction of AHR2 in Elizabeth River Fundulus and statistically indistinguishable basal levels of expression may indicate that resistance is conferred through an alteration of AHR2 activation. Recent work also supports the conclusion that an alteration at the top of the AHR pathway confers resistance on the Elizabeth River population fish. Wills et al. (2010) demonstrated that multiple AHR pathway genes (CYP1A, CYP1B1, and CYP1C1) are induced in reference Fundulus embryos, but not in Elizabeth River embryos, suggesting suppression via the shared transcription factor AHR.
In contrast to Meyer et al., Powell et al. (2000) observed no differences in basal or contaminant-induced mRNA expression of AHR1 or AHR2 between embryos from DLC-resistant New Bedford Harbor Fundulus and a reference population. Interestingly, AHR1 expression was distributed in all tissues investigated in wild-caught adults from the New Bedford Harbor population, but only in brain, heart, and ovary of reference Fundulus. However, the unusual pattern of AHR1 expression was not maintained in lab-reared offspring of New Bedford Harbor Fundulus, indicating that the altered AHR1 expression pattern was probably not responsible for the heritable resistance to DLCs. Subsequently, the same lab investigated polymorphisms of the Fundulus AHR1 locus and reported differences in frequency of the major AHR1 allele types between the New Bedford Harbor population and a reference population (Hahn et al., 2004). They expressed representative proteins from the two most divergent allele groups and compared their functional properties in vitro. Both AHR1 alleles exhibited similar TCDD binding and ability to activate a luciferase reporter upon TCDD-binding. Although there are a number of potential explanations for these findings, including that the activity of the AHR1 alleles differs in ways that were not assessed by the functional assays, it is possible that AHR1 polymorphism is not driving the resistance. Hahn et al. (2004) also point out that AHR1 could be linked to another polymorphic gene and they identify AHR2 as one candidate. AHR2 and AHR1 are arranged in tandem in the genome of Takifugu rubripes (pufferfish) and zebrafish (Hahn et al., 2006), but the arrangement of these genes is not yet determined in Fundulus. Given our findings on the role of Fundulus AHR2 in aryl hydrocarbon response, it is interesting to speculate that polymorphisms in AHR2 could be driving the observed variation in AHR1 allele frequency via linkage disequilibrium. Currently, an assessment of variation of AHR2 allele frequency in the New Bedford Harbor population has not been published.
4.5. Conclusions
In summary, we have shown through targeted morpholino gene knockdown that AHR2 mediates PCB-126 and PAH responses in Fundulus, including cardiac teratogenesis and CYP1A protein expression. Although our results do not eliminate the possibility that AHR1 or a combination of AHR1 and AHR2 may play a role in response to other aryl hydrocarbons or through other endpoints, we did not find any evidence to support this conclusion. Results from this study aid in further characterization of the mechanism of aryl hydrocarbon toxicity in Fundulus and other fish and will enhance investigation of the molecular mechanisms underlying adaptation of Fundulus populations to severely contaminated environments.
5. Acknowledgements
We thank laboratory members Lauren Wills, Lindsey Van Tiem, and Carrie Fleming for advice and assistance conducting morpholino injection and deformity screening and Autumn Bernal for assistance in developing Western blot methodology. Antibodies were generously provided by Dr. Mark Hahn (AHR1 and AHR2) and Dr. John Stegeman (CYP1A) of the Woods Hole Oceanographic Institution. This work was funded by the NIEHS-supported Duke University Superfund Basic Research Program (P42ES010356) and Duke Integrated Toxicology and Environmental Health Program (T32ES07031).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen EA, Hahn ME, Heideman W, Peterson RE, Tanguay RL. The zebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol Pharmacol. 2002;62:234–249. doi: 10.1124/mol.62.2.234. [DOI] [PubMed] [Google Scholar]
- Arzuaga X, Elskus A. Evidence for resistance to benzo[a]pyrene and 3,4,3 ′ 4 ′-tetrachlorobiphenyl in a chronically polluted Fundulus heteroclitus population. Mar Environ Res. 2002;54:247–251. doi: 10.1016/s0141-1136(02)00184-8. [DOI] [PubMed] [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: In vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol. Sci. 2001;60:77–91. doi: 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Querbach K, Hodson PV. Toxicity of retene to early life stages of two freshwater fish species. Environ. Toxicol. Chem. 1999;18:2070–2077. [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, Di Giulio RT, Evans DH, Gomez-Chiarri M, Hahn ME, Hoover CA, Karchner SI, Katoh F, MacLatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL. Fundulus as the premier teleost model in environmental biology: Opportunities for new insights using genomics. Comp Biochem Physiol D Genomics Proteomics. 2007;2:257–286. doi: 10.1016/j.cbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunson WA, Fricano P, Sadinski WJ. Variation in tolerance to abiotic stresses among sympatric salt-marsh fish. Wetlands. 1993;13:16–24. [Google Scholar]
- Elskus AA, Monosson E, McElroy AE, Stegeman JJ, Woltering DS. Altered CYP1A expression in Fundulus heteroclitus adults and larvae: a sign of pollutant resistance? Aquat. Toxicol. 1999;45:99–113. [Google Scholar]
- Evans BR, Karchner SI, Franks DG, Hahn ME. Duplicate aryl hydrocarbon receptor repressor genes (ahrr1 and ahrr2) in the zebrafish Danio rerio: Structure, function, evolution, and AHR-dependent regulation in vivo. Arch Biochem Biophys. 2005;441:151–167. doi: 10.1016/j.abb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Fortin MG, Couillard CM, Pellerin J, Lebeuf M. Effects of salinity on sublethal toxicity of atrazine to mummichog (Fundulus heteroclitus) larvae. Mar Environ Res. 2008;65:158–170. doi: 10.1016/j.marenvres.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem.-Biol.Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Evans BR, Franks DG, Merson RR, Lapseritis JM. Unexpected diversity of aryl hydrocarbon receptors in non-mammalian vertebrates: Insights from comparative genomics. J Exp Zool Part A Comp Exp Biol. 2006;305A:131–131. doi: 10.1002/jez.a.323. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Franks DG, Merson RR. Aryl hydrocarbon receptor polymorphisms and dioxin resistance in Atlantic killifish (Fundulus heteroclitus) Pharmacogenetics. 2004;14:131–143. doi: 10.1097/00008571-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ. Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Jonsson ME, Jenny MJ, Woodin BR, Hahn ME, Stegeman JJ. Role of AHR2 in the expression of novel cytochrome p450 1 family genes, cell cycle genes, and morphological defects in developing zebra fish exposed to 3,3 ′,4,4 ′,5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2007;100:180–193. doi: 10.1093/toxsci/kfm207. [DOI] [PubMed] [Google Scholar]
- Jung D, Di Giulio RT. Identification of mitochondrial cytochrome P450 induced in response to polycyclic aromatic hydrocarbons in the mummichog (Fundulus heteroclitus) Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2010;151:107–112. doi: 10.1016/j.cbpc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Hahn ME. AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: tandem arrangement of ahr1b and ahr2 genes. Biochem. J. 2005;392:153–161. doi: 10.1042/BJ20050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Powell WH, Hahn ME. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AHR repressor, AHR1, and AHR2. J Biol Chem. 2002;277:6949–6959. doi: 10.1074/jbc.M110779200. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Powell WH, Hahn ME. Identification and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleost Fundulus heteroclitus - Evidence for a novel subfamily of ligand-binding basic helix loop helix-Per-ARNT-Sim (bHLH-PAS) factors. J Biol Chem. 1999;274:33814–33824. doi: 10.1074/jbc.274.47.33814. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Di Giulio RT. Developmental toxicity of estrogenic alkylphenols in killifish (Fundulus heteroclitus) Environ. Toxicol. Chem. 2000;19:2564–2570. [Google Scholar]
- Kneib RT. THE ROLE OF FUNDULUS-HETEROCLITUS IN SALT-MARSH TROPHIC DYNAMICS. American Zoologist. 1986;26:259–269. [Google Scholar]
- Lotrich VA. Summer home range and movements of Fundulus heteroclitus (Pisces-Cyprinodontidae) in a tidal creek. Ecology. 1975;56:191–198. [Google Scholar]
- Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT. Development of the morpholino gene knockdown technique in Fundulus heteroclitus: A tool for studying molecular mechanisms in an established environmental model. Aquat. Toxicol. 2008;87:289–295. doi: 10.1016/j.aquatox.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith WH, Lotrich VA. Production dynamics of a tidal creek population of Fundulus heteroclitus (Linnaeus) Estuarine and Coastal Marine Science. 1979;8:99–118. [Google Scholar]
- Merson RR, Franks DG, Karchner SI, Hahn ME. Development and characterization of polyclonal antibodies against the aryl hydrocarbon receptor protein family (AHR1, AHR2, and AHR repressor) of Atlantic killifish Fundulus heteroclitus. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2006;142:85–94. doi: 10.1016/j.cbpc.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Meyer J, Di Giulio R. Patterns of heritability of decreased EROD activity and resistance to PCB 126-induced teratogenesis in laboratory-reared offspring of killifish (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, VA, USA. Mar Environ Res. 2002;54:621–626. doi: 10.1016/s0141-1136(02)00170-8. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Nacci DE, Di Giulio RT. Cytochrome P4501A (CYP1A) in killifish (Fundulus heteroclitus): Heritability of altered expression and relationship to survival in contaminated sediments. Toxicol. Sci. 2002;68:69–81. doi: 10.1093/toxsci/68.1.69. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Wassenberg DM, Karchner SI, Hahn ME, Di Giulio RT. Expression and inducibility of aryl hydrocarbon receptor pathway genes in wild-caught killifish (Fundulus heteroclitus) with different contaminant-exposure histories. Environ. Toxicol. Chem. 2003;22:2337–2343. doi: 10.1897/02-495. [DOI] [PubMed] [Google Scholar]
- Nacci D, Coiro L, Champlin D, Jayaraman S, McKinney R, Gleason TR, Munns WR, Specker JL, Cooper KR. Adaptations of wild populations of the estuarine fish Fundulus heteroclitus to persistent environmental contaminants. Mar. Biol. 1999;134:9–17. [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlie FG. Physicochemical environments and tolerances of cyprinodontoid fishes found in estuaries and salt marshes of eastern North America. Rev Fish Biol Fish. 2006;16:51–106. [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ. Toxicol. Chem. 2002;21:1897–1902. [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of diosins and related compounds - cross-species comparisons. Crit. Rev. Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Pollenz RS. The mechanism of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chem.-Biol. Interact. 2002;141:41–61. doi: 10.1016/s0009-2797(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Powell WH, Bright R, Bello SM, Hahn ME. Developmental and tissue-specific expression of AHR1, AHR2, and ARNT2 in dioxin-sensitive and - resistant populations of the marine fish Fundulus heteroclitus. Toxicol. Sci. 2000;57:229–239. doi: 10.1093/toxsci/57.2.229. [DOI] [PubMed] [Google Scholar]
- Powell WH, Karchner SI, Bright R, Hahn ME. Functional diversity of vertebrate ARNT proteins: Identification of ARNT2 as the predominant form of ARNT in the marine teleost, Fundulus heteroelitus. Arch Biochem Biophys. 1999;361:156–163. doi: 10.1006/abbi.1998.0992. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prince R, Cooper KR. Comparisons of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on chemically impacted and nonimnpacted subpopulations of Fundulus heteroclitus 2. Metabolic considerations. Environ. Toxicol. Chem. 1995a;14:589–595. [Google Scholar]
- Prince R, Cooper KR. Comparisons of the Effects of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin on Chemically Impacted and Nonimpacted Subpopulations of Fundulus-Heteroclitus .1. Tcdd Toxicity. Environ. Toxicol. Chem. 1995b;14:579–587. [Google Scholar]
- Roling JA, Bain LJ, Gardea-Torresdey J, Bader J, Baldwin WS. Hexavalent chromium reduces larval growth and alters gene expression in mummichog (Fundulus heteroclitus) Environmental Toxicology and Chemistry. 2006;25:2725–2733. doi: 10.1897/05-659r.1. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu. Rev. Cell. Dev. Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Schulte PM, Glemet HC, Fiebig AA, Powers DA. Adaptive variation in lactate dehydrogenase-B gene expression: Role of a stress-responsive regulatory element. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6597–6602. doi: 10.1073/pnas.97.12.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shute JR. Fundulus heteroclitus Linnaeus. Pages 517. In: Lee DS, Gilbert CR, Hocutt VH, Jenkins RE, McAllister DC, Stauffer JR, editors. Atlas of North American Freshwater Fishes. North Carolina State Museum of Natural History; Raleigh, NC: 1980. [Google Scholar]
- Smith KJ, Able KW. Dissolved oxygen dynamics in salt marsh pools and its potential impacts on fish assemblages. Marine Ecology-Progress Series. 2003;258:223–232. [Google Scholar]
- Stierhoff KL, Targett TE, Grecay PA. Hypoxia tolerance of the mummichog: the role of access to the water surface. J Fish Biol. 2003;63:580–592. [Google Scholar]
- Tanguay RL, Abnet CC, Heideman W, Peterson RE. Cloning and characterization of the zebrafish (Danio rerio) aryl hydrocarbon receptor. Biochimica Et Biophysica Acta-Gene Structure and Expression. 1999;1444:35–48. doi: 10.1016/s0167-4781(98)00252-8. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem. Biophys. Res. Commun. 2003;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Van Veld PA, Westbrook DJ. Evidence for depression of cytochrome P4501A in a population of chemically resistant mummichog (Fundulus heteroclitus) Environ Sci. 1995;3:221–234. [Google Scholar]
- Vogelbein WK, Fournie JW, Vanveld PA, Huggett RJ. Hepatic Neoplasms in the Mummichog Fundulus-Heteroclitus from a Creosote-Contaminated Site. Cancer Res. 1990;50:5978–5986. [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor Agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ. Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis JS, Weis P. A Rapid Change in Methylmercury Tolerance in a Population of Killifish, Fundulus-Heteroclitus, from a Golf Course Pond. Mar Environ Res. 1984;13:231–245. [Google Scholar]
- Wills LP, Jung D, Koerhn K, Zhu SQ, Willett KL, Hinton DE, Di Giulio RT. Comparative chronic liver toxicity of benzo[a]pyrene in two populations of the Atlantic killifish (Fundulus heteroclitus) with different exposure histories. Environ. Health Perspect. doi: 10.1289/ehp.0901799. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills LP, Matson CW, Landon CD, Di Giulio RT. Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquat. Toxicol. 2010 doi: 10.1016/j.aquatox.2010.03.015. doi:10.1016/j.aquatox.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CM, Marshall WS. Ion balance, acid-base regulation, and chloride cell-fucntion in the common killifish Fundulus heteroclitus - a euryhaline estuarine teleost. Estuaries. 1994;17:34–52. [Google Scholar]