Abstract
As the mainstay treatment for advanced prostate cancer, androgen-deprivation therapy (ADT) targets the action of androgen receptor (AR) by reducing androgen level and/or by using anti-androgen to compete with androgens for binding to AR. Albeit effective in extending survival, ADT is associated with dose-limiting toxicity and the development of castration-resistant prostate cancer (CRPC) after prolonged use. Since CRPC is lethal and incurable, developing effective strategies to enhance the efficacy of ADT and circumvent resistance becomes an urgent task. Continuous AR signaling constitutes one major mechanism underlying the development of CRPC. The present study showed that methylseleninic acid (MSA), an agent that effectively reduces AR abundance, could enhance the cancer-killing efficacy of the anti-androgen bicalutamide in both androgen-dependent and castration-resistant prostate cancer cells. We found that combination of MSA and bicalutamide produced a robust downregulation of prostate-specific antigen and a recently identified AR target, telomerase and its catalytic subunit, telomere reverse transcriptase (hTERT). The downregulation of hTERT occurs mainly at the transcriptional level, and reduced AR occupancy of the promoter contributes to the downregulation. Furthermore, apoptosis induction by the two agents is significantly mitigated by restoration of hTERT. Our findings thus indicate that MSA in combination with anti-androgen could represent a viable approach to improve the therapeutic outcome of ADT. Given the critical role of hTERT/telomerase downregulation in mediating the combination effect and the fact that hTERT/telomerase could be measured in blood and urine, hTERT/telomerase could serve as an ideal tumor-specific biomarker to monitor the efficacy of the combination therapy non-invasively.
Keywords: anti-androgen, methylseleninic acid, androgen receptor, telomerase, prostate cancer
INTRODUCTION
Telomerase is a ribonucleoprotein enzyme charged with attaching telomeric DNA to the ends of chromosomes, and thus preventing chromosomes from degradation and end-to-end fusion (1). The role of telomerase in tumorigenesis has been well documented. It is activated in 85-90% of malignant tumors, but generally not in normal somatic cells (2). The reactivation of telomerase allows cells to overcome replicative senescence, thereby leading to cellular immortalization and oncogenesis. Inhibition of telomerase has been shown to limit life span, impair cell growth, and suppress the tumorigenic potential of cancer cells of different organs, including prostate (3,4).
Human telomerase consists of a telomerase RNA component, a telomere reverse transcriptase (hTERT), and telomerase-associated proteins (2). The telomerase RNA component and telomerase-associated proteins are constitutively expressed in cancer and normal cells, irrespective of the presence or absence of telomerase activity. However, a strong correlation has been observed between hTERT mRNA expression and telomerase activity (5,6).
The expression of hTERT and the activity of telomerase are regulated by androgen receptor (AR) signaling. In LNCaP human prostate cancer cells that express a mutant but functional AR, androgen was demonstrated to induce telomerase activity by recruiting AR to the hTERT promoter and increasing its transcription (7,8). However, exogenously-expressed wild-type AR was shown to inhibit hTERT transcription in an AR-null prostate cancer cell line (8). The data, nonetheless, cannot be generalized to the conclusion that wild-type suppresses hTERT expression. Activated AR is known to inhibit the growth of AR-null cells, as opposed to the growth stimulatory effect seen in prostate cancer cells expressing endogenous AR (9). In fact, evidence from both xenograft and human prostate cancer studies have indicated that endogenously-expressed wild-type AR positively regulates hTERT expression (7,10). Castration was shown to decrease hTERT expression and telomerase activity in CWR22 xenograft tumors (7), which express a wild-type AR (11), and these effects were reversed by the subsequent administration of androgen (7). A study of 30 prostate cancer patients underwent ADT before prostatectomy showed that hTERT expression dropped to 36% of pre-treatment level as a result of ADT (10). AR genotyping was not performed in this study. However, since AR mutations occur at low levels in prostate carcinomas (12), it is reasonable to believe that the majority of the prostate cancer cells in the patients express a wild-type AR. Collectively, the data demonstrate a positive regulation of hTERT/telomerase by endogenously-expressed AR in prostate tumors, and suppression of AR signaling could represent a viable approach for blocking telomerase activation in the tumor.
Our previous studies demonstrated that methylated forms of selenium are very effective in reducing AR abundance and thereby AR trans-activation (13-16). Selenium has been shown to be an effective anticancer agent in cancers of various sites, including the prostate, by numerous preclinical studies, epidemiological observations, and clinical trials (17,18). Although the interim analysis of the Selenium and Vitamin E Chemoprevention Trial (SELECT) indicated that supplementation of healthy individuals with nutritional dose of selenium did not reduce prostate cancer risk (19), the finding should not have been entirely unexpected. The anticancer efficacy of selenium depends on the form and dosage of selenium administered and the stage of the disease progression (17,20-23). In fact, animal studies using xenograft models have shown that selenomethionine (Se-Met), the form of selenium used in the SELECT, is ineffective against prostate cancer growth, whereas “second-generation” selenium compounds, including methylseleninic acid (MSA), are very effective in inhibiting tumor growth (21,23). This is in concordance with the fact that Set-Met is converted to the active metabolite, methylselenol, much less efficiently than the much more potent new selenium compounds (17,20,24). Additionally, in most of the preclinical studies that showed a positive association between selenium administration and tumor inhibition, the experiments were conducted by using pharmacological doses of selenium, not the nutritional dose that was used in the SELECT. Therefore, the negative SELECT finding underscores the urgency of studying the efficacy of the new selenium compounds, particularly at pharmacological doses, for prostate cancer intervention. Selenium has a low toxicity profile. While the recommended dietary allowance and the tolerable upper intake level for selenium for adults are set at 55 and 400 μg/day, respectively (25), patients with biopsy-proven prostate cancer supplemented with 3,200 μg selenium per day for an average of one year did not develop any serious selenium-related toxicities (26). Therefore, selenium supplementation at pharmacological doses will be well tolerated.
We have previously reported that treatment of prostate cancer cells with MSA, at a concentration between 2.5 and 10 μM, could markedly decrease the levels of AR mRNA and protein (13,14). In mice given a non-toxic dose of MSA, serum selenium level reaches a peak level of 12.5 μM at 1 hr and gradually declines to basal level by 24 hr (27). Therefore, the concentrations of MSA that could suppress AR expression are physiologically relevant. We have also found that the reduced abundance of AR results in downregulated AR trans-activation. Ectopic expression of AR reverses MSA suppression of both AR trans-activation and cell growth, suggesting that AR signaling is a key mediator of MSA effect (14). The findings lend credence to the idea that MSA should also be effective in suppressing androgen-induced hTERT expression and telomerase activity. In addition, since MSA and anti-androgen target AR signaling at different levels, through reducing receptor availability and disrupting ligand binding respectively, combining these two drugs would be expected to produce a more pronounced suppression of AR trans-activation and subsequently hTERT/telomerase, thereby inhibiting the growth/survival of prostate cancer cells. Therefore, the present study was designed to determine the potential of using MSA to increase the efficacy of anti-androgen by characterizing the effects of MSA and an anti-androgen, bicalutamide (Casodex®), either alone or in combination, on hTERT expression, telomerase activation, and apoptosis in both androgen-dependent and castration-resistant prostate cancer cells.
MATERIALS AND METHODS
Prostate Cancer Cell Lines and Reagents
The LNCaP, 22Rv1, and DU-145 human prostate cancer cell lines were obtained from American Type Culture Collection (Manassas, VA) at Passage 4. Cells used in this study were within 20 passages (~ 3 months of non-continuous culturing). The LAPC-4 cell line (28) was provided by Dr. Charles L. Sawyers at the UCLA Jonsson Comprehensive Cancer Center. The two castration-resistant LNCaP sublines, LN3 (29) and C81 (30), were obtained from Dr. Curtis A. Pettaway (University of Texas M.D. Anderson Cancer Center) and Dr. Ming-Fong Lin (University of Nebraska Medical Center), respectively. The cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin. In some experiments, cells were cultured in an androgen-defined condition by using 10% charcoal-stripped fetal bovine serum in the absence or presence of dihydrotestosterone (DHT). MSA and bicalutamide were purchased from PharmaSe (Lubbock, TX) and Toronto Research Chemicals Inc. (Ontario, Canada), respectively.
Apoptosis Detection
Detached cells were precipitated by centrifugation and pooled with attached cells. Cytoplasmic histone-associated DNA fragments were quantified by using the Cell Death Detection ELISAPLUS Kit (Roche Applied Science, Indianapolis, IN) as per manufacturer's protocol. The absorbance was measured at 405 nm (reference wavelength at 490 nm) and normalized by the SRB value for cell content.
Quantitative Reverse Transcription-PCR (qRT-PCR)
The analysis was performed as described previously (13) using RNA isolated with the PerfectPure RNA Cultured Cell Kit (Fisher Scientific, LLC). The PCR primers and TaqMan® probes for β-actin (a housekeeping gene), prostate-specific antigen (PSA), and hTERT were products of Applied Biosystems (Foster City, CA).
Western Blot Analysis
Details of the procedure for Western blot analysis were described previously (31). Immunoreactive bands were quantitated by volume densitometry and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following antibodies were used in this study (source): anti-GAPDH (Millipore, Billerica, MA), anti-AR (Millipore, Billerica, MA), and anti-HA (Covance Inc., Princeton, NJ).
Telomerase Activity Assay
Telomerase activity was analyzed by using the TRAPeze Telomerase Detection kit (Millipore, Billerica, MA) per manufacturer's protocol with minor modification. In brief, 2×105 cells were lysed in 200 μl of CHAPS lysis buffer. Protein concentration of the lysate was determined with the bicinchoninic acid protein assay kit (Pierce, Rockford, IL), and an amount of 1.5 μg was used in the PCR reaction. The extract was incubated at 30°C for 60 min in 50 μL of reaction solution containing the TS primer, reverse primer, dNTPs, TRAP reaction buffer (provided by the kit) and Titanium Taq DNA polymerase (Clontech). The products were then subjected to PCR amplification with one cycle at 94°C for 2 min and followed by 29 cycles at 94°C for 30 sec and 59°C for 45 sec. A pair of internal-control primers was included to monitor the amplification efficiency of each PCR reaction. For each sample, a heat inactivation telomerase control was tested to ensure the specificity of the reaction. The PCR products were separated on a 28 cm × 20 cm 12% non-denaturing polyacrylamide gel, and visualized by SYBR Green staining. The signal of the TRAP product ladder bands for each sample was quantitated by volume densitometry using the NIH ImageJ software, and normalized to that of the internal control band.
AR Knockdown
LNCaP cells stably transfected with a doxycycline-inducible AR-shRNA lentiviral system was generated as previously described (32). Doxycycline at a concentration of 2 μg/ml was added to the cells 24 hr prior to the addition of MSA to induce AR knockdown. The MSA treatment continued for 16 hr.
Transient Transfection of an hTERT Expression Vector
The hTERT expression vector, hTERT/pCI-Neo, was purchased from Addgene (# Plasmid 1782). The transfection was carried out in a 10-cm culture dish as described previously (33), and the cells were subsequently trypsinized and replated in triplicate onto a 96-well plate for apoptosis analysis. Cells were allowed to recover for an additional 48 hr before treatment.
Reporter Gene Assay
The hTERT promoter construct, hTERT-3915/pGL4, was generated by subcloning a ~4 kb (−3915 to +40) hTERT promoter region from the pBT-3915 plasmid (34) into the pGL4.19 [luc2CP/neo] rapid response luciferase expression vector (Promega). Transfection and luciferase activity analyses were performed as previously described (33). In order to obtain uniform transfection efficiency, transfection was conducted in a 10-cm culture dish, and the cells were subsequently trypsinized and replated in triplicate onto 6-well plates for treatments. Luciferase activity was normalized by the protein concentration of the sample.
mRNA Stability Assay
Actinomycin D (5 μg/mL) was added to the cultures to stop new RNA synthesis at the time of MSA treatment, and hTERT mRNA levels were measured by qRT-PCR at the 2, 4 and 6 hr time points. The turnover of hTERT mRNA was determined by comparing mRNA levels over time in cells treated with or without MSA.
Quantitative Nuclear Run-On Assay
Run-on transcription was performed according to a previously described method with biotin-16-UTP (Roche) (35). Biotin-labeled nascent transcripts were then isolated by using streptavidin particle beads, and quantitated by qRT-PCR.
Chromatin Immunoprecipitation (ChIP) Assay
The ChIP assay was carried out by using the ChIP-IT™ Express kit (Active Motif, Carlsbad, CA) per instruction of the manufacturer. Briefly, cells were treated with 1% formaldehyde for 15 min at room temperature. Nuclei were isolated, and chromatin was sheared to a size of 500-1500 bp by sonication at 25% power for ten pulses of 10 seconds each. Immunoprecipitation was performed with an AR antibody (PG-21, Millipore) or rabbit IgG (as a negative control) overnight at 4°C. DNA released from the antibody-bound protein/DNA complexes was subjected to regular PCR and quantitative PCR (qPCR) analyses. The sequences of the primers used in regular PCR, −4 kb TERT s and −4 kb TERT as, were as described (8). The sequences of the primers and probes used in the qPCR experiments were as follows. hTERT Primer-1: 5′-TCACAGTGAAGAGGAACATGCCGT-3′ (−3844 to −3820); hTERT Primer-2: 5′-TCAGTATCCCATGGAGGTGGCAGTTT-3′ (−3778 to −3752); hTERT Probe: 5′/56-FAM/AAGCCTGCAGGCATCTCAAGGGAATT /3BHQ_1/−3′ (−3815 to −3789).
Statistical Analysis
Mean activities were calculated from at least three independent experiments performed in triplicate. The Student's two-tailed t test was used to determine significant differences between two groups.
RESULTS
Downregulation of hTERT by androgen-signaling suppression
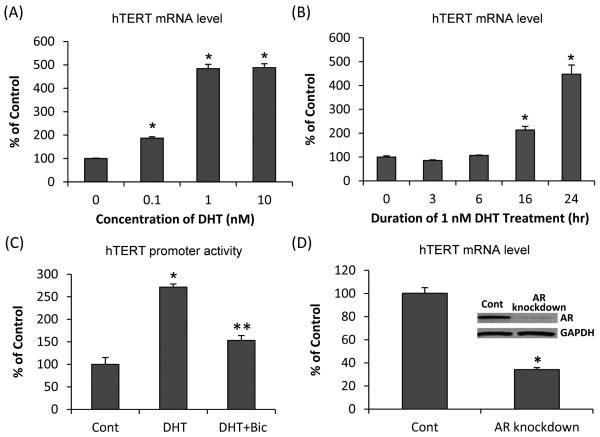
We first performed dose-response and time-course experiments on LNCaP cells cultured in a medium containing charcoal-stripped fetal bovine serum to study the effect of androgen (in the form of DHT) on the expression of hTERT. Consistent with previous reports (7,8), DHT treatment of LNCaP cells led to a marked induction of hTERT mRNA (Fig. 1A and 1B). The induction was dose dependent between 0.1 nM and 1 nM DHT, and no further increase was observed when DHT was raised to 10 nM (Fig. 1A). On the basis of this finding, we chose 1 nM DHT for the subsequent experiments. Fig. 1B shows the data of the time-course experiment. Expression of hTERT mRNA was increased by 2-fold or 4.5-fold at 16 hr or 24 hr, respectively. We also examined the effect of DHT on hTERT promoter activity by transiently transfecting LNCaP cells with the hTERT-3915/pGL4 construct, which contains nucleotides −3915 to +40 of the hTERT promoter region. A significant increase of hTERT promoter activity was detected after treating the cells with DHT for 6 hr. The induction was almost completely blocked by 5 μM bicalutamide, which is an anti-androgen (Fig. 1C). We then studied the consequence of AR knockdown on hTERT expression. As shown in Fig. 1D, the knockdown of AR by treating LNCaP cells stably transfected with a doxycycline-inducible AR-shRNA lentiviral system with doxycycline led to a significant reduction of hTERT mRNA. The data therefore suggest that suppression of AR signaling by either an anti-androgen or AR knockdown could efficiently inhibit hTERT expression.
Figure 1. Androgen-AR regulation of hTERT.
(A & B) qRT-PCR analysis of hTERT mRNA level in LNCaP cells treated with different doses of DHT for 24 hr (A), or 1 nM DHT for 3, 6, 16 or 24 hr (B). (C) Change in hTERT promoter activity, as determined by reporter gene assay, in LNCaP cells as a response to treatment with 1 nM DHT or 1 nM DHT + 5 μM bicalutamide (Bic) for 6 hr. (D) Decrease of hTERT mRNA level, as assessed by qRT-PCR, in response to AR knockdown in LNCaP cells stably transfected with a doxycycline-inducible AR shRNA. Western blot confirmation of AR protein level is shown in the inset. Data are presented as % of ethanol-treated control cells cultured in charcoal-stripped fetal bovine serum (A-C) or untreated (no doxycycline) LNCaP cells stably transfected with a doxycycline-inducible AR shRNA (D). Bars represent SEM. *, P<0.01 compared to control. **, P<0.01 compared to DHT-treated sample.
Downregulation of hTERT/telomerase by MSA
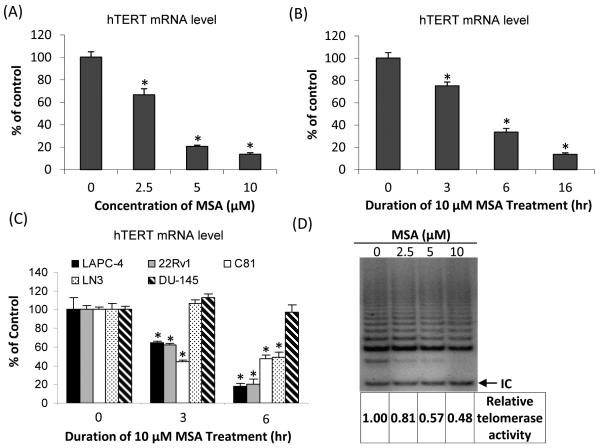
Since MSA reduces AR abundance (13,14), it would be important to find out whether AR repression by MSA could also lead to hTERT downregulation. We treated LNCaP cells with various concentrations of MSA for 16 hr, and determined hTERT mRNA levels by qRT-PCR. As shown in Fig. 2A, as little as 2.5 μM MSA depressed hTERT mRNA level by 40%. Increasing the concentration of MSA to 5 or 10 μM further reduced the level by 80% or more. The downregulation of hTERT mRNA was also time-dependent (Fig. 2B). The level of hTERT mRNA declined gradually from 80% of control at 3 hr to 10% of control at 16 hr in response to 10 μM MSA treatment. It is of note that the experiment was done on LNCaP cells cultured in a medium containing regular fetal bovine serum and treated with MSA for various durations. As shown in Fig. 1B, a 16 hr of treatment was required for androgen to induce hTERT mRNA level. The use of regular fetal bovine serum allowed us to determine the time necessary for MSA to downregulate hTERT in a condition where AR is already activated.
Figure 2. MSA suppression of hTERT expression and telomerase activity.
(A) Changes in hTERT mRNA, as determined by qRT-PCR, as a function of MSA concentration at 16 hr in LNCaP cells. (B & C) Expression of hTERT mRNA in response to treatment with 10 μM MSA for various durations in LNCaP cells (B), or in LAPC-4, 22Rv1, C81, LN3, and DU-145 cells (C). Data are presented as % of untreated control cells cultured in regular fetal bovine serum. *, P<0.01, significantly different from untreated control. (D) TRAP assay of changes in telomerase activity as a function of MSA concentration in LNCaP cells. IC, internal control. The numbers below the gel are normalized telomerase activities relative to untreated control.
Similar decreases of hTERT mRNA were also observed in another androgen-dependent cell line, LAPC-4, as well as in three AR-positive castration-resistant cell lines, 22Rv1, C81 and LN3, but not in the AR-negative cell line, DU-145 (Fig. 2C). The data shown in Fig. 2C were collected at 3 and 6 hr. We also analyzed hTERT mRNA level in DU-145 cells at 16 and 24 hr, and still could not detect any decrease by MSA (data not shown). Our results thus suggest a universal effect of MSA inhibition of hTERT in AR-positive prostate cancer cells. We wanted to examine the effect of MSA on hTERT protein level. However, none of the three hTERT antibodies we tested (Rockland Immunochemicals for Research, Cat# 600-401-252; Santa Cruz Biotechnology, Cat# sc-7212; and GeneTex, Cat# GTX30410) produced a specific signal on Western blots, even with the use of nuclear extracts (see the supplementary data). This could be due to the relatively low level of hTERT protein expressed by the cells. To circumvent this problem, we analyzed telomerase activity in response to MSA treatment in LNCaP cells. As shown in Fig. 2D, MSA treatment led to a dose-dependent inhibition of telomerase activity, although the magnitudes of inhibition are smaller than that observed on hTERT transcript level (refer to Fig. 2A for the transcript data). The disparity could be due to different sensitivity of the two assays and/or inherent difference between the extents of effect on hTERT mRNA versus hTERT protein or telomerase activity. Nonetheless, the data collectively showed a marked downregulation of hTERT and telomerase by MSA.
Inhibition of hTERT transcription by MSA
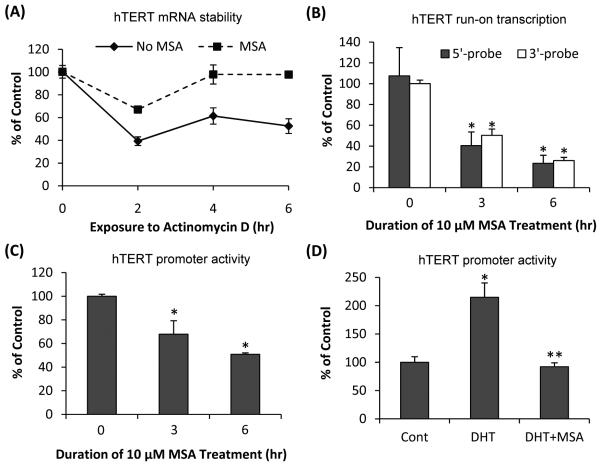
To determine whether MSA downregulation of hTERT was due to increased mRNA degradation or decreased transcription, we performed the mRNA stability assay. Actinomycin D (5 μg/ml) was added to the LNCaP cultures to stop new RNA synthesis at the time of MSA treatment, and hTERT mRNA levels were measured by qRT-PCR during a 6-hr treatment duration. The decay of hTERT mRNA was compared in cells treated with or without MSA. Since actinomycin D could be cytotoxic, we monitored cell growth for up to 8 hr and did not observe cell death or significant growth inhibition during this period. Treatment with 10 μM MSA actually increased the stability of hTERT mRNA (Fig. 3A). We then studied the effect of MSA on hTERT transcription by quantitative nuclear run-on assay. Biotin-labeled nascent transcripts obtained by run-on transcription were isolated by using streptavidin particle beads. To differentiate the effect of MSA on transcription initiation versus transcription elongation, qRT-PCR analysis was conducted with a primer-probe set corresponding to either the 5′-end or the 3′-end of hTERT mRNA. For both sets of primer-probe, MSA treatment resulted in ~50% and ~80% inhibition of hTERT transcription at 3 hr and 6 hr, respectively (Fig. 3B). The data thus indicate that MSA-mediated decrease of hTERT mRNA level is accounted for largely by a block of hTERT transcription initiation. Gene transcription is generally controlled by promoter regions. We next characterized the effect of MSA on the activity of the ~4 kb hTERT promoter in LNCaP cells cultured in a medium containing regular fetal bovine serum. Treatment with 10 μM MSA caused a time-dependent inhibition of the promoter activity (Fig. 3C). When we repeated the experiment by culturing the cells in charcoal-stripped fetal bovine serum and adding DHT to the culture, we also found that MSA was able to block DHT induction of hTERT promoter activity (Fig. 3D).
Figure 3. MSA inhibition of hTERT transcription.
(A) Effect of MSA on hTERT mRNA stability. (B) Effect of MSA on hTERT transcription initiation as assessed by quantitative nuclear run-on analysis. (C) Effect of MSA on hTERT promoter activity. (D) Effect of MSA on DHT-induced hTERT promoter activity. The results are expressed as % of untreated control cells cultured in regular fetal bovine serum (A-C) or ethanol-treated control cells cultured in charcoal-stripped fetal bovine serum (D). Bars represent SEM. *, P<0.01, significantly different from untreated control. **, P<0.01, significantly different from DHT-treated sample.
Reduction of AR occupancy of the hTERT promoter by MSA
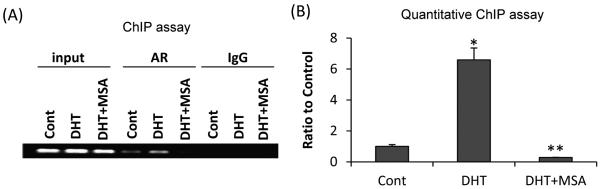
In order to demonstrate that suppressed hTERT promoter activity is attributable to reduced AR occupancy of the hTERT promoter as a consequence of MSA downregulation of AR, we performed the ChIP assay on the hTERT promoter region that was reported to associate with AR (8). Fig. 4A shows the result of PCR amplification of DNA co-immunoprecipitated with either an AR antibody or IgG using primers specific for the hTERT promoter region. The specificity of the ChIP assay is evident from the difference in band intensity between the AR-antibody-immunoprecipitated samples and the IgG-immunoprecipitated samples. In concordance with a previous report (8), AR is only weakly associated with the hTERT promoter in the absence of androgen in LNCaP cells. DHT significantly increased AR occupancy of the promoter, and the increase was blocked by 10 μM MSA. We then conducted qPCR of the co-immunoprecipitated DNA to quantify the change in AR occupancy of hTERT promoter in response to different treatments. As shown in Fig. 4B, DHT treatment induced a ~6-fold increase of AR occupancy of the promoter, and the increase was completely suppressed by MSA.
Figure 4. MSA reduction of AR occupancy of hTERT promoter as determined by ChIP assay (A) or quantitative ChIP assay (B).
The assay was conducted at 16 hr after treatment with 1 nM DHT and/or 10 μM MSA. Input, total input chromatin for each treatment sample. AR, DNA co-immunoprecipitated with an AR antibody. IgG, DNA co-immunoprecipitated with an IgG. *, P<0.01, significantly different from ethanol-treated control cells cultured in charcoal-stripped fetal bovine serum. **, P<0.01, significantly different from DHT-treated sample.
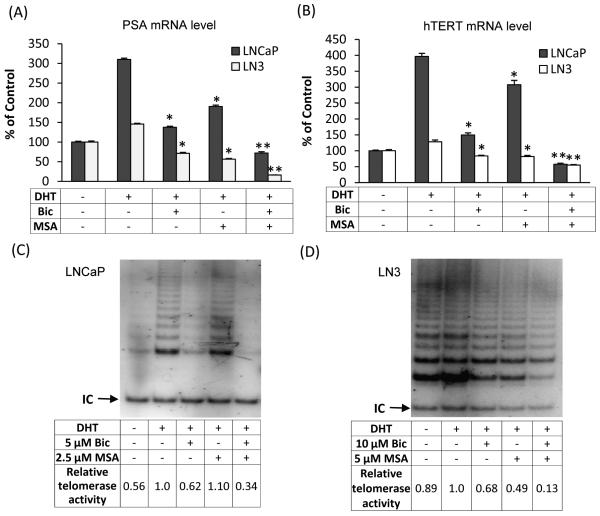
Combinatorial effects of bicalutamide and MSA on PSA and hTERT/telomerase
Bicalutamide and MSA target AR through two distinct mechanisms. As an anti-androgen, bicalutamide suppresses AR trans-activation by competing with androgens for binding to AR. MSA also disrupts AR signaling mainly through reducing the availability of AR (13,14). Therefore, it is reasonable to believe that the two drugs in combination would produce a more pronounced effect on AR-signaling suppression. We quantified the mRNA level of PSA, a well-characterized AR target, by qRT-PCR in both LNCaP and LN3 cell models. As shown in Fig. 5A, incubation of LNCaP cells with DHT for 6 hr led to a 3-fold increase of PSA mRNA compared to the vehicle control. The level was reduced to ~1.4- or 1.9-fold of the vehicle control as a result of treatment with 5 μM bicalutamide or 2.5 μM MSA, respectively. Combining bicalutamide and MSA at these concentrations brought down DHT-induced PSA expression to ~70% of the vehicle control. We chose to use low doses of bicalutamide or MSA individually in order to leave room for an enhanced effect with the combination. Consistent with the castration-resistant characteristics of LN3 cells, only a marginal induction of PSA mRNA by DHT was observed (Fig. 5A). Treating LN3 cells with 10 μM bicalutamide or 5 μM MSA reduced DHT-induced PSA level to ~60% and 50% of the vehicle control, respectively. The combination, on the other hand, almost completely blocked the expression of PSA mRNA. The data thus demonstrate a robust suppression of AR trans-activation by bicalutamide and MSA in both androgen-dependent and castration-resistant prostate cancer cells.
Figure 5. Bicalutamide (Bic) and MSA downregulation of PSA and hTERT/telomerase.
LNCaP cells were treated with 5 μM Bic and/or 2.5 μM MSA, and LN3 cells with 10 μM Bic and/or 5 μM MSA. The levels of PSA mRNA (A) and hTERT mRNA (B) were determined by qRT-PCR. The activity of telomerase in LNCaP (C) and LN3 (D) cells were analyzed by the TRAP assay. Data in Panels A and B are expressed as % of ethanol-treated control cells cultured in charcoal-stripped fetal bovine serum. Bars represent SEM. *, P<0.01 compared to DHT-treated sample. **, P<0.01 compared to Bic- or MSA-treated sample. IC in Panels C and D represents internal control. The numbers in Panels C and D are normalized telomerase activities relative to the DHT-treated sample.
We proceeded to evaluate the effect of bicalutamide in combination with MSA on hTERT expression and telomerase activity. The hTERT mRNA data (Fig. 5B) essentially mirror the PSA mRNA result. In both LNCaP and LN3 cells, treatment with bicalutamide or MSA alone inhibited DHT-induced hTERT expression; the inhibitory effect was much more striking when the drugs were used in combination. We then analyzed telomerase activity as a result of the combination treatment in the two cell lines. As shown in Fig. 5C, telomerase activity in LNCaP cells was markedly induced by DHT. Bicalutamide or MSA alone produced minimal inhibition of DHT-induced telomerase activity. The two drugs in combination, however, almost completely blocked DHT induction of telomerase activity. Comparing to the data presented in Fig. 2D, the effect of MSA alone seems to be smaller here (0% vs. 19% inhibition). This could be due to the different culturing conditions that were used for the two experiments (charcoal-stripped fetal bovine serum for Fig. 5C vs. regular fetal bovine serum for Fig. 2D). The combination effect on telomerase activity was also manifested in LN3 cells (Fig. 5D). Thus, the telomerase activity data were qualitatively similar to the hTERT mRNA result.
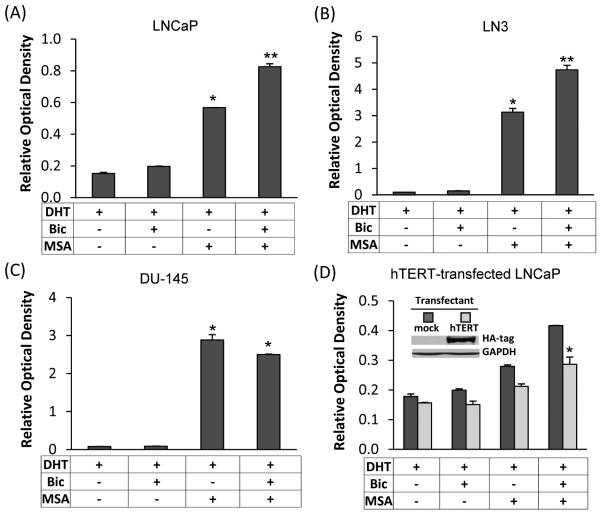
Combinatorial effects of bicalutamide and MSA on apoptosis
Given the critical role of hTERT/telomerase in regulating cell survival, we assessed the effect of bicalutamide and MSA on apoptosis induction in the absence or presence of hTERT overexpression. LNCaP and LN3 cells were treated with bicalutamide and/or MSA for 48 hr, and apoptosis was analyzed by using the Cell Death ELISA assay. As shown in Fig. 6A and 6B, in both cell models, bicalutamide and MSA in combination led to a more striking increase of apoptosis than either agent alone. Conversely, the combination efficacy was not observed in the AR-negative DU-145 cell line (Fig. 6C). In concordance with the previously-reported AR-independent activity of MSA in prostate cancer cells (31,36-39), MSA treatment of DU-145 cells resulted in a marked induction of apoptosis. However, the combination with bicalutamide was not able to lead to further induction of apoptosis.
Figure 6. Bicalutamide (Bic) and MSA induction of apoptosis.
(A - C) Induction of apoptosis as determined by the Cell Death ELISA assay in LNCaP cells treated with 5 μM Bic, 2.5 μM MSA, or Bic and MSA in combination (A), in LN3 cells treated with 10 μM Bic, 5 μM MSA, or Bic and MSA in combination (B), or in DU-145 cells treated with 10 μM Bic, 2.5 μM MSA, or Bic and MSA in combination (C) for 48 hr. The data obtained by ELISA were normalized by SRB results for cell content. *, P<0.01 compared to DHT-treated sample. **, P<0.01 compared to Bic- or MSA-treated sample. (D) Attenuation of Bic/MSA induction of apoptosis by ectopic expression of hTERT in LNCaP cells. The apoptosis assay was conducted at 24 hr after Bic/MSA treatments. Western blot confirmation of the level of transfected HA-tagged hTERT is shown in the inset. *, P<0.01 compared to mock-transfectants.
In order to delineate the functional significance of hTERT downregulation in mediating the effect of bicalutamide and MSA, we transiently transfected LNCaP cells with an hTERT expression construct and assessed the response of the hTERT-overexpressing cells to the induction of apoptosis. The apoptosis assay was conducted at 24 hr after treatments, and the data are presented in Fig. 6D. An abundant presence of hTERT not only weakened the apoptosis-inducing ability of bicalutamide or MSA alone, but also significantly inhibited the apoptosis-inducing efficacy of the combination treatment. One reason that the difference was seemingly compressed was due to the fact that only a fraction of cells was successfully transfected, and apoptosis was assessed using the whole cell population. It is of importance to point out that, in order to obtain uniform transfection efficiency, the transfection was carried out in a 10-cm culture dish, and the cells were subsequently trypsinized and replated onto a 96-well plate for apoptosis analysis. Since the cells were allowed to recover for an additional 48 hr before treatment, and transiently transfected plasmid generally could not maintain expression beyond 72 hr after transfection, the apoptosis assay in this experiment was conducted at 24 hr after treatments, not at 48 hr as in Fig. 6A. This could contribute to the difference in the magnitude of apoptosis induction observed in the experiments presented in Fig. 6D and Fig. 6A.
DISCUSSION
ADT is the mainstay treatment for advanced prostate cancer (40). However, relapse with CRPC invariably occurs. Since CRPC is lethal and incurable (40), developing effective strategies to enhance the efficacy of ADT and circumvent resistance becomes an urgent task. AR expression and signaling are maintained in CRPC although the tumor is no longer responsive to ADT. Prostate cancer can adapt to androgen deprivation by AR mutation, AR overexpression, androgen-independent activation of AR, and/or by increasing intra-tumoral androgens through de novo steroidogenesis (41-43). Xenograft studies have shown that knocking down AR expression by shRNA could delay the progression of prostate cancer to CRPC and suppress the growth of prostate tumor that has already progressed to the castration-resistant state (32,44). Therefore, rationally designed therapies aimed at diminishing the availability of AR would be helpful not only in enhancing the efficacy of ADT, but also in inhibiting the development of CRPC. In this study, we reported for the first time that MSA, an agent that could effectively reduce AR abundance, has the ability to potentiate the cancer-killing efficacy of bicalutamide in both androgen-dependent and castration-resistant prostate cancer cells.
Since bicalutamide and MSA suppress AR signaling at different points, a combination of the two drugs would be expected to deliver a more powerful punch in knocking out AR signal transduction. In support of this notion, our data showed that the combination produced a stronger suppression of PSA and hTERT than either agent alone. We also demonstrated that apoptosis induction by the two agents was mitigated by restoration of hTERT, thus confirming the critical involvement of hTERT downregulation in mediating the combination effect. The downregulation of hTERT occurred mainly at the transcriptional level, and decreased AR occupancy of the hTERT promoter contributes to the reduction of hTERT transcription. These findings, however, cannot exclude the possibility of the existence of an AR-independent mechanism by which MSA diminishes hTERT expression. This possibility deserves further investigation.
The role of telomerase is generally regarded as maintaining telomere length. However, accumulating evidence has indicated that telomerase may have additional functions in regulating cell growth and survival, and thereby oncogenesis (45). Telomeric DNA repeats are associated with an array of telomere-specific proteins in forming a chromosome-protective cap (45). Functional telomeres switch stochastically between capped and uncapped states (46). Telomerase plays an essential role in telomere recapping. Uncapped telomeres, if left uncorrected too long, are recognized as DNA breaks and will trigger a DNA damage response, leading to cell cycle arrest and/or apoptosis (46). Additionally, the non-homologous end-joining of dysfunctional telomeres may result in telomere-telomere fusions and contribute to genomic instability. In the present study, we show that bicalutamide and MSA induce a marked increase of apoptosis at 48 hr. Telomerase suppression by bicalutamide and MSA is unlikely to result in appreciable telomere shortening within such a short period of time. This is actually confirmed by our experiment (data not shown). Therefore, a mechanism independent of telomere shortening should be considered. It is likely that inhibiting telomerase by bicalutamide and MSA could lead to telomere uncapping, which would then trigger a rapid DNA damage response and lead to cell cycle arrest and apoptosis. Experiments are ongoing to test the hypothesis.
The identification of hTERT/telomerase as an important AR target mediating the bicalutamide/MSA effect has great clinical implications. Telomerase activation has been reported in >90% of prostate cancer samples, but not in normal or benign prostatic hyperplasia tissues (47). Telomerase activation has been well documented to play an essential role in cell survival and oncogenesis, and inhibition of telomerase has been shown to suppress growth and tumorigenic potential of prostate cancer cells (3,48). Blocking telomerase activation by anti-androgen and MSA through suppressing AR signaling could thus represent an effective and selective treatment modality to target prostate cancer cells. In addition, hTERT/telomerase could be measured in blood and urine (49,50), and therefore could serve as a non-invasive, tumor-specific, functionally relevant molecular biomarker for monitoring the efficacy of the intervention.
Supplementary Material
ACKNOWLEDGMENTS
We would like to extend our sincere gratitude to Dr. Charles L. Sawyers at the UCLA Jonsson Comprehensive Cancer Center, Dr. Curtis A. Pettaway at the University of Texas M.D. Anderson Cancer Center, and Dr. Ming-Fong Lin at the University of Nebraska Medical Center for the generous gift of the LAPC-4, LN3, and C81 cell line, respectively.
Grant support: This work was supported by the Department of Defense Prostate Cancer Training grant No. W81XWH-08-1-0291 (SL) and New Investigator grant No. W81XWH-05-1-0598 (HZ); the National Cancer Institute grant No. K01CA114252 (YD); the American Cancer Society grant No. RSG-07-218-01-TBE (YD); Jilin Provincial Scholarship for Outstanding Scientists from Jilin Province, China (YD); the Louisiana Cancer Research Consortium Start-up Fund (YD and HZ); and partial support provided from developmental funds of the Tulane Cancer Center (YD).
The abbreviations used are
- ADT
androgen-deprivation therapy
- AR
androgen receptor
- CRPC
castration-resistant prostate cancer
- PSA
prostate-specific antigen
- hTERT
human telomere reverse transcriptase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- DHT
dihydrotestosterone
- ChIP
chromatin immunoprecipitation
- qRT-PCR
quantitative reverse transcription-PCR
- Se-Met
selenomethionine
- MSA
methylseleninic acid
- SELECT
Selenium and Vitamin E Chemoprevention Trial
REFERENCE LIST
- 1.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–7. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 2.Harley CB, Kim NW, Prowse KR, et al. Telomerase, cell immortality, and cancer. Cold Spring Harb Symp Quant Biol. 1994;59:307–15. doi: 10.1101/sqb.1994.059.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Guo C, Geverd D, Liao R, et al. Inhibition of telomerase is related to the life span and tumorigenicity of human prostate cancer cells. J Urol. 2001;166:694–8. [PubMed] [Google Scholar]
- 4.Hahn WC, Stewart SA, Brooks MW, et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–70. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 5.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 6.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 7.Guo C, Armbruster BN, Price DT, Counter CM. In vivo regulation of hTERT expression and telomerase activity by androgen. J Urol. 2003;170:615–8. doi: 10.1097/01.ju.0000074653.22766.c8. [DOI] [PubMed] [Google Scholar]
- 8.Moehren U, Papaioannou M, Reeb CA, et al. Wild-type but not mutant androgen receptor inhibits expression of the hTERT telomerase subunit: a novel role of AR mutation for prostate cancer development. FASEB J. 2008;22:1258–67. doi: 10.1096/fj.07-9360com. [DOI] [PubMed] [Google Scholar]
- 9.Yuan S, Trachtenberg J, Mills GB, et al. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–11. [PubMed] [Google Scholar]
- 10.Iczkowski KA, Huang W, Mazzucchelli R, et al. Androgen ablation therapy for prostate carcinoma suppresses the immunoreactive telomerase subunit hTERT. Cancer. 2004;100:294–9. doi: 10.1002/cncr.20002. [DOI] [PubMed] [Google Scholar]
- 11.Tepper CG, Boucher DL, Ryan PE, et al. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002;62:6606–14. [PubMed] [Google Scholar]
- 12.Steinkamp MP, O'Mahony OA, Brogley M, et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009;69:4434–42. doi: 10.1158/0008-5472.CAN-08-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y, Lee SO, Zhang H, et al. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64:19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- 14.Dong Y, Zhang H, Gao AC, Marshall JR, Ip C. Androgen receptor signaling intensity is a key factor in determining the sensitivity of prostate cancer cells to selenium inhibition of growth and cancer-specific biomarkers. Mol Cancer Ther. 2005;4:1047–55. doi: 10.1158/1535-7163.MCT-05-0124. [DOI] [PubMed] [Google Scholar]
- 15.Lee SO, Yeon CJ, Nadiminty N, et al. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA) Prostate. 2006;66:1070–5. doi: 10.1002/pros.20329. [DOI] [PubMed] [Google Scholar]
- 16.Cho SD, Jiang C, Malewicz B, et al. Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol Cancer Ther. 2004;3:605–11. [PubMed] [Google Scholar]
- 17.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–54. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 18.Meuillet E, Stratton S, Prasad CD, et al. Chemoprevention of prostate cancer with selenium: an update on current clinical trials and preclinical findings. J Cell Biochem. 2004;91:443–58. doi: 10.1002/jcb.10728. [DOI] [PubMed] [Google Scholar]
- 19.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–6. [PubMed] [Google Scholar]
- 21.Li GX, Lee HJ, Wang Z, et al. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis. 2008;29:1005–12. doi: 10.1093/carcin/bgn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Stampfer MJ, Giovannucci EL, et al. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst. 2004;96:696–703. doi: 10.1093/jnci/djh125. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Bonorden MJ, Li GX, et al. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev Res (Phila Pa) 2009;2:484–95. doi: 10.1158/1940-6207.CAPR-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohta Y, Kobayashi Y, Konishi S, Hirano S. Speciation analysis of selenium metabolites in urine and breath by HPLC- and GC-inductively coupled plasma-MS after administration of selenomethionine and methylselenocysteine to rats. Chem Res Toxicol. 2009;22:1795–801. doi: 10.1021/tx900202m. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine FaNB . Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 26.Reid ME, Stratton MS, Lillico AJ, et al. A report of high-dose selenium supplementation: response and toxicities. Journal of Trace Elements in Medicine and Biology. 2004;18:69–74. doi: 10.1016/j.jtemb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Hu H, Li GX, Wang L, et al. Methylseleninic acid enhances taxane drug efficacy against human prostate cancer and down-regulates antiapoptotic proteins Bcl-XL and survivin. Clin Cancer Res. 2008;14:1150–8. doi: 10.1158/1078-0432.CCR-07-4037. [DOI] [PubMed] [Google Scholar]
- 28.Klein KA, Reiter RE, Redula J, et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–8. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 29.Pettaway CA, Pathak S, Greene G, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–36. [PubMed] [Google Scholar]
- 30.Lin MF, Meng TC, Rao PS, et al. Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. J Biol Chem. 1998;273:5939–47. doi: 10.1074/jbc.273.10.5939. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y, Zhang H, Hawthorn L, Ganther HE, Ip C. Delineation of the Molecular Basis for Selenium-induced Growth Arrest in Human Prostate Cancer Cells by Oligonucleotide Array. Cancer Research. 2003;63:52–9. [PubMed] [Google Scholar]
- 32.Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66:10613–20. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Zhang H, Zhu L, Zhao L, Dong Y. Kruppel-like factor 4 is a novel mediator of selenium in growth inhibition. Mol Cancer Res. 2008;6:306–13. doi: 10.1158/1541-7786.MCR-07-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horikawa I, Cable PL, Mazur SJ, et al. Downstream E-box-mediated regulation of the human telomerase reverse transcriptase (hTERT) gene transcription: evidence for an endogenous mechanism of transcriptional repression. Mol Biol Cell. 2002;13:2585–97. doi: 10.1091/mbc.E01-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrone G, Puppo F, Cusano R, et al. Nuclear run-on assay using biotin labeling, magnetic bead capture and analysis by fluorescence-based RT-PCR. Biotechniques. 2000;29:1012–7. doi: 10.2144/00295st02. [DOI] [PubMed] [Google Scholar]
- 36.Jiang C, Wang Z, Ganther H, Lu J. Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001;61:3062–70. [PubMed] [Google Scholar]
- 37.Jiang C, Wang Z, Ganther H, Lu J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol Cancer Ther. 2002;1:1059–66. [PubMed] [Google Scholar]
- 38.Wu Y, Zhang H, Dong Y, Park YM, Ip C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005;65:9073–9. doi: 10.1158/0008-5472.CAN-05-2016. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Zu K, Warren MA, Wallace PK, Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–52. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- 40.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Culig Z, Hobisch A, Hittmair A, et al. Expression, structure, and function of androgen receptor in advanced prostatic carcinoma. Prostate. 1998;35:63–70. doi: 10.1002/(sici)1097-0045(19980401)35:1<63::aid-pros9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 42.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 43.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 44.Snoek R, Cheng H, Margiotti K, et al. In vivo knockdown of the androgen receptor results in growth inhibition and regression of well-established, castration-resistant prostate tumors. Clin Cancer Res. 2009;15:39–47. doi: 10.1158/1078-0432.CCR-08-1726. [DOI] [PubMed] [Google Scholar]
- 45.Blasco MA. Telomerase beyond telomeres. Nat Rev Cancer. 2002;2:627–33. doi: 10.1038/nrc862. [DOI] [PubMed] [Google Scholar]
- 46.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–6. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Kapusta LR, Slingerland JM, Klotz LH. Telomerase activity in prostate cancer, prostatic intraepithelial neoplasia, and benign prostatic epithelium. Cancer Res. 1998;58:619–21. [PubMed] [Google Scholar]
- 48.Kondo Y, Koga S, Komata T, Kondo S. Treatment of prostate cancer in vitro and in vivo with 2-5A-anti-telomerase RNA component. Oncogene. 2000;19:2205–11. doi: 10.1038/sj.onc.1203538. [DOI] [PubMed] [Google Scholar]
- 49.Botchkina GI, Kim RH, Botchkina IL, et al. Noninvasive detection of prostate cancer by quantitative analysis of telomerase activity. Clin Cancer Res. 2005;11:3243–9. doi: 10.1158/1078-0432.CCR-04-1919. [DOI] [PubMed] [Google Scholar]
- 50.Dasi F, Martinez-Rodes P, March JA, et al. Real-time quantification of human telomerase reverse transcriptase mRNA in the plasma of patients with prostate cancer. Ann N Y Acad Sci. 2006;1075:204–10. doi: 10.1196/annals.1368.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.