Abstract
The development and validation of disease specific, patient reported outcomes have become increasingly relevant in the care of cancer patients, especially for assessing symptoms from the patient’s perspective. Recently, two patient symptom questionnaires were developed for kidney cancer patients, the Renal Cell Carcinoma-Symptom Index (RCC-SI) and the Functional Assessment for Cancer Therapy – Kidney Symptom Index (FKSI). This paper describes the development of the revised FKSI scale (FKSI-19) and reconciles its use with the RCC-SI. Fifty participants with advanced kidney cancer commented on their symptoms and concerns about kidney cancer and this input was used to revise FKSI items. These patients also completed the RCC-SI, the Functional Assessment for Cancer Therapy-General (FACT-G), and an older version of the FKSI scale. We qualitatively reviewed item wording and content coverage across the two instruments, examined correlations between the scales, and calculated basic psychometrics on each scale. We found that the FKSI-19 and the RCC-SI addressed similar symptom. Qualitative and descriptive statistical analyses demonstrated considerable overlap between the two instruments. The FKSI-19 has some advantages over the RCC-SI. The FKSI-19 has more clarity in item phrasing, is shorter in length, and covers a similar breadth of disease-based symptoms when compared to the RCC-SI.
Keywords: Kidney Cancer, Renal Cell Carcinoma, measurement, patient-reported outcomes
Introduction
The American Cancer Society estimates that more than 54,000 people will receive a new diagnosis of kidney cancer and 13,000 will die of the disease in 20081. Until recently, biologic agents such as interleukin-2 (IL-2) or interferon-alpha were the first line of treatments for people with kidney cancer. However, these parenteral treatments are often associated with severe side effects such as fatigue and other constitutional symptoms, or hypotension in the case of IL-2. More recently, targeted oral therapies such as sorafenib and sunitinib have been approved for use by the Food and Drug and Administration. These agents have a different spectrum of treatment associated side effects2,3,4,5. Along with drugs that are currently in development, these recent advancements potentially offer improvement in length and quality of life2,6,7.
Disease specific patient reported outcomes, particularly those for symptom assessment, have become increasingly important in cancer treatment research8,9,10. Recent reports have suggested that symptom patterns reflect an underlying pathogenic process in which symptom burden can signal disease progression11,12,13. In addition, patient perspectives on symptom relevance and severity can differ widely from provider perspectives, signaling the need for valid methods of assessing symptoms from the patient’s perspective14,15,16,17. Furthermore, most cancer symptoms do not have biochemical markers or other objective tests to measure them, and thus, assessment of these symptoms primarily rely upon patient self-report. As such, providers are in need of targeted instruments that accurately assess patient self-reported symptom burden in order to determine treatment impact and evaluate clinical benefit.
Available kidney cancer symptom instruments
Recently, two patient questionnaires have been developed to assess the symptom burden of kidney cancer patients, the Renal Cell Carcinoma-Symptom Index (RCC-SI)18 and the Functional Assessment for Cancer Therapy – Kidney Symptom Index (FKSI)19,20. The questionnaires were developed at the same time using similar methodologies that incorporate information gathered from patients and expert clinicians to guide the content and wording of items.
The 15-item FKSI (FKSI-15) and its 9-item subset of disease related symptoms (FKSI-DRS), were developed by combining existing Functional Assessment for Cancer Therapy-General (FACT-G) items with new items derived from surveys of physician and nurse experts in kidney cancer at 17 National Comprehensive Cancer Network (NCCN) member institutions19,20,21. Furthermore, FACT-G items were developed as part of the Functional Assessment for Chronic Illness Therapy (FACIT) system of measurement22,23 using a standardized procedure in which patients and experts (3 patients for every 1 expert) nominated and prioritized important symptoms and concerns of each disease24. Items from the Functional Assessment for Chronic Illness Therapy (FACIT) measurement system have gone through content development and verification, been validated and translated into multiple languages, and construct validated in various cancer patient populations22. While the FACT-G and original FKSI items were all derived from patient input, we more recently sought to verify adequate coverage of items by soliciting open-ended input from advanced kidney cancer patients.
Concurrent with this effort, another group developed the 30-item RCC-SI18 using the FACIT measurement system model of scale development22,23. Harding and colleagues conducted a narrative literature review on the health-related quality of life of patients with Renal Cell Carcinoma (RCC), the most prevalent clinical sub-type of kidney cancer. Qualitative data from this review was used to develop a pilot index that would be used in obtaining patient and caregiver feedback. They then conducted structured interviews with 30 RCC patients and caregivers who were members of the Kidney Cancer Association. The pilot items were refined based on patient and caregiver feedback. Nurses and physicians with experience treating RCC patients subsequently evaluated the revised items. Finally, the study investigators incorporated expert information with the patient and caregiver-derived revised index, and the resulting instrument is the RCC-SI.
Our aim in the present paper is to describe the development of the revised FKSI scale (FKSI-19), reconcile its use with the RCC-SI, and provide recommendations on the best use of the revised FKSI instrument and the RCC-SI. In order to accomplish this goal, we qualitatively reviewed item wording and content coverage across the instruments and calculated basic psychometrics on the scales. Finally, we provided guidelines and recommendations for use of these similarly-constructed kidney cancer instruments.
Method
As part of a larger study25, fifty (50) people with stage 3 or 4 kidney cancer from Illinois (Northwestern University/Evanston Northwestern Healthcare, N=13) Washington (Fred Hutchinson Cancer Center, N=8) North Carolina (Duke University, N=19), and Florida (H. Lee Moffitt Cancer Center, N=10) were recruited into the scale development study between May 2005 and August 2006. Participants were asked to generate and rank up to 10 important symptoms and concerns that physicians should monitor when assessing the value of chemotherapy. In addition, patients were asked to select the five most important symptoms and concerns related to kidney cancer from a list of concerns derived from published quality of life measures. Finally, patients were able to list symptoms and health issues arising from their disease that did not appear on the provided list of questions. Participant responses to open-ended and structured questions were used to modify the original FKSI instrument. Open-ended responses provided qualitative information that was evaluated systematically. The responses were content analyzed, themes emerged from the data, and consensus on these themes was reached through independent expert judgment and group discussion26. When a patient-identified symptom or concern reflected a concept covered by an item in the FACIT system, the FACIT wording was used in the revised FKSI instrument. Where no FACIT item existed, additional items were written to cover the patient-identified symptoms/concerns. In addition to providing feedback on prominent symptoms and concerns, patients were asked to complete the Functional Assessment for Cancer Therapy-General (FACT-G), the RCC-SI, and the FKSI-15.
Qualitative and Quantitative Comparisons between the RCC-SI and FKSI-19
Qualitative comparisons between the RCC-SI and the FKSI-19 were made based on item wording, content, and domain areas measured by the two instruments. We compared items on both instruments using the same systematic qualitative analysis techniques described above (independent expert judgment and consensus discussions). Quantitative comparisons were made based on the questionnaires completed by participants (FACT-G, FKSI-15, and RCC-SI). We prorated total scores on the 17 items from the FACT-G and FKSI-15 that carried over to the revised FKSI in order to examine the means and standard deviations as they would appear on the 19-item version of the FKSI instrument. Consistent with FACIT scoring convention, scores were prorated by taking the mean score on each item and multiplying by 19, the number of items on the revised FKSI instrument.
We calculated descriptive statistics and internal consistency reliability for the FKSI-15, FKSI-DRS, and the RCC-SI, along with Spearman correlation coefficients that examined the relationships between the FKSI-15, FKSI-DRS, FKSI-19 (prorated) and the RCC-SI. In addition, several patient socio-demographic variables were captured through self-report (age, gender ethnicity, education, occupational status). We obtained patient and physician ratings of the patients’ performance status: normal activity with no symptoms (0), normal activity with some symptoms (1), bed rest required for less than half of the waking day (2), bed rest required for more than half of the waking day (3) and unable to get out of bed (4)27. All institutions provided IRB approval and all patients provided informed consent. All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
Fifty participants with advanced kidney cancer completed the RCC-SI and 17 items of the FKSI-19. Table 1 provides details on the sample characteristics. The mean age of participants was 59 years (SD = 10.5), and participants were predominantly White (94%). They were geographically dispersed, with North Carolina and Illinois contributing 38% and 26% of the cohort, respectively. Most patients were not working (62%), being either retired, unemployed, or on disability. Twenty-two percent of the patients rated their performance as ‘normal activity’, 44% as ‘some symptoms’, 26% as ‘require bed rest less than 50% of waking day’, and 8% as ‘requiring bed rest more than 50% of waking day’.
Table 1.
Sample Characteristics (Total N=50)
| N (%) | ||
|---|---|---|
| Sex | Male | 32 (64) |
| Race/Ethnicity | White | 47 (94) |
| Black/African-American | 3 (6) | |
| Education | Some high school or less | 3 (6) |
| High school graduate/GED | 14 (28) | |
| Vocational/some college | 11 (22) | |
| College degree | 13 (26) | |
| Professional/graduate degree | 9 (18) | |
| Occupational Status | Retired/Unemployed | 19 (38) |
| On disability | 12 (24) | |
| On leave of absence | 5 (10) | |
| Full time employed | 10 (20) | |
| Part time employed | 4 (8) | |
| Patient-Reported Performance Status | Normal Activity | 11 (22) |
| Some Symptoms | 22 (44) | |
| Bed rest > 50% of waking day | 13 (26) | |
| Bed rest < 50% of waking day | 4 (8) | |
| Region (State) | Illinois | 13 (26) |
| Florida | 10 (20) | |
| North Carolina | 19 (38) | |
| Washington | 8 (16) |
Participants provided rich information on important symptoms and concerns affecting people with kidney cancer. On the basis of this open-ended input from advanced kidney cancer patients, four items were added to the original FKSI-15 in order to create the FKSI-19. The additional items included: (1) “I feel weak all over;” (2) “I have nausea;” (3) “I have diarrhea;” and (4) “I am content with the quality of my life right now.” Like the FACT-G, the FKSI-DRS, FKSI-15, and FKSI-19 used five Likert-type response categories ranging from ‘not at all’ (0) to ‘very much’ (4). All items from the FKSI-15 were retained, and the same scoring procedure was used for the revised instrument such that once certain items were reverse scored, a higher score indicated better health-related quality of life.
Qualitative Item Comparison
The RCC-SI and FKSI-19 have many similarities. The RCC-SI and the FKSI-19 use the same five Likert-type response categories previously mentioned. . However, with 30 items the RCC-SI is a lengthier symptom measurement tool than the FKSI-19. Although both instruments measure the domains of pain, fatigue, and urinary/bowel symptoms, the RCC-SI has more items assessing symptoms within these domains. For example, the FKSI-19 has two items assessing pain “I have pain” and “I have bone pain”, and the RCC-SI has 4 items that assess pain “I have pain”, “I have pain in my back”, “I have discomfort or pain in my stomach area”, and “Pain interfered with my daily activities”.
The phrasing of items on the FKSI-19 may be somewhat clearer and unbiased than the phrasing used in the RCC-SI. On the FKSI-19, patients are asked simply about the presence of blood in the urine rather than if it bothered them, thus removing a potential coping or adjustment bias in responses. Furthermore, diarrhea is referred as such rather than “bowel control”, which may improve the item’s clarity and respondents’ interpretation of the item.
As Table 2 depicts, 8 of the 9 domains measured by the RCC-SI and the FKSI-19 contain items that use the exact or similar wording on the RCC-SI and FKSI-19. The one domain that differs is the domain with items that measure treatment side effects. Within this domain, the FKSI-19 measures treatment side effects more generally whereas the RCC-SI measures treatment side effects more specifically. For example, the FKSI-19 contains the items “I have nausea” and “I am bothered by side effects of treatment”. The RCC-SI contains more specifically worded items such as “I feel lightheaded”, “I have difficulty remembering things”, and “I have trouble concentrating”.
Table 2.
Domain comparison of item wording
| DOMAIN | RCC-SI Items | FKSI-19 Items |
|---|---|---|
| Pain | I have pain | I have pain |
| I have discomfort or pain in my stomach area | I have bone pain | |
| Pain interfered with my daily activities | ||
| I have pain in my back | ||
| Fatigue | I have a lack of energy | I have a lack of energy |
| I feel fatigued | I feel fatigued | |
| I have had trouble sleeping | I am sleeping well | |
| I have felt weak | I feel weak all over | |
| I feel tired | ||
| I have trouble starting things because I am tired | ||
| I have trouble finishing things because I am tired | ||
| Pulmonary Symptoms | I have been short of breath | I have been short of breath |
| I have been coughing | ||
| Bowel/Bladder Symptoms | I am bothered by blood in my urine | I have had blood in my urine |
| I have control of my bowels | I have diarrhea | |
| I have trouble moving my bowels | ||
| I urinate more frequently than usual | ||
| I have difficulty urinating | ||
| Nutritional Health | I am losing weight | I am losing weight |
| I have a good appetite | I have a good appetite | |
| I have lacked appetite | ||
| Psychosocial Functioning | I worry that my condition will get worse | I worry that my condition will get worse |
| I am able to enjoy life | I am able to enjoy life | |
| I have emotional ups and downs | I am able to work (include work at home) | |
| I feel depressed | I am content with the quality of my life right now | |
| Treatment Side Effects | (Neurologic symptoms) I feel lightheaded | I have nausea |
| (Cognitive symptoms)I have difficulty remembering things | I am bothered by side effects of treatment | |
| (Cognitive symptoms) I have trouble concentrating | ||
| (Flu-like symptoms) I have had fevers | (Flu-like symptoms) I am bothered by fevers | |
| (Flu-like symptoms) I have had chills | ||
| (Flu-like symptoms) I have had sweats |
Bolded text indicates exact wording
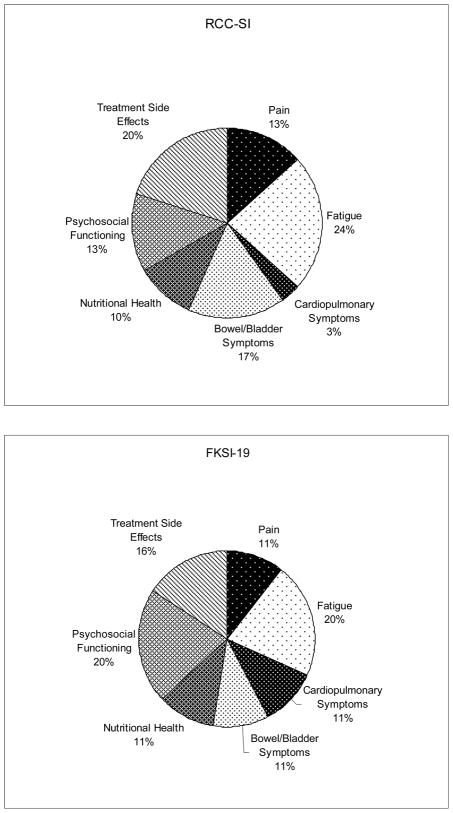
Despite these differences, the percentages of items that measure each domain for the RCC-SI and the FKSI-19 are similar (Figure 1). For example, 13% of the items on the RCC-SI measure symptoms of pain, whereas 11% of the items on the FKSI-19 measure symptoms of pain. Along the same lines, 22% of the items on the RCC-SI measure symptoms of fatigue and 22% of the items on the FKSI-19 measure symptoms of fatigue. One important difference in domain content on the two instruments is that 22% of the FKSI-19 items measure the psychosocial functioning domain, while only 13% of items on the RCC-SI measure these symptoms. Consequently, the FKSI-19 focuses more on assessing the psychosocial concerns of patients with kidney cancer than the RCC-SI.
Figure 1.
Percentage of scale items within each domain for the RCC-SI and the FKSI-19.
Quantitative Item Comparison
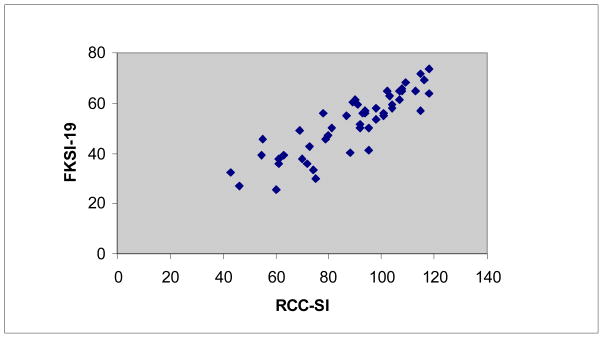
The means, standard deviations, and Cronbach’s alpha for the four instruments analyzed are listed in Table 3. All three Spearman rho correlations calculated between the RCC-SI and the FKSI-19 (rho = 0.88, p<0.001), FKSI-15 (rho = 0.89, p < 0.001), and FKSI-DRS (rho = 0.84, p < 0.001) were statistically significant. The scatterplot depicting participants’ scoring patterns on the RCC-SI and the FKSI-19 is shown in Figure 2. Scatterplots of scoring patterns on the RCC-SI with the FKSI-15 and FKSI-DRS are not shown, but appeared similar to the scatterplot shown in Figure 2.
Table 3.
Means, Standard Deviations, and Cronbach’s alpha for total scores on the FKSI-19, FKSI-15, FKSI-DRS, and the RCC-SI.
| Cronbach’s Alpha | Mean Total Score | Standard Deviation | |
|---|---|---|---|
| FKSI-191 | 0.86 | 51.8 | 12.2 |
| FKSI-15 | 0.84 | 40.9 | 9.8 |
| FKSI-DRS (9 items) | 0.78 | 26.2 | 6.0 |
| RCC-SI (30 items) | 0.92 | 88.4 | 19.7 |
Cronbach’s alpha was based on 17 items of the FKSI-19, and FKSI-19 means were derived by prorating responses to the 17 administered items.
Figure 2.
Scatterplot depicting the relationship between participants’ responses on the RCC-SI and the FKSI-19 (Spearman rho = 0.88).
Discussion
The FKSI and RCC-SI are both instruments that assess symptoms important to patients with kidney cancer. Of note, the scales used a similar methodology to derive candidate items. In fact, the FKSI and the RCC-SI address similar symptom domains, and in many cases, both instruments use items of exact or similar wording. The correlations between the instruments suggest that the shared variance is on the order of 80% or higher, indicating that the FKSI and the RCC-SI capture much of the same information from patients.
However, there are differences between the scales that may guide a preference for using one instrument over the other in future studies of kidney cancer symptoms. For instance, the FKSI-19 is highly correlated with RCC-SI, but the FKSI-19 is nearly half the length of the RCC-SI. Therefore, use of the FKSI-19 may capture relevant information while reducing patient burden and the amount of time needed to administer the questionnaire. With brevity, of course, comes a potential reduction in the information provided by the FKSI. Therefore, researchers requiring more depth of information may benefit from using the RCC-SI. However, the clear and unbiased phrasing of certain items in the FKSI-19 may be preferable to the phrasing used in the RCC-SI, particularly regarding hematuria and especially diarrhea as this adverse event is commonly observed with the new oral multi-kinase inhibitors.
On both the RCC-SI and FKSI-19, multiple items were used to define each symptom domain, and as such, a weighting scheme that characterizes the importance of each domain is implied. Unfortunately, there is little empirical evidence to guide instrument construction with regard to weighting schemes for instruments such as the FKSI and RCC-SI. For most domains assessed by these instruments, this is not an issue. However, the FKSI places approximately 10% more importance on psychosocial functioning than does the RCC-SI. In a separate study, we found that psychosocial functioning was a “top 5” concern among a group of advanced kidney cancer patients28. These findings guided our construction of the current instrument and the relative important of the psychosocial functioning domain.
Our study had some limitations. First, we did not collect detailed clinical information on participants other than the site of their cancer and their performance status. Therefore, we could not determine how many patients within our sample had been diagnosed with renal cell carcinoma and how many had been diagnosed with other renal tumors (such as renal pelvic urothelial cancer), although >80% of kidney cancers are considered to be parenchymal renal cell cancers1. Similarly, subset analysis by disease stage could not be conducted and concomitant medication use was not captured. Although the participants were well-distributed in terms of geography and education, the sample lacked ethnic/racial diversity, and thus caution should be taken in applying the results of the present study to populations that are more heterogeneous with respect to ethnicity and race.
Given these limitations, we found considerable conceptual and statistical overlap between the FKSI-19 and the RCC-SI. Several features of the FKSI-19 provide an advantage over the RCC-SI in clinical and observational research. Its breadth in disease-based symptom coverage, clarity in the phrasing of certain symptoms questions, and brief length will be beneficial in both research and practice.
Acknowledgments
The authors would like to thank the study participants for their participation in this study and Jennifer Beaumont for her assistance with the statistical analysis for this manuscript.
References
- 1.American Cancer Society. Kidney Cancer (Adult) - Renal Cell Carcinoma. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 2.Bukowski R, Cella D, Gondek K, Escudier B. Effects of sorafenib on symptoms and quality of life: results from a large randomized placebo-controlled study in renal cancer. American Journal of Clinical Oncology. 2007;30:220–227. doi: 10.1097/01.coc.0000258732.80710.05. [DOI] [PubMed] [Google Scholar]
- 3.Dhanda R, Gondek K, Song J, Cella D, Bukowski R, Escudier B. A comparison of quality of life and symptoms in kidney cancer patients receiving sorafenib versus placebo. Journal of Clinical Oncology. 2006;24(18S):225s. [Google Scholar]
- 4.Cella D, Cappelleri JC, Bushmakin A, Kim ST, Chen I, Motzer RJ. Effects of sunitinib versus interferon-alfa in health-related quality of life in patients with metastatic renal cell carcinoma (MRCC): results from a randomized multinational phase III trial. European Urology Supplement. 2007;6(2):238. [Google Scholar]
- 5.Cella D, Li J, Cappelleri JC, Bushmakin A, Charbonneau C, Kim S, et al. Quality of life (QOL) predicts for progression-free survival (PFS) in patients with metastatic renal cell carcinoma (mRCC) treated with sunitnib compared to interfereon-alpha (IFN) Journal of Clincial Oncology. 2007;25(18S):345S. [Google Scholar]
- 6.Eisen T, Bukowski R, Staehler S, et al. Randomized phase III trial of sorafenib in advanced renal cell carcinoma (RCC): impact of crossover on survival. Journal of Clinical Oncology. 2006;24 (Supplement 18):4524. [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New England Journal of Medicine. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Williams G, Pazdur R, Temple R. Assessing tumor-related signs and symptoms to support cancer drug approval. Journal of Biopharmaceutical Statistics. 2004;14:5–21. doi: 10.1081/BIP-120028503. [DOI] [PubMed] [Google Scholar]
- 9.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. National Cancer Institute Monograph. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 10.Illman J, Corringham R, Robinson D, Davis H, Rossi J-F, Cella D, Trikha M. Are inflammatory cytokines the common link between cancer-associated cachexia and depression? Journal of Supportive Oncology. 2005;3(1):37–50. [PubMed] [Google Scholar]
- 11.Kim HL, Belldegrun AS, Freitas DG, Bui MH, Han KR, Dorey FJ, et al. Paraneoplastic signs and symptoms of renal cell carcinoma: Implications for prognosis. Journal of Urology. 2003;170:1742–1746. doi: 10.1097/01.ju.0000092764.81308.6a. [DOI] [PubMed] [Google Scholar]
- 12.Cleeland C, Gennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 13.Victorson D, Soni M, Cella D. Metaanalysis of the correlation between radiographic tumor response and patient-reported outcomes. Cancer. 2006;106:494–504. doi: 10.1002/cncr.21637. [DOI] [PubMed] [Google Scholar]
- 14.Brunelli C, Costantini M, Di Giulio P, Gallucci M, Fusco F, Miccinesi G, et al. Quality-of-life evaluation: When do terminal cancer patients and health- care providers agree? Journal of Pain and Symptom Management. 1998;15:151–158. doi: 10.1016/s0885-3924(97)00351-5. [DOI] [PubMed] [Google Scholar]
- 15.Stromgren AS, Groenvold M, Pedersen L, Olsen AK, Spile M. Does the medical record cover the symptoms experienced by cancer patients receiving palliative care? A comparison of the record and patient self-rating. Journal of Pain and Symptom Management. 2001;21:189–196. doi: 10.1016/s0885-3924(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 16.Lampic C, von Essen L, Peterson VW, Larsson G, Sjoden PO. Anxiety and depression in hospitalized patients with cancer: Agreement in patient-staff dyads. Cancer Nursing. 1996;19:419–428. doi: 10.1097/00002820-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists’ recognition of depression in their patients with cancer. Journal of Clinical Oncology. 1998;16:1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- 18.Harding G, Cella D, Robinson D, Jr, Mahadevia PJ, Clark J, Revicki DA. Symptom burden among patients with renal cell carcinoma (RCC): content for a symptom index. Health and Quality of Life Outcomes. 2007;5:34. doi: 10.1186/1477-7525-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella D, Yount S, Du H, Dhanda R, Gondek K, Langefeld K, et al. Development and validation of the Functional Assessment of Cancer Therapy - Kidney Symptom Index (FKSI) Supportive Oncology. 2006;4:191–199. [PubMed] [Google Scholar]
- 20.Cella D, Yount S, Brucker PS, Du H, Bukowski R, Vogelzang N, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value in Health. 2007;10:285–293. doi: 10.1111/j.1524-4733.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- 21.Cella D, Paul D, Yount S, Winn R, Chang CH, Banik D, et al. What are the most important symptom targets when treating advanced cancer? A survey of providers in the National Comprehensive Cancer Network (NCCN) Cancer Investigation. 2003;21:526–535. doi: 10.1081/cnv-120022366. [DOI] [PubMed] [Google Scholar]
- 22.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health and Quality of Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella D, Nowinski C. Measuring quality of life in chronic illness: the functional assessment of chronic illness therapy measurement system. Archives of Physical Medicine and Rehabilitation. 2002;83 (12 Supplement 2):S10–S17. doi: 10.1053/apmr.2002.36959. [DOI] [PubMed] [Google Scholar]
- 24.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbloom SK, Yount SE, Yost KJ, Hampton D, Paul D, Abernethy AP, Jacobsen PB, Syrjala K, Von Roenn JH, Cella D. Development and Validation of Eleven Symptom Indices to Evaluate Response to Chemotherapy for Advanced Cancer: Measurement Compliance with Regulatory Demands. In: Farquhar I, Summers K, Sorkin A, editors. The Value of Innovation: Impacts on Health, Life Quality, and Regulatory Research. Oxford: Elsevier; 2007. [Google Scholar]
- 26.Ryan G, Bernard HR. Data Management and Analysis Methods. In: Denzin N, Lincoln Y, editors. Handbook of Qualitative Research. 2. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- 27.Zubrod C, Schneiderman M, Frei E, Brindley C, Gold GL, Shnider B, et al. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene. Journal of Chronic Disease. 1960;11:7–33. [Google Scholar]
- 28.Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala K, Von Roenn JH, Cella D. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. Journal of the National Comprehensive Cancer Network. doi: 10.6004/jnccn.2008.0036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]