Abstract
The importance of identifying VEGF-independent pathways in pathological angiogenesis is increasingly recognized as a result of the emerging drug resistance to anti-VEGF therapies. PDGF-CC is the third member of the PDGF family discovered after more than two decades of studies on PDGF-AA and PDGF-BB. The biological function of PDGF-CC and the underlying cellular and molecular mechanisms remain largely unexplored. Here, using different animal models, we report that PDGF-CC inhibition by neutralizing antibody, shRNA, or genetic deletion suppressed both choroidal and retinal neovascularization. Importantly, we revealed that PDGF-CC targeting acted not only on multiple cell types important for pathological angiogenesis, such as vascular mural and endothelial cells, macrophages, choroidal fibroblasts and retinal pigment epithelial cells, but also on the expression of other important angiogenic genes, such as PDGF-BB and PDGF receptors. At a molecular level, we found that PDGF-CC regulated glycogen synthase kinase (GSK)–3β phosphorylation and expression both in vitro and in vivo. Activation of GSK3β impaired PDGF-CC–induced angiogenesis, and inhibition of GSK3β abolished the antiangiogenic effect of PDGF-CC blockade. Thus, we identified PDGF-CC as an important candidate target gene for antiangiogenic therapy, and PDGF-CC inhibition may be of therapeutic value in treating neovascular diseases.
Keywords: choroidal neovascularization, glycogen synthase kinase-3β, vascular biology, retinal neovascularization, ocular disease
Antiangiogenic therapy has shown great therapeutic efficacy. However, the clinical needs are still unmet and many problems remain. One difficulty in antiangiogenic therapy is the selective up-regulation of other angiogenic factors leading to resistance to anti-VEGF therapy (1). Another issue is that mature blood vessels covered by pericytes (PCs) or smooth muscle cells (SMCs) are more resistant to antiangiogenic therapy and difficult to prune (2). Antiangiogenic strategies targeting not only vascular endothelial cells (ECs), but also PCs, SMCs and other proangiogenic cells are therefore desired. In addition, emerging drug resistance (3) and side effects (4) have also become challenging obstacles to overcome. Thus, the identification additional pleiotropic factors affecting multiple vascular and nonvascular cell types important for pathological angiogenesis, together with a better understanding of the molecular and cellular mechanisms, is warranted.
Neovascular age-related macular degeneration (AMD) is a major cause of blindness in aged population in the Western society as a result of outgrowth of new blood vessels from the choroid, if uncontrolled, resulting in retinal detachment and vision loss. Current anti-VEGF therapies can halt vision loss in some patients with AMD. However, not all patients with neovascular AMD respond to the currently available treatments, implicating the existence of other angiogenic factors involved. Retinopathy of prematurity (ROP), one of the most common causes of vision loss in childhood, is a condition caused primarily by the overgrowth of abnormal blood vessels throughout the retina. Current treatments for ROP include laser therapy and cryotherapy, both of which destroy the side vision to a certain extent, and not all patients with ROP respond to such treatments. Thus, more effective antineovascularization therapies are still needed.
PDGF-CC was discovered (5, 6) after many years of intensive studies on PDGF-AA and PDGF-BB. The biological function of PDGF-CC remains largely unexplored. PDGF-CC protein is produced as a secreted homodimer and needs to be proteolytically processed for receptor binding (5). PDGF-CC binds to and activates PDGF receptor (PDGFR)–α (5). When PDGFR-β is coexpressed with PDGFR-α in the same cells, it can also be activated by PDGF-CC (7–9). PDGF-CC is critically required for embryonic development, as PDGF-CC–deficient mice die postnatally as a result of developmental defects when the mice are bred on a 129 background (10). It is recently shown that PDGF-CC induces blood–brain barrier permeability during ischemic stroke (11, 12). PDGF-CC is abundantly expressed in the eye (13) and induces proliferation and migration of retinal pigment epithelial (RPE) cells (14). Furthermore, PDGF-CC plays important roles in recruiting fibroblasts associated with drug resistance in tumors (15–17). In addition, PDGF-CC induces monocyte migration and up-regulates matrix metalloproteinase (MMP)–2 and MMP-9 expression (18). Thus, accumulating data have suggested important roles of PDGF-CC in pathological angiogenesis. However, this hypothesis has not been well studied thus far.
In this work, by using comprehensive approaches and different animal models, we found that PDGF-CC is an important player in neovessel formation by affecting multiple cellular and molecular targets and by regulation of glycogen synthase kinase (GSK)–3β phosphorylation. PDGF-CC targeting inhibited both choroidal and retinal angiogenesis. PDGF-CC inhibition might offer new therapeutic options to treat neovascular diseases.
Results
Increased PDGF-CC Expression in Choroidal Neovascularization.
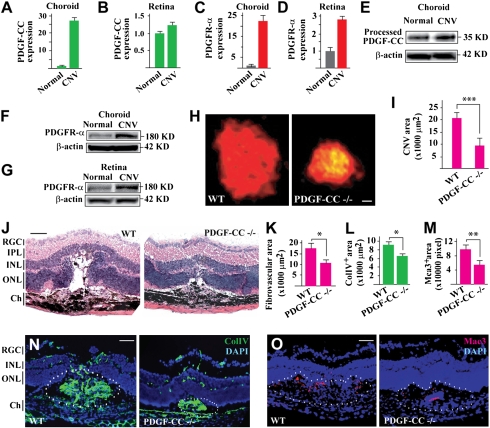
The potential role of PDGF-CC in choroidal neovascularization (CNV) is thus far unknown. Real-time PCR showed up-regulated expression of PDGF-CC and PDGFR-α in both choroids and retinas 7 d after laser-induced CNV (Fig. 1 A–D; n = 8). The up-regulated expression of PDGF-CC and PDGFR-α in CNV was confirmed by Western blot analysis (Fig. 1 E–G). Immunofluorescence staining showed abundant PDGF-CC expression within the CNV area (Fig. S1A Center, lined area), whereas PDGF-CC was detected mainly in the RPE cells in normal retina (Fig. S1A Left). In addition, Western blot detected PDGF-CC expression in different types of choroidal and retinal cells, i.e., mouse choroidal fibroblasts (mCF), rat retinal endothelial cells (TR-iBRB), rat retinal ganglion cell (RGC)–derived cell line (RGC5), rat retinal pericytes (TR-rPCT), and human fetal retinal pigment cells (hRPE; Fig. S1B). The elevated expression of PDGF-CC and PDGFR-α following CNV induction suggested a role of PDGF-CC in CNV.
Fig. 1.
Up-regulated PDGF-CC expression in CNV and reduced CNV formation in PDGF-CC–deficient mice. (A–D) Real-time PCR showed up-regulated expression of PDGF-CC and PDGFR-α in choroids and retinas 7 d after laser-induced CNV. Arbitrary unit was used for gene expression with the normal controls set to 1. (E–G) Western blot confirmed the up-regulated expression of PDGF-CC and PDGFR-α in CNV. (H and I) PDGF-CC deficiency reduced CNV area to approximately 47% of control 7 d after laser treatment as measured by IB4 staining. (J and K) Histological analysis showed less edema formation in the CNV lesions in PDGF-CC–deficient mice as indicated by the reduced empty space around the CNVs. PDGF-CC deficiency also reduced fibrovascular formation. (L and N) PDGF-CC deficiency decreased ColIV+ (vascular marker) areas within the CNV lesions. (M and O) PDGF-CC deficiency decreased Mac3+ (macrophage marker) areas following CNV induction. Blue color in N and O indicates nuclei stained by DAPI. IPL, inner plexiform layer; INL/ONL, inner/outer nuclear layer; Ch, choroid. (Scale bars: 250 μm in H; 50 μm in J, N, and O.) *P < 0.05, **P < 0.01, ***P < 0.001.
PDGF-CC Deficiency Reduced CNV Formation.
We next tested the potential role of PDGF-CC in CNV formation by using PDGF-CC–deficient mice and a laser-induced CNV model. PDGF-CC–deficient mice die at postnatal d 1 on a 129S1 background (10). However, when bred on a C57Bl6 background, these mice are largely healthy without obvious abnormalities. Histological analysis did not show any obvious morphological difference between PDGF-CC–deficient and WT retinas and choroids (Fig. S2). PDGF-CC deficiency reduced CNV area to approximately 47% of the control group 7 d after laser treatment as measured by isolectin B4 (IB4) staining (Fig. 1 H and I; n = 8, P < 0.001). Histological analysis showed less edema formation within the CNVs in PDGF-CC–deficient mice as indicated by the reduced empty space around the CNVs (Fig. 1J). PDGF-CC deficiency also reduced fibrovascular formation (Fig. 1 J and K; n = 7, P < 0.05) and ColIV+ areas within the CNVs (Fig. 1 L and N; n = 8, P < 0.05), which are common indicators of blood vessel presence. Furthermore, PDGF-CC deficiency decreased Mac3+ (macrophage marker) areas following CNV induction (Fig. 1 M and O; n = 8, P < 0.01), indicating reduced inflammation. Thus, PDGF-CC deficiency reduced CNV formation.
PDGF-CC Blockade Inhibited CNV Formation and Blood Vessel Leakage.
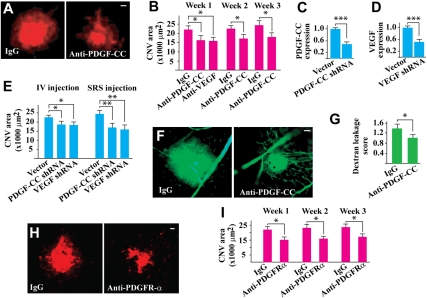
We subsequently tested whether PDGF-CC targeting by neutralizing antibody or shRNA could inhibit CNV. One single dose of intravitreal injection of PDGF-CC–neutralizing antibody (2 μg per eye) after laser treatment reduced CNV areas at different time points with a comparable effect of VEGF neutralizing antibody (Fig. 2 A and B; n = 8, P < 0.05). Further, treatment with PDGF-CC shRNA, which reduced the PDGF-CC expression level to approximately 40% of normal level (Fig. 2C; n = 8, P < 0.001), reduced CNV area 7 d after laser treatment when injected into vitreous or subretinal space (Fig. 2E; n = 8, P < 0.05 or P < 0.01). The effect of PDGF-CC shRNA was comparable to that of VEGF shRNA (Fig. 2E; n = 8, P < 0.05 or P < 0.01), which reduced VEGF expression to approximately 40% of normal level (Fig. 2D; n = 8, P < 0.001). Moreover, fluorescein angiography showed that PDGF-CC–neutralizing antibody treatment decreased blood vessel leakage in the CNVs 1 wk after laser treatment (Fig. 2 F and G; n = 7, P < 0.05). Histological analysis showed that treatment with PDGF-CC–neutralizing antibody decreased fibrovascular tissue formation (Fig. S3 A and B; n = 8, P < 0.05), α-smooth muscle cell actin (SMA; vascular smooth muscle cell marker)–positive areas (SMA+, Fig. S3 C and D; n = 7, P < 0.01), and Mac3 (macrophage marker)–positive areas (Fig. S3 E and F; n = 8, P < 0.01). In addition, intravitreal injection of PDGFR-α–neutralizing antibody decreased CNV areas at different time points after laser treatment (Fig. 2 H and I; n = 7, P < 0.05). It is noteworthy that the inhibitory effect of PDGFR-α–neutralizing antibody on CNV formation was similar to that of PDGF-CC–neutralizing antibody (Fig. 2B; n = 8, P < 0.05), indicating that PDGF-CC is a major ligand of PDGFR-α that plays an important role in CNV formation.
Fig. 2.
PDGF-CC targeting inhibited CNV formation and blood vessel leakage. (A and B) PDGF-CC–neutralizing antibody treatment reduced CNV areas at different time points with a comparable effect of a VEGF-neutralizing antibody. (C–E) Treatment with PDGF-CC shRNA, which reduced PDGF-CC expression level to approximately 40% of normal level (C), reduced CNV areas 7 d after laser treatment when injected into vitreous (IV) or subretinal space (SRS) (E). The effect of the PDGF-CC shRNA was comparable to that of a VEGF shRNA (E), which reduced VEGF expression level to approximately 40% of normal level (D). (F and G) Fluorescein angiography showed reduced blood vessel leakage in the PDGF-CC–neutralizing antibody–treated CNVs 1 wk after laser treatment. (H and I) Intravitreal injection of PDGFR-α–neutralizing antibody decreased CNV areas at different time points after laser treatment. The inhibitory effect of PDGFR-α–neutralizing antibody was similar to that of PDGF-CC–neutralizing antibody. (Scale bar: 50 μm in A, F, and H.) *P < 0.05, **P < 0.01, ***P < 0.001.
PDGF-CC Regulated Expression of Proangiogenic and Proapoptotic Genes.
We next investigated the genes regulated by PDGF-CC. In cultured primary human macrophages and RPE cells, which play important roles in CNV, PDGF-CC protein treatment up-regulated the expression of several important proangiogenic genes, such as PDGF-BB, PDGFR-α, and PDGFR-β, as measured by real-time PCR (Fig. S4 A and B). PDGF-BB is one of the most potent stimuli of pathological angiogenesis (19). The up-regulated expression of these genes was confirmed by Western blot assays (Fig. S4 D and E). Indeed, intravitreal injection of PDGF-CC neutralizing antibody inhibited their expression in the retinas with CNV in vivo as measured by real-time PCR (Fig. S4C). Moreover, intravitreal injection of PDGF-CC–neutralizing antibody up-regulated the expression of TNF-α, an inhibitor of PDGF/PDGFR–induced cell proliferation, survival, and migration, and also the expression of Bmf, a potent apoptosis inducer, in both choroids and retinas with CNV (Fig. S4 F and G). On the contrary, PDGF-CC protein treatment inhibited their expression in cultured human umbilical vein smooth muscle cells (HUVSMCs; Fig. S4H). Furthermore, PDGF-CC deficiency down-regulated the expression of PDGF-D, PDGFR-β, PDGF-A, and PDGF-B in mouse choroids (Fig. S4I; n = 3), and down-regulated the expression of PDGF-B and PDGF-A in the retinas (Fig. S4J; n = 3), similar to what was observed in the PDGF-CC–neutralizing antibody–treated samples (Fig. S4C).
PDGF-CC Deficiency and Blockade Reduced Retinal Neovascularization.
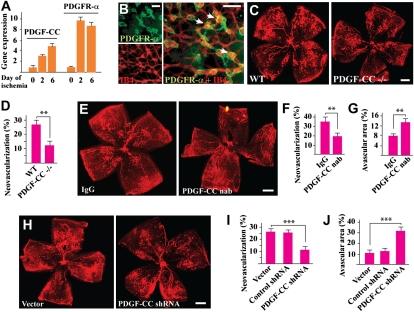
It is thus far unknown whether PDGF-CC plays a role in retinal neovascularization. Real-time PCR showed up-regulated expression of PDGF-CC and PDGFR-α in the neovascular retinae in an ROP mouse model (Fig. 3A; n = 7). Immunofluorescence staining detected PDGFR-α expression on the neovessels in the retinas, with a higher expression level in the neovascular tufts (Fig. 3B, arrows). PDGF-CC deficiency reduced retinal neovascularization 5 d after retinal ischemia (Fig. 3 C and D; n = 8, P < 0.01), demonstrating a role of PDGF-CC in retinal neovascularization. Intravitreal injection of PDGF-CC–neutralizing antibody (2 μg per eye) inhibited retinal neovascularization 5 d after injection (Fig. 3 E and F; n = 8, P < 0.01), leading to larger avascular areas in the retinas (Fig. 3 E and G; n = 8, P < 0.01). Intravitreal injection of PDGF-CC shRNA (1 μg per eye) also inhibited retinal neovascularization (Fig. 3 H and I; n = 8, P < 0.001), leading to greater avascular areas in the retinas (Fig. 3 H and J; n = 8, P < 0.001). Moreover, real-time PCR showed decreased expression of many proangiogenic genes in the PDGF-CC shRNA-treated neovascular retinas (Fig. S5A; n = 7), and increased expression of many proapoptotic genes (Fig. S5B; n = 7). The expression of many proapoptotic genes was also increased in the PDGF-CC–neutralizing antibody–treated neovascular retinas (Fig. S5C; n = 8).
Fig. 3.
PDGF-CC deficiency and blockades inhibited ischemia-induced retinal neovascularization. (A) Real-time PCR showed up-regulated expression of PDGF-CC and PDGFR-α during retinal ischemia. Arbitrary unit was used for gene expression with the d 0 control set to 1. (B) Immunofluorescence staining showed PDGFR-α expression by the neovessels in the retina, with a higher expression level in the neovascular tufts. (C and D) PDGF-CC deficiency reduced retinal neovascularization 5 d after retinal ischemia. (E–G) PDGF-CC nAb intravitreal injection inhibited retinal neovascularization (E and F), leading to greater avascular areas in the retinas (E and G). (H–J) Intravitreal injection of PDGF-CC shRNA decreased retinal neovascularization (H and I), leading to greater avascular areas in the retinas (H and J). (Scale bars: 100 μm in B; 500 μm in C, E, and H.) ** P < 0.01, *** P < 0.001.
PDGF-CC Promoted Proliferation, Survival, and Migration of Multiple Cell Types.
To investigate the cellular targets of PDGF-CC, we tested its effect on different cell types. PDGF-CC protein (50 ng/mL) increased proliferation/survival of TR-rPCT, TR-iBRB, and mCF fibroblasts at different time points (Fig. S6 A–C; n = 5, P < 0.05, P < 0.01, or P < 0.001). In a monolayer cell migration assay, PDGF-CC promoted TR-rPCT cell migration at different concentrations (Fig. S6 D and E; n = 7, P < 0.01 or P < 0.001). PDGF-CC thus displayed pleiotropic effects on multiple cell types. Moreover, in a chick chorioallantoic membrane (CAM) assay, administration of two different PDGF-CC–neutralizing antibodies from goat or mouse (gAnti-PDGF-CC and mAnti-PDGF-CC, respectively, 500 ng per /egg) reduced blood vessel branch points 2 d after administration (Fig. S6 F and G; n = 8–10, P < 0.01).
PDGF-CC Activated PDGFR-α and Akt and Regulated GSK3β Phosphorylation and Expression.
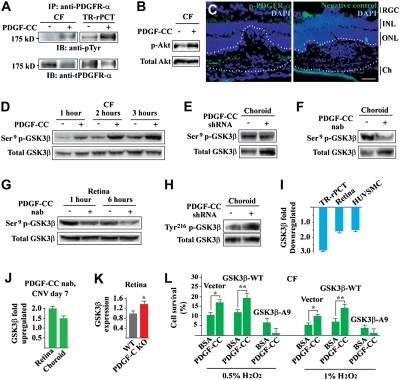
Little is known about the PDGF-CC–induced signaling pathway in ocular cells thus far. Immunoprecipitation (IP) followed by immunoblot (IB) detected PDGFR-α activation in choroidal fibroblasts (CFs) and TR-rPCTs induced by PDGF-CC (Fig. 4A). PDGF-CC also induced Akt activation in the CFs (Fig. 4B). Immunofluorescence staining detected phosphorylated PDGFR-α (p-PDGFR-α) within the CNV area (Fig. 4C, lines). Moreover, PDGF-CC protein stimulation led to GSK3β serine9 (Ser9) phosphorylation in the CFs in a time-dependent manner (Fig. 4D). This observation was confirmed by loss-of-function assays with both PDGF-CC shRNA and neutralizing antibody (nAb). Intravitreal injection of PDGF-CC shRNA or neutralizing antibody decreased GSK3β Ser9 phosphorylation in choroids and retinas (Fig. 4 E–G), and increased tyrosine216 (Tyr216) phosphorylation in the choroids in vivo (Fig. 4H). In addition, PDGF-CC protein stimulation inhibited GSK3β expression in different vascular cells in vitro (e.g., TR-rPCTs, HUVSMCs), and in the retinas in vivo (Fig. 4I). On the contrary, PDGF-CC–neutralizing antibody treatment increased GSK3β expression in the retinas and choroids with CNV in vivo (Fig. 4J). Indeed, real-time PCR showed that GSK3β expression level was approximately 35% higher in the PDGF-CC–deficient retinas than in normal control (Fig. 4K). Furthermore, PDGF-CC deficiency decreased GSK3β Ser9 phosphorylation in PDGF-CC-null hearts (Fig. S8 A–B). These data thus indicated a role of GSK3β in the PDGF-CC–induced signaling pathway in ocular and other types of cells.
Fig. 4.
PDGF-CC activated PDGFR-α, Akt, regulated GSK3β phosphorylation/expression, and GSK3β Ser9 phosphorylation was required for the survival effect of PDGF-CC. (A and B) IP followed by IB showed that PDGF-CC protein stimulation activated PDGFR-α in mCFs and TR-rPCTs (A) and activated Akt in mCFs (B). (C) Immunofluorescence staining detected p-PDGFR-α (green) within the CNV area (lined). Blue color indicates nuclei stained by DAPI. IPL, inner plexiform layer; ONL, outer nuclear layer; Ch, choroid. (Scale bar: 50 μm.) (D) PDGF-CC protein increased Ser9 GSK3β phosphorylation in mCFs in a time-dependent manner. (E and F) PDGF-CC shRNA (E) and nAb (F) treatment reduced GSK3β Ser9 phosphorylation in the choroids in vivo. (G) PDGF-CC–neutralizing antibody treatment reduced GSK3β Ser9 phosphorylation in the mouse retina in vivo. (H) PDGF-CC shRNA treatment increased GSK3β Tyr216 phosphorylation in mouse choroids in vivo. (I) PDGF-CC protein treatment inhibited GSK3β expression in cultured vascular cells in vitro (e.g., TR-rPCT, HUVSMC) and in the retina in vivo as measured by real-time PCR. (J) PDGF-CC–neutralizing antibody treatment increased GSK3β expression in choroids and retinas with CNV as measured by real-time PCR. (K) Real-time PCR showed that GSK3β expression level was approximately 35% higher in the PDGF-CC–deficient retinas than that of normal control. (L) PDGF-CC protein treatment protected CFs from H2O2-induced cell death in the WT GSK3β– and vector-transfected cells. The survival effect of PDGF-CC diminished in the GSK3β-A9–transfected cells. *P < 0.05, **P < 0.01.
GSK3β Serine9 Phosphorylation Was Required for the Survival Effect of PDGF-CC.
It is known that inhibition of GSK3β activity by Ser9 phosphorylation or down-regulation of its expression promotes cell survival. To verify the role of GSK3β in PDGF-CC function, we expressed a mutant form of human GSK3β in which the Ser9 was mutated to alanine (GSK3β-A9), thus lacking the ability of Ser9 phosphorylation, in CFs. The WT GSK3β (GSK3β-WT)– and vector-transfected cells were used as controls. PDGF-CC protein (100 ng/mL) protected CFs from H2O2-induced cell death in the GSK3β-WT– and vector-transfected cells (Fig. 4L; n = 6, P < 0.01 or 0.05). However, in the GSK3β-A9–transfected cells, the protective effect of PDGF-CC was abolished (Fig. 4L; n = 6, P < 0.01 or P < 0.05), demonstrating that GSK3β Ser9 phosphorylation was required for the survival effect of PDGF-CC.
GSK3β Activation Impaired PDGF-CC–Induced Angiogenesis.
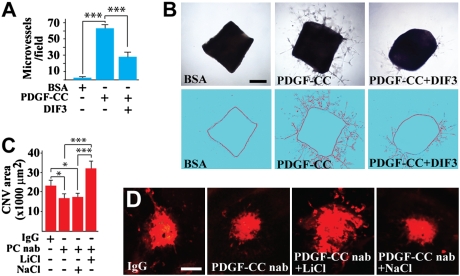
To further investigate the involvement of GSK3β in PDGF-CC–induced angiogenesis, we used a GSK3β activator, the differentiation-inducing factor–3 (DIF-3), in combination with a mouse aortic ring assay. PDGF-CC protein induced vascular cell proliferation, migration, and microvessel formation in the aortic ring assay (Fig. 5 A and B; n = 10, P < 0.001). Cotreatment of the aortic rings with the GSK3β activator DIF3 impaired PDGF-CC–induced microvessel formation (Fig. 5 A and B; n = 10, P < 0.001), demonstrating that inhibition of GSK3β activity was required for PDGF-CC–induced angiogenesis.
Fig. 5.
Involvement of GSK3β in PDGF-CC–induced angiogenesis and in the antiangiogenic effect of PDGF-CC targeting. (A and B) PDGF-CC protein induced vascular cell proliferation, migration, and microvessel formation in the aortic ring assay (B, Middle). Cotreatment of the aortic rings with the GSK3β activator DIF3 impaired PDGF-CC–induced microvessel formation (B, Right). (Scale bar: 500 μm.) (C and D) Intravitreal injection of PDGF-CC nAb (PC nAb) inhibited CNV formation. Coinjection of the GSK3β inhibitor LiCl abolished the PDGF-CC–neutralizing antibody induced reduction of CNV. NaCl was used as a control and had no effect. (Scale bars: 100 μm.) *P < 0.05, ***P < 0.001.
GSK3β Inhibition Abolished the Antiangiogenic Effect of PDGF-CC Blockade.
We next studied the importance of GSK3β activity in the antiangiogenic effect of PDGF-CC blockade by using a GSK3β inhibitor, lithium chloride (LiCl), and a laser-induced CNV model. Intravitreal injection of PDGF-CC–neutralizing antibody inhibited CNV formation (Fig. 5 C and D; n = 9, P < 0.05). Coinjection of the GSK3β inhibitor LiCl abolished the PDGF-CC–neutralizing antibody–induced reduction of CNV (Fig. 5 C and D; n = 9, P < 0.001). NaCl was used as a control and had no effect (Fig. 5 C and D; n = 9, P > 0.05). These data demonstrated that GSK3β activity was required for the antiangiogenic effect induced by the PDGF-CC–neutralizing antibody.
Discussion
Antiangiogenic therapy has proven to be successful in treating certain neovascular diseases. VEGF and its receptors have been the major targets in antiangiogenic therapy. Several Food and Drug Adminstration–approved antiangiogenic reagents, such as bevacizumab, sorafenib, and sunitinib, all target the VEGF pathway. However, not all patients with neovascular diseases show a response to these antiangiogenic therapies, and those whose disease is responsive to these therapies can become less responsive during the later stage of the treatment. This has indicated the presence of other important angiogenic factors in pathological angiogenesis (1, 20). Indeed, VEGF has been shown to be responsible for only approximately 50% of vascular growth in pathological angiogenesis (21). Moreover, in patients whose disease is responsive to anti-VEGF therapy, VEGF inhibition may result in different side effects, such as neuronal apoptosis (22), ultrastructural changes in choriocapillaris (23), mitochondrial disruption in the inner segments of photoreceptors (24), and intraocular inflammation (25). In addition, the costs of the current clinically available anti-VEGF reagents are formidable. Thus, there is still an urgent need to identify other non–VEGF-driven angiogenic pathways (1).
In this study, we found that PDGF-CC may be one such new target gene for antiangiogenic therapy. The expression of PDGF-CC and PDGFR-α increased considerably during choroidal and retinal neovascularization, indicating a role of PDGF-CC in pathological angiogenesis. Indeed, PDGF-CC inhibition by different means, such as neutralizing antibody, shRNA, or genetic deletion, suppressed both choroidal and retinal neovascularization. It is noteworthy that PDGF-CC targeting by neutralizing antibody or shRNA showed a similar antiangiogenic effect as VEGF-neutralizing antibody or shRNA in the CNV model. Several cellular mechanisms are involved in the antiangiogenic effect of PDGF-CC inhibition. First, PDGF-CC promoted the proliferation, survival, and migration of fibroblasts and pericytes, which are known to play multiple roles in neovascular diseases. Second, PDGF-CC increased the survival of vascular endothelial cells, which is critical for pathological angiogenesis. Third, PDGF-CC inhibition reduced macrophages infiltration during CNV formation. Indeed, it is known that macrophages play important roles in pathological angiogenesis, and PDGF-CC is abundantly expressed by macrophages and promotes macrophage migration (18). Collectively, PDGF-CC inhibition has versatile effects on multiple cell types critical for pathological angiogenesis. In addition, PDGF-CC significantly up-regulated the expression of many important angiogenic genes, such as PDGF-BB, PDGFR-β, and VEGF, and PDGF-CC blockade inhibited their expression. VEGF has been the major target in antiangiogenic therapy. The role of PDGF-BB in pathological angiogenesis has also been intensively studied, and PDGF-BB inhibitors are currently being tested in clinical trials for antiangiogenic therapy (26). Thus, by downregulating the expression of PDGF-BB, PDGFR-β, and VEGF, the antiangiogenic effect of PDGF-CC blockade was amplified. Thus, PDGF-CC appears to be a promising target molecule in antiangiogenic therapy. Future studies using other PDGF-CC antagonists and animal models are needed to assess the therapeutic potential of PDGF-CC targeting in inhibiting pathological angiogenesis.
Thus far, little is known about the PDGF-CC–induced signaling pathways in ocular cells. In this study, we found that PDGF-CC activated PDGFR-α in both ocular fibroblasts and vascular cells. Treatment with PDGFR-α–neutralizing antibody inhibited CNV to a similar extent as PDGF-CC–neutralizing antibody, indicating that the effect of PDGF-CC was mediated by PDGFR-α, and PDGF-CC is a major ligand of PDGFR-α that plays an important role in CNV. Furthermore, PDGF-CC activated Akt and increased GSK3β Ser9 phosphorylation and GSK3β Tyr216 dephosphorylation in CFs. It is known that inhibition of GSK3β by Ser9 phosphorylation or Tyr216 dephosphorylation promotes vascular cell survival and proliferation (27, 28). Indeed, the critical involvement of GSK3β in PDGF-CC–induced angiogenesis was confirmed in the aortic ring assay in vitro, and in the laser-induced CNV model in vivo. Activation of GSK3β by its activator DIF3 inhibited PDGF-CC–induced microvessel formation, and inhibition of GSK3β by its inhibitor LiCl abolished the suppressing effect of PDGF-CC–neutralizing antibody on CNV formation. Thus far, little is known about the role of GSK3β in pathological angiogenesis, and whether GSK3β is involved in the neovessel formation induced by other angiogenic factors. Future studies will verify this.
In summary, our data derived from different in vivo and in vitro models advocate that PDGF-CC appears to be a promising target molecule for antiangiogenic therapy, and PDGF-CC inhibition might offer therapeutic opportunities to treat neovascular diseases.
Materials and Methods
Animals, Tissues, and Retinal Thickness Measurement.
All animal experiments were approved by the Animal Care and Use Committee at the National Eye Institute/National Institutes of Health (NEI-553), and were performed according to National Institutes of Health regulations. More details can be found in SI Materials and Methods.
Laser-Induced CNV Model and Fluorescein Angiography.
The CNV model was used as described previously (29). More details can be found in SI Materials and Methods .
ROP Model.
The ROP model was described previously (29). More details can be found in the SI Materials and Methods. SI Materials and Methods also provides details on cell culture, cell proliferation/survival, migration, and real-time PCR assays; CAM assay and immunofluorescence staining; PDGFR-α, Akt, and GSK3β expression/phosphorylation assay; Western blot; and aortic ring assay.
Supplementary Material
Acknowledgments
This research was supported in part by the Macular Degeneration Research, a program of the American Health Assistance Foundation, and the Intramural Research Program of the National Institutes of Health, National Eye Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004143107/-/DCSupplemental.
References
- 1.Ferrara N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev. 2010;21:21–26. doi: 10.1016/j.cytogfr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 3.Crawford Y, Ferrara N. Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol Sci. 2009;30:624–630. doi: 10.1016/j.tips.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Maharaj AS, et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 6.Kazlauskas A. A new member of an old family. Nat Cell Biol. 2000;2:E78–E79. doi: 10.1038/35010508. [DOI] [PubMed] [Google Scholar]
- 7.Cao R, et al. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16:1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- 8.Li X, et al. Revascularization of ischemic tissues by PDGF-CC via effects on endothelial cells and their progenitors. J Clin Invest. 2005;115:118–127. doi: 10.1172/JCI19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimmeler S. Platelet-derived growth factor CC—a clinically useful angiogenic factor at last? N Engl J Med. 2005;352:1815–1816. doi: 10.1056/NEJMcibr050670. [DOI] [PubMed] [Google Scholar]
- 10.Ding H, et al. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet. 2004;36:1111–1116. doi: 10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- 11.Su EJ, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieckmann P. Imatinib buys time for brain after stroke. Nat Med. 2008;14:712–713. doi: 10.1038/nm0708-712. [DOI] [PubMed] [Google Scholar]
- 13.Lei H, et al. A potential role for PDGF-C in experimental and clinical proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2007;48:2335–2342. doi: 10.1167/iovs.06-0965. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Maminishkis A, Wang FE, Miller SS. PDGF-C and -D induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2007;48:5722–5732. doi: 10.1167/iovs.07-0327. [DOI] [PubMed] [Google Scholar]
- 15.Crawford Y, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Francia G, Emmenegger U, Kerbel RS. Tumor-associated fibroblasts as “Trojan Horse” mediators of resistance to anti-VEGF therapy. Cancer Cell. 2009;15:3–5. doi: 10.1016/j.ccr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Anderberg C, et al. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009;69:369–378. doi: 10.1158/0008-5472.CAN-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wågsäter D, Zhu C, Björck HM, Eriksson P. Effects of PDGF-C and PDGF-D on monocyte migration and MMP-2 and MMP-9 expression. Atherosclerosis. 2009;202:415–423. doi: 10.1016/j.atherosclerosis.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 19.Cao R, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 20.Bhandarkar SS, et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119:2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aiello LP, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avci B, Avci R, Inan UU, Kaderli B. Comparative evaluation of apoptotic activity in photoreceptor cells after intravitreal injection of bevacizumab and pegaptanib sodium in rabbits. Invest Ophthalmol Vis Sci. 2009;50:3438–3446. doi: 10.1167/iovs.08-2871. [DOI] [PubMed] [Google Scholar]
- 23.Peters S, et al. Tübingen Bevacizumab Study Group. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007;143:995–1002. doi: 10.1016/j.ajo.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Inan UU, et al. Preclinical safety evaluation of intravitreal injection of full-length humanized vascular endothelial growth factor antibody in rabbit eyes. Invest Ophthalmol Vis Sci. 2007;48:1773–1781. doi: 10.1167/iovs.06-0828. [DOI] [PubMed] [Google Scholar]
- 25.Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–261. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Cao Y, Cao R, Hedlund EM. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86:785–789. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- 27.Allard D, Figg N, Bennett MR, Littlewood TD. Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem. 2008;283:19739–19747. doi: 10.1074/jbc.M710098200. [DOI] [PubMed] [Google Scholar]
- 28.de Jesus Perez VA, et al. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol. 2009;184:83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang F, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.