Abstract
Template-directed synthesis of complementary strands is pivotal for life. Nature employs polymerases for this reaction, leaving the ability of DNA itself to direct the incorporation of individual nucleotides at the end of a growing primer difficult to assess. Using 64 sequences, we now find that any of the four nucleobases, in combination with any neighboring residue, support enzyme-free primer extension when primer and mononucleotide are sufficiently reactive, with ≥93% primer extension for all sequences. Between the 64 possible base triplets, the rate of extension for the poorest template, CAG, with A as templating base, and the most efficient template, TCT, with C as templating base, differs by less than two orders of magnitude. Further, primer extension with a balanced mixture of monomers shows ≥72% of the correct extension product in all cases, and ≥90% incorporation of the correct base for 46 out of 64 triplets in the presence of a downstream-binding strand. A mechanism is proposed with a binding equilibrium for the monomer, deprotonation of the primer, and two chemical steps, the first of which is most strongly modulated by the sequence. Overall, rates show a surprisingly smooth reactivity landscape, with similar incorporation on strongly and weakly templating sequences. These results help to clarify the substrate contribution to copying, as found in polymerase-catalyzed replication, and show an important feature of DNA as genetic material.
Cells from all kingdoms of life store their genes in the form of DNA sequences. Before growing cells divide, semiconservative replication ensures that the daughter cells are provided with complete genomes. On a molecular level, replication relies on the stepwise copying of the template through extension of a primer on a DNA template. Each extension step must include formation of a Watson–Crick base pair, and a transphosphorylation step, in which a leaving group is replaced by a nucleophile from the primer (1). The same type of reaction occurs during transcription, except that an RNA strand is formed (2). Copying lies at the heart of our understanding of genetics, but this reaction with its multiple substrates continues to be enigmatic (3). For replication to be successful, any of the four canonical nucleobases must direct the incorporation of its complementary base, in any given sequence context. To achieve this, differences in templating efficiency and monomer reactivity must either be sufficiently small or effectively suppressed to avoid blocks at specific loci of the genome.
In today’s cells, replication is catalyzed by polymerases with deoxynucleoside triphosphates as substrates (Fig. 1, compounds 1 and 2). These reactions occur with high fidelity (1, 4). Polymerases also achieve high processivity on leading and lagging strands, despite differences in rate for individual substrates/templates (5). The mechanism of enzymatic primer extension is complex. It involves monomer binding, transphosphorylation, conformational changes, translocation, and proofreading. Strategies for dissecting individuals are known (6), but determining substrate contributions to fidelity and processivity is difficult.
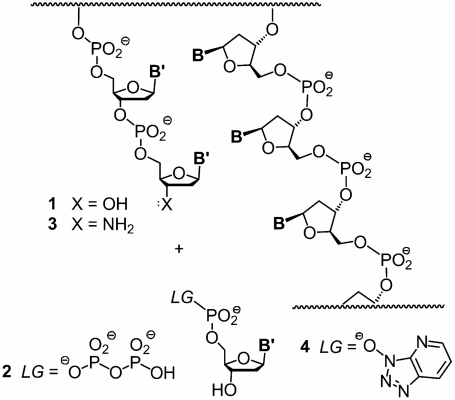
Fig. 1.
Copying of DNA involves the stepwise extension of a primer as directed by the sequence of the template. The grahic shows the molecular components for polymerase-catalyzed copying (1 and 2) or enzyme-free copying (3 and 4). B, bases of template; B′, bases of primer/monomer.
Insights into how much catalysis is needed for primitive replication processes on DNA templates may be gained from enzyme-free primer extension on naked templates. In search for the origin of life (10), enzyme-free copying of nucleic acids has been studied since the 1960s. Chemically activated nucleoside monophosphates were shown to oligomerize on template strands in the absence of polymerases (11). Copying is efficient on templates rich in cytidylic acid with monomers featuring organic leaving groups (12, 13). Oligomers (substrates experiencing stronger template effects) can undergo several rounds of enzyme-free self-replication, particularly when reversible immobilization is involved (14, 15). But, early studies with monomers and polymer templates suggested that there is an enormous diversity in the templating chemistry of oligonucleotides (16). Using defined DNA templates, sequences such as AT, TA, AA, GA, and AG were reported not to support enzyme-free copying (17). Isolated A or T residues in an oligo(G) sequence were called “blocks” to copying (18), suggesting that there is a very strong intrinsic sequence dependence of copying reactions, caused by the structure of DNA.
Copying is faster when the primer terminus is an amine, particularly when the amine is at the 2′ position (19) or part of an alternative structure (20–22). Successful extensions on mixed sequences have been achieved with amines (20, 21), but only when the template was RNA or a close analog that favors A-type helices, and when replacements for uracil and adenine were used. This leaves open the question how good a template DNA is for primer extension, and thus replication. Sequence-dependent effects are important for replication and (23) for improving single-molecule sequencing (24) and genotyping by chemical primer extension (25).
To separate templating efficiency of DNA from enzymatic catalysis, and to better understand the fundamental self-replicating capabilities of DNA, we decided to systematically measure primer extension using a simple, but close system of the fundamental reaction of replication: the extension of a primer by individual nucleotides. We measured rates of incorporation and fidelity on DNA templates possessing any of the 64 possible combinations of templating base and neighboring residues. Our study was made feasible by the availability of chemically activated monomers (26), amino primers (27) (Fig. 1, 3), and mass spectrometrically monitored assays for quantitative monitoring (28), unbiased by the effect of labels (29). The amino group at the primer terminus is isosteric and isoelectronic to the hydroxy group of natural DNA, and can be expected not to bias the templating efficiency. More than one extension of amino primers may be induced (25).
Results
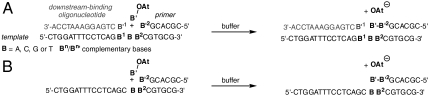
We reacted primers on DNA templates with activated mononucleotides, as shown in Scheme 1. The 3′-amino terminus of the primer gives increased nucleophilicity toward oxyazabenzotriazolides (OAt) (26) as monomers (Fig. 1, 3 and 4). Primer extension produces phosphoramidate linkages that are isoelectronic to phosphodiesters of DNA. The 3′-hydroxy group of the monomer is unreactive enough to make a second extension (that would complicate kinetics) insignificant. For the kinetic studies, only the monomer complementary to the template base was used to keep the system as simple as possible. During the first phase of our study, we employed a downstream-binding oligonucleotide that provides additional stacking surface to the monomer (Scheme 1A) (26).
Scheme 1.
Sequences of assays with (A) or without (B) downstream-binding strand. Core triplets are given as B1BB2, where B is the templating base.
We assayed all 64 combinations of templating base (B), and neighboring bases (B1/B2). To our surprise, > 99% primer conversion was observed for all sequences tested. Fig. S2 (SI Appendix) shows mass spectra for the fastest and the slowest reaction. Monoexponential fits (Fig. 2) gave the rate constants listed in Table 1.* The rates of the fastest reaction (TCT as core template) and the slowest reaction (CAG as core template) differed by a factor of 81, i.e., by less than two orders of magnitude. The hydrolysis of the most slowly reacting monomer (dTMP-OAt) has a half-life time of 16.2 h in assay buffer (26). It is thus more than one order of magnitude slower than even the slowest of the primer extensions (template CAG, t1/2 = 80 min).
Fig. 2.
Representative kinetics of primer extension for template core sequences B1BB2 (A) TCT, and (B) AAA. Lines are monoexponential fits (symbols); gray, extended primer; black, primer.
Table 1.
Rate constants for primer extension with matched OAt-esters of dNMPs as directed by the central nucleobase of any of the 64 possible core triplets of templates
| Template B1B B2 | k′, h-1 M-1 | Template B1B B2 | k′, h-1 M-1 | Template B1B B2 | k′, h-1 M-1 | Template B1B B2 | k′, h-1 M-1 |
| AAA | 220 ± 50 | AAC | 230 | AAG | 150 | AAT | 490 |
| ACA | 1,250 ± 20 | ACC | 1,780 ± 160 | ACG | 2,310 | ACT | 5,730 |
| AGA | 810 | AGC | 2,200 | AGG | 990 | AGT | 2,780 |
| ATA | 610 | ATC | 590 | ATG | 530 | ATT | 1,740 |
| CAA | 200 | CAC | 150 | CAG | 100 | CAT | 290 |
| CCA | 2,970 | CCC | 1,390 ± 180 | CCG | 780 | CCT | 4,370 |
| CGA | 390 ± 100 | CGC | 1,580 ± 180 | CGG | 530 | CGT | 1,980 |
| CTA | 280 | CTC | 600 ± 90 | CTG | 380 | CTT | 1,460 |
| GAA | 390 | GAC | 450 | GAG | 220 | GAT | 880 |
| GCA | 2,210 | GCC | 2,820 ± 420 | GCG | 820 ± 110 | GCT | 3,850 |
| GGA | 1,010 | GGC | 1,320 | GGG | 1,050 | GGT | 1,070 |
| GTA | 760 | GTC | 1,120 | GTG | 800 | GTT | 1,800 |
| TAA | 210 | TAC | 180 | TAG | 140 | TAT | 360 |
| TCA | 1,280 | TCC | 2,590 ± 150 | TCG | 3,620 | TCT | 8,310 ± 240 |
| TGA | 1,210 | TGC | 2,480 | TGG | 1,290 | TGT | 3,410 |
| TTA | 550 | TTC | 630 | TTG | 740 | TTT | 1,700 |
Conditions: 200 mM Hepes buffer (400 mM NaCl, 80 mM MgCl2, pH 8.9), 3.6 mM monomer, 36 μM primer, 54 μM template, and downstream-binding oligonucleotide, 20 °C. Conversion of the primers > 99% at the end of the assay in all cases. Rate constants are averages of at least two independent assays. Errors are standard deviations and are given for sequence motifs that were studied in at least three independent assays. Estimated errors for the remaining rate constants are < 25%. See SI Appendix, Table S4 for representative values from individual assays.
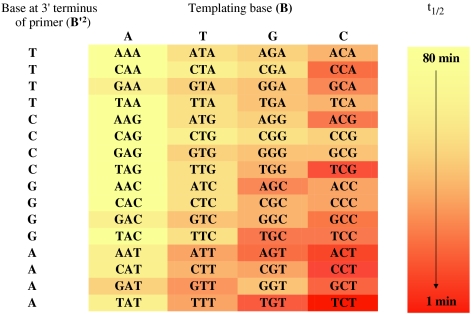
Clear trends are discernible, as shown in color code in Fig. 3. The first is the effect of the base pair formed. Averaging over all 16 triplets with the same B, incorporation of G opposite C is 10-fold faster than that of T opposite A, whereas incorporation of C opposite G and A opposite T are 5-fold and 3-fold faster than that of T apposite A, respectively (SI Appendix, Table S1). So, three hydrogen bond base pairs give faster extensions than two hydrogen bond base pairs, and within each group, purines are incorporated faster than pyrimidines. But, on average, the absolute differences are quite small. Adenine at the 3′ terminus of the primer favors rapid extensions (SI Appendix, Fig. S5a). Adenine is the most lipophilic of the bases and may thus provide the most favorable stacking interactions, combined with a strong hydrophobic effect upon binding of the monomer. Guanine as base of the incoming monomer seems to benefit most from these interactions.
Fig. 3.
“Heat map” representation of the rates of primer extension for different core triplets of the template. The deeper red the color, the faster the reaction. The legend on the right shows the color code used for each box; t1/2, time for extension of 50% of the primer.
The modulating effect of the 5′-terminal base of the downstream-binding oligonucleotide, the other stacking partner for the monomer, is not as strong (SI Appendix, Fig. S5b). Again adenine usually induces the fastest reaction. Thymine is also beneficial, possibly because it offers slightly more stacking surface than C, which lacks the exocyclic methyl group. Why G as stacking partner at the terminus of the third strand does poorly is not immediately clear. These general trends accurately predict TCT as the core sequence giving the fastest primer extension. The poorest templates, CAG, TAG, and AAG are not predicted as easily by these rules, but the rates they induce are within a factor or two of that induced by CAG, expected to support copying most poorly.
We then measured primer extension in the absence of a downstream-binding oligonucleotide (Scheme 1B). Cytosine was chosen as the unpaired nucleotide immediately downstream of the templating base, because this dangling base provides the weakest stabilization via stacking. Primer extension on the 16 resulting sequences was measured both at 20 and 10 °C (Table 2 and SI Appendix, Fig. S11). At 20 °C, ≥97% primer conversion was observed for 12 of the 16 sequences. Three of the templates directing incorporation of T, and one template directing incorporation of A gave incomplete conversion. Successful incorporation was found for five sequences tested with a different dangling base, including AAA (SI Appendix, Table S5). Overall, the rate constants were 2.2–6.5-fold smaller than those with downstream-binding oligonucleotide. Lowering the temperature to 10 °C to strengthen monomer binding gave near-complete conversion for all templates. Only two sequences gave slightly less than 99% primer extension, with CAG again being the poorest, inducing 93% conversion. This data established that, given reactive primers and monomers, the base pairing system of DNA provides a strong enough template effect to drive high-yielding copying of any of its four bases without the need for a downstream-binding strand.
Table 2.
Results of primer extension assays without downstream-binding oligonucleotide (Scheme 1B) for the 16 core template sequences CBB2, as determined by fitting
| Core of template | Conversion* 20 °C, % | k′, 20 °C, h-1 M-1 | Conversion* 10 °C, % | k′, 10 °C, h-1 M-1 |
| CAA | 80 | 30 | 99 | 20 |
| CAC | 81 | 40 | 96 | 20 |
| CAG | 69 | 30 | 93 | 20 |
| CAT | 99 | 90 | 99 | 100 |
| CCA | 99 | 530 | 99 | 490 |
| CCC | 99 | 550 | 99 | 340 |
| CCG | 97 | 360 | 99 | 320 |
| CCT | 99 | 1,480 | 99 | 1,310 |
| CGA | 98 | 150 | 99 | 70 |
| CGC | 99 | 310 | 99 | 150 |
| CGG | 99 | 240 | 99 | 160 |
| CGT | 99 | 660 | 99 | 420 |
| CTA | 87 | 70 | 99 | 70 |
| CTC | 99 | 150 | 99 | 190 |
| CTG | 98 | 90 | 99 | 100 |
| CTT | 99 | 280 | 99 | 370 |
Conditions: 3.6 mM OAt-activated monomer, 36 μM aminoprimer, 54 μM template, 200 mM HEPES buffer (400 mM NaCl, 80 mM MgCl2, pH 8.9).
*Maximum conversion of primer, as determined by fit.
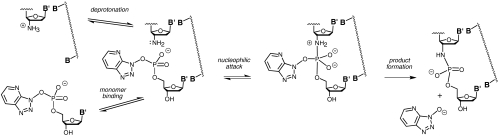
Next, we probed the mechanism of enzyme-free primer extension. Reactions on poorer templates showed a modest lag phase at the beginning of assays (Fig. 2), suggesting that there are at least two consecutive steps in the mechanism; one that is rate limiting during the early phase and one that is rate limiting in the late phase of the assay. We propose the mechanism shown in Scheme 2 with two fast equilibrium processes, namely the deprotonation of the primer and the binding of the monomer, and two chemical steps, the first of which is the nucleophilic attack of the amino group on the phosphodiester, and the second is the collapse of the pentavalent intermediate that releases the leaving group. The last step also encompasses the deprotonation of the initial iminium group, linking the 3′-carbon of the primer and the phosphorus atom of the monomer, a step that is unlikely to be rate limiting. We note that a pentavalent intermediate of the type shown in Scheme 2 may require pseudorotation for the leaving group to reach an apical position (30). This mechanism led to the kinetic scheme of Eq. 1, where M is the monomer, P1 is the primer/template duplex, M-P1 is the pentavalent intermediate, and P2 is the duplex of extended primer and template.
Scheme 2.
Proposed mechanism of primer extension.
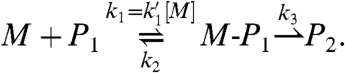 |
[1] |
All three rate constants may be determined from fits to kinetics measured at different monomer concentrations by using the time dependence [P2]t of the concentration of P2.
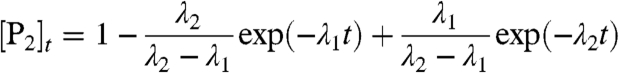 |
[2] |
with
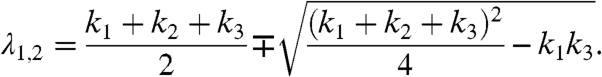 |
[3] |
The results are independent of whether the smaller of the two λ values is assigned to λ1 and the larger one to λ2 or vice versa, as Eq. 2 is invariant with respect to an interchange of λ1 and λ2.
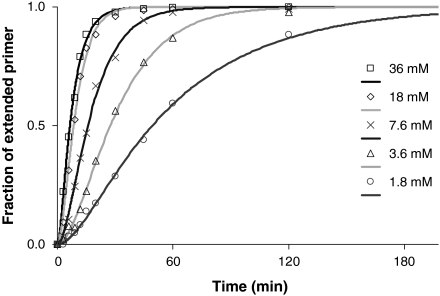
Additional observations support the proposed mechanism. Lowering the concentration of the monomer leads to a more pronounced lag phase (Fig. 4), as expected, if this shifts the binding equilibrium for the monomer to the unbound side, lowering the concentration of the kinetically competent species, and thus lengthening the phase during which the first step is rate limiting. Lowering the pH also leads to a more pronounced lag phase (SI Appendix, Fig. S7), as less of the active amino form of the primer becomes available, lowering the rate for the first chemical step, as known for the attack of amines on other monomers (31). The pKa of the protonated form of the 3′-amine of 3′-amino-2′,3′-dideoxythymdine is 7.7, as determined by 13C NMR (SI Appendix, Figs. S11 and S12). The drop in rate upon lowering the pH appears to be independent of the concentration of the monomer and of the nucleobase (SI Appendix, Table S2), as expected for protonation of the primer. A lag phase is also observed for assays performed with 2-methylimidazolides as activated monomers (SI Appendix, Fig. S8), suggesting that the kinetic scheme holds for other monomers.
Fig. 4.
Influence of concentration of the incoming monomer (here TMP-OAt) on the lag phase: kinetics of primer extension on template GAA at 20 °C. Lines are fits using Eqs. 2 and 3 with one common set of kinetic parameters k1, k2, k3.
Fits to data from assays with A as templating base at five different monomer concentrations gave the individual rate constants in Table 3. Details of the fitting procedure can be found in the SI Appendix. The first chemical step appears to be essentially irreversible, possibly because deprotonation of the iminium bridge is fast, and the first of the two chemical steps is more strongly modulated by the sequence context than the second. This finding is consistent with the notion that the first step is affected by the monomer binding equilibrium, which is more strongly dependent on neighboring residues than the release of the leaving group.
Table 3.
Kinetic values (sorted by increasing k1 values) for two-step primer extension as obtained by fitting the model of Eq. 1 to kinetics at different monomer concentrations (1.8, 3.6, 7.2, 18, and 36 mM TMP-OAt) for the 16 different B1AB2 templates
| Template |
 , mM-1 min-1 , mM-1 min-1
|
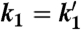 M*, min-1 M*, min-1
|
k3, min-1 |
| CAG | 0.0029 | 0.0104 | 0.13 |
| AAG | 0.0032 | 0.0115 | 0.10 |
| TAG | 0.0033 | 0.0119 | 0.11 |
| CAC | 0.0046 | 0.0166 | 0.17 |
| CAA | 0.0047 | 0.0169 | 0.16 |
| TAA | 0.0052 | 0.0187 | 0.17 |
| TAC | 0.0054 | 0.0194 | 0.16 |
| AAA | 0.0059 | 0.0212 | 0.17 |
| GAG | 0.0064 | 0.0230 | 0.16 |
| CAT | 0.0072 | 0.0259 | 0.21 |
| GAA | 0.0088 | 0.0317 | 0.20 |
| AAC | 0.0098 | 0.0353 | 0.15 |
| TAT | 0.0101 | 0.0364 | 0.21 |
| GAC | 0.0148 | 0.0533 | 0.13 |
| AAT | 0.0221 | 0.0796 | 0.28 |
| GAT | 0.0265 | 0.0954 | 0.36 |
Note, k2 was found to be smaller than 0.0001 min-1 in all cases.
*Calculated for the default concentration of 3.6 mM TMP-OAt.
We then varied the monomer concentration in assays with the four CBC templates (Table 4). Again, k3 values varied by no more than a factor of 3, whereas the 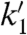 values varied by almost an order of magnitude, being low for monomers that bind weakly, and high for those forming strong base pairs. This effect is expected, if the second chemical step, where the monomer is already covalently linked to the primer, is affected in no more than subtle ways by the sequence (e.g., through the dynamics of the structure and local dipoles), but the first step is directly modulated by the binding/templating capability of the sequence. Leaving out the downstream-binding strand and dropping the temperature to 10 °C led to the k1 and k3 values reported in the lower part of Table 4. Values of k1 drop more steeply than those of k3, and the extent to which the nature of the base pair modulates k1 is greater than in the presence of the third strand.
values varied by almost an order of magnitude, being low for monomers that bind weakly, and high for those forming strong base pairs. This effect is expected, if the second chemical step, where the monomer is already covalently linked to the primer, is affected in no more than subtle ways by the sequence (e.g., through the dynamics of the structure and local dipoles), but the first step is directly modulated by the binding/templating capability of the sequence. Leaving out the downstream-binding strand and dropping the temperature to 10 °C led to the k1 and k3 values reported in the lower part of Table 4. Values of k1 drop more steeply than those of k3, and the extent to which the nature of the base pair modulates k1 is greater than in the presence of the third strand.
Table 4.
Rates for two-step primer extension on each of the four different CBC templates either in the presence of a downstream-binding oligomer (third strand) at 20 °C or in its absence at 10 °C, based on fits to data obtained at 1.8, 3.6, 7.2, 18, and 36 mM monomer concentration, using Eqs. 1 and 2; k2 was smaller than 0.0001 min-1 in all cases
| Template | Third strand | °C |
 M*, min-1 M*, min-1
|
k3, min-1 |
| CAC | + | 20 | 0.0166 | 0.170 |
| CTC | + | 20 | 0.0508 | 0.220 |
| CCC | + | 20 | 0.1127 | 0.460 |
| CGC | + | 20 | 0.1188 | 0.330 |
| CAC | − | 10 | 0.0014 | 0.035 |
| CTC | − | 10 | 0.0119 | 0.022 |
| CCC | − | 10 | 0.0263 | 0.088 |
| CGC | − | 10 | 0.0126 | 0.140 |
*Calculated for the default concentration of 3.6 mM monomer.
Polymerase-catalyzed primer extension occurs with extremely low misincorporation frequencies of approximately 1 in 104–105 extensions, even in the absence of proofreading. It was believed that this is much more selective than what base pairing in naked DNA can provide (32), and several models have been proposed to explain the fidelity-enhancing effect of the active site (6, 33). To shed light on the intrinsic fidelity of primer extension, we used a mix of the four activated monomers and the assay of Scheme 1A. Monomer concentrations were first set to 3.6 mM dGMP-OAt, 7.2 mM dCMP-OAt, 10.8 mM dAMP-OAt, and 22 mM TMP-OAt, roughly reflecting the relative reactivities of the monomers. Incorporation of individual nucleotides opposite the given templating base was determined from relative peak intensities in mass spectra acquired after 3 h. The data are believed to represent the lower limit of fidelity, because the resolution of our spectra does not always suffice to fully correct for residual salt adducts (e.g., [M + Na-2H]- ions) that have similar masses as expected extension products, which may be significant for low-level misincorporations.
On all 64 templates, the complementary nucleotide was incorporated preferentially (Table 5). For 12 templates, misincorporation was < 1%. In 46 cases, ≥90% of the extended primer held the correct nucleobase. In 10 out of 18 cases, where the correct extension product was formed in less than 90% yield, G was the templating base. This result may be due, in part, to the underrepresentation of the complementary nucleotide in the “reactivity-adjusted” mixture, as assays with a 1∶1∶1∶1 mixture of monomers often gave higher absolute fidelity (SI Appendix, Table S3). For sequences such as GGC and GGG, the apparent incorporation of 10–15% of A instead of C may also be due to the limitations of the detection method, as the Na+ adduct of correctly extended primer has almost the same mass as the +A extension product. Mass spectra acquired in high-resolution mode suggested that the contribution of the salt adduct was < 20% of the peak intensity, though. For GGG, the sum of the misincorporations was 5–9% for the 1∶1∶1∶1 mixture, with the lowest error rate measured at 10 °C in the absence of a downstream-binding oligonucleotide (SI Appendix, Table S3). Averaging over all 64 cases reported in Table 5 gives a fidelity of incorporation of 92%. On average, 98% T was incorporated opposite A, 90% G was incorporated opposite C, 85% C was incorporated opposite G, and 95% A was incorporated opposite T.
Table 5.
Product distribution for primer extension with a reactivity-adjusted balanced mixture of OAt-esters of the four canonical deoxynucleoside monophosphates (A/C/G/T) for any of the 64 possible core triplets of templates
| Template B1B B2 | Product distribution +C/+T/+A/+G | Template B1B B2 | Product distribution +C/+T/+A/+G | Template B1B B2 | Product distribution +C/+T/+A/+G | Template B1B B2 | Product distribution +C/+T/+A/+G |
| AAA | 0/ > 99/0/0 | AAC | 0/ > 99/0/0 | AAG | 0/ > 99/0/0 | AAT | 0/ > 99/0/0 |
| ACA | 1/1/5/93 | ACC | 2/10/4/84 | ACG | 0/10/6/84 | ACT | 0/2/2/96 |
| AGA | 86/7/5/2 | AGC | 84/8/6/2 | AGG | 83/8/8/1 | AGT | 91/6/3/0 |
| ATA | 0/7/93/0 | ATC | 0/5/94/1 | ATG | 0/10/90/0 | ATT | 0/3/97/0 |
| CAA | 0/ > 99/0/0 | CAC | 0/98/0/2 | CAG | 0/93/7/0 | CAT | 0/ > 99/0/0 |
| CCA | 0/2/0/98 | CCC | 2/11/8/79 | CCG | 3/15/10/72 | CCT | 0/0/3/97 |
| CGA | 92/5/2/1 | CGC | 87/8/4/1 | CGG | 78/15/7/0 | CGT | 91/7/2/0 |
| CTA | 0/5/95/0 | CTC | 0/9/90/1 | CTG | 0/11/89/0 | CTT | 0/3/97/0 |
| GAA | 0/ > 99/0/0 | GAC | 0/ > 99/0/0 | GAG | 2/98/0/0 | GAT | 0/ > 99/0/0 |
| GCA | 0/4/3/93 | GCC | 1/3/1/95 | GCG | 4/9/6/81 | GCT | 0/0/2/98 |
| GGA | 84/6/9/1 | GGC | 73/10/15/3 | GGG | 75/9/16/0 | GGT | 87/6/5/2 |
| GTA | 0/4/96/0 | GTC | 0/3/97/0 | GTG | 2/6/91/1 | GTT | 0/1/99/0 |
| TAA | 0/97/3/0 | TAC | 0/ > 99/0/0 | TAG | 0/94/6/0 | TAT | 0/96/4/0 |
| TCA | 1/4/2/93 | TCC | 0/7/7/86 | TCG | 3/5/5/87 | TCT | 0/2/2/96 |
| TGA | 90/6/4/0 | TGC | 81/11/7/1 | TGG | 92/4/3/1 | TGT | 93/4/2/1 |
| TTA | 0/2/98/0 | TTC | 0/5/93/2 | TTG | 0/0/ > 99/0 | TTT | 0/0/ > 99/0 |
Conditions: Hepes buffer pH 8.9 (200 mM), 3.6 mM dGMP-OAt, 7.2 mM dCMP-OAt, 10.8 mM dAMP-OAt, 22 mM TMP-OAt, 54 μM template and downstream-binding oligonucleotide, 36 μM primer, 20 °C. Conversion of the primers > 99% at the end of the assay in all cases.
Discussion
Polymerases are encoded in the DNA whose copying they catalyze. This fact begs the question of what was first: the encoding system or the catalyst encoded? At the core of the problem is the question whether today’s genetic system supports self-replication “by itself,” i.e., in the absence of proteins (34). Previous work on monomer-based enzyme-free replication has come to the conclusion that the templating efficiency of too many sequences is too poor to allow for replication (35). Our results suggest that there is a strong enough template effect, at least if one allows for sufficiently reactive nucleophiles at the primer terminus and organic leaving groups on the monomers. Our data show that any DNA sequence context, as defined by templating base and either of its neighboring residues, can attract the complementary nucleotide and promote its addition to a primer. This finding allays concerns that a template may have to contain at least 60% residues (36) or nonnatural base pairs (37) to support replication. As noted for other backbones (38), sufficient base pairing strength may be achieved, but the extent of misincorporation on some templates is a reason for concern (Table 5). For longer, mixed sequence templates, a very high mutation frequency can be expected, if errors are not corrected or if miselongated primers are not unreactive enough to shut down further extension.
We speculate that the reactivity of amino-terminal primers and OAt ester of dNMPs is not critical for nucleic acid-based self-replication (21). Primer extension with natural, hydroxy-terminated primers and dNTPs may be induced by the deprotonation of the 3′-hydroxy group (producing a very strongly nucleophilic oxyanion) or metal binding to the leaving group, or both. Perhaps less complex catalysts than known ribozyme polymerases (39) are sufficient to provide this level of catalysis. If no sequence is generally refractory to copying, and even poor templates, such as AAA, promote primer extension in DNA and all-RNA systems at low temperatures (40), a search for well replicating sequences, as proposed by others (18), may not be required for finding self-replicating systems (22).
For the two simplest cases, templates consisting either of alternating C/G residues (CG)n or of alternating T/A residues (TA)n, rate constants ka and kb measured for GCG and CGC or ATA and TAT can be combined to a compounded rate constant kab according to the rules for the first passage time of two first-order rate processes: kab = kakb/(ka + kb) (SI Appendix). One thus obtains a first glimpse at how fast multiple extensions may be occurring. In the absence of a downstream-binding strand at 10 °C (Table 2), the first passage time values for CG and TA are 541 and 227, respectively. The difference is less than one order of magnitude. Even on the level of homopolymers, the differences in rate are surprisingly small, with extension on AAA (220 h-1 M-1) being only 6-fold slower than that on CCC (1390 h-1 M-1). For the two-strand system at 10 °C, the difference in rates is 15-fold (Table 2), demonstrating that homosequences, such as d(C)n are not the best templates for copying of DNA and that DNA is well suited for semiconservative replication.
For DNA, differences in rate of less than two orders of magnitude make the molecular feat of smoothing out the reactivity landscape from one sequence to another less daunting than it may have seemed, based on binding constants for A∶T and G∶C in lipophilic environments (41). The reactivity landscape of RNA may not be as smooth as that of DNA. Primer extension on RNA templates, catalyzed by ribozyme polymerases, can be strongly sequence dependent (42), though the order or reactivity is generally similar to that found here (43). Quantitative date like those provided here may help to better isolate substrate reactivity from catalysis. Further, enzyme-free primer extension provides an opportunity to test nonnatural base pairs (44) without the biasing effects of enzymes. The literature suggests that shape complementarity and stacking are critical for base pairing (45). The effect of the neighboring residues on chemical primer extension (SI Appendix, Fig. S5) show that stacking is indeed an important factor. Still, the differences are not so strong as to prevent copying in less favorable cases. Chemical genotyping with fluorescent nucleotides (25) should benefit from this property of DNA.
Independent of this, the following rules can be derived from our results: (i) G and C are incorporated faster than A and T; (ii) purines are incorporated faster than pyrimidines; and (iii) the neighboring bases to the newly formed base pair modulate the rate of primer extension to a smaller extent than the nature of the templating base, but together, these factors modulate the rate by less than two orders of magnitude. All core triplets can template copying of their central base, at least in a sequence context that does not contain inhibitory secondary or tertiary structures. Further, neither a nucleophile at the 2′ position (20) nor a downstream-binding oligomer are absolutely necessary for successful individual copying steps. The very nature of DNA appears to aid primer extension by any of the four natural base pairs, even in the absence of enzymes.
Materials and Methods
For primer extension, template, primer (and downstream-binding oligonucleotide) were dissolved to give stock solutions, aliquots of which (0.5 μL) were added to 1 μL of Hepes buffer (1 M), NaCl (2 M), and MgCl2 (0.4 M) plus water, to give a final solution at pH 8.9 (5 μL). The oligonucleotides were annealed by cooling from 90 to 20 °C at a rate of 0.1 °C/s, and a solution of the activated monomer(s) in water was added to start the assay. Samples (0.4 μL) were drawn at stated intervals and diluted with water (10 μL). The resulting solution was kept over a few grains of Dowex cation exchange resin (50WX8, ammonium form) for 3 min. A sample of the supernatant was then used for MALDI-TOF MS, performed under conditions allowing for quantitative detection (28). The desalting step ensures that salt adducts are reduced to a minimum (compare Fig. 2 to Fig. S2, SI Appendix). Spectra from assays with mixtures of monomers were acquired both in linear and reflectron mode (near-isotopic resolution) to test for salt adducts.
Supplementary Material
Acknowledgments.
The authors thank M. Röthlingshöfer, C. Deck, and A. Kaiser for discussions. This work was supported by Deutsche Forschungsgemeinschaft Grant RI 1063/8-1 (to C.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.A.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914872107/-/DCSupplemental.
*As detailed below, in-depth kinetic analysis showed a slightly biphasic behavior for some templates, explaining why some standard deviations are larger than others.
References
- 1.Joyce C-M, Benkovic S-J. DNA polymerase fidelity: Kinetics structure and checkpoints. Biochemistry. 2004;43:14317–14324. doi: 10.1021/bi048422z. [DOI] [PubMed] [Google Scholar]
- 2.Nudler E. RNA Polymerase active center: The molecular engine of transcription. Annu Rev Biochem. 2009;78:335–361. doi: 10.1146/annurev.biochem.76.052705.164655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berdis A-J. Mechanisms of DNA polymerases. Chem Rev. 2009;109:2862–2879. doi: 10.1021/cr800530b. [DOI] [PubMed] [Google Scholar]
- 4.Kunkel T-A, Bebenek K. DNA replication fidelity. Annu Rev Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Zhuang Z, Roccasecca R-M, Trakselis M-A, Benkovic S-J. The dynamic processivity of the T4 DNA polymerase during replication. Proc Natl Acad Sci USA. 2004;101:8289–8294. doi: 10.1073/pnas.0402625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuchta R-D, Benkovic P, Benkovic S-J. Kinetic mechanism whereby DNA-polymerase I (Klenow) replicates DNA with high fidelity. Biochemistry. 1988;27:6716–6725. doi: 10.1021/bi00418a012. [DOI] [PubMed] [Google Scholar]
- 7.Mendelman L-V, Boosalis M-S, Petruska J, Goodman M-F. Nearest neighbor influences on DNA polymerase insertion fidelity. J Biol Chem. 1989;264:14415–14423. [PubMed] [Google Scholar]
- 8.Patel S-S, Wong I, Johnson K-A. Pre-steady-state kinetic-analysis of processive DNA-replication including complete characterization of an exonuclease-deficient mutant. Biochemistry. 1991;30:511–525. doi: 10.1021/bi00216a029. [DOI] [PubMed] [Google Scholar]
- 9.Minetti C-A-S-A, et al. The thermodynamics of template-directed DNA synthesis: Base insertion and extension enthalpies. Proc Natl Acad Sci USA. 2003;100:14719–14724. doi: 10.1073/pnas.2336142100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce G-F, Orgel L-E. In: The RNA World. Gesteland R-F, Cech T-R, Atkins J-F, editors. Plainview, NY: Cold Spring Harbor Laboratory Press; 2006. pp. 23–56. [Google Scholar]
- 11.Sulston J, Lohrmann R, Orgel L-E, Miles H-T. Nonenzymatic synthesis of oligoadenylates on a polyuridylic acid template. Proc Natl Acad Sci USA. 1968;59:726–733. doi: 10.1073/pnas.59.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozlov I-A, Orgel L-E. Nonenzymatic template-directed synthesis of RNA from monomers. Mol Biol. 2000;34:781–789. [PubMed] [Google Scholar]
- 13.Kanavarioti A, Bernasconi C-F, Baird E-E. Effects of monomer and template concentration on the kinetics of nonenzymatic template-directed oligoguanylate synthesis. J Am Chem Soc. 1998;120:8575–8581. [Google Scholar]
- 14.Sievers D, von Kiedrowski G. Self-Replication of complementary nucleotide-based oligonucleotides. Nature. 1994;369:221–224. doi: 10.1038/369221a0. [DOI] [PubMed] [Google Scholar]
- 15.Luther A, Brandsch R, von Kiedrowski G. Surface-promoted replication and exponential amplification of DNA analogues. Nature. 1998;396:245–248. doi: 10.1038/24343. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Orgel L-E. A nonenzymatic RNA polymerase model. Science. 1983;219:859–862. doi: 10.1126/science.6186026. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Orgel L-E. Non enzymatic template-directed synthesis on hairpin oligonucleotides. 3. Incorporation of adenosine and uridine residues. J Am Chem Soc. 1992;114:7963–7969. doi: 10.1021/ja00047a001. [DOI] [PubMed] [Google Scholar]
- 18.Hill A-R, Orgel L-E, Wu T. The limits of template-directed synthesis with 5′-phosphoro-(2-methyl)imidazolides. Origins Life Evol B. 1993;23:285–290. doi: 10.1007/BF01582078. [DOI] [PubMed] [Google Scholar]
- 19.Lohrmann R, Orgel L-E. Template-directed synthesis of high molecular weight polynucleotide analogues. Nature. 1976;261:342–344. doi: 10.1038/261342a0. [DOI] [PubMed] [Google Scholar]
- 20.Chen J-J, Cai X, Szostak J-W. N2′ → P3′ Phosphoramidate glycerol nucleic acid as a potential alternative genetic system. J Am Chem Soc. 2009;131:2119–2121. doi: 10.1021/ja809069b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrum J-P, Ricardo A, Krishnamurthy M, Blain J-C, Szostak J-W. Efficient and rapid template-directed nucleic acid copying using 2′-amino-2′,3′-dideoxyribonucleoside-5′-phosphorimidazolide monomers. J Am Chem Soc. 2009;131:14560–14570. doi: 10.1021/ja906557v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansy S-S, et al. Template-directed synthesis of a genetic polymer in a model protocell. Nature. 2008;454:122–125. doi: 10.1038/nature07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wuite G-J, Smith S-B, Young M, Keller D, Bustamante C. Single-molecule studies of the effect of template tension on T7 DNA polymerase activity. Nature. 2000;404:103–106. doi: 10.1038/35003614. [DOI] [PubMed] [Google Scholar]
- 24.Eid J, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 25.Griesang N, Giessler K, Lommel T, Richert C. Four color enzyme-free interrogation of DNA sequences with chemically activated 3′-fluorophore-labeled nucleotides. Angew Chem Int Edit. 2006;45:6144–6148. doi: 10.1002/anie.200600804. [DOI] [PubMed] [Google Scholar]
- 26.Hagenbuch P, Kervio E, Hochgesand A, Plutowski U, Richert C. Chemical primer extension: Efficiently determining single nucleotides in DNA. Angew Chem Int Edit. 2005;44:6588–6592. doi: 10.1002/anie.200501794. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhuth R, Richert C. Convenient syntheses of 3′-amino-2′3′-dideoxynucleosides their 5′-monophosphates and 3′-aminoterminal oligodeoxynucleotide primers. J Org Chem. 2009;74:26–37. doi: 10.1021/jo8018889. [DOI] [PubMed] [Google Scholar]
- 28.Sarracino D, Richert C. Quantitative MALDI-TOF spectrometry of oligonucleotides and a nuclease assay. Bioorg Med Chem Lett. 1996;6:2543–2548. [Google Scholar]
- 29.Rojas Stütz J-A, Richert C. A steroid cap adjusts the selectivity and accelerates the rates of nonenzymatic single nucleotide extensions of an oligonucleotide. J Am Chem Soc. 2001;123:12718–12719. doi: 10.1021/ja011448i. [DOI] [PubMed] [Google Scholar]
- 30.Fersht A. Structure and Mechanism in Protein Science. New York: Freeman; 1999. pp. 259–266. [Google Scholar]
- 31.Kanavarioti A, Stronach M-W, Ketner R-J, Hurley T-B. Large steric effect in the substitution reaction of amines with phosphoimidazolide-activated nucleosides. J Org Chem. 1995;60:632–637. doi: 10.1021/jo00108a027. [DOI] [PubMed] [Google Scholar]
- 32.Loeb L-A, Kunkel T-A. Fidelity of DNA-synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- 33.Goodman M-F, Fygenson K-D. DNA polymerase fidelity: From genetics toward a biochemical understanding. Genetics. 1998;148:1475–1482. doi: 10.1093/genetics/148.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lincoln T-A, Joyce G-F. Self-sustained replication of an RNA enzyme. Science. 2009;323:1229–1232. doi: 10.1126/science.1167856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zielinski M, Kozlov I-A, Orgel L-E. A comparison of RNA with DNA in template-directed synthesis. Helv Chim Acta. 2000;83:1678–1684. doi: 10.1002/1522-2675(20000809)83:8<1678::AID-HLCA1678>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 36.Orgel L-E. Molecular replication. Nature. 1992;358:203–209. doi: 10.1038/358203a0. [DOI] [PubMed] [Google Scholar]
- 37.Hartel C, Göbel M-W. Substitution of adenine by purine-2,6-diamine improves the nonenzymatic oligomerization of ribonucleotides on templates containing thymidine. Helv Chim Acta. 2000;83:2541–2549. [Google Scholar]
- 38.Beier M, Reck F, Wagner T, Krishnamurthy R, Eschenmoser A. Chemical etiology of nucleic acid structure: Comparing pentopyranosyl (2′-4′) oligonucleotides with RNA. Science. 1999;283:699–703. doi: 10.1126/science.283.5402.699. [DOI] [PubMed] [Google Scholar]
- 39.Johnston W-K, Unrau P-J, Lawrence M-S, Glasner M-E, Bartel D-P. RNA-catalyzed RNA polymerization: Accurate and general RNA-templated primer extension. Science. 2001;292:1319–1325. doi: 10.1126/science.1060786. [DOI] [PubMed] [Google Scholar]
- 40.Vogel S-R, Richert C. Adenosine residues in the template do not block spontaneaous replication steps of RNA. Chem Commun. 2007:1896–1898. doi: 10.1039/b702768k. [DOI] [PubMed] [Google Scholar]
- 41.Kyogoku Y, Lord R-C, Rich A. An infrared study of hydrogen-bonding specificity of hypoxanthine and other nucleic acid derivatives. Biochim Biophys Acta. 1969;179:10–17. doi: 10.1016/0005-2787(69)90116-6. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence M-S, Bartel D-P. Processivity of ribozyme catalyzed RNA polymerization. Biochemistry. 2003;42:8748–8755. doi: 10.1021/bi034228l. [DOI] [PubMed] [Google Scholar]
- 43.Bartel D-P, Doudna J-A, Usman N, Szostak J-W. Template-directed primer extension catalyzed by the Tetrahymena ribozyme. Mol Cell Biol. 1991;11:3390–3394. doi: 10.1128/mcb.11.6.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Switzer C, Moroney S-E, Benner S-A. Enzymatic incorporation of a new base pair into DNA and RNA. J Am Chem Soc. 1989;111:8322–8323. [Google Scholar]
- 45.Krueger A-T, Kool E-T. Model systems for understanding DNA base pairing. Curr Opin Chem Biol. 2007;11:588–594. doi: 10.1016/j.cbpa.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.