Abstract
High survival and breeding philopatry was previously confirmed for the Adélie penguin (Pygoscelis adeliae) during a period of stable environmental conditions. However, movements of breeding adults as a result of an unplanned natural experiment within a four-colony meta-population provided interesting insights into this species’ population dynamics. We used multistate mark-recapture models to investigate apparent survival and dispersal of breeding birds in the southwestern Ross Sea during 12 breeding seasons (1996–2007). The natural experiment was facilitated by the temporary grounding of two immense icebergs that (i) erected a veritable fence separating colonies and altering migration routes and (ii) added additional stress by trapping extensive sea ice in the region during 5 of 12 y. Colony size varied by orders of magnitude, allowing investigation of apparent survival and dispersal rates in relation to both environmental conditions and colony size within this meta-population. Apparent survival was lowest for the smallest colony (4,000 pairs) and similar for the medium (45,000 pairs) and large colonies (155,000 pairs), despite increased foraging effort expended by breeders at the largest colony. Dispersal of breeding birds was low (<1%), except during years of difficult environmental conditions when movements increased, especially away from the smallest colony (3.5%). Decreased apparent survival at the smallest colony could reflect differences in migration chronology and winter habitat use compared with the other colonies, or it may reflect increased permanent emigration to colonies outside this meta-population. Contrary to current thought, breeding penguins are not always philopatric. Rather, stressful conditions can significantly increase dispersal rates.
Keywords: Antarctica, avian demography, climate change, philopatry, population dynamics
Changes in animal populations are the result of a variety of demographic processes, including survival and dispersal. Dispersal, and its complement philopatry, can be difficult to study, particularly for mobile species like birds, but statistical methods now allow the estimation of these parameters from data collected on marked individuals (1, 2). Nevertheless, studies are rare in which sufficient search effort away from the original marking location is possible to acquire the data needed for modeling. Dispersal and philopatry can be considered at two life stages: natal, concerning movements of young animals before breeding, and breeding, for movement of individuals that have bred at least one time in a known location (3, 4). Dispersal or philopatry during these two stages can reflect multiple, even opposite, selective pressures, because both involve proximate and ultimate costs and benefits to moving or remaining (5, 6) and a wide variety of factors can affect the cost–benefit balance, including habitat quality and environmental variation (7), intra- or interspecific competition (8), predation (9), individual plasticity (10), and variation in physiology or life stage (6, 11). Dispersal affects gene flow and thus, is integral to understanding population genetics, meta-population dynamics (12), and ultimately, the process of evolution (13).
For many long-lived bird species with low reproductive success, breeding philopatry is believed to be very high, although actual dispersal rates or degree of philopatry has been rigorously quantified for relatively few such species (14, 15). For penguins, demographic models have been constructed assuming that emigration is nonexistent or low enough to be ignored (16–18). The Adélie penguin (Pygoscelis adeliae) is a well-studied seabird exhibiting delayed maturation, low reproductive output, and high adult survival. Thus, a high degree of natal and breeding philopatry is predicted (19) and has been confirmed for a period of stable environmental conditions (20). However, genetic homogeneity across the world population of Adélie penguins argues for dispersal at least over a millennial time scale (21, 22), and increased between-colony visitation among subadults and adults has been observed recently in distinct but infrequent episodes (22). The contribution that these movements make to overall meta-population dynamics has yet to be quantified.
The use of multistate mark-recapture models that allow the estimation of movement probabilities among states, in addition to apparent survival and detection rates (23, 24), allowed us to investigate apparent survival and dispersal patterns of adult breeding birds among three colonies of a four-colony meta-population in the southwestern Ross Sea, Southern Ocean, during 12 breeding seasons (1996–2007), although some data were available from the harder-to-reach fourth colony. Multistate models allowed us to refine estimates of apparent survival by incorporating movement rates of breeding individuals between a subset of breeding colonies (hereafter referred to as dispersal), thus reducing negative bias associated with undetected permanent emigration (24, 14).
We define breeding colony, using the intention of Ainley (19) (note that radius was inadvertently doubled in the original source), as all Adélie penguins breeding within a 4-km radius that are strongly related demographically, because this is the size of the largest colonies for this species. Here, we investigate the temporary visitations and longer-distance recruitment of adult breeders between breeding colonies rather than short-distance movements within a breeding colony. The colonies in the meta-population vary in size by orders of magnitude, with Cape Royds as the smallest (peak of 4,000 breeding pairs), Cape Bird as the intermediate (45,000 breeding pairs), and Cape Crozier as the largest (155,000 breeding pairs). The fourth infrequently searched colony is at Beaufort Island (35,000 pairs), 30 km to the north of Ross Island. The next closest breeding colonies to this cluster are at Franklin and Inexpressible Islands 100 and 230 km away, respectively, which is an appreciable gap relative to the distribution of penguin colonies in the Ross Sea (19).
Previous research showed that foraging effort and energy expended by breeding penguins was highest at the largest colony (Crozier) because of intra- and interspecific competition (25, 26). Individuals breeding at Cape Royds have much lower foraging trip duration and energetic requirements than those breeding at the other colonies during typical environmental conditions (27). However, the fact that the Cape Crozier colony is by far the largest in the meta-population (and one of the largest in the world for this species) implies that survival, recruitment, or immigration must be higher there compared with the smaller colonies. This could be because of a more reliable physical location with respect to access to open water (and thus, food) (19, 28) associated with an adjacent polyna (area of persistent open water within the regional sea ice). Thus, a tradeoff between the negative effects of competition and the benefits of a reliable foraging habitat may be responsible for maintaining the size of the largest colony. To better understand how biological (competitive) and physical (location/access to open water) factors interact to determine the size of Adélie penguin colonies, we measured apparent survival and dispersal at all three intensively studied colonies within the meta-population over a 12-y period. We hypothesized that apparent survival is higher at larger colonies than at the smallest colony (29) and/or that dispersal away from the smallest colony is higher than dispersal away from the largest colony, particularly during adverse environmental conditions (30). To test our hypothesis, we took advantage of a natural experiment. Extreme physical environmental conditions were caused by the temporary presence of two enormous icebergs during the middle portion of our study period (one iceberg was 165 km long) (22, 31, 32). These icebergs created a physical barrier between the easternmost colony on Ross Island and the western colonies (including that on Beaufort) (22) and in association with temporary (episodic) reductions in winter winds, resulted in more extensive sea ice during the austral spring of some years in that western area (33). Based on previous analyses implying increased movement among colonies (22) and heightened differences between low- and high-quality individuals in terms of apparent survival and foraging success during years of stressful conditions (31, 32), we predicted that the more difficult conditions would heighten differences in apparent survival and dispersal among individuals as affected by colony location, size, and environmental severity.
Results
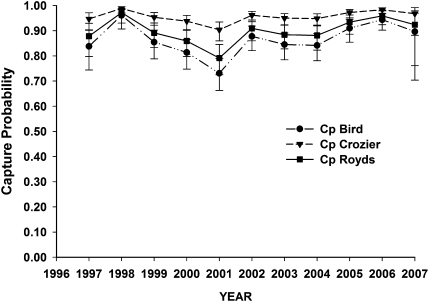
Our mark-recapture dataset included resighting histories for 475 individuals at Cape Royds, 970 at Cape Bird, and 1,236 at Cape Crozier (n = 2,681), all of whom were recorded breeding at least one time during the study (1996–2007). The best model included resighting rate differences by colony and year, with the highest resighting rates observed at Cape Crozier and the lowest at Cape Bird (Fig. 1). The additive effect of annual variation (t) was also important, and this best model [p(col + t)] was supported by a model weight of 0.75; no other models were competitive [i.e., quasi-Akaike's Information Criteria (QAICc) < 2.0]. Thus, we retained this resighting-rate structure as we continued with apparent survival and movement probability modeling.
Fig. 1.
Estimated resighting rate, with 95% confidence limits, for breeding adult Adélie penguins at Capes Royds, Bird, and Crozier, Ross Island, Antarctica from 1996 to 2007.
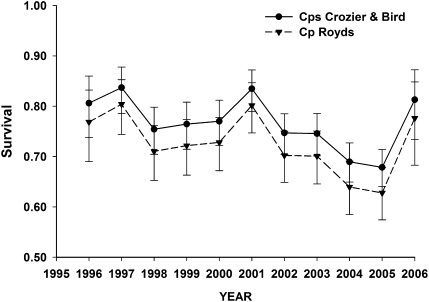
Apparent survival rates of breeders also varied annually and by colony (Fig. 2), and our best model suggested that rates for Capes Bird and Crozier were similar and higher every year compared with those for breeding adult birds at Cape Royds [S (B = C, R + t); model weight = 0.61] (Table 1). This top model had two times as much support as a competitive model including apparent survival differences for each of the three colonies [S(B, C, R + t)]. In addition, confidence limits on the slope coefficient reflecting the Bird/Crozier colony effect in our top model did not include zero [β = 0.23; 95% confidence interval (CI) = 0.06–0.39], providing additional support for this effect. Neither the presence of the icebergs (ΔQAICc best iceberg model = 33.52) or years with extensive sea ice (ΔQAICc best sea ice model = 36.94) during the breeding season received any support for an effect on apparent survival compared with the general time-dependent models (Table 1). Apparent survival exhibited substantial variation among years, with rates >70% in all but 2 y and declines observed since 2003 (Fig. 2). Estimates ranged from highs of 0.80 at Cape Royds and 0.84 at Capes Bird and Crozier in 1997 to lows of 0.63 and 0.68 for Royds (Fig. 2), and apparent survival rates for birds breeding at Cape Royds were 3–5% lower than for birds breeding at Bird or Crozier.
Fig. 2.
Estimated apparent survival, with 95% confidence limits, for breeding adult Adélie penguins at Capes Royds, Bird, and Crozier, Ross Island, Antarctica from 1996 to 2007.
Table 1.
Model selection results for breeding Adélie penguin survival
| Model | ΔQAICc* | K | QAICc wt | QDeviance |
| S(B = C,R + t) | 0.00 | 88 | 0.61 | 1,126.46 |
| S(B,C,R + t) | 1.64 | 89 | 0.27 | 1,126.04 |
| S(B,C = R + t) | 4.30 | 88 | 0.07 | 1,130.76 |
| S(t) | 5.42 | 87 | 0.04 | 1,133.93 |
| S(B = R,C + t) | 7.16 | 88 | 0.02 | 1,133.62 |
| S(.) | 47.88 | 80 | 0.00 | 1,190.78 |
Model selection results for five best models relating colony (B, Cape Bird; C, Cape Crozier; R, Cape Royds), general time-dependence (t), linear (T), pseudothreshold (lnT), and quadratic (TT) time trends, the presence of the iceberg (Ice), and the effect of ice cover late into the nesting season (Ice2) to apparent survival (S) of breeding Adélie penguins at three colonies in a Ross Sea meta-population during 1996–2007. Models were ranked according to Akaike's Information Criterion adjusted for small sample size and overdispersion (QAICc). The model deviance, number of parameters (k), ΔQAICc, and QAICc weights are given for all models. The best structure for resighting rates was retained from a previous model step [p (B,C,R + t)], and a general structure on movement probabilities [psi (B,C,R + t)] was included while modeling survival. A model that included no effect on survival (.) was included for comparison. An equal sign means that colonies were combined, and a comma indicates that they were modeled separately. Asterisks denote interactions, and plus signs denote additive effects.
*Lowest QAICc = 7,779.12.
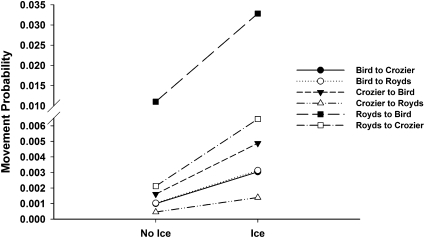
Movement probabilities for breeders varied by colony and unlike apparent survival, also varied in response to the presence of the icebergs. Our best model garnered 84% of the total model weight and included differences in movement probabilities by colony and between years when the icebergs were or were not present (Tables 2 and 3). Movement of breeding adult penguins was consistently higher in the years affected by the icebergs for all colonies (Fig. 3) (β = 0.41; 95% CI = 0.30–1.92), but movements increased the most from Cape Royds to Cape Bird when the iceberg was present. In general, movement probabilities of breeding adults were low (<1%). However, during the iceberg years, movement rates from Cape Royds to Cape Bird more than tripled (>3%).
Table 2.
Model selection results for breeding Adélie penguin movement probabilities
| Model | ΔQAICc* | K | QAICc wt | QDeviance |
| ψ (B,C,R + Ice) | 0.00 | 32 | 0.84 | 1,167.38 |
| ψ (B,C,R + Ice2) | 4.99 | 32 | 0.07 | 1,172.37 |
| ψ (B,C,R*Ice) | 5.85 | 37 | 0.05 | 1,163.12 |
| ψ (B,C,R) | 6.29 | 31 | 0.04 | 1,175.70 |
| ψ (B,C,R*Ice) | 10.43 | 37 | 0.01 | 1,167.70 |
| ψ (.) | 30.44 | 26 | 0.00 | 1,209.93 |
Model selection results for the five best models relating colony (B, Cape Bird; C, Cape Crozier; R, Cape Royds), general time-dependence (t), linear (T), pseudothreshold (lnT), and quadratic (TT) time trends, the presence of the iceberg (Ice), and the effect of ice cover late into nesting season (Ice2) to movement probabilities (ψ) of breeding Adélie penguins at three colonies in a Ross Sea meta-population during 1996–2007. Models were ranked according to Akaike's Information Criterion adjusted for small sample size and overdispersion (QAICc). The model deviance, number of parameters (k), ΔQAICc, and QAICc weights are given for all models. The best structure for resighting rates [p (B,C,R + t)] and apparent survival [S(B = C,R + t)] was retained from previous modeling stages. A model that included no effect on movement rates (.) was included for comparison. An equal sign means that colonies were combined, and a comma indicates that they were modeled separately. Asterisks denote interactions, and plus signs denote additive effects.
*Lowest QAICc = 7,705.88.
Table 3.
Numbers of Adélie penguins originally observed breeding at a particular colony (n = total number of breeding birds) in our Ross Sea meta-population and subsequently, resighted at another location during 1996–2007
| Original breeding colony | Subsequent resighting colony |
|||
| Royds | Bird | Crozier | Beaufort Island | |
| Royds (n = 475) | — | 17 | 4 | 3 |
| Bird (n = 970) | 5 | — | 4 | 1 |
| Crozier (n = 1,236) | 1 | 6 | — | 1 |
Fig. 3.
Movement probability estimates, with 95% confidence limits, for breeding adult Adélie penguins at Capes Royds, Bird, and Crozier, Ross Island, Antarctica from 1996 to 2007.
Discussion
Contrary to current thought, our results show that breeding penguins are not always philopatric and that environmental perturbations can increase dispersal rates. Our results confirm that, in years of little environmental adversity (ice conditions normal), up to 1% of breeding adult birds move from the colony where they are currently breeding to another colony in the meta-population the following year (a rate noted earlier during a period also of little environmental variation and stress) (20). However, we have found that when faced with great environmental adversity (extensive sea ice or blockage to usual migration patterns), movement rates of breeding birds at all colonies in all directions increased; in the case of the birds breeding at Cape Royds, the most severely affected by altered sea ice, movements rates more than tripled. Thus, the perceived costs of moving to an unknown location to breed were outweighed by perceived benefits associated with improved habitat conditions gained by making such movements. Movement rates between colonies also seemed to reflect colony distance, with the lowest rates being between colonies that were the farthest from each other (i.e., Cape Royds to Cape Crozier), consistent with breeding dispersal patterns observed in black-headed gulls (Chroicocephalus ridibundus) (29). Irrespective of environmental conditions, breeding birds at Cape Bird were equally as likely to move to Cape Royds as Cape Crozier, perhaps because of the location of Cape Bird between these two colonies. Therefore, when birds chose to move, they tried to make the shortest movements possible.
In good years and bad, the highest movement probabilities were always from the smallest colony, Cape Royds, to the larger colonies (Cape Bird and Cape Crozier). This was despite the lower foraging effort required at Cape Royds in years of usual conditions (27) compared with Cape Crozier in particular. This is consistent with the meta-population dynamics of the lesser kestrel (Falco naumanni), in which movement rates were always highest from the small colony to the larger colonies; the larger kestrel colonies also had higher apparent survival rates and increased nest success (34). In our case, although the largest colony had the most competition among individuals (as indicated by the need to expand foraging area) (26, 27, 32), it also had the most consistent access to ocean resources because of the adjacent polynya. Thus, in difficult years when extensive sea ice was present, birds breeding at Crozier had a substantially shorter walk to open ocean-food resources than birds breeding at either Royds or Bird.
The 0.5% annual movement rate that we measured means that as many as 1,550 birds breeding at Cape Crozier (i.e., 0.5% of 310,000 breeding birds) or 450 birds breeding at Cape Bird (0.5% of 90,000) move to another colony within the meta-population in any given year. These breeding dispersal rates can be compared with those observed for Audouin's gull (Larus audouinii) on the Ebro Delta, western Mediterranean Sea (1–8%) (15). In that study, movement rates from another colony to the Ebro Delta colony were much higher (20–70%), a rate that the authors believed reflected the higher reproductive success and higher-quality food resources at Ebro Delta (15). This supported the hypothesis that individuals select habitat where their fitness potential is increased (34–36). Despite the decreased energetic costs of foraging at Cape Royds during most years when ice conditions were normal, productivity (chicks fledged per pair) was slightly lower than at other colonies (27). Furthermore, at Cape Royds during the iceberg/extensive sea-ice years, unusual weather conditions, high rates of nest desertion, and disproportionate predation (relative to the other colonies) from skuas (Stercorarious maccormicki) resulted in near-complete nesting failure. We conjecture that the high-movement rates that we observed for Cape Royds are a response to the poor reproductive success most breeders experienced during years of adverse conditions.
As predicted, despite lower foraging trip durations and energetic requirements for individuals breeding at Royds compared with the other colonies (27), apparent survival was lowest at Royds compared with Bird and Crozier. This lower apparent survival rate likely contributes to Royds remaining the smallest of the study colonies in the meta-population. The tradeoff between decreased energetic requirements for breeding at Royds during typical environmental conditions may not be enough to compensate for increased energetic demands (resulting in decreased reproductive success) during years that the iceberg and/or extensive seas ice were present and decreased apparent survival during all years.
Apparent survival differences between birds breeding at Royds compared with Bird or Crozier could be related to differences in migration chronology and/or winter habitat use (37). Egg laying occurs later at Royds compared with Bird and Crozier (∼1 wk), and many birds remain at the Royds colony to molt, which does not happen appreciably at either Crozier or Bird (38). This means that postbreeding birds from Cape Royds find a different sea-ice regimen than that encountered by Crozier or Bird breeders after they depart the colony at season end (37). In particular, the delay in departure for wintering areas means that Royds penguins sometimes have to cross an extensive and growing expanse of pack ice (hundreds of kilometers). Both situations may result in increased mortality. It is common for small penguin colonies to be in areas of extensive fast ice (e.g., Cape Royds, Edmonson Point, Bechervaise, and Syowa; all intensively studied) (19), whereas larger colonies usually have better access to open water (e.g., Cape Crozier and Cape Adare) (19, 39). However, apparent survival rates reported for Edmonson Point are higher (0.77–0.95; mean = 0.85 ± 0.01; 1994–2005) (17) than those for the Ross Island penguins (uncorrected for potential band loss), especially compared with Cape Royds. Thus, a small colony size does not necessarily imply lower survival rates. It is probably important that the amount of fast ice that Royds penguins have had to cross during years when the iceberg was present or when winds were reduced is more than any other Adélie colony in the world. For example, the Edmonson Point colony enjoys the unusually persistent Terra Nova Bay Polynya, which likely decreases annual variation in habitat availability or quality.
An alternative explanation for observed survival-rate differences is that because we are measuring apparent survival, Royds breeders may be moving to other colonies, outside our study area, at a higher rate than breeders from Cape Bird or Cape Crozier, even if just to Beaufort (Table 3 and Fig. 3); 3 of 475 (0.6%) adult breeders from Royds did emigrate to Beaufort Island over the 12 y of this study compared with 0.1% and 0.08% for Bird and Crozier, respectively. Although we coded these specific birds as losses on capture, so as not to bias survival-rate estimates after they moved out of our three-colony analysis area, it is possible that true emigration rates from Royds to Beaufort are substantially higher given that our resighting effort at Beaufort was often quite low (i.e., we may have missed the movements of at least this many birds). Thus, higher movement rates in general for Royds birds may translate to higher rates of permanent emigration outside our survey area, which may contribute to decreased apparent survival estimates at Royds compared with Bird and Crozier. However, the differences in apparent survival we noted between Royds birds and those birds breeding at the other colonies were generally much higher (4%) than the observed dispersal rate from Royds to Beaufort. Most likely, all three factors (wintering location, migration chronology, and permanent emigration) contribute to the lower apparent survival rates observed for breeding birds at Cape Royds.
Annual variation in apparent survival of Adélie penguins is high (40, 17). However, incorporating estimates of movement probabilities and increasing the sample of breeding birds to include those banded as adults and those banded as chicks that return to breed has resulted in less annual variation than observed in an earlier study that incorporated a smaller subset of adult breeding birds (40). The general range in survival rates observed in this study were comparable with those for banded birds from a specific subcolony at Cape Crozier between 1996 and 2004, although very high apparent survival rates during 1996 and 1997 were observed for those birds and not in this study (40). The amount of variation seen in movement and apparent survival, in retrospect, is not surprising in the face of the extreme environmental variation seen at these colonies, the most southern colonies of Adélie penguins.
Materials and Methods
A long-term study of demography and foraging ecology was initiated in 1996 at four adjacent colonies on Ross and Beaufort Islands in the southern Ross Sea. These colonies varied in size by orders of magnitude, with the Cape Royds the smallest (1,200–4,000 breeding pairs), Cape Bird (35,000–50,000 breeding pairs), and Beaufort Island (35,000–50,000 pairs) intermediate in size; Cape Crozier is one of the six largest Adélie penguin colonies in the world (118,000–155,000 breeding pairs). Beaufort Island was difficult to access regularly and so, is not included in the modeling. Breeding birds observed at Beaufort Island who had previously been observed breeding elsewhere were coded in the mark-recapture histories as a loss on capture, because we knew they had left our three-colony modeling space. If these birds were ever observed back at one of the intensively studied colonies, they were added back to the database as a new bird. Thus, we lost some survival data on these birds for the years that they were at Beaufort Island, but this occurred for relatively few birds during the study and this general approach reduced the potential negative bias in apparent survival associated with birds that we knew emigrated to Beaufort Island.
Penguin chicks were banded on the left flipper with a numbered stainless steel flipper band following the Boersma design from Porzana (40). These were applied just before fledging at all four colonies beginning in 1996. In addition, 1,274 adult (but otherwise unknown age) breeding birds were banded (three colonies combined) between 1996 and 2003. Each colony was searched on 2- to 7-d intervals (depending on the colony) throughout the breeding season, and bands were read with binoculars from a distance (<10 m). Thus, birds were not physically recaptured. Here, we report resighting, apparent survival and movement rates for breeding adult Adélie penguins between 1996 and 2007 in relation to environmental change. Birds banded as chicks first occurred in our dataset when they recruited into the breeding population (1,407 total). We did not include adjustments for band loss, which we have documented occasionally over the 12 y of this study. At this time, we have no estimates of this potential bias, although it is a factor we are trying to quantify using birds both banded and tagged with a microchip.
Environmental Conditions.
Two large icebergs, called B-15 and C-16, calved from the Ross Ice Shelf in March 2000 and by January 2001, had lodged against Ross Island, physically separating Cape Crozier from the other colonies. Positioned as they were, these icebergs (i) served as physical barriers to penguin movement between Cape Crozier and the other colonies, (ii) significantly altered the spring migration route of all but the Crozier colony, and (iii) in some years, restricted the normal annual diminution of spring/summer pack ice (31). Figure 3 in ref. 22 shows the layout of colonies and icebergs. This scenario exacerbated the effects of unusual winter wind patterns that resulted in compacted ice conditions and delayed pack ice break-up in some years in waters adjacent to the western colonies (2004 and 2005 in particular but not 2003). When extensive ice conditions persisted into the chick-rearing season, penguin foraging effort was increased, and productivity was decreased (32), which might reflect increased costs of reproduction. Thus, the presence of the icebergs, and resulting extensive sea ice, was expected to impact both penguin movements between colonies and apparent survival.
We tested the direct effect of icebergs and extensive sea ice as barriers or facilitators of dispersal and movement. Following the study by Lescroël et al. (31), years 1996–2000 and 2006 were considered not impacted by the cumulative effects of the icebergs, and years 2001–2005 were considered iceberg years. In addition, extensive spring/summer pack ice persistence in McMurdo Sound, western side of Ross Island (shores of Capes Royds and Bird), occurred at least one time in the years before the iceberg (1996–2000), and normal spring/summer ice conditions were present in 1 y when the icebergs were present (2003). Therefore, in addition to the iceberg covariate that incorporated the physical presence of the icebergs as a barrier to dispersal, we looked at the effects of extensive sea ice cover alone as a proxy of increased reproductive costs that we might predict to decrease apparent survival. Extensive sea ice could also discourage breeders from returning to, for instance, Cape Royds. Under this scenario, the 5 y with extensive sea ice during the breeding season included 1999, 2001–2002, and 2004–2005.
Finally, we might expect increased movements or impacts on breeding propensity associated with the iceberg effects to decrease resighting rates, perhaps through a decrease in time spans of visitation. Thus, we used the iceberg covariate to model capture rates as well.
Statistical Analysis.
We used multistate mark-recapture modeling (23, 24) to estimate apparent survival (S), resighting rates (p), and the probability of moving from one colony to another (R = Royds, B = Bird, C = Crozier) between years (ψ). The fourth colony in our meta-population (Beaufort Island) was visited by us infrequently, and thus, resightings were incomplete. Undetected permanent emigration to this fourth colony, or other colonies outside our surveyed study area, likely did result in reduced estimates of survival (true survival × site fidelity = apparent survival), but the use of a larger geographic scale and movement probabilities from multistate models decreased this bias. We used program MARK to generate estimates and model-selection results (41). We used UCARE (42) to generate goodness-of-fit statistics for the more general model (JMV) (43), which allows capture probabilities to vary by state of arrival in addition to full-time dependence. The JMV model also includes full-time dependent apparent survival and movement probabilities. Goodness-of-fit results suggested that we had some memory structuring in our data (non-Markovian transitions) and some temporary emigration, both of which can be addressed by the use of an overdispersion parameter that inflates SEs and adjusts for lack of independence in the data (42). We used the median c-hat procedure available in Program MARK to generate estimates of the overdispersion parameter  , because estimates closer to the truth are believed to result particularly when temporary emigration is present.
, because estimates closer to the truth are believed to result particularly when temporary emigration is present.
In addition to covariates associated with ice conditions, we investigated the effects of colony and time on apparent survival, movement, and resighting rates. In addition to general time dependence, we investigated trends in resighting rates and apparent survival. We did not hypothesize any time trends on movement probabilities but rather, only general time effects or the influence of the iceberg. Because of the large number of parameters and covariates of interest, we modeled parameters in stages. Resighting rates were modeled first, and the best structure was retained as we moved on to model apparent survival rates. The best model for resighting rates and apparent survival was retained as we modeled movement probabilities. At each stage, the best models were cross-checked against competitive models from a previous stage to be sure that we apportioned variation correctly among parameters and effects.
We used an information theoretic approach to generate a priori model sets and then, selected best models and effects at each stage (44). For model selection, we used Akaike's Information Criteria adjusted for small sample size and overdispersion (QAICc), differences between model QAICc and the model with the lowest QAICc (ΔQAICc = model QAICc − minimum QAICc), and Akaike weights (44). Generally, we selected the best model for inference based on the lowest QAICc, but we also used estimates of regression coefficients (β) and their 95% confidence limits to provide additional information (strength of evidence) for specific effects.
Acknowledgments
We thank the following people for help in the field: S. Allen, I. Gaffney, C. Gjerdrum, D. Hardesty, S. Heath, M. Hester, R. Orben, C. Ribic, B. Saenz, V. Toniolo, L. Blight, J. Blum, A. Lescroël, C. McCreedy, R. Orben, V. Patil, L. Sheffield., B. Karl, W. Cook, K. Drew, and P. Dilks. We also thank G. White and K. Burnham for statistical consultation and S. Olmastroni, who provided regular band-searching effort at Terra Nova Bay colonies from 1996 to 2006. Critical comments from two anonymous reviewers were greatly appreciated. This is PRBO Conservation Science Contribution 1734. Fieldwork was conducted with logistic support provided by the US Antarctic Program and Antarctica New Zealand. Permits were provided under the Antarctic Conservation Act. National Science Foundation Office of Polar Programs (2006-10) (04-40643) and Landcare Research Animal Ethics Committee Permit (0509/01) Programme. Funding was provided by National Science Foundation Grants OPP 9526865, 9814882, 0125608, and 0440643 (to K.M.D., D.G.A., and G.B.) and New Zealand Foundation for Research, Science, and Technology Grant C09X0510 with addenda (to P.O’B.L. and K.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Bennetts RE, et al. Methods for estimating dispersal probabilities and related parameters using marked animals. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 3–17. [Google Scholar]
- 2.Schwarz CJ. In: Modeling Demographic Processes in Marked Populations. Thomson DL, Cooch EG, Conroy MJ, editors. New York: Springer; 2009. pp. 323–347. [Google Scholar]
- 3.Greenwood PJ. Mating systems, philopatry, and dispersal in birds and mammals. Anim Behav. 1980;28:1140–1162. [Google Scholar]
- 4.Greenwood PJ, Harvey PH. The natal and breeding dispersal of birds. Annu Rev Ecol Syst. 1982;13:1–21. [Google Scholar]
- 5.Gandon S, Michalakis Y. Multiple causes of the evolution of dispersal. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 155–167. [Google Scholar]
- 6.Ims RA, Hjermann DØ. Condition-dependent dispersal. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 203–216. [Google Scholar]
- 7.Stamps JA. Habitat selection by dispersers: integrating proximate and ultimate approaches. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 230–242. [Google Scholar]
- 8.Lambin X, Aars J, Piertney SB. Dispersal, intraspecific competition, kin competition and kin facilitation: a review of the empirical evidence. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 110–122. [Google Scholar]
- 9.Weisser WW. The effects of predation on dispersal. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 180–188. [Google Scholar]
- 10.Murren CJ, Julliard R, Schlichting CD, Clobert J. Dispersal, individual phenotype, and phenotypic plasticity. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 261–272. [Google Scholar]
- 11.Dufty AM, Jr, Belthoff JR. Proximate mechanisms of natal dispersal: the role of body condition and hormones. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 217–229. [Google Scholar]
- 12.Hanski I. Population dynamic consequences of dispersal in local populations and in metapopulations. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 283–298. [Google Scholar]
- 13.Roff DA, Fairbairn DJ. The genetic basis of dispersal and migration, and its consequences for the evolution of correlated traits. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 191–202. [Google Scholar]
- 14.Spendelow JA, et al. Estimating annual survival and movement rates of adults within a metapopulation of roseate terns. Ecology. 1995;76:2415–2428. [Google Scholar]
- 15.Cam E, Oro D, Pradel R, Jimenez R. Assessment of hypotheses about dispersal in a long-lived seabird using multistate capture-recapture models. J Anim Ecol. 2004;73:723–736. [Google Scholar]
- 16.Le Bohec C, et al. King penguin population threatened by Southern Ocean warming. Proc Natl Acad Sci USA. 2008;105:2493–2497. doi: 10.1073/pnas.0712031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballerini T, Tavecchia G, Olmastroni S, Pezzo F, Focardi S. Nonlinear effects of winter sea ice on the survival probabilities of Adélie penguins. Oecologia. 2009;161:253–265. doi: 10.1007/s00442-009-1387-9. [DOI] [PubMed] [Google Scholar]
- 18.Jenouvrier S, et al. Demographic models and IPCC climate projections predict the decline of an emperor penguin population. Proc Natl Acad Sci USA. 2009;106:1844–1847. doi: 10.1073/pnas.0806638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ainley DG. The Adélie Penguin: Bellwether of Climate Change. New York: Columbia University Press; 2002. [Google Scholar]
- 20.Ainley DG, LeResche RE, Sladen WJL. The Breeding Biology of the Adélie Penguin. Berkeley, CA: University of California Press; 1983. [Google Scholar]
- 21.Roeder AD, et al. Gene flow on the ice: Genetic differentiation among Adélie penguin colonies around Antarctica. Mol Ecol. 2001;10:1645–1656. doi: 10.1046/j.0962-1083.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd LD, et al. Microevolution and mega-icebergs in the Antarctic. Proc Natl Acad Sci USA. 2005;102:16717–16722. doi: 10.1073/pnas.0502281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hestbeck JB, Nichols JD, Malecki RA. Estimates of movement and site fidelity using mark-resight data of wintering Canada geese. Ecology. 1991;72:523–533. [Google Scholar]
- 24.Brownie C, Hines JE, Nichols JD, Pollock KH, Hestbeck JB. Capture-recapture studies for multiple strata including non-Markovian transitions. Biometrics. 1993;49:1173–1187. [Google Scholar]
- 25.Ainley DG, et al. Diet and foraging effort of Adélie penguins in relation to pack-ice conditions in the southern Ross Sea. Polar Biol. 1998;20:311–319. [Google Scholar]
- 26.Ballance LT, Ainley DG, Ballard G, Barton K. Colony size and foraging effort in seabirds: Is there an energetic correlate? J Avian Biol. 2009;40:279–288. [Google Scholar]
- 27.Ainley DG, et al. Geographic structure of Adélie penguin populations: Overlap in colony-specific foraging areas. Ecol Monogr. 2004;74:159–178. [Google Scholar]
- 28.Ballard G. Biotic and physical forces as determinants of Adélie penguin population location and size. 2010 PhD thesis (University of Auckland, Auckland, New Zealand) [Google Scholar]
- 29.Brown CR, Covas R, Anderson MD, Brown MB. Multistate estimates of survival and movement in relation to colony size in the sociable weaver. Behav Ecol. 2003;14:463–471. [Google Scholar]
- 30.Péron G, Lebreton J-D, Crochet P-A. Breeding dispersal in black-headed gull: The value of familiarity in a contrasted environment. J Anim Ecol. 2010;79:317–326. doi: 10.1111/j.1365-2656.2009.01635.x. [DOI] [PubMed] [Google Scholar]
- 31.Lescroël A, Dugger KM, Ballard G, Ainley DG. Effects of individual quality, reproductive success and environmental variability on survival of a long-lived seabird. J Anim Ecol. 2009;78:798–806. doi: 10.1111/j.1365-2656.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- 32.Lescroël A, et al. Working less to gain more: When breeding quality relates to foraging efficiency. Ecology. doi: 10.1890/09-0766.1. in press. [DOI] [PubMed] [Google Scholar]
- 33.Arrigo KR, van Dijken GL, Ainley DG, Fahnestock MA, Markus T. The impact of the B-15 iceberg on productivity and penguin breeding success in the Ross Sea, Antarctica. Geophys Res Lett. 2002 doi:10.1029/2001GL014160. [Google Scholar]
- 34.Serrano D, Oro D, Ursua E, Tella JL. Colony size selection determines adult survival and dispersal preferences: Allee effects in a colonial bird. Am Nat. 2005;166:E22–E31. doi: 10.1086/431255. [DOI] [PubMed] [Google Scholar]
- 35.Danchin E, Bouilinier T, Massot M. Conspecific reproductive success and breeding habitat selection: Implications for the study of coloniality. Ecology. 1998;79:2415–2428. [Google Scholar]
- 36.Holt RD, Barfield M. On the relationship between the ideal free distribution and the evolution of dispersal. In: Clobert J, Danchin JE, Dhondt AA, Nichols JD, editors. Dispersal. London: Oxford University Press; 2001. pp. 83–95. [Google Scholar]
- 37.Ballard G, et al. Responding to climate change: Adélie penguins confront astronomical and ocean boundaries. Ecology. doi: 10.1890/09-0688.1. in press. [DOI] [PubMed] [Google Scholar]
- 38.Taylor RH. The Adélie Penguin Pygoscelis adeliae at Cape Royds. Ibis. 1962;104:176–204. [Google Scholar]
- 39.Arrigo KR, van Dijken GL. Impact of iceberg C-19 on Ross Sea primary production. Geophys Res Lett. 2003 doi:10.1029/2003GL017721. [Google Scholar]
- 40.Dugger KM, Ballard G, Ainley DG, Barton KJ. Effects of flipper bands on foraging behaviour and survival of Adélie penguins (Pygoscelis adeliae) Auk. 2006;123:858–869. [Google Scholar]
- 41.White GC, Kendall WL, Barker RJ. Multistate survival models and their extensions in Program MARK. J Wildl Manage. 2006;70:1521–1529. [Google Scholar]
- 42.Choquet R, Lebreton J-D, Gimenez O, Reboulet A-M, Pradel R. U-CARE: Utilities for performing goodness of fit tests and manipulating capture-recapture data. Ecography. 2009;32:1071–1074. [Google Scholar]
- 43.Pradel R. In: Marked Individuals in the Study of Bird Populations. Lebreton J-D, North PM, editors. Basel: Birkaüser; 1993. pp. 9–28. [Google Scholar]
- 44.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd Ed. New York: Springer; 2002. [Google Scholar]