Abstract
The number of memory CD8 T cells generated by infection or vaccination correlates strongly with the degree of protection observed in infection and tumor models. Therefore, rapid induction of protective numbers of effector and memory CD8 T cells may be crucial in the case of malignancy, pandemic infection, or bioterrorism. Many studies have shown that amplifying T-cell numbers by prime-boost vaccination is most effective with a substantial time interval between immunizations. In contrast, immunization with peptide-coated mature dendritic cells (DCs) results in a CD8 T-cell response exhibiting accelerated acquisition of memory characteristics, including the ability to respond to booster immunization within days of initial priming. However, personalized DC immunization is too costly, labor intensive, and time-consuming for large-scale vaccination. Here, we demonstrate that in vivo cross-priming with cell-associated antigens or antigen-coated, biodegradable microspheres in the absence of adjuvant quickly generates CD8 T cells that display the phenotype and function of long-term memory populations. Importantly, cross-primed CD8 T cells can respond to booster immunization within days of the initial immunization to generate rapidly large numbers of effector and memory T cells that can protect against bacterial, viral, and parasitic infections, including lethal influenza and malaria-causing Plasmodium infection. Thus, accelerated CD8 T-cell memory after in vivo cross-priming in the absence of adjuvant is generalizable and can be exploited to generate protective immunity rapidly.
Keywords: protective immunity, vaccination
CD8 T cells are critical in protecting the host from infection by intracellular pathogens. During infection, antigen-specific CD8 T cells undergo proliferative expansion to increase in number, followed by contraction and generation of a stable pool of long-lived memory cells that provide enhanced resistance to reinfection (1). The number of memory CD8 T cells correlates strongly with the level of protection in experimental models of infection (2–5). To date, prime-boost immunization remains the most successful approach to generate high numbers of memory T cells and enhanced resistance (6, 7). However, most current prime-boost strategies, which are based on the use of adjuvants to amplify initial T-cell responses, require several months between each immunization to achieve the greatest amplification of immunological memory. Clearly, reducing the time interval between priming and boosting would be beneficial in the case of pandemic outbreaks or in immunotherapy of cancer, when time is of the essence.
Infection of mice with intracellular pathogens stimulates robust CD8 T-cell responses that initially exhibit an “effector” phenotype and acquire memory phenotype and function relatively slowly after the infection is cleared (5, 8). Similarly, subunit vaccines that use adjuvants to mimic the inflammatory conditions of infection also induce T-cell responses that are slow to acquire memory function (9–11). In contrast, immunization with peptide-coated mature dendritic cells (DC) in the absence of additional adjuvant evokes CD8 T cells that display memory characteristics within days of the initial priming (12). Importantly, systemic inflammatory cytokines induced by infection or adjuvant prevent accelerated memory differentiation by DC-primed CD8 T cells. Thus, priming of naïve CD8 T cells by mature DC in the absence of systemic inflammation is key to evoking a response that can respond rapidly to booster immunization. However, the laborious and personalized nature of DC immunization is a major hurdle for translating this approach to large-scale vaccination of outbred humans. Overcoming this limitation requires an “off-the-shelf” approach to immunization that induces little systemic inflammation but still results in maturation of DC capable of stimulating CD8 T-cell responses. Here, we demonstrate an alternative vaccination strategy that exploits the cross-priming pathway in the absence of adjuvants to generate rapidly protective CD8 T-cell immunity against multiple pathogens.
Results
Cross-Priming with Cell-Associated Antigen Accelerates CD8 T-Cell Memory.
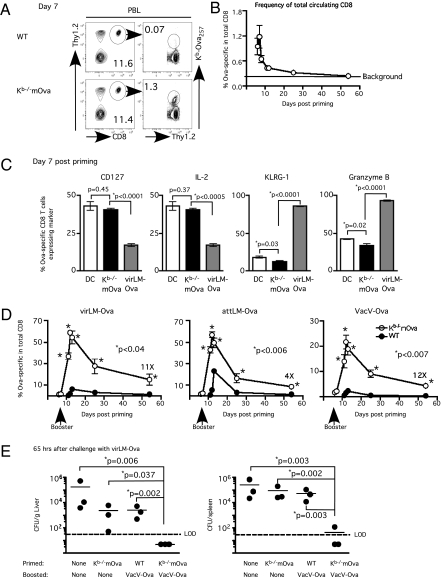
Disposal of apoptotic cells by DC limits inflammation and provides a mechanism for cross-priming CD8 T cells against cell-associated antigens (13). Immunization of naïve C57BL/6 (B6) mice with irradiated Act-mOva.Kb−/− splenocytes, which cannot directly present the ovalbumin (Ova) epitope (14), cross-primed functional H-2Kb–restricted Ova257-specific CD8 T cells that are detectable in both peripheral blood and spleen (Fig. 1 A and B and Fig. S1A) in the absence of overt systemic inflammation (Fig. S1B). More importantly, Ova257-specific CD8 T cells that were primed with either DC-Ova257 or irradiated Act-mOva.Kb−/− splenocytes acquired phenotypic (CD127hi, KLRG-1lo) and functional (∼35–40% produced IL-2 after antigen stimulation and exhibited low granzyme B) memory characteristics at day 7 after immunization (Fig. 1C and Fig. S1C) (3, 12). This result contrasts sharply with the effector phenotype (CD127lo, KLRG-1hi) and function (reduced IL-2, increased frequency of granzyme B-expressing cells) of Ova-specific CD8 T cells stimulated by infection with Listeria monocytogenes expressing Ova (LM-Ova). Thus, similar to DC immunization, cross-priming CD8 T cells with cell-associated antigen results in accelerated acquisition of memory phenotype and function.
Fig. 1.
Cross-priming with cell-associated antigen followed by short-interval booster immunization rapidly generates protective CD8 T-cell immunity. Naïve C57BL/6 (B6) mice received ∼107 irradiated WT or Kb−/−mOva splenocytes (i.v.). (A) Detection by Kb/Ova257 tetramer staining. (B) Kinetics of Ova257-specific CD8 T-cell response (mean frequency ± SEM, n = 3) in PBL. (C) Phenotypic and functional status of Ova257-specific CD8 T cells at day 7 after DC immunization, cross-priming, or virLM-Ova infection (mean ± SEM, n = 3). (D) Kinetics of Ova257-specific CD8 T-cell response (mean frequency ± SEM, n = 3) in PBL with different booster immunizations as indicated. Numbers indicate fold difference at day 54. (E) Bacteria count (mean ± SEM, n = 3) in spleen and liver ∼65 h after a lethal dose of virLM-Ova. LOD, limit of detection. *Statistical analysis was performed using an unpaired, two-tailed t test.
Cross-Primed CD8 T Cells Respond Vigorously to Short-Interval Boosting.
A cardinal feature of memory CD8 T cells is their robust proliferative response upon reexposure to antigen (1). Consistent with their accelerated memory phenotype, Ova257-specific CD8 T cells in cross-primed mice underwent vigorous secondary expansion in response to three different booster regimens: virulent Listeria monocytogenes expressing Ova (virLM-Ova), attenuated actA-deficient Listeria monocytogenes expressing Ova (attLM-Ova), and Vaccinia virus expressing the Ova257–264 epitope (VacV-Ova) delivered at day 7 after initial immunization (short-interval booster immunization) (Fig. 1D). Listeria boosting induced an enormous response in cross-primed mice: ∼60% of circulating CD8 T cells in peripheral blood were specific for the Ova257 epitope within 1 wk after boosting. Importantly, this enormous CD8 T-cell response was not observed in mice that received the booster immunizations after initial priming with irradiated WT splenocytes without Ova (Fig. 1D) and thus is a function of the presence of initially cross-primed CD8 T cells. As observed in our DC immunization model, inducing systemic inflammation with CpG oligodeoxynucleotide, a Toll-like receptor 9 agonist, prevented rapid acquisition of memory characteristics by cross-primed CD8 T cells (Fig. S2 A–C). Thus, cross-priming accelerates memory CD8 T-cell differentiation and secondary potential response to booster immunizations only in the absence of adjuvant-induced inflammation.
To assess protection, we challenged mice cross-primed with the Act-mOvaKb−/− splenocyte and boosted with VacV-Ova memory with a lethal dose of virLM-Ova; the only shared antigen was the Ova257 epitope. Mice cross-primed and boosted with VacV-Ova cleared the bacterial challenge much more efficiently than naïve mice, mice immunized with irradiated Act-mOva.Kb−/− splenocytes alone, or mice irradiated with WT splenocytes and boosted with VacV-Ova (Fig. 1E). Thus, initial cross-priming against cell-associated antigen plus short-interval booster immunization stimulates large numbers of effector and memory CD8 T cells capable of long-term protection against bacterial challenge.
Cross-Priming with Autologous Peripheral Blood Mononuclear Cells.
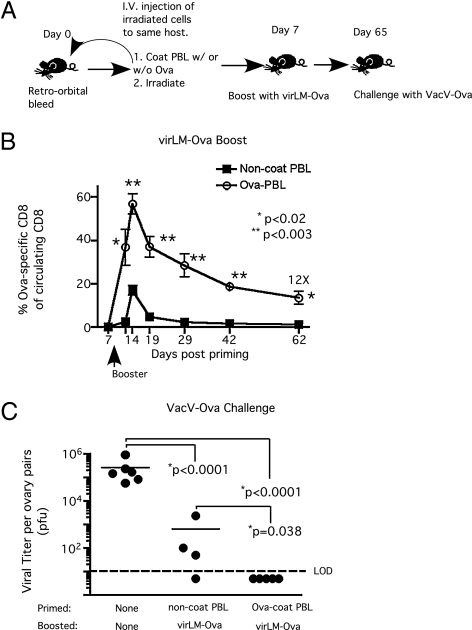
To avoid alloreactivity, cross-priming CD8 T-cell responses in humans would require reinfusion of syngeneic antigen-coated PBMC. To address the feasibility of this approach, we first determined that 106 irradiated Act-mOva.Kb−/− splenocytes (Fig. S3 A and B) or 106 irradiated Ova-coated syngeneic splenocytes (Fig. S4 A–C) primed CD8 T cells capable of responding to short-interval boosting. We were able to obtain 106 peripheral blood mononuclear cells (PBMC) from ∼150 μL of mouse blood. Next, we isolated PBMC from individual mice, coated the cells with Ova protein, and irradiated the cells before reinjecting them into the same donor (Fig. 2A). Mice initially immunized with autologous Ova-coated PBMC generated enormous numbers of Ova257-specific effector CD8 T cells (>50% of the circulating CD8 T-cell compartment) in response to short-interval booster immunization. Importantly, compared with control mice, this autologous “cross-prime plus short-interval booster” approach also generated ≈12-fold higher numbers of memory cells 62 d later (Fig. 2B), which led to enhanced clearance of VacV-Ova infection from the ovaries after challenge (Fig. 2C). Thus, initial vaccination with antigen-coated autologous PBMC followed by short-interval booster immunization provides a potentially useful strategy to generate quickly individualized long-term antiviral CD8 T-cell immunity.
Fig. 2.
Protective CD8 T-cell immunity can be achieved rapidly by cross-priming with antigen-coated, irradiated autologous PBMC followed by short-interval booster immunization. (A) Experimental design: PBMC were obtained from individual mice via retro-orbital bleeding, coated with full-length Ova protein in PBS or with PBS only, irradiated, and returned to the same donor mouse. Control mice received irradiated autologous PBMC without Ova coating. Mice received a virLM-Ova (∼105 cfu/mouse) booster immunization 7 d after priming. (B) Kinetics of Ova257-specific CD8 T-cell response (mean frequency ± SEM, n = 5) in PBL. (C) Vaccinia viral titer per ovary pair 3 d after a high-dose VacV-Ova challenge (∼5 × 107 pfu/mouse, i.v.). Naïve or memory mice were challenged with VacV-Ova on day 65 after priming. *Statistical analysis was performed using an unpaired, two-tailed t test.
A Universal Cross-Priming Vehicle.
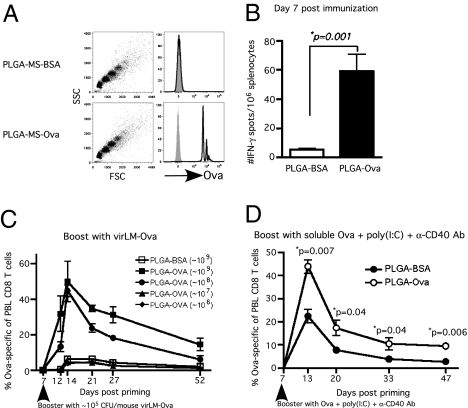
In the case of a pandemic outbreak, rapid formulation and deployment of an effective vaccine would be critical for protecting the population. This issue has been underscored by the delay in producing sufficient vaccine to immunize the entire population against the H1N1 pandemic of 2009 (15). Pathogen subunit antigens produced by recombinant DNA technology or purified from infected cells or cultures provide an attractive target for rapid vaccine formulation. In addition, professional antigen-presenting cells (APCs) present exogenous particulate antigen to CD8 T cells much more efficiently than soluble antigen (16). Particulate formulations of antigen encapsulated in biodegradable particles such as poly(lactic-coglycolic) acid (PLGA) microspheres or nanospheres have been explored to improve the efficiency of cross-priming CD8 T cells both in vitro and in vivo (17–19). Importantly, the prevailing notion in the field is that adjuvants are absolutely essential to induce T-cell responses to antigens delivered by PLGA microspheres. To determine whether a particulate antigen cross-primes CD8 T cells with an accelerated secondary response potential in the absence of adjuvant, we adsorbed PLGA microspheres with full-length Ova protein (Fig. 3A). Immunizing mice with ∼109 or 108, but not lower numbers, of Ova-coated PLGA microspheres in the absence of adjuvant cross-primed low numbers of Ova257-specific CD8 T cells, which were detectable only using the highly sensitive IFN-γ ELISPOT assay (Fig. 3B). However, these cross-primed CD8 T cells were again capable of enormous secondary CD8 T-cell responses to short-interval virLM-Ova booster (Fig. 3C). Thus, Ova-coated PLGA microspheres cross-primed weak Ova257-specific CD8 T-cell responses that can be amplified massively by short-interval booster immunization. Importantly, this result is clearly based on cross-priming against particulate antigen, because immunizing mice with twice the amount of soluble Ova did not prime a boostable CD8 T-cell response (Fig. S5). Commercial preparations of Ova protein may be contaminated with endotoxin. However, immunizing mice with EndoGrade [essentially lipopolysaccharide (LPS) free] Ova-coated PLGA microspheres also cross-primed CD8 T cells that responded vigorously to short-interval booster immunization, whereas addition of LPS to the EndoGrade Ova-coated PLGA microspheres abrogated the robust booster response (Fig. S5). Thus, cross-priming with antigen-coated PLGA microspheres is not an artifact of LPS contamination, and, when followed by short-interval booster immunization offers an attractive, potentially off-the-shelf approach to generate a high number of antigen-specific CD8 T cells rapidly.
Fig. 3.
Cross-priming with Ova-adsorbed, biodegradable PLGA microspheres followed by short-interval booster immunization quickly generates robust Ova257-specific CD8 T cells. (A) Adsorbed Ova protein on the surface of PLGA microspheres was detected with Ova-specific antibody by flow cytometry before mice were immunized (shaded histograms: isotype controls). (B–D) Naïve B6 mice were immunized with ∼109 (B and D) or different (C) doses of Ova-adsorbed PLGA microspheres (∼109, ∼108, ∼107, or ∼106) or with ∼109 BSA-adsorbed PLGA microspheres as control and were analyzed at day 7 after priming (B) for Ova257-specific CD8 T cells by mouse IFN-γ ELISPOT assay or were boosted i.p. with either (C) virLM-Ova (∼105 cfu/mouse) or (D) 500 μg full-length Ova protein plus poly(I:C) (100 μg) plus anti-CD40 mAb (clone 1C10). C and D show kinetics of Ova257-specific CD8 T-cell response in PBL as detected by Kb/Ova257 tetramer staining (mean frequency ± SEM, n = 4). *Statistical analysis was performed using an unpaired, two-tailed t test.
Boosting with infectious agents may complicate translation of this approach to humans. To determine if noninfectious booster immunizations were effective, we primed mice with Ova-coated PLGA microspheres and boosted with soluble Ova protein plus poly(I:C) plus α-CD40 monoclonal antibody (20). This noninfectious booster regimen also massively amplified the Ova257-specific CD8 T-cell effector and memory responses in Ova-PLGA–immunized mice compared with control mice (Fig. 3D). Together, these data demonstrate that cross-priming with antigen-coated biodegradable microspheres followed by short-interval boosting can rapidly generate extremely high numbers of effector and memory CD8 T cells against both infectious and noninfectious booster immunizations.
Cross-Priming Plus Short-Interval Boosting and Immunity Against Influenza.
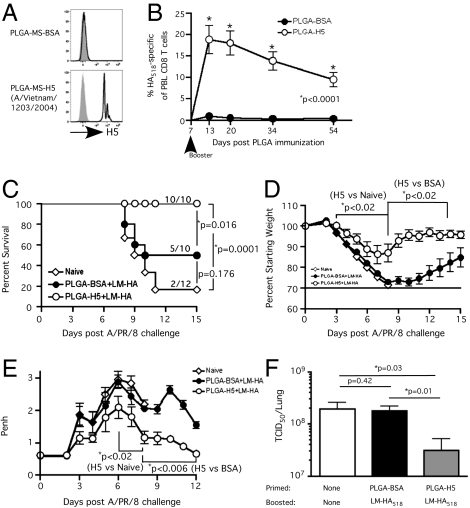
CD8 T cells are critical in controlling and eliminating respiratory infections, especially those caused by highly pathogenic strains of influenza viruses (21, 22). Furthermore, CD8 T cells specific for conserved or cross-reactive epitopes have been shown to mediate heterosubtypic cell-mediated immunity against influenza strains that differ in HA serotypes and thus are not subject to clearance by preexisting antibodies (23–25). To test the utility of our approach in a model of pandemic infection, we immunized mice with either H5- [from A/Vietnam/1203/2004 (H5N1)] or BSA-coated PLGA microspheres (Fig. 4A) and boosted with attenuated Listeria monocytogenes expressing the HA-IYSTVASSL epitope (attLM-HA518) at day 7 after immunization. Both the avian H5 protein and the HA derived from influenza strain A/PR/8/34 (H1N1) encode the H-2Kd–restricted epitope, IYSTVASSL. Booster immunization elicited robust IYSTVASSL-specific effector (∼20% of circulating CD8 T cells within 13 d after initial priming) and memory (∼10% of circulating CD8 T cells at >50 d after priming) CD8 T cells in mice immunized with H5-coated PLGA microspheres as compared with control-immunized mice (Fig. 4B). Naïve and memory-immune mice then were challenged with a lethal dose of the serologically distinct (H1N1) influenza strain A/PR/8/34. In this scenario, the immune mice had never seen the H1 protein and therefore lacked serotype-specific neutralizing antibodies. Thus, the model system mimics heterosubtypic CD8 T-cell–dependent immunity. Importantly, the majority of naïve mice and 50% of control immune mice succumbed to the influenza infection, whereas all the mice in the cross-prime plus short-interval boost group survived (Fig. 4C). Furthermore, mice in the cross-prime plus short-interval boost group exhibited less morbidity (weight loss) and better lung function and recovered faster after influenza infection (Fig. 4 D and E). Additionally, the mice in the cross-prime plus short-interval boost group had significantly reduced lung viral titer at day 3 after influenza challenge than either naïve or control immune mice, suggesting that higher numbers of IYSTVASSL-specific memory cells were able to control the infection (Fig. 4F).
Fig. 4.
Protective heterosubtypic immunity against lethal influenza. (A) Detection of recombinant HA H5 (A/Vietnam/1203/2004) on PLGA microspheres by flow cytometry before mice were immunized. Shaded histograms indicate isotype controls. (B–F) Naïve BALB/c mice were immunized with ∼109 H5- or BSA-coated PLGA microspheres and received booster immunization with attLM-HA518 (Kd/IYSTVASSL) (∼107 cfu/mouse) on day 7. (B) Kinetics of HA518-specific CD8 T-cell response as detected by Kd/HA518 tetramer staining and expressed as mean frequency ± SEM (n = 5) of CD8 T cells (CD8+Thy1.2+) in PBL. (C–F) Naïve BALB/c mice and cross-prime-boost mice from B were challenged with a lethal dose (∼5 LD50) of influenza A/PR/8 (H1N1). (C) Kaplan–Meier survival curve; mortality is defined as > 30% loss of starting weight, mice are euthanized at this endpoint per IACUC guidelines. Numbers on the graph indicate number surviving mice/total number of mice. Log-rank test was used to generate P values for the survival curves. (D) Morbidity is measured by weight loss and expressed as percent of starting weight. (E) Airway resistance (Penh value) was measured before and at indicated time points after influenza A/PR/8/34 challenge. (F) Viral titer was determined from the lung 3 d after influenza challenge (n = 4). *Statistical analysis was performed using an unpaired, two-tailed t test.
One advantage of the cross-prime plus short-interval boost approach involves rapid induction of protective immunity. To assess this advantage, we primed mice with H5-coated PLGA microspheres, boosted the mice with attLM-HA, and challenged the mice with a lethal dose of A/PR/8/34 7 d later (Fig. S6A). Three of four naïve mice succumbed to the lethal viral challenge, but all the immunized mice survived. The immunized mice exhibited only slight weight loss and mildly compromised airway function and recovered rapidly (Fig. S6 B and C). Such a striking result usually is associated only with the presence of substantial neutralizing HA-specific antibody at the time of infection (26, 27). Thus, a period of 7 d after booster immunization (a total of 14 d after initial immunization) was sufficient to provide almost sterilizing protective heterosubtypic immunity against high-dose influenza infection. Taken together, these results show that both rapid and long-term protective heterosubtypic immunity against substrains of influenza virus can be achieved by cross-priming with antigen-coated PLGA microspheres followed by short-interval boosting.
Cross-Priming Plus Short-Interval Boosting and Protection Against Plasmodium.
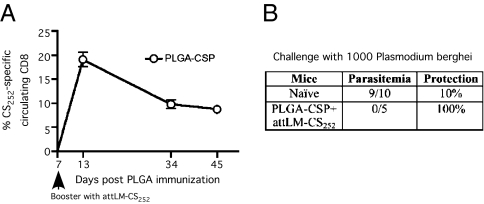
Malaria is a global health problem (28), and substantial efforts have focused on improving vaccine development using rodent models of Plasmodium infection (29). We recently showed that sterile immunity against liver-stage Plasmodium berghei infection in BALB/c mice requires extremely large numbers of circumsporozoite (CS)-specific memory CD8 T cells (30). Importantly, the P. falciparum CS protein is the antigen in the RTS,S vaccine currently shown to have some efficacy in human clinical trials in Africa (31). To determine the effectiveness of our microsphere-based cross-prime plus short-interval boost approach against P. berghei challenge, we immunized naïve BALB/c mice with PLGA microspheres coated with a 40-mer synthetic polypeptide containing the Kd-restricted CS252–260 epitope derived from P. berghei and boosted the mice with L. monocytogenes expressing the CS252–260 epitope (attLM-CS252) 7 d later. We observed robust expansion in the number of CS252-specific effector CD8 T cells and in the frequency of memory cells (8–10% of circulating CD8 T cells) at day 45 after immunization (Fig. 5A). Importantly, our previous work had shown that the CD8 T-cell response to attLM-CS252 vaccination alone was insufficient to provide sterilizing immunity (32). Mice subjected to cross-priming with CS-coated PLGA microspheres and short-interval booster immunization exhibited sterile immunity against P. berghei sporozoite challenge (i.e., none developed blood-stage parasitemia), whereas 9 of 10 naïve controls developed blood-stage parasitemia (Fig. 5B). Thus, cross-priming with CS-coated PLGA microspheres and short-interval boosting provides an exciting alternative strategy to amplify antigen-specific CD8 T cells for protective immunity against malaria infection.
Fig. 5.
Cross-priming followed by short-interval boost generates sterile immunity against liver-stage P. berghei. Naïve BALB/c mice or day-46 immune mice that were cross-primed with ∼5 × 109 PLGA microspheres coated with a 40-mer synthetic peptide containing the P. berghei-derived, Kd-restricted CS252–260 epitope and received attLM-CS252 (∼2 × 107 cfu/mouse) booster immunization on day 7 after cross-priming were challenged with 1,000 P. berghei parasites. (A) Kinetics of CS252-specific CD8 T-cell response as detected by CS252/Kd tetramer staining and expressed as mean frequency ± SEM (n = 5) in PBL. (B) Table summarizes number of mice that had blood-stage infection (parasitemia) and percent protection.
Discussion
Here, we show that cross-priming with cell-associated antigen or antigen-coated biodegradable microspheres in the absence of adjuvant generates CD8 T cells that can be amplified massively by short-interval booster immunization to achieve rapid protective immunity against multiple pathogens. Most importantly, the absence of systemic inflammation in this vaccination strategy is key to evoking such a response. Thus, our results illustrate a dichotomy in vaccine approaches aimed at eliciting cell-mediated immunity. Should a vaccine stimulate a robust primary response with full effector differentiation or a primary response of potentially lesser magnitude that can be amplified vigorously within a short interval after priming? The former can be achieved by coadministration of strong adjuvants to induce systemic proinflammatory cytokines that promote effector differentiation at the expense of memory development and also aid in increasing effector CD8 T-cell numbers (12, 33). For safety and production reasons, attention in vaccine development has shifted toward defined protein antigens as subunit vaccines. However, these vaccines alone stimulate very weak and often undetectable immune responses, and thus much attention has been focused on adjuvants, which are thought to augment the immune response against poorly immunogenic subunit antigens (34). For example, both the direct coupling of protein antigen to adjuvant and the coencapsulation of antigen with adjuvant such as Toll-like receptor agonists in biodegradable microspheres have been shown to elicit CD8 T-cell–mediated responses against subunit antigens (35). However, in addition to activating the APCs, adjuvant also induces inflammatory cytokines that promote expansion and enforce effector differentiation of antigen-specific CD8 T cells while delaying memory development (12, 33). Because fully differentiated effector CD8 T cells are refractory to further proliferative expansion (33), short-interval booster immunization cannot be used to amplify the number of memory T-cells (12, 33). Consequently, priming CD8 T cells in the presence of adjuvant-induced inflammatory cytokines requires a substantial time interval after initial immunization to boost the response to achieve protection. To shorten the interval between initial priming and booster immunization, we propose the alternative strategy of cross-priming in the absence of adjuvant to generate antigen-specific CD8 T cells with accelerated memory function that can be amplified to achieve protective levels within a very short time, on the order of days. We believe this strategy may be beneficial in the case of a pandemic outbreak and even in tumor immunotherapy, where time is of the essence.
Seasonal influenza vaccination requires annual administration to provide effective protection against homologous viral strains by induction of antibodies against viral-coat proteins (36). However, such antibodies are not effective against serologically distinct influenza strains. Moreover, antibody-mediated protection eventually is lost when sufficient mutations are accumulated in the homologous strain as the result of antigenic drift (36, 37). The emergence of highly pathogenic strains such as avian H5N1 or pandemic strains such as 2009 H1N1 underscores the urgent need for rapidly deployable vaccines that provide protective heterosubtypic immunity (36, 37). In this regard, influenza-specific CD8 T cells control and limit the progression of severe influenza infection in murine models (38–41). Here, we show that protective heterosubtypic immunity can be achieved by cross-priming with antigen from a heterologous influenza strain followed by short-interval booster immunization. This proof-of-principle finding can be applied to other conserved CD8 T-cell epitopes derived from internal, largely invariant proteins common to multiple heterologous influenza strains. Moreover, our microsphere-based cross-prime plus short-interval boost approach is amenable to stimulating CD8 T-cell responses against multiple invariant antigens, such as the conserved NP and M proteins of influenza, to increase the breadth of protective heterosubtypic immunity.
Mass immunization approaches based on DC priming are not tenable for widespread application, particularly in developing nations. Here, we show that antigen coupled with biodegradable microspheres elicits antigen-specific CD8 T cells that can be amplified massively by short-interval booster immunization. The advantage of this strategy includes the use of full-length protein as antigen to increase the potential that individuals in outbred populations can respond to the chosen antigens and the possibility of formulation with multiple antigens. In addition, biodegradable microspheres could be engineered to improve targeting the antigen to DCs for more efficient cross-priming of CD8 T cells. Thus, this strategy theoretically could amplify CD8 T-cell responses against multiple epitopes in a single short-interval prime-boost sequence. Importantly, PLGA microspheres or nanospheres as antigen delivery vehicles offer an attractive, potentially off-the-shelf formulation because of their extensive safety record in human clinical applications such as drug delivery (42, 43). Finally, we believe that the potency of this cross-prime plus short-interval boost strategy in rodents merits evaluation in humans.
Materials and Methods
Mice.
C57BL/6 (B6) and BALB/c mice were from the National Cancer Institute (Frederick, MD). Transgenic Act-mOva.Kb−/− mice were a generous gift from Stephen Schoenberger (La Jolla Institute for Immunology and Allergy, La Jolla, CA).
Pathogen-infected mice were housed in appropriate biosafety conditions. All experiments were approved by the University of Iowa Institutional Animal Care and Use Committee.
Dendritic Cells, Recombinant Bacteria, and Viruses.
Peptide-coated splenic DC were prepared as described (32). virLM-Ova (44), attLM-Ova (45), attLM-HA518, and actA-, intB-deficient L. monocytogenes expressing the H-2Kd-restricited P. berghei circumsporozoite protein epitope CS252 (attLM-CS252) (Aduro Biotech) were grown, injected i.v. at the indicated dose per mouse, and quantified as described (12). VacV-OVA has been described previously (46). Mouse-adapted A/PuertoRico/8/34 (H1N1) influenza virus was propagated and stored as previously described (47). For influenza infection, BALB/c mice were anesthetized by isofluorane and were infected intranasally with a tissue culture infectious dose 50 (∼6.4–8 × 104 of virus) in 50 μL of Iscoves medium. Three days after infection, lungs were homogenized, and viral titers were determined as previously described (48).
Protection After L. Monocytogenes or Vaccinia Challenge.
Naive or Ova-immune mice were injected i.v. with virLM-Ova (∼5 × 105 cfu/mouse) or VacV-Ova (∼5 × 107 pfu/mouse). Bacterial numbers were determined in the spleen and liver 3 d later, as described (49). Ovaries from Vaccinia-challenged mice were homogenized and subjected to three freeze–thaw cycles, and viral titers were determined by plaque assaying on Vero cells (50).
PLGA Microspheres, Ovalbumin, Recombinant HA, CpG, and Peptides.
PLGA microspheres (mean diameter, 2.0 μm) were purchased from Phosphorex, Inc. Hen Ovalbumin protein (Sigma) or EndoGrade Ovalbumin protein (Profos AG) was dissolved in sterile PBS at 1 mg/mL for coating cells and PLGA microspheres. Recombinant HA protein H5 (A/Vietnam/1203/2004) was purchased from Protein Sciences Corporation at a concentration of ∼0.6 mg/mL. Adsorption of protein onto cells or PLGA microspheres was carried out at 37 °C with occasional mixing for 1 h. CpG oligonucleotide 1826 was purchased from IDT.
Quantification and Phenotypic Analysis of Antigen-Specific T Cells.
The magnitude of the epitope-specific CD8 T-cell response was determined either by intracellular IFN-γ staining or MHC class I peptide tetramer staining as described (12). A small volume (∼50 μL) of blood was obtained via retro-orbital bleeding for analysis of circulating antigen-specific CD8 T cells in peripheral blood lymphocytes (PBL). MHC class I tetramers (Kd) specific for HA518–526 (IYSTVASSL) were obtained from the National Institute of Allergy and Infectious Diseases MHC Tetramer Core Facility (Atlanta, GA).
Serum Cytokine Quantification and IFN-γ ELISPOT Assay.
Serum (∼25 μL) was obtained via retro-orbital bleeding 20 h after mice were immunized with Ova257-coated DC (∼106 DC/mouse), irradiated Kb−/−mOva splenocytes (∼107 cells/mouse), or virLM-Ova (∼105 cfu/mouse), and IL-6 and IFN-γ were measured using Bio-Plex Mouse Cytokines Assays (Bio-Rad) and were read on the Bio-Rad Bioplex 200 system. For the IFN-γ ELISPOT assay (eBioscience), ∼106 splenocytes/well from day 7 immunized (BSA- or EndoGrade Ova-Coated PLGA microspheres) mice were plated on a Millipore MultiScreen Filter 96-well plate (0.45-μm Immobilon-P membrane) and incubated with or without Ova257 peptide for 24 h before developing according to the manufacturer's protocol. Spots were analyzed by Cellular Technology, Ltd. ImmunoSpot instrument and software.
Measurement of Airway Resistance.
Airway resistance was measured using a whole-body plethysmograph (Buxco Electronics) and expressed as maximal enhanced pause (Penh) values. Baseline Penh values for each mouse were recorded before and at the indicated time points after influenza A/PR/8/34 challenge.
Statistical Analysis.
Statistical analysis was performed using an unpaired, two-tailed t test. Log10-transformed data were used for statistical analysis in the challenge studies.
Supplementary Material
Acknowledgments
We thank members of the Harty laboratory for helpful discussion, Stanley Perlman for critical comments, Stephen Schoenberger (La Jolla Institute for Allergy and Immunology) for providing transgenic Act-mOva.Kb−/− mice, Jack Bennink and Jon Yewdell (National Institutes of Health) for providing VacV-Ova, and Aduro Biotech for providing recombinant Listeria. This work was supported by National Institute of Health Grants AI46653, AI150073, and AI42767 (to J.T.H.) and AI83286 (to V.P.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004661107/-/DCSupplemental.
References
- 1.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 2.Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 4.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 6.Woodland DL. Jump-starting the immune system: Prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Ramshaw IA, Ramsay AJ. The prime-boost strategy: Exciting prospects for improved vaccination. Immunol Today. 2000;21:163–165. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 9.Heit A, et al. Protective CD8 T cell immunity triggered by CpG-protein conjugates competes with the efficacy of live vaccines. J Immunol. 2005;174:4373–4380. doi: 10.4049/jimmunol.174.7.4373. [DOI] [PubMed] [Google Scholar]
- 10.Celis E. Toll-like receptor ligands energize peptide vaccines through multiple paths. Cancer Res. 2007;67:7945–7947. doi: 10.1158/0008-5472.CAN-07-1652. [DOI] [PubMed] [Google Scholar]
- 11.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 13.Bellone M. Apoptosis, cross-presentation, and the fate of the antigen specific immune response. Apoptosis. 2000;5:307–314. doi: 10.1023/a:1009671105696. [DOI] [PubMed] [Google Scholar]
- 14.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 15.Anonymous Lessons learned. Nat Immunol. 2009;10:1133. doi: 10.1038/ni1109-1133. [DOI] [PubMed] [Google Scholar]
- 16.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 17.Fischer S, et al. Concomitant delivery of a CTL-restricted peptide antigen and CpG ODN by PLGA microparticles induces cellular immune response. J Drug Target. 2009;17:652–661. doi: 10.1080/10611860903119656. [DOI] [PubMed] [Google Scholar]
- 18.Shen H, et al. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlosser E, et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine. 2008;26:1626–1637. doi: 10.1016/j.vaccine.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Ahonen CL, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen JP, Doherty PC, Branum KC, Riberdy JM. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J Virol. 2000;74:11690–11696. doi: 10.1128/jvi.74.24.11690-11696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bender BS, Small PA., Jr Influenza: Pathogenesis and host defense. Semin Respir Infect. 1992;7:38–45. [PubMed] [Google Scholar]
- 23.Effros RB, Doherty PC, Gerhard W, Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977;145:557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zweerink HJ, Courtneidge SA, Skehel JJ, Crumpton MJ, Askonas BA. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977;267:354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]
- 25.Braciale TJ. Immunologic recognition of influenza virus-infected cells. I. Generation of a virus-strain specific and a cross-reactive subpopulation of cytotoxic T cells in the response to type A influenza viruses of different subtypes. Cell Immunol. 1977;33:423–436. doi: 10.1016/0008-8749(77)90170-8. [DOI] [PubMed] [Google Scholar]
- 26.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 27.Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol. 1997;71:4347–4355. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwood B, Mutabingwa T. Malaria in 2002. Nature. 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji M, Zavala F. T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol. 2003;19:88–93. doi: 10.1016/s1471-4922(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt NW, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci USA. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacarlal J, et al. Long-term safety and efficacy of the RTS,S/AS02A malaria vaccine in Mozambican children. J Infect Dis. 2009;200:329–336. doi: 10.1086/600119. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt NW, Butler NS, Harty JT. CD8 T cell immunity to Plasmodium permits generation of protective antibodies after repeated sporozoite challenge. Vaccine. 2009;27(44):6103–6106. doi: 10.1016/j.vaccine.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heit A, Busch DH, Wagner H, Schmitz F. Vaccine protocols for enhanced immunogenicity of exogenous antigens. Int J Med Microbiol. 2008;298:27–32. doi: 10.1016/j.ijmm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Heit A, Schmitz F, Haas T, Busch DH, Wagner H. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur J Immunol. 2007;37:2063–2074. doi: 10.1002/eji.200737169. [DOI] [PubMed] [Google Scholar]
- 36.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown LE, Kelso A. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol Cell Biol. 2009;87:300–308. doi: 10.1038/icb.2009.16. [DOI] [PubMed] [Google Scholar]
- 38.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998;160:322–327. [PubMed] [Google Scholar]
- 40.Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 42.Foged C, Sundblad A, Hovgaard L. Targeting vaccines to dendritic cells. Pharm Res. 2002;19:229–238. doi: 10.1023/a:1014474414097. [DOI] [PubMed] [Google Scholar]
- 43.Little SR, et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci USA. 2004;101:9534–9539. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pope C, et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 45.Corbin GA, Harty JT. Duration of infection and antigen display have minimal influence on the kinetics of the CD4+ T cell response to Listeria monocytogenes infection. J Immunol. 2004;173:5679–5687. doi: 10.4049/jimmunol.173.9.5679. [DOI] [PubMed] [Google Scholar]
- 46.Restifo NP, et al. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 47.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Meyerholz DK, et al. Chronic alcohol consumption increases the severity of murine influenza virus infections. J Immunol. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 50.Shen H, et al. Compartmentalization of bacterial antigens: Differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.