Abstract
Rodents can localize odor sources by comparing odor inputs to the right and left nostrils. However, the neuronal circuits underlying such odor localization are not known. We recorded neurons in the anterior olfactory nucleus (AON) while administering odors to the ipsilateral or contralateral (ipsi- or contra-) nostril. Neurons in the AON pars externa (AONpE) showed respiration phase-locked excitatory spike responses to ipsinostril-only stimulation with a category of odorants, and inhibitory responses to contranostril-only stimulation with the same odorants. Simultaneous odor stimulation of the ipsi- and contranostrils elicited significantly smaller responses than ipsinostril-only stimulation, indicating that AONpE neurons subtract the contranostril odor inputs from ipsinostril odor inputs. An ipsilateral odor source induced larger responses than a centrally located source, whereas an odor source at the contralateral position elicited inhibitory responses. These results indicate that individual AONpE neurons can distinguish the right or left position of an odor source by referencing signals from the two nostrils.
Keywords: olfactory cortex, binasal inputs, odor localization
Olfactory neuronal circuits translate odor cues into a variety of behavioral responses that enable rodents to find and locate food, mates, and predators. For the directional localization of sound sources, the central auditory system has neuronal circuit mechanisms that compare auditory inputs from the right and left cochleas (1). Similarly, rodents can localize odor sources by comparing odor inputs through the right and left nostrils (2). However, the neuronal circuits and mechanisms subserving the right or left localization of odor sources are not yet known.
In the auditory system, binaural sound localization relies on central neuronal mechanisms that compare auditory inputs from the two ears. Interaural differences in the intensity of the sound pressure level arriving at the two ears are important cues used by the mammalian auditory system to localize higher-frequency sounds (3). Neurons in the lateral superior olive are sensitive to interaural intensity differences, being excited by stimulation of the ipsilateral ear and suppressed by stimulation of the contralateral ear (4). These neurons are thus referred to as ipsi-excitation and contrainhibition (E-I) neurons and play a key role in sound source localization (5).
Odorants are inhaled through the two nostrils into two segregated nasal passages. Because the two passages are relatively well isolated, odorants inhaled through one nostril activate olfactory sensory neurons only in the ipsilateral olfactory epithelium (6). Therefore, to detect the right or left localization of odor sources, the central olfactory system needs only to compare afferent odor signals originating from the right and left olfactory epithelia (OEs). Olfactory sensory neurons in the epithelium project their axons to the ipsilateral olfactory bulb (OB), and mitral and tufted cells in the OB project their axons to the ipsilateral olfactory cortex (7). Therefore, odor signals originating from the right and left olfactory sensory epithelia are largely segregated at the level of the right and left OBs, and their afferents are segregated to the right or left olfactory cortex.
The anterior olfactory nucleus (AON) is the most rostral region of the olfactory cortex and receives excitatory axonal inputs from the ipsilateral OB (8–11). In addition, AON neurons receive inputs from the contralateral olfactory cortex via the anterior commissure (12, 13), suggesting that individual AON neurons receive odor information originating from both ipsilateral and contralateral OEs. The AON is composed of two separate structures, the pars principalis (AONpP) and pars externa (AONpE) (12).
We report here that AONpE neurons show ipsilateral (ipsi)-nostril excitation and contralateral (contra)-nostril inhibition (E-I) responses, and compare the magnitude of responses to ipsinostril odor inputs with those of contranostril inputs.
Results
We recorded single-unit spike responses of individual AON neurons in urethane-anesthetized rats in response to nasal stimulation with a panel of odors consisting of 10 categories of odorant molecules, each containing five separate odorants (Fig. 1A and Table S1). A thermoplastic external nasal septum that fitted the external shape of the rat nose was used to prevent odors delivered in front of one nostril from spreading to the contralateral nostril (Fig. 1A), which enabled the selective stimulation of either the ipsilateral or contralateral olfactory epithelium (6). We examined spike responses of individual neurons in the rostral part of the AON (Fig. 1B) to ipsinostril-only and contranostril-only stimulation with the panel of odorants (52 cells in 36 rats). AON neurons that showed excitatory spike responses to ipsinostril stimulation with an odorant category showed three types of responses to contranostril stimulation with the same odorant category: excitatory responses (ipsi-excitatory and contraexcitatory response, or an E-E-type response), no response (ipsi-excitatory and contranull response, E-0-type response), and suppressive responses (ipsi-excitatory and contrainhibitory response, E-I-type response) (6).
Fig. 1.
E-I-type neurons in the AON. (A) Experimental procedure used for uninostril odor stimulation. An external nasal septum was used to ensure delivery of odorants selectively to unilateral olfactory epithelium. Single-unit recordings were obtained from AON neurons. (B) Lateral view of a 3D reconstruction of the olfactory peduncle of a rat brain. AONpE (black); AONpP (green); MOB, main olfactory bulb; AOB, accessory olfactory bulb; NC, neocortex. (C) Spike responses of an E-I-type AON neuron to ipsinostril (Upper left trace) and contranostril (Upper right trace) odor stimulation. Resp., the trace of the respiration monitor. The ascending and descending phases of the trace indicate inspiration and expiration, respectively. Raster, raster representation of the spike responses. Each row corresponds to a single odor stimulation. Peristimulus time histograms of the response are shown at the bottom. Bar, duration of odor stimulation (sulfide category, 3 s). F.R., firing rate (Hz). (D) Locations of E-I type neurons in the AONpE. Dr, Di, Dc: rostral, intermediate, and caudal regions of the dorsal AONpE, respectively. Vr, Vi, Vc: rostral, intermediate and caudal regions of the ventral AONpE, respectively. D-R, dorso-rostral; V-C, ventro-caudal. Red and yellow dots represent individual E-I type neurons. Numbered cells correspond to those in Fig. 2. (E) A raster representation of the spike responses (Upper) and peristimulus time histograms (Lower) from a single cell. Black bar, duration of odor stimulation (sulfide category, 3 s). (F) Morphology of the E-I-type AON neuron whose responses are shown in E. The cell was labeled with biotinylated dextran amine by juxtacellular electroporation (SI Materials and Methods). This cell emitted an axon collateral (single arrowhead) that reached the superficial part of layer II of the AONpP. AC, anterior commissure (double arrowhead).
Individual AON neurons that showed E-I responses to ipsi- and contranostril stimulation with one odorant category did not show E-I responses to the other odorant categories (Fig. 2), suggesting that their E-I response is specific to a single odorant category. In addition, these AON neurons did not show E-E-type responses to any of the 10 odorant categories (Fig. 2). We thus classified these neurons as single-category E-I-type neurons. Because we preferentially searched for E-I-type neurons, we analyzed 31 E-I-type neurons, 13 E-E-type neurons, and 8 E-0-type neurons. Fig. 1C shows a representative example of a single-category E-I-type AON neuron. This cell showed high-frequency burst spike responses to ipsinostril stimulation with sulfide odorants and suppressive responses to contranostril stimulation with the same odorants. Because single-category E-I AON neurons are candidate neurons subserving the comparison between ipsinostril and contranostril odor inputs, we focused our analysis on these neurons in this study (31 cells in 23 rats).
Fig. 2.
AONpE neurons showed an E-I-type response to only a single odorant category. The odorant category selectivity of each neuron (#1–16) is represented by the columns above and below each line. The colored and gray bars indicate excitatory and inhibitory responses, respectively, to stimulation with individual odorant categories. Bars above and below the line indicate responses to ipsinostril and contranostril stimulation, respectively. In all of the recorded neurons, only a single odorant category induced an E-I-type response (shown by asterisks). The numbers indicate the relative magnitude of responses.
Single-Category E-I-Type Neurons Are Located in the Pars Externa of the AON.
To examine whether the single-category E-I-type neurons were in the AONpP or AONpE, we located the positions of these neurons by depositing a marker dye at the recording sites. All single-category E-I-type AON neurons (n = 17) were localized to the AONpE (Fig. 1 D and F). In striking contrast, all E-E-type and E-0-type AON neurons (n = 9) were localized to the AONpP (Fig. S1). Although our recordings from AONpE neurons were obtained mostly in the ventro-caudal part of the AONpE (Fig. 1D), the above results suggest that a majority of AONpE neurons are single-category E-I-type neurons.
AONpE neurons receive massive afferent inputs from mitral and tufted cells of the ipsilateral OB. Thus, the E-I response might be generated at the level of the mitral and tufted cells in the OB and transmitted to the AONpE neurons. To examine this possibility, we recorded the response of mitral and tufted cells in the OB to ipsinostril-only and contranostril-only stimulation with the 10 odorant categories in rats fitted with the external nasal septum. Mitral and tufted cells (n = 21) showed E-0 responses but did not show E-I-type responses to any of the 10 odorant categories (Fig. S2), suggesting that the synaptic interactions between ipsinostril and contranostril inputs in the OB do not play a major role in generating the E-I-type response of AONpE neurons.
Each AONpE Neuron Shows a Selective E-I Response to a Single Odorant Category.
For sound localization, individual neurons in the lateral superior olive compare ipsilateral and contralateral sound inputs of equivalent frequency (14). Similarly, if AONpE neurons are involved in the localization of odor sources, they might compute the difference between ipsinostril and contranostril input signals of the same odor quality. As a first step to address this question, we examined the odorant category selectivity of individual E-I AON neurons using the 10 different categories of odorant molecules. We performed detailed quantitative analysis of ipsinostril and contranostril responses using a computer-controlled olfactometer in 16 of the 31 E-I-type neurons.
As shown in Fig. 2, AONpE neurons showed ipsinostril-induced excitatory responses (colored bars above the line) to either a single odorant category (cells #1–3, #15, #16) or a specific combination of odorant categories (#4–14). In contrast, they showed contranostril-induced inhibitory responses (gray bars below the line) to only a single odorant category. Because most E-I neurons showed spontaneous discharges in the absence of odor stimulation, the inhibitory responses were clearly detected and distinguished from null responses (Fig. 1C). Among 16 E-I-type AON neurons, all cells showed E-I responses to only a single odorant category (Fig. 2, shown by asterisks). The single-category E-I response was selective to the sulfide category in 14 neurons, selective to the terpene hydrocarbon category in one neuron (#6), and selective to the aldehyde category in one neuron (#10). Neuron #10 was exceptional in that it showed an I-E-type response to sulfides in addition to the E-I response to the aldehyde category. These results suggest that individual E-I-type neurons are specialized to compare the ipsinostril and contranostril inputs within an odorant category, although we do not rule out the possibility that different component odorants within an odorant category induced the ipsiexcitatory or contrasuppressive responses.
Spike Responses of E-I-Type AON Neurons Are Phase-Locked to the Respiration Cycle.
External odor information is detected intermittently by right and left OEs during the inhalation phase of each respiration cycle. Because of the possible movement of odor sources or the animal's head position during two successive inhalations, single-category E-I neurons in the AONpE might need to compare ipsi- and contranostril inputs within each respiration cycle. In support of this hypothesis, the ipsinostril odor responses of all single-category E-I neurons were strictly phase-locked to the respiration cycle (Fig. 3 A and B and Fig. S3A). In addition, we observed that the odor-induced respiration phase-locked spike responses ended just after the cessation of the odor stimulus (Figs. 1C and 3 A and B, and Fig. S4). We also observed that the inhibitory responses of single-category E-I-type neurons to contranostril-only stimulation ended just after the cessation of the stimulation (Fig. 1C and Fig. S4).
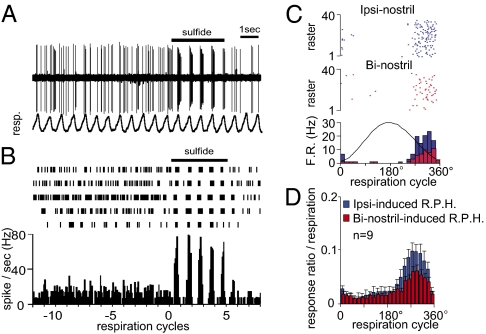
Fig. 3.
Respiration phase-locked spike responses of AONpE neurons. (A) Spontaneous spike discharges and spike responses to ipsinostril odor stimulation of an AONpE neuron. Resp, respiration. Black bar, duration of odor stimulation (sulfide category, 3 s). (B) A raster plot and a respiration-phase histogram of the spike responses of the neuron shown in A. Note the respiration phase-locked discharges during odor stimulation. (C) Raster representation and respiration-phase histograms (Bottom) of spike responses of an E-I-type AON neuron to ipsinostril-only stimulation (blue dots and bars) and binostril odor stimulation (red dots and bars). (D) The averaged respiration-phase histograms for ipsinostril responses (blue bars) and binostril responses (red bars). R.P.H., respiration phase histogram. The vertical axis indicates response probability at each phase of a respiration cycle.
In 30 single-category E-I neurons, we generated respiration phase histograms of spike responses to ipsinostril stimulation. The ipsinostril-induced spike responses of the cell in Fig. 3 occurred consistently during the late part of the expiration phase. In a majority of single-category E-I neurons (28 of 30 cells), the peak of ipsinostril odor responses occurred during the expiration phase (255 ± 9.0°, mean ± SEM) (Fig. S3B). Averaged spike responses to ipsinostril odor stimulation in these 30 single-category E-I neurons also showed that their responses were phase-locked to expiration, with the peak of responses during the middle part of the expiration phase (blue, Fig. 3 C and D and Fig. S3). In addition, simultaneous odor inputs to both nostrils caused the suppression of respiration phase-locked spike responses (red, Fig. 3 C and D). These results suggest that the comparison of inputs from ipsi- and contranostrils in the AONpE occurs within a short time window during the expiration phase of each respiration cycle.
Computation of Differences Between Ipsinostril and Contranostril Odor Inputs.
In all single-category E-I neurons examined in detail (n = 9), ipsinostril-only stimulation evoked significantly larger responses than binostril odor stimulation (paired t test between the peak of ipsinostril- and binostril-induced responses, P = 0.0009, n = 9), suggesting that single-category E-I neurons subtract the contranostril inputs from ipsinostril inputs of single-category odorants.
The external nasal septum enabled us to independently control the odor concentrations used for ipsi- and contranostril stimulation. To examine whether odorant concentration affects the decrease in ipsinostril odor responses of single-category E-I neurons caused by simultaneous stimulation of the contranostril, we examined the neuronal responses to simultaneous ipsi- and contranostril stimulation at the following odorant concentration ratios: 1:0, 1:1, 1:2, 0:1, and 0:2 (ipsinostril:contranostril, n = 5 cells in five rats) (Fig. 4 A and B).
Fig. 4.
AONpE neurons compare ipsinostril odor inputs with contranostril inputs. (A) Responses of an E-I neuron to different ratios of odor concentration for ipsi- and contranostril stimulation. Upper diagrams indicate the ratio (1:0, 1:1, 1:2, 0:1, and 0:2) of odor concentration (sulfide category) between ipsi- and contranostril stimulation. Bars above the zero line indicate excitatory responses (mean ± SEM). Bars below the zero line indicate inhibitory responses (ANOVA among the different ratios, significant differences, five of five cells; posthoc analysis with Student-Newman-Keuls, *P < 0.05; **P < 0.01). (B) Differential responses to stimulation of ipsi- and contranostrils with different concentration ratios were observed at each respiration cycle (ANOVA, significant differences for each respiration cycle, 20 of 20 respiration cycles in five cells; posthoc analysis with Student-Newman-Keuls, *P < 0.05; **P < 0.01). (C) E-I-type neurons differentiate the odor source at three different positions relative to the two nostrils. Upper diagram indicates the three positions for odor stimulation: a, an ipsilateral position; b, a central position; c, a contralateral position. Each bar indicates the magnitude of the responses (mean ± SEM) to odor stimulation at the corresponding positions (a–c; ANOVA across the three positions, significant differences, five of five cells; posthoc analysis with Student-Newman-Keuls, **P < 0.01). (D) The differential responses to distinct positions of odor source were observed at each respiration cycle (ANOVA, significant differences for each respiration cycle, 19 of 20 respirations in five cells; posthoc analysis with Student-Newman-Keuls *P < 0.05; **P < 0.01).
Ipsinostril-only stimulation (1:0) with sulfide-category odorants produced excitatory responses in the neuron shown in Fig. 4A. Simultaneous stimulation of the contranostril at the same concentration (1:1) greatly reduced the magnitude of the excitatory response. The suppression was observed in each respiration phase-locked response during the simultaneous odor stimulation (Fig. 4B). Furthermore, increasing the odorant concentration for contranostril stimulation (1:2 in Fig. 4A) completely suppressed the excitatory response. This suppression was also observed in each respiration phase-locked response during odor stimulation (Fig. 4B). These results suggest that the degree of suppression of excitatory responses to ipsinostril stimulation is proportional to the concentration of odorants used to stimulate the contranostril. We also noted that the concentration of odorants for contranostril-only stimulation was proportional to the amount of suppression of spontaneous spike discharges in E-I neurons (Fig. 4A, 0:1 and 0:2). The suppression was again observed in each respiration phase-locked response during the contranostril odor stimulation (Fig. 4B). Thus, the single-category E-I neurons appear to subtract the contranostril inputs from the ipsinostril inputs or ongoing activity during each respiration cycle.
The external nasal septum might produce artificial nasal airflow on both sides. To address whether the single-category E-I neurons detect the right or left localization of odor sources in natural conditions without the external nasal septum, we examined the responses of these neurons when the odor source was placed at an ipsilateral, central, or contralateral position with regard to the two nostrils (five cells in four rats) (Fig. 4C). We first identified a single-category E-I-type neuron with the external nasal septum in place. We then removed the external nasal septum and presented odorants of the same category at the above three positions. The single-category E-I-type neuron shown in Fig. 4C showed an excitatory response to nasal stimulation from the ipsilateral position and a smaller excitatory response to stimulation from the central position. Odor stimulation from the contralateral position elicited inhibitory responses (Fig. 4C). The differential responses to distinct positions of the odor source were observed at each respiration cycle (Fig. 4D) and in all of the E-I neurons examined (n = 5 cells). These results indicate that single-category E-I neurons differentiate between odor sources at different positions with reference to the right and left nostrils, suggesting that E-I neurons participate in the right or left localization of odor sources.
Discussion
The present results revealed that individual AONpE neurons showed an E-I-type response to only a single category among 10 categories of odorant molecules. Each AONpE neuron was strongly activated by ipsinostril and inhibited by contranostril stimulation. Among all of the AONpE neurons examined, simultaneous odor stimulation of ipsi- and contranostrils elicited a significantly smaller response than that elicited by ipsinostril-only stimulation. Increasing the odor concentration of contranostril stimulation caused a larger suppression of the ipsinostril response. These results indicate that AONpE neurons detect differences in the concentration of odorants of a single category between ipsi- and contranostril inputs.
By analogy with the auditory E-I neurons in the lateral superior olive (3), the above results suggest that AONpE neurons participate in the localization of odor sources to the right or left side. Our results without the external nasal septum support this hypothesis. AONpE neurons were activated most strongly when the odor source was placed at the ipsilateral position, were activated moderately when the odor source was at the central position, and were inhibited when the odor source was placed at the contralateral position (Fig. 4). Thus, the closer the position of the odor source is to the ipsilateral nostril and the more distant from the contralateral nostril, the stronger the response of AONpE neurons. However, behavioral studies with selective inactivation of AONpE using lesions or pharmacological blockers are necessary to determine whether AONpE is essential for the directional localization of odor sources. The right and left nostrils are very close to each other and thus might not be suitable for the ipsi- and contranostril comparison of odor concentration. However, the anatomical configuration of the rat external nose produces laterally directed respiratory airflows, such that lateral inhalation might produce a functional widening of the internostril distance (15). The lateral inhalation may thus help AONpE neurons to localize odor sources.
Most of the AONpE neurons recorded in the present study were located in the ventro-caudal region of the AONpE (Fig. 1D) and showed E-I-type responses selectively to sulfide-category odorants, suggesting that computation of the directional localization of sulfide odors is performed by neurons in the ventro-caudal region. In agreement with this finding, ipsinostril-only stimulation with sulfide odorants resulted in c-fos expression in the ventro-caudal region of the AONpE (Fig. S5). These results suggest that distinct parts of the AONpE might be specialized for E-I-type responses to different categories of odorants, although we do not rule out the possibility that AONpE neurons are tuned to other groupings of odorants, or even to one odorant in a category. Further experiments are necessary to record from neurons in the rostral and intermediate regions of the AONpE and examine their odorant specificity.
AONpE receives topographical axonal projections from the ipsilateral OB (11, 16–18), raising the possibility that the odorant category tuning of ipsinostril input to individual AONpE neurons is a result of the topography of afferent projection from the ipsilateral OB. In accordance with this hypothesis, Johnson et al. demonstrated that sulfide-category odorants activate glomeruli at the ventro-caudal region in the ventral zone of the OB (19), and mitral/tufted cells in these regions project axons to the ventro-caudal region of the AONpE (11, 18).
Fig. 5 A and B illustrates candidate neuronal circuits that could be responsible for the odorant category tuning of inhibitory responses of ipsilateral AONpE neurons to contranostril inputs. In one candidate pathway (Fig. 5A), contralateral AONpE neurons send topographically organized commissural projections to the ipsilateral OB (17, 18, 20, 21) and form excitatory synaptic inputs on granule cells (“G” in Fig. 5A) that inhibit mitral and tufted cells (22). Ipsinostril excitatory responses and contranostril inhibitory responses (E-I-type responses) are first generated in mitral/tufted cells (M/T cells in Fig. 5A) through neuronal circuits in the ipsilateral OB including granule cells, and the E-I-type responses of mitral/tufted cells are then transmitted to ipsilateral AONpE neurons via the topographic afferent connection. However, the mitral/tufted cells did not show an E-I-type response to any of the 10 odorant categories. The results thus argue against this model.
Fig. 5.
Candidate neuronal pathways underlying the right or left localization of odor sources. (A) One candidate pathway in which E-I-type responses are first generated in mitral/tufted (M/T) cells in the ipsilateral OB (ipsi-OB) and then transmitted to ipsilateral AONpE. G, granule cells; AC, anterior commissure. (B) Another candidate pathway in which E-I-type responses are generated by neuronal circuits within the AON. See text for details.
Another candidate neuronal pathway underlying the E-I responses of ipsilateral AONpE neurons is the projection of contralateral AONpE neurons to as-yet unidentified inhibitory neurons in the ipsilateral AON via the anterior commissure (Fig. 5B) (10). The activated inhibitory neurons then inhibit the ipsilateral AONpE neurons. Further intracellular recordings from AONpE neurons are necessary to examine whether they receive inhibitory synaptic inputs following contranostril odor stimulation. It is also necessary to identify the inhibitory neurons that would be responsible for the synaptic inhibition. AONpE neurons have substantial reciprocal connections with the contralateral AONpP pars dorsalis and receive additional input from the contralateral AONpP pars ventralis (23), suggesting close synaptic interactions between AONpE and AONpP. Thus, a possible source of the inhibitory input might be local inhibitory neurons within the AONpP.
A characteristic feature of the olfactory system is that sampling of external odor information is intermittent. Consistent with the respiration phase-dependent intermittent sampling, the spike responses of AONpE neurons to ipsinostril odor stimulation were strictly phase-locked to the respiration cycle. No AONpE neurons showed sustained spike responses that outlasted the odor stimulus for more than a few respiration cycles. These results suggest that AONpE neurons compare ipsi- and contranostril odor inputs within each respiration cycle. In addition, the intermittent sampling may help reduce sensory adaptation of olfactory sensory neurons and central olfactory circuits, thus maximizing the amount of ipsi- and contranostril odor information available to the AONpE for comparison at each successive respiration (24), which may enable the AONpE neurons to temporally follow changes in the position of odor sources.
In the auditory system, information detected by the lateral superior olive concerning differences in the interaural intensity of sound is transmitted to higher auditory centers, including the inferior colliculus (25). Similarly, we speculate that information about the right and left localization of odor sources detected by single-category E-I neurons in the AONpE may be transmitted to higher olfactory centers, in addition to the feedback connection to the contralateral OB. In agreement with this idea, behavioral studies have shown that rats can properly respond to odor localization cues (2, 26). Single-cell labeling revealed that AONpE neurons send an axon collateral to AONpP (Fig. 1F), raising the possibility that AONpE neurons send information to specific subsets of AONpP neurons via their axon collaterals, which in turn project their axons to higher olfactory centers.
In contrast to the extensive invertebrate research on odor localization using bilateral comparisons of odors at antennae (27, 28), relatively little is known about the neuronal mechanisms for orientation to an odor source in mammals (2). Further studies on AONpE neurons and their connection to higher olfactory centers will provide additional clues for understanding the role of stereo-olfaction in odor source localization in the mammalian brain.
The olfactory cortex contains many regions including the AONpE, AONpP, anterior piriform cortex, posterior piriform cortex, olfactory tubercle, and cortical amygdaloid nucleus. This study is unique in providing evidence that a specific region of olfactory cortex, AONpE, is involved in the right and left localization of odor sources. Further analysis of each region of the olfactory cortex may elucidate the functional differentiation between different regions of the olfactory cortex.
Materials and Methods
Animals.
All experiments were performed in accordance with the guidelines of the Physiological Society of Japan and were approved by the Experimental Animal Research Committee of the University of Tokyo. Wistar rats (male, 280–350 g; Japan SLC) were anesthetized with urethane (1.2 g/kg) and placed in a stereotaxic apparatus (SR-6R; Narishige) (29). Respiration was monitored with a strain gauge to measure chest movement (TR-651T; Nihon Kohden).
Odor Delivery.
For uninostril odor stimulation, thermoplastic material was used to construct an external nasal septum that fit the external shape of the rat snout. To examine the odorant category selectivity of individual AON neurons, we used the method of placing test tubes containing diluted odorants (5% in odorless mineral oil) in front of a nostril at a distance of 2 cm. To examine the response magnitude of individual AON neurons, we used a custom air-dilution olfactometer fitted with Teflon tubing, controlled by a program written in Labview software (National Instruments). Ipsinostril-only stimulation (ipsi:contra, 1:0), contranostril-only stimulation (0:1), and binostril stimulation (1:1) were delivered in pseudorandom order. Differential concentrations of odorants were produced by differential airflow dilution using a pair of mass flow controllers. The outlet of the Teflon tube from the olfactometer was placed 2 cm in front of the ipsinostril (recorded side) or the contranostril. An exhaust pipe was placed over the head of the rat to remove any stray odorants.
Odorants.
Ten categories of odorant molecules (sulfide, ester, terpene hydrocarbon, acid, ether, ketone, aldehyde, alcohol, lactone, and phenol) were used (Table S1). A mixture of five representative odorants was used for stimulation with each category.
Electrophysiology.
A glass micropipette (10–20 MΩ) filled with 2% Chicago Sky Blue 6B (Tocris Bioscience) in 0.5 M sodium acetate was inserted vertically into the AON. After single-unit recordings, a negative current (20 μA) was applied for 5 min so that the recorded sites could be marked by the dye. The dye-marked sites were examined histologically (SI Materials and Methods). The signals of single-unit activity were amplified (AB-610J; Nihon Kohden), filtered (150–10 kHz; EW-610J; Nihon Kohden), and stored on a computer.
Supplementary Material
Acknowledgments
We thank Drs. M. Yamaguchi and H. Nagao for critically reading the manuscript, and members of the Departments of Otolaryngology and Physiology for useful discussion. This work was supported by grants-in-aid for scientific research from Japan Science and Technology Agency, Core Research for Evolutional Science and Technology (to K.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003999107/-/DCSupplemental.
References
- 1.Konishi M. Coding of auditory space. Annu Rev Neurosci. 2003;26:31–55. doi: 10.1146/annurev.neuro.26.041002.131123. [DOI] [PubMed] [Google Scholar]
- 2.Rajan R, Clement J-P, Bhalla U-S. Rats smell in stereo. Science. 2006;311:666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T-T. The neural coding of auditory space. J Exp Biol. 1989;146:307–322. doi: 10.1242/jeb.146.1.307. [DOI] [PubMed] [Google Scholar]
- 4.Boudreau J-C, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- 5.van Adel B-A, Kelly J-B. Kainic acid lesions of the superior olivary complex: Effects on sound localization by the albino rat. Behav Neurosci. 1998;112:432–446. doi: 10.1037//0735-7044.112.2.432. [DOI] [PubMed] [Google Scholar]
- 6.Kikuta S, Kashiwadani H, Mori K. Compensatory rapid switching of binasal inputs in the olfactory cortex. J Neurosci. 2008;28:11989–11997. doi: 10.1523/JNEUROSCI.3106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori K, Nagao H, Yoshihara Y. The olfactory bulb: Coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- 8.Neville K-R, Haberly L-B. Olfactory cortex. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York: Oxford University Press; 2004. pp. 415–454. [Google Scholar]
- 9.Lei H, Mooney R, Katz L-C. Synaptic integration of olfactory information in mouse anterior olfactory nucleus. J Neurosci. 2006;26:12023–12032. doi: 10.1523/JNEUROSCI.2598-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Carlos J-A, Lopez-Mascaraque L, Valverde F. Connections of the olfactory bulb and nucleus olfactorius anterior in the hedgehog (Erinaceus europaeus): Fluorescent tracers and HRP study. J Comp Neurol. 1989;279:601–618. doi: 10.1002/cne.902790408. [DOI] [PubMed] [Google Scholar]
- 11.Scott J-W, Ranier EC, Pemberton JL, Orona E, Mouradian LE. Pattern of rat olfactory bulb mitral and tufted cell connections to the anterior olfactory nucleus pars externa. J Comp Neurol. 1985;242:415–424. doi: 10.1002/cne.902420309. [DOI] [PubMed] [Google Scholar]
- 12.Brunjes P-C, Illig K-R, Meyer E-A. A field guide to the anterior olfactory nucleus (cortex) Brain Res Brain Res Rev. 2005;50:305–335. doi: 10.1016/j.brainresrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Wilson D-A. Binaral interactions in the rat piriform cortex. J Neurophysiol. 1997;78:160–169. doi: 10.1152/jn.1997.78.1.160. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchitani C, Boudreau J-C. Single unit analysis of cat superior olive S segment with tonal stimuli. J Neurophysiol. 1966;29:684–697. doi: 10.1152/jn.1966.29.4.684. [DOI] [PubMed] [Google Scholar]
- 15.Wilson D-A, Sullivan R-M. Respiratory airflow pattern at the rat's snout and an hypothesis regarding its role in olfaction. Physiol Behav. 1999;66:41–44. doi: 10.1016/s0031-9384(98)00269-8. [DOI] [PubMed] [Google Scholar]
- 16.Haberly L-B, Price J-L. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- 17.Yan Z, et al. Precise circuitry links bilaterally symmetric olfactory maps. Neuron. 2008;58:613–624. doi: 10.1016/j.neuron.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld T-A, Macrides F. Topographic organization of connections between the main olfactory bulb and pars externa of the anterior olfactory nucleus in the hamster. J Comp Neurol. 1984;227:121–135. doi: 10.1002/cne.902270113. [DOI] [PubMed] [Google Scholar]
- 19.Johnson B-A, Ong J, Leon M. Glomerular activity patterns evoked by natural odor objects in the rat olfactory bulb are related to patterns evoked by major odorant components. J Comp Neurol. 2010;518:1542–1555. doi: 10.1002/cne.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis B-J, Macrides F. The organization of centrifugal projections from the anterior olfactory nucleus, ventral hippocampal rudiment, and piriform cortex to the main olfactory bulb in the hamster: An autoradiographic study. J Comp Neurol. 1981;203:475–493. doi: 10.1002/cne.902030310. [DOI] [PubMed] [Google Scholar]
- 21.Luskin M-B, Price J-L. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- 22.Green JD, Mancia M, Mancia M von BAUMGARTEN. Recurrent inhibition in the olfactory bulb. II. Effects of antidromic stimulation of commissural fibers. J Neurophysiol. 1962;25:489–500. doi: 10.1152/jn.1962.25.4.489. [DOI] [PubMed] [Google Scholar]
- 23.Illig K-R, Eudy J-D. Contralateral projections of the rat anterior olfactory nucleus. J Comp Neurol. 2009;512:115–123. doi: 10.1002/cne.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen T-A, Heinbockel T, Hildebrand J-G. Olfactory information processing in the brain: Encoding chemical and temporal features of odors. J Neurobiol. 1996;30:82–91. doi: 10.1002/(SICI)1097-4695(199605)30:1<82::AID-NEU8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 25.Glendenning K-K, Baker B-N, Hutson K-A, Masterton R-B. Acoustic chiasm V: Inhibition and excitation in the ipsilateral and contralateral projections of LSO. J Comp Neurol. 1992;319:100–122. doi: 10.1002/cne.903190110. [DOI] [PubMed] [Google Scholar]
- 26.Wallace D-G, Gorny B, Whishaw I-Q. Rats can track odors, other rats, and themselves: Implications for the study of spatial behavior. Behav Brain Res. 2002;131:185–192. doi: 10.1016/s0166-4328(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Marin A, Duistermars B-J, Frye M-A, Louis M. Mechanisms of odor-tracking: Multiple sensors for enhanced perception and behavior. Front Cell Neurosci. 2010;4:6. doi: 10.3389/fncel.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei H, Riffell J-A, Gage S-L, Hildebrand J-G. Contrast enhancement of stimulus intermittency in a primary olfactory network and its behavioral significance. J Biol. 2009;8:21. doi: 10.1186/jbiol120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagayama S, Takahashi Y-K, Yoshihara Y, Mori K. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol. 2004;91:2532–2540. doi: 10.1152/jn.01266.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.