Abstract
How do fluctuations in the level of generalized arousal of the brain affect the performance of specific motivated behaviors, such as sexual behaviors that depend on sexual arousal? A great deal of previous work has provided us with two important starting points in answering this question: (i) that histamine (HA) serves generalized CNS arousal and (ii) that heightened electrical activity of neurons in the ventromedial nucleus of the hypothalamus (VMN) is necessary and sufficient for facilitating the primary female sex behavior in laboratory animals, lordosis behavior. Here we used patch clamp recording technology to analyze HA effects on VMN neuronal activity. The results show that HA acting through H1 receptors (H1R) depolarizes these neurons. Further, acute administration of estradiol, an estrogen necessary for lordosis behavior to occur, heightens this effect. Hyperpolarization, which tends to decrease excitability and enhance inhibition, was not affected by acute estradiol or mediated by H1R but was mediated by other HA receptor subtypes, H2 and H3. Sampling of mRNA from individual VMN neurons showed colocalization of expression of H1 receptor mRNA with estrogen receptor (ER)-α mRNA but also revealed ER colocalization with the other HA receptor subtypes and colocalization of different subtypes with each other. The latter finding provides the molecular basis for complex “push-pull” regulation of VMN neuronal excitability by HA. Thus, in the simplest causal route, HA, acting on VMN neurons through H1R provides a mechanism by which elevated states of generalized CNS arousal can foster a specific estrogen-dependent, aroused behavior, sexual behavior.
Keywords: estrogen, arousal, lordosis, receptor colocalization, whole-cell patch clamp
The concept of a function called “generalized CNS arousal” has been presented and defined (1). This function is served by several classical neurotransmitters and certain peptides, such as orexin/hypocretin. Among them, histamine (HA), an arousal transmitter par excellence (2), was used here in biophysical studies of ventromedial hypothalamic neurons because these neurons are at the top of the neural circuit that produces female sexual arousal and behavior (3). Our goal is to explicate how a force for generalized CNS arousal, HA, affects neurons that regulate a specific, aroused behavior, sexual behavior.
Rodent female sexual behavior, lordosis, is dependent on genomic actions of estrogens. In addition to having genomic actions, estrogens can also act nongenomically (e.g., ref. 4). Nongenomic actions on the hypothalamic ventromedial nucleus (VMN), a brain region which is crucial for estrogenic induction of lordosis (5), can facilitate genomic actions in the induction of lordosis (6) and also can potentiate the excitability of VMN neurons in response to HA (7). HA is not only an important arousal neurotransmitter but also can facilitate lordosis (8). Thus, by potentiating HA action, estrogens may act nongenomically to facilitate lordosis by heightening the effect of a generalized CNS arousal transmitter.
The relationships among estrogens, HA action and the facilitation of lordosis mentioned above are complicated because, first, HA evokes not just one but three types of responses from VMN neurons: excitation, inhibition, and biphasic responses (7). Second, all of these responses could be modulated by an acute applied estrogen, estradiol (E2), in ways consistent with E2 acting through VMN to facilitate lordosis behavior but opposite for different responses: potentiation of excitation and attenuation of inhibition (7). Third, in the hypothalamus, there are at least three HA receptor (HAR) subtypes and probably one ligand-gated ionotropic receptor (2, 9). These subtypes couple to different G protein systems and could mediate different types of HA responses. Thus, one needs to know the identity of the HAR subtype mediating each type of HA responses and how its signaling is modulated by the nongenomic action of acutely applied E2.
In the current study we used both pharmacological analyses of whole-cell recording results and reverse transcription quantitative PCR (RT-qPCR) to (i) identify which receptor subtypes mediate HA's depolarizing or hyperpolarizing actions; (ii) determine whether there are colocalizations among H1, H2, and H3 subtypes and whether they colocalize with estrogen receptor (ER) subtypes ERα and /or ERβ; and (iii) to identify HAR subtype(s), whose response(s) to HA can be modulated by E2. These findings have helped us to elucidate a complex, push-pull hormonal regulation of the process by which an arousal-related transmitter facilitates performance of a specific, sexually aroused behavior.
Results
Electrophysiology.
HA responses are due to HA acting directly on the recorded neurons and not through any interneuron.
HA and its agonists can evoke three types of responses: depolarization, hyperpolarization, and biphasic response (Fig. 1A and Fig. S1). In 12 neurons depolarized and five hyperpolarized by HA, synaptic blockade with TTX (1 μM) or Ca2+-free ACSF neither blocked nor attenuated the responses. The amplitudes of the depolarizations and the hyperpolarizations during synaptic blockade were, respectively, 99.2 ± 4.3% (n = 12, P > 0.7) and 98.3 ± 1.7% (n = 5, P > 0.3, paired t test, two-tailed) of those before the blockade. Apparently, HA caused different responses by acting on different receptor subtypes, rather than on a single type whose effect was subsequently reversed by an interneuron(s).
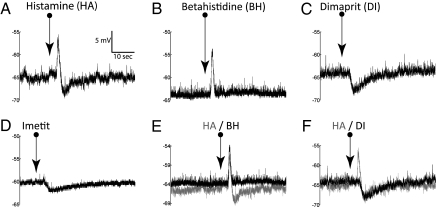
Fig. 1.
HA caused biphasic responses that could be mimicked by agonists for its different receptor subtypes. Here and in the similar figures hereafter, the traces represent membrane potentials recorded under current clamp. The arrows underneath the names of agonist indicate the time points when the agonists were ejected. With respect to HA-evoked biphasic membrane voltage shifts (A), BH (histamine receptor H1 agonist) mimicked only the depolarization portion (B), whereas H2 agonist dimaprit (C) and H3 agonist imetit (D) mimicked only the hyperpolarization. Superposition of HA biphasic response (lighter traces) with either BH depolarization (E) or dimaprit hyperpolarization (F) shows that the time courses of the respective responses resembled closely but differed between the two types of responses. The voltage/time scale in A applies to all traces.
HA evoked depolarizations are mediated mainly by H1 receptors and partly by H2 receptors, but unlikely by H3 receptors.
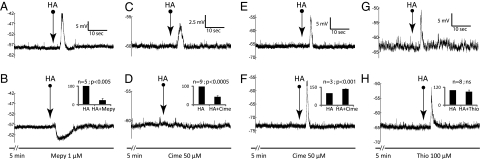
HA evoked most frequently (69% or 25 of 36 neurons tested) depolarization, followed by biphasic response (28% or 10/36) and rarely hyperpolarization alone (3% or 1/36) (Fig. S1). This action was mimicked by H1 agonist, betahistidine [or betahistine (10)] (BH), which evoked mainly (85% or 17/20) depolarization (Figs. 1B and Fig. S1) and, to a much lesser extent, biphasic responses (15% or 3/20) but never hyperpolarization alone (Fig. S1). Conversely, the H1 antagonist mepyramine, at 1 μM [used by others at 10 (11) and 30 μM (12)], attenuated or blocked HA depolarizations or reversed them into hyperpolarizations in five over seven neurons (with no effect on the remaining two) (Fig. 2 A and B), and, in all three neurons showing biphasic HA response, mepyramine attenuated the depolarization phase, shifted it into hyperpolarization, and/or enhanced the hyperpolarization phase. Mepyramine also had similar antagonistic effects on the depolarization by BH, the H1 agonist. At 1 μM, it diminished BH depolarization by more than 80% in two out of two neurons. At 10 μM, it abolished BH depolarization (Fig. 3 A and B) in all four neurons tested. These consistent actions of the H1 agonist and antagonist clearly indicate that HA depolarizations, at least the majority of them, are mediated by H1 receptors (H1R) subtype.
Fig. 2.
Effects of histamine (HA) receptor antagonists on HA evoked responses. Responses to agonists before and during application of modulators are in the upper and lower rows, respectively, in this figure and the next two figures. The interval between upper and lower traces in each pair is approximately 10 min. (B, D, F, and H, Insets) The summaries of the results (error bars represent SEM) of each modulator treatment, with the pretreatment amplitude served as the control. The modulator and the dose used are shown at the bottom. The voltage and time scales apply to both traces in each pair. (A and B) mepyramine (Mepy) not only blocked but reversed HA depolarization (n = 2), revealing a hidden hyperpolarization (note the difference in time courses). It attenuated HA depolarization in three others to reduce it down to 22 ± 25% (Inset). (C and D) on some neurons, cimetidine (Cime) could block or attenuate HA depolarizations down to 39 ± 24% (Inset). (E and F) On other neurons, it enhanced the depolarization to 135 ± 2%. (G and H) thioperamide (Thio) had no significant effect on HA response. Statistical significance is indicated above every histogram. ns indicates P > 0.05.
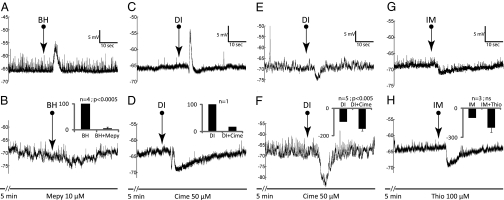
Fig. 3.
Effects of antagonists for H1, H2, and H3 subtypes on responses evoked by respective agonists. (A and B) mepyramine (Mepy) was able to block BH depolarization to 5 ± 10% of pretreatment amplitude. (C and D) depolarization by DI (dimaprit) occurred only in one unit, with an average amplitude of 9 mV over three stimulations. It was reversed 3 min after cimetidine (Cime) perfusion into a hyperpolarization. During washout, the hyperpolarization was reduced to zero and the depolarization recovered to 3 mV. (E and F) when DI caused hyperpolarizations, cimetidine enhanced the amplitude to 140 ± 36%. (G and H) thioperamide (Thio) had a tendency to enhance the hyperpolarizations caused by IM (imetit).
The role of H2 receptors (H2R) is ambiguous. Its agonist, dimaprit (13), in contrast to H1 agonist, very rarely evoked depolarization (1/28) (Fig. 3C and Fig. S1) and never biphasic response (0/28) (Fig. S1). Its antagonist, cimetidine [50 μM, Yamaura et al. used 10 μM (14) and Saccomani et al. used 100 μM (15) successfully], on the other hand, acted more like the H1 antagonist, mepyramine. It blocked or attenuated HA depolarization in 9 of 14 neurons (Fig. 2 C and D), attenuated the depolarizing phase of a biphasic response (1/2), and reversed the only depolarization by the H2 agonist, dimaprit (Fig. 3 C and D). Although controversial, a role, at least a minor one, of H2R in mediating HA depolarization cannot be ruled out.
The H3 receptors (H3R) subtype plays, at best, only a minor role. Its agonist, imetit (16), in six neurons, evoked no depolarization, only one biphasic response, and five hyperpolarizations (Fig. 1D and Fig. S1). The H3 antagonist, thioperamide, at 100 μM [Yamaura et al. found it effective at 10 μM (17)] had no effect on HA response in seven neurons (Fig. 2 G and H) but enhanced HA depolarization in one neuron.
HA evoked hyperpolarizations are not mediated by H1 but may be by H2R and/or H3R.
HA and the H1 agonist practically never evoked hyperpolarization alone (Fig. S1). Consistent with this, the H1 antagonist, mepyramine, never blocked the hyperpolarization phase of HA-evoked biphasic responses in any of the three neurons tested. Clearly, H1 receptors do not mediate HA hyperpolarization.
The H2 and H3 agonists, dimaprit (Fig. 1C) and imetit (Fig. 1D), hyperpolarized 96% (27/28 neurons) and 83% (5/6), respectively, of the neurons tested (Fig. S1), suggesting that HA hyperpolarization was mediated by H2 and H3 receptors. However, the results from their antagonists were not consistent with this suggestion. In six neurons that responded with biphasic or hyperpolarizing responses to HA, neither cimetidine (50 μM) or thioperamide (100 μM), each on three neurons, attenuated the hyperpolarization.
This inconsistency was also obvious in the following tests. In seven neurons, the hyperpolarization by dimaprit was slightly reduced (down to 78% of the control) by cimetidine in only one, not affected in another, but enhanced to 162.9 ± 4.3% in the remaining five neurons (Fig. 3 E and F). In six neurons, the hyperpolarization by the H3 agonist, imetit, was not affected by 0.01, 1, or 10 μM of the H3 antagonist, thioperamide. At the supramaximal 100 μM, thioperamide still did not block but instead enhanced the imetit hyperpolarization to 198.9 ± 103.3% (Fig. 3 G and H; n = 3; range: 80–267%) of the control.
Two phases of biphasic HA responses are mediated by different HAR subtypes.
In eight neurons that responded biphasically to HA, none had both phases simultaneously attenuated or abolished by any of the HAR antagonists, indicating that no biphasic response was mediated by a single HAR subtype. The depolarizing phase could be attenuated or even abolished (often accompanied by enhancement of hyperpolarizing phase) by mepyramine or cimetidine (n = 4) but not thioperamide, indicating that this phase, like the depolarization described above, is mediated by H1R and H2R but not by H3R. The hyperpolarizing phase, on the other hand, like the hyperpolarizations, has never been observed to be abolished or attenuated by cimetidine or thioperamide, suggesting that both types of response are mediated by the same subtype of receptors. These suggestions are also supported by the similarities in time courses as revealed by superimposing a depolarization or hyperpolarization trace on a biphasic response (Fig. 1 E and F).
Estrogen potentiated HA depolarization but had no effect on HA hyperpolarization.
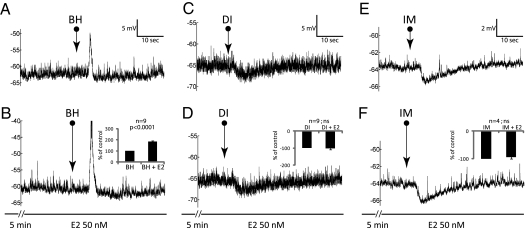
Three groups of neurons were either repeatedly depolarized with BH (n = 11) or hyperpolarized by dimaprit (n = 9) or imetit (n = 4) and treated with E2 (10 or 50 nM for up to 15 min) during the repeated agonist application (Fig. 4). As summarized in Table 1, E2 treatment reversibly potentiated the depolarizations (Fig. 4 A and B) in 9 of the 11 neurons stimulated with BH (Table 1, first and second rows). The potentiation occurred within 5 min and lasted throughout the entire 15 min of the E2 treatment. Hyperpolarization by dimaprit (Fig. 4 C and D) or imetit (Fig. 4 E and F) were not affected by E2, except for one neuron. This neuron was hyperpolarized with dimaprit, and the response amplitude decreased to 78.7% at 10 and 15 min after E2 treatment. The specificity of E2 action was assessed by replacing E2 with vehicle (ACSF, on neurons depolarized with BH) or testosterone (n = 7, five depolarized with BH and two hyperpolarized with dimaprit). No modulation of depolarization or hyperpolarization by testosterone or vehicle was ever observed (Table 1).
Fig. 4.
Typical examples of the effects or lack of an effect of acutely applied estradiol (E2) on responses evoked by histamine agonists. (A and B) E2 potentiated betahistidine (BH) depolarization to 186 ± 17% of the pretreatment amplitude, but neither potentiated nor attenuated hyperpolarizations caused by dimaprit (DI in C and D) or imetit (IM in E and F).
Table 1.
Estradiol effects on responses to histamine agonists
| Amplitude of responses to agonists (%, during hormone treatment over prehormone baseline) | |||||
| Treatment | Agonist | Range | Avg ± SEM | n | P* |
| BH | 126.3–272.3 | 185.6 ± 17.0 | 9 | <0.0001 | |
| E2 10 or 50 nM | BH† | 98.1–102.3 | 100.2 ± 2.1 | 2 | ns |
| DI | 91.8–116.7 | 102.5 ± 2.8 | 9 | ns | |
| IM | 83.8–109.4 | 94.2 ± 6.2 | 4 | ns | |
| Testosterone 50 nM | DI | 93.1–99.4 | 96.2 ± 3.1 | 2 | ns |
| BH | 100.0–105.5 | 102.4 ± 1.1 | 5 | ns | |
| Vehicle | BH | 100 | 100 | 1 | — |
Estradiol (E2), but not testosterone or vehicle, can potentiate BH depolarization but not the hyperpolarizations by dimaprit (DI) or imetit (IM).
*Match-paired two-tailed t test.
†Note that in plotting the magnitude of the E2 effect we found a bimodal distribution, in which these two neurons were not affected by E2.
Colocalization of histamine receptor subtypes and estrogen receptors: Single-neuron RT-qPCR evidence.
Of 250 cells examined, only 97 were positive for at least one of the gene examined. Such a low rate of expression, together with precautions taken during cell sampling, makes contamination or false positives unlikely.
H1R, H2R, H3R, and ERα mRNA were detected, but not ERβ (Fig. S2). Of the HAR positive cells, H3R was, surprisingly, the most frequently expressed histamine receptor (49%, 48/97), followed by H1R (28%), and lastly H2R (9%). ERα was detected in 53% of the positive cells.
Fig. S2B summarizes coexpressions between the different types of receptors in single cells and Fig. S2 B–D show their distribution. In the 97 positive cells, histaminergic receptors colocalizing the most frequently were H1R with H3R (10%, 10/97 cells). H1R colocalized with H2R and/or H3R in 11% of the 97 cells and this combination of receptors may be responsible for the biphasic membrane voltage shift caused by HA. Among the cells that expressed each subtype of HA receptor, the percentages where H1R, H2R, and H3R colocalize with ERα were 37%, 22%, and 33%, respectively (Fig. S2B).
The expressions and coexpressions of HARs by RT-qPCR are lower than the corresponding responses and signs of colocalization by whole-cell recording. Membrane receptors in general are encoded by low abundance genes, and the levels of their mRNA are very likely to be low. Consequently, false negatives are highly likely to occur and could explain the observed discrepancies.
Discussion
Using biophysical analyses of whole-cell patch clamp recording and single-cell RT-qPCR assays of individual rat VMN cells, we have found evidence for (i) colocalization of depolarization-mediating and hyperpolarization-mediating HA receptor subtypes, (ii) possible existence of other H2- and/or H3-like subtypes, (iii) Selective E2 potentiation of HA depolarizing actions without affecting HA hyperpolarization. These results reveal both simple, clear (E2 on H1R, depolarization), and complex (H2R, H3R) regulation by an arousal transmitter of excitability in neurons that govern a sexually aroused behavior, lordosis.
Colocalization of Depolarizing and Hyperpolarizing HA receptors with ERα.
Throughout the present study, many lines of evidence led us to reach the above conclusion. First of all, the presence of biphasic responses strongly supports colocalization of different HAR subtypes by the following facts: (i) because HA acts directly on the neurons, the opposite depolarizing (excitatory) and hyperpolarizing (inhibitory) phases by HA are due to the activation of different receptor subtypes; (ii) simultaneous blockade of both phases was never observed, an antagonist either reduced the depolarizing phase and/or enhanced the hyperpolarizing phase; (iii) the time courses of the two phases are different, and each tightly matches corresponding depolarization or hyperpolarization by agonists (Fig. 1 E and F).
In addition to biphasic responses, the colocalization was also evidenced by: (i) the reversal of depolarizations into hyperpolarizations by mepyramine in two cases (Fig. 2 A and B), indicating the coexistence of “hidden” or “masked” inhibitory receptors with H1 receptors in these neurons; (ii) similar but conversely, enhancement of HA depolarizations by cimetidine or thioperamide (Fig. 2 E and F) in four neurons, again, indicate the existence of masked inhibitory HA receptors. Colocalization of two excitatory subtypes is also evident. In nine neurons depolarized by HA, cimetidine abolished the response in one (Fig. 2 C and D) but caused only partial blockade in the remaining eight (Fig. 2 Inset), suggesting that the eight neurons possess two types of excitatory receptors: cimetidine-sensitive and -insensitive. In all, of 32 VMN neurons that responded to HA, at least 23, or 72%, showed evidence of receptor colocalization.
Single-cell RT-qPCR confirmed the above mentioned electrophysiological findings regarding receptor colocalization. Each of the three HAR subtypes coexpressed with one or the other subtype (Fig. S2 B and C). Each HAR subtype also coexpressed with ERα (Fig. S2 B and D). Thus, with evidence from both functional and gene expression approaches, we demonstrated that colocalizations among different HAR subtypes and with ERα do occur in individual VMN neurons.
Colocalization of receptor subtypes in individual neurons in the medial basal hypothalamus appears to be the rule rather than the exception. It has been reported for several neurotransmitter systems, including adrenergic (18, 19), serotonergic (20), and cholinergic systems (21). It has also been reported for HA, but in brain regions other than VMN (22, 23).
Implications.
In the majority of cases, colocalized receptor subtypes mediate opposite responses (excitation and inhibition). This would allow for a fine tuning (“push-pull”) control of VMN neuronal responses to HA. Because the net response of a neuron with such receptor colocalization would be the sum of the opposing actions, modulation of either receptor subtype alone would affect the net outcome. This push-pull regulation arguably economizes modulatory mechanisms such as the estrogen potentiation of HA depolarization, to be discussed below. It is also important for pharmacological considerations. For example, application of an antagonist not only can block the targeted action but can also enhance the opposite action.
Possible Existence of Other H2- and/or H3-Like Subtypes in VMN Neurons.
This possibility is raised by careful examination of the unexpected inconsistencies between the actions of respective agonists and antagonists for H2R and H3R, as well as between agonist actions and gene expression. For H2 receptors, there are several revealing observations. First, although dimaprit evoked overwhelmingly hyperpolarization (Fig. 3 E and F), it could also evoke, albeit rarely, depolarization (Fig. 3C). Second, in the sole neuron depolarized by dimaprit, the depolarization was not only abolished but converted into hyperpolarization by cimetidine (Fig. 3 C and D), indicating that the agonist not only could stimulate a cimetidine-sensitive excitatory receptor but could also do so to a hidden, cimetidine-insensitive inhibitory receptor. Third, in nine neurons excited by HA and another showing biphasic response, the depolarization or depolarizing phase was blocked by cimetidine, indicating that there are depolarization-mediating cimetidine-sensitive HA receptors. Fourth, cimetidine did not block dimaprit hyperpolarization in any of the five neurons tested but, instead, enhanced it (Fig. 3 E and F). Fifth, although dimaprit was very effective in inducing hyperpolarization, eliciting that response from 38 of the 39 neurons tested (Fig. S1), the expression of H2R, in contrast, was unexpectedly sparse (Fig. S2), suggesting that the dimaprit-responsive inhibitory H2Rs were not detected by the present RT-qPCR protocol. All of these observations can be explained by the hypothesis that there are two subtypes of H2 receptors: one mediates dimaprit depolarization and is sensitive to cimetidine, less frequently observed and low expressed; and the other mediates hyperpolarization and is insensitive to cimetidine and not detected by present RT-qPCR protocol. Our finding of a dimaprit-responsive and cimetidine-insensitive inhibitory receptor may be a revelation of another HAR subtype.
Consistent with the above hypothesis, H2R has been reported to mediate excitation/depolarization in (e.g., refs. 2, 24), as well as inhibition/hyperpolarization (e.g., refs. 25, 26, also see 27).
As in the case of H2, H3 agonist and antagonist actions are not consistent. Like dimaprit, the presumed H3 agonist, imetit, induced hyperpolarization in almost all six neurons tested (five hyperpolarizations and one biphasic response; Fig. S1). Also, similar to cimetidine, the H3 antagonist, thioperamide, did not block HA-evoked depolarizations, hyperpolarizations, biphasic responses (Fig. 2 G and H; n = 8), or imetit-induced hyperpolarizations (Fig. 3 G and H; n = 3). Imetit was defined as a H3 agonist because it can inhibit the binding of a prototypic H3 ligand and inhibit depolarization-induced HA release from brain slices (16). The latter action was blocked by the selective H3 antagonist, thioperamide, and was presumed to be due to the inhibition of presynaptic H3 receptors (16). Because imetit hyperpolarization was not blocked by thioperamide, and because synaptic blockade with TTX showed that HA response was not due to a presynaptic action, it is very likely that imetit acted on a thioperamide-insensitive postsynaptic version of H3 receptors or even another subtype of HA receptor.
Selective E2 Potentiation of HA Depolarization.
In a previous study, we found that E2 could both potentiate HA-induced excitation and attenuate HA-induced inhibition (7), raising the possibility that E2 can act through two independent mechanisms to modulate excitatory and inhibitory HA responses. In the present study, E2 was found only to potentiate depolarization induced by the H1 agonist and had no effect, either potentiation or attenuation, on hyperpolarization induced by H2 or H3 agonists (Table 1). The failure to modulate hyperpolarization is, first, not because the agonist doses used were too high, as we have adjusted the position of the ejecting pipette to obtain submaximal responses or doses (Materials and Methods). This was reflected by the facts that the hyperpolarizations induced by dimaprit (Fig. 3 E and F) or imetit (Fig. 3 G and H) with such doses could still be enhanced by cimetidine or thioperamide, respectively and that by dimaprit is weak enough to be masked by its depolarizing action (Fig. 3 C and D). Second, a lack of ERα is not a cause either because H2R and especially H3R were found to be coexpressed with ERα (Fig. S2 B and D). Thus, in the HA system at least, E2 appears to modulate neuronal responses via a single mechanism.
The selective modulation by E2 has another implication. HA depolarization is mediated mainly by H1 receptors, which couple to Gq/11 systems (2), and the activation of Gq/11 by HA leading to the break down of the membrane phosphoinositide, phosphatidylinositol 4,5-bisphosphate (PIP2) is a crucial step in inducing depolarization/excitation. The break down of PIP2, which binds electrostatically to a wide variety of K+ channels thereby keeping the channels functioning (28, 29), would inhibit K+ channels by hydrolyzing PIP2 and thereby induces depolarization. Thus, a likely mechanism of E2 modulation is to further the inhibition of certain K+ channels by facilitating the hydrolysis of PIP2 (for more detailed discussion, see ref. 30). This possibility is consistent with our earlier finding that acutely applied E2 alone could inhibit whole-cell K+ currents (31).
Behavioral Implications.
The implications are driven as follows. An increase in generalized arousal would be accompanied by increased release of HA, which, having bound to H1 receptors in VMN neurons, depolarizes them. Furthermore, E2 increases depolarizing actions and does not increase hyperpolarizing actions of HA, further heightening the excitability of VMN neurons. HA stimulation facilitates sexual arousal (8), and heightened electrical activity in VMN neurons is necessary and sufficient for activating the neural circuit for the female sex behavior, lordosis (30, 32). Thus, present results provide a simple biophysical mechanism by which generalized arousal heightens electrical excitability of VMN neurons and thereby fosters female sexual behavior.
The same experiments also revealed more complex phenomena: push-pull regulation of VMN neuronal electrical activity by HA and the complexities of HA receptor antagonist effects that either show their unexpected multiple actions or provide the harbinger of other HA receptor subtypes.
Materials and Methods
Materials.
HA, its agents, E2, testosterone, and chemicals for electrophysiology were purchased from Sigma; and those for RT-qPCR from Applied Biosystems. Details about materials and the rest of this section are presented in SI Materials and Methods.
Electrophysiology.
All procedures in handling and treating the animals were approved by The Rockefeller University's Animal Care and Use Committee in accordance with the Animal Welfare Act and the Department of Health and Human Services Guide for the Care and Use of Laboratory Animals.
Neurons exclusively from ventrolateral VMN in brain slices (300 μm) were prepared as described previously (7, 31) from 12- to 30-d-old female rats capable of responding to E2 (33), perifused with ACSF at room temperature, and patched using conventional methods as described in a previous study (31). Once obtained, the quality of the patch was checked with a membrane test, and if it met the criteria [access resistance (Ra) ≤ 15 mΩ, leak current ≤30 pA and membrane voltage (Vm) equal or more negative than −45 mV] the recording was switched to current clamp (holding potential = −65 mV) for experiments. When in doubt, the criteria were checked again, and, if not met, the recording was terminated.
Experiments began with applying HA or its agonists (10 mM in ACSF in ejecting pipette) repeatedly at a fixed interval with a picospritzer as the position of the pipette was adjusted to obtain a modulable and stable response. Following this, antagonists or E2 were applied through the bath for up to 15 min while the stimulation continued. Any change in responses caused by these agents was regarded as an effective modulation if it was beyond ±20% of the pretreatment control. Ejection of ACSF, even accompanied with mechanical disturbances, never evoked any response.
Single Cell RT-qPCR.
Collection of single cells.
Cells from ventrolateral VMN were carefully aspirated into a pipette (tip diameter approximately 3.5 μm) filled with 15 μL of RNase-free water (Ambion). The pipette with the cell was then removed and its tip broken into a 0.2-mL PCR tube (Molecular BioProducts) where its content was released and immediately frozen on dry ice.
Twenty-four cells in one slice per animal were collected. As a negative control, ACSF was collected instead of the cell. As expected, the collected ACSF did not result in any amplification. In another control, portion of each sample was subjected to NO-RT reaction where the enzyme is replaced by an equal volume of RNase-free water. In 79 NO-RT reactions only one produced amplification before cycle 40, the cycle limit for expression validation.
RT-qPCR.
mRNA was reverse-transcribed into cDNA following the manufacturer's protocol (High Capacity cDNA Reverse Transcription Kit; Applied Biosystems). RT was followed by quantitative PCR on a Prism 7700 (Applied Biosystems) using the following TaqMan gene expression assays: Rn00566691_s1 for H1R, Rn00564216_s1 for H2R, Rn00585276_m1 for H3R, Rn00562166_m1 for ERα (34), and Rn00562610_m1 for ERβ (35). A cell was regarded as positive and included in analyses if it showed expression of at least one of the receptors (H1R, H2R, H3R, or ERα or β).
Statistics.
Match-paired, two-tailed t test was used to test the significance of the effects of E2 on histamine agonists (Table 1) and the significance of the effects of histamine receptors antagonists (Figs. 2–4).
Supplementary Material
Acknowledgments
We thank Drs. Ana Ribeiro and Nino Devidze for technical advice. This study was funded by National Institutes of Health Grants HD05751 and MH38273.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006049107/-/DCSupplemental.
References
- 1.Pfaff DW. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge, MA: Harvard University Press; 2006. [Google Scholar]
- 2.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, et al. Genetic mechanisms in neural and hormonal controls over female reproductive behaviors. In: Pfaff DW, editor. Hormones, Brain and Behavior. 2nd Ed. Vol. 2. San Diego: Academic Press/Elsevier; 2009. pp. 1163–1186. [Google Scholar]
- 4.Vasudevan N, Kow L-M, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Pfaff DW. Estrogen and Brain Function, Neural Analysis of a Hormone-Controlled Mammalian Reproductive Behavior. New York: Springer-Verlag; 1980. [Google Scholar]
- 6.Kow L-M, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kow L-M, Easton A, Pfaff DW. Acute estrogen potentiates excitatory responses of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2005;1043:124–131. doi: 10.1016/j.brainres.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 8.Donoso AO, Broitman ST. Effects of a histamine synthesis inhibitor and antihistamines on the sexual behavior of female rats. Psychopharmacology (Berl) 1979;66:251–255. doi: 10.1007/BF00428315. [DOI] [PubMed] [Google Scholar]
- 9.Haas HL, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 10.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Hatton GI. Histamine suppresses non-NMDA excitatory synaptic currents in rat supraoptic nucleus neurons. J Neurophysiol. 2000;83:2616–2625. doi: 10.1152/jn.2000.83.5.2616. [DOI] [PubMed] [Google Scholar]
- 12.Fioretti B, et al. Histamine hyperpolarizes human glioblastoma cells by activating the intermediate-conductance Ca2+-activated K+ channel. Am J Physiol Cell Physiol. 2009;297:C102–C110. doi: 10.1152/ajpcell.00354.2008. [DOI] [PubMed] [Google Scholar]
- 13.Whyment AD, Blanks AM, Lee K, Renaud LP, Spanswick D. Histamine excites neonatal rat sympathetic preganglionic neurons in vitro via activation of H1 receptors. J Neurophysiol. 2006;95:2492–2500. doi: 10.1152/jn.01135.2004. [DOI] [PubMed] [Google Scholar]
- 14.Yamaura K, Yonekawa T, Nakamura T, Yano S, Ueno K. The histamine H2-receptor antagonist, cimetidine, inhibits the articular osteopenia in rats with adjuvant-induced arthritis by suppressing the osteoclast differentiation induced by histamine. J Pharmacol Sci. 2003;92:43–49. doi: 10.1254/jphs.92.43. [DOI] [PubMed] [Google Scholar]
- 15.Saccomani G, Psarras CG, Smith PR, Kirk KL, Shoemaker RL. Histamine-induced chloride channels in apical membrane of isolated rabbit parietal cells. Am J Physiol. 1991;260:C1000–C1011. doi: 10.1152/ajpcell.1991.260.5.C1000. [DOI] [PubMed] [Google Scholar]
- 16.Garbarg M, et al. S-[2-(4-imidazolyl)ethyl]isothiourea, a highly specific and potent histamine H3 receptor agonist. J Pharmacol Exp Ther. 1992;263:304–310. [PubMed] [Google Scholar]
- 17.Takeshita Y, et al. Histamine modulates high-voltage-activated calcium channels in neurons dissociated from the rat tuberomammillary nucleus. Neuroscience. 1998;87:797–805. doi: 10.1016/s0306-4522(98)00152-3. [DOI] [PubMed] [Google Scholar]
- 18.Kow L-M, Pfaff DW. Responses of ventromedial hypothalamic neurons in vitro to norepinephrine: Dependence on dose and receptor type. Brain Res. 1987;413:220–228. doi: 10.1016/0006-8993(87)91013-4. [DOI] [PubMed] [Google Scholar]
- 19.Kow L-M, Weesner GD, Pfaff DW. α1-adrenergic agonists act on the ventromedial hypothalamus to cause neuronal excitation and lordosis facilitation: Electrophysiological and behavioral evidence. Brain Res. 1992;588:237–245. doi: 10.1016/0006-8993(92)91581-x. [DOI] [PubMed] [Google Scholar]
- 20.Kow L-M, Tsai YF, Wang L, Pfaff DW. Electrophysiological analyses of serotonergic actions on neurons in hypothalamic ventromedial nucleus in vitro:Receptor subtypes involved and implications for regulation of feeding and lordosis behaviors. Chin J Physiol. 1992;35:105–121. [PubMed] [Google Scholar]
- 21.Kow L-M, Tsai YF, Weiland NG, McEwen BS, Pfaff DW. In vitro electro-pharmacological and autoradiographic analyses of muscarinic receptor subtypes in rat hypothalamic ventromedial nucleus: Implications for cholinergic regulation of lordosis. Brain Res. 1995;694:29–39. doi: 10.1016/0006-8993(95)00747-e. [DOI] [PubMed] [Google Scholar]
- 22.Jorgenson KL, Kow L-M, Pfaff DW. Histamine excites arcuate neurons in vitro through H1 receptors. Brain Res. 1989;502:171–179. doi: 10.1016/0006-8993(89)90473-3. [DOI] [PubMed] [Google Scholar]
- 23.Kow L-M, Pfaff DW. Responses of hypothalamic paraventricular neurons in vitro to norepinephrine and other feeding-relevant agents. Physiol Behav. 1989;46:265–271. doi: 10.1016/0031-9384(89)90266-7. [DOI] [PubMed] [Google Scholar]
- 24.Shen B, Li H-Z, Wang J-J. Excitatory effects of histamine on cerebellar interpositus nuclear cells of rats through H(2) receptors in vitro. Brain Res. 2002;948:64–71. doi: 10.1016/s0006-8993(02)02950-5. [DOI] [PubMed] [Google Scholar]
- 25.Geller HM, Springfield SA, Tiberio AR. Electrophysiological actions of histamine. Can J Physiol Pharmacol. 1984;62:715–719. doi: 10.1139/y84-118. [DOI] [PubMed] [Google Scholar]
- 26.Hatton GI, Yang QZ. Ionotropic histamine receptors and H2 receptors modulate supraoptic oxytocin neuronal excitability and dye coupling. J Neurosci. 2001;21:2974–2982. doi: 10.1523/JNEUROSCI.21-09-02974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas HL. Electrophysiology of histamine-receptors. In: Schwartz JC, Haas HL, editors. The Histamine Receptor. New York: Wiley-Liss; 1992. pp. 161–177. [Google Scholar]
- 28.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:re19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 29.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: How and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kow L-M, Pfaff DW. Membrane-initiated estrogen actions on ion channels and the induction of lordosis, the rodent female sexual behavior. Chin J Physiol. 2009;52:175–195. [Google Scholar]
- 31.Kow L-M, Devidze N, Pataky S, Shibuya I, Pfaff DW. Acute estradiol application increases inward and decreases outward whole-cell currents of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2006;1116:1–11. doi: 10.1016/j.brainres.2006.07.104. [DOI] [PubMed] [Google Scholar]
- 32.Kow L-M, Mobbs CV, Pfaff DW. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: A review. Neurosci Biobehav Rev. 1994;18:251–268. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 33.Kow L-M, Bogun M, Zhang Q, Pfaff DW. Hormonal induction of lordosis and ear wiggling in rat pups: Gender and age differences. Endocrine. 2007;32:287–296. doi: 10.1007/s12020-008-9046-1. [DOI] [PubMed] [Google Scholar]
- 34.Wu W, et al. Network analysis of temporal effects of intermittent and sustained hypoxia on rat lungs. Physiol Genomics. 2008;36:24–34. doi: 10.1152/physiolgenomics.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiik A, et al. Activation of estrogen response elements is mediated both via estrogen and muscle contractions in rat skeletal muscle myotubes. Am J Physiol Cell Physiol. 2009;296:C215–C220. doi: 10.1152/ajpcell.00148.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.