Abstract
Experimental autoimmune encephalomyelitis (EAE) is an animal model of multiple sclerosis (MS). Although prostaglandin (PG) concentrations are increased in cerebrospinal fluid of MS patients, the role of PGs in MS is unknown. We examined this issue by subjecting mice deficient in each PG receptor type or subtype to EAE induction and using agonists or antagonists selective for each of the four PGE receptor (EP) subtypes. Among PG receptor-deficient mice, only EP4−/− mice manifested significant suppression of EAE, which was mimicked in wild-type mice and to a greater extent, in EP2−/− mice by administration of the EP4 antagonist ONO-AE3-208 during the immunization phase. EP4 antagonism during immunization also suppressed the generation of antigen-specific T helper (Th) 1 and Th17 cells in wild-type mice and to a greater extent, in EP2−/− mice. ONO-AE3-208 administration at EAE onset had little effect on disease severity, and its administration throughout the experimental period did not cause significant reduction of the peak of disease, suggesting that, in addition to its facilitative action during the immunization phase, EP4 exerts a preventive action in the elicitation phase. Administration of the EP4 agonist ONO-AE1-329 at EAE onset delayed and suppressed disease progression as well as inhibited the associated increase in permeability of the blood–brain barrier. Thus, PGE2 exerts dual functions in EAE, facilitating Th1 and Th17 cell generation redundantly through EP4 and EP2 during immunization and attenuating invasion of these cells into the brain by protecting the blood–brain barrier through EP4.
Keywords: disease model, knockout mice, prostaglandin, prostaglandin receptor, multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS that primarily affects young adults (1, 2). The main pathological characteristics of MS include cell infiltration, demyelination, and axonal loss in the CNS. Although its etiology remains unclear, MS is thought to be a T cell-mediated autoimmune disease of the CNS in genetically susceptible individuals. Experimental autoimmune encephalomyelitis (EAE) is an animal model of MS that can be induced in susceptible animals by immunization with myelin proteins or by injection of myelin protein-specific CD4+ T cells (3). EAE has been studied intensively in investigations of the autoimmune response in the CNS, with recent studies having shown that T effector helper (Th) 17 or both Th17 and Th1 cells play a key role in disease pathogenesis (4–6). These Th cell subsets are also implicated in human MS (7–9).
The concentrations of arachidonate metabolites such as prostaglandin (PG) E2 and leukotriene C4 are increased in the cerebrospinal fluid (CSF) of individuals with MS (10, 11). Arachidonic acid is liberated from phospholipids by the action of phospholipase A2 and converted to PGs or leukotrienes by the action of cyclooxygenase (COX) and 5-lipoxygenase, respectively. Cytosolic phospholipase A2 is a major form of phospholipase A2 that is activated and liberates arachidonic acid in response to extracellular stimuli, and inhibition of this enzyme or deletion of its gene prevents EAE development in C57BL/6 mice (12, 13). In contrast, deletion of the 5-lipoxygenase gene did not prevent, but instead aggravated, EAE progression (14). These findings, thus, implicate PGs in the pathogenesis of EAE. Given their antiinflammatory actions, COX inhibitors have been examined for their effects on EAE in many studies. However, these drugs have been shown to exacerbate or ameliorate the disease (15–20), leaving the role of PGs an enigma. Prophylactic use of these drugs—that is, their administration beginning the day of immunization or before—seems to suppress EAE symptoms, whereas their therapeutic administration after or immediately before disease onset has only a mildly suppressant or an aggravating effect. Such opposite actions have been clearly shown for piroxicam (16) for example. These findings led us to hypothesize that PGs have dual actions, one facilitative and the other preventive, during EAE development and that the former is exerted in the immunization phase and the latter is exerted in the elicitation phase. However, the precise nature of these actions and the underlying signaling pathways remain unknown.
Prostaglandins include PGD2, PGE2, PGF2α, PGI2, and thromboxane (TX) A2, and they interact with eight types or subtypes of PG receptors to exert their actions. These receptors include the PGD receptor (DP), four subtypes of PGE receptor (EP1, EP2, EP3, and EP4), the PGF receptor (FP), the PGI receptor (IP), and the TXA receptor (TP) (21). We have generated mice deficient in each of these receptors individually as well as developed agonists or antagonists selective for each EP subtype. With these tools, we have examined the roles of each PG receptor in various pathological processes (21). We recently found that PGE2 acting at EP2 and EP4 collaborates with relevant cytokines to facilitate Th1 cell differentiation and Th17 cell expansion in vitro and that administration of an EP4 antagonist attenuated the development of EAE in vivo (22). However, genetic evidence for a role of EP4 in EAE has been lacking, and the roles of other PG receptors have not been tested, leaving the dual actions of PGs in EAE, suggested by the effects of COX inhibitors, a puzzle. We have now examined these issues by subjecting mice deficient in each PG receptor to EAE induction and applying agonists and antagonists selective for each EP subtype at different phases of EAE.
Results
Effects of Loss of Each PG Receptor on EAE Development.
The protocol for EAE induction is shown in Fig. 1A. We immunized mice with a peptide corresponding to amino acid residues 35–55 of mouse myelin oligodendrocyte glycoprotein (MOG35–55) in complete Freund's adjuvant on day 0 and injected pertussis toxin on days 0 and 2. Clinical signs were scored and monitored up to day 25. With this protocol, MOG-reactive T cells begin to accumulate in regional lymph nodes on day 7, and mice begin to develop clinical signs between days 8 and 10, with a peak around day 14 (22). We, therefore, defined days −1 to 7 as the immunization phase and days 7–25 as the elicitation phase. To verify a role for PGs in EAE and identify the receptors involved and their actions, we induced EAE in mice deficient in each of the eight types or subtypes of PG receptor individually. C57BL/6 mice were studied as controls, with the exception that the progeny of wild-type (WT) littermates (EP4+/+) in the mixed background of C57BL/6 and 129/Ola were used as controls for EP4−/− mice. We found that only EP4−/− mice showed significant attenuation of EAE signs compared with control mice, with the other seven lines of receptor-deficient mice developing EAE to an extent similar to that in the control animals (Fig. 1B). No aggravation or facilitation of EAE was apparent in any of the receptor-deficient mice.
Fig. 1.
Effects of loss of each PG receptor on EAE development. (A) Experimental protocol for EAE induction. PTX, pertussis toxin. (B) Mice lacking knockout (KO) DP, FP, IP, TP, EP1, EP2, EP3, or EP4 individually were subjected to EAE induction, and disease development was scored and compared with that in control (WT) mice. Data are means ± SEM for 5–12 mice per group. *P < 0.05 vs. the corresponding value for WT mice.
Effects of an EP4 Antagonist on EAE Development.
These results indicated that PGE2-EP4 signaling facilitates the EAE response, and thus, they provided genetic evidence in support of our previous data obtained with the EP4 antagonist ONO-AE3-208 in EAE (22). However, the fact that none of the other receptor-deficient mouse lines showed an exaggerated EAE response did not provide a clue as to the presumed protective action of PGs in EAE suggested by previous studies with COX inhibitors (15–20). Given that it was unclear from these experiments whether EP4 signaling functions other than during the immunization phase, we administered ONO-AE3-208 to C57BL/6 mice at a final dose of 10 mg/kg body mass per day in drinking water. We administered the drug in the immunization phase (days −1 to 7) only to examine its effects on initial T-cell development, in the elicitation phase to examine its effects on continued T-cell development after day 7 and on elicitation (days 7–25), or throughout the experimental period (days −1 to 25) to examine its combined effects on immunization and elicitation. Consistent with our previous finding (22), we found that ONO-AE3-208 significantly suppressed EAE signs throughout elicitation when administered only in the immunization phase. However, it elicited no significant suppression in the peak and only a suppressive effect in the downgrade of disease when administered from days −1 to 25, and it has no effect when administered only in the elicitation phase (Fig. 2). These results confirmed those obtained with EP4−/− mice and verified a facilitative role for PGE2-EP4 signaling in the immunization phase. However, the reduced extent of the effect of EP4 antagonist administration during the entire experimental period compared with that observed for administration during the immunization phase alone indicated that EP4 signaling may also exert a preventive action during elicitation.
Fig. 2.
Effects of the EP4 antagonist ONO-AE3-208 on EAE development. ONO-AE3-208 was administered per os at 10 mg/kg per day to WT C57BL/6 mice from days −1 to 25, days −1 to 7, or days 7 to 25. Control mice received vehicle. Data for clinical score are means ± SEM for six mice per group. *P < 0.05 vs. the corresponding value for control mice.
EP2 and EP4 Function Additively in EAE Development.
We previously showed that PGE2-EP2/EP4 signaling facilitates interleukin-12 (IL-12)–induced Th1 cell differentiation and IL-23–induced Th17 cell expansion in vitro (22). We also found that administration of ONO-AE3-208 attenuated the development of EAE in vivo with concomitant suppression of the accumulation of Th1 and Th17 cells in regional lymph nodes (22). However, whereas the effects of PGE2-EP4 signaling on T cells are likely to underlie the apparent role of EP4 in EAE development, whether EP2 redundantly contributes to the development of EAE has remained unknown. We, therefore, examined possible redundancy of EP2 and EP4 in EAE development and immune activation by comparing the dose dependence of the effects of ONO-AE3-208 in WT and EP2−/− mice. Furthermore, we compared the dose dependence of the suppressive effect of this compound on EAE with that of its effects on Th1 and Th17 cells and examined the possible relation between these parameters. To this end, we administered ONO-AE3-208 orally two times per day from days 3 to 7 in our EAE model. Treatment with ONO-AE3-208 reduced the EAE clinical score in a dose-dependent manner in WT mice, although some clinical signs still remained, even at the highest dose of 100 mg/kg per day (Fig. 3A). In contrast, ONO-AE3-208 suppressed EAE signs almost completely in EP2−/− mice, even at the lowest dose of 10 mg/kg per day (Fig. 3A). This effect of ONO-AE3-208 on the clinical score was confirmed by histological analysis (Fig. 3 B and C). H&E staining of the spinal cord at the disease peak revealed that the extent of infiltration of inflammatory cells was similar in WT and EP2−/− mice without treatment and that treatment with ONO-AE3-208 suppressed infiltration of these cells in a dose-dependent manner in both mouse lines (Fig. 3B and Fig. S1A). However, suppression of inflammatory cell infiltration by ONO-AE3-208 was more effective in EP2−/− mice than in WT animals (Fig. 3B). Demyelination of the spinal cord was also ameliorated by treatment with ONO-AE3-208 in both WT and EP2−/− mice (Fig. 3C and Fig. S1B), but again, this effect was more pronounced in EP2−/− mice (Fig. 3C). To examine the roles of EP2 and EP4 in effector T-cell development, we collected (on day 7) cells from the draining lymph nodes of both WT and EP2−/− mice treated with various doses of ONO-AE3-208 and examined their responsiveness to the MOG antigen. Suppression of MOG-specific cell proliferation and production of IFN-γ and IL-17, the signature cytokines of Th1 and Th17 cells, respectively, was observed in both WT and EP2−/− mice treated with ONO-AE3-208 (Fig. 3 D and E). However, the extent of the suppression of cytokine production was greater in EP2−/− mice than in WT animals. These results thus suggested that EP2 functions redundantly with EP4 in facilitation of effector T-cell development and elicitation of EAE in vivo.
Fig. 3.
Dose-dependent effects of ONO-AE3-208 on EAE development in WT and EP2−/− mice. (A) ONO-AE3-208 was administered per os at the indicated daily doses to WT C57BL/6 mice (Left) or EP2−/− (EP2KO) mice (Right) from days 3 to 7, and EAE development was examined for up to 25 d. Control mice received vehicle. (B and C) Histological analysis of WT and EP2−/− mice treated with the indicated doses of the EP4 antagonist as in A. The extents of cell infiltration (B) and demyelination (C) in the spinal cord were scored at day 14. Representative preparations stained with H&E or Luxol fast blue are shown in Fig. S1. (D and E) MOG-dependent proliferation of lymph node cells (D) and their production of IFN-γ and IL-17 (E). Lymph node cells were prepared on day 7 from WT and EP2−/− mice treated with the indicated doses of the EP4 antagonist as in A. All data are means ± SEM for eight mice per group. *P < 0.05; **P < 0.01; ***P < 0.005 vs. the corresponding value for control mice.
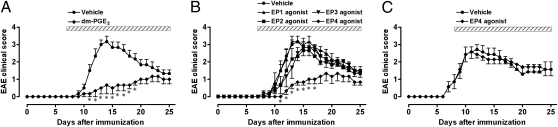
Preventive Effect of an EP4 Agonist on the Onset of EAE.
Our observation that administration of the EP4 antagonist from days −1 to 25, unlike that from days −1 to 7, did not significantly affect the peak of EAE (Fig. 2) suggested that PGE2 may exert a preventive action in EAE through interaction with EP4 during elicitation. To test this notion, we administered EP agonists during the elicitation phase and examined their effects on EAE development. We first administered 16,16-dimethyl PGE2 (dm-PGE2), a stable PGE2 analog, at an s.c. dose of 200 μg/kg per day from day 7 and examined its effects on the onset and progression of EAE. Administration of dm-PGE2 not only significantly slowed EAE development but also reduced its severity (Fig. 4A). Given that dm-PGE2 binds to three of four mouse EP subtypes—EP2, EP3, and EP4 (23)—we next examined the effects of agonists selective for each EP subtype (24). Among these selective agonists, only the EP4-selective agonist ONO-AE1-329 showed significant inhibition of EAE development similar to that observed with dm-PGE2 (Fig. 4B). Administration of the EP2- or EP3-selective agonists ONO-AE1-259 and ONO-AE-248, respectively, tended to suppress the severity of disease at its peak, but these effects were not statistically significant and the EP1 agonist ONO-DI-004 was without effect (Fig. 4B). Histological examination of the spinal cord at the peak of EAE (day 14) revealed that administration of the EP4 agonist inhibited both the infiltration of inflammatory cells and demyelination (Fig. 5 A–D). Given that administration from days 7 to 25 encompassed the periods of both cell infiltration into the CNS and active disease, we next administered the EP4 agonist after the onset of disease, when mice exhibited a clinical score of 1 (on average from day 8), and examined its effects. Administration of the EP4 agonist according to this schedule was without effect (Fig. 4C), indicating that this drug delays the onset of EAE but does not suppress ongoing CNS inflammation.
Fig. 4.
Effects of dm-PGE2 and EP subtype-selective agonists on the development of EAE. (A and B) Either dm-PGE2 at 200 μg/kg (A) or the EP1 agonist ONO-DI-004, the EP2 agonist ONO-AE1-259, the EP3 agonist ONO-AE-248, or the EP4 agonist ONO-AE1-329 at 100 μg/kg (B) was administered s.c. to WT C57BL/6 mice two times per day from days 7 to 25, and EAE development was monitored. (C) ONO-AE1-329 (100 μg/kg, two times a day) was administered s.c. after the onset of EAE signs (clinical score of 1) from day 8 on average, and EAE development was monitored. Shaded bars represent the period of drug treatment in each panel. All data are means ± SEM for six (A and B) or seven (C) mice per group. *P < 0.05 vs. the corresponding value for vehicle-treated mice.
Fig. 5.
Effects of ONO-AE1-329 on cell infiltration and demyelination in the spinal cord as well as BBB permeability during EAE development. (A–D) ONO-AE1-329 or vehicle (Veh) was administered to WT C57BL/6 mice from day 7 as described in Fig. 4B, the spinal cord was dissected at day 14 and stained with H&E (A) or Luxol fast blue (C), and both cell infiltration (B) and demyelination (D) were scored. The arrow in A indicates inflammatory-cell infiltration, and the arrow in C indicates demyelination. (Scale bars, 100 μm.) (E) ONO-AE1-329 was administered to mice as in A, sodium fluorescein was injected intraperitoneally on day 14, and the spinal cord was dissected 30 min later and evaluated for fluorescein incorporation; data are expressed as milligrams of sodium fluorescein per milligrams of spinal-cord protein. All quantitative data are means ± SEM for six mice per group. *P < 0.05; **P < 0.01 vs. vehicle-treated mice.
A pivotal step in triggering of CNS inflammation is disruption of the blood–brain barrier (BBB) (25). We, therefore, next administered the EP4 agonist to mice from days 7 to 14, injected sodium fluorescein intraperitoneally on day 14, and then, quantified fluorescein incorporation into the spinal cord 30 min later. The increase in the uptake of fluorescein into the CNS because of EAE was significantly inhibited by administration of the EP4 agonist (Fig. 5C), suggesting that stimulation of EP4 either directly or indirectly preserves BBB integrity and thereby impedes the entry of inflammatory substances and cells into the CNS.
Discussion
We have studied mice deficient in each of the eight types or subtypes of PG receptor individually and found that the development of EAE is suppressed in EP4−/− mice. This result provides genetic corroboration for our previous finding that administration of an EP4 antagonist in the immunization phase reduced the severity of EAE. Together, our observations have clarified the importance of PGE2-EP4 signaling in this model of MS. A role for PGE2 in the pathogenesis of EAE was also recently shown by the observation that disease development was suppressed in mice deficient in microsomal prostaglandin E synthase-1 (mPGES-1) (26), an inducible form of PGE synthase. Whereas this finding is complementary to our results, the kinetics of EAE suppression differ between EP4−/− and mPGES-1−/− mice. EP4 deficiency, thus, both delayed the onset and reduced the extent of EAE, whereas EAE developed to its peak in mPGES-1−/− mice with a time course similar to that in WT mice and was then suppressed. The reason for this difference remains unknown. In contrast to mPGES-1−/− mice, COX-2−/− mice were found not to differ from WT animals in the development of EAE (19), indicating the importance of COX-1 or redundancy of COX-1 and COX-2 in disease pathogenesis.
Our examination of the effects of an EP4-selective agonist and antagonist revealed that PGE2 exerts dual actions through EP4 in mouse EAE. First, consistent with our previous results (22), we found that administration of the EP4 antagonist in the immunization phase suppressed disease progression with concomitant suppression of Th1 and Th17 cell development, thus confirming that PGE2-EP4 signaling promotes disease progression by facilitating antigen-specific Th1 and Th17 cell development during this phase. However, we also found that administration of the EP4 antagonist at the onset of disease had little effect, whereas that of the EP4 agonist at this time markedly reduced disease severity. This protective action of the EP4 agonist was not observed when the drug was administered 1 d after disease onset, suggesting that the EP4 agonist inhibits an initiating event in CNS inflammation. We further found that administration of the EP4 agonist at disease onset inhibited the increase in BBB permeability. These results together indicate that PGE2-EP4 signaling suppresses EAE onset by preserving BBB integrity. These dual actions of PGE2-EP4 signaling can explain the puzzling effects of COX inhibitors on EAE observed previously. The effect of EP4 signaling on BBB integrity might be exerted either directly in endothelial cells or indirectly through regulation of T cells. A recent study showed that human Th17 cells preferentially migrate across a layer of human BBB endothelial cells in vitro, that human BBB endothelial cells in MS lesions express both IL-17 receptors and IL-22 receptors in vivo, and that IL-17 down-regulates expression of tight junction molecules, such as occludin and zonula occludens-1 (ZO-1), in BBB endothelial cells in vitro (27). Reduction of occludin and ZO-1 was also noted in the spinal cord of EAE mouse (27). Agents capable of suppressing Th17 cell function may, thus, preserve BBB integrity at disease onset. However, PGE2-EP4 signaling enhances rather than inhibits Th17 cell function, making it difficult to explain the observed promotion of BBB integrity by the EP4 agonist in the present study through inhibition of Th17 cells. On the other hand, previous studies have shown that cAMP as well as PGs such as PGE2 and prostacyclin that induce cAMP accumulation potentiate cadherin-mediated cell–cell contact and enhance endothelial barrier function (28, 29). Given that EP4 activation results in cAMP accumulation, EP4 signaling is more likely to promote BBB integrity through a direct action in endothelial cells.
In addition to the dual actions of PGE2-EP4 signaling, we have also clarified the redundant actions of EP2 and EP4 in EAE by comparing the dose-response relations of the EP4 antagonist in WT and EP2-deficient mice. The EP4 antagonist was more potent in suppressing CNS inflammation in EP2−/− mice than in WT mice, and this increased potency was also apparent in suppression of the development of effector T cells. These results suggest that an EP2-EP4 dual antagonist might be more effective in suppressing EAE than an antagonist selective for EP4 alone. However, administration of the EP4 antagonist alone to WT mice markedly suppressed disease onset and progression at high doses, suggesting that EP4 plays the dominant role in the in vivo response to EAE antigen, at least in mice. With regard to whether administration of an EP4 antagonist is able to suppress ongoing CNS inflammation, we did not observe an inhibitory effect of ONO-AE3-208 on EAE when the drug was administered after disease onset, at least under the present experimental conditions. However, further studies are required to clarify this point, given that such a therapeutic action is more properly evaluated with a relapsing model of EAE induced by a proteolipid protein peptide. Given the importance of Th17 and Th1 cells in human MS, our present findings are likely relevant to the pathogenesis of MS. Indeed, PGE2 in collaboration with IL-23 or the combination of IL-1β and IL-23 was recently shown to facilitate human Th17 cell expansion in vitro (30, 31), with this action of PGE2 being mediated through EP2 and EP4 (31). Our present findings may facilitate the development of drugs for MS as well as other immune diseases in which Th17 cells play a key role.
Materials and Methods
Materials.
EP1−/−, EP2−/−, EP3−/−, EP4−/−, DP−/−, FP−/−, IP−/−, and TP−/− mice were generated as described (32–38). With the exception of EP4−/− mice, each mutant was backcrossed >10 times to the C57BL/6 background, and we used WT C57BL/6 (Japan SLC) mice as their controls. Most EP4−/− mice die postnatally as a result of patent ductus arteriosus and do not survive on the C57BL/6 background (34). Survivors of the F2 progeny of EP4−/− mice in the mixed genetic background of 129/Ola and C57BL/6 were, therefore, intercrossed, and the resulting female survivors were studied. Mice were maintained at the Institute of Laboratory Animals of Kyoto University on a 12-h light and 12-h dark cycle under specific pathogen-free conditions. All experimental procedures were approved by the Committee on Animal Research of Kyoto University Faculty of Medicine. The EP1 agonist ONO-DI-004, the EP2 agonist ONO-AE1-259, the EP3 agonist ONO-AE-248, and the EP4 agonist ONO-AE1-329 were supplied by Ono Pharmaceutical. The ligand-binding specificities of these compounds for PG receptors have been described (24, 39). The EP4 antagonist ONO-AE3-208 was also provided by Ono Pharmaceutical, and its structure, binding affinities, selectivity, and pharmacokinetic properties have also been described (40). PGE2 and dm-PGE2 were obtained from Sigma.
Induction of EAE.
Ten- to 11-wk-old female mice were immunized s.c. in both flanks with the MOG35–55 peptide (200 μg per animal; Operon) in 200 μL of an emulsion consisting of a 1:1 (volume/volume) mixture of physiological saline and complete Freund's adjuvant, the latter of which contained Mycobacterium tuberculosis H37RA at 5 mg/mL (Difco). The clinical severity of EAE was scored as follows: score 0, no sign; score 1, tail paralysis; score 2, mild hindlimb weakness; score 3, moderate to severe hindlimb paresis or mild forelimb weakness (or both); score 4, complete hindlimb paralysis or moderate to severe forelimb weakness (or both); score 5, quadriplegia or moribund state; score 6, death. To induce an average clinical score of between 3 and 4 in control animals, we injected mice with 100–500 ng of pertussis toxin (List Biological Laboratories) intraperitoneally on days 0 and 2 after immunization; given that we found that the activity of the pertussis toxin preparation can change during storage, we adjusted its dose in each experiment to achieve the desired effect. Mice were examined every day for signs of EAE. We administered dm-PGE2 (200 μg/kg) or selective EP agonists (100 μg/kg) s.c. two times per day. ONO-AE3-208 was either administered per os in drinking water or injected two times per day by gavage at the indicated doses.
Histology.
The spinal cord was removed, fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned at a thickness of 6 μm. The sections were stained with either H&E or Luxol fast blue for assessment of inflammation and demyelination, respectively. Semiquantitative histological evaluation of inflammatory-cell infiltration was scored in a blinded manner as follows: score 0, no inflammation; score 1, cellular infiltrates present only in perivascular areas and meninges; score 2, mild cellular infiltration in the parenchyma; score 3, moderate cellular infiltration in the parenchyma; and score 4, severe cellular infiltration in the parenchyma. Myelin breakdown was also scored in a blinded manner as follows: score 0, no demyelination; score 1, mild demyelination; score 2, moderate demyelination; and score 3, severe demyelination. The average score for five sections from each spinal cord was determined for each animal.
T Cell Proliferation and Cytokine Production.
Lymph node cells were prepared from axillary and inguinal lymph nodes at day 7 after immunization and were cultured in triplicate in 96-well plates at a density of 2 × 106 cells/mL in RPMI 1640 medium supplemented with 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, and 10% FBS in the absence or presence of the MOG35–55 peptide (5 μg/mL). After 48 h, the cells were exposed to 0.5 μCi [3H]thymidine (PerkinElmer) for 16 h in the continued absence or presence of MOG35–55, and then, they were collected with the use of a cell harvester (Skatron) and assayed for [3H]thymidine incorporation with a liquid scintillation counter (Aloka). For measurement of cytokine production, culture supernatants were collected at 48 h and stored at −80 °C until assay of IFN-γ (Endogen) and IL-17 (R&D Systems) with the use of ELISA kits.
Determination of BBB Permeability.
BBB permeability was assessed as described previously (41) by measurement of tissue content of sodium fluorescein as a tracer molecule. In brief, mice were injected intraperitoneally with 200 μL of 5% sodium fluorescein (Sigma) in PBS and 30 min later, were anesthetized and perfused transcardially with a minimum of 50 mL of heparin (1,000 U/L) in PBS. The spinal cord was then removed and homogenized, and the homogenate was mixed with an equal volume of 80% trichloroacetic acid before centrifugation at 10,000 × g for 10 min. The resulting supernatant was diluted with 0.8 volume of 5 M NaOH and then measured for fluorescence with the use of a Fluoroskan Ascent FL instrument (Labsystems) at excitation and emission wavelengths of 485 and 538 nm, respectively. Sodium fluorescein standard solutions (1–1,000 ng/mL) were used to calculate the tissue content, which was normalized by the amount of total protein in the homogenate.
Statistical Analysis.
Data are presented as means ± SEM and were analyzed by Student t test. All experiments were repeated at least two times, and representative data are shown. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Ono Pharmaceutical for supplying EP agonists and the EP4 antagonist, A. Mizutani and T. Fujiwara for animal care, and T. Arai for assistance. This work was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, a grant from the National Institute of Biomedical Innovation of Japan, and a collaborative research grant to Kyoto University from Ono Pharmaceutical.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0915112107/-/DCSupplemental.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: A complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Weiner HL. The challenge of multiple sclerosis: How do we cure a chronic heterogeneous disease? Ann Neurol. 2009;65:239–248. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- 3.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 4.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 6.Steinman L. A rush to judgment on Th17. J Exp Med. 2008;205:1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 8.Tzartos JS, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moldovan IR, et al. Interferon gamma responses to myelin peptides in multiple sclerosis correlate with a new clinical measure of disease progression. J Neuroimmunol. 2003;141:132–140. doi: 10.1016/s0165-5728(03)00221-2. [DOI] [PubMed] [Google Scholar]
- 10.Bolton C, Turner AM, Turk JL. Prostaglandin levels in cerebrospinal fluid from multiple sclerosis patients in remission and relapse. J Neuroimmunol. 1984;6:151–159. doi: 10.1016/0165-5728(84)90002-x. [DOI] [PubMed] [Google Scholar]
- 11.Dore-Duffy P, Ho SY, Donovan C. Cerebrospinal fluid eicosanoid levels: Endogenous PGD2 and LTC4 synthesis by antigen-presenting cells that migrate to the central nervous system. Neurology. 1991;41:322–324. doi: 10.1212/wnl.41.2_part_1.322. [DOI] [PubMed] [Google Scholar]
- 12.Kalyvas A, David S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron. 2004;41:323–335. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 13.Marusic S, et al. Cytosolic phospholipase A2 alpha-deficient mice are resistant to experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerson MR, LeVine SM. Experimental allergic encephalomyelitis is exacerbated in mice deficient for 12/15-lipoxygenase or 5-lipoxygenase. Brain Res. 2004;1021:140–145. doi: 10.1016/j.brainres.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 15.Ovadia H, Paterson PY. Effect of indomethacin treatment upon actively-induced and transferred experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin Exp Immunol. 1982;49:386–392. [PMC free article] [PubMed] [Google Scholar]
- 16.Weber F, Meyermann R, Hempel K. Experimental allergic encephalomyelitis-prophylactic and therapeutic treatment with the cyclooxygenase inhibitor piroxicam (Feldene) Int Arch Allergy Appl Immunol. 1991;95:136–141. doi: 10.1159/000235418. [DOI] [PubMed] [Google Scholar]
- 17.Reder AT, Thapar M, Sapugay AM, Jensen MA. Prostaglandins and inhibitors of arachidonate metabolism suppress experimental allergic encephalomyelitis. J Neuroimmunol. 1994;54:117–127. doi: 10.1016/0165-5728(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 18.Moon C, et al. Sodium salicylate-induced amelioration of experimental autoimmune encephalomyelitis in Lewis rats is associated with the suppression of inducible nitric oxide synthase and cyclooxygenases. Neurosci Lett. 2004;356:123–126. doi: 10.1016/j.neulet.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto K, et al. Selective COX-2 inhibitor celecoxib prevents experimental autoimmune encephalomyelitis through COX-2-independent pathway. Brain. 2006;129:1984–1992. doi: 10.1093/brain/awl170. [DOI] [PubMed] [Google Scholar]
- 20.Ni J, et al. COX-2 inhibitors ameliorate experimental autoimmune encephalomyelitis through modulating IFN-γ and IL-10 production by inhibiting T-bet expression. J Neuroimmunol. 2007;186:94–103. doi: 10.1016/j.jneuroim.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Narumiya S. Physiology and pathophysiology of prostanoid receptors. Proc Jpn Acad Ser B. 2007;83:296–319. doi: 10.2183/pjab/83.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao C, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 23.Kiriyama M, et al. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzawa T, et al. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: An analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 25.Fabis MJ, Scott GS, Kean RB, Koprowski H, Hooper DC. Loss of blood–brain barrier integrity in the spinal cord is common to experimental allergic encephalomyelitis in knockout mouse models. Proc Natl Acad Sci USA. 2007;104:5656–5661. doi: 10.1073/pnas.0701252104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kihara Y, et al. Targeted lipidomics reveals mPGES-1-PGE2 as a therapeutic target for multiple sclerosis. Proc Natl Acad Sci USA. 2009;106:21807–21812. doi: 10.1073/pnas.0906891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kebir H, et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farmer PJ, et al. Permeability of endothelial monolayers to albumin is increased by bradykinin and inhibited by prostaglandins. Am J Physiol Lung Cell Mol Physiol. 2001;280:L732–L738. doi: 10.1152/ajplung.2001.280.4.L732. [DOI] [PubMed] [Google Scholar]
- 29.Fukuhara S, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chizzolini C, et al. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boniface K, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ushikubi F, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 33.Hizaki H, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci USA. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segi E, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 35.Matsuoka T, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto Y, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 37.Murata T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 38.Kabashima K, et al. Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nat Immunol. 2003;4:694–701. doi: 10.1038/ni943. [DOI] [PubMed] [Google Scholar]
- 39.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabashima K, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenzsér G, et al. Contribution of poly(ADP-ribose) polymerase to postischemic blood-brain barrier damage in rats. J Cereb Blood Flow Metab. 2007;27:1318–1326. doi: 10.1038/sj.jcbfm.9600437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.