Abstract
Addicts repeatedly relapse to drug seeking even after years of abstinence, and this behavior is frequently induced by the recall of memories of the rewarding effects of the drug. Established memories, including those induced by drugs of abuse, can become transiently fragile if reactivated, and during this labile phase, known as reconsolidation, can be persistently disrupted. Here we show that, in rats, a morphine-induced place preference (mCPP) memory is linked to context-dependent withdrawal as disrupting the reconsolidation of the memory leads to a significant reduction of withdrawal evoked in the same context. Moreover, the hippocampus plays a critical role in linking the place preference memory with the context-conditioned withdrawal, as disrupting hippocampal protein synthesis and cAMP-dependent-protein kinase A after the reactivation of mCPP significantly weakens the withdrawal. Hence, targeting memories induced by drugs may represent an important strategy for attenuating context-conditioned withdrawal and therefore subsequent relapse in opiate addicts.
Keywords: addiction, reconsolidation, hippocampus morphine
The environmental context of an experience induced by a drug of abuse is a powerful determinant of drug-seeking behavior and relapse in addicts (1, 2), and a wealth of evidence indicates that learning and memory, and particularly contextual memories, play a critical role in establishing conditioned responses to drugs of abuse (3–6). Contextual representations and memories are known to critically recruit the hippocampus, a brain region that plays a major role in processing the learning of associations between the environmental context and unconditioned stimuli (7) (e.g., drugs of abuse). However, the precise role of the hippocampus in the development of drug-induced conditioning and addiction is still unclear. Furthermore, it is unknown how and whether contextual memories of the effect of the drugs are linked to other drug-induced behaviors such as relapse and withdrawal.
A general feature of memory is that, initially, it exists in a labile state and, over time, it undergoes a process of stabilization known as consolidation (8). Established memories can however become again transiently fragile if reactivated for example by retrieval or reexposure to reinforced stimuli (9–12). This phase of postreactivation vulnerability is known as reconsolidation, because during this period the memory returns to a stable state. Reconsolidation, like consolidation, requires new RNA and protein synthesis, as well as the functional role of specific molecular pathways, including those activated by cAMP and cAMP-dependent protein kinase A (PKA) (9–12). Thus, the disruption of these mechanisms following memory reactivation represents a strategy for weakening pathogenic memories including those involved in drug conditioning (12). Morphine- or cocaine-conditioned place preference, as well as the conditioned reinforcing properties of a cocaine-associated stimulus, can be disrupted by postreactivation inhibition of protein synthesis, ERK, or of the expression of the immediate-early gene Zif268 (13–16). We previously showed that, in rats, a conditioned place preference evoked by morphine [m-chlorophenylpiperazine (mCPP)] is persistently disrupted by systemic or stereotactic injections of protein synthesis inhibitors into the hippocampus, amygdala, or nucleus accumbens following its reactivation by a conditioning trial (14).
In this study, we investigated three questions. First, is the contextual memory induced by experiencing a drug of abuse directly linked to motivational withdrawal, a response known to play a major role in relapse and the establishment of addiction? Second, if such a link exists, what is the role of the hippocampus? Finally, is this region involved in establishing a contextual link between the representation of the reinforcing effects of the drug and the withdrawal?
Results
Disrupting the Contextual Memory Evoked by Morphine Weakens Subsequent Withdrawal in the Same Context.
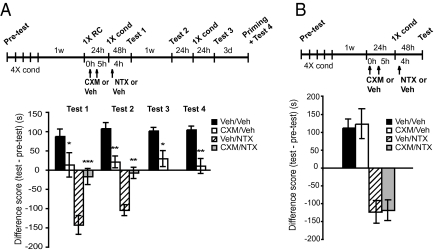
We first tested whether disrupting mCPP by inhibiting protein synthesis systemically after its reactivation affects subsequent motivational withdrawal. The protocol consisted of three parts: morphine conditioning (i.e., mCPP), reactivation of mCPP, and induction of withdrawal (Fig. 1A). Rats were conditioned for four consecutive days to 10 mg/kg morphine in a counterbalanced fashion. One week later, mCPP memory was reactivated with a single 10-mg/kg conditioning trial (1XRC), and immediately after, half the animals received two systemic cycloheximide (CXM) injections at 2.2 mg/kg, 5 h apart, and the other half received two injections of vehicle solution. This cycloheximide treatment has been shown to block more than 70% of protein synthesis in the rat brain for at least 6 h and to persistently disrupt mCPP reconsolidation (14). The next day, both groups of rats were divided in two subgroups, which underwent 0.3 mg/kg naltrexone (NTX)–precipitated withdrawal or a control vehicle treatment (see Materials and Methods for details) (17). The withdrawal protocol consisted of NTX administration 4 h after a single morphine conditioning (17). This protocol elicits a significant conditioned place aversion, which is known to be a sensitive and accurate index of the aversive motivational consequences of withdrawal (18). Two days later, all four groups (vehicle/vehicle; CXM/vehicle; vehicle/NTX; CXM/NTX) were tested (test 1) for place preference or place aversion as indices of morphine seeking or morphine withdrawal, respectively. To test whether the effect was persistent, all four groups were retested 1 wk later (test 2). To determine whether place conditioning could be recovered following the reexperience of a new morphine conditioning trial, 24 h after test 2, animals from vehicle/vehicle and CXM/vehicle groups underwent a single conditioning trial and were tested 24 h later (saving; test 3). Finally, to test whether place preference could be recovered in a state-dependent condition, 3 d later, the same animals received an injection of morphine and were then immediately tested (priming injection; test 4).
Fig. 1.
Disrupting mCPP reconsolidation disrupts subsequent NTX-precipitated withdrawal. Experimental timelines are shown above each experiment. The score values are shown in Table S1. Values of preference or avoidance are expressed in seconds as differences (test vs. pretest) and shown as means ± SEM (A) Cycloheximide significantly disrupts mCPP compared with vehicle (tests 1–4, n = 6–8; *P < 0.05, **P < 0.01). The animals with disrupted CPP also show a significantly disrupted NTX-precipitated withdrawal (tests 1 and 2, n = 7–8; **P < 0.01, ***P < 0.001). (B) Same as in A, except that 1XRC was omitted and testing was completed at test 1; n = 8 per group.
A two-way ANOVA comparing preference scores across treatment (vehicle/vehicle, CXM/vehicle) and test (tests 1–4) revealed a significant effect of treatment (F1,48 = 42.50; P < 0.0001), no effect of test (F3,48 = 0.30; P = 0.81), and no test–treatment interaction (F3,48 = 0.17; P = 0.91). A Bonferroni post hoc test revealed that, compared with vehicle, cycloheximide significantly disrupts mCPP at test 1 (P < 0.05; Fig. 1A). The disruption was persistent at test 2 (P < 0.01), test 3 (P < 0.05), and test 4 (P < 0.01), suggesting that the loss of mCPP is persistent and not state-dependent.
Notably, the rats that had a disrupted mCPP by cycloheximide and subsequently received NTX also showed a significant loss of withdrawal compared with the group that had an intact mCPP and received NTX, which, as expected, underwent significant withdrawal at test 1 (Fig. 1A). A two-way ANOVA across treatment (CXM/NTX, vehicle/NTX) and test (tests 1 and 2) revealed a significant effect of treatment (F1,26 = 33.33; P < 0.0001), no effect of test (F1,26 = 1.54; P = 0.23), and no test–treatment interactions (F1.26 = 0.55; P = 0.46). Bonferroni post hoc test revealed that, compared with vehicle, cycloheximide disrupted NTX-precipitated avoidance at test 1 (P < 0.001) and the disruption was persistent at test 2 (P < 0.01). Furthermore, the effect on both mCPP and withdrawal was dependent on the reactivation of mCPP. Indeed, rats that underwent the same conditioning and testing schedule described above but received cycloheximide injections in the absence of the reactivating conditioning (Fig. 1B) had normal mCPP (Student t test, P = 0.76) and normal withdrawal (Student t test, P = 0.88).
The effect of disrupting mCPP on withdrawal was maintained over time. We performed the same experiment as described earlier, except that the NTX-precipitated withdrawal was elicited 1 wk after the reactivation session followed by cycloheximde or vehicle. As shown in Fig. S1, mCPP was significantly disrupted by cycloheximide (vehicle/vehicle vs. CXM/vehicle; t test, P < 0.001) and this disruption also led to a significant loss of withdrawal (CXM/NTX vs. vehicle/NTX; t test, P < 0.001).
Thus, disrupting mCPP with postreactivation injection of protein synthesis inhibitors also significantly weakens withdrawal and the effect is contingent on the reactivation of the memory. This suggests that an associative link must exist between the conditioned contextual representation (i.e., mCPP) and a subsequent withdrawal response.
Drug Conditioning Is Required for Linking Motivational Withdrawal to Memory.
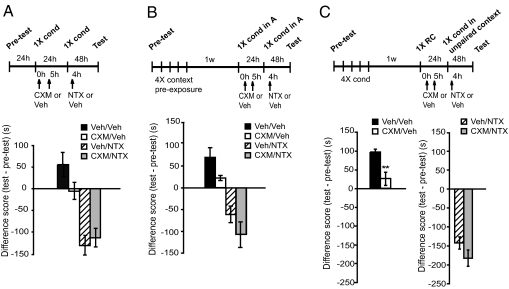
If a link exists between an established conditioned place preference and withdrawal and it is the result of repeated contextual conditioning, omitting the conditioning (i.e., no mCPP memory) should produce a normal withdrawal. Furthermore, this withdrawal should not be affected by prior inhibition of protein synthesis following a single conditioning. To test this hypothesis, rats received a single 10-mg/kg morphine conditioning followed by two 2.2-mg/kg cycloheximide injections and, 24 h later, underwent the NTX-precipitated withdrawal protocol. As depicted in Fig. 2A, a single morphine conditioning induced only a small preference at test 1, which was disrupted, although not significantly, by cycloheximide (t test, P = 0.1). Cycloheximide did not affect NTX-induced withdrawal (CXM/NTX vs. vehicle/NTX; t test, P = 0.58, Fig. 2A). Hence, an established conditioning appears to be essential for making a link between mCPP memory and subsequent withdrawal. Furthermore, these data exclude that the effect on withdrawal seen in Fig. 1 results from a direct effect of cycloheximide on the withdrawal.
Fig. 2.
The contextual conditioning to morphine is necessary for linking a context-precipitated withdrawal. Experimental timelines are shown above each experiment. The score values are shown in Table S1. Preference or avoidance are expressed in seconds as differences (test vs. pretest) and shown as means ± SEM (A) Cycloheximide treatment following a single morphine conditioning (n = 7–8 per group) shows no effect on withdrawal. (B) Four vehicle-context exposures were experienced instead of morphine conditioning. One week later, one morphine conditioning was administered in one context (context A); 24 h later, the withdrawal protocol was carried out in the same context. No effect on withdrawal is found; n = 6–8 per group. (C) Morphine conditioning (4×) 1 wk later: 1XRC was administered following cycloheximide or vehicle treatment. Twenty-four hours later 1× conditioning followed 4 h later by NTX was performed in the vehicle-paired (unpaired) context. Cycloheximide significantly disrupts mCPP (n = 7–8; **P < 0.01), but no effect on withdrawal was found.
All together, these results lead to a twofold conclusion. First, the representation of a place memory of the rewarding effects of a drug is linked to and critically influences the representations of other types of drug-induced behaviors such as withdrawal. Second, disrupting the memory, in fact, weakens withdrawal.
Given the fact that the withdrawal is linked to the contextual memory, we then asked the question: is a repeated preexposure to a context (without drug pairing) sufficient to create a contextual representation that becomes linked to withdrawal when precipitated by NTX in the same context? Or is the contextual conditioning to the drug necessary? As depicted in Fig. 2B, rats underwent the same experimental protocol as described in Fig. 1A except that they received a 4-d exposure to a conditioning box but were injected with vehicle and not morphine. A week later, they received one morphine conditioning in the same context followed by cycloheximide or vehicle treatment, and the following day were exposed to the withdrawal (one morphine conditioning followed by NTX and context exposure) or relative control protocol (one morphine conditioning followed by vehicle and context exposure), as detailed in Materials and Methods. Two morphine exposures induced only a small preference which was disrupted, although not significantly, by cycloheximide (t test, P = 0.08). Furthermore, cycloheximide did not affect NTX-precipitated withdrawal, as both cycloheximide and vehicle-injected groups showed a strong and comparable aversion (t test, P = 0.2; Fig. 2B). Hence, the withdrawal linked to the contextual memory is established as a consequence of the place conditioning to morphine.
Given these results, we then asked whether disrupting the mCPP memory only affects withdrawal evoked in the conditioned context. We repeated the experiment described earlier in Fig. 1A, but carried out the withdrawal protocol in the vehicle-paired compartment. Specifically, rats received 4-d morphine conditioning and, 1 wk later, the 1XRC followed by cycloheximide or vehicle injections. Twenty-four hours later, they underwent the NTX-precipitated withdrawal protocol or vehicle-control treatment in the context that during conditioning was paired with vehicle. Therefore, rats experienced this context for the same duration and frequency as that paired with morphine, but this context was never paired with the drug during the 4 d of conditioning; thus, the rats experienced the drug only once during the withdrawal protocol. Student t test revealed that, compared with vehicle, cycloheximide significantly disrupted mCPP (P < 0.01; Fig. 2C), as expected. However, the rats that received cycloheximide showed a NTX-precipitated withdrawal (CXM/NTX) in the vehicle-paired context comparable to that of vehicle-treated controls (vehicle/NTX).
These data show that the withdrawal that is precipitated in the morphine-conditioned environment has also become conditioned to this context and it is different from the withdrawal precipitated by NTX in a new context that was not previously conditioned to the drug. Hence, our findings indicate that, following contextual conditioning to a drug of abuse, the drug-paired contextual representation is a critical component of the withdrawal elicited in the same context. When the memory of the drug-associated contextual experience is disrupted, the subject fails to show withdrawal in the same context.
Finally, we investigated whether the effect of mCPP memory disruption on withdrawal impact physical signs. Rats received morphine conditioning and, 1 wk later, mCPP reactivation followed by cycloheximide or vehicle treatments as described earlier for the experiment in Fig. 1A. NTX-precipitated withdrawal was induced 24 h later by one morphine conditioning followed, 4 h later, by 3 mg/kg of NTX s.c. Immediately after, the rats were confined to the morphine-conditioned compartment and videotaped for 15 min to score their physical signs of withdrawal (19). As shown in Table S2, no difference in global rating or severity of individual somatic signs was seen in cycloheximide versus vehicle-injected groups. Thus, postreactivation disruption of mCPP, which leads to significant weakening of context-dependent withdrawal, targets motivational but not physical withdrawal.
Hippocampal Mechanisms Play an Essential Role in Linking Morphine-Induced Contextual Conditioning and Withdrawal.
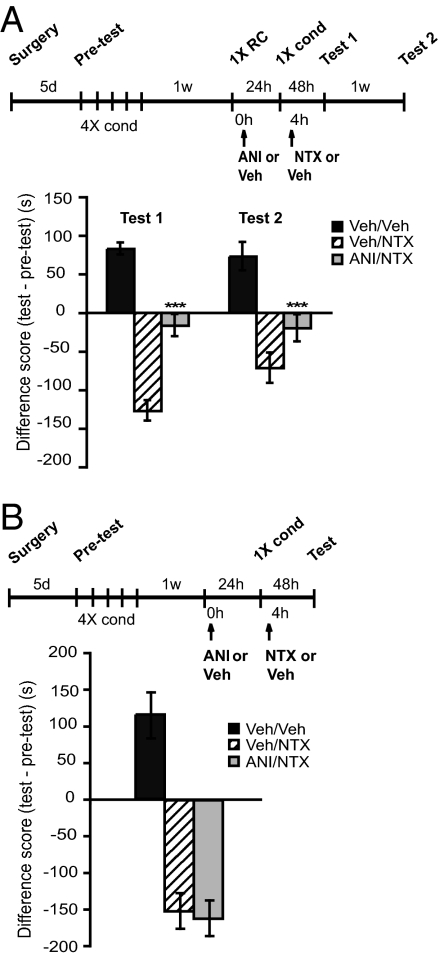
Given the relevant contribution of the contextual representation in both mCPP and withdrawal, we tested whether the hippocampus, a brain region known to process contextual and place memories (20), including mCPP, is involved in linking memories to withdrawal. Dorsal or complete hippocampal lesions impair rat mCPP (21), and we have previously shown that an injection of the protein synthesis inhibitor anisomycin into the dorsal hippocampus following mCPP reactivation persistently disrupts an established mCPP memory (14). Here we tested whether blocking protein synthesis in the dorsal hippocampus affects the link between mCPP and withdrawal evoked as in the previous experiments. Rats bilaterally implanted with cannulas targeting the hippocampus (Fig. S2) were conditioned with 10 mg/kg of morphine for 4 d and, 1 wk later, intrahippocampally infused with 1 μL containing 125 μg of anisomycin per side or 1 μL vehicle following 1XRC (Fig. 3A). A two-way ANOVA across treatment and test revealed a significant effect of treatment (F2,40 = 70.34; P < 0.0001), no effect of test (F1,40 = 1.31; P = 0.26), and no test–treatment effect (F2,40 = 2.89; P = 0.07). Bonferroni post hoc test showed that, as seen with systemic cycloheximide, postreactivation anisomycin injection significantly disrupted withdrawal at test 1 (P < 0.001). This disruption persisted at test 2 (P < 0.001). The effect of anisomycin on withdrawal was contingent on reactivation as the omission of the 1XRC resulted in normal withdrawal (Fig. 3B). A one-way ANOVA comparing avoidance and preference scores revealed an effect of NTX (F = 4.2; P < 0.05). Bonferroni post hoc test revealed no significant differences between vehicle/NTX and anisomycin/NTX.
Fig. 3.
Inhibition of hippocampal protein synthesis significantly disrupts both mCPP and subsequent withdrawal. Experimental timelines are shown above each experiment. Scores are shown in Table S1. Values of preference or avoidance are expressed in seconds as differences (test vs. pretest) and shown as means ± SEM. (A) Anisomycin injected into the dorsal hippocampus significantly disrupts NTX-precipitated withdrawal (n = 7–8 per group; ***P < 0.001). (B) The effect of anisomycin is contingent upon reactivation: same as in A, except that 1XRC was omitted and testing was completed after the first test. Anisomycin did not affect withdrawal (n = 8 per group).
Previous studies showed that opiate treatment can modulate synaptic plasticity in the hippocampus and that this reward-related learning is dependent on the activation of the cAMP- PKA pathway (22), which is also known to critically mediate long-term memory formation in a variety of species (23). Therefore, we investigated whether the activation of PKA in the hippocampus occurs as a result of mCPP reactivation and whether this activation is functionally required for the reconsolidation of mCPP and/or its link to subsequent withdrawal. Rats were conditioned to morphine for 4 d, as described earlier, and 1 wk later, they were divided in two subgroups. One subgroup underwent 1XRC and was euthanized 30 min later; the other, which remained in the home cage and served as a nonreactivated control (NR), was euthanized in parallel. The hippocampi were dissected and extracted for detection of PKA activity. Extracts within each group were pooled. As depicted in Fig. S3A, the hippocampal extracts from rats that underwent reactivation exhibited increased PKA activity compared with the NR group.
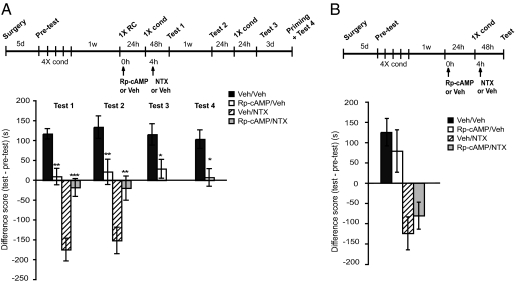
We then tested the effect of inhibiting this PKA activation. Rats were conditioned for 4 d and, 1 wk later, intrahippocampally infused (Fig. S3B) with either 36 μg in 1 μL per side of the PKA inhibitor Rp-cAMP or vehicle immediately after 1XRC (Fig. 4A). A two-way ANOVA across treatment (vehicle/vehicle, Rp-cAMP/vehicle) and test (tests 1–4) revealed a significant effect of treatment (F1,56 = 41.17; P <0 .0001), no effect of test (F3,56 = 0.39; P = 0.76) and no test–treatment interactions (F3,56 = 0.14; P = 0.94). A Bonferroni post hoc test revealed that Rp-cAMP treatment disrupted mCPP at test 1 (P < 0.01) and the disruption was persistent at test 2 (P < 0.01). Furthermore, there was no significant saving of mCPP at test 3 after a conditioning trial (P < 0.05), and, finally, priming the animals with a single morphine injection did not produce any significant recovery of mCPP at test 4 (P < 0.05; Fig. 4A). Notably, the rats that had a disrupted mCPP by Rp-cAMP and subsequently received NTX also showed a significant reduction of withdrawal compared with those that had intact mCPP and received NTX. A two-way ANOVA across treatment (Rp-cAMP/NTX, vehicle/NTX) and test (tests 1 and 2) revealed a significant effect of treatment (F1,28 = 34.18; P < 0.0001), no effect of test (F1,28 = 0.19; P = 0.67) and no test–treatment interactions (F1,28 = 0.24; P = 0.63; Fig. 4A). Bonferroni post hoc test revealed that Rp-cAMP disrupted NTX-induced avoidance at test 1 (P < 0.001) and the disruption was persistent 1 wk later at test 2 (P < 0.01).
Fig. 4.
Hippocampal PKA is required for the link between mCPP memory and NTX-precipitated withdrawal. Experimental timelines are shown above each experiment. Scores are shown in Table S1. Values of preference or avoidance are expressed in seconds as differences (test vs. pretest) and shown as means ± SEM. (A) Bilateral hippocampal injections of Rp-cAMP significantly disrupts mCPP (n = 8 per group; **P < 0.01, *P < 0.05). These rats also showed a significant reduction in NTX-precipitated withdrawal (n = 8 per group; ***P < 0.001, **P < 0.01). (B) The Rp-cAMP effect is contingent upon reactivation. Same as in A, except that 1XRC was omitted and testing was completed after the first test (n = 8 per group).
As seen with protein synthesis inhibitors, the effect of Rp-cAMP on both mCPP and withdrawal was contingent on mCPP reactivation (Fig. 4B): In fact, when 1XRC was omitted, both mCPP (Student t test, P = 0.31) and withdrawal (Student t test, P = 0.26) occurred normally. Together, these results show that inhibiting reactivation-induced hippocampal protein synthesis and PKA activation disrupts mCPP, which, in turn, prevents the precipitation of withdrawal.
Discussion
Our results show that, first, a link is established between the contextual conditioning of the incentive effect of morphine and the subsequent motivational withdrawal response precipitated in the same context. Second, this withdrawal is disrupted by interfering with the reconsolidation of the drug-induced contextual memory. Third, the hippocampus, and specifically hippocampal protein synthesis and PKA, play a critical role in establishing the contextual conditioning to morphine as well as its link to the withdrawal precipitated in the same context. Finally, this withdrawal is different from a withdrawal that is precipitated in a new or nonconditioned context. Thus, disrupting the reconsolidation of drug-induced memories may represent a unique strategy for attenuating context-dependent withdrawal in opiate addicts.
Establishing a Link Between Memory and Withdrawal.
Our data, in agreement with a large body of evidence, suggests that memory plays an essential part in establishing and maintaining behavioral responses induced by the intake of drugs of abuse. Furthermore, we show that the memory of the reinforcing effect of a drug of abuse is directly linked to the development of other drug-induced responses, specifically context-specific withdrawal. In fact, disrupting the former also weakens the latter. This outcome has important implications for developing therapeutic strategies to treat or prevent addiction, and in line with previous literature, underscores the importance of context and cue-dependent conditioning in eliciting morphine dependence and motivational withdrawal (24–27).
Memory formation and retrieval are tightly interconnected with addiction responses at many levels. Drug addicts relapse to drug seeking and activate the same mesolimbic circuitry after the retrieval of environmental conditioned cues that recall the rewarding effects of the drug (12). Reexposure to drug-paired cues increases dopamine release in the dorsal striatum; this increase positively correlates with craving (28) and can elicit conditioned responses including changes in heart rate and skin conductance (29), suggesting that drugs act as unconditioned reinforcers. Both memory and addiction use overlapping neural systems, particularly the limbic system (30, 31), as well as common cellular and molecular mechanisms (31–34).
Established memories, including those induced by drugs of abuse, can be disrupted if, after their reactivation, their reconsolidation process is disrupted (9, 12, 35). In this study, we show that the disruption of the reconsolidation of a place memory induced by morphine also leads to the loss of a withdrawal response elicited in the same context. This effect is likely to be caused by the disruption of the reconsolidation of the memory (12), as the impairment of both place conditioning and withdrawal are dependent on the reactivation of mCPP. This is in agreement with the results of our previous study (14) that also demonstrated that reexperiencing morphine in a different context or in the home cage does not evoke any lability in mCPP. Furthermore, we show that the disruption of mCPP is persistent and the memory does not return after further conditioning or testing in the presence of morphine, suggesting that the disruption is not caused by state dependency. Finally, the effect appears to target psychological and motivational, rather than physical, withdrawal, although more studies are needed to fully dissect this issue.
In a previous study (14), we have excluded that the disruptive effect of protein synthesis inhibitors on mCPP is the result of nonspecific effects of the inhibitor used, as different inhibitors administered systemically or intracerebrally similarly disrupts mCPP reconsolidation. We also showed that systemic administration(s) of the protein synthesis inhibitors, per se, do not induce place avoidance response, thus excluding that the disruption of mCPP is caused by an avoidance elicited by nonspecific effects of the treatment (14).
Critical Role of Drug Context Conditioning on the Conditioned Withdrawal Response.
The link of mCPP and withdrawal was not observed in animals that (i) did not undergo previous conditioning, (ii) underwent a repeated exposure to the context without the drug, and (iii) received NTX in a context different from the conditioning place. These results indicate that, first, a previously established place conditioning memory is essential for creating a link between the drug-induced reinforced response and the withdrawal, and, second, that the withdrawal that is precipitated in a conditioned context is different from the one precipitated in a novel or nonconditioned context. Thus, conditioning to a drug of abuse renders the conditioning context prone to a withdrawal response that is context-specific. Our results agree with the hypothesis proposed by several authors (25, 36–39) that opiate withdrawal symptoms frequently reflect classically conditioned responses. Evidence of context-specific withdrawal exists in both humans and animals, and this conditioned withdrawal is believed to be an important factor that precipitates craving and relapse in abstinent ex-addicts (1, 37, 40). Our results support the same conclusions proposed by these authors that withdrawal symptoms, like tolerance, are the result of associative processes and that withdrawal in a conditioned context becomes context-specific. Our data also show that context-conditioned withdrawal is distinct from withdrawal precipitated by NTX in a novel or nonconditioned context, as only the former but not the latter critically recruits the hippocampus and is linked to the drug-conditioned memory of the context. In fact, disrupting this memory selectively eliminates the contextual-conditioned withdrawal but leaves a withdrawal precipitated in a novel or nonconditioned context intact. Moreover, the context-conditioned withdrawal is not produced by a previous, repeated history of context exposure in the absence of the drug. These results, together with previous reports of a context-specific withdrawal, provide substantial evidence that addiction results from a drug-induced conditioning response, and emphasizes the importance of understanding conditioning processes and environmental associations in treating opiate addiction, dependence, withdrawal, and relapse (36, 41).
Role of the Hippocampus.
Changes in the neural substrates and mechanisms underlying learning and memory are believed to contribute to the development and maintenance of addiction (30, 31). Furthermore, imaging studies in humans show that craving is associated with activation of memory circuits, including, in addition to the amygdala and the dorsal striatum, the hippocampus, which is known to process declarative, contextual, and spatial memories (20), and, like amygdala and dorsal striatum, receives dopamine innervation.
The hippocampus has been hypothesized to process contextual drug associations (14, 21) that contribute to context-evoked craving and drug-seeking behavior (1, 2), but its role in addiction is still poorly understood. Here we provide evidence that hippocampal mechanisms are critical not only for establishing a place preference but also for linking mCPP with context-conditioned withdrawal. These mechanisms include the protein synthesis–dependent cascade induced during reconsolidation and the activation of the cAMP-dependent PKA, which is known to play an evolutionarily conserved role in long-term synaptic plasticity and memory formation (42). Interestingly, PKA appears to mediate long-lasting changes in many brain areas implicated in addiction, as it is also recruited in the nucleus accumbens during addiction responses like relapse and sensitization (43–45) and in the amygdala during reward-related learning (46). Furthermore, in the amygdala, PKA bidirectionally modulates the reconsolidation of a fear memory (47).
We speculate that experiencing the rewarding effect of a drug of abuse in a given environment critically recruits the hippocampus to establish a contextual associative memory that includes the representation of the incentive features of the drug (i.e., conditioned context). This hippocampal representation is then recruited whenever other contextual responses are created, for example, when experiencing withdrawal in the same context. This view is in line with the findings of Fuchs et al. (48), who showed that inactivation of the hippocampus prevents context-stimulated reinstatement of cocaine seeking behavior, and Vorel et al. (49), who reported that the stimulation of hippocampal traces can induce relapse. Thus, we speculate that disrupting drug-induced contextual memories may result in the weakening of other context-linked responses. If this is true, then context-elicited relapse and drug seeking might be thwarted if the initial incentive contextual memory is disrupted.
Several additional important questions arise from our results: is the effect extendable to chronic conditioning? Can the present findings be generalized to other incentive and aversive behaviors? Further studies are needed to address these issues. Nevertheless, based on the present findings, we propose that disruption of place memories induced by drugs of abuse may represent an important overall strategy for attenuating context-specific withdrawal symptoms and therefore subsequent relapse in opiate-conditioned subjects.
Materials and Methods
Methods Summary.
Adult male Long-Evans rats underwent 10 mg/kg mCPP for 4 d using a counterbalanced procedure. 1XRC occurred 1 wk after conditioning and consisted of a single morphine conditioning (14). Morphine withdrawal was induced with one morphine conditioning followed, 4 h later, by s.c. NTX injection (0.3 or 3 mg/kg) and placement into the conditioned compartment for 30 min (17), counterbalanced with vehicle 24 h later. During testing, the amount of time the animals spent in each chamber over 10 min was recorded and data were expressed as the difference (in s) between the time spent in the drug-paired compartment after conditioning and the time spent in this compartment after conditioning. Cycloheximide was injected s.c. at 2.2 mg/kg. Intrahippocampal injections were performed as described in Milekic et al. (14) with anisomycin at 125 μg/μL and Rp-cAMP at 36 μg/μL (1 μL per hippocampus).
Supplementary Material
Acknowledgments
We thank Gabriella Pollonini, Sarah Stern, Klaude Weiss, Reginald Miller, and the Center for Comparative Medicine and Surgery facility at Mount Sinai School of Medicine (New York, NY). This work was supported by National Institutes of Health Cutting-Edge Basic Research Award DA017672 (to C.M.A.) and Grants MH074736 and MH065635 (to C.M.A.); the Hirschl Foundation; Philoctetes Foundation (C.M.A.); and National Institute on Drug Abuse Grant T32 DA0713525 (to S.M.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003152107/-/DCSupplemental.
References
- 1.Childress AR, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 4.Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- 5.Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- 6.White NM. Addictive drugs as reinforcers: Multiple partial actions on memory systems. Addiction. 1996;91:921–949. discussion 951–965. [PubMed] [Google Scholar]
- 7.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 8.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 9.Alberini CM. Mechanisms of memory stabilization: Are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Dudai Y. Reconsolidation: The advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White DA, Hwang ML, Holtzman SG. Naltrexone-induced conditioned place aversion following a single dose of morphine in the rat. Pharmacol Biochem Behav. 2005;81:451–458. doi: 10.1016/j.pbb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- 19.Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- 20.Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 21.Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- 22.Sharifzadeh M, et al. Intra-hippocampal inhibition of protein kinase AII attenuates morphine-induced conditioned place preference. Pharmacol Biochem Behav. 2006;85:705–712. doi: 10.1016/j.pbb.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 24.Kelsey JE, Aranow JS, Matthews RT. Context-specific morphine withdrawal in rats: Duration and effects of clonidine. Behav Neurosci. 1990;104:704–710. doi: 10.1037//0735-7044.104.5.704. [DOI] [PubMed] [Google Scholar]
- 25.Schulteis G, Liu J, Amitai N, Tzeng S. Context- and cue-conditioned potentiation of acute morphine dependence and withdrawal. Pharmacol Biochem Behav. 2005;82:82–89. doi: 10.1016/j.pbb.2005.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulteis G, Morse AC, Liu J. Conditioning processes contribute to severity of naloxone-precipitated withdrawal from acute opioid dependence. Psychopharmacology (Berl) 2004;175:463–472. doi: 10.1007/s00213-004-1843-5. [DOI] [PubMed] [Google Scholar]
- 27.Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: The associative basis of tolerance. Exp Clin Psychopharmacol. 2000;8:276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, et al. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- 30.Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 31.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 32.Kauer JA. Learning mechanisms in addiction: Synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- 33.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 34.McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- 35.Diergaarde L, Schoffelmeer AN, De Vries TJ. Pharmacological manipulation of memory reconsolidation: Towards a novel treatment of pathogenic memories. Eur J Pharmacol. 2008;585:453–457. doi: 10.1016/j.ejphar.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Hinson RE, Siegel S. Nonpharmacological bases of drug tolerance and dependence. J Psychosom Res. 1982;26:495–503. doi: 10.1016/0022-3999(82)90089-7. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien CP. Experimental analysis of conditioning factors in human narcotic addiction. Pharmacol Rev. 1975;27:533–543. [PubMed] [Google Scholar]
- 38.Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien CP, Testa T, O'Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–1002. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- 40.Falls WA, Kelsey JE. Procedures that produce context-specific tolerance to morphine in rats also produce context-specific withdrawal. Behav Neurosci. 1989;103:842–849. doi: 10.1037//0735-7044.103.4.842. [DOI] [PubMed] [Google Scholar]
- 41.Siegel S. The role of conditioning in drug tolerance and addiction. In: Keehn JD, editor. Psychopathology in Animals: Research and Clinical Implications. New York: Academic Press; 1979. pp. 143–168. [Google Scholar]
- 42.Abel T, Nguyen PV. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog Brain Res. 2008;169:97–115. doi: 10.1016/S0079-6123(07)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Self DW, et al. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnsten AF, Ramos BP, Birnbaum SG, Taylor JR. Protein kinase A as a therapeutic target for memory disorders: Rationale and challenges. Trends Mol Med. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 45.DiRocco DP, Scheiner ZS, Sindreu CB, Chan GC, Storm DR. A role for calmodulin-stimulated adenylyl cyclases in cocaine sensitization. J Neurosci. 2009;29:2393–2403. doi: 10.1523/JNEUROSCI.4356-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jentsch JD, Olausson P, Nestler EJ, Taylor JR. Stimulation of protein kinase a activity in the rat amygdala enhances reward-related learning. Biol Psychiatry. 2002;52:111–118. doi: 10.1016/s0006-3223(02)01358-6. [DOI] [PubMed] [Google Scholar]
- 47.Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- 48.Fuchs RA, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 49.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.