Abstract
The rapidly increasing number of diabetes patients across the world poses a great challenge to the current therapeutic approach. The traditional method of exogenous supply of insulin is not sufficient and often causes lethal hypoglycemia that demands to develop a novel strategy. Recent investigations on regeneration of insulin producing cells (IPCs) revealed that in addition to primary source i.e., pancreatic beta cells, IPCs can be derived from several alternative sources including embryonic, adult, mesenchymal, and hematopoietic stem cells via the process of proliferation, dedifferentiation, neogenesis, nuclear reprogramming and trans-differentiation. There is considerable success in insulin independency of diabetes patient after transplantation of whole pancreas and/or the islets cell. However, the major challenge for regenerative therapy is to obtain a large source of islet/beta cells donor. Recent advances in the directed differentiation of stem cells generated a promising hope for a better and permanent insulin independency for diabetes patients. In this review we discussed stem cells as a potential future therapeutic target for the treatment of diabetes and associated diseases.
Keywords: Diabetes mellitus, Mesenchymal stem cells, adult stem cells, mesenchymal stem cell, insulin producing cells, induced pluripotent stem cells, human embryonic stem cells, nuclear reprogramming, MicroRNAs, stem cell therapy, pancreas development, beta-cells regeneration, Review
2. INTRODUCTION
Diabetes mellitus (DM) is characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or both that affects more than 200 million of adult populations worldwide and is projected to affect at least 5 % of global adult population by the year 2025 (1;2). Diabetes can be categorized into three major types- (a) Type 1 diabetes: it is also known as juvenile-onset diabetes and is characterized by beta-cell destruction, typically by an autoimmune T cell-mediated mechanism, which usually leads to an absolute deficiency of insulin in the body required for glucose metabolism. About 5–10% of Americans who were diagnosed with diabetes have type1 diabetes. (b) Type 2 diabetes: it is also known as adult onset diabetes and is characterized by inability of insulin to properly metabolize glucose. Combined with insulin deficiency, it scored about 90–95% of diabetes patients in USA. It is commonly linked to obesity, which can cause insulin resistance. Despite the different pathogenic mechanisms of Type 1 and Type 2 diabetes, they share common symptoms including glucose intolerance, hyperglycemia, hyperlipidaemia and similar complications, and (c) Gestational diabetes: it appears during the second trimester of gestation causing high blood glucose level and disappears after the birth of the baby. It is uncontrolled and affects both the baby and the mother. However, proper diet, exercise, and medication can reduce its effect. Gestational diabetes is reported in approximately 5-10 % of pregnant women. The total number of diabetes patient in USA is approximately 23.6 millions (http://www.diabetes.org/about-diabetes.jsp). It stands sixth in the leading causes of mortality in USA even after current medication of insulin injection and oral hypoglycemic pills. Additionally, it is also implicated in the other pathologies such as adult blindness, kidney failure, amputation of leg and feet, pregnancy complications and heart attack (http://www.kellogg.umich.edu/patientcare/conditions/diabetes.html). The association of diabetes with micro-and macrovascular complications and cardiomyopathy makes it a major cause of morbidity and mortality in the world (3–5). The alarming rate of increase in the incidence of type 1 diabetes is not only limited to Europe and America (6) but also includes other countries of the world (7).
The major strategy of the current medication for decreasing the blood glucose level in diabetes is exogenous supply of insulin. Although it is successful in decreasing the blood glucose level in hyperglycemic patients, it is neither capable of completely mimicking endogenously secreted insulin released from pancreatic beta-cells, which is tightly regulated for maintaining the optimum level of blood glucose nor is safe as it often causes hypoglycemic coma. Thus, strategies to promote either the expansion of existing beta-cells within the body or the supply of stem cell derived insulin-producing cells would provide a future treatment options for the patients with complicated diabetes. Stem cells are self-renewing, clonogenic and multipotent cells having tremendous potential for the treatment of several human diseases and potential source for regenerative medicine and tissue replacement after injury or disease. They are classified as embryonic and adult stem cells based on their respective origins; from blastocyst -stage embryos and from niches of mature adult tissues and bone marrow (8). Since these cells can be used to replenish the dead cells of different organs, they can be used in therapy of diseases such as myocardial infarction in heart where cardiomyocytes dies and diabetes where insulin producing pancreatic beta-cells either die or become defective. This review aims to provide an overview of the most current progress in this exciting area and will cover development of pancreatic beta cells, beta-cell regeneration from different stem cell lineages, regulatory role of microRNAs in diabetes, therapeutic challenges and the strategies to deal with it.
3. PANCREAS AND beta-CELL DEVELOPMENT
Before discussing stem cell based therapies for diabetes, it is important to understand how pancreas develops. Pancreas is a complex endoderm-derived organ, which consists of two major functional entities namely exocrine cells and duct cells that exert exocrine and endocrine activities. The exocrine cells constitute more than 90–95 % of the total pancreatic cell mass including acinar cells that produce digestive enzymes such as lipases, carbohydrases and amlyases, and duct cells that provide conduits to the gut for the enzymes (9). In the pancreatic tissue 1–2% of the endocrine organ consists of hundreds of thousands endocrine clusters that ranges from less than 50 to more than 500µM in diameter and scattered into the tissue. They play a key role in establishing normoglycemia in the body.
Five different endocrine cell types are known in the pancreas and each specialized in production and secretion of specific pancreatic hormone that are essential for the regulation of glucose homeostasis in the blood. They are alpha-cells secreting glucagon, beta-cells producing insulin, delta-cells producing somatostatin, PP-cells secreting pancreatic polypeptide, and ε-cells producing ghrelin (10, 11). In human pancreas islet cells contains approximately 50 to 63 % beta-cells, 15 to 20 % alpha-cells, 3 to 5 % delta-cells, ~1 % ghrelin cells, and ~1 % PP cells (12). Pancreas is a combination of lobulated, branched acinar gland that forms the exocrine pancreas and embedded in the acinar gland, the Islets of Langerhans, which constitute the endocrine pancreas. Considerable progress has been made over the last century to understand the cellular organization of the adult pancreas and the morphological changes that occur during pancreas development. In recent years, tremendous work has been done to gather information about the molecular mechanisms that regulate pancreas organogenesis, epithelial cell differentiation, and beta-cell replacement therapy. The development of pancreas includes generation of endoderm/gut endothelium, pancreatic differentiation, endocrine specification, and ultimately beta-cell differentiation. Pancreas development is controlled by a complex interaction of signaling pathways and transcription factors that determine early pancreatic specification as well as the later differentiation of exocrine and endocrine lineages (figure 1).
Figure 1.
A Schematic overview of cell lineage determination during pancreas development. The pancreas has two distinctly different tissues. It is composed of exocrine tissue and endocrine tissues. The exocrine tissue is made up of acinar cells that secrete pancreatic enzymes delivered to the intestine to facilitate the digestion of food. Scattered throughout the exocrine tissue are many thousands of clusters of endocrine cells known as islets of Langerhans. Within the islet, alpha cells produce glucagon; beta cells, insulin; deltacells, somatostatin; ε-cells produce Ghrelin and γ cells, pancreatic polypeptide. Transcription factors involved in the specification of the various lineages are shown in italic.
During development the three germ layers- ectoderm, mesoderm and endoderm are formed through intensive cell migration at the stage of gastrulation (13, 14). The definitive endoderm from which the pancreas arises begins as a flat sheet of cells that is specified during gastrulation. The anterior part of the definitive endoderm gives rise to the foregut, liver and lungs, while the posterior part becomes the midgut and hindgut (13). Genes required for definitive endoderm formation include Wnt/beta-catenin, Nodal, GATA4/6, FoxA2 and Mix (15; 16) and several members of the Sox family including sox17 (17).
Specification of the pancreatic field occurs around embryonic day 8.5 (E8.5) in mouse and 3 weeks in human. After the domains are specified and initiate morphogenetic budding, the dorsal and ventral pancreatic buds merge to create the gland. The development of the pancreas is orchestrated by a series of inductive interactions between endoderm and mesoderm-derived tissues, including the notochord, blood vessels and gut mesoderm (18). These interactions can lead to the differentiation of endoderm to a pancreatic fate. Pancreatic epithelial cells proliferate, branch and differentiate toward several types of cells in the pancreas. Insulin and glucagon can be detected as early as E9.5 and other hormone-secreting cells become first evident at E13. Pdx1-expressing cells give rise to endocrine, exocrine and ductal cells, demonstrating that Pdx1 represents a marker of all pancreatic lineages. Inactivation of Pdx-1 after bud formation prevents both islet and acinar cell differentiation. The expansion and differentiation of pancreatic progenitor cells is regulated by Notch signaling. Further, the notch-signaling pathway determines endocrine fate by the expression of the ‘pro-endocrine’ gene, neurogenin3 (Ngn3). At the end stage of islet formation and maturation, mutual interaction between vascular endothelial cells and endocrine cells promotes islet angiogenesis that is vital for the functional islets. Many transcription factors such as Pdx-1, ISL LIM homeobox 1 (Isl-1), Ngn-3, NK2 homeobox 2 (Nkx2.2), NK6 homeobox 1 (Nkx6.1), neurogenic differentiation factor (NeuroD), Hlxb9, paired box gene (Pax)-4, MafA and (Pax)-6 have been reported as islet differentiation factors. Ngn-3 is a key transcription factor required for islet cell development. Nkx2.2 is required for the final differentiation of beta-cells and production of insulin. Nkx6.1. and Pax-4 act as beta-cell determining factors. Pax- 6 is required for islet cell proliferation, morphology and beta-cell function. Transcriptional regulator Islet-1 (Isl-1) is essential for the maturation, proliferation and survival of the endocrine pancreas (19). MafA is a basic-leucine zipper transcription factor (20–22), that controls beta-cell-specific expression of the insulin gene through RIPE3b1 and thus acts as a potent transactivator for the insulin gene (22, 23) and involved in the function and development of beta-cells as well as in the pathogenesis of diabetes (20, 21). MafB an activator of the glucagon gene expressed in developing islet alpha- and beta-cells and regulates transcription of key factors during development that are required for the production of mature alpha and beta cells (21). Heparan sulfate binds with several signaling molecules and regulates ligand-receptor interactions. It thus plays an essential role in embryonic development. It is also involved in the regulation of postnatal islet maturation, which is required to ensure normal insulin secretion (24). A recent study suggests that Dicer1 is important for maintaining the adult pancreas and regulates the differentiation of endocrine precursor cells (25). A number of signaling pathways including the Hedgehog, Fgf, Notch, Wnt, and TGF-beta control various aspects of pancreas and endocrine cell development, proliferation and differentiation. Activin and growth differentiation factors (GDF) are involved in the endocrine and exocrine lineage specification (26–28). Vascular endothelial growth factor (VEGF) regulates insulin gene expression and beta -cell proliferation through laminin and maintains adult islet function. Tremendous progress has been made on pancreatic development, transcriptional regulation of pancreatic endocrine specification, growth and lineage allocation that contributes to our understanding of how endogenous beta-cells develop and differentiate. Understanding pancreas organogenesis will provide a clue for translational research for beta-cell regeneration.
4. DEVELOPMENT OF STEM CELL THERAPY FOR DIABETES
Stem cells are self-renewing, unspecialized cells that give rise to multiple specialized cell types through a process of differentiation. The adult endocrine pancreas has for a long time been considered a quiescent cell population. Recent studies have shown that beta-cell mass is dynamically regulated like the other tissues. The interplay of cell expansion and reduction mechanisms determines the amount of the beta-cell mass. Expansion can occur through beta-cell hypertrophy, self-replication, trans-differentiation and neogenesis. In contrast, reduction can results from beta-cell atrophy, death or loss of phenotypic stability. Thus, the development of strategies to avoid beta-cell mass reduction or to enhance beta-cell mass expansion, both in vivo and in vitro could provide a promising option for cell-based therapy of type 1 and type 2 diabetes.
The major approach to ameliorate the hyperglycemic condition is either by exogenous supply of insulin or induction of insulin producing cells (pancreatic beta cells) either by differentiation of stem cells in vivo or transplantation of ex vivo differentiated cells in pancreas. The fact that exogenous insulin cannot maintain the optimum physiological level of glucose and is often accompanied by hypoglycemia, pancreas/pancreatic islet replacement therapy is considered as a better alternative. The transplantation of intact pancreas or the beta cell mass can fulfill the need for achieving life long normoglycemia. Although there are several promising advancements in this direction (6), the major limiting factor is shortage of functional beta-cells from available donors (29). Therefore, the current strategy is focused mainly on regeneration of pancreatic beta- cells where the basic need is identification of biomarkers for these cells. The micro environmental cues required for differentiation of stem cells into pancreatic beta- cells either in vitro, ex vivo or in vivo will promote the regeneration of large number of the cells required for therapy of diabetes. Recently, the success of mesenchymal stem cells to achieve this goal and mitigate the effect of hyperglycemia is quite enthusiastic (29–32). Several sources of stem / precursor cells have been suggested that can repopulate the damaged beta- cells and that include ES cells, HSC, MSC, resident stem cells, dedifferentiation, nuclear reprogramming, induced pluripotent cells differentiation, transdifferentiation, and neogenesis (figure 2)
Figure 2.
Schematic diagram depicting Possible sources of beta-cells for cell replacement therapy. During type 1 and type 2 diabetes condition most of the beta cells are destroyed and no or very low insulin is produced. These conditions are treated and beta cells can be regenerated by several sources. Details are given in the text.
4.1. Tradition approach of treatment of diabetes
The regenerative therapy targets type 1 diabetes, where beta-cells die and inadequate production of insulin causes diabetes. The best criteria to characterize type 1 diabetes are to assess the presence of anti-islet cell- antibody (6). The other symptoms are severe insulitis and autoimmune destruction of pancreatic beta-cells leading to hyperglycemia (6). The traditional approach to treat this disease is injection of exogenous insulin and subsequent follow up of blood glucose level. However, the major drawback of this method is frequent incidence of hypoglycaemia in the patients occurred due to inability of the exogenous insulin to mimic the physiology of secretion of endogenous insulin (6). The other promising approach is transplantation of pancreas (33, 34) and islet cells (35–37) for beta-cell replacement therapy. There was considerable success to treat diabetic patient from this approach. The follow up studies after transplantation of beta-cells from 2 to 5 years in different studies show great achievement for insulin independency (38–40). Nevertheless, this method has several limiting factors like need of immunosuppressant, which always adds several side effects, difficulty of obtaining transplant material and getting access to suitable organ donar (41).
4.2. Therapeutic potential of stem cells in diabetes
The latest approach using stem cells for treatment of diabetes was started in a clinical trials using autologous nonmyeloablative hematopoietic stem cell transplantation by exploiting the immunomodulatory properties of stem cells (42, 43). The main strategy of their treatment was to inhibit the autoimmune destruction of beta-cells with immunosuppressive drugs and to replenish the destroyed immune cells by using autologous hematopoietic stem cells, which will reconstitute the normal immune system (42). More than a year and half follow up studies of patients with nonmyeloablative hematopoietic stem cell transplantation revealed that the patients are insulin free. Further, the constant monitoring of their c-peptide level corroborated that insulin free condition of patients was due to the preservation of beta-cell mass (43). The important caveat for this therapy was promotion of beta-cell regeneration to overcome autoimmunity and to ameliorate endogenous insulin secretion. Mesenchymal stem cells having immunomodulatory properties and their power to differentiate into insulin-secreting cells make it a promising therapeutic target for diabetes (6).
A number of studies have suggested the existence of stem cells within the pancreas that can give rise to insulin producing cells (44–64). There are other evidences suggesting that trans-differentiation of liver cells can generate beta-cells (65–71). Several other studies reported that bone marrow derived stem cells can be differentiated into insulin-expressing cells (72–78). Neural progenitor cells from the brain also have the capacity to differentiate into insulin expressing cells (45). In addition to these cells, there are other highly proliferative and pluripotent cells derived from inner cell mass of the blastocyst called ES cells. They have the capacity to differentiate into all three embryonic germ layers. Accumulating evidences suggest that ES cells can differentiate into cells with an insulin-expressing phenotype (79–88). Other sources for beta-cell regeneration are pancreasderived multipotent progenitor (60, 89, 90); pancreatic duct cells (91); splenocytes (92, 93); and umbilical cord blood cells (94–96).
Stem cell-derived insulin-producing cells could be a renewable source of insulin-producing cells for cell transplantation. To enhance the maturation process of human embryonic stem cells (hESCs)-derived insulin-producing cells, recent studies used genetic manipulation methodologies to deliver specific pancreatic transcription factors or developmental control genes to hESCs (49, 97). hESCs are derived from the inner cell mass of pre-implantation blastocyst and have potential for self-renewal, differentiation into all embryonic cell types, and unlimited expansion without compromising its differentiation capacity. Previous studies on beta-cells generation from hESCs were focused on the selection of cells positive for nestin (98, 99). It served as a biomarker for stem/progenitor cell populations in other tissues (100). However, recently it turns out to be a biomarker for neural and pancreatic exocrine progenitors and does not mark endocrine progenitor cells (100, 101). The first report that insulin-secreting cells can be generated from spontaneous differentiation using hESCs come from Assady et al. (102). Later on Lavon et al. (97) demonstrated that the constitutive expression of Pdx1 enhances the differentiation of hESCs toward pancreatic endocrine and exocrine cell types. The expression of Pdx1 also increased the expression of several transcription factors that are downstream to it such as Ngn3, PAX4, NKX2.2, and ISL1. Further, by reprogramming rat hepatic stem cell into functional insulin-producing cells by over expression of Pdx1 and their delivery into diabetic mice with a lentivirus demonstrate that Pdx1 is effective in converting hepatic stem cells into pancreatic endocrine precursor cells and it is able to generate insulinproducing cells and restore euglycemia (103).
4.3. Transcription factors involved in converting MSC to insulin producing cells
Transplantation of adult human bone marrow-derived mesenchymal stem cells (hMSCs) could be a promising source to replenish insulin-producing cells because hMSCs have the suppressive effects on T cell responses to alloantigen and thus offer a novel cell-based approach for the prevention of autoimmune diabetes and for islet cell transplantation (6;104–107). Dedifferentiation is the process whereby mature cells become less differentiated and acquire the ability to differentiate into different cell types. As opposed to dedifferentiation, transdifferentiation is the process through which differentiated cells are stimulated to become a different mature cell type. hMSCs can be induced to differentiate into functional insulin-producing cells when Pdx1 is introduced via recombinant adenoviral vector (108). Furthermore, Pdx1 modified hMSCs seemed to contribute to the regeneration of pancreatic islets after cell transplantation in STZ-induced diabetic mice. Mouse bone marrow derived stem cells when treated with fetal calf serum and high concentrations of glucose for 4 months, were differentiated (or transdifferentiated) into functional beta-cells (109;110). Contrary to this, negative results are also documented (109). Thus genetically modified hMSCs are a potential cell source for cell replacement therapy for diabetes. It is also reported that progenitor cells in close proximity to ductal epithelium can differentiate into beta-cells because of cues from the large number of beta- cells in the pancreas (55,104,106,111). By using adenovirus to mediate Pdx-1, Neurogenin3 (Ngn3), NeuroD or Pax4 expression in duct cells, Noguchi et al. (55) demonstrate that NeuroD was the most effective inducer of insulin expression in primary duct cells and suggested that the over expression NeuroD facilitates pancreatic stem/progenitor cell differentiation into insulin-producing cells in pancreas. Kodama et al. (93) have shown that live donor male or labeled splenocytes administered to diabetic NOD females contain cells that rapidly differentiate into islet and ductal epithelial cells within the pancreas. They found that treatment with irradiated splenocytes was also followed by islet regeneration, but at a slower rate and were persistent, functional, and apparent in all NOD hosts with permanent disease reversal. Nagaya, et al. (112) recently demonstrated that a sub-population of intra-hepatic biliary epithelial cells (IHBECs) can be induced to a beta-like phenotype. Recently, research has been focused on stem cells like human umbilical cord blood (UCB-MSCs). The use of UCB-MSCs for development as a universal donor cell source for beta-cell replacement offers several advantages over other cells such as it can be obtained at higher frequencies and it has an unusually broad differentiation potential (113;114). Recently, Gao et al. (95) reported that mesenchymal stem cells derived from UCB-MSCs can be used as new and potential stem cells in the treatment of diabetes.
The beta-cell populations of the endocrine pancreas may expand by either of two processes- replication or neogenesis. While replication requires the existence of an already differentiated beta-cell, neogenesis depends on the presence of active stem cells. Dor et al. (115) observed cell lineage using a transgenic mouse strain, in which the insulin promoter regulates the expression of a tamoxifen-dependent Cre recombinase to mark adult progenitor cells. Using this system, they were able to distinguish between existing beta-cells and new beta-cells that differentiated from stem cells. They found that beta-cells are derived only from the duplication of existing beta-cells and suggested that only beta-cells can produce new beta-cells rather than being derived from pluripotent adult precursor cells (115). This was subsequently confirmed by Teta et al. (116) who used a DNA analogue- based lineage-tracing technique as well as other investigators (117–119). The autopsy studies in humans provide strong supportive evidence that beta-cell replication is the primary mechanism underlying beta-cell expansion (120). Recently, it has been also documented that all beta-cells contribute equally to islet growth and maintenance. It is speculated that for tissues lacking an adult stem cell can be replenished equally by replication of all differentiated cells (121). Although, beta-cell replication alone may be sufficient to account for maintaining the mass of the pancreas, there are strong evidences supporting that new beta-cells can be generated by a process of neogenesis from a stem-cell population residing in the pancreatic duct (91). Al-Abdullah et al. (122) reported that copper deprivation contributes to the neogenesis of pancreatic alpha- and beta- cells in the ductules and acinar tissue of adult pancreas in rat model; and that transplanted stem cells maintain their functional capacity in the recipient after transplantation. Several other studies demonstrated that transcriptional regulation involving pdx-1 is essential for endocrine neogenesis in vivo and in vitro and that ectopic expression of pdx-1 in the pancreas could induce endocrine neogenesis (84, 97,108). Taniguch et al. (123) demonstrated that adenovirus-mediated expression of pdx-1 can activate the endogenous pdx-1 gene, leading to beta-cell neogenesis and ductal proliferation. It has been shown that new beta-cell can be formed from non-beta-cells located in the lining of the duct during regeneration of the pancreas in response to duct ligation. Further, it was found that duct ligation induces an increased number of cells expressing Ngn3 (124). Recently, PaSCs (pancreatic stellate cells) have been identified in the pancreas that express the ABCG2 transporter and are able to secrete insulin after cell differentiation (125).
4.4. Generation of insulin-secreting cells through nuclear reprogramming and induced pluripotent stem (iPS) cells
Accumulative evidence suggests that islet cell transplantation for patients with diabetes holds great promise for achieving insulin independency. However, the extreme shortage of matched organ donors and the immuno-rejection has made it difficult for this treatment to be used for the general diabetic population. Recent success in generating insulin-secreting islet-like cells from human embryonic stem (ES) cells coupled with the success in deriving human ES cell-like induced pluripotent stem (iPS) cells from human fibroblasts have opened an emerging possibility of patient-specific treatment where insulin-secreting isletlike cells could be derived from the patient's somatic cells by reprogramming the cell fate through defined factors. Induced pluripotent stem cells (iPS cells) are a type of pluripotent stem cell artificially derived by reprogramming a somatic cell. iPS cells are morphologically similar to embryonic stem cells and are capable of differentiating into a variety of different somatic cell types (126, 127). Takahashi and Yamanaka (127) was the first to discovered that viral transfection of four genes (Oct 3/4, Sox2, c-Myc, and KLF4) into an adult mouse fibroblast population that can lead to the appearance of some cells with the characteristics of ES cells. Tateishi et al. (128) demonstrated that skin fibroblast-derived iPS cells have the potential to be differentiated into islet-like clusters through definitive and pancreatic endoderm. Zhou et al. (129) identify a specific combination of three transcription factors (Ngn3, Pdx1 and MafA) that reprograms differentiated pancreatic exocrine cells in adult mice into cells that closely resemble beta-cells. The induced beta-cells are indistinguishable from endogenous islet beta-cells in size, shape and ultrastructure. Stadtfeld et al. (130) used inducible lentiviruses to express Oct4, Sox2, c-myc, and Klf4 in pancreatic beta-cells to assess whether a defined terminally differentiated cell type remains amenable to reprogramming. Their results provide evidence that terminally differentiated cells can be reprogrammed into pluripotent cells, suggesting that in vitro reprogramming is not restricted to certain cell types or differentiation stages. Recently, Zhang et al. (131) reported a highly efficient approach to induce human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells to differentiate into mature insulin-producing cells in a chemical-defined culture system. The differentiated human ES cells obtained by this approach comprised nearly 25% insulin-positive cells as assayed by flow cytometry analysis, which released insulin/C-peptide in response to glucose stimuli in a manner comparable to that of adult human islets. Most of these insulin-producing cells co-expressed mature beta-cell -specific markers, such as NKX6-1 and PDX1 indicating a similar gene expression pattern to adult islet beta-cell in vivo. Further they demonstrated that EGF facilitates the expansion of PDX1-positive pancreatic progenitors. The above studies confirmed that insulin-secreting cells can be generated from skin fibroblasts, raising the possibility that patient-specific iPS cells could potentially provide a treatment for diabetes in the future.
5. microRNAs IN DIABETES
MicroRNAs (miRNAs) are a novel group of highly conserved, endogenous, 22–23 nucleotide non-coding RNAs that are involved in precise regulation of biological functions by negatively modulating the gene expression either through promotion of mRNA degradation or through translational repression of proteins (132,133). The tremendous potential of these tiny regulators has been recently documented in many cellular pathways including development, cell differentiation, proliferation and apoptosis, and are also manifested in diverse diseases including cardiovascular, different types of cancer as well as diabetes (133–137). It has been reported that miRNAs are critical in regulation of these complex diseases and they may be exploited as targets for therapeutic intervention. Understanding the regulatory mechanisms of miRNAs in insulin secretion and glucose homeostasis may unravel better understanding of pancreatic cell biology and diabetes pathophysiology opening a new window for novel therapeutic targets that includes the strategies to manipulate in the development and progression of diabetes and its complications (138,139).
Accumulative evidence suggests that miRNAs play an important role in insulin secretion pancreatic islet development, beta-cell differentiation, and indirectly control glucose and lipid metabolism (134,140–145). Poy et al. (143,144) identified a novel islet-specific miRNA, miRNA-375, which is highly expressed in pancreatic islets, essential for normal glucose homeostasis, alpha- and beta-cell turnover and adaptive beta-cell expansion in response to increasing insulin demand in insulin resistance. Joglekar et al. (141) provide evidence for miRNA-mediated silencing of ngn3, which inhibits endocrine cell development via the classical 'stem cell pathway' during mouse pancreatic regeneration, thereby favoring beta-cell regeneration. Manipulation of the miR-221-c-kit pathway may offer a novel strategy for treatment of vascular dysfunction in diabetic patients (146). High levels of miR-29 led to insulin resistance and overexpression of miR-29 caused a decrease in the levels of Insig1 (insulin-induced gene 1), and Cav2 proteins (caveolin 2). Insulin receptor substrate (IRS) proteins are important components of the insulin signaling pathway. There are three IRS proteins in humans and mice such as IRS1 and IRS2, and IRS-4, IRS1 knockout mice insulin resistant, whereas IRS2 deficient mice develop diabetes (147). Although IRS2 is involved in the type-2 diabetes, only IRS1 has been identified to be a direct target of miR-145 (148). Recently Tang et al. (149) in a screen identified 61 glucose-regulated miRNAs including miR-124a, miR-107, and miR-30d, up-regulated in the presence of high glucose and some of the miRNAs, including miR-296, miR-484, and miR-690 were significantly down-regulated in the presence of high glucose. Interestingly, they found that overexpression of miR-30d, increased insulin gene expression, while inhibition of miR-30d abolished glucose-stimulated insulin gene transcription and suggested that miR-30d may be negative regulators of insulin gene expression. Recently, it has been reported that miR-30 family miRNAs confer epithelial phenotype to human pancreatic cells (142).
Growing evidence suggests that miRNAs play an important role in insulin production, secretion and action. Diabetes leads to changes in miRNA expression profiles in many tissues. The roles of miRNAs in diabetes are very complex as changes in miRNA levels may lead to diabetes in both early and late stages. MiRNAs provide a new class of biomarkers for various diseases including cancer, and will be useful biomarkers for diabetes as well. Furthermore, recent progress in the development and use of synthetic miRNAs such as antagomiRs to silence miRNAs in vivo such as miR-375 in case of diabetes (150) may provide novel therapeutic tools for the treatment of diabetes and other diseases in the future.
6. THERAPEUTIC CHALLENGES
Although there are several evidences to corroborate that stem cells and islet cells have tremendous capability to treat diabetic patient and maintain normoglycemic condition/insulin independency for several years (38, 57,151–153), the precise mechanism for differentiation of stem cells into IPCs is still nebulous. The genetic manipulations and micro- environmental conditions required for differentiation of stem cells into IPCs are major issues to be elucidated with concrete evidences. It will facilitate the generation of functional IPCs from mesenchymal stem cells in large scale, which is one of the major challenges ahead for the treatment of diabetes. As usual with the most of the therapy, there are several drawbacks/side effects associated with treatment, which needs to be taken seriously before going into clinical trials. Recent investigations showed the association of mesenchymal stem cell expansion with tumor development (151, 154–156), which cautions us to understand meticulously the side effects and their remedy before using it for therapy.
7. FUTURE DIRECTIONS
Stem cells have been identified in many of the adult organs and across the animal and plant kingdom (157–165). They are maintained in a specialized microenvironment known as the stem-cell niche. Two fundamental questions in stem cell research are what controls stem cell number and which signal pathways regulate its self-renewal (157–165). Accumulative evidence suggests that the niche maintain the stem cell number and multiple signals are required to maintain a balanced control of stem cell self-renewal (157–165). An interesting method for generating beta-cells in bulk is to understand the signaling pathway that promotes differentiation of any stem cell into beta-cells. Technological advancement is required for proper transplantation of beta-cells into suitable niches for maximum success of treatment. Recent progress on pancreatic stem cell research has revealed that the putative multipotent pancreatic stem cells and /or beta-cell precursors may reside in the pancreatic gland in the adult life. The presence of undifferentiated pancreatic cells with stem cell-like properties opens the possibility of stimulating their expansion and differentiation for beta-cells replacement-based therapies for type 1 or 2 diabetes. In addition, the transplantation of either insulin-producing beta-cell from embryonic, fetal and other tissue-resident adult stem/progenitor cells or genetically modified adult stem/progenitor cells may also combine alternative promising therapies for treating diabetic patients and associated diseases including diabetic cardiomyopathy. The most important issue is to understand the side effects associated with transplantation of beta-cells and how to regulate it. The precise regulatory role of microRNAs in several pathological conditions (132,133,137) tempted us to speculate that they can provide an impetus in investigating the regulatory mechanisms underlying differentiation of stem cells into beta-cells.
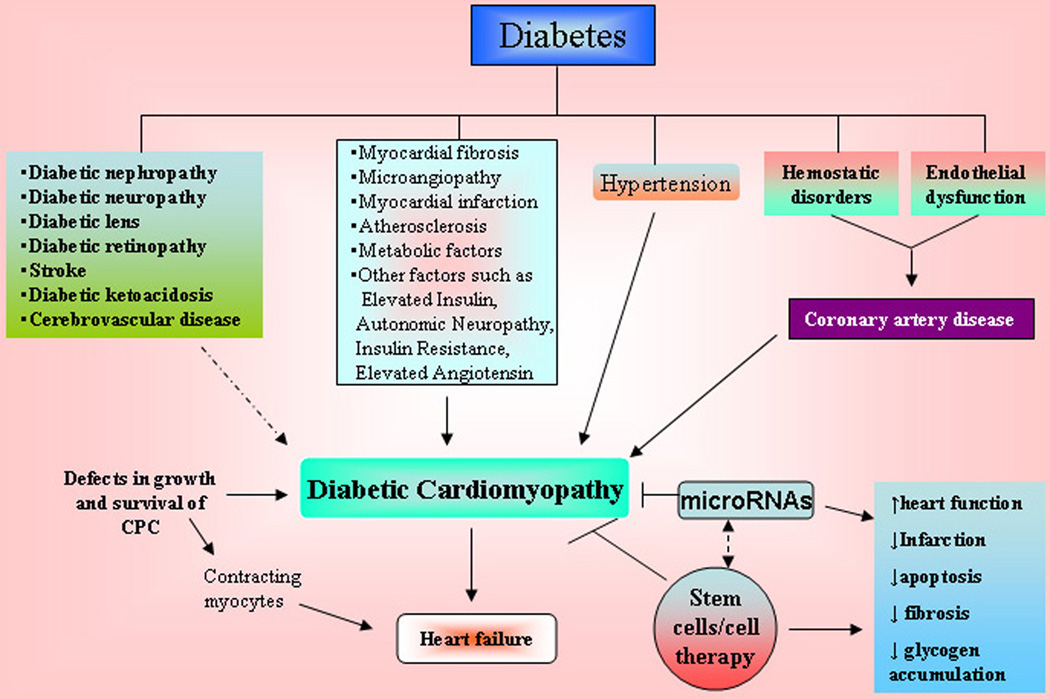
DM is a well known and important risk factor for cardiac disease ((166–174). Although the most common cardiac manifestation in diabetic patients is coronary artery disease, DM is also strongly linked to heart failure (HF). Approximately 15 to 25% of patients with HF are diabetic. It has been known that hyperglycaemia and hyperinsulinemia increase the risk of death due to premature and accelerated coronary artery disease. Hyperglycaemia over time can lead to increased deposits of fatty materials on the insides of the blood vessel walls that affect blood flow, increasing the chance of clogging and hardening of blood vessels result in diabetic cardiomyopathy and heart failure (166–174). Diabetic cardiomyopathy can be clinically defined by the presence of abnormal myocardial performance or structure in the absence of epicardial coronary artery disease, hypertension, and significant valvular disease (Figure 3). It has been demonstrated that following an ischemic insult to the heart, neural stem cells participated in sympathetic fiber innervation of the peri-infarct/infarct region, de novo blood vessel formation and maladaptive healing following ischemic injury (166–174). The cardiac function can be improved following MSCs transplantation, which significantly increased myocardial arteriolar density and decreased the collagen volume in diabetic myocardium. MSCs transplantation increased MMP-2 activity and decreased transcriptional level of MMP-9 (173). Zhang et al. (173) suggests that MSCs transplantation improved cardiac function, possibly through angiogenesis and attenuation of cardiac remodeling. The growing evidence suggest that the heart acquire a compartment of multipotent progenitor cells (MPCs) that differentiate into myocytes, endothelial cells, and smooth muscle cells. The heart cells are continuously self-renew and any alteration between cell death and regeneration following diabetes could be mediated by defects in growth and survival of MPCs resulting in an excessive number of old, dying and poorly contracting myocytes and eventually heart failure. A recent study also suggests that diabetes promotes cardiac stem cell aging and heart failure (174). However, this can be prevented by deletion of the p66shc gene (174). These studies suggest that stem cells can be a potential therapeutic target for the diabetic cardiomyopathy that eventually restores cardiac function (figure 3). Furthermore, since miRNAs play important roles in myocardial dysfunction associated with insulin resistance it may provide novel therapeutic approaches for the management of diabetes-induced cardiomyopathy.
Figure 3.
Effects of diabetes on diabetic cardiomyopathy. Diabetes mellitus is associated with multiple physiopathological changes in the heart and other organ system. Diabetic cardiomyopathy results in heart failure that occurred due to defects in growth and survival of cardiac progenitor cells (CPC) or other possible causes such as myocardial fibrosis, abnormal myocardial metabolism, hypertension, and coronary artery disease (CAD). These physiopathological changes in the heart can be corrected by supplying the stem cells that may restore the heart function. Details are provided in the text.
ACKNOWLEDGEMENTS
Paras Kumar Mishra, Shree Ram Singh contributed equally to this work. This work was supported in part by the NIH grants HL-74185, HL-71010, HL-88012 and NS-51568. We would like to thank Lindsey Draper and Avinash S. Yadava for their help during preparation of the manuscript.
Abbreviations
- BM
bone marrow
- CAD
coronary artery disease
- CPC
cardiac progenitor cells
- DM
Diabetes mellitus
- GDF
growth differentiation factors
- HSC
hematopoietic stem cell
- HF
heart failure
- hMSCs
human bone marrow-derived mesenchymal stem cells
- hESCs
human embryonic stem cells
- MSCs
mesenchymal stem cells
- MPCs
multipotent progenitor cells
- UCB-MSCs
mesenchymal stem cells derived from human umbilical cord blood
- IPCs
insulin producing cells
- IRS
Insulin receptor substrate
- iPS
induced pluripotent stem cells
- IHBECs
intra-hepatic biliary epithelial cells
- MSCs
mesenchymal stem cells
- miRNAs
MicroRNAs
- Nkx2.2
NK2 homeobox 2
- Nkx6.1
NK6 homeobox 1
- NeuroD
neurogenic differentiation factor
- Ngn3
Neurogenin3
- Pax-4
paired box gene 4
- PaSCs
pancreatic stellate cells
- VEGF
Vascular endothelial growth factor.
REFERENCES
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Joshua IG, Zhang Q, Falcone JC, Bratcher AP, Rodriguez WE, Tyagi SC. Mechanisms of endothelial dysfunction with development of type 1 diabetes mellitus: role of insulin and C-peptide. J Cell Biochem. 2005;96:1149–1156. doi: 10.1002/jcb.20620. [DOI] [PubMed] [Google Scholar]
- 4.Pearson ER. Pharmacogenetics and future strategies in treating hyperglycaemia in diabetes. Front Biosci. 2009;14:4348–4362. doi: 10.2741/3532. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi SC, Rodriguez W, Patel AM, Roberts AM, Falcone JC, Passmore JC, Fleming JT, Joshua IG. Hyperhomocysteinemic diabetic cardiomyopathy: oxidative stress, remodeling, and endothelial-myocyte uncoupling. J Cardiovasc Pharmacol Ther. 2005;10:1–10. doi: 10.1177/107424840501000101. [DOI] [PubMed] [Google Scholar]
- 6.Vija L, Farge D, Gautier JF, Vexiau P, Dumitrache C, Bourgarit A, Verrecchia F, Larghero J. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35:85–93. doi: 10.1016/j.diabet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Majaliwa ES, Elusiyan BE, Adesiyun OO, Laigong P, Adeniran AK, Kandi CM, Yarhere I, Limbe SM, Iughetti L. Type 1 diabetes mellitus in the African population: epidemiology and management challenges. Acta Biomed. 2008;79(3):255–259. [PubMed] [Google Scholar]
- 8.Nelson TJ, ZD Ge, Van OJ, Barron M, Rudy-Reil D, Hacker TA, Misra R, Duncan SA, Auchampach JA, Lough JW. Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1216–1224. doi: 10.1002/ar.a.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: A comparative study. Islets. 2009;1:1–8. doi: 10.4161/isl.1.2.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 14.Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- 15.Zorn AM, Wells JM. Molecular basis of vertebrate endoderm development. Int Rev Cytol. 2007;259:49–111. doi: 10.1016/S0074-7696(06)59002-3. [DOI] [PubMed] [Google Scholar]
- 16.Grapin-Botton A, Constam D. Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev. 2007;124:253–278. doi: 10.1016/j.mod.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 17.de Santa BP, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60:1322–1332. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev Biol. 2006;290:189–199. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Du A, Hunter CS, Murray J, Noble D, Cai CL, Evans SM, Stein R, May CL. Islet-1 is required for the maturation, proliferation and survival of the endocrine pancreas. Diabetes. 2009 doi: 10.2337/db08-0987. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem. 2002;277:49903–49910. doi: 10.1074/jbc.M206796200. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman DA, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olbrot M, Rud J, Moss LG, Sharma A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci USA. 2002;99:6737–6742. doi: 10.1073/pnas.102168499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyatsuka T, Matsuoka TA, Kaneto H. Transcription factors as therapeutic targets for diabetes. Expert Opin Ther Targets. 2008;12:1431–1442. doi: 10.1517/14728222.12.11.1431. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, Mizumoto S, Ikeda T, Sugihara K, Asano M, Yoshikawa T, Yamauchi A, Shervani NJ, Uruno A, Kato I, Unno M, Sugahara K, Takasawa S, Okamoto H, Sugawara A. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem Biophys Res Commun. 2009;383:113–118. doi: 10.1016/j.bbrc.2009.03.140. [DOI] [PubMed] [Google Scholar]
- 25.Morita S, Hara A, Kojima I, Horii T, Kimura M, Kitamura T, Ochiya T, Nakanishi K, Matoba R, Matsubara K, Hatada I. Dicer is required for maintaining adult pancreas. PLoS One. 2009;4:e4212. doi: 10.1371/journal.pone.0004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharfmann R, Duvillie B, Stetsyuk V, Attali M, Filhoulaud G, Guillemain G. Beta-cell development: the role of intercellular signals. Diabetes Obes Metab. 2008;4:195–200. doi: 10.1111/j.1463-1326.2008.00953.x. [DOI] [PubMed] [Google Scholar]
- 27.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barajas M, Principe RM, Escalada J, Prosper F, Salvador J. New therapeutic strategies for type 1 diabetes mellitus. An Sist Sanit Navar. 2008;31:219–234. doi: 10.4321/s1137-66272008000500002. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Wang Z, Zhu M. Human bone marrow mesenchymal stem cells transfected with human insulin genes can secrete insulin stably. Ann Clin Lab Sci. 2006;36:127–136. [PubMed] [Google Scholar]
- 31.Xu J, Lu Y, Ding F, Zhan X, Zhu M, Wang Z. Reversal of diabetes in mice by intrahepatic injection of bone-derived GFP-murine mesenchymal stem cells infected with the recombinant retrovirus-carrying human insulin gene. World J Surg. 2007;31:1872–1882. doi: 10.1007/s00268-007-9168-2. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Zhu MY, Lu YH, Lu Y, Wang Z. Treatment of type 1 diabetes by transplantation of bone-derived mesenchymal stem cells expressing human insulin gene: experiment with mice. Zhonghua Yi Xue Za Zhi. 2007;87:2557–2560. [PubMed] [Google Scholar]
- 33.Lam VW, Wong K, Hawthorne W, Ryan B, Lau H, Robertson P, Allen RD, Pleass H. The linear cutting stapler for enteric anastomosis: a new technique in pancreas transplantation. Transpl Int. 2006;19:915–918. doi: 10.1111/j.1432-2277.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 34.Robertson P, Davis C, Larsen J, Stratta R, Sutherland DE. Pancreas transplantation in type 1 diabetes. Diabetes Care. 2004;27:S105. doi: 10.2337/diacare.27.2007.s105. [DOI] [PubMed] [Google Scholar]
- 35.Ricordi C, Hering BJ, Shapiro AM. Beta-cell transplantation for diabetes therapy. Lancet. 2008;372:27–28. doi: 10.1016/S0140-6736(08)60984-8. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro AM. Islet transplantation--the imperative need for continued clinical trials. Nat Clin Pract Nephrol. 2008;4:662–663. doi: 10.1038/ncpneph0964. [DOI] [PubMed] [Google Scholar]
- 38.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 39.Ryan EA, Paty BW, Senior PA, Bigam D, Shapiro AM. Beta-score:an assessment of beta-cell function after islet transplantation. Diabetes Care. 2005;28:343–347. doi: 10.2337/diacare.28.2.343. [DOI] [PubMed] [Google Scholar]
- 40.Street CN, Lakey JR, Shapiro AM, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T, Korbutt GS. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107–3114. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 42.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simões BP, Foss MC, Squiers E, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 43.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simões BP, Martinez EZ, Foss MC, Burt RK, Voltarelli JC. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 44.Abraham EJ, Leech CA, Lin JC, Zulewski H, Habener JF. Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producing cells. Endocrinology. 2002;143:3152–3161. doi: 10.1210/endo.143.8.8973. [DOI] [PubMed] [Google Scholar]
- 45.Burns CJ, Persaud SJ, Jones PM. Diabetes mellitus: a potential target for stem cell therapy. Curr Stem Cell Res Ther. 2006;1:255–266. doi: 10.2174/157488806776956832. [DOI] [PubMed] [Google Scholar]
- 46.Docherty K, Bernardo AS, Vallier L. Embryonic stem cell therapy for diabetes mellitus. Semin Cell Dev Biol. 2007;18:827–838. doi: 10.1016/j.semcdb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Furth ME, Atala A. Stem cell sources to treat diabetes. J Cell Biochem. 2009;106:507–511. doi: 10.1002/jcb.22000. [DOI] [PubMed] [Google Scholar]
- 48.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo T, Hebrok M. Stem cells to pancreatic beta-cells: new sources for diabetes cell therapy. Endocr Rev. 2009;30:214–227. doi: 10.1210/er.2009-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardikar AA, Lees JG, Sidhu KS, Colvin E, Tuch BE. Stem-cell therapy for diabetes cure: how close are we? Curr Stem Cell Res Ther. 2006;1:425–436. doi: 10.2174/157488806778226830. [DOI] [PubMed] [Google Scholar]
- 51.Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet. 2004;364:203–205. doi: 10.1016/S0140-6736(04)16635-X. [DOI] [PubMed] [Google Scholar]
- 52.Kraitchman DL, Bulte JW. In vivo imaging of stem cells and Beta cells using direct cell labeling and reporter gene methods. Arterioscler Thromb Vasc Biol. 2009;29:1025–1030. doi: 10.1161/ATVBAHA.108.165571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lock LT, Tzanakakis ES. Stem/Progenitor cell sources of insulin-producing cells for the treatment of diabetes. Tissue Eng. 2007;13:1399–1412. doi: 10.1089/ten.2007.0047. [DOI] [PubMed] [Google Scholar]
- 54.Minami K, Seino S. Pancreatic acinar-to-beta cell transdifferentiation in vitro. Front Biosci. 2008;13:5824–5837. doi: 10.2741/3119. [DOI] [PubMed] [Google Scholar]
- 55.Noguchi H, Xu G, Matsumoto S, Kaneto H, Kobayashi N, Bonner-Weir S, Hayashi S. Induction of pancreatic stem/progenitor cells into insulin-producing cells by adenoviral-mediated gene transfer technology. Cell Transplant. 2006;15:929–938. doi: 10.3727/000000006783981431. [DOI] [PubMed] [Google Scholar]
- 56.Peck AB, Ramiya V. In vitro-generation of surrogate islets from adult stem cells. Transpl Immunol. 2004;12:259–272. doi: 10.1016/j.trim.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 58.Sahu S, Tosh D, Hardikar AA. New sources of beta-cells for treating diabetes. J Endocrinol. 2009;202:13–16. doi: 10.1677/JOE-09-0097. [DOI] [PubMed] [Google Scholar]
- 59.Santana A, Ensenat-Waser R, Arribas MI, Reig JA, Roche E. Insulin-producing cells derived from stem cells: recent progress and future directions. J Cell Mol Med. 2006;10:866–883. doi: 10.1111/j.1582-4934.2006.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 60.Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 61.Sordi V, Bertuzzi F, Piemonti L. Diabetes mellitus: an opportunity for therapy with stem cells? Regen Med. 2008;3:377–397. doi: 10.2217/17460751.3.3.377. [DOI] [PubMed] [Google Scholar]
- 62.Stanley EG, Elefanty AG. Building better beta cells. Cell Stem Cell. 2008;2:300–301. doi: 10.1016/j.stem.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Sumi S, Gu Y, Hiura A, Inoue K. Stem cells and regenerative medicine for diabetes mellitus. Pancreas. 2004;29:e85–e89. doi: 10.1097/00006676-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 64.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 65.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 66.Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 67.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 68.Tuch BE, Szymanska B, Yao M, Tabiin MT, Gross DJ, Holman S, Swan MA, Humphrey RK, Marshall GM, Simpson AM. Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Ther. 2003;10:490–503. doi: 10.1038/sj.gt.3301911. [DOI] [PubMed] [Google Scholar]
- 69.Yang Q, Yamagata K, Fukui K, Cao Y, Nammo T, Iwahashi H, Wang H, Matsumura I, Hanafusa T, Bucala R, Wollheim CB, Miyagawa J, Matsuzawa Y. Hepatocyte nuclear factor-1alpha modulates pancreatic beta-cell growth by regulating the expression of insulin-like growth factor-1 in INS-1 cells. Diabetes. 2002;51:1785–1792. doi: 10.2337/diabetes.51.6.1785. [DOI] [PubMed] [Google Scholar]
- 70.Tosh D, Shen CN, Slack JM. Differentiated properties of hepatocytes induced from pancreatic cells. Hepatology. 2002;36:534–543. doi: 10.1053/jhep.2002.35060. [DOI] [PubMed] [Google Scholar]
- 71.Tosh D, Shen CN, Slack JM. Conversion of pancreatic cells to hepatocytes. Biochem Soc Trans. 2002;30:51–55. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 72.Butler AE, Huang A, Rao PN, Bhushan A, Hogan WJ, Rizza RA, Butler PC. Hematopoietic stem cells derived from adult donors are not a source of pancreatic beta-cells in adult nondiabetic humans. Diabetes. 2007;56:1810–1816. doi: 10.2337/db06-1385. [DOI] [PubMed] [Google Scholar]
- 73.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jahr H, Bretzel RG. Insulin-positive cells in vitro generated from rat bone marrow stromal cells. Transplant Proc. 2003;35:2140–2141. doi: 10.1016/s0041-1345(03)00747-4. [DOI] [PubMed] [Google Scholar]
- 75.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 76.Kang EM, Zickler PP, Burns S, Langemeijer SM, Brenner S, Phang OA, Patterson N, Harlan D, Tisdale JF. Hematopoietic stem cell transplantation prevents diabetes in NOD mice but does not contribute to significant islet cell regeneration once disease is established. Exp Hematol. 2005;33:699–705. doi: 10.1016/j.exphem.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 78.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gitelman SE, Haller MJ, Schatz D. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;302:624–625. doi: 10.1001/jama.2009.1098. [DOI] [PubMed] [Google Scholar]
- 80.Kutlu B, Burdick D, Baxter D, Rasschaert J, Flamez D, Eizirik DL, Welsh N, Goodman N, Hood L. Detailed transcriptome atlas of the pancreatic beta cell. BMC Med Genom. 2009;2:3. doi: 10.1186/1755-8794-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kutlu B, Kayali AG, Jung S, Parnaud G, Baxter D, Glusman G, Goodman N, Behie LA, Hayek A, Hood L. Meta analysis of gene expression in human pancreatic islets after in vitro expansion. Physiol Genom. 2009 doi: 10.1152/physiolgenomics.00063.2009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 82.Parekh VS, Joglekar MV, Hardikar AA. Differentiation of human umbilical cord blood-derived mononuclear cells to endocrine pancreatic lineage. Differentiation. 2009 doi: 10.1016/j.diff.2009.07.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 83.Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030–1037. doi: 10.2337/diabetes.53.4.1030. [DOI] [PubMed] [Google Scholar]
- 85.Moritoh Y, Yamato E, Yasui Y, Miyazaki S, Miyazaki J. Analysis of insulin-producing cells during in vitro differentiation from feeder-free embryonic stem cells. Diabetes. 2003;52:1163–1168. doi: 10.2337/diabetes.52.5.1163. [DOI] [PubMed] [Google Scholar]
- 86.Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265–274. doi: 10.1634/stemcells.22-3-265. [DOI] [PubMed] [Google Scholar]
- 87.Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K, Takahashi Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells. 2002;20:284–292. doi: 10.1634/stemcells.20-4-284. [DOI] [PubMed] [Google Scholar]
- 88.Sipione S, Eshpeter A, Lyon JG, Korbutt GS, Bleackley RC. Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia. 2004;47:499–508. doi: 10.1007/s00125-004-1349-z. [DOI] [PubMed] [Google Scholar]
- 89.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 90.Gershengorn MC, Geras-Raaka E, Hardikar AA, Raaka BM. Are better islet cell precursors generated by epithelial-to-mesenchymal transition? Cell Cycle. 2005;4:380–382. doi: 10.4161/cc.4.3.1538. [DOI] [PubMed] [Google Scholar]
- 91.Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, Toschi E, Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36:353–356. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- 92.Chong AS, Shen J, Tao J, Yin D, Kuznetsov A, Hara M, Philipson LH. Reversal of diabetes in non-obese diabetic mice without spleen cell-derived beta cell regeneration. Science. 2006;311:1774–1775. doi: 10.1126/science.1123510. [DOI] [PubMed] [Google Scholar]
- 93.Kodama S, Kuhtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- 94.Ende N, Chen R, Reddi AS. Transplantation of human umbilical cord blood cells improves glycemia and glomerular hypertrophy in type 2 diabetic mice. Biochem Biophys Res Commun. 2004;321:168–171. doi: 10.1016/j.bbrc.2004.06.121. [DOI] [PubMed] [Google Scholar]
- 95.Gao F, Wu DQ, Hu YH, Jin GX. Extracellular matrix gel is necessary for in vitro cultivation of insulin producing cells from human umbilical cord blood derived mesenchymal stem cells. Chin Med J (Engl) 2008;121:811–818. [PubMed] [Google Scholar]
- 96.Naruse K, Hamada Y, Nakashima E, Kato K, Mizubayashi R, Kamiya H, Yuzawa Y, Matsuo S, Murohara T, Matsubara T, Oiso Y, Nakamura J. Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes. 2005;54:1823–1828. doi: 10.2337/diabetes.54.6.1823. [DOI] [PubMed] [Google Scholar]
- 97.Lavon N, Yanuka O, Benvenisty N. The effect of overexpression of Pdx1 and Foxa2 on the differentiation of human embryonic stem cells into pancreatic cells. Stem Cells. 2006;24:1923–1930. doi: 10.1634/stemcells.2005-0397. [DOI] [PubMed] [Google Scholar]
- 98.Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:16105–16110. doi: 10.1073/pnas.252618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 100.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 101.Esni F, Stoffers DA, Takeuchi T, Leach SD. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121(1):15–25. doi: 10.1016/j.mod.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 102.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, TzukermanL M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 103.Tang DQ, Lu S, Sun YP, Rodrigues E, Chou W, Yang C, Cao LZ, Chang LJ, Yang LJ. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab Invest. 2006;86:83–93. doi: 10.1038/labinvest.3700368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 105.Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004;84:607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- 106.Zorina TD, Subbotin VM, Bertera S, Alexander AM, Haluszczak C, Gambrell B, Bottino R, Styche AJ, Trucco M. Recovery of the endogenous beta cell function in the NOD model of autoimmune diabetes. Stem Cells. 2003;21:377–388. doi: 10.1634/stemcells.21-4-377. [DOI] [PubMed] [Google Scholar]
- 107.Madec AM, Mallone R, Afonso G, Abou ME, Mesnier A, Eljaafari A, Thivolet C. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52:1391–1399. doi: 10.1007/s00125-009-1374-z. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Zhang R, Qiao H, Zhang H, Wang Y, Yuan H, Liu Q, Liu D, Chen L, Pei X. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol. 2007;211:36–44. doi: 10.1002/jcp.20897. [DOI] [PubMed] [Google Scholar]
- 109.Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes. 2004;53:616–623. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- 110.Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 112.Nagaya M, Katsuta H, Kaneto H, Bonner-Weir S, Weir GC. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrino. 2009;201:37–47. doi: 10.1677/JOE-08-0482. [DOI] [PubMed] [Google Scholar]
- 113.Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton's Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. doi: 10.1371/journal.pone.0001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Feldmann RE, Jr, Bieback K, Maurer MH, Kalenka A, Burgers HF, Gross B, Hunzinger C, Klüter H, Kuschinsky W, Eichler H. Stem cell proteomes: a profile of human mesenchymal stem cells derived from umbilical cord blood. Electrophoresis. 2005;26:2749–2758. doi: 10.1002/elps.200410406. [DOI] [PubMed] [Google Scholar]
- 115.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 116.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 117.Joglekar MV, Joglekar VM, Joglekar SV, Hardikar AA. Human fetal pancreatic insulin-producing cells proliferate in vitro. J Endocrinol. 2009;201:27–36. doi: 10.1677/JOE-08-0497. [DOI] [PubMed] [Google Scholar]
- 118.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 119.Russ HA, Ravassard P, Kerr-Conte J, Pattou F, Efrat S. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS One. 2009;4:e6417. doi: 10.1371/journal.pone.0006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brennand K, Huangfu D, Melton All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Al-Abdullah IH, Ayala T, Panigrahi D, Kumar AM, Kumar MS. Neogenesis of pancreatic endocrine cells in copper-deprived rat models. Pancreas. 2000;21:63–68. doi: 10.1097/00006676-200007000-00053. [DOI] [PubMed] [Google Scholar]
- 123.Taniguchi H, Yamato E, Tashiro F, Ikegami H, Ogihara T, Miyazaki J. beta-cell neogenesis induced by adenovirus-mediated gene delivery of transcription factor pdx-1 into mouse pancreas. Gene Ther. 2003;10:15–23. doi: 10.1038/sj.gt.3301846. [DOI] [PubMed] [Google Scholar]
- 124.Xu X, D'Hoker J, Stange G, Bonne S, De LN, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 125.Mato E, Lucas M, Petriz J, Gomis R, Novials A. Identification of a pancreatic stellate cell population with properties of progenitor cells: new role for stellate cells in the pancreas. Biochem J. 2009;421:181–191. doi: 10.1042/BJ20081466. [DOI] [PubMed] [Google Scholar]
- 126.Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science. 2008;322:1811–1815. doi: 10.1126/science.1160810. [DOI] [PubMed] [Google Scholar]
- 127.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 128.Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 129.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol. 2008;18:890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 132.Liu Z, Sall A, Yang D. MicroRNA: an Emerging Therapeutic Target and Intervention Tool. Int J Mol Sci. 2008;9:978–999. doi: 10.3390/ijms9060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular disease. J Cell Mol Med. 2009;13:778–789. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hennessy E, O'Driscoll L. Molecular medicine of microRNAs: structure, function and implications for diabetes. Expert Rev Mol Med. 2008;10:e24. doi: 10.1017/S1462399408000781. [DOI] [PubMed] [Google Scholar]
- 135.Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RH. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007;5:e203. doi: 10.1371/journal.pbio.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perron MP, Provost P. Protein interactions and complexes in human microRNA biogenesis and function. Front Biosci. 2008;13:2537–2547. doi: 10.2741/2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang V, Wu W. MicroRNA-based therapeutics for cancer. BioDrugs. 2009;23:15–23. doi: 10.2165/00063030-200923010-00002. [DOI] [PubMed] [Google Scholar]
- 138.Poy MN, Spranger M, Stoffel M. microRNAs and the regulation of glucose and lipid metabolism. Diabetes Obes Metab. 2007;2:67–73. doi: 10.1111/j.1463-1326.2007.00775.x. [DOI] [PubMed] [Google Scholar]
- 139.Tavintharan S, Chi LS, Fang SC, Arunmozhiarasi A, Jeyaseelan K. Riboregulators and metabolic disorders: getting closer towards understanding the pathogenesis of diabetes mellitus? Curr Mol Med. 2009;9:281–286. doi: 10.2174/156652409787847245. [DOI] [PubMed] [Google Scholar]
- 140.Correa-Medina M, Bravo-Egana V, Rosero S, Ricordi C, Edlund H, Diez J, Pastori RL. MicroRNA miR-7 is preferentially expressed in endocrine cells of the developing and adult human pancreas. Gene Expr Patterns. 2009;9:193–199. doi: 10.1016/j.gep.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 141.Joglekar MV, Parekh VS, Mehta S, Bhonde RR, Hardikar AA. MicroRNA profiling of developing and regenerating pancreas reveal post-transcriptional regulation of neurogenin3. Dev Biol. 2007;311:603–612. doi: 10.1016/j.ydbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 142.Joglekar MV, Joglekar VM, Hardikar AA. Expression of islet-specific microRNAs during human pancreatic development. Gene Expr Patterns. 2009;9:109–113. doi: 10.1016/j.gep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 143.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 144.Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci USA. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, Rutter GA, Van Obberghen E. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J Biol Chem. 2007;282:19575–19588. doi: 10.1074/jbc.M611841200. [DOI] [PubMed] [Google Scholar]
- 146.Li Y, Song YH, Li F, Yang T, Lu YW, Geng YJ. MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem Biophys Res Commun. 2009;381:81–83. doi: 10.1016/j.bbrc.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Laustsen PG, Michael MD, Crute BE, Cohen SE, Ueki K, Kulkarni RN, Keller SR, Lienhard GE, Kahn Lipoatrophic diabetes in Irs1(−/−)/Irs3(−/−) double knockout mice. Genes Dev. 2002;16:3213–3222. doi: 10.1101/gad.1034802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, deAngelis T, Baserga R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem. 2007;282:32582–32590. doi: 10.1074/jbc.M702806200. [DOI] [PubMed] [Google Scholar]
- 149.Tang X, Muniappan L, Tang G, Ozcan S. Identification of glucose-regulated miRNAs from pancreatic {beta} cells reveals a role for miR-30d in insulin transcription. RNA. 2009;15:287–293. doi: 10.1261/rna.1211209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 151.Chaudhari M, Cornelius JG, Schatz D, Peck AB, Ramiya VK. Pancreatic stem cells: a therapeutic agent that may offer the best approach for curing type 1 diabetes. Pediatr Diabetes. 2001;2:195–202. doi: 10.1034/j.1399-5448.2001.20410.x. [DOI] [PubMed] [Google Scholar]
- 152.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, Shapiro AM. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 153.Sun Y, Chen L, Hou XG, Hou WK, Dong JJ, Sun L, Tang KX, Wang B, Song J, Li H, Wang KX. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl) 2007;120:771–776. [PubMed] [Google Scholar]
- 154.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 155.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis MA, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai k, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 156.Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J, McNiece IK. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 157.Hou SX, Singh SR. Germline stem cells. Methods Mol Biol. 2008;450 doi: 10.1007/978-1-60327-214-8. [DOI] [PubMed] [Google Scholar]
- 158.Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell stem cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh SR, Hou SX. Multipotent stem cells in the Malpighian tubules of adult Drosophila melanogaster. J Exp Biol. 2009;212:413–423. doi: 10.1242/jeb.024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Singh SR, Hou SX. Lessons learned about adult kidney stem cells from the malpighian tubules of Drosophila. J Am Soc Nephrol. 2008;19:660–666. doi: 10.1681/ASN.2007121307. [DOI] [PubMed] [Google Scholar]
- 161.Scheres B. Stem-cell niches: nursery rhymes across kingdoms. Nature Reviews Molecular Cell Biology. 2007;8:345–354. doi: 10.1038/nrm2164. [DOI] [PubMed] [Google Scholar]
- 162.Fuchs E. Finding one's niche in the skin. Cell Stem Cell. 2009;2009(4):499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yamashita YM. Regulation of asymmetric stem cell division: spindle orientation and the centrosome. Front Biosci. 2009;14:3003–3011. doi: 10.2741/3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Meirelles Lda S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009;14:4281–4298. doi: 10.2741/3528. [DOI] [PubMed] [Google Scholar]
- 165.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bauters C, Lamblin N, Mc Fadden EP, Van Belle E, Millaire A, de Groote P. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol. 2003;2:1. doi: 10.1186/1475-2840-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ. Coronary artery disease in IDDM. Gender differences in risk factors but not risk. Arterioscler Thromb Vasc Biol. 1996;16:720–726. doi: 10.1161/01.atv.16.6.720. [DOI] [PubMed] [Google Scholar]
- 168.Aneja A, Tang WH, Bansilal S, et al. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–757. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 169.Solang L, Malmberg K, Ryden L. Diabetes mellitus and congestive heart failure. Further knowledge needed. Eur Heart J. 1999;20:789–795. doi: 10.1053/euhj.1998.1472. [DOI] [PubMed] [Google Scholar]
- 170.El-Helou V, Beguin PC, Assimakopoulos J, Clement R, Gosselin H, Brugada R, Aumont A, Biernaskie J, Villeneuve L, Leung TK, Fernandes KJ, Calderone A. The rat heart contains a neural stem cell population; role in sympathetic sprouting and angiogenesis. J Mol Cell Cardiol. 2008;45:694–702. doi: 10.1016/j.yjmcc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 171.El-Helou V, Proulx C, Gosselin H, Clement R, Mimee A, Villeneuve L, Calderone A. Dexamethasone treatment of post-MI rats attenuates sympathetic innervation of the infarct region. J Appl Physiol. 2008;104:150–156. doi: 10.1152/japplphysiol.00663.2007. [DOI] [PubMed] [Google Scholar]
- 172.El-Helou V, Proulx C, Béguin P. The cardiac neural stem cell phenotype is compromised in streptozotocin-induced diabetic cardiomyopathy. J Cell Physiol. 2009;220:440–449. doi: 10.1002/jcp.21785. [DOI] [PubMed] [Google Scholar]
- 173.Zhang N, Li J, Luo R, Jiang J, Wang JA. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp Clin Endocrinol Diabetes. 2008;116:104–111. doi: 10.1055/s-2007-985154. [DOI] [PubMed] [Google Scholar]
- 174.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Lüscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]