Abstract
Human blood eosinophils exposed ex vivo to hematopoietic cytokines (e.g., IL-5 or GM-CSF) subsequently display enhanced responsiveness to numerous chemoattractants, such as chemokines, PAF or FMLP, through a process known as priming. Airway eosinophils, obtained by bronchoalveolar lavage after segmental antigen challenge, also exhibit enhanced responsiveness to selected chemoattractants, suggesting that they are primed during cell trafficking from the blood to the airway. Earlier work has shown that chemoattractants stimulate greater activation of ERK1 and ERK2 following IL-5-priming in vitro, thus revealing that ERK1/ERK2 activity can be a molecular readout of priming under these circumstances. Because few studies have examined the intracellular mechanisms regulating priming as it relates to human airway eosinophils, we evaluated the responsiveness of blood and airway eosinophils to chemoattractants (FMLP, PAF, CCL11, CCL5, CXCL8) with respect to degranulation, adherence to fibronectin, or Ras-ERK signaling cascade activation. When compared to blood eosinophils, airway eosinophils exhibited greater FMLP-stimulated EDN release as well as augmented FMLP- and CCL11-stimulated adherence to fibronectin. In airway eosinophils, FMLP, CCL11 and CCL5 stimulated greater activation of Ras or ERK1/ERK2 when compared to baseline. Ras activation by FMLP in blood eosinophils was also enhanced following IL-5-priming. These studies are consistent with a model of in vivo priming of eosinophils by IL-5 or related cytokines following allergen challenge, and further demonstrate the key role of priming in the chemoattractant-stimulated responses of eosinophils. The data also demonstrate the importance of the Ras-ERK signaling pathway to the regulation of eosinophil responses to chemoattractants in the airway.
Keywords: Eosinophils, Allergy, Lung, Signal Transduction
INTRODUCTION
Allergic asthma is generally described as an inflammatory disease of the airways and is characterized by increased numbers of eosinophils in the affected tissues. Considerable evidence supports the view that eosinophils are key effector cells in asthma (reviewed in(1)). Eosinophils acquired from the airway of human allergic or allergic asthmatic subjects 48 hours after segmental bronchoprovocation with antigen (SBP-Ag3) display a phenotype that is altered in several respects relative to their counterparts in the peripheral blood. Among the phenotypic changes observed in airway eosinophils are 1) altered expression of several membrane receptors and cell surface epitopes (2-6), 2) increased capacity to produce superoxide anion and to adhere to endothelial cells when stimulated with the chemoattractant FMLP (2, 7) and 3) the increased expression of genes encoding proteins related to cell survival or responsiveness to the inflammatory microenvironment (4, 6). Although airway eosinophils display an enhanced capacity to respond to several ligands relevant to allergic inflammation, compared to blood eosinophils, the mechanisms regulating this change in phenotype are poorly understood and are one of the aims of the current study.
Human eosinophils may encounter a variety of ligands of chemoattractant receptors in the asthmatic airway, such as chemokines (8), lipid-derived mediators (9, 10) and ligands of formyl peptide receptors (11, 12). As a consequence of in vitro exposure to chemoattractants, eosinophils display altered adherence to matrix proteins and cells (2, 7, 13, 14), undergo directed migration (15, 16), release pre-formed enzymes and cytotoxic proteins (17, 18), synthesize reactive oxygen species (2, 19), elaborate arachadonic acid metabolites (20, 21) and release cytokines and chemokines (22-24). Therefore, numerous studies have documented that stimulation of eosinophil chemoattractant receptors can have profound effects on the accumulation of eosinophils in the airway and their cytotoxic effector functions in the inflammatory milieu.
In leukocytes and other mammalian cells, a variety of heterotrimeric-G-protein-coupled receptors mediate responsiveness to chemoattractants. In addition, the responsiveness to chemoattractants can be further modulated by other factors present in the inflammatory microenvironment. In particular, IL-5 and related cytokines augment eosinophil responsiveness to chemoattractants via a process referred to as “priming”. Previous studies have shown the importance of this process to eosinophil recruitment, accumulation in tissues and activation (7, 21, 25-30). Certain aspects of priming are seen within minutes of IL-5 exposure, suggesting that non-transcriptional processes can participate in priming, and the phenotypic characteristics of priming may persist for many hours after the cytokine is removed. For example, IL-5 priming of human blood eosinophils for 5 to 90 min enhances FMLP-stimulated leukotriene C4 (LTC4) generation (21, 29), as well as platelet activating factor- (PAF-) induced Ca++ fluxes (31), β2 integrin activation (16, 32) and chemotaxis to FMLP and CCL5 (30). Collectively these data suggest the existence of mechanisms that rapidly and persistently integrate the activities of the IL-5 receptor and the G protein-coupled chemoattractant receptors, resulting in enhanced cytotoxic effector functions, migration and inflammatory capacity.
The present study is unique in that we have utilized human airway eosinophils, acquired from the bronchoalveolar lavage (BAL) fluid 48 hours after SBP-Ag, to test the hypothesis that these cells display the enhanced responsiveness characteristic of priming without the requiring exposure to IL-5 or a related cytokine. To address this goal, we evaluated blood and airway eosinophils from the same donor for the ability to adhere to fibronectin-coated plates and to release eosinophil derived neurotoxin (EDN) after exposure to chemoattractants. In addition, we have elaborated upon our earlier studies of signaling events associated with priming (21) and observed increased activation of Ras and ERK1/ERK2 following stimulation of airway eosinophils with the chemoattractants FMLP, CCL5 and CCL11. These findings suggest that, during migration of eosinophils from the blood to the airway, stable phenotypic changes may occur. This in vivo priming eliminates the need for additional stimulation by IL-5 and related cytokines before the cells can respond vigorously to chemoattractants. Furthermore, intracellular mechanisms enhancing the activation of the Ras-ERK signaling cascades may contribute to the enhanced inflammatory capacity of airway eosinophils.
MATERIALS AND METHODS
Reagents
Anti-CD16-conjugated paramagnetic microbeads and the Automacs System were obtained from Miltenyi Biotechnology (Auburn, CA). Chemiluminescence substrate reagents were obtained from Kirkegaard and Perry Laboratories (Gaithersburg, MD) and Pierce Biotechnology (Rockford, IL). Immunoblotting antibodies included anti-Ras, anti ERK1/ERK2 (Upstate Biotechnology, Lake Placid, NY), and anti phospho-ERK1 (Invitrogen-Biosource, Carlsbad California). Antibodies used for flow cytometry included PE-conjugated monoclonal antibodies to the human formyl peptide receptor FPR1, ERK2, dually phosphorylated ERK1/ERK2, and relevant isotype controls (Becton Dickenson, San Jose, CA). Anti-human FPR2 (a rabbit polyclonal antibody) was a generous gift from Arena Pharmaceuticals. We acquired IL-5, CCL11, CCL5 and CXCL8 from R&D Systems (Minneapolis, MN) and GM-CSF from PeproTech (Rocky Hill, NJ). Human serum albumin, tissue fibronectin and the chemoattractants FMLP and PAF were obtained from Sigma (St. Louis MO).
Human Subjects
Subjects supplying blood for eosinophil studies ranged in age from 18 to 55 years and included non-allergic individuals, atopic subjects and individuals with physician-diagnosed allergic asthma. Subjects were not taking any medications other than short-acting β-agonists as needed. Airway eosinophils were also acquired from atopic or mild asthma subjects, following SBP-Ag and BAL, using protocols described below. Written, informed consent was obtained from all participants before inclusion in the study. All protocols used in the collection of human blood and airway eosinophils were approved by the University of Wisconsin Human Subjects Committee Internal Review Board.
Isolation of Human Eosinophils from the Peripheral Blood and BAL Fluid
Eosinophils were purified from heparinized peripheral blood of volunteer human donors as previously described (4). Briefly, blood was density fractionated over a 1.090 g/ml Percoll monolayer, the granulocyte-rich pellets were collected and the erythrocytes were lysed by hypotonic shock. The resulting granulocyte mixture was depleted of neutrophils by incubation with anti-CD16-conjugated paramagnetic microbeads and passage through an Automacs column. Airway eosinophils were obtained from BAL fluid 48h following SBP-Ag of atopic human subjects with relevant antigen. Antigen dose for SBP was defined, and SBP-Ag and BAL were performed as described previously (3). Airway eosinophils were purified by centrifugation of BAL cells through a Percoll bilayer (1.085/1.100 g/ml) and recovery of the eosinophil population at the interface between the Percoll layers. The recovered blood and airway eosinophils were resuspended in Hanks’ Balanced Salt Solution (HBSS) supplemented with 2% newborn calf serum. Eosinophil preparations were at least 96% eosinophils and greater than 95% viable, based on morphological examination of cytofuge preparations stained with Giemsa’s-based Diff-Quik stain (Baxter Scientific Products, McGaw Park, IL) and trypan blue exclusion.
Eosinophil Stimulation, Preparation of Lysates and Immunoblotting
Eosinophils were resuspended in HBSS + 0.1% human serum albumin at a cell density of 5 × 106/ml and incubated for 30 min at 37°C. The cell suspension was stimulated by addition of control buffer or chemoattractant. After stimulation in vitro, cells were pelleted by centrifugation, and lysed as previously described (21). Cell lysate proteins were resolved by SDS PAGE by loading lysate of equivalent numbers of eosinophils in each lane of the gel. Equal protein loading was assured by immunoblotting for control proteins (usually ERK1/ERK2) in the same samples. Images were captured using X-ray film or digital photography, scanned and quantified using densitometry and NIH Image software. Signal intensities from immunoblotting with anti-phospho-ERK1/2 antibodies were normalized to the signal from the corresponding loading control.
Detection of Active, GTP-bound Ras
Active Ras was affinity precipitated from eosinophil cell lysates as previously described (33). Briefly, following stimulation with FMLP, cells were lysed, vortexed and clarified by centrifugation to remove insoluble cellular debris. The clarified lysates were incubated with glutathione-conjugated agarose beads coupled to a GST-fusion protein carrying the Ras-binding domain of Raf-1. The beads were washed five times with lysis buffer and the captured (active) Ras was visualized by SDS-Page and immunoblotting with anti-Ras mab. Images were captured by X-ray film or digital photography and quantified using densitometry (NIH Image software).
Adherence to Fibronectin
Purified blood or airway eosinophils were incubated in fibronectin-coated tissue culture plates for 30 min following the addition of various chemoattractants. After vigorous washing to remove non-adherent cells, eosinophil adhesion was measured as residual eosinophil peroxidase activity (EPO) of the adherent cells (34).
Degranulation
Purified blood and airway eosinophils were cultured in HBSS + 0.03% gelatin with various chemoattractants in 24-well tissue culture plates for 4h at 37°C in 5% CO2 (3). The cell-free culture supernates were frozen at −80°C until assayed for EDN by radioimmunoassay (3). Total cellular EDN was measured for each cell type and data were expressed as % of total EDN.
Statistical Analysis
Data are presented in toto, or summarized as medians (25 and 75 quartiles) for data that are not normally distributed, or as mean (SEM) for normally distributed data, as indicated in the figure legends. A paired t test or Wilcoxon signed rank test was used to compare results between chemoattractant-stimulated and control-stimulated samples or between blood and BAL samples acquired from the same subject at the same time. In some cases, the data were log transformed prior to statistical analysis. A 2-tailed p value of < 0.05 was considered significant.
Results
Effect of Chemoattractants on EDN Release from Blood and Airway Eosinophils
Previous studies have demonstrated that chemoattractants stimulate the release of the granule protein EDN from eosinophils (17) and elevated levels of EDN is present in BAL fluid after allergen challenge (35, 36). However, EDN release by airway eosinophils exposed to chemoattractants has not been examined. To determine if chemoattractants have a similar effect on degranulation of airway eosinophils as blood eosinophils, EDN release was measured from eosinophils purified from blood and BAL fluid 48 h after SBP-Ag. The eosinophils from both compartments were purified and then stimulated with FMLP, PAF, CCL5 or CCL11 and the % of total EDN release was evaluated in the supernates (3). As can be seen in Fig. 1, FMLP and PAF stimulated release of EDN from both blood and airway eosinophils, that was significantly higher than the release stimulated by control buffer. However CCL5 and CCL11 did not promote significant EDN release above levels stimulated by control buffer alone. Interestingly, EDN release was significantly greater from airway eosinophils than from blood eosinophils following stimulation with control buffer and with FMLP, CCL11 and CCL5 but not PAF (Fig. 1). This increase did not appear to be the result of a greater mass of EDN protein in BAL cells because the two cell populations contained comparable cellular amounts of EDN. Total cellular EDN (mean (SEM)) of blood and airway eosinophils was 2540 (330) ng/106 cells and 2280 (271) ng/106 cells respectively, and the difference between the samples was not statistically significant. These data demonstrate that both basal and FMLP-stimulated EDN release is greater in airway eosinophils than in blood eosinophils, suggesting that airway eosinophils have an increased inflammatory capacity when stimulated with some, but not all, chemoattractants.
Figure 1. EDN release by purified blood and airway eosinophils.
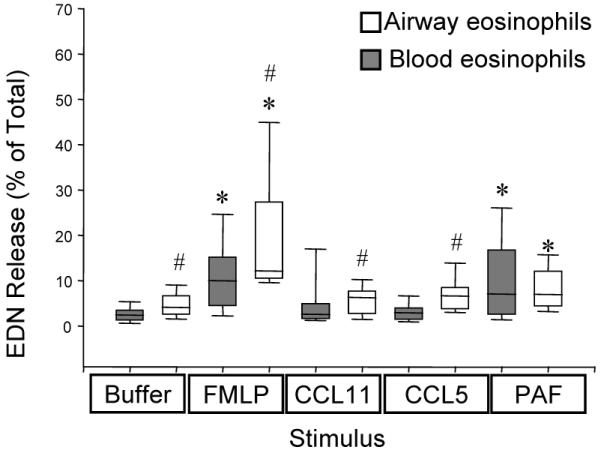
Release of EDN was measured 4 h. after in vitro exposure of blood (darker bars) and airway (white bars) eosinophils from the same subjects to buffer alone, 100 nM FMLP, 100 ng/ml CCL11, 100 ng/ml CCL5 or 100 nM PAF. Boxes represent medians with 25 and 75 % quartiles; whiskers are 10 and 90 percentiles. N = 15-21, *, p< 0.05 compared with spontaneous (buffer-stimulated) release; # p < 0.05 relative to blood cells. Total cellular EDN (mean (SEM)) of blood and airway eosinophils was 2543 (330) ng/106 cells and 2283 (271) ng/106 cells respectively and was not significantly different between the two populations.
Eosinophil Adherence to Fibronectin
Fibronectin, a component of the extracellular matrix, is increased in BAL fluid 48 h. following SBP-Ag (37). The adherence of blood eosinophils to fibronectin-coated wells is enhanced by stimulation with chemoattractants in IL-5- or GM-CSF-primed blood eosinophils and greater in eosinophils from atopic individuals (data not shown and (38, 39)). Furthermore, adherence to fibronectin, which is mediated by integrins, enhances eosinophil viability, increases degranulation and modulates the activity of several intracellular signaling molecules (40-44). Following in vitro stimulation with FMLP or CCL11, unprimed blood eosinophils showed no significant increase in adhesion to fibronectin (Fig. 2A). In contrast, airway eosinophils were significantly more adherent, both at baseline and following stimulation with chemoattractants, than matched blood eosinophils isolated 48h post SBP-Ag. (Fig. 2A, p< 0.05, N= 5).
Figure 2. Adherence of purified blood or airway eosinophils to fibronectin.
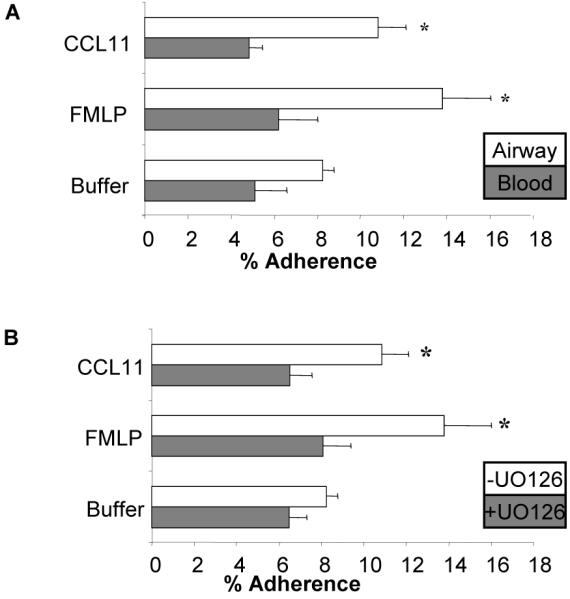
Blood or airway eosinophils from the same subjects were incubated for 30 min. in tissue culture wells pre-coated with 10 μg/ml tissue fibronectin in the presence of control buffer, 100 nM FMLP or 100 ng/ml CCL11. Data are summarized as the mean (SEM). A. Relative adherence of blood eosinophils (dark bars) or airway eosinophils (white bars) N = 5, * p<0.005 vs. blood cells. B Purified airway eosinophils were preincubated with 0 μM (white bars) or 10 μM (dark bars) of the MEK antagonist UO126 for 30 minutes and subsequently assessed for adherence to fibronectin as described above. * p < 0.02, 0 μM UO126 vs..10 μM UO126, N = 5.
Because previous studies demonstrated that integrin-mediated eosinophil adherence stimulated by chemoattractants was sensitive to inhibition of the MEK/ERK signaling cascade (40, 45), we assessed the effect of the MEK1 and MEK2 antagonist, UO126 (10 μM), on FMLP- and CCL11-stimulated adherence of airway eosinophils to fibronectin. This concentration of UO126 effectively inhibits FMLP-stimulated ERK1/ERK2 activation in blood and airway eosinophils ((21) and data not shown). As can be seen in Fig 2B, UO126 significantly inhibited the stimulus-induced adherence of airway eosinophils to fibronectin coated wells to levels comparable to unstimulated airway eosinophils. These data suggest that the MEK/ERK signaling cascade is an intracellular regulator of chemoattractant-stimulated adherence to fibronectin in airway eosinophils.
Expression of Receptors for Chemoattractant Ligands on Blood and Airway Eosinophils
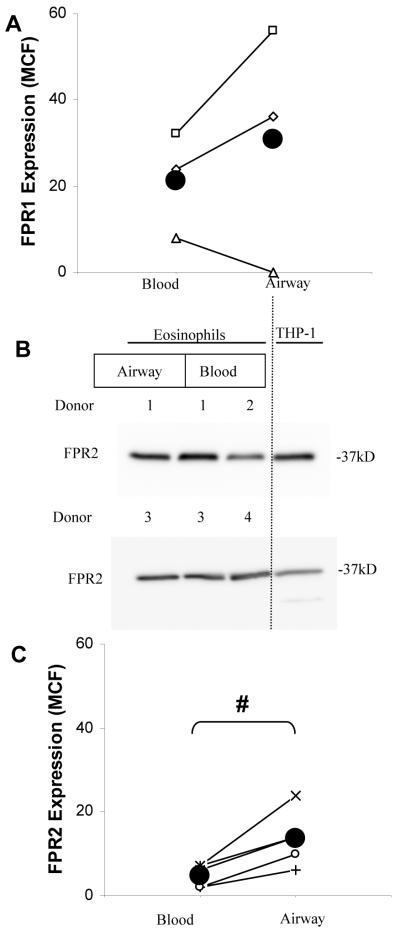
One mechanism that may contribute to the enhanced responsiveness of airway eosinophils to chemoattractants is the modulation of cell surface receptor expression. Therefore, we evaluated whether airway eosinophils exhibit greater expression of cell surface receptors for ligands, such as CCL11 and FMLP, which have been shown in previous studies (7, 35) or in this report (Fig. 1 and Fig. 2A) to stimulate the differential responses of eosinophils obtained from the peripheral and airway compartments. Given that CCL11 binds predominantly to CCR3 in eosinophils (46), it is noteworthy that the cell surface expression of CCR3 and numerous other chemokine receptors on human blood and airway eosinophils have been found to be significantly lower on airway eosinophils relative to blood eosinophils (47, 48). However, the expression of receptors binding FMLP on airway eosinophils has not been previously reported. In leukocytes, FMLP is believed to act primarily through two receptors, FPR1 and FPR2 (49). Our previous work has revealed that, in blood eosinophils, the mRNA for these two receptors is expressed at very low levels (see online supplement in (4)). We examined the expression of FPR1 protein on blood and airway eosinophils of three patients by flow cytometry and observed very low cell surface expression relative to blood neutrophils from the same subjects. In addition, FPR1 expression was not significantly different between blood and airway eosinophils (Fig. 3A, p NS, n =3). A polyclonal antiserum to FPR2, which immunodetects a protein of expected size in blood and airway eosinophils and THP-1 monocytes by immunoblot (Fig 3B), was used to evaluate cell surface FPR2 protein by flow cytometry. FPR2 was abundantly detected on the surface of THP-1 monocytes (not shown) but was expressed at low levels on blood eosinophils, and the surface expression was slightly but significantly greater on airway eosinophils than in the blood eosinophils from the same patients (Fig 3C, p < 0.02, n = 5). Taken together, these data suggest that the enhanced responsiveness of airway eosinophils to FMLP may result, at least in part, from marginally greater cell surface expression of FPR2.
Figure 3. Expression of FPR receptors on blood and airway eosinophils.
A. Expression of cell surface receptors for FPR1 was evaluated by flow cytometry, summarized as the MCF with labeling by isotype control antibody subtracted. Values are plotted for 3 separate donors and, as an aid to visualization, the blood and airway eosinophil expression levels for each subject are connected with a line. The mean values for each cell type are shown as a large filled circle. p = NS. B. FPR2 was evaluated in lysates of human blood and airway eosinophils for 2 separate donors, from blood eosinophils from 2 additional donors and in THP-1 monocytes as a positive control. Lysate representing equal numbers of each eosinophil preparation was loaded into gel lanes. C. Expression of FPR2 was evaluated by flow cytometry using the polyclonal rabbit anti-human FPR2 and PE-conjugated anti-Rabbit IgG, summarized as the MCF and the MCF labeling by control antisera was subtracted. Values are plotted for 5 separate donors and, as an aid to visualization, the blood and airway eosinophil expression levels for each subject are connected with a line. The mean values for each cell type are plotted with a large filled circle. (# p < 0.02 blood vs. airway eosinophils)
Effect of Chemoattractants on ERK1/ERK2 Activation
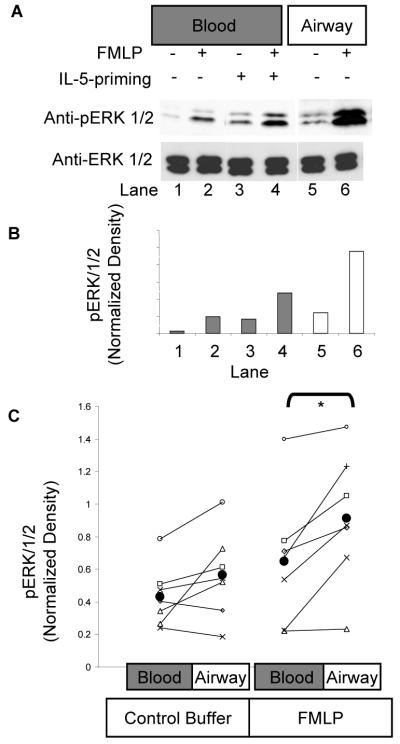
Because chemoattractant-stimulated adherence to fibronectin (Fig. 2B and (40, 45)) and degranulation (18) can be attenuated by inhibition of MEKs 1 and 2, the upstream activators of ERK1/ERK2, we examined the effect of various chemoattractants on ERK1/ERK2 phosphorylation in blood and airway eosinophils. Previous studies from our laboratory had revealed that priming of blood eosinophils with IL-5 enhanced the capacity of FMLP to stimulate ERK1 and ERK2 activity in blood eosinophils (21). Therefore, we hypothesized that airway eosinophils would respond to FMLP in a similar manner to in vitro-primed blood eosinophils and demonstrate increased activity of ERK1/ERK2. Purified blood eosinophils were primed with control buffer or 1 nM IL-5 for 1 h. Subsequently, airway eosinophils as well as unprimed and primed blood eosinophils were stimulated with control buffer or 100 nM FMLP. As can be seen in Fig. 4A, low levels of immunodetectable phospho-ERK1/ERK2 are present in unprimed blood eosinophils stimulated by FMLP (Fig. 4A, lane 2 vs. lane 1). IL-5 priming enhances the phosphorylation of ERK1/ERK2 stimulated by FMLP (Fig. 4A, lane 4 vs. lane 2) as described previously (21). By comparison, FMLP was a powerful activator of ERK1/ERK2 in airway eosinophils (Fig. 4A, lane 6 vs. lane 5) even in the absence of prior in vitro exposure to IL-5. These data demonstrate that, although the three cell populations have similar mass of total ERK1/ERK2 protein (Fig 4A, lower immunoblot panel), airway eosinophils have a greater capacity to activate ERK1/ERK2 in response to FMLP than either unprimed or IL-5-primed blood eosinophils. The immunoblot density of the images in Fig 4A was determined, expressed as a ratio of pERK density to ERK1/ERK2 density and plotted in Fig. 4B. The densitometric quantification shows greater pERK1/2 labeling in both the medium-treated and FMLP-treated lysates of airway eosinophils relative to blood eosinophils (Fig. 4B). When the FMLP-stimulated activation of ERK1/ERK2 was examined in blood and airway eosinophils of multiple subjects 48 h. following SBP-Ag, and summarized by densitometry in a similar manner as for Fig. 4A/4B, airway eosinophils showed significantly higher ERK1/2 phosphorylation in response to 100 nM FMLP than their blood derived counterparts (Fig. 4C, N=7).
Figure 4. ERK1/2 phosphorylation in blood and airway eosinophils stimulated with FMLP.
Purified blood eosinophils were stimulated for 2 min with control buffer or 100 nM FMLP and cell lysates were prepared. SDS PAGE gels were loaded with protein equivalent to 106 cells per lane and immunoblotted with antiserum raised against the dually-phosphorylated activation motif of ERK1 (pERK1/2) as well as total ERK1/2. A. Blood eosinophils were primed for 1 h. with 0 nM (lanes 1, 2) or 1 nM (lanes 3, 4) IL-5 and then the blood eosinophils and airway eosinophils (lanes 5,6) were stimulated for 2 min with 0 nM (lanes 1,3,5) or 100 nM (lanes 2,4,6) FMLP. Lysates were immunoblotted for anti-phospho-ERK1/2 (upper panel) and anti ERK1/2 (lower panel). B. The phosphorylation of ERK1/ERK2 seen in the representative experiment of panel A. above was quantified by densitometry and the data normalized to the loading control (ERK1/ERK2). Relative densities for blood eosinophils are plotted with dark bars and for airway eosinophils with light bars. C. ERK1/ERK2 phosphorylation stimulated with FMLP or control buffer was evaluated on both blood and airway eosinophils of 7 subjects, quantified as the normalized density, in the same way as panel 3B above, and plotted with a different symbol for each subject. As an aid to visualization, the blood and airway eosinophil densitometry values for each subject are connected with a line. The population mean for each cell/treatment group is shown as a larger filled circle. * p<0.05 for blood vs. airway eosinophils following FMLP stimulation.
Because different methods of cell purification were used to isolate blood and airway eosinophils, we assessed if the robust responsiveness of airway eosinophils can be attributed to the technique of purification via Percoll density gradient centrifugation. To address this issue, we conducted an analysis of ERK1/ERK2 phosphorylation by intracellular FACS following in vitro stimulation of the cells with FMLP (see Supplemental Information, Supplemental Figs. 1-3). This technique permits the evaluation of eosinophil ERK1/ERK2 phosphorylation in mixed populations of cells by allowing for the identification of eosinophils based on their size, light scattering properties and green autofluorescence. We observed that the robust activation of ERK1/ERK2 was seen in airway eosinophils following stimulation with FMLP, even without prior purification of the airway cells by centrifugation through a Percoll bilayer (N = 3, Supplemental Fig. 2). This robust activation was markedly greater that that seen in blood eosinophils purified by immunomagnetic negative selection as outlined under Materials and Methods. Furthermore, when blood eosinophils were stimulated by FMLP and assessed for ERK1/ERK2 phosphorylation in a mixed population of leukocytes obtained after centrifugation through a Percoll bilayer (the technique routinely used to purify airway eosinophils), their activation was not significantly different from eosinophils isolated from the same blood donor using immunomagnetic negative selection (Supplemental Fig. 3). Taken together, these findings confirm that the enhanced ERK1/2 activation detected in airway eosinophils following SBP-Ag is an intrinsic property of the cells from the airway compartment and not a feature of the different isolation methods used to acquire the purified populations of cells.
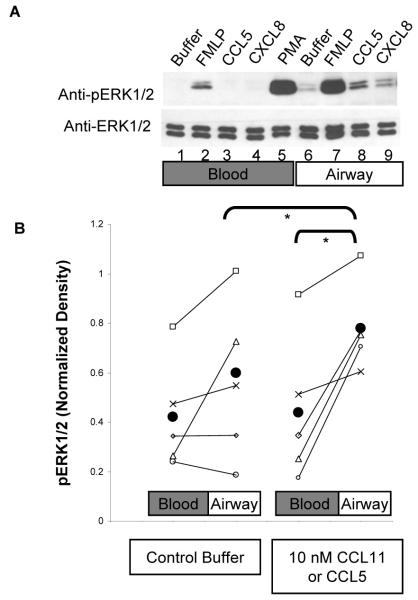
We next determined if this potent activation of ERK1 and ERK2 was a general feature of airway eosinophil responsiveness to chemoattractants, as was demonstrated for blood eosinophils following in vitro priming with IL-5 (21). In that study, IL-5-primed blood eosinophils displayed greater ERK activation when stimulated with FMLP, CCL5 or CXCL8 than did unprimed blood eosinophils. Therefore, blood and airway eosinophils from the same patient 48 h after SBP-Ag were stimulated for 2 min. with various agonists of chemoattractant receptors, as well as PMA, a potent stimulus of ERK1/ERK2 phosphorylation in eosinophils (50). PMA stimulated marked and approximately equivalent activation in blood and airway eosinophils (p is NS, N = 12). In this experiment, the chemoattractants FMLP, CCL5 and CXCL8 stimulated greater ERK activation in airway eosinophils than in blood eosinophils (Fig. 5A, upper immunoblot panel) although the mass of ERK protein was similar in all samples (Fig. 5A, lower immunoblot panel). Identical experiments using blood and airway eosinophils from multiple subjects stimulated with FMLP, CXCL8, CCL5 or CCL11 revealed that ERK activation in airway eosinophils was equal to or greater than that in blood eosinophils in the majority of subjects and with most stimuli, although there was some heterogeneity in the responses, both in control-stimulated and chemoattractant stimulated eosinophils. It is noteworthy that ligands that bind CCR3, namely CCL11 and CCL5 stimulate significantly greater ERK1/ERK2 phosphorylation in airway than in blood eosinophils (Fig. 5B, p < 0.05, N =5). An identical conclusion was reached based on 4 additional experiments where, due to the limited availability of post SBP-Ag blood cells, blood eosinophils from an unrelated donor, who did not undergo SBP-Ag, were compared to BAL eosinophils from the antigen-challenged subjects (data not shown).
Figure 5. ERK1/2 phosphorylation in blood and airway eosinophils stimulated by additional chemoattractants.
A. Purified blood eosinophils (lanes 1-5) and airway eosinophils (lanes 6-9) were stimulated for 2 min. with buffer control (lanes 1, 6), 100 nM FMLP (lanes 2, 7), 10 nM CCL5 (lanes 3, 8), 10 ng/ml CXCL8 (lanes 4, 9) or 50 nM PMA (lane 5). The cell lysates were immunoblotted for pERK1/2 (upper panel) and ERK1/2 (lower panel) to validate the presence of equal mass of ERK1/ERK2 protein in the various cell preparations. B ERK1/2 phosphorylation was evaluated on both blood and airway eosinophils from 5 subjects following stimulation for 2 min. with control buffer, 10 nM CCL5 or 10 nM CCL11. ERK1/ERK2 phosphorylation was quantified by densitometry and normalized by the densitometry of the corresponding ERK1/2 immunoblot. Each subject’s data is plotted with a different symbol and the values for the blood and airway eosinophils are connected by a line as an aid to visualization. The population mean for each cell/treatment category is shown as a solid black circle. Data were log transformed prior to statistical analysis to compensate for lack of normalcy in the distribution. * p<0.05
Ras Activation in Blood and Airway Eosinophils Following FMLP Stimulation
To evaluate a potential mechanism of the priming described above, we examined the ability of IL-5 priming to affect the activation of the small molecular weight G-protein Ras, given that Ras is an upstream activator of the ERK kinase cascade in many cellular contexts including FMLP-(45) and IL-5-stimulated blood eosinophils (33, 45, 50). Blood eosinophils were primed for 1 h. with 0 pM or 100 pM IL-5 and subsequently stimulated with FMLP (100 nM for 2 or 5 min.). Active, GTP-bound Ras was affinity precipitated from the cell lysates by incubation with the Ras-binding domain of Raf-1 coupled to agarose, as previously described (33). Although both primed and unprimed blood eosinophils contained a small amount of GTP-Ras in the absence of FMLP stimulation, the mass of active Ras was increased by FMLP stimulation (p<0.05) and enhanced by IL-5 priming, as pictured in Fig. 6A and quantified in Fig. 6B, (p<0.004, N = 5, Fig. 6B). Furthermore, airway eosinophils stimulated with FMLP showed greater Ras activation than did blood eosinophils acquired at the same time in all of the 5 subjects tested (Figs. 7A and 7B, N=5). These data demonstrate that the increased activation of ERK1 and ERK2 seen in airway eosinophils and in blood eosinophils following IL-5-priming is accompanied by increased accumulation of GTP-bound Ras. These findings, therefore, support a model of airway eosinophil function that encompasses in vivo priming of eosinophil responses to chemoattractants, possibly because of exposure to IL-5 or related cytokines during migration to, or residence in, the airway.
Figure 6. The effect of IL-5-priming on FMLP-stimulated Ras activity in blood eosinophils.
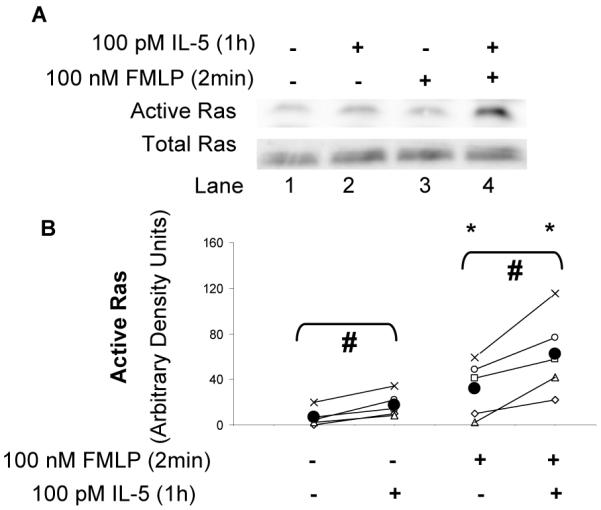
A. Blood eosinophils were primed for 1h with 0 pM or 10 pM IL-5 and subsequently stimulated for 2 or 5 min with 0 nM or 100 nM FMLP. Cell lysates were assessed for Ras activity by capturing the active Ras from the cell lysates with agarose beads coupled to the Ras-binding domain of Raf1. Active Ras was subsequently visualized by immunoblotting with anti-Ras (see (33) and Material and Methods). Equivalent protein content of the cell lysates is demonstrated by an anti-Ras immunoblot of the corresponding cell lysates (lower immunoblot panel) B. Ras activity following in vitro priming with 0 or 10 pM IL-5 was evaluated in the blood eosinophils of 5 separate subjects and quantified by densitometry analysis. Each subject’s data is plotted with a different symbol and the values for the primed and unprimed eosinophils are connected by a line as an aid to visualization. The population mean for each cell/treatment category is shown as a solid black circle.* p < 0.05 0 nM FMLP vs. 100 nM FMLP; # p < 0.02 unprimed vs. primed.
Figure 7. FMLP-stimulated Ras activity in blood and airway eosinopils.
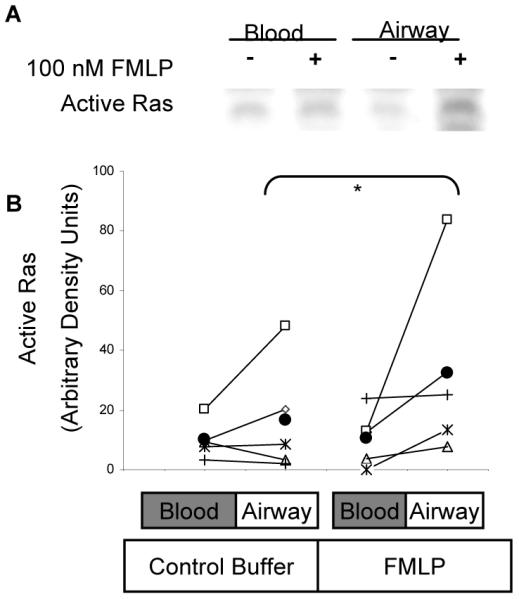
A. Purified blood eosinophils and airway eosinophils from the same subjects were stimulated for 2 min with 0 nM or 100 nM FMLP. Active Ras was captured from the cell lysates and visualized by immunblotting as described for Fig. 5, above. B. Ras activity was assessed in blood and airway eosinophils from 5 subjects and quantified by densitometry of the Ras immunoblots. Each subject’s data are plotted with a different symbol and the values for the blood and BAL eosinophils are connected by a line as an aid to visualization. The population mean for each cell/treatment category is shown as a solid black circle.* p < 0.05, 100 nM FMLP vs. 0 nM FMLP (data were transformed to square root values prior to statistical analysis).
Discussion
Previous studies have shown that airway eosinophils respond to chemoattractants with greater adherence, migration and superoxide anion generation than do blood eosinophils (2, 7, 51). This process is recapitulated in many respects by treating blood eosinophils with IL-5 or related cytokines prior to exposure to chemoattractants, a process known as priming (7). Given the prominent occurrence of chemoattractants in the airway lumen following antigen challenge, the current study was initiated to further examine important biological responses of airway and blood eosinophils to chemoattractants, including degranulation and adherence to fibronectin (Figs. 1 and 2). In addition, because of our previous work indicating that eosinophil priming with IL-5 and related cytokines is associated with enhanced activity of the Ras/ERK signaling cascade ((21) and data not shown), we tested the hypothesis that the enhanced responsiveness to chemoattractants seen in airway eosinophils following SBP-Ag is associated with increased activity of the ERK1/ERK2 signal transduction cascade following chemoattractant exposure. We report here that multiple chemoattractants activate ERK1 and ERK2 in airway eosinophils to a greater extent than blood eosinophils and in a manner similar to that seen in IL-5-primed blood eosinophils (Fig. 4 and 5). Furthermore, the enhanced activation of the ERK signaling cascade observed in airway eosinophils was associated with a parallel increase in Ras activation (Fig. 7A and 7B), also a feature of IL-5-primed blood eosinophils (Fig. 6A and 6B). This report therefore suggests that airway eosinophils are primed in vivo and that this process is an important mechanism regulating eosinophil responsiveness to chemoattractants in the airway. Furthermore, this study extends our previous observations (21) suggesting that, as with in vitro-primed eosinophils, the greater activation of the Ras-ERK signaling cascade may be an intracellular marker of priming.
Release of cytotoxic granule proteins is an important mechanism through which eosinophils contribute to innate immunological defense and may contribute to the pathobiology of airway diseases. Previous studies have shown that the release of EDN by blood eosinophils is stimulated by IL-5 and related cytokines but that chemokines such as CCL5 and CXCL8 did not cause the elaboration of significant amounts of EDN or superoxide anion (52). However, some studies suggest that priming with IL-5 at very low concentrations promoted significant release of EDN following stimulation with CCL11, but only after prior exposure of the cells to cytochalasin B (17). To our knowledge, no previous reports have examined granule protein release in airway eosinophils stimulated by chemoattractants. The finding, that airway eosinophils release greater amounts of the granule protein EDN constitutively and following stimulation with FMLP (Fig. 1), is consistent with the model of in vivo priming of FMLP-stimulated responses and our previous studies showing that airway eosinophils produce markedly greater amounts of superoxide anion than do blood eosinophils following exposure to FMLP (2, 7). As seen in Fig. 1, PAF also stimulated EDN release from both blood and airway eosinophils but in approximately equal amounts, and in this respect EDN release was also reflective of the behavior of airway eosinophils with respect to superoxide generation. PAF did not stimulate greater superoxide release from airway eosinophils than from blood eosinophils although there was some heterogeneity in the responses (2). It is notable therefore, that airway eosinophils are not more responsive to all stimuli since another potent activator of blood eosinophil EDN release, namely IL-5, was less robust in promoting EDN release from airway eosinophils than from blood eosinophils (3). With respect to the chemokines CCL5 and CCL11, however, we did not observe EDN release above that stimulated by buffer alone from either blood or airway eosinophils demonstrating that following eosinophil transit into the airway, CCR3 ligands are not potent activators of EDN release as are PAF and FMLP.
In current paradigms of allergic airway inflammation, the recruitment and retention of eosinophils in the airway is an adherence-dependent process. This model predicts that with the coordinated action of chemoattractants, integrins and adherence molecules such as ICAM1 and VCAM, eosinophils migrate to inflammatory foci where adherence to physiological substrates such as fibronectin can prolong eosinophil viability and enhance superoxide production (4, 13, 16, 32, 34, 37, 44, 51, 53-55). The current finding that airway eosinophils show greater adherence to fibronectin than do blood eosinophils following stimulation with FMLP or CCL11 (Fig. 2A) is consistent with studies showing that in vitro priming of eosinophils with IL-5-family cytokines enhances adherence to fibronectin stimulated by CCL11 (data not shown and (38)) and that chemoattractant-stimulated adherence is a process dependent on the RAS/MEK/ERK signaling network (Fig. 2).
Earlier investigations have revealed that, even in the absence of in vitro stimulation, airway eosinophils specifically adhere to a wide variety of physiologically-relevant ligands, including VCAM, ICAM, albumin, vitronectin, fibrinogen and laminin. Blood eosinophils express at least seven integrin heterodimers, namely α4β1, α6β1, αLβ2, αMβ2, αXβ2, αDβ2 and α4β7 (56, 57). Of these receptors, adherence to tissue fibronectin appears to be primarily mediated by α4β1 (58, 59). When the cell surface expression of integrin epitopes was examined on blood and airway eosinophils following SBP-Ag, the α4 subunit was not significantly different in the two eosinophil populations. However, a subpopulation of asthmatic patients did show discernibly increased expression of the β1 subunit in airway eosinophils as well as enhanced labeling by an antibody specific for an activation-sensitive motif of β1. This subpopulation of asthmatics consisted of those individuals who were characterized by a late-phase reaction to inhaled antigen challenge (60). Therefore, these data suggest that, with respect to adherence to fibronectin, the enhanced cell surface expression of β1 integrin and in vivo cellular priming (which promotes the appearance of an activation epitpoe for β1 integrins) can cooperate to increase the ability of airway eosinophils to bind fibronectin. This process could then contribute to the enhanced adherence-dependent physiological responses observed when the cells are exposed to chemoattractants.
The intracellular mechanisms regulating the primed phenotype are likely multifaceted. Previous work has shown that priming of eosinophils with IL-5 or related cytokines can increase the ability of these cells to undergo degranulation (61), directed migration (30), superoxide anion generation (7) and LTC4 (21) production in response to selected chemoattractants. The enhancement of these effector functions was found to be accompanied by an increase in ERK1/ERK2 activation and an increase in the association of the adapter protein GRB2 with tyrosine phosphorylated proteins (21). This priming process could be initiated by IL-5 and GM-CSF, but not by other eosinophil-active cytokines such as IFNγ, SCF, TNFα or IL-4. The current study identifies enhanced Ras activation as an additional potential intracellular marker of priming in vitro and in vivo (Figs. 6 and 7).
The effector functions stimulated in eosinophils by a given chemoattractant in the airway is governed by several processes including the concentration of the ligand in the airway and expression of the ligand’s receptor(s) on the eosinophil cell surface. Previous flow cytometry studies have reported equivalent and very low, or non-detectable, levels of receptors for several of the chemoattractants used in this study (CXCR1, CXCR2, CCR1 and CCR5) on blood and airway eosinophils. Furthermore, markedly decreased expression of CCR3 on human airway eosinophils relative to blood cells was seen (47, 48). Therefore, the enhanced signaling and adherence responses of airway eosinophils to CCL5 and CCL11 are not likely the result of increased cell surface expression of CCR3, suggesting that additional mechanisms such as in vivo priming are regulating the behavior of airway eosinophils. With respect to eosinophil responsiveness to FMLP, two receptors, namely Formyl Peptide Receptors (FPR) 1 and 2 have been shown to be expressed and to transduce signals in blood eosinophils (4, 62). Flow cytometric analysis revealed that expression of FPR1 on eosinophils was low relative to expression in neutrophils and approximately equivalently expressed on blood and airway eosinophils (Fig 3A). FPR2 was also expressed at low levels on the eosinophil cell surface, relative to its surface expression on THP-1 monocytic cells, and was slightly, but significantly, more abundant on airway eosinophils than blood eosinophils. Therefore, the mechanisms by which priming of FMLP-stimulated responses are achieved likely encompass multiple intracellular events besides the expression of cognate receptors on the eosinophil cell surface.
It should be noted that priming also contributes to the enhanced responsiveness of additional receptor classes; notably the Fc receptors for immunoglobulins. In this regard, IL-5 and GM-CSF can increase the expression of CD32 mRNA and protein on blood eosinophils (4, 63). IgG is a potent stimulus for eosinophil degranulation, and the ability of blood eosinophils to bind IgG-coated beads is enhanced by in vitro priming with cytokines, a process that also appears to be dependent on the MEK-ERK signaling cascade (64). Indeed, we have reported that a priming-associated motif of FcγRII (CD32) can be detected with the monoclonal phage antibody A17 on airway eosinophils (5). Eosinophils in the blood have greater expression of this motif following allergen challenge, and the magnitude of the increase in expression in airway eosinophils correlates with a measure of the patients’ bronchial hyper-responsiveness (5). Taken together, these previous reports and the current study suggest a model of airway eosinophil activation by chemoattractants and IgG-coated targets that encompasses several levels of regulation that may include 1) exposure to one or more cytokines, 2) expression of cognate receptors on the eosinophil cell surface, 3) inside-out signaling that modulates receptor affinity for its ligand(s), and 4) post receptor signaling pathway alteration. Although IgG has not been utilized as a stimulus in the current study, the model described above predicts that airway eosinophils would demonstrate a greater capacity to bind immunoglobulin-coated targets and release cytotoxic granule proteins with subsequent ramifications for tissue homeostasis.
The present work, together with previous studies from our laboratories and others, reveals that agonists of diverse chemoattractant receptors, namely FMLP, CCL11 and CCL5, stimulate greater adherence to several substrates such as collagen, endothelial cells and fibronectin (Fig. 2 and (2, 7)), and that FMLP promotes enhanced superoxide anion release (2, 7) and degranulation (Fig 1) in airway eosinophils relative to blood-derived eosinophils. Intracellular markers of this enhanced responsiveness to FMLP include the activation of ERK1/ERK2 and the low molecular weight G-protein Ras, as well as prolonged elevations in intracellular free calcium concentrations (2). The increased responsiveness to CCL11 and CCL5, seen with respect to activation of ERK1/2 in airway eosinophils, was modest in comparison to that stimulated by FMLP (Fig. 5A), and variable among the subjects examined. It is likely however, that any increase in ERK activation promoted in airway eosinophils by CCR3 ligands, resulting from in vivo exposure to priming agents such as IL-5 and related cytokines (GM-CSF, IL-3), may be partially offset by the concurrent reduction in the cell surface expression of CCR3 (47, 48) detected in airway eosinophils. In contrast, FMLP, a synthetic ligand of the formyl peptide receptors, is a potent activator of airway eosinophils. Enhanced responsiveness to FMLP and CCR3 ligands by airway eosinophils may be mediated in part by mechanisms impinging on intracellular signaling through the Ras-ERK cascade. Altogether, these studies support a model of in vivo priming of eosinophils by IL-5 or related cytokines following allergen challenge, and reveal an important role of priming and the Ras-ERK network in the differences in chemoattractant-stimulated responses of blood and airway eosinophils.
Capsule Summary.
Human airway eosinophils respond to several chemoattractants with increased activation of the RAS/ERK cascade, EDN release and adherence to fibronectin relative to blood eosinophils.
Supplementary Material
Footnotes
This work was supported by NIH grants AI34486, AI070503, HL56396, HL088594, and HL069116 and by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
- SBP-Ag
- segmental bronchoprovocation with antigen
- LTC4
- leukotriene C4
- PAF
- platelet activating factor
- EDN
- eosinophil-derived neurotoxin
- BAL
- bronchoalveolar lavage
- FPR
- formyl peptide receptor
- MCF
- median channel fluorescence
Disclosures
The authors have no financial conflict of interest.
References
- 1.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 2.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. Journal of Immunology. 1992;149:3710–3718. [PubMed] [Google Scholar]
- 3.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. Journal of Immunology. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 4.Bates ME, Liu LY, Esnault S, Stout BA, Fonkem E, Kung V, Sedgwick JB, Kelly EA, Bates DM, Malter JS, Busse WW, Bertics PJ. Expression of interleukin-5- and granulocyte macrophage-colony-stimulating factor-responsive genes in blood and airway eosinophils. American Journal of Respiratory Cell & Molecular Biology. 2004;30:736–743. doi: 10.1165/rcmb.2003-0234OC. [DOI] [PubMed] [Google Scholar]
- 5.Luijk B, Lindemans CA, Kanters D, van der Heijde R, Bertics P, Lammers JWJ, Bates ME, Koenderman L. Gradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challenge. Journal of Allergy & Clinical Immunology. 2005;115:997–1003. doi: 10.1016/j.jaci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Brooks AM, Bates ME, Vrtis RF, Jarjour NN, Bertics PJ, Sedgwick JB. Urokinase-type plasminogen activator modulates airway eosinophil adhesion in asthma. Am J Respir Cell Mol Biol. 2006;35:503–511. doi: 10.1165/rcmb.2006-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedgwick JB, Quan SF, Calhoun WJ, Busse WW. Effect of interleukin-5 and granulocyte-macrophage colony stimulating factor on in vitro eosinophil function: comparison with airway eosinophils. Journal of Allergy & Clinical Immunology. 1995;96:375–385. doi: 10.1016/s0091-6749(95)70057-9. [DOI] [PubMed] [Google Scholar]
- 8.Ko FW, Lau CY, Leung TF, Wong GW, Lam CW, Lai CK, Hui DS. Exhaled breath condensate levels of eotaxin and macrophage-derived chemokine in stable adult asthma patients. Clinical & Experimental Allergy. 2006;36:44–51. doi: 10.1111/j.1365-2222.2006.02398.x. [DOI] [PubMed] [Google Scholar]
- 9.Kivity S, Argaman A, Onn A, Shwartz Y, Man A, Greif J, Fireman E. Eosinophil influx into the airways in patients with exercise-induced asthma. Respir Med. 2000;94:1200–1205. doi: 10.1053/rmed.2000.0951. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese C, Triggiani M, Marone G, Mazzarella G. Arachidonic acid metabolism in inflammatory cells of patients with bronchial asthma. Allergy. 2000;55(Suppl 61):27–30. doi: 10.1034/j.1398-9995.2000.00504.x. [DOI] [PubMed] [Google Scholar]
- 11.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 12.Le Y, Zhou Y, Tao H, Wang JM. Formylpeptide receptors and their potential roles in inflammatory airway diseases. Clinical & Experimental Allergy Reviews. 2004;4:155–161. [Google Scholar]
- 13.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol. 2006;35:378–386. doi: 10.1165/rcmb.2006-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulfman LH, Alblas J, van Aalst CW, Zwaginga JJ, Koenderman L. Differences in potency of CXC chemokine ligand 8-, CC chemokine ligand 11-, and C5a-induced modulation of integrin function on human eosinophils. Journal of Immunology. 2005;175:6092–6099. doi: 10.4049/jimmunol.175.9.6092. [DOI] [PubMed] [Google Scholar]
- 15.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, Bacon KB. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. Journal of Immunology. 1999;163:1611–1618. [PubMed] [Google Scholar]
- 16.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunological Reviews. 2001;179:163–172. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujisawa T, Kato Y, Nagase H, Atsuta J, Terada A, Iguchi K, Kamiya H, Morita Y, Kitaura M, Kawasaki H, Yoshie O, Hirai K. Chemokines induce eosinophil degranulation through CCR-3. Journal of Allergy & Clinical Immunology. 2000;106:507–513. doi: 10.1067/mai.2000.108311. [DOI] [PubMed] [Google Scholar]
- 18.Kampen GT, Stafford S, Adachi T, Jinquan T, Quan S, Grant JA, Skov PS, Poulsen LK, Alam R. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 2000;95:1911–1917. [PubMed] [Google Scholar]
- 19.Shin MH, Lee YA, Bae YS, Kita H, Kim Y, Ryu SH. The synthetic chemoattractant peptide WKYMVm induces superoxide production by human eosinophils via the phosphoinositide 3-kinase-mediated activation of ERK1/2. International Archives of Allergy & Immunology. 2005;137(Suppl 1):21–26. doi: 10.1159/000085428. [DOI] [PubMed] [Google Scholar]
- 20.Mishra RK, Scaife JE, Harb Z, Gray BC, Djukanovic R, Dent G. Differential dependence of eosinophil chemotactic responses on phosphoinositide 3-kinase (PI3K) Allergy. 2005;60:1204–1207. doi: 10.1111/j.1398-9995.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- 21.Bates ME, Green VL, Bertics PJ. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C(4) biosynthesis. Journal of Biological Chemistry. 2000;275:10968–10975. doi: 10.1074/jbc.275.15.10968. [DOI] [PubMed] [Google Scholar]
- 22.Miyamasu M, Hirai K, Takahashi Y, Iida M, Yamaguchi M, Koshino T, Takaishi T, Morita Y, Ohta K, Kasahara T, et al. Chemotactic agonists induce cytokine generation in eosinophils. Journal of Immunology. 1995;154:1339–1349. [PubMed] [Google Scholar]
- 23.Nakajima T, Yamada H, Iikura M, Miyamasu M, Izumi S, Shida H, Ohta K, Imai T, Yoshie O, Mochizuki M, Schroder JM, Morita Y, Yamamoto K, Hirai K. Intracellular localization and release of eotaxin from normal eosinophils. FEBS Letters. 1998;434:226–230. doi: 10.1016/s0014-5793(98)00863-1. [DOI] [PubMed] [Google Scholar]
- 24.Rossi AG, Haslett C, Hirani N, Greening AP, Rahman I, Metz CN, Bucala R, Donnelly SC. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. Journal of Clinical Investigation. 1998;101:2869–2874. doi: 10.1172/JCI1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coffer PJ, Koenderman L. Granulocyte signal transduction and priming: cause without effect? Immunology Letters. 1997;57:27–31. doi: 10.1016/s0165-2478(97)00067-9. [DOI] [PubMed] [Google Scholar]
- 26.Koenderman L, Tool AT, Roos D, Verhoeven AJ. Priming of the respiratory burst in human eosinophils is accompanied by changes in signal transduction. Journal of Immunology. 1990;145:3883–3888. [PubMed] [Google Scholar]
- 27.Koenderman L, Yazdanbakhsh M, Roos D, Verhoeven AJ. Dual mechanisms in priming of the chemoattractant-induced respiratory burst in human granulocytes. A Ca2+-dependent and a Ca2+-independent route. Journal of Immunology. 1989;142:623–628. [PubMed] [Google Scholar]
- 28.Schweizer RC, Welmers BA, Raaijmakers JA, Zanen P, Lammers JW, Koenderman L. RANTES- and interleukin-8-induced responses in normal human eosinophils: effects of priming with interleukin-5. Blood. 1994;83:3697–3704. [PubMed] [Google Scholar]
- 29.Takafuji S, Bischoff SC, De Weck AL, Dahinden CA. IL-3 and IL-5 prime normal human eosinophils to produce leukotriene C4 in response to soluble agonists. Journal of Immunology. 1991;147:3855–3861. [PubMed] [Google Scholar]
- 30.Warringa RA, Schweizer RC, Maikoe T, Kuijper PH, Bruijnzeel PL, Koendermann L. Modulation of eosinophil chemotaxis by interleukin-5. American Journal of Respiratory Cell & Molecular Biology. 1992;7:631–636. doi: 10.1165/ajrcmb/7.6.631. [DOI] [PubMed] [Google Scholar]
- 31.Coeffier E, Joseph D, Vargaftig BB. Modulation of the enhanced migration of eosinophils from the airways of sensitized guinea pigs: role of IL-5. Annals of the New York Academy of Sciences. 1994;725:274–281. doi: 10.1111/j.1749-6632.1994.tb39810.x. [DOI] [PubMed] [Google Scholar]
- 32.Fernvik E, Lundahl J, Hallden G. The impact of eotaxin- and IL-5-induced adhesion and transmigration on eosinophil activity markers. Inflammation. 2000;24:73–87. doi: 10.1023/a:1006940109869. [DOI] [PubMed] [Google Scholar]
- 33.Hall DJ, Cui J, Bates ME, Stout BA, Koenderman L, Coffer PJ, Bertics PJ. Transduction of a dominant-negative H-Ras into human eosinophils attenuates extracellular signal-regulated kinase activation and interleukin-5-mediated cell viability. Blood. 2001;98:2014–2021. doi: 10.1182/blood.v98.7.2014. [DOI] [PubMed] [Google Scholar]
- 34.Nagata M, Sedgwick JB, Kita H, Busse WW. Granulocyte macrophage colony-stimulating factor augments ICAM-1 and VCAM-1 activation of eosinophil function. American Journal of Respiratory Cell & Molecular Biology. 1998;19:158–166. doi: 10.1165/ajrcmb.19.1.3001. [DOI] [PubMed] [Google Scholar]
- 35.Sedgwick JB, Calhoun WJ, Gleich GJ, Kita H, Abrams JS, Schwartz LB, Volovitz B, Ben-Yaakov M, Busse WW. Immediate and late airway response of allergic rhinitis patients to segmental antigen challenge. Characterization of eosinophil and mast cell mediators. American Review of Respiratory Disease. 1991;144:1274–1281. doi: 10.1164/ajrccm/144.6.1274. [DOI] [PubMed] [Google Scholar]
- 36.Kelly EA, Busse WW, Jarjour NN. Inhaled budesonide decreases airway inflammatory response to allergen. American Journal of Respiratory & Critical Care Medicine. 2000;162:883–890. doi: 10.1164/ajrccm.162.3.9910077. [DOI] [PubMed] [Google Scholar]
- 37.Meerschaert J, Kelly EA, Mosher DF, Busse WW, Jarjour NN. Segmental antigen challenge increases fibronectin in bronchoalveolar lavage fluid. American Journal of Respiratory & Critical Care Medicine. 1999;159:619–625. doi: 10.1164/ajrccm.159.2.9806053. [DOI] [PubMed] [Google Scholar]
- 38.Lundahl J, Moshfegh A, Gronneberg R, Hallden G. Eotaxin increases the expression of CD11b/CD18 and adhesion properties in IL5, but not fMLP-prestimulated human peripheral blood eosinophils. Inflammation. 1998;22:123–135. doi: 10.1023/a:1022379821130. [DOI] [PubMed] [Google Scholar]
- 39.Anwar AR, Moqbel R, Walsh GM, Kay AB, Wardlaw AJ. Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993;177:839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sano H, Zhu X, Sano A, Boetticher EE, Shioya T, Jacobs B, Munoz NM, Leff AR. Extracellular signal-regulated kinase 1/2-mediated phosphorylation of cytosolic phospholipase A2 is essential for human eosinophil adhesion to fibronectin. Journal of Immunology. 2001;166:3515–3521. doi: 10.4049/jimmunol.166.5.3515. [DOI] [PubMed] [Google Scholar]
- 41.Shin EH, Osada Y, Sagara H, Takatsu K, Kojima S. Involvement of complement and fibronectin in eosinophil-mediated damage to Nippostrongylus brasiliensis larvae. Parasite Immunol. 2001;23:27–37. doi: 10.1046/j.1365-3024.2001.00352.x. [DOI] [PubMed] [Google Scholar]
- 42.Sung KP, Yang L, Kim J, Ko D, Stachnick G, Castaneda D, Nayar J, Broide DH. Eotaxin induces a sustained reduction in the functional adhesive state of very late antigen 4 for the connecting segment 1 region of fibronectin. J Allergy Clin Immunol. 2000;106:933–940. doi: 10.1067/mai.2000.110797. [DOI] [PubMed] [Google Scholar]
- 43.Xu X, Hakansson L. Regulation of the release of eosinophil cationic protein by eosinophil adhesion. Clin Exp Allergy. 2000;30:794–806. doi: 10.1046/j.1365-2222.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 44.Meerschaert J, Vrtis RF, Shikama Y, Sedgwick JB, Busse WW, Mosher DF. Engagement of alpha4beta7 integrins by monoclonal antibodies or ligands enhances survival of human eosinophils in vitro. Journal of Immunology. 1999;163:6217–6227. [PubMed] [Google Scholar]
- 45.Myou S, Zhu X, Boetticher E, Myo S, Meliton A, Lambertino A, Munoz NM, Leff AR. Blockade of focal clustering and active conformation in beta 2-integrin-mediated adhesion of eosinophils to intercellular adhesion molecule-1 caused by transduction of HIV TAT-dominant negative Ras. Journal of Immunology. 2002;169:2670–2676. doi: 10.4049/jimmunol.169.5.2670. [DOI] [PubMed] [Google Scholar]
- 46.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. Journal of Allergy & Clinical Immunology. 2003;112:556–562. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- 48.Nagase H, Kudo K, Izumi S, Ohta K, Kobayashi N, Yamaguchi M, Matsushima K, Morita Y, Yamamoto K, Hirai K. Chemokine receptor expression profile of eosinophils at inflamed tissue sites: Decreased CCR3 and increased CXCR4 expression by lung eosinophils. Journal of Allergy & Clinical Immunology. 2001;108:563–569. doi: 10.1067/mai.2001.118292. [DOI] [PubMed] [Google Scholar]
- 49.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends in Immunology. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 50.Bates ME, Bertics PJ, Busse WW. IL-5 activates a 45-kilodalton mitogen-activated protein (MAP) kinase and Jak-2 tyrosine kinase in human eosinophils. Journal of Immunology. 1996;156:711–718. [PubMed] [Google Scholar]
- 51.Sedgwick JB, Busse WW. Adhesion proteins on airway eosinophils in allergy and asthma. Agents & Actions - Supplements. 1993;43:163–172. doi: 10.1007/978-3-0348-7324-6_14. [DOI] [PubMed] [Google Scholar]
- 52.Horie S, Gleich GJ, Kita H. Cytokines directly induce degranulation and superoxide production from human eosinophils. Journal of Allergy and Clinical Immunology. 1996;98:371–381. doi: 10.1016/s0091-6749(96)70161-6. [DOI] [PubMed] [Google Scholar]
- 53.Nagata M, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. Journal of Immunology. 1995;155:2194–2202. [PubMed] [Google Scholar]
- 54.Teixeira MM, Williams TJ, Hellewell PG. Description of an in vivo model for the assessment of eosinophil chemoattractants in the mouse. Mem Inst Oswaldo Cruz. 1997;92(Suppl 2):211–214. doi: 10.1590/s0074-02761997000800029. [DOI] [PubMed] [Google Scholar]
- 55.Wardlaw AJ, Walsh GM, Symon FA. Adhesion interactions involved in eosinophil migration through vascular endothelium. Ann N Y Acad Sci. 1996;796:124–137. doi: 10.1111/j.1749-6632.1996.tb32574.x. [DOI] [PubMed] [Google Scholar]
- 56.Tachimoto H, Bochner BS. The surface phenotype of human eosinophils. Chem Immunol. 2000;76:45–62. doi: 10.1159/000058780. [DOI] [PubMed] [Google Scholar]
- 57.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol. 2008;83:1–12. doi: 10.1189/jlb.0607344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anwar AR, Walsh GM, Cromwell O, Kay AB, Wardlaw AJ. Adhesion to fibronectin primes eosinophils via alpha 4 beta 1 (VLA-4) Immunology. 1994;82:222–228. [PMC free article] [PubMed] [Google Scholar]
- 59.Foster AP, McCabe PJ, Sanjar S, Cunningham FM. Agonist-induced adherence of equine eosinophils to fibronectin. Vet Immunol Immunopathol. 1997;56:205–220. doi: 10.1016/s0165-2427(96)05740-6. [DOI] [PubMed] [Google Scholar]
- 60.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol. 2008;180:7622–7635. doi: 10.4049/jimmunol.180.11.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Badewa AP, Heiman AS. Inhibition of CCL11, CCL24, and CCL26-induced degranulation in HL-60 eosinophilic cells by specific inhibitors of MEK1/MEK2, p38 MAP kinase, and PI 3-kinase. Immunopharmacol Immunotoxicol. 2003;25:145–157. doi: 10.1081/iph-120020466. [DOI] [PubMed] [Google Scholar]
- 62.Svensson L, Dahlgren C, Wenneras C. The chemoattractant Trp-Lys-Tyr-Met-Val-D-Met activates eosinophils through the formyl peptide receptor and one of its homologues, formyl peptide receptor-like 1. Journal of Leukocyte Biology. 2002;72:810–818. [PubMed] [Google Scholar]
- 63.Koenderman L, Hermans SW, Capel PJ, van de Winkel JG. Granulocyte-macrophage colony-stimulating factor induces sequential activation and deactivation of binding via a low-affinity IgG Fc receptor, hFc gamma RII, on human eosinophils. Blood. 1993;81:2413–2419. [PubMed] [Google Scholar]
- 64.Bracke M, Coffer PJ, Lammers JW, Koenderman L. Analysis of signal transduction pathways regulating cytokine-mediated Fc receptor activation on human eosinophils. Journal of Immunology. 1998;161:6768–6774. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.