Abstract
Thyroid cancer is the most common malignancy of the endocrine system. There are several variants, ranging from well-differentiated cancers, such as papillary carcinomas, to poorly differentiated types, which carry a worse prognosis. Many patients with well-differentiated thyroid cancers are cured by surgical intervention alone, while others require adjuvant therapy. For those patients with more aggressive tumors, such as metastatic and anaplastic thyroid cancers, surgery rarely offers a definitive cure and alternative treatment methods such as chemotherapy do not improve survival. Due to the difficulty in treating aggressive thyroid cancers, other novel therapies are needed. In this paper, we will review current strategies for managing the various types of thyroid carcinomas. We will also address many of the studied genetic pathways and new therapeutic drug targets for treating individual thyroid malignancies.
Introduction
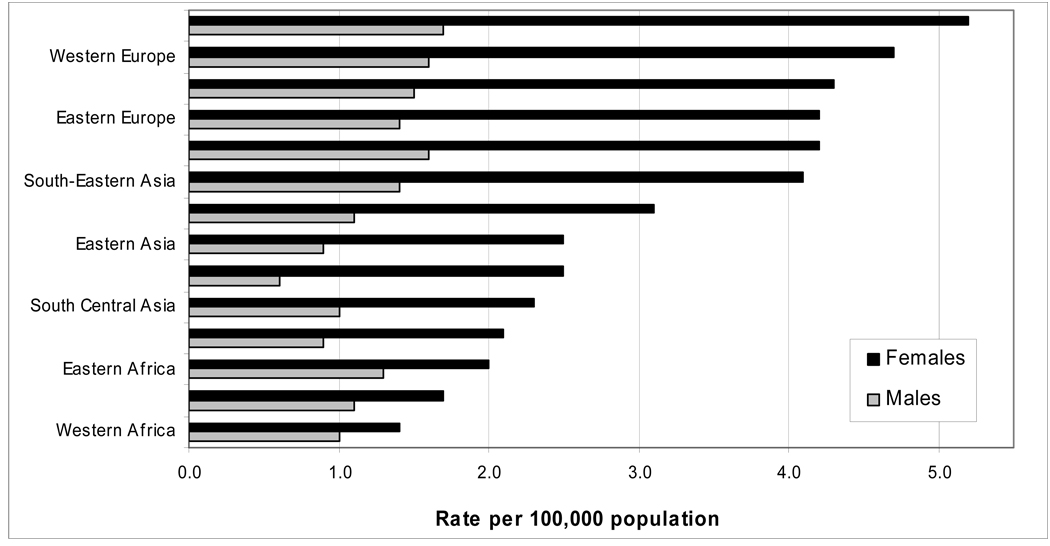
Thyroid cancers account for approximately 2% of all new cancer diagnoses (1). The worldwide incidence of thyroid cancer varies, with estimates of 12 cases per 100,000 in North America to areas of Europe seeing 5.6 new cases per 100,000 (Fig. 1) (2). The incidence of thyroid cancer has also doubled over the last 30 years, due to improved imaging modalities (3). The majority of thyroid cancers are of the well differentiated type. Cancers arising from follicular epithelial cells include papillary carcinoma, follicular carcinoma, and Hurthle cell carcinoma. Papillary cancers account for up to 85% of thyroid cancers, follicular carcinomas comprise another 10%, and Hurthle cell carcinoma up to 3% (4). Medullary thyroid carcinoma arises from parafollicular c-cells and accounts for another 3–5% of thyroid cancers (5). Anaplastic thyroid carcinoma, an undifferentiated type of thyroid cancer, only accounts for 1% of all types of thyroid cancer (6).
Figure 1.
Age-standardized incidence rates of thyroid cancer, 2002 estimates (2). (adapted from www.cancerresearchuk.org)
The less differentiated cancers tend to be more aggressive and carry a worse prognosis. A study of nearly 16,000 patients in the United States estimated the survival rates for the various types of cancer to be 98% for papillary, 92% for follicular, 80% for medullary, and 13% for anaplastic (7). As with most cancers, tumor size, lymph node involvement and widespread metastases may indicate a worse prognosis. Increased age at the time of diagnosis is also an important prognostic indicator in differentiated thyroid cancer (8). Many studies have found that the prognosis for those with differentiated thyroid cancer is improved for those younger than 40–45 years of age (8, 9). This is widely accepted and age is included in the familiar TNM staging system for differentiated thyroid cancer (Table 1).
Table 1. Staging of Differentiated Thyroid Carcinoma (10).
STAGING: TUMOR, NODE, METASTASIS STAGING SYSTEM FOR DIFFERENTIATED THYROID CARCINOMA (PAPILLARY AND FOLLICULAR TYPES)
| Stage | Age < 45 years | Age > 45 years |
|---|---|---|
| I | Tany Nany M0 | T1 N0 M0 |
| II | Tany Nany M1 | T2 N0 M0 T3 N0 M0 |
| III | T4 N0 M0 Tany N1 M0 |
|
| IV | Tany Nany M1 |
T1, ±1 cm; T2,>1 cm,±4 cm; T3, >4 cm; T4, extrathyroidal extension; N0, no nodal involvement; N1, regional nodal metastases; M0, no distant metastases; M1, distant metastases.
There are several treatment options available for patients with thyroid carcinoma. Surgical resection, radioactive iodine ablation, and TSH suppression therapy are all well accepted treatment modalities for differentiated thyroid cancers by the medical community. Yet, in spite of the multiple treatments that exist for differentiated thyroid cancer, survival rates have not improved over the last few decades (6). In contrast, undifferentiated thyroid cancer is much more aggressive and surgery provides little benefit to these patients. Chemotherapy and radiation treatment have been utilized to treat anaplastic cancer, but with minimal effectiveness (11). The need to develop new treatment options for both differentiated and undifferentiated thyroid carcinoma is of paramount importance.
Current management strategies
Primary surgical management
Differentiated Cancer
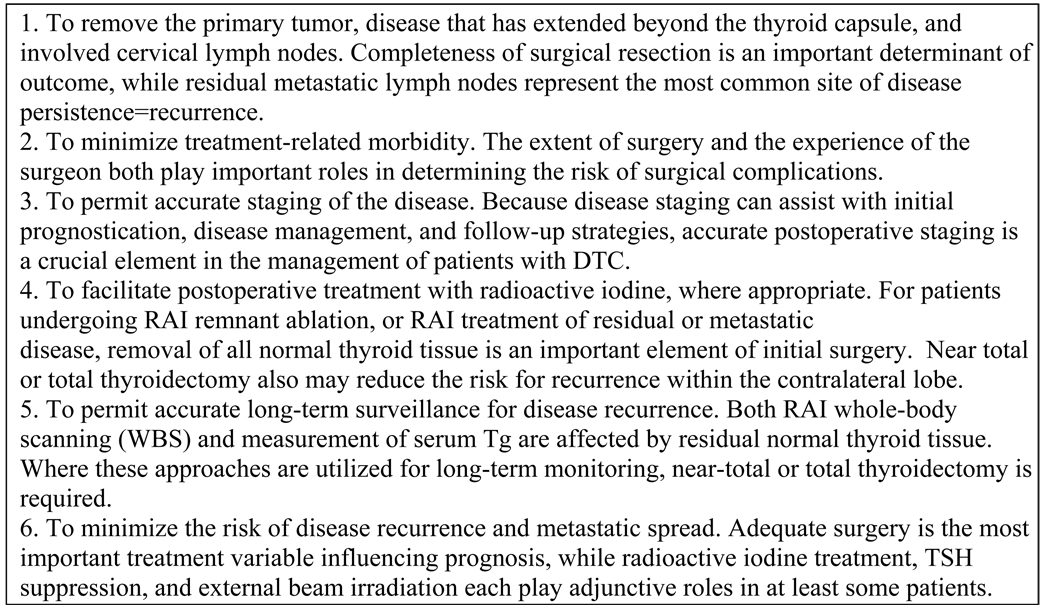
The standard of care for the initial treatment of papillary, follicular, and Hurthle cell carcinomas is thyroidectomy (12). The American Thyroid Association recently released guidelines for the management of differentiated thyroid cancer, which includes goals for initial therapy of the disease (Fig. 2).
Fig. 2.
ATA goals of initial therapy for differentiated thyroid cancer (13)
It is widely accepted that if the primary tumor is greater than 1cm in size, there are nodules present in the contralateral lobe or the patient has a history of head and neck radiation, a total thyroidectomy should be performed (14). Increased extent of the initial surgery may increase survival for both high and low risk patients (14, 15, 16). Thyroid lobectomy may be adequate in patients with a papillary carcinoma <1cm in size that is unifocal (13).
In patients with papillary carcinoma anywhere from 20–90% will have regional lymph node metastases at the time of diagnosis (17, 18). In contrast, follicular thyroid cancers rarely present with lymph node metastases. Sanders and colleagues reviewed survival rates in over 1000 patients with differentiated thyroid cancer and noted no significant difference among survival in low risk patients with and without nodal metastases (9). While there are studies that confirm the findings of the Sanders’ study, others have demonstrated an increased risk of local tumor recurrence when positive lymph nodes were noted on initial presentation (6, 19). More recently, a study of the Surveillance, Epidemiology, and End Results (SEER) database found that lymph node metastases was a significant predictor of a poor outcome in patients with papillary thyroid cancer (20). Given these findings, many centers now perform ipsilateral modified radical neck dissections and central neck dissections for clinically evident lymph node metastases.
Medullary Thyroid Cancer
A total thyroidectomy with bilateral central neck dissection is the recommended initial treatment for clinically evident medullary thyroid carcinoma. It has been demonstrated that addition of bilateral central neck dissection at the initial surgery can increase the cure rate for these patients (21). Some surgeons advocate the removal of all four parathyroid glands during the central neck dissection to insure that all disease has been eliminated (22). The parathyroid tissue can then be reimplanted in the sternocleidomastoid if the patients have sporadic or familial medullary thyroid carcinoma or MEN-2B. If the patients have been diagnosed with MEN-2A, then the parathyroid needs to be reimplanted in the forearm due to the risk of subsequent hyperparathyroidism. However, some surgeons advocate a more conservative approach for the parathyroid glands, leaving them in-situ.
Anywhere from 14–80% of patients diagnosed with medullary thyroid cancer will have ipsilateral lateral lymph node metastases at presentation (23, 24). Due to the high incidence of lymph node disease, some surgeons advocate performing a bilateral neck dissection at the time of thyroidectomy regardless of tumor size, while others prefer a more conservative approach (23). Many surgeons will agree that if physical exam or pre-operative imaging (ultrasound or CT) suggest the presence of lateral lymph node metastases, an ipsilateral lateral lymphadenectomy can be performed (22).
Prophylactic surgery is recommended for family members carrying a RET mutation (25). The age at which to perform prophylactic surgery for patients with hereditary medullary thyroid cancer is based on the type of RET mutation that is present (Table 2).
Table 2.
Recommendations for Prophylactic Surgery in Hereditary MTC
| Risk level for MTC |
Codon mutation |
Age of prophylactic surgery |
|---|---|---|
| Level 3 (highest) |
883 918 922 |
Within the first 6 months of life (preferably in the first month) |
| Level 2 (higher) |
611 618 620 634 |
By age 5 |
| Level 1 (high) | 609 630 768 790 791 804 891 |
By age 5–10 |
Adapted from Brandi ML, et al: Guidelines for Diagnosis and Therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86:5658–5671, 2001.
For those diagnosed with MEN-2A, thyroidectomy is advocated prior to age 6 (26). Although everyone agrees that all patients should prophylactically undergo a total thyroidectomy, controversy exists whether a central neck dissection should also be performed. Proponents of prophylactic central neck dissection state that the incidence of occult lymph node disease is too high not to perform lymphadenectomy, but opponents argue that the incidence of occult disease in children under the age of 10 is rare and therefore does not warrant central neck dissection (22).
Anaplastic Thyroid Carcinoma
Patients with anaplastic thyroid carcinoma usually present with local invasion or distant metastases, making surgical resection with a chance for cure highly unlikely (27). When surgical resection is possible, studies have shown that adjuvant radiotherapy and chemotherapy improves survival (28). Unfortunately, for most patients their disease is beyond the extent of resection at presentation and median survival ranges from 3 to 7 months (27). In many patients death occurs due to upper airway obstruction and surgery can be performed for palliative purposes. Thus, most patients with anaplastic thyroid cancer are treated with radiation and/or chemotherapy.
131 I adjuvant therapy
There are three reasons for using adjuvant radioiodine ablation therapy after thyroidectomy for papillary and follicular carcinomas. The first is to destroy any residual cancer, the second is to eliminate uptake of 131 I by normal tissue, therefore making detection of recurrent disease easier, and finally to allow for measurement of serum thyroglobulin as a serum marker. Multiple studies have demonstrated that radioiodine ablation reduces disease specific mortality in patients whose tumors are greater than 1 cm, have capsular or soft tissue invasion, or are multicentric (6, 29). Others have shown no benefit of radioiodine ablation in preventing recurrence in patients with microscopic papillary carcinoma (30, 31). Radioiodine ablation has also been shown to have minimal effectiveness for patients with hurthle cell carcinoma (32). It is also of no benefit to patients with medullary thyroid cancer since C cells do not take up iodine.
Thyroid-stimulating hormone suppression therapy
Thyroid-stimulating hormone (TSH) suppression therapy is used as an adjuvant treatment for differentiated thyroid carcinomas. The rationale for its use stems from studies that have demonstrated that thyroid cell growth is dependent on TSH (33). Investigators have also shown that by suppressing TSH with LT4 in high-risk thyroid patients, disease progression and recurrence rates are decreased (34). The level of TSH suppression required to achieve a benefit remains controversial. In order to suppress TSH, patients develop a subclinical thyrotoxicosis that can lead to bone and cardiac disease (35). A study evaluating patients undergoing TSH suppression found that those patients with a greater degree of suppression (TSH ≤0.05mU/L) had a longer relapse free survival (36). Yet another study done by Cooper and colleagues demonstrated that greater degrees of suppression did not improve outcomes for low risk patients and demonstrated questionable benefit for high risk patients (37). After taking these various studies into consideration, the American Thyroid Association in their 2009 guidelines on the management of thyroid cancer recommended initial TSH suppression to below 0.1mU/L in high and intermediate risk patients and maintenance of TSH at below the lower limit of normal (0.1–0.5mU/L) in low risk patients (13).
Novel molecular therapeutic strategies
According to the National Cancer Institute’s SEER database over the last decade there has been a 6.2% increase in the incidence of thyroid cancer in the United States (38). It is in this same time period that survival rates have remained unchanged. In order to improve upon the treatments available for patients with thyroid cancer researchers have been working to uncover the molecular pathways that lead to the development of well differentiated, medullary, and anaplastic thyroid cancers. One of the most promising pathways involves the RET receptor tyrosine kinase (RTK). Multiple researchers have demonstrated its importance in the development of both papillary and medullary thyroid cancer (39). Another important area of study involves the B-type Raf kinase (BRAF) gene and its role in papillary thyroid cancer (40). Cell cycle progression of differentiated thyroid cancers is thought to be regulated in part by epidermal growth factor receptor (EGFR) activation (41). Other pathways involved include activation of glycogen synthase kinase 3-β, overexpression of the Notch 1 intracellular domain, and mutations of genes such as PPARy (42, 43, 44). Research into these various pathways has lead to the initiation of multiple clinical trials testing the efficacy and safety of numerous drugs in the treatment of thyroid cancer.
RET receptor tyrosine kinase (RTK)
The RET gene codes for a cell membrane receptor kinase. In medullary thyroid cancer it is activated by a point mutation, which can be found in nearly all patients with familial forms of the disease. In MEN 2A and familial medullary thyroid cancer, mutations are found the cysteine-rich extracellular domain (45). In MEN 2A 90% of the mutations affect codon 634, while in familial medullary thyroid cancer the mutations are evenly distributed (46). These mutations have been shown to lead to unpairing of the cysteine residues, which then leads to activation of RET kinase (47). In MEN 2B, most of the mutations occur in codon 918 in the intracellular domain of RET leading to phosphorylation and subsequent activation of intracellular proteins (47). RET somatic mutations have also been found in sporadic medullary thyroid cancers.
In papillary thyroid carcinoma RET is activated by a chromosomal rearrangement known as RET/PTC. The most common rearrangements that have been discovered have been named RET/PTC1 and RET/PTC3. Each is formed by fusion with another gene leaving the tyrosine kinase domain of the RET receptor intact and activating the RAS-RAF-MAPK cascade downstream (48). Several studies have demonstrated that RET/PTC requires the presence of a functional BRAF kinase in order to be oncogenic (49, 50). In general RET/PTC rearrangement is found in patients presenting at a younger age and having a higher rate of lymph node metastases (51).
Many tyrosine kinase inhibitors directed specifically at RET kinase have been tested as treatments for thyroid cancer. ZD6474 (Vandetanib) is a tyrosine kinase inhibitor that also inhibits vascular endothelial growth factor receptor 2 (VEGFR-2) (52). ZD6474 has been shown to block phosphorylation in the most common MEN2A and MEN2B mutations in vitro and to inhibit growth in papillary cancer cell lines with the RET/PTC1 mutation (53, 54). A phase II clinical trials of patients with metastatic familial medullary thyroid carcinoma demonstrated confirmed partial response in 17% of patients and stable disease in another 33% at 24 weeks (55). Multiple phase II clinical trials testing the efficacy of ZD6474 in patients with metastatic medullary thyroid cancer, as well as metastatic papillary and follicular cancer are currently underway (55).
AMG 706 (Motesanib) is another tyrosine kinase inhibitor that targets VEGF receptors and has been shown to decrease autophosphorylation of RET in vitro (56). A phase I study confirmed that of 6 patients enrolled who had thyroid cancer, 3 experienced at least a 30% reduction in tumor size (57). Based on those findings a phase II trial was initiated for patients with advanced differentiated thyroid cancers and medullary thyroid cancer (58). Of the patients with differentiated thyroid carcinoma, 14% were considered partial responders and another 35% had stable disease after 24 weeks on motesanib. Only 2% of patients with medullary thyroid cancer partially responded, but 47% experienced stable disease at 24 weeks.
BRAF mutations
BRAF is a serine/threonine kinase belonging to the family of RAF proteins, that when activated turn on MEK, ERK, and the MAPK cascade (39). Mutations of this gene are most commonly found in papillary thyroid carcinoma (59). The mutations involve a valine-to-glutamine substitution at residue 600 (V600E) that leads to activation of the BRAF kinase (60). Importantly, many studies have demonstrated that BRAF mutations correlate with aggressive tumor characteristics such as lymph node and distant metastases (61,62). The BRAF V600E has also been found to be a predictor of tumor recurrence, possibly due to the decreased ability of the tumors to trap radioiodine (63, 64). BAY 43-9006 (Sorafenib) is a multikinase inhibitor of RTK and VEGFR, also shown to inhibit BRAF signaling and reduce growth of all thyroid cell lines carrying the BRAF mutation (65). This drug has been shown to have a partial response in some patients with progressive papillary carcinoma (66, 67). Currently, phase II clinical trials are underway using BAY 43-9006 in the treatment of anaplastic and metastatic medullary thyroid cancer (39).
Targeting over-expression of EGFR
The epidermal growth factor receptor (EGFR) family consists of four structurally similar receptors. Her2/neu, one of the better known receptors, and EGFR or Her 1, are most often found in thyroid cancers (68). The over-expression of EGFR correlates with a worse prognosis in differentiated thyroid cancers and Her2/neu has been associated with a poor prognosis in solid tumors (69). Specifically, the EGFR pathway, once activated, leads to cell cycle progression, but when blocked cells are arrested and undergo apoptosis, resulting in decreased metastases (68).
Antibodies have been developed against both EGFR and Her2/neu. Anti-EGFR antibodies bind to the extracellular domain, decreasing the number of available receptors for activation. Two studies have examined the use of anti-EGFR antibodies in thyroid cancer cell lines and both showed a reduction in growth (70, 71). Herceptin is an anti-Her2/neu antibody that has been shown to decrease the growth of cancer cells, but no trials testing it on thyroid cancer have been performed (72).
Notch 1 signaling pathway
Notch 1 is a membrane receptor involved in cell differentiation, proliferation, and survival (73). Notch 1 plays both the role of an oncogene and tumor suppressor, depending on tumor type. In pancreatic, colon, and non-small cell lung cancers it is upregulated, but in neuroendocrine tumors such as carcinoids and medullary thyroid carcinomas it has been shown to be downregulated or absent (43).
Notch is cleaved into its active Notch intracellular domain (NICD) after binding with one of its ligands. NICD then translocates to the nucleus, resulting in activation of target genes. In a study done by Kunnimalaiyaan, et al, they created a doxycycline-inducible NICD in a medullary thyroid cancer cell line (74). When these cells were treated with doxycycline, Notch 1 protein levels increased and tumor markers were reduced in a dose dependent fashion. In this study, medullary thyroid cancer cell growth was also markedly inhibited. From this work, other Notch 1 activators, such as valproic acid (VPA) and suberoyl bis-hyrdroxamine acid (SBHA) have been studied and found to inhibit growth and decrease the expression of neuroendocrine markers (75, 76).
The role of the Notch 1 activation has also been studied in papillary and follicular cancers. Xiao and colleagues demonstrated that although there was minimal Notch 1 expression in the papillary and follicular cancer cell lines they studied, but transfection with a Notch 1 plasmid led to growth inhibition (77). When the cells were treated with either VPA or SBHA, cell growth was inhibited in a dose dependent manner and Notch 1 protein expression was induced.
Inactivation of glycogen synthase kinase-3β (GSK-3β)
Glycogen synthase kinase-3β (GSK-3β) is a serine/threonine protein kinase that has previously been shown to regulate cellular metabolism, cell fate determination, proliferation, and survival (78). Inhibition of GSK-3β has been shown to decrease tumor growth in several cancers, including pancreas, colorectal, and prostate adenocarcinomas. Studies have demonstrated that unlike most kinases, GSK-3β is active in the non-phosphorylated state and becomes inhibited when phosphorylation occurs at a single serine residue (Ser9) (79). Kunnimalaiyaan and colleagues demonstrated that in medullary thyroid cancer cells (TT cells) activation of the raf-1 signaling pathway resulted in phosphorylation of GSK-3β (42). They found that by treating TT cells with lithium chloride (LiCl) and SB216763, both well known inhibitors of GSK-3β, suppressed growth both in vitro and in vivo. Both drugs also caused a reduction in neuroendocrine tumor markers, human-achaete-scute complex-like 1 (ASCL 1) and chromogranin A (CgA). They were also able to demonstrate that growth inhibition occurred due to cell cycle arrest in the medullary thyroid cancer cells. Due to these findings phase II clinical trials with lithium chloride for medullary thyroid cancer are now ongoing.
More recently, researchers at utilized the antifungal antibiotics, tautomycetin and tautomycin to suppress growth of medullary thyroid cancer cells via inhibition of GSK-3β (80). They found that these drugs decreased hormonal secretion, reduced neuroendocrine markers, and inhibited growth via apoptosis. Given the findings of inhibition by both cell cycle arrest and apoptosis it appears that GSK-3β regulates growth of medullary thyroid cancer via multiple mechanisms.
Mutations of RAS
The RAS genes encode for G-proteins located on the cell membrane that play a crucial role in the transduction of signals from both tyrosine kinases and G-protein coupled receptors. When inactivated RAS is bound to guanosine diphosphate (GDP), but when activated it binds guanosine triphosphate (GTP) and activates downstream signaling pathways. RAS-GTP quickly becomes inactivated due to an intrinsic GTPase that converts GTP back to GDP. When point mutations of the RAS gene occur, it remains in the active, RAS-GTP, position, and consequently, the downstream signaling pathway is permanently switched on.
One are of research directed at inhibiting the RAS pathway revolves around antisense compounds. These compounds are engineered DNA sequences that are complementary to a target mRNA sequence and can block gene expression, ribosomal assembly, and protein synthesis (68). Two of these drugs, ISIS 2503 and ISIS 5132, showed promise in being able to interfere with the RAS pathway. One trial, found decreased RAS mRNA levels in patients treated with ISIS 2503 who had advanced solid tumors, but no patient demonstrated a partial or complete response to treatment (81).
Mutations of PAX8-PPARγ
The PAX8- PPARγ mutation occurs due to a translocation that results in fusion of the PAX8 gene to the peroxisome poliferator-activated receptor (PPARγ) (82). PAX8-PPARγ has been shown to occur in approximately 35% of follicular carcinomas and in Hurthle cell cancers as well (44, 83, 84). The downstream effects of this mutation are not yet fully understood, but research has shown that there is excess PPARγ protein present (82). Some researchers have demonstrated that this translocation results in inhibition of normal PPARγ function (82, 85), while others have shown increased activation of known PPAR targets (86). One area of study that appears to be consistent is the rare development of both RAS and PAX8-PPARγ mutations in the same follicular carcinoma, signifying that these cancers develop through at least two different molecular pathways (44).
A study was undertaken to evaluate the role of PPARγ agonists in patients with metastatic disease (87). Rosiglitazone, a known PPARγ agonist, was administered to 10 patients in the hopes that it would re-establish radioiodine uptake. Of the 10 patients, 4 had an increase in uptake, but with a limited clinical response.
Conclusion
Although many thyroid cancers, such as papillary and follicular types, have good prognoses, survival rates for the various subtypes have not improved over the last two decades. Surgery and radioactive iodine ablation have been the mainstays of treatment, with radiation therapy being used as an adjunct for unresectable disease. Chemotherapy has played a minor role in the palliative treatment of patients with undifferentiated or anaplastic carcinomas. Thus, novel therapeutic strategies addressing the various molecular pathways by which these cancers develop are a necessity.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.UK Cancer Research. [Accessed December 10, 2009]; Available at: http://info.cancerresearchuk.org/cancerstats/types/thyroid/incidence/uk-thyroid-cancer-incidence-statistics.
- 3.Davies L, Welch H. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Tomoda C, Uruno T, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004;28(5):498–501. doi: 10.1007/s00268-004-7192-z. [DOI] [PubMed] [Google Scholar]
- 5.Ball DW. Medullary Thyroid Cancer. In: Wondisford FERS, editor. Clinical Management of Thyroid Disease: Saunders. 2009. pp. 399–405. [Google Scholar]
- 6.Sherman SI. Thyroid Carcinoma. The Lancet. 361:501–511. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 7.Gilliland F, Hunt W, Morris D, et al. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79(3):564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Dean D, Hay I. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control. 7(3):229–239. doi: 10.1177/107327480000700302. [DOI] [PubMed] [Google Scholar]
- 9.Sanders L, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133(4):419–425. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 10.Verburg F, Mader U, Kruitwagen C, et al. A comparison of prognostic classification systems for differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03734.x. [DOI] [PubMed] [Google Scholar]
- 11.Haugen B. Management of the patient with progressive radioiodine non-responsive disease. Semin Surg Oncol. 16(1):34–41. doi: 10.1002/(sici)1098-2388(199901/02)16:1<34::aid-ssu7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Udelsman R, Chen H. The current management of thyroid cancer. Adv Surg. 1999;33:1–27. [PubMed] [Google Scholar]
- 13.Cooper D, Doherty G, Haugen B, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria K, Bentrem D, Ko C, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246(3):375–381. doi: 10.1097/SLA.0b013e31814697d9. discussion 381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzaferri E, Young R. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70(3):511–518. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 16.DeGroot L, Kaplan E, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71(2):414–424. doi: 10.1210/jcem-71-2-414. [DOI] [PubMed] [Google Scholar]
- 17.Grebe S, Hay I. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5(1):43–63. [PubMed] [Google Scholar]
- 18.Kouvaraki M, Shapiro S, Fornage B, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134(6):946–954. doi: 10.1016/s0039-6060(03)00424-0. discussion 954-5. [DOI] [PubMed] [Google Scholar]
- 19.Simon D, Goretzki P, Witte J, et al. Incidence of regional recurrence guiding radicality in differentiated thyroid carcinoma. World J Surg. 1996;20(7):860–866. doi: 10.1007/s002689900131. discussion 866. [DOI] [PubMed] [Google Scholar]
- 20.Podnos Y, Smith D, Wagman L, et al. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71(9):731–734. doi: 10.1177/000313480507100907. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt D, Elson D, Mack E, et al. Initial lymph node dissection increases cure rates in patients with medullary thyroid cancer. Asian J Surg. 2007;30(2):108–112. doi: 10.1016/S1015-9584(09)60141-X. [DOI] [PubMed] [Google Scholar]
- 22.Sippel R, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13(5):539–547. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 23.Moley J, DeBenedetti M. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. 1999;229(6):880–887. doi: 10.1097/00000658-199906000-00016. discussion 887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machens A, Hauptmann S, Dralle H. Increased risk of lymph node metastasis in multifocal hereditary and sporadic medullary thyroid cancer. World J Surg. 2007;31(10):1960–1965. doi: 10.1007/s00268-007-9185-1. [DOI] [PubMed] [Google Scholar]
- 25.Brandi M, Gagel R, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 26.Chi D, Moley J. Medullary thyroid carcinoma: genetic advances, treatment recommendations, and the approach to the patient with persistent hypercalcitoninemia. Surg Oncol Clin N Am. 1998;7(4):681–706. [PubMed] [Google Scholar]
- 27.Sherrman SI. Anaplastic carcinoma: clinical aspects. In: L W, editor. Thyroid cancer: a comprehensive guide to clinical management. Totowa, NJ: Humana Press; 1999. pp. 319–325. [Google Scholar]
- 28.Pierie J, Muzikansky A, Gaz R, et al. The effect of surgery and radiotherapy on outcome of anaplastic thyroid carcinoma. Ann Surg Oncol. 9(1):57–64. doi: 10.1245/aso.2002.9.1.57. [DOI] [PubMed] [Google Scholar]
- 29.Mazzaferri E, Jhiang S. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 30.Hay I, Hutchinson M, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144(6):980–987. doi: 10.1016/j.surg.2008.08.035. discussion 987-8. [DOI] [PubMed] [Google Scholar]
- 31.Ross D, Litofsky D, Ain K, et al. Recurrence after treatment of micropapillary thyroid cancer. Thyroid. 2009;19(10):1043–1048. doi: 10.1089/thy.2008.0407. [DOI] [PubMed] [Google Scholar]
- 32.Pinchot SNSR, Chen H. Multi-targeted approach in the treatment of thyroid cancer. Therapeutics and Clinical Risk Management. 4(5):935–947. doi: 10.2147/tcrm.s3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiba E, Miyauchi K, Kobayashi T, et al. The effect of thyroid hormone on the growth of thyroid cancer. Gan To Kagaku Ryoho. 1989;16(12):3678–3684. [PubMed] [Google Scholar]
- 34.Biondi B, Filetti S, Schlumberger M. Thyroid-hormone therapy and thyroid cancer: a reassessment. Nat Clin Pract Endocrinol Metab. 2005;1(1):32–40. doi: 10.1038/ncpendmet0020. [DOI] [PubMed] [Google Scholar]
- 35.Bauer D, Nevitt M, Ettinger B, et al. Low thyrotropin levels are not associated with bone loss in older women: a prospective study. J Clin Endocrinol Metab. 1997;82(9):2931–2936. doi: 10.1210/jcem.82.9.4229. [DOI] [PubMed] [Google Scholar]
- 36.Pujol P, Daures J, Nsakala N, et al. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab. 1996;81(12):4318–4323. doi: 10.1210/jcem.81.12.8954034. [DOI] [PubMed] [Google Scholar]
- 37.Cooper D, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8(9):737–744. doi: 10.1089/thy.1998.8.737. [DOI] [PubMed] [Google Scholar]
- 38.National Cancer Institute Surveillance Epidemiology and End Results Database. [Accessed December 8, 2009];US National Institutes of Health. Available at: http://seer.cancer.gov/statfacts/html/thyro.html.
- 39.Nikiforov Y. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21 Suppl 2:S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura E, Nikiforova M, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63(7):1454–1457. [PubMed] [Google Scholar]
- 41.Yeh M, Rougier J, Park J, et al. Differentiated thyroid cancer cell invasion is regulated through epidermal growth factor receptor-dependent activation of matrix metalloproteinase (MMP)-2/gelatinase A. Endocr Relat Cancer. 2006;13(4):1173–1183. doi: 10.1677/erc.1.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunnimalaiyaan M, Vaccaro A, Ndiaye M, et al. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6(3):1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 43.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 44.Nikiforova M, Lynch R, Biddinger P, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88:2318–2326. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 45.Mulligan L, Marsh D, Robinson B, et al. Genotype-phenotype correlation in multiple endocrine neoplasia type 2: report of the International RET Mutation Consortium. J Intern Med. 1995;238(4):343–346. doi: 10.1111/j.1365-2796.1995.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 46.Hansford J, Mulligan L. Multiple endocrine neoplasia type 2 and RET: from neoplasia to neurogenesis. J Med Genet. 2000;37(11):817–827. doi: 10.1136/jmg.37.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santoro M, Melillo R, Carlomagno F, et al. Molecular biology of the MEN2 gene. J Intern Med. 1998;243(6):505–508. doi: 10.1046/j.1365-2796.1998.00330.x. [DOI] [PubMed] [Google Scholar]
- 48.Knauf J, Kuroda H, Basu S, et al. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003;22(28):4406–4412. doi: 10.1038/sj.onc.1206602. [DOI] [PubMed] [Google Scholar]
- 49.Melillo R, Castellone M, Guarino V, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115(4):1068–1081. doi: 10.1172/JCI22758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Mitsutake N, Knauf J, Mitsutake S, et al. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. 2005;65(6):2465–2473. doi: 10.1158/0008-5472.CAN-04-3314. [DOI] [PubMed] [Google Scholar]
- 51.Adeniran A, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30(2):216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 52.Herbst R, Heymach J, O'Reilly M, et al. Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opin Investig Drugs. 2007;16(2):239–249. doi: 10.1517/13543784.16.2.239. [DOI] [PubMed] [Google Scholar]
- 53.Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62(24):7284–7290. [PubMed] [Google Scholar]
- 54.Santoro M, Carlomagno F. Drug insight: Small-molecule inhibitors of protein kinases in the treatment of thyroid cancer. Nat Clin Pract Endocrinol Metab. 2006;2(1):42–52. doi: 10.1038/ncpendmet0073. [DOI] [PubMed] [Google Scholar]
- 55.Sherman S. Early clinical studies of novel therapies for thyroid cancers. Endocrinol Metab Clin North Am. 2008;37(2):511–524. doi: 10.1016/j.ecl.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Coxon A, Bready J, Fiorino M, et al. Anti-tumor activity of AMG 706, an oral multi-kinase inhibitor, in human medullary thyroid carcinoma xenografts. Thyroid. 2006;16(9):920. [Google Scholar]
- 57.Rosen L, Kurzrock R, Mulay M, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25(17):2369–2376. doi: 10.1200/JCO.2006.07.8170. [DOI] [PubMed] [Google Scholar]
- 58.Schlumberger M, Elisei R, Sherman SI, et al. Phase 2 trial of motesanib diphosphate (AMG 706) in patients with medullary thyroid cancer (MTC). Presented at 89th Annual Meeting of the Endocrine Society; June 2007; Toronto (ON). [Google Scholar]
- 59.Smallridge R, Marlow L, Copland J. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16(1):17–44. doi: 10.1677/ERC-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciampi R, Nikiforov Y. Alterations of the BRAF gene in thyroid tumors. Endocr Pathol. 2005;16(3):163–172. doi: 10.1385/ep:16:3:163. [DOI] [PubMed] [Google Scholar]
- 61.Nikiforova M, Kimura E, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88(11):5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 62.Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88(9):4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 63.Xing M, Westra W, Tufano R, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90(12):6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 64.Kim T, Kim W, Rhee Y, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65(3):364–368. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 65.Salvatore G, De Falco V, Salerno P, et al. BRAF is a therapeutic target in aggressive thyroid carcinoma. Clin Cancer Res. 2006;12(5):1623–1629. doi: 10.1158/1078-0432.CCR-05-2378. [DOI] [PubMed] [Google Scholar]
- 66.Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8(2):148–156. doi: 10.1016/S1470-2045(07)70034-7. [DOI] [PubMed] [Google Scholar]
- 67.Espinosa A, Porchia L, Ringel M. Targeting BRAF in thyroid cancer. Br J Cancer. 2007;96(1):16–20. doi: 10.1038/sj.bjc.6603520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braga-Basaria M, Ringel M. Clinical review 158: Beyond radioiodine: a review of potential new therapeutic approaches for thyroid cancer. J Clin Endocrinol Metab. 2003;88(5):1947–1960. doi: 10.1210/jc.2002-021863. [DOI] [PubMed] [Google Scholar]
- 69.Akslen L, Myking A, Salvesen H, et al. Prognostic impact of EGF-receptor in papillary thyroid carcinoma. Br J Cancer. 1993;68(4):808–812. doi: 10.1038/bjc.1993.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holting T, Siperstein A, Clark O, et al. Epidermal growth factor (EGF)- and transforming growth factor alpha-stimulated invasion and growth of follicular thyroid cancer cells can be blocked by antagonism to the EGF receptor and tyrosine kinase in vitro. Eur J Endocrinol. 1995;132(2):229–235. doi: 10.1530/eje.0.1320229. [DOI] [PubMed] [Google Scholar]
- 71.Gabler B, Aicher T, Heiss P, et al. Growth inhibition of human papillary thyroid carcinoma cells and multicellular spheroids by anti-EGF-receptor antibody. Anticancer Res. 17(4B):3157–3159. [PubMed] [Google Scholar]
- 72.Pietras R, Fendly B, Chazin V, et al. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9(7):1829–1838. [PubMed] [Google Scholar]
- 73.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8(6):709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 74.Kunnimalaiyaan M, Haymart M, Chen H. Tumor suppressor role of Notch-1 and Raf-1 signaling in medullary thyroid cancer cells. Translational Ocogenomics. 2007;2:43–47. [PMC free article] [PubMed] [Google Scholar]
- 75.Ning L, Greenblatt D, Kunnimalaiyaan M, et al. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008;13(2):98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- 76.Greenblatt D, Cayo M, Adler J, et al. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008;247(6):1036–1040. doi: 10.1097/SLA.0b013e3181758d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao X, Ning L, Chen H. Notch1 mediates growth suppression of papillary and follicular thyroid cancer cells by histone deacetylase inhibitors. Mol Cancer Ther. 2009;8(2):350–356. doi: 10.1158/1535-7163.MCT-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hardt S, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90(10):1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 79.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2(10):769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 80.Adler J, Cook M, Luo Y, et al. Tautomycetin and tautomycin suppress the growth of medullary thyroid cancer cells via inhibition of glycogen synthase kinase-3beta. Mol Cancer Ther. 2009;8(4):914–920. doi: 10.1158/1535-7163.MCT-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cunningham C, Holmlund J, Geary R, et al. A Phase I trial of H-ras antisense oligonucleotide ISIS 2503 administered as a continuous intravenous infusion in patients with advanced carcinoma. Cancer. 2001;92(5):1265–1271. doi: 10.1002/1097-0142(20010901)92:5<1265::aid-cncr1447>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 82.Kroll T, Sarraf P, Pecciarini L, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected] Science. 2000;289(5483):1357–1360. doi: 10.1126/science.289.5483.1357. [DOI] [PubMed] [Google Scholar]
- 83.French C, Alexander E, Cibas E, et al. Genetic and biological subgroups of low-stage follicular thyroid cancer. Am J Pathol. 2003;162(4):1053–1060. doi: 10.1016/S0002-9440(10)63902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dwight T, Thoppe S, Foukakis T, et al. Involvement of the PAX8/peroxisome proliferator-activated receptor gamma rearrangement in follicular thyroid tumors. J Clin Endocrinol Metab. 2003;88(9):4440–4445. doi: 10.1210/jc.2002-021690. [DOI] [PubMed] [Google Scholar]
- 85.Gregory Powell J, Wang X, Allard B, et al. The PAX8/PPARgamma fusion oncoprotein transforms immortalized human thyrocytes through a mechanism probably involving wild-type PPARgamma inhibition. Oncogene. 2004;23(20):3634–3641. doi: 10.1038/sj.onc.1207399. [DOI] [PubMed] [Google Scholar]
- 86.Giordano T, Au A, Kuick R, et al. Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8-PPARG translocation. Clin Cancer Res. 2006;12(7 Pt 1):1983–1993. doi: 10.1158/1078-0432.CCR-05-2039. [DOI] [PubMed] [Google Scholar]
- 87.Kebebew E, Peng M, Reiff E, et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery. 2006;140(6):960–966. doi: 10.1016/j.surg.2006.07.038. discussion 966-7. [DOI] [PubMed] [Google Scholar]