Abstract
Mast cell-derived prostaglandin D2 (PGD2) is the major prostanoid found within the airway of asthmatics immediately following allergen challenge. PGD2 has been shown to have chemokinetic effects on eosinophils and T helper type 2 (Th2) cells in vitro. This occurs through the interaction of PGD2 with the G-protein-coupled chemokine receptor homologous molecule expressed on Th2 lymphocytes (CRTH2). The expression of CRTH2 has been shown to be highly selective for Th2 cells. Using flow cytometry we have studied the expression of CRTH2 on T cells in blood and bronchoalveolar lavage fluid in asthmatics and normal subjects. CRTH2 expression was confined to a small percentage of blood T cells in asthmatics (1·8% ± 0·2) and normal (1·6% ± 0·2) subjects. CRTH2 was enriched significantly on interleukin (IL)-4+/IL-13+ T cells compared to interferon (IFN)-γ+ T cells (P < 0·001). There was a small population of CRTH2+ T cells in the bronchoalveolar lavage (BAL) of asthmatics (2·3% ± 0·6) and normal subjects (0·3% ± 0·1), and there was a significant difference between the two groups (P < 0·05). There were similar amounts of PGD2 in the BAL of asthma and normal subjects. Within paired blood–BAL samples from the same subject there was no increase in CRTH2+ T cells in the BAL compared to blood in asthmatics. Enrichment of CRTH2 on IL-4+ and IL-13+ T cells compared to IFN-γ+ T cells was also seen in BAL from asthmatics (P < 0·001). CRTH2 is expressed preferentially by IL-4+/IL-13+ T cells compared to IFN-γ+ T cells. However, given their small numbers they are unlikely to have a significant involvement in the pathogenesis of asthma. CRTH2 antagonism may not diminish T cell accumulation in the asthmatic lung.
Keywords: asthma, CRTH2, lung T cell
Introduction
The release of preformed mediators from resident lung cells is thought to contribute to the typical early asthma response following allergen encounter. The arachidonic acid metabolite prostaglandin D2 (PGD2) is a lipid mediator known to be released rapidly by immunoglobulin (Ig)E-activated mast cells in significant quantities upon allergen exposure [1]. Dendritic cells are also known sources of PGD2[2]. In asthmatics, large quantities of PGD2 are detected in bronchoalveolar lavage fluid within minutes of allergen encounter. PGD2 not only exerts local effects within the asthmatic airway, but may also play a role in recruiting and activating leucocytes that could then contribute to the late asthma response [3].
The proinflammatory effects of PGD2 include induction of local vasodilatation which also contributes to oedema, recruitment of eosinophils and T helper type 2 (Th2) cells, induction of Th2 cytokine synthesis and in asthma causes bronchoconstriction [4,5]. PGD2 is known to exert its biological effects via three G-protein coupled receptors; D prostanoid 1 (DP1), TP (thromboxane A2 receptor) and the recently characterized receptor CRTH2 (chemokine receptor homologous molecule expressed on T helper type 2 cells) [3]. Despite their local effects, neither DP1 nor TP receptor had any effect on inflammatory cell activation or recruitment. The recruitment and activation of eosinophils and Th2 cells by PGD2 appears to be mediated primarily via CRTH2 [6]. The CRTH2 receptor also interacts with many of the PGD2 metabolites, including 15d-PGJ2, which has an anti-inflammatory role [7].
In vitro, activation of CRTH2 (also known as the DP2 receptor) was shown to induce migration of eosinophils [8], Th2 cells and basophils [9–11]. A selective CRTH2 agonist was shown to exacerbate pathology in a mouse model of asthma [12] and Ramatroban, a moderate CRTH2 and strong TP antagonist, was shown to attenuate eosinophil accumulation into the mouse airway [13]. PGD2 was shown to induce interleukin (IL)-4, IL-5 and IL-13 production by Th2 cells in humans via a CRTH2-dependent mechanism [14]. This effect was also inhibited by Ramatroban.
Cytokines from Th2 cells are recognized to relate closely to a number of aspects of asthma pathophysiology [15]. CRTH2 has been suggested to be the most reliable marker of circulating Th2 cells [16]. The selective recruitment of Th2 cells to the airway by mast cell-derived PGD2 acting via CRTH2 provides a convenient mechanism by which the innate immune system links with the adaptive immune response in propagating the inflammatory response. Functional polymorphisms of the CRTH2 gene within certain populations have been linked with severe forms of asthma [17]. A short-term human asthma study has shown that treatment with Ramatroban resulted in reduced airway hyper-responsiveness [18]. In some animal models of asthma, eosinophil accumulation and IL-5 production was shown to be enhanced in CRTH2-deficient mice [19]; however, this may be explained in part by the fact that mouse Th2 cells do not express CRTH2 preferentially.
Given the possible involvement of CRTH2 in asthmatic inflammation and its potential to lead to future pharmacological intervention, further evidence is needed for the direct involvement of CRTH2 in human asthma. There have been no studies to date demonstrating the direct role of CRTH2 in the recruitment of Th2 cells in human asthma. Using a commercially available monoclonal antibody (mAb) to CRTH2, we have studied the role of CRTH2 in T cell recruitment to the lung in asthma.
Materials and methods
Subjects
A total of 24 subjects with asthma and 20 normal subjects were included in the study. Patients were recruited from Glenfield Hospital in Leicester, UK. Subjects were characterized using clinical history and lung function. Asthma was diagnosed as described previously [20]. Asthma subjects had a suggestive history with recurrent cough, wheeze, sputum production and either airway hyperresponsiveness (Pc20 < 8 mg/ml) or reversible airflow obstruction [forced expiratory volume in 1 s (FEV1) > 12%]. Normal subjects had no history of respiratory disease. Methacholine challenge was performed using a Wright's nebulizer and results are expressed as the provoking concentration of methacholine required to elicit a 20% decrease in FEV1 (Pc20). Atopic status was assessed by measurement of total IgE concentration and skin prick tests to common aeroallergens (a wheal diameter > 3 mm compared to control was defined as positive). Subject characteristics are given in Table 1. Not all subjects had a bronchoscopy. Characteristics of those who had a bronchoscopy are listed separately within Table 1. The patients in this study are also the subject of a separate report on CCR8 expression in asthma (in press).
Table 1.
Characteristics of all asthmatics and normal subjects who were studied are given in the first two columns.
| Normal | Asthma | Bronchoscopy normal | Bronchoscopy asthma | |
|---|---|---|---|---|
| n | 20 | 24 | 7 | 11 |
| Age† | 30 (19–56) | 42 (20–66) | 24 (21–49) | 44 (36–60) |
| Male | 7 | 16 | 3 | 5 |
| Atopy | 6 | 17 | 1 | 4 |
| IgE‡ (kU/l) | 17 (7·2) | 513 (190) | 101 (34) | 613 (322) |
| FEV1%Pred‡ | 94 (9) | 83 (3·9) | 100 (6·3) | 74 (7·2) |
| Pc20§ | > 16 | 1·0 (0·8) | > 16 | 0·6 (0·8) |
| On OCS | 0 | 8 | 0 | 5 |
The characteristics of only those who underwent bronchoscopy are given separately in the last two columns.
Median (range).
Mean (standard error of the mean).
Geometric mean (log standard deviation). FEV1, forced expiratory volume in 1 s; Ig, immunoglobulin; OCS, oral glucocorticosteroids.
All but five asthmatics (mild intermittent asthmatics) were treated with regular inhaled corticosteroids. Eight asthmatics were using oral and inhaled corticosteroids. Among the asthmatics, 12 had refractory asthma according to the American Thoracic Society (ATS) criteria. Bronchoscopy was performed on only 11 of the asthmatics and seven normal subjects. Of the 11 asthmatics investigated with bronchoscopy, nine had severe asthma, and of these five were on oral corticosteroids. The study was approved by the local ethics committee and written informed consent was obtained from all participants.
Bronchoscopy
Ten ml of venous blood was obtained prior to the procedure for isolation of peripheral blood mononuclear cells (PBMC). Bronchoscopy was performed using local anaesthesia according to standard techniques. Asthmatic subjects were premedicated with 2·5 mg of nebulized Salbutamol 20 min prior to the procedure. Mild sedation with midazolam was used in some subjects. Bronchoalveolar lavage (BAL) was performed by sequential instillation and aspirations of three aliquots of 60 ml of warmed sterile 0·9% saline. BAL fluid was placed into and transported in ice prior to analysis. Biopsies were first fixed in 4% paraformaldehyde and then embedded in paraffin.
Isolation of PBMC and BAL T cells
Heparinized venous blood (10–15 ml) was obtained from all subjects. PBMC were isolated from whole blood using density gradient centrifugation (Histopaque 1077; Sigma, Poole, UK). PBMC were washed and suspended in phosphate-buffered saline (PBS) (Invitrogen, Paisley, UK) + 0·5% bovine serum albumin (BSA) (Sigma) during staining and subsequent flow cytometry. First, BAL samples were filtered through 48-µm nylon gauze to remove debris. Cells were pelleted and red cell lysis was performed if significant blood contamination had occurred. Supernatants were collected and stored at −80°C for estimation of PGD2. Red cell lysis was performed by incubating the cellular pellet with a small volume of ice-cold distilled water for 20 s followed by quenching with a large volume of isotonic PBS.
Flow cytometry
All samples were labelled with anti-CRTH2 and anti-CD3. Anti-CD4, anti-CD8 (Becton Dickinson Biosciences, Oxford, UK) and anti-CD45RO (Caltag, Buckingham, UK) were also used in some samples. PBMC and BAL cells were incubated with biotinylated anti-CRTH2 mAb (Mitenyi Biotec, Bergisch Gladbach, Germany) at 4°C while suspended in PBS and 0·5% BSA. Streptavidin–phycoerythrin (Caltag) was used as the secondary agent. Intracellular cytokine was studied in some of the samples. Where intracellular cytokine was studied, cells were first incubated with anti-CRTH2 and then stimulated using 25 ng/ml of phorbol myristate acetate (PMA) (Sigma) and 500 ng/ml of calcium ionophore A2317 (Sigma) in the presence of 10 µg/ml Brefeldin (Sigma) for 6 h at 37°C. Cells were then washed and fixed using 4% paraformaldehyde before being permeabilized with a 0·1% solution of Saponin. Cells were then stained for intracellular IL-4, IL-13 and interferon (IFN)-γ using directly labelled mAbs followed by CD3-peridinin chlorophyll (PerCP). Samples were analysed on a fluorescence activated cell sorter (FACS) Canto using FACS Diva software (Becton Dickinson immunocytometry system). At least 10 000 live cells were acquired from each sample for the analysis of surface CRTH2. The percentage of CRTH2+ cells was estimated within the CD3 gated population. The percentages of CD3+ cells that were cytokine-positive were low, and on average 30 000–40 000 PBMC, and BAL cells were analysed from each sample when intracellular cytokines were studied. Isoytpe controls were included for all tests. Samples showing non-specific binding with isotype control (> 0·1%) were eliminated from analysis.
Prostaglandin D2 enzyme-linked immunosorbent assay (ELISA)
Detectable quantities of PGD2 were found in BAL samples from asthma and normal subjects without the need for concentration. PGD2 was measured in BAL samples using a commercially available PGD2 ELISA kit [prostaglandin-D2 MOX enzyme immunoassay kit from Cayman Chemicals (Tallinn, Estonia)]. The detection limit for this product was 6 pg/ml.
Statistical analysis
Subject characteristics are described using descriptive statistics. Means are expressed together with standard error of the mean (s.e.m.). Median values were expressed with interquartile ranges (IQR). Statistical analysis was performed with GraphPad Prism (GraphPad Software Inc.). Differences between groups were assessed using the Mann–Whitney U-test. Pearson's or Spearman's tests were used to assess correlation. A P value < 0·05 was considered statistically significant. This was an explorative study and power calculation had not been possible, as the level and variation of the expression of CRTH2 in human blood or BAL was not known.
Results
The percentage of CRTH2+ T cells in blood was similar in subjects with asthma and normal controls
Among PBMC, CRTH2 expression was seen mainly on non-T cells (∼3%) (Fig. 1a). The non-T cells were confirmed as basophils using IgεR1 (results not shown). Only a small number of CRTH2+ T cells were seen in blood from asthmatics (1·8% ± 0·2) and normal subjects (1·6% ± 0·2), with no significant difference between the groups (two asthma subjects were not included in the analysis (because of what appeared to be high levels of non-specific staining) (Fig. 1b). The majority were CD4+ T cells (62·8% ± 2·1; n = 5). CRTH2+ T expression was seen on 30·9% ± 1·5 (n = 5) of CD8 T cells. 68% ± 9·8 (n = 5) were CD45RO+. Given the association of CRTH2 with allergy, we also investigated whether CRTH2+ T cells were associated with the allergy serum markers. CRTH2+ T cell numbers did not show any significant correlation with atopy, serum IgE levels (r2 = 0·2; P = 0·28) or blood eosinophil numbers (r2 = 0·1; P = 0·5).
Fig. 1.
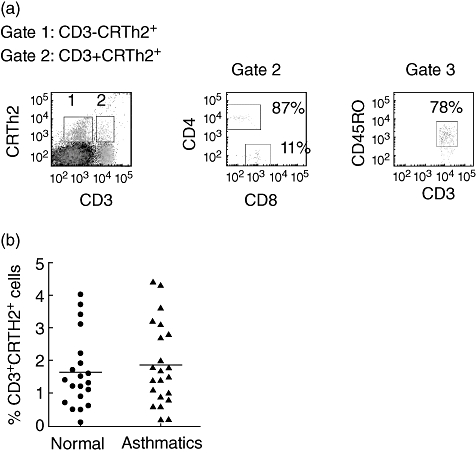
(a) Representative fluorescence activated cell sorter (FACS) dot plots showing association of chemokine receptor homologous molecule expressed on T helper type 2 (Th2) lymphocytes (CRTH2) with CD3, CD4 and CD8, and CD45RO. The gates were set with reference to the isotype control for each antibody. There was no difference in the degree of staining (mean fluorescence) on CRTh2-positive T cells from asthmatics and controls. (b) Scatter-plot of the percentages of CRTH2+ T cells in blood of normal subjects (•) and asthmatics (▴). Bars represent the mean. No significant difference in the percentage of CRTH2+ T cells was seen between the groups.
CRTH2 is enriched on IL-4 and IL-13 expressing T cells in blood compared to IFN-γ-expressing T cells
The association of blood T cell CRTH2 expression with intracellular cytokine was studied following in vitro stimulation of PBMC with PMA and calcium ionophore for 6 h. CRTH2 was expressed by a higher percentage of IL-4+ and IL-13+ T cells than IFN-γ+ T cells. In asthma, 26·5% ± 4·5 and 35·5% ± 3·3 of the IL-4+- and IL-13+-producing T cells also expressed CRTH2, while only 2·4% ± 0·5 of IFN-γ+ cells also expressed CRTH2 (P < 0·001) (Fig. 2a). A similar pattern of expression of CRTH2 on IL-4+ and IL-13+ T cells relative to IFN-γ+ T cells was also seen in blood of normal subjects (Fig. 2b).
Fig. 2.
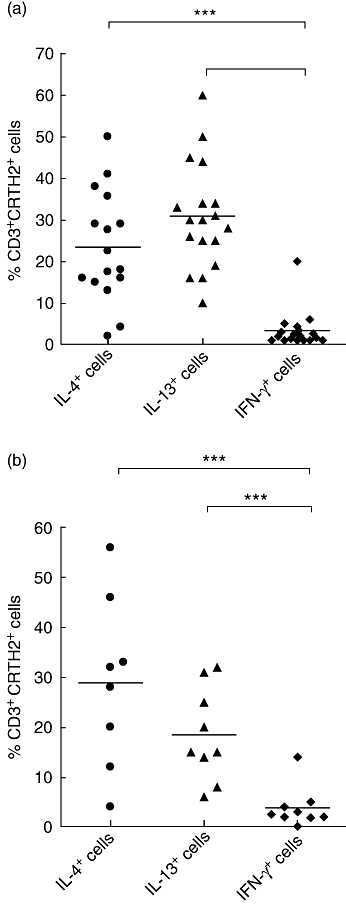
(a) Scatter-plot showing chemokine receptor homologous molecule expressed on T helper type 2 (Th2) lymphocytes (CRTH2) expression on (♦) interferon (IFN)-γ+, (•) interleukin (IL)-4+ and (▴) IL-13+ T cells derived from peripheral blood mononuclear cells (PBMC) from asthmatic subjects. Cells were stimulated for 6 h with phorbol myristate acetate (PMA) and calcium ionophore. ***P < 0·001 using the Mann–Whitney U-test. Bars represent mean percentages. (b) Scatter-plot showing CRTH2 expression on (♦) IFN-γ+, (•) IL-4+ and (▴) IL-13+ T cells derived from PBMC from normal subjects. Cells were stimulated for 6 h with PMA and calcium ionophore. ***P < 0·001 using the Mann–Whitney U-test. Bars represent mean percentages.
There were more CRTH2+ T cells in the BAL of asthmatics compared to normal subjects
Although BAL was taken from 12 asthma subjects, one sample had be eliminated due to very poor quality and non-specific staining. There was only a small number of CRTH2+ T cells in the BAL in both groups. Nevertheless, there was a significant difference between the two groups (2·3% ± 0·6 in asthmatics versus 0·3% ± 0·1 in normal subjects; P < 0·05) (Fig. 3a). We also compared the percentages of CRTH2+ T cells in paired blood and BAL samples from the same subject. No significant difference was seen in asthmatics; however, there was a significant difference in normal subjects (0·8% ± 0·1 in blood versus 0·3% ± 0·1 in BAL; P < 0·05) (Fig. 3b).
Fig. 3.
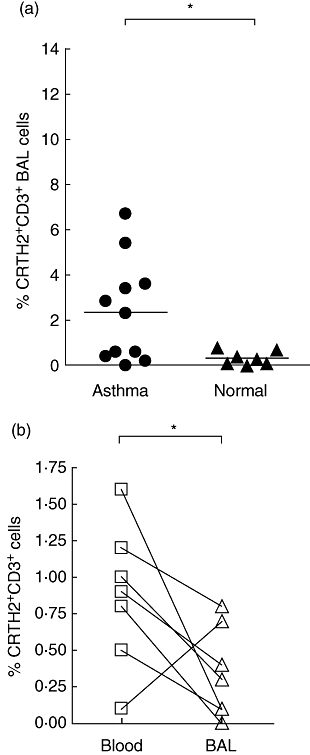
(a) Chemokine receptor homologous molecule expressed on T helper type 2 (Th2) lymphocytes (CRTH2)+ T cells in bronchoalveolar lavage (BAL) from asthmatic (•) and normal (▴) subjects. There was a significant difference between asthmatic and normal controls. *P < 0·05 using the Mann–Whitney U-test. Bars represent mean percentages. (b) CRTH2+ T cells in paired blood (□) and BAL (▵) samples from normal subjects. Lines connect samples from the same subject. *P < 0·05 using the Mann–Whitney U-test.
CRTH2 expression was enriched on IL-4 and IL-13 expressing BAL T compared to IFN-γ-expressing T cells
Intracellular cytokine content in BAL T cells was studied in only five of the 11 subjects who had BAL samples taken, due to lack of sufficient cell numbers from some of the samples. Cells were stimulated with PMA and calcium ionophore for 6 h. In asthmatic subjects CRTH2 was expressed by 17·3% ± 5·3 of the IL-4+ cells and 16% ± 6·4 of the IL-13+ cells. In contrast, only 1·2% ± 0·1 of IFN-γ+ cells expressed CRTH2 (P < 0·01; Fig. 4). The expression of CRTH2 on BAL T cells from normal subjects was too small for this assessment to be made accurately.
Fig. 4.
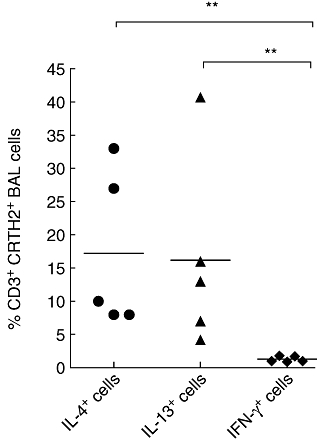
Scatter-plot of the percentage of chemokine receptor homologous molecule expressed on T helper type 2 (Th2) lymphocytes (CRTH2)+ bronchoalveolar lavage (BAL) T cells from asthmatic subjects expressing interferon (IFN)-γ (♦), interleukin (IL)-4 (•) and IL-13 (▴). **P < 0·01 using the Mann–Whitney U-test. Bars represent mean percentages.
There was no difference in the concentration of PGD2 in BAL between and asthmatics and normal subjects
Although there was no difference in the percentage of CRTH2+ T cells between BAL and blood in asthma, there was a higher percentage of these cells in asthmatic BAL compared to normal controls. We have therefore measured the ligand for CRTH2, PGD2 in BAL. Although a significant difference was not seen between the two groups, a bimodal distribution of PGD2 levels was seen within the asthma group (Fig. 5). A similar distribution was seen with CRTH2+ T cells in BAL in asthma (Fig. 3a). We therefore explored whether there was a correlation between the percentages of CRTH2+ T cells and PGD2 within the same BAL sample. Due to the small numbers, Spearman's correlation test was used. A significant correlation was not seen (r2 = 0·4; P = 0·07).
Fig. 5.
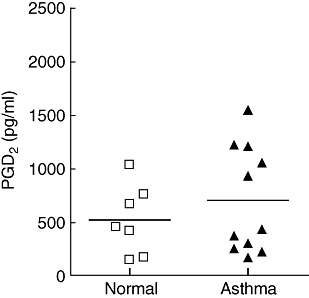
Levels of prostaglandin D2 (PGD2) in bronchoalveolar lavage (BAL) fluid from asthmatic (□) and normal subjects (▴). Bars represent mean percentages. No significant difference was seen between the two groups.
Discussion
The inflammatory process in asthma is considered to be driven in part by cytokines derived from Th2 lymphocytes recruited into the airway. The mechanisms controlling migration of Th2 cells into the airway are therefore of interest. CRTH2 has been suggested to be a marker for Th2 cells [16]. It binds to the ligand PGD2 known to be released in large quantities by mast cells in response to allergen exposure [1]. PGD2 has therefore attracted considerable interest due its potential for recruiting Th2 cells to the asthma lung. We believe that this is the first study to report expression of CRTH2 on T cells in asthma. We found that CRTH2 is expressed by a greater percentage of IL-4+ T cells than IFN-γ+ T cells. However, we found only very low levels of expression of CRTH2 in both blood and BAL T cells; nevertheless, a significant difference was seen in CRTH2+ BAL T cells in asthmatics compared to controls. Asthmatics had a greater percentage of CRTH2+ T cells in their BAL than normal controls.
We found that CRTH2 was expressed by only a small percentage of blood T cells in asthma (∼1·8%) and there was no difference in the percentage of these cells in blood from asthma compared to normal subjects. The percentages of IL-4+/IL-13+ T cells in our study also included CD8+ T cells (Tc2). Furthermore, cells in our study were single-stained with anti-IL-4 or anti-IFN-γ, and some of the IL-4+ T cells may also be IFN-γ+ (Th0 cells). Given these limitations, it would be difficult to suggest a definite association of CRTH2 with Th2 cells. None the less, our data provide some evidence that they are associated with Th2 cells. CRTH2 was expressed on ∼8–10-fold higher percentages of IL-4+ T cells than IFN-γ+ T cells.
The absolute numbers of IL-4+ or IL-13+ T cells that are CRTH2+ were small and only one sample was taken from each subject in our study, but the data for PBMC is from a reasonable number of subjects and the range of expression small, so we feel the lack of a difference between asthmatics and normal subjects is a robust finding. The small numbers of patients included for BAL analysis is a limitation of the study but, again, the spread of the data is small, demonstrating that the low-level expression seen is a consistent finding.
The expression of CRTH2 on BAL T cells in asthma has the appearance of being bimodal. While this could be an artefact of the small numbers of subjects and there was no significant correlation between BAL PGD2 concentrations and the percentage of BAL CRTH2 cells, the five subjects within the group who had low numbers of CRTH2+ T cells in BAL also had lower levels of PGD2 (< 500 pg/ml). This may indicate that the PGD2 may be playing a role in recruiting CRTH2+ T cells to the lung in some asthmatics. Some of the asthmatics were taking oral corticosteroids; however, there was no significant difference in the percentages of BAL CRTH2+ T cells or PGD2 levels between this group and those not on oral corticosteroids.
We did not see a significant increase in CRTH2+ T cells in BAL compared to blood within the same subject. In fact, in the normal group there was a lower percentage of CRTH2 expressing T cells in the BAL compared with blood. We cannot explain this readily. It is possible that this could be due to down-regulation of the receptor due to interaction with PGD2 in the lung, or could be related to more activated cells being present in the lung. However, only low levels of PGD2 would be expected to be present in the normal lung, and we have shown that short-term activation with PMA and calcium ionophore did not affect expression of CRTH2 (data not shown).
PGD2 has been shown to be able to modulate T cells locally and encourage Th2 cell polarization through interaction with the DP1 and CRTH2 receptor [3]. The DP1 receptor is expressed mainly by Th1 cells, and its interaction with PGD2 results in the inhibition of cytokines by these cells. CRTH2, on the other hand, has been shown to be involved in the induction of cytokines by Th2 cells [14]. CRTH2+ T cells were shown to be able to produce more PGD2, providing an autocrine amplification mechanism. It has been suggested that PGD2, by acting on DP1 and CRTH2 receptors, could drive a state of Th2 polarization within the tissue [14]. However, given the small number of CRTH2+ cells we had observed among BAL T cells, it would seem unlikely for CRTH2 to have a significant functional role in modulating T cells in asthma pathogenesis.
CRTH2 has been suggested to be the most reliable marker for circulating human Th2 cells [16]. In this study ∼26% of IL-4+ T cells and ∼35% of IL-13+ T cells in blood expressed CRTH2 compared to ∼2% of IFN-γ+ T cells, indicating a degree of specificity for IL-4+/IL-13+ T cells. In a previous study we have shown that ∼40% of IL-4+ blood and BAL T cells express CCR4; however, in the same study ∼15% of IFN-γ+ T cells also expressed CCR4, which may suggest that CCR4 is less specific than CRTH2 as a marker for IL-4+ T cells [21]. The downside for CRTH2 as a Th2 marker in asthma is that its expression was seen in only ∼17% of IL-4+ T cells in the BAL of asthmatics. Conversely, we have shown that ∼30% of IL-4+ T cells in the BAL of asthmatics express CCR8, and this receptor also showed a similar degree of specificity for Th2 cells as CRTH2 (paper under submission).
In summary, although we found a significant difference in the percentage of CRTH2+ T cells in the lung between asthmatics and normal subjects, the percentage of these cells in BAL in both groups is very low. It is therefore difficult to conclude whether they have a significant biological role in the pathogenesis of asthma. There was no evidence of enrichment of CRTH2 within the lung compared to blood in asthmatics or normal subjects, and furthermore we had not been able to demonstrate a difference in the level of PGD2 in the BAL fluid between asthmatics and normal controls. Our data therefore do not provide evidence to support CRTH2 playing a role in recruiting T cells to the lung.
Acknowledgments
This study was part-funded by the Wellcome Trust. This study was also part-funded by Glaxo Smith Kline.
Disclosure
None.
References
- 1.Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ., II Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol. 1982;129:1627–31. [PubMed] [Google Scholar]
- 2.Gosset P, Pichavant M, Faveeuw C, Bureau F, Tonnel AB, Trottein F. Prostaglandin D2 affects the differentiation and functions of human dendritic cells: impact on the T cell response. Eur J Immunol. 2005;35:1491–500. doi: 10.1002/eji.200425319. [DOI] [PubMed] [Google Scholar]
- 3.Pettipher R, Hansel TT, Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007;6:313–25. doi: 10.1038/nrd2266. [DOI] [PubMed] [Google Scholar]
- 4.Hirai H, Tanaka K, Yoshie O, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gervais FG, Cruz RP, Chateauneuf A, et al. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108:982–8. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- 6.Shiraishi Y, Asano K, Nakajima T, et al. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther. 2005;312:954–60. doi: 10.1124/jpet.104.078212. [DOI] [PubMed] [Google Scholar]
- 7.Pettipher R. The roles of the prostaglandin D(2) receptors DP(1) and CRTH2 in promoting allergic responses. Br J Pharmacol. 2008;153(Suppl 1):S191–S199. doi: 10.1038/sj.bjp.0707488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyles SL, Xue L, Townsend ER, Wettey F, Pettipher R. A dominant role for chemoattractant receptor-homologous molecule expressed on T helper type 2 (Th2) cells (CRTH2) in mediating chemotaxis of CRTH2+ CD4+ Th2 lymphocytes in response to mast cell supernatants. Immunology. 2006;119:362–8. doi: 10.1111/j.1365-2567.2006.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata K, Hirai H. The second PGD(2) receptor CRTH2: structure, properties, and functions in leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:169–77. doi: 10.1016/s0952-3278(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 10.Nagata K, Hirai H, Tanaka K, et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s) FEBS Lett. 1999;459:195–9. doi: 10.1016/s0014-5793(99)01251-x. [DOI] [PubMed] [Google Scholar]
- 11.Nagata K, Tanaka K, Ogawa K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–86. [PubMed] [Google Scholar]
- 12.Spik I, Brenuchon C, Angeli V, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–8. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto H, Shichijo M, Iino T, et al. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J Pharmacol Exp Ther. 2003;305:347–52. doi: 10.1124/jpet.102.046748. [DOI] [PubMed] [Google Scholar]
- 14.Xue L, Gyles SL, Wettey FR, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 15.Robinson DS. Th-2 cytokines in allergic disease. Br Med Bull. 2000;56:956–68. doi: 10.1258/0007142001903625. [DOI] [PubMed] [Google Scholar]
- 16.Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972–9. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Huang JL, Gao PS, Mathias RA, et al. Sequence variants of the gene encoding chemoattractant receptor expressed on Th2 cells (CRTH2) are associated with asthma and differentially influence mRNA stability. Hum Mol Genet. 2004;13:2691–7. doi: 10.1093/hmg/ddh279. [DOI] [PubMed] [Google Scholar]
- 18.Aizawa H, Shigyo M, Nogami H, Hirose T, Hara N. BAY u3405, a thromboxane A2 antagonist, reduces bronchial hyperresponsiveness in asthmatics. Chest. 1996;109:338–42. doi: 10.1378/chest.109.2.338. [DOI] [PubMed] [Google Scholar]
- 19.Chevalier E, Stock J, Fisher T, et al. Cutting edge: chemoattractant receptor-homologous molecule expressed on Th2 cells plays a restricting role on IL-5 production and eosinophil recruitment. J Immunol. 2005;175:2056–60. doi: 10.4049/jimmunol.175.4.2056. [DOI] [PubMed] [Google Scholar]
- 20.Brightling BE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. Th2 cytokine expression in bronchoalveolar lavage T-lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2002;110:899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 21.Morgan AJ, Symon FA, Berry MA, Pavord ID, Corrigan CJ, Wardlaw AJ. IL-4-expressing bronchoalveolar T cells from asthmatic and healthy subjects preferentially express CCR 3 and CCR 4. J Allergy Clin Immunol. 2005;116:594–600. doi: 10.1016/j.jaci.2005.03.052. [DOI] [PubMed] [Google Scholar]


