Abstract
While mechanical loading is known to be essential in maintaining tendon homeostasis, repetitive mechanical loading has also been implicated in the etiology of tendon overuse injuries. The purpose of this study was to determine whether cyclic mechanical stretching regulates inflammatory responses induced by interleukin-1β (IL-1β) treatment in human patellar tendon fibroblasts (HPTFs). HPTFs were grown in microgrooved silicone dishes, where they became elongated in shape and aligned with the microgrooves, which is similar to the shape and organization of tendon fibroblasts in vivo. Cyclic uniaxial stretching was then applied to silicone culture dishes with a 4% or 8% stretch at a stretching frequency of 0.5 Hz for a duration of 4 h in the presence or absence of 10 pM IL-1β treatment. Non-stretched cells in the presence or absence of IL-1β were used for controls, respectively. The expression of cyclooxygenase-2 (COX-2), matrix metalloproteinase-1 (MMP-1), and the production of prostaglandin E2 (PGE2) were measured. In the absence of stretching, it was found that 10 pM of IL-1β markedly induced higher levels of COX-2, MMP-1 gene expression, and PGE2 production than non-treated cells. Furthermore, cells with 4% stretching decreased the COX-2 and MMP-1 gene expression and PGE2 production that were stimulated by IL-1β, whereas cells with 8% stretching further increased these gene products and/or expression levels in addition to the effects of IL-1β stimulation. Thus, the results suggest that repetitive, small-magnitude stretching is anti-inflammatory, whereas large-magnitude stretching is pro-inflammatory. Therefore, moderate exercise may be beneficial to reducing tendon inflammation.
Keywords: Stretching, Tendon fibroblasts, IL-1β, COX-2, MMP-1, PGE2
1. Introduction
In occupational and athletic settings, tendon overuse injuries are a common problem. The clinical symptoms of tendon overuse injuries include pain, regional swelling, and soreness (Jozsa LG, 1997; Smith, 2000). Tendon injuries are accompanied with inflammation, which is characterized by an upregulation of IL-1β, COX-2 expression, and PGE2 production. IL-1β is a potent pro-inflammatory cytokine produced mainly by macrophages in injured tissues and upregulates the expression of other inflammatory mediators, including COX-2, IL-6, MMP-1, and MMP-3. In fibroblasts, for example, IL-1β induces the expression of MMPs (Tewari et al., 1994; Alvares et al., 1995), which could initiate the degradation and remodeling of tissues (e.g., tendons) in response to mechanical loading (Vailas et al., 1985). COX-2 is a major marker of tissue inflammation (Gilroy et al., 1998; Lipsky, 1999) that converts arachidonic acid into prostaglandins, such as PGE2. PGE2 is one of the major lipid mediators of pain and acute inflammation in tendons and other tissues (Narumiya et al., 1999; Fiorucci et al., 2001).
While tendon overuse injury implies that repetitive mechanical loading can be detrimental to tendons, numerous studies have shown that appropriate mechanical stimulation is essential for tendon homeostasis. This is also true for those tissues that bear and transmit mechanical loads, including muscles, bone, cartilage, and ligaments (Banes et al., 1995). Previous studies have shown that low tensile strains (≤6%) down-regulate the IL-1β stimulated expression of pro-inflammatory genes, COX-2 and MMP-1, and the production of PGE2, whereas large tensile strains had the opposite effect in osteoblast-like periodontal ligament cells (Long et al., 2002; Agarwal et al., 2003) and in rabbit chondrocytes (Xu et al., 2000; Agarwal et al., 2001). From these studies, we hypothesized that in HPTFs, cyclic mechanical stretching is anti-inflammatory for low stretching magnitudes and pro-inflammatory for large stretching magnitudes. To test this hypothesis, we measured the gene expression of COX-2 and MMP-1 and the secreted production of PGE2 by HPTFs in the presence of IL-1β and cyclic mechanical stretching conditions. We found that cyclic uniaxial stretching at a low stretching magnitude (4%) reduced the mRNA levels of COX-2 and MMP-1 as well as the production of PGE2 that were stimulated by IL-1β (10 pM). On the other hand, 8% stretching amplified the IL-1β-mediated stimulation of both COX-2 and MMP-1, and PGE2 production. Herein, we provide a detailed report.
2. Materials and methods
2.1. Culture of human tendon fibroblasts
HPTFs were isolated from fresh tendon samples donated by three healthy male patients (15, 21, and 32 years old) who underwent ACL reconstruction using a patellar tendon autograft. Tendon samples were obtained following a protocol approved by the University of Pittsburgh Institutional Review Board (IRB #0407060). Tendon samples were minced aseptically, transferred to a polystyrene Petridish, and cultured with 10 mL of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) (50 units/ml). The culture was maintained at 37 °C in an incubator with a humidified atmosphere of 5% CO2. To obtain a larger number of tendon fibroblasts for the stretching experiments described below, cells were subcultured up to five passages. Under our culture conditions, the subcultured HPTFs did not show any apparent changes in cell morphology or doubling time (Li et al., 2004).
2.2. In vitro cell stretching model studies
To apply cyclic mechanical stretching to cultured tendon fibroblasts, we used an in vitro stretching system (Wang and Grood, 2000). In this system, custom-made silicone dishes are used to grow and stretch cells. The culture surfaces of these dishes were fabricated with microgrooves, which were 10 μm wide and 3 μm deep. The tendon fibroblasts were grown on the microgrooved surface of the silicone dish, which was pre-coated with 10 μg/mL of ProNectin-F (Sigma, St Louis, MO) to promote the attachment of tendon fibroblasts to the silicone surface. In the microgrooved silicone dishes, HPTFs were highly elongated and aligned parallel to the direction of microgrooves, along which the cyclic uniaxial stretching was applied. In this stretching system, the shape, alignment, and mechanical stretching conditions are similar to those in vivo (Wang et al., 2004).
For all cell stretching experiments, a total of 2×105 tendon fibroblasts were plated on each dish and grown in DMEM containing 10% FBS and 1% P/S for 36 to 48 h. Then the medium was replaced with DMEM containing 1% FBS and 10 pM IL-1β (Sigma, St. Louis, MO) to impose inflammatory conditions. The concentration of IL-1β applied for this study was determined based on our preliminary studies of a mild cellular inflammatory response by HPTFs (see Results below). After incubation of HPTFs for 1 h, the cells were then cyclically stretched at either 4% or 8% with a constant stretching frequency (0.5 Hz) for a duration of 4 h. Non-stretched HPTFs were used as the control. Note that the stretching magnitude was defined to be the percentage change in the length of the dish between the two clamps; therefore, they do not represent the strains experienced by cells on the dish surface, which are generally much smaller than the applied substrate strains (Wang et al., 2001).
Immediately after stretching, using the RNAeasy Mini Kit (QUIAGEN, Valencia, CA), cells were subjected to total RNA extraction for RT-PCR as described below, and the media were collected to measure PGE2 production by ELISA (R&D Systems, Minneapolis, MN).
2.3. RT-PCR analysis of COX-2 and MMP-1 gene expression
For RT-PCR, oligonucleotide primers (Table 1) were designed according to the published human sequences of COX-2 (Nakatani et al., 2002), MMP-1 (Orlando et al., 1998), and GAPDH (Adam et al., 1999) as an internal control. The primers were synthesized by the University of Pittsburgh DNA Synthesis Facility.
Table 1.
Primer sequence for RT-PCR
| Genes | Primer sequences (forward/reverse) | PCR product size (bp) | Annealing temperature (°C) | Reference |
|---|---|---|---|---|
| COX-2 | 5′-TTCAAATGAGATTGTGGGAAAT-3′ 5′-AGATCATCTCTGCCTGAGTATCTT-3′ |
305 | 51 | (Nakatani et al., 2002) |
| MMP-1 | 5′-CACAGCTTTCCTCCACTGCTGCTGC-3′ 5′-GGCATGGTCCACATCTGCTCTTGGC-3′ |
395 | 63 | (Orlando et al., 1998) |
| MMP-1 for real-time PCR | 5′-AGTGACTGGGAAACCAGATGCTGA-3′ 5′-GCTCTTGGCAAATCTGGCCTGTAA-3′ |
160 | 62 | |
| GAPDH | 5′-TCACCATCTTCCAGGAGCG-3′ 5′-CTGCTTCACCACCTTCTTGA-3′ |
570 | 56 | (Adam et al., 1999) |
| GAPDH for real-time PCR | 5′-TCGACAGTCAGCCGCATCTTCTTT-3′ 5′-GCCCAATACGACCAAATCCGTTGA-3′ |
148 | 62 |
Samples for RT-PCR analyses were made to have a final volume of 20 μL, containing 1 μg of total RNA, 5 mM of MgCl2, 1X reverse transcription buffer, 1 mM of dNTP, and 1 U/μL of recombinant RNaseOUT™ (Invitrogen Life Technologies, Carlsbad, CA). The synthesis of cDNA was carried out at 42 °C for 55 min, followed by termination at 70 °C for 15 min. The synthesized cDNA was amplified using the listed primers (Table 1). For COX-2, the amplification was done at 94 °C for 50 s, 51 °C for 40 s, and 72 °C for 40 s in each reaction cycle. For MMP-1, the amplification was carried out at 94 °C for 50 s, 63 °C for 40 s, and 72 °C for 40 s in each cycle. For GAPDH, the amplification was carried out at 94 °C for 50 s, 56 °C for 40 s, and 72 °C for 40 s in each cycle. A total of 25 cycles were used, which is within the linear region of the assay (Nakatani et al., 2002). Electrophoresis was performed in a 2% agarose gel containing ethidium bromide. The RT-PCR products were visualized, scanned and quantified by image analysis (NIH Image J). The density of each band was normalized to that of GAPDH. For real-time PCR, the human MMP-1 and GAPDH primer sets were designed to generate <200 bp coding regions, spanning two exons and an intron. The primer sequences and optimized annealing temperature for real-time PCR is shown in Table 1.
2.4. ELISA for measuring PGE2 levels in media
An enzyme immunoassay kit (R&D Systems Inc., Minneapolis MN USA) was used to measure the PGE2 production levels in the medium. Briefly, 100 μL of either the medium from a sample group or the standard solution sequences for the standard curve was loaded into each well of an antibody-coated 96-well plate. Then, 50 μL of PGE2 conjugate (PGE2 conjugated to alkaline phosphatase) and 50 μL of PGE2 antibody (mouse monoclonal antibody to PGE2) solution were added into the wells and incubated at 4 °C for 18 to 24 h. After incubation, the solution was removed and the wells were washed four times with wash buffer. Next, 200 μL of pNPP (p-Nitrophenly Phosphate) substrate solution was added to each well and incubated at 37 °C for 1 h. Following the addition of 50 μL of stop solution (Trisodium Phosphate solution), the solution absorbance was measured at 405 nm on a microplate reader (Spectra MAX 190, Molecular Devices, Sunnyvale, CA) and was then converted to PGE2 concentration using a standard curve.
2.5. Statistical analysis
One-way ANOVA was used for statistical data analysis, followed by Fisher’s PLSD test for multiple comparisons with the significance level set at α =0.05.
3. Results
3.1. IL-1β induced COX-2 expression in a dose-dependent manner by HPTFs
When HPTFs grown in microgrooved silicone dishes were incubated with IL-1β in various doses (10 pM to 100 pM), COX-2 mRNA levels were markedly increased in a dose-dependent manner compared to that of non-treated cells (Fig. 1). This stimulation of COX-2 gene expression was noticed within 4 h and 5 pM of IL-1β was sufficient enough to observe 100% induction in the mRNA level of COX-2. At 10 pM of IL-1β, COX-2 gene expression was increased by 200%, and at 50 to 100 pM, COX-2 expression was increased by >300% compared with those of tendon fibroblasts without IL-1β treatment, which resulted in minimal COX-2 expression. At 50 pM of IL-1β, COX-2 gene expression levels were so high that they obscured the changes in COX-2 expression induced by cyclic mechanical stretching (data not shown). Based on these results, we chose 10 pM as the IL-1β concentration for further studies, which is in the linear range of COX-2 expression in response to IL-1β treatment.
Fig. 1.
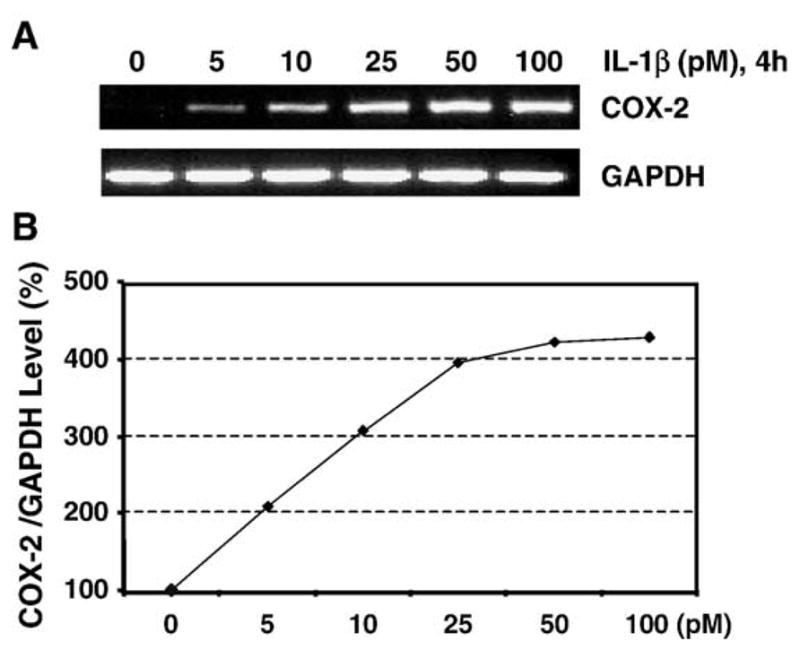
A. A representative RT-PCR result showing COX-2 mRNA expression levels of HPTFs in response to various doses of IL-1β. HPTFs were cultured on microgrooved silicone surfaces and were treated with five doses of IL-1β (5, 10, 25, 50, and 100 pM) for 4 h. Cells without IL-1β were used as the control. B. IL-1β dose-dependent COX-2 gene expression. IL-1β at 10 pM induced a significantly higher expression level of COX-2 mRNA levels compared to non-treated cells and it appears to be in the linear range of COX-2 gene expression in response to IL-1β treatment.
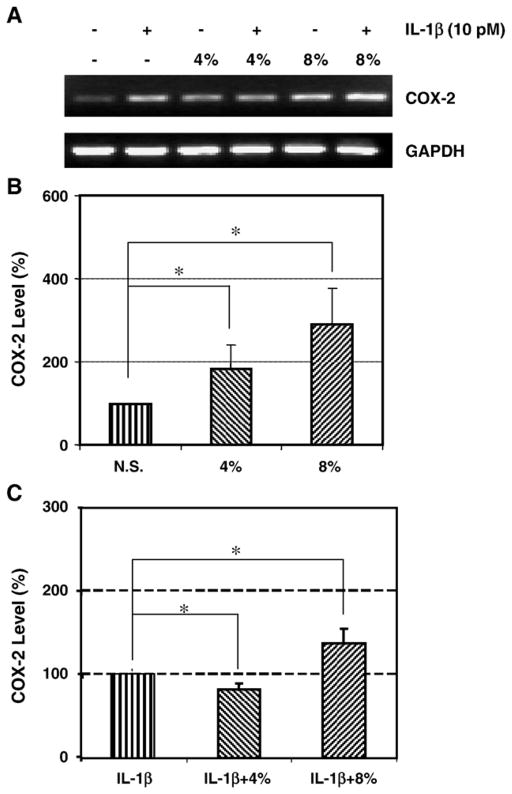
3.2. The effect of IL-1β and mechanical stretching on COX-2 expression
In the absence of IL-1β stimulation, we found that increases in COX-2 expression by HPTFs were stretching magnitude-dependent (Fig. 2A). Specifically, 4% and 8% stretching stimulated COX-2 mRNA levels by 83% (p<0.015) and 188% (p<0.002), respectively, compared to that of non-stretched cells (Fig. 2B). Interestingly, 4% cyclic stretching of HPTFs appeared to decrease the IL-1β-stimulated COX-2 expression by 27% (p<0.01) compared to non-stretched cells. IL-1β treated cells at 8% stretching, however, additively increased COX-2 mRNA levels by 38% (p<0.001) (Fig. 2C).
Fig. 2.
A. A representative RT-PCR result showing the effect of IL-1β treatment and mechanical stretching on mRNA expression of COX-2 in HPTFs. B. The effect of cyclic mechanical stretching on COX-2 gene expression. COX-2 expression levels in the 4% stretched group had an 83% increase over non-stretched cells and 8% stretched cells had an 188% increase. (N=6; *p<0.05; N. S.: non-stretched cells). C. The effect of IL-1β and mechanical stretching on COX-2 gene expression. Compared to the IL-1β treated, non-stretched fibroblasts, COX-2 mRNA expression levels decreased by 27% in the 4% stretched fibroblasts but increased by 38% in the 8% stretched fibroblasts. (N=6, *p<0.05).
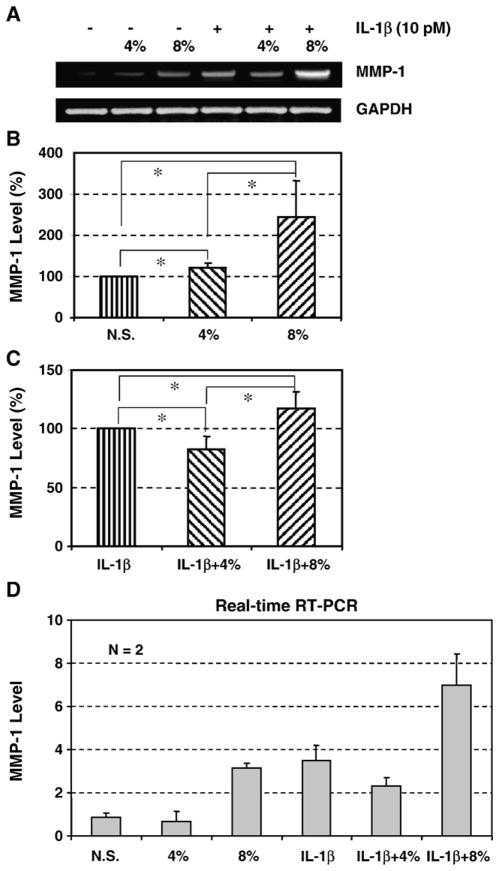
3.3. The effect of IL-1β and mechanical stretching on MMP-1 expression
In response to IL-1β and mechanical stretching, the pattern of MMP-1 gene expression was similar to that of COX-2 gene expression (Fig. 3A). In the absence of IL-1β, cyclic stretching at 4% increased the expression of MMP-1 by 20%, whereas 8% stretching increased it by 145% (p<0.05), compared to that of non-stretched cells. A significant differential induction of MMP-1 gene expression between 4% and 8% (p<0.05) was noticed (Fig. 3B). In the presence of IL-1β (10 pM), however, 4% stretching decreased IL-1β-induced MMP-1 expression by 17% (p<0.05), whereas 8% stretching increased MMP-1 expression by 18% (Fig. 3C). These results were further verified by performing real-time RT-PCR (Fig. 3D).
Fig. 3.
A. A representative RT-PCR result showing the effect of IL-1β treatment and mechanical stretching on MMP-1 gene expression in HPTFs. B. The effect of cyclic mechanical stretching on MMP-1 gene expression. In response to 4% stretching, HPTFs slightly increased MMP-1 mRNA expression levels as well as 8% stretch, compared to non-stretched cells. C. The effect of IL-1β and mechanical stretching on MMP-1 gene expression. Compared to the IL-1β treated, non-stretched fibroblasts, MMP-1 mRNA expression level decreased by 18% in the 4% stretched fibroblasts, whereas it slightly increased by 17% in the 8% stretched fibroblasts (N.S.: non-stretched cells; N=5; *p<0.05). D. Semi-quantitative RT-PCR results were further confirmed by real-time PCR using the same RNA samples that were used for the semi-quantitative RT-PCR data (Fig. 3A). The results from both analyses were consistent.
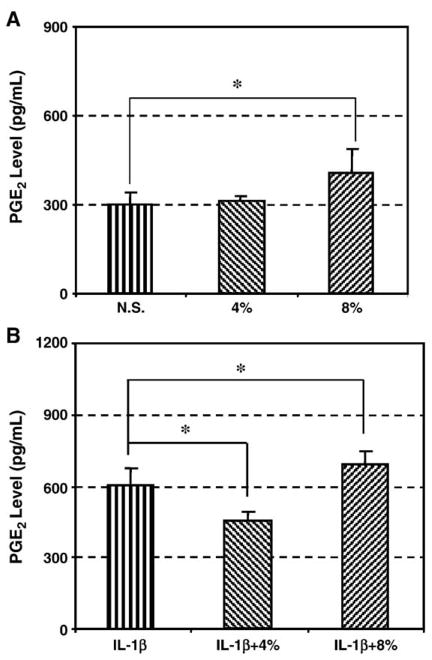
3.4. The effect of IL-1β and mechanical stretching on PGE2 production
Since PGE2 is one of the major lipid mediators of pain and acute inflammation in tendons and other tissues (Narumiya et al., 1999; Fiorucci et al., 2001), we tested for an interactive effect of IL-1β and mechanical stretching on PGE2 production by HPTFs. In the absence of stimulation with IL-1β, we found that 8% stretching of HPTFs significantly increased PGE2 production by 37% (p<0.01), whereas 4% stretching did not change PGE2 production (Fig. 4A). In the presence of IL-1β (10 pM), however, the cells with 4% stretching demonstrated decreased PGE2 production by 25% (p < 0.01), suggesting the antagonistic effect of 4% stretching on the IL-1β-stimulated PGE2 production by HPTFs. On the other hand, we observed that the cells with 8% stretching further increased the IL-1β-stimulated PGE2 production by 15% (p<0.01) (Fig. 4B).
Fig. 4.
A. PGE2 production of HPTFs in response to cyclic mechanical stretching. There was a 37% increase in 8% stretched fibroblasts compared to non-stretched cells, but 4% stretched cells showed little effect. (N=4; *p<0.05). B. PGE2 production of HPTFs in response to both IL-1β (10 pM) treatment and 4% or 8% stretching. It is shown that compared to those cells treated with IL-1β alone, 4% stretching decreased PGE2 levels by 25%, whereas 8% increased PGE2 levels by 15%. (N =4; *p < 0.05).
4. Discussion
Tendons are load-bearing tissues, responsible for transmitting muscular forces to bone. Tendons respond to mechanical loads by changing their metabolism as well as structural and mechanical properties (Kjaer, 2004). These biological changes are largely brought about by tendon fibroblasts that reside in the tendon matrix.
Therefore, it is important to determine how these cells respond to mechanical loads in order to better understand tendon cell mechanobiology as well as common pathologies, including tendinopathy (Khan et al., 2000). This study investigated the effect of mechanical loads and IL-1β, a potent inducer of COX-2 and MMP-1 expression and PGE2 production in many types of cells, on the inflammatory response of HPTFs (Newton and Covington, 1987; Fukuda et al., 1995; Manfield et al., 1996; Dubois et al., 1998). The major finding of this study is that 4% cyclic uniaxial stretching decreased inflammation-associated gene expression (COX-2 and MMP-1) and PGE2 production in HPTFs stimulated by IL-1β treatment, whereas 8% stretching further enhanced all these cellular responses to IL-1β treatment. Thus, cyclic mechanical stretching modulates IL-1β induced inflammatory response in HPTFs.
In tendon fibroblasts, it has been shown that mechanical stretching and IL-1β have a synergistic effect on the cellular inflammatory response (Archambault et al., 2002). Rabbit Achilles tendon cells were subjected to 5% cyclic biaxial stretching at 0.33 Hz for 6 h, treated with IL-1β (1000 pM), or exposed to cyclic stretching and IL-1β in combination. It was found that stretching alone did not increase COX-2 gene expression but IL-1β treatment slightly increased its expression. The combined treatment with stretching and IL-1β caused a further increase in MMP-1 and MMP-3 but not COX-2 gene expression. In this study, we found that in the presence of a lower dose of IL-1β(10 pM), cyclic stretching at 0.5 Hz for 4 h had either a reductive or additive effect on COX-2 and MMP-1 gene expression, depending on the stretching magnitude (4% or 8%). The differences between these two studies may stem from the source of tendon fibroblasts (rabbit Achilles paratenon vs. human patellar tendon) as well as the experimental conditions, including biaxial stretching in the previous study versus uniaxial stretching in this study.
Previous studies have also investigated the association of mechanical loading and IL-1β in other types of cells. For example, in the human synovial cell line, 2% cyclic stretching at 0.1 Hz for 1 h decreased both mRNA and protein levels of MMP-1 and MMP-13 and both enzymatic activities were decreased. The moderate stretching also upregulated the mRNA level of tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2 (Sun and Yokota, 2002). In human articular chondrocytes, a physiologically relevant dynamic compression suppresses the synthesis of both nitric oxide and PGE2 (Chowdhury et al., 2001). In other studies, cyclic tensile strain (CTS) at a low magnitude (3~6%) was found to inhibit IL-1β-induced COX-2, nitric oxide synthase, and collagenase gene expression, as well as decrease production of PGE2 in rabbit chondrocytes (Xu et al., 2000; Agarwal et al., 2001) and human periodontal ligament cells (Long et al., 2001; Long et al., 2002; Agarwal et al., 2003). Furthermore, it was found that to observe the CTS regulatory effects, the presence of an inflammatory signal in cells is required as CTS alone had little effect on these inflammatory gene expressions (Xu et al., 2000). CTS-regulated anti-inflammatory effects are not attributed to down-regulation of IL-1β itself; rather, CTS functions as an effective antagonist against the action of IL-1β by disrupting and regulating the signal transduction cascade of IL-1β. Whether a similar mechanism applies to the HPTFs of this study is not clear yet and further studies will be necessary.
PGE2, which is one of the most abundant prostaglandins in many tissues, mediates tendon inflammation (Almekinders and Temple, 1998). Previous studies showed that fibroblasts can produce high levels of PGE2 in response to mechanical loading (Baracos et al., 1983; Yamaguchi et al., 1994; Wang et al., 2003). The result of this study is consistent with these results. We recently showed that the exogenous addition of PGE2 decreased the proliferation and collagen production by HPTFs in a dose-dependent manner (Cilli et al., 2004). The results of this study suggest that the application of small mechanical stretching (≤4%) to tendons is beneficial in terms of reducing tendon inflammation and restoring tendon homeostasis by decreasing PGE2 production. However, large mechanical stretching (>4%) may be detrimental because it amplifies tendon inflammation-associated gene expression and PGE2 secretion and thus, may lead to tendon matrix degradation, a hallmark of late stage tendinopathy.
In the current study, we examined the effect of stretching magnitude on COX-2 and MMP-1 gene expression and PGE2 secretion by HPTFs. Future studies should investigate the effect of stretching frequency and duration on these inflammatory gene expressions, PGE2 production, the expression of other inflammatory mediators such as cPLA2 and sPLA2 (Li et al., 2004), and other extracellular matrix-degrading enzymes such as MMP-3 and MMP-13. All these factors could participate in mechanical loading-induced development of tendinopathy by initiating matrix destruction. Finally, the molecular mechanisms that are responsible for the modulation of IL-1β induced inflammatory gene expression by repetitive mechanical stretching conditions in HPTFs should be investigated.
Acknowledgments
This study was supported in part by the Arthritis Investigator Award, Whitaker Foundation Biomedical Engineering Grant, and NIH grant 049921 (JHW).
References
- Adam RM, Borer JG, Williams J, Eastham JA, Loughlin KR, Freeman MR. Amphiregulin is coordinately expressed with heparin-binding epidermal growth factor-like growth factor in the interstitial smooth muscle of the human prostate. Endocrinology. 1999;140:5866–5875. doi: 10.1210/endo.140.12.7221. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Long P, Gassner R, Piesco NP, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Long P, Seyedain A, Piesco N, Shree A, Gassner R. A central role for the nuclear factor-kappaB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J. 2003;17:899–901. doi: 10.1096/fj.02-0901fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almekinders LC, Temple JD. Etiology, diagnosis, and treatment of tendonitis: an analysis of the literature. Med Sci Sports Exerc. 1998;30:1183–1190. doi: 10.1097/00005768-199808000-00001. (see comments) [DOI] [PubMed] [Google Scholar]
- Alvares O, Klebe R, Grant G, Cochran DL. Growth factor effects on the expression of collagenase and TIMP-1 in periodontal ligament cells. J Periodontol. 1995;66:552–558. doi: 10.1902/jop.1995.66.7.552. [DOI] [PubMed] [Google Scholar]
- Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36–39. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Banes AJ, et al. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J Biomech. 1995;28:1505–1513. doi: 10.1016/0021-9290(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Baracos V, Rodemann HP, Dinarello CA, Goldberg AL. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983;308:553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Chowdhury TT, Bader DL, Lee DA. Dynamic compression inhibits the synthesis of nitric oxide and PGE (2) by IL-1beta-stimulated chondrocytes cultured in agarose constructs. Biochem Biophys Res Commun. 2001;285:1168–1174. doi: 10.1006/bbrc.2001.5311. [DOI] [PubMed] [Google Scholar]
- Cilli F, Khan M, Fu F, Wang JH. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin J Sport Med. 2004;14:232–236. doi: 10.1097/00042752-200407000-00006. [DOI] [PubMed] [Google Scholar]
- Dubois RN, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- Fiorucci S, Meli R, Bucci M, Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol. 2001;62:1433–1438. doi: 10.1016/s0006-2952(01)00747-x. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Ohtani K, Dan H, Tanaka S. Interleukin-1 inhibits keratan sulfate production by rabbit chondrocytes: possible role of prostaglandin E2. Inflamm Res. 1995;44:178–181. doi: 10.1007/BF01782816. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Tomlinson A, Willoughby DA. Differential effects of inhibition of isoforms of cyclooxygenase (COX-1, COX-2) in chronic inflammation. Inflamm Res. 1998;47:79–85. doi: 10.1007/s000110050285. [DOI] [PubMed] [Google Scholar]
- Jozsa LG, KP Overuse injuries of tendons. Human Tendons Anatomy, Physiology, and Pathology. 1997;(Part III) [Google Scholar]
- Khan KM, Maffuli N, Coleman BD, Cook JL, Taunton JE. Patellar tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2000;32:346–355. doi: 10.1136/bjsm.32.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Li Z, Yang G, Khan M, Stone D, Woo SL, Wang JH. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med. 2004;32:435–440. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- Lipsky PE. Role of cyclooxygenase-1 and -2 in health and disease. Am J Orthop. 1999;28:8–12. [PubMed] [Google Scholar]
- Long P, Hu J, Piesco N, Buckley M, Agarwal S. Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res. 2001;80:1416–1420. doi: 10.1177/00220345010800050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P, Liu F, Piesco NP, Kapur R, Agarwal S. Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone. 2002;30:547–552. doi: 10.1016/s8756-3282(02)00673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfield L, Jang D, Murrell GA. Nitric oxide enhances cyclooxygenase activity in articular cartilage. Inflamm Res. 1996;45:254–258. doi: 10.1007/BF02259612. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1. J Orthop Res. 2002;20:1380–1386. doi: 10.1016/S0736-0266(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Newton RC, Covington M. The activation of human fibroblast prostaglandin E production by interleukin 1. Cell Immunol. 1987;110:338–349. doi: 10.1016/0008-8749(87)90127-4. [DOI] [PubMed] [Google Scholar]
- Orlando C, Pinzani P, Pazzagli M. Developments in quantitative PCR. Clin Chem Lab Med. 1998;36:255–269. doi: 10.1515/CCLM.1998.045. [DOI] [PubMed] [Google Scholar]
- Smith LL. Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Med Sci Sports Exerc. 2000;32:317–331. doi: 10.1097/00005768-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Sun HB, Yokota H. Reduction of cytokine-induced expression and activity of MMP-1 and MMP-13 by mechanical strain in MH7A rheumatoid synovial cells. Matrix Biol. 2002;21:263–270. doi: 10.1016/s0945-053x(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Tewari DS, et al. Mechanistic features associated with induction of metalloproteinases in human gingival fibroblasts by interleukin-1. Arch Oral Biol. 1994;39:657–664. doi: 10.1016/0003-9969(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Vailas AC, Pedrini VA, Pedrini-Mille A, Holloszy JO. Patellar tendon matrix changes associated with aging and voluntary exercise. J Appl Physiol. 1985;58:1572–1576. doi: 10.1152/jappl.1985.58.5.1572. [DOI] [PubMed] [Google Scholar]
- Wang JH, Grood ES. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connect Tissue Res. 2000;41:29–36. doi: 10.3109/03008200009005639. [DOI] [PubMed] [Google Scholar]
- Wang JH, Goldschmidt-Clermont P, Wille J, Yin FC. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech. 2001;34:1563–1572. doi: 10.1016/s0021-9290(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Wang JH, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–133. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- Wang JH, Yang G, Li Z, Shen W. Fibroblast responses to cyclic mechanical stretching depend on cell orientation to the stretching direction. J Biomech. 2004;37:573–576. doi: 10.1016/j.jbiomech.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000;165:453–460. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, et al. Effect of different magnitudes of tension force on prostaglandin E2 production by human periodontal ligament cells. Arch Oral Biol. 1994;39:877–884. doi: 10.1016/0003-9969(94)90019-1. [DOI] [PubMed] [Google Scholar]