Abstract
OBJECTIVES
We tested the hypothesis that gene therapy using apolipoprotein A-I Milano (apoA-IMilano) is more effective than that using wild-type apolipoprotein A-I (apoA-I) in reducing atherosclerosis.
BACKGROUND
Apolipoprotein A-I Milano is a naturally occurring mutant with established antiatherogenic activity; however, its relative antiatherogenic efficacy compared with that of wild-type apoA-I remains unclear.
METHODS
We performed bone marrow transplantation in female double-knockout mice lacking both the apoE and apoA-I genes using male donor mice–derived bone marrow that had been transduced with a retroviral vector alone or retroviral vector expressing wild-type apoA-I or apoA-IMilano gene under the control of macrophage-specific scavenger receptor A promoter. Mice were fed a high-cholesterol diet and killed 24 weeks after transplantation, at which time the extent of aortic atherosclerosis was determined.
RESULTS
Compared with vector control (n = 12), apoA-IMilano gene therapy (n = 15) reduced aortic atherosclerosis by 65% (p < 0.001) and plaque macrophage immunoreactivity by 58% (p < 0.0001), whereas wild-type apoA-I (n = 11) reduced atherosclerosis by 25% (p = 0.1) and plaque macrophage immunoreactivity by 23% (p < 0.05). The apoA-IMilano gene therapy was significantly more effective in reducing atherosclerosis (p < 0.05) and macrophage immunoreactivity (p < 0.001) compared with wild-type apoA-I. The circulating levels of cholesterol, lipoprotein profile, and apoA-IMilano or wild-type apoA-I were comparable among the groups. Apolipoprotein A-I Milano was more effective than wild-type apoA-I in promoting macrophage cholesterol efflux.
CONCLUSIONS
Macrophage-specific expression of the apoA-IMilano gene is more effective than wild-type apoA-I in reducing atherosclerosis and plaque inflammation despite comparable circulating levels of the transgene and lipid profile.
Substantial efforts have been made to promote cholesterol efflux to prevent or treat atherosclerosis. For example, liver-specific overexpression of the apolipoprotein A-I (apoA-I) transgene was found to increase high-density lipoprotein (HDL) cholesterol and apoA-I levels, reducing atherosclerosis in C57BL/6 mice (1) and hyperlipidemic mice (2,3). A significant reduction in atherosclerosis was found after somatic gene transfer of human apoA-I into low-density lipoprotein (LDL) receptor knockout mice fed a high-fat diet (4,5). Conversely, mice lacking the apoA-I gene had significantly increased atherosclerosis (6). Collectively, these data show that apoA-I has antiatherogenic activity in animal models.
Apolipoprotein A-I Milano (apoA-IMilano) is a naturally occurring mutant of apolipoprotein A-I characterized by Arg173 → Cys173 substitution (7). The human carriers of this mutation have very low levels of HDL cholesterol and high levels of triglycerides and yet do not show increased cardiovascular risk (8). We and others have previously shown that intravenous recombinant apoA-IMilano inhibits progression and induces rapid regression and remodeling of atherosclerosis in hypercholesterolemic rabbits and mice while attenuating endothelial dysfunction (9–13). Based on these promising preclinical results, a small phase II human trial of patients with acute coronary syndromes was conducted in which once-weekly injections of recombinant apoA-IMilano were shown to induce rapid coronary atheroma regression within 5 weeks (14). Although these results suggest that intravenous infusions of recombinant apoA-IMilano could be used to rapidly induce atherosclerosis regression/remodeling, the need for repeated intravenous injections and the logistical issues associated with large-scale production of recombinant apoA-IMilano could pose a significant challenge to the widespread use of this novel approach. An attractive or complementary approach could be the use of gene transfer to exploit the atheroprotective effect of apoA-IMilano. In addition, the relative atheroprotective effects of apoA-IMilano compared with those of wild-type apoA-I are unknown.
In this study, we used transplantation of retrovirally transduced bone marrow cells to provide proof of the concept that apoA-IMilano gene transfer is effective in markedly reducing atherosclerosis in a murine model. Furthermore, we also compared the atheroprotective effects of apoA-IMilano and wild-type apoA-I gene transfer.
METHODS
Animals
The apoA-I/apoE double-knockout mice were kindly provided by Dr. Linda Curtiss (Department of Immunology, The Scripps Research Institute, La Jolla, California) and housed in microisolator cages. Mice were healthy throughout these studies and showed no differences in feeding pattern or body weight. Animal care and experimental procedures were performed with the approval of and according to the regulations of the Cedars-Sinai Medical Center Institutional Animal Care and Usage Committee.
Sample size calculations showed that the number of mice needed per group was 7 based on a statistical ability to detect a 30% relative difference in percent of aorta covered by plaque with 90% power and p < 0.05 assuming that controls will have 10% of aortic surface covered by plaque. Therefore, we have used 8 to 10 mice per group.
Construction of retroviral vector
To generate double-knockout mice with the ability to express human apoA-I and apoA-IMilano, bone marrow was harvested from male mice, marrow cells were transduced with retrovirus expressing apoA-I or apoA-IMilano, and then female mice were reconstituted with the transduced bone marrow cells. To target the expression of transgenes to macrophages, we used macrophage-specific promoter, essentially as described previously (15). We received the retroviral construct containing the 868-bp cDNA of human apoA-I gene under the control of scavenger receptor A (SR-A) promoter from Dr. Sergio Fazio (Vanderbilt University). The PT67 viral packaging cell line was used to package the plasmid and generate retrovirus-expressing wild-type human apoA-I (15).
We used the SR-A–apoA-I plasmid to generate SR-A–apoA-IMilano by site-directed mutagenesis, using a Stratagene (La Jolla, California) kit essentially as described by the manufacturer. The DNA sequencing confirmed missense mutation of the apoA-I gene (173 arg:cys). Because promoter and noncoding sequences of the 2 constructs are identical, this allowed us to express comparable levels of apoA-I and apoA-IMilano in vivo and in vitro. This is further confirmed by enzyme-linked immunosorbent assay (ELISA).
Western blot analysis for human apoA-I and apoA-IMilano
To investigate the expression of apo A-I and apoA-IMilano by retroviral vector, the conditioned media from PT67 cells were collected, concentrated by Amicon Microcon-100 columns (Millipore, Waltham, Massachusetts), and analyzed by 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis. This is followed by transferring the separated proteins to a nitrocellulose membrane and then incubating the membrane with a polyclonal rabbit antihuman apoA-I (Calbiochem, San Diego, California) antibody and a horseradish peroxidase conjugated antirabbit immunoglobulin G (Sigma, St. Louis, Missouri) antibody. The visualization of the bound antibody was performed by chemiluminescence using an ECL kit (Amersham Pharmacia Biotech, Piscataway, New Jersey).
Bone marrow transplantation
Bone marrow transduction was performed essentially as described previously (15). Briefly, bone marrow was taken from the femurs and tibias of 6- to 8-week-old apoA-I/apoE double-knockout male mice, 3 days after tail vein injection of 150 mg/kg of 5-fluorouracil (American Pharmaceutical Partners, Los Angeles, California). The bone marrow was resuspended at 1 to 2 × 106 nucleated cells/ml in RPMI 1640 supplemented with 15% fetal bovine serum, 2 mmol/l L-glutamine, penicillin-streptomycin, 50 ng/ml mouse stem cell factor, 10 ng/ml mouse interleukin-6, and 10 ng/ml interleukin-3 (all from R&D Systems, Minneapolis, Minnesota). After 48 h, bone marrow cells were diluted 1:1 with RPMI and Dulbeco's Modified Eagle Medium supplemented with 15% fetal bovine serum, cytokines, and 4 µg/ml polybrene. The diluted cells were co-cultured with irradiated (40 Gy) viral producer cells expressing apoA-I or apoA-IMilano for 48 h. Empty vector was used as a control. On the day of transplantation, the bone marrow cells were gently flushed and collected, washed, and counted. The cells were resuspended in RPMI supplemented with 5 U/ml heparin and 1 × 106 cells were injected into each female recipient mouse that had been lethally irradiated (1,000 Gy). At 4 weeks after transplantation, mice were placed on a high-fat diet for 20 weeks and then killed. Blood was collected 1 day before being fed a high-fat diet and then at intervals, 4 weeks, 8 weeks, 12 weeks, and 20 weeks afterward.
Bone marrow engraftment assessment using Y-chromosome polymerase chain reaction (PCR)
The DNA was isolated from the bone marrow of recipient mice using a UNIQ-10 universal DNA-purifying kit (Gentaur Molecular Products, Brussels, Belgium). Oligonucleotide primers for the syr locus were as follows: forward, 5′-CTG CTG TGA ACA GAC ACT AC-3′; reverse 5′-GAC TCC TCT GAC TTC ACTTG-3′. Amplification of beta-actin served as an internal control. All PCR amplifications also included DNA from a male C57BL/6 mouse (positive control) and DNA from a female C57BL/6 mouse (negative control). Amplification cycles consisted of an initial denaturation step of 96°C for 4 min; followed by 35 cycles of denaturation at 94°C for 60 s, annealing at 62°C for 60 s, and extension at 72°C for 2 min; and a final 72°C extension for 7 min. These primers amplified a DNA fragment of 395 bp. The PCR products were analyzed by agarose electrophoresis using a 1.5% gel.
ApoA-I expression
The reverse transcriptase (RT)-PCR was performed on the total RNA isolated from liver of recipient mice reconstituted with apoA-I or apoA-IMilano expressing retroviruses. The PCR was performed with the following primer sequences for apoA-I: 5′-AAGGACCTGGCCACTGTGTA-3′ sense and 5′-TCTCCTCCTGCCACTTCTTC-3′ antisense (301 bp). Primers for beta-actin were as follows: forward, 5′-GAG ACC TTC AAC ACC CCA GCC-3′; reverse, 5′-AAT GTC ACG CAC GAT TTC CC-3′ (401 bp). The cycling conditions were an initial denaturation for 10 min at 94°C followed by 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min.
Lipoprotein analysis
Fasting blood samples were collected from bone marrow transplant recipient mice by retro-orbital venous plexus puncture using heparinized tubes under isoflurane anesthesia (Medeva Pharmaceuticals, Rochester, New York). Total cholesterol was measured using a Cholesterol Reagent Enzymatic Kit (Thermo Electron, Louisville, Colorado). Lipoprotein profile was assessed by fast protein liquid chromatography.
ELISA for the detection of human apoA-I and apoA-IMilano
Serum levels of human apoA-I or apoA-IMilano in the bone marrow transplant recipients were determined by ELISA (15). Briefly, ELISA plates were coated with an antihuman apoA-I monoclonal antibody (Calbiochem) at a concentration of 1 µg/ml and incubated at 4°C overnight. The wells were washed, blocked with fetal bovine serum, and diluted standards (Calbiochem) or serum samples were added to the wells and incubated at 4°C overnight. After washing, rabbit antihuman apoA-I antibody was added and incubated at room temperature for 1 h. After washing, a peroxidase-conjugated goat-antirabbit antibody (Calbiochem) was added and the color was developed by addition of 2,2′ -azino-bis-3-ethylbenz-thiazoline-6-sulfonic acid (Sigma). Absorbance was measured at 405 nm on a microplate reader (Molecular Devices, Sunnyvale, California). Serum human apoA-I and apoA-IMilano levels were calculated by comparison with the standard curve.
Assessment of atherosclerosis
All image acquisitions and measurements were made by an observer blinded to treatment assignment. The extent of the atherosclerotic lesions in the aorta and innominate artery were quantified after oil red O staining, as described previously (10,11). Briefly, the thoracic cavity was opened; the aortic arch as well as the aorta were exposed, and were photographed under a dissecting microscope. After capturing the images, most of the vascular tree was dissected intact. The luminal surface was exposed, and then the aortic tissue was fixed with Histochoice tissue fixative (Amresco, Solon, Ohio). After tissue fixation, the adventitial tissue was removed and aortic tissue was stained with oil red O. The aortas were pinned to a dark surface, and images were captured by using a Spot digital camera (Diagnostic Instruments, Sterling Heights, Michigan). The extent of the atherosclerotic lesions was quantified using Image-Pro Plus (version 4, Media Cybernetics, Silver Spring, Maryland). Lesions were reported as percentage of the total aortic area consisting of thoracic aorta (ending at the final intercostal artery), and abdominal aorta ending at the iliac bifurcation.
For innominate artery sections, aortic arch was fixed in 4% paraformaldehyde overnight and then embedded in Optimal Cutting Temperature (OCT). The frozen tissues were serially sectioned into 8-µm sections from the aortic arch using a Leica cryostat and kept in a − 70°C freezer for <1 month before use. The lesion areas were quantified by Image-Pro Plus using the hematoxylin-stained sections (mean of 8 sections per mouse; 5 mice per group). Aortic sinus plaque area was determined by measuring the cross-sectional lesion area from serial sections of aortic sinus that were stained with oil red O and measured by manual tracing of the lesion, which was identified by a combination of lipid staining and histologic morphology.
Immunostaining
Macrophage immunoreactivity was detected by immunostaining of frozen sections (8 µm) with a 1:100 dilution of rat antimouse murine monocyte/ macrophage (MOMA-2) monoclonal antibody (Accurate Chemical and Scientific, Westbury, Connecticut). The expression of apoA-I or apoA-IMilano proteins in the reconstituted mice was determined by immunostaining of frozen sections with a 1:25 dilution of rabbit antihuman apoA-I antibody (Calbiochem) essentially as described previously (10,11). After washing, the sections were incubated with the appropriate secondary antibodies, followed by color development. The sections were counterstained with hematoxylin. Photomicroscopy was performed using an Olympus microscope. The percentage of plaque area occupied by MOMA-2 staining, reflecting macrophage immunoreactivity, was calculated from the captured images using the Image-Pro Plus software.
Laser microdissection
For laser microdissection, 7-µm-thick OCT aortic root sections were used. The macrophage-rich areas were defined by MOMA-2 staining (5 mice per group). The macrophage-rich areas of serial sections were dissected (PixCell LCM System Arcturus, Sunnyvale, California), and the fragments were captured and used for total RNA extraction using a Strataperp Total RNA Microprep Kit (Stratagene).
Cholesterol efflux
Cholesterol efflux was measured in macrophages expressing wild-type apoA-I or apoA-IMilano genes as wells as in macrophages treated with exogenous apoA-I or apoA-IMilano proteins. To measure cholesterol efflux by macrophages that produce apoA-I or apoA-IMilano, RAW cells (American Type Culture) were transduced with retroviral vector expressing apoA-I or apoA-IMilano, essentially as described above. The ELISA analysis of culture medium from transduced cells revealed that the cells transduced with wild-type apoA-I produced 171 ng/ml of protein and cells transduced with apoA-IMilano produced 167 ng/ml of protein. Control cells were transduced with vector-expressing green fluorescent protein (GFP). The transduced cells were plated in 6-well plates (1 × 105 cells per well). After 24 h, media were removed and replaced with loading media containing 1 µci/well of 3H-cholesterol (Amersham, Piscataway, New Jersey) and 70 µg/ml of oxidized LDL. Cells were loaded for 48 h, after which the monolayers were washed and equilibrated for 18 to 20 h in DMEM/1% bovine serum albumin (BSA). After washing, cells were exposed to DMEM/1% BSA efflux media for 4 h and then 100 µl was used for scintillation counting.
To further compare the effects of apoA-I and apoA-IMilano proteins on cholesterol efflux, we also used peritoneal macrophages isolated from the double-knockout mice as well as untransduced RAW cells. Cells were loaded with 3H-cholesterol as described above. The cholesterol loaded cells were then incubated with efflux media (DMEM + 1% BSA) supplemented with 200 µg/ml of either apoA-I or apoA-IMilano proteins that were complexed with phospholipid, dipalmitoyl phosphatidylcholine (DPPC) at the protein to phospholipid ratio of 1:2.7 for 4 h. Cholesterol efflux was measured as previously described.
Statistical evaluation
Statistical analyses were performed by analysis of variance followed by the Bonferroni Multiple Comparison test. Each post-hoc comparison was done at the significance level of 0.01 with 3 groups each at p < 0.05. The Prism 4.0 software (GraphPad, San Diego, California) was used for all calculations. All data are represented as mean ± SD. Values with p < 0.05 were considered statistically significant.
RESULTS
Expression of the transgenes in the packaging cell line
To establish that the retroviral construct expresses the gene, medium collected from the PT67 packaging cell line was used for Western blot analysis (data not shown). We found that the construct expresses both the monomeric (28 kDa) and dimeric (56 kDa) forms of apoA-IMilano. The level of the dimer is approximately 30% higher than that of the monomer. The construct expressing wild-type apoA-I only expressed the monomeric form of apoA-I transduced bone marrow cells. The empty vector was used for control. These data indicate that the retroviruses are capable of expressing the transgenes in vitro.
Engraftment of the transplanted bone marrow
The engraftment of the transplanted bone marrow cells was determined by examining the expression of the Y chromosome (provided by the male donors of the bone marrow) in the recipient female mice. The DNA was extracted from the bone marrow of female recipient mice, and the Y chromosome engraftment was determined by PCR (data not shown). We found that the livers from 3 randomly selected mice injected with apoA-IMilano, apoA-I, and control vector expressed the Y chromosome. The female and male chromosomes were used as negative and positive controls. These data show that the transplanted bone marrow cells were engrafted.
Expression of the transgene in liver and circulating monocytes of recipient mice
To determine whether the engraftment is associated with transgene expression, the expression of the transgene was determined by RT-PCR using total RNA isolated from the livers of the recipient mice (data not shown). The liver was chosen because approximately 70% of the transplanted bone marrow cells are known to be retained in the liver (16). We found that a 301-bp PCR product corresponding with the wild-type and Milano variants of apoA-I were detected in the randomly selected mice transplanted with apoA-IMilano or wild-type apoA-I transduced bone marrow cells. The 3 mice transplanted with bone marrow cells transduced with the control empty vector did not express the transgene. These data showed that the engrafted bone marrow cells expressed the transgenes.
We used the SR-A promoter, a monocyte/macrophage-specific promoter, to express the transgene. To determine whether the circulating monocytes express the transgenes, blood was collected from the recipient mice and the expression of transgenes was determined by RT-PCR in isolated monocytes (data not shown). We found that monocytes of mice receiving bone marrow cells transduced with the retrovirus expressing apoA-I or apoA-IMilano expressed the genes, whereas the monocytes from the recipients of bone marrow cells transduced with the empty vector did not express the transgene. These data indicate that the circulating monocytes from the recipient mice express the transgene.
Expression of transgenes in circulating blood
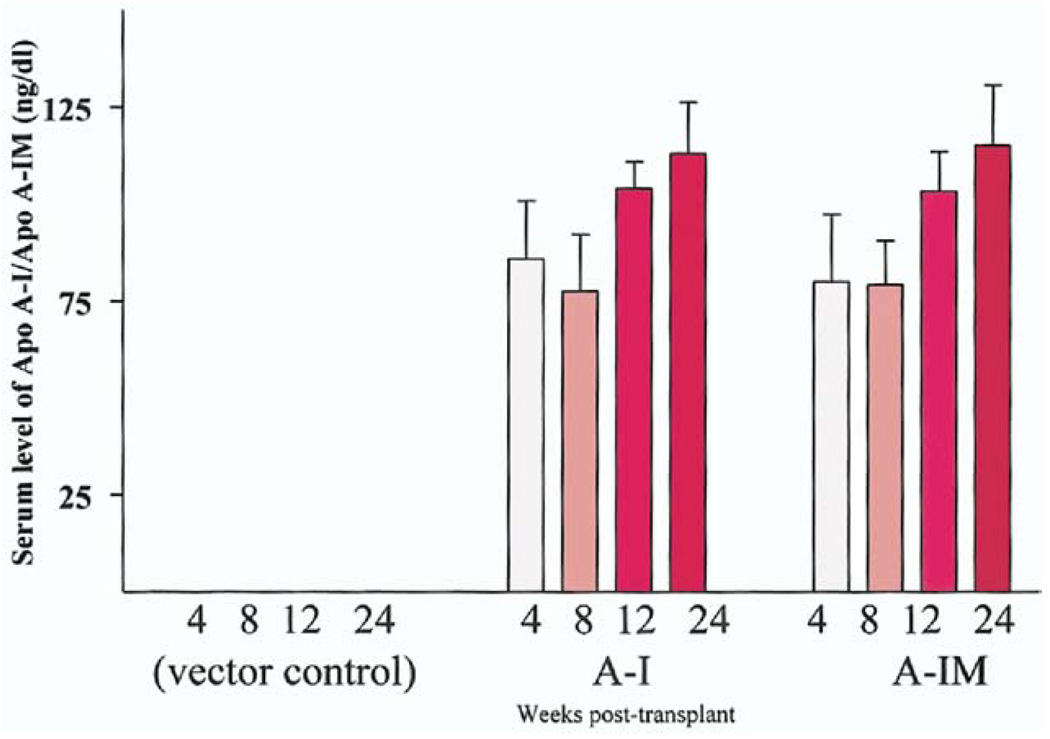
To compare the expression levels of the transgenes in vivo, we examined the serum level of apoA-I and apoA-IMilano in the recipient mice using an ELISA (Fig. 1). As expected, apoA-I was not detected in the serum before transplantation. The apoA-I was detected as early as 4 weeks post-transplantation (103 ± 69 ng/ml, n = 5), at 8 weeks post-transplantation (i.e., 4 weeks on high-fat diet) the level was 93 ± 58 ng/dl (n = 5), at 12 weeks post-transplantation (8 weeks on high-fat diet) the level was 124 ± 34 ng/dl (n = 5), and at 24 weeks posttransplantation (20 weeks on high-fat diet) the level was 113 ± 23 ng/dl (n = 5). These levels of serum apoA-I were similar to those of apoA-IMilano. At 4 weeks post-transplantation the level was 96 ± 66 ng/ml, at 8 weeks 95 ± 55 ng/dl, at 12 weeks 124 ± 44 ng/dl, and at 24 weeks 115 ± 35 ng/dl. Collectively, these data show there was no statistically significant difference between the serum levels of apoA-I and apoA-IMilano.
Figure 1.
Expression of apolipoprotein A-I (apoA-I) or apolipoprotein A-I Milano (apoA-IM) in the serum of recipient double-knockout mice. Serum levels of wild-type apoA-I or apoA-IM at 4, 8, 12, and 24 weeks after bone marrow transplantation are shown. Serum from a group of mice transplanted with the empty vector is used as a control. The data represent mean ± SD from 5 mice per group.
Circulating lipoprotein profile
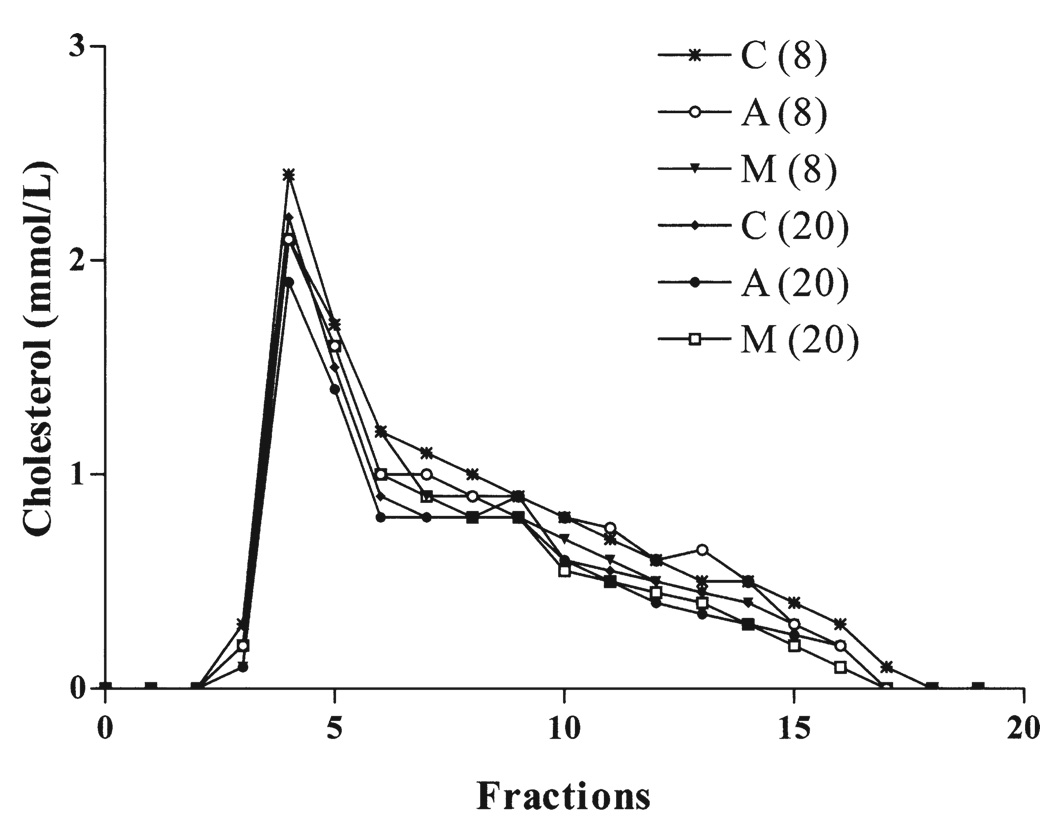
The total circulating cholesterol levels at 24 weeks were comparable in the 3 groups, averaging 1,123 ± 53 mg/dl in vector control groups, 1,127 ± 269 mg/dl in the wild-type apoA-I group, and 1,173 ± 237 mg/dl in apoA-IMilano group. Most of the cholesterol was found to be associated with the very-low-density lipoprotein fraction, followed by the intermediate-density lipoprotein/LDL fractions (Fig. 2). No difference in the distribution of cholesterol was found among the 3 groups of mice. Both the apoA-IMilano and wild-type apoA-I were primarily found to be associated with the HDL fractions.
Figure 2.
Comparative serum lipoprotein profile of mice on a high-fat diet for 8 and 20 weeks. Distribution of cholesterol was determined in serum from the recipient mice transplanted with wild-type apolipoprotein A-I (A), apolipoprotein A-I Milano (M) or vector control (C) using standard superose-6 chromatography.
Localization of apoA-I and apoA-IMilano in the atherosclerotic plaque
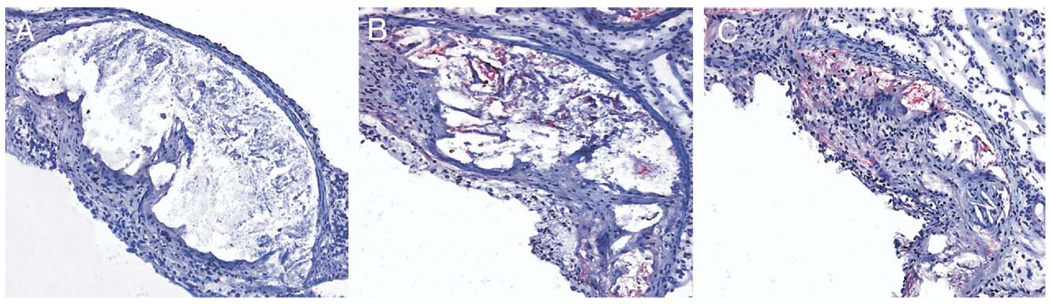
Anti–apoA-I antibody was used to determine whether the transgenes were expressed in the lesions. The atherosclerotic lesions of mice receiving empty vector consisted mainly of lipid core and foam cells, and no apoA-I or apoA-IMilano was detected (Fig. 3A). In contrast, mice expressing apoA-I (Fig. 3B) or apoA-IMilano (Fig. 3C) showed strong immunoreactivity for the corresponding proteins, mostly at the base of the lesions and around and within the lipid cores. The wild-type and mutant forms of apoA-I found in the plaque may have originated from circulating proteins, because we have detected the proteins in serum. In addition, because the circulating monocytes expressed the genes for wild-type and mutant forms of the protein, it is conceivable that at least some of the proteins may have originated from the local monocyte-derived macrophages found in the plaque.
Figure 3.
Representative aortic sinus lesions showing apolipoprotein A-I (apoA-I) or apolipoprotein A-I Milano (apoA-IMilano) expression in mice samples reconstituted with cells expressing apoA-I (B) or apoA-IMilano (C) compared with no expression in vector controls (A). Data were consistent in all 5 mice per group examined.
Effect on aortic atherosclerosis
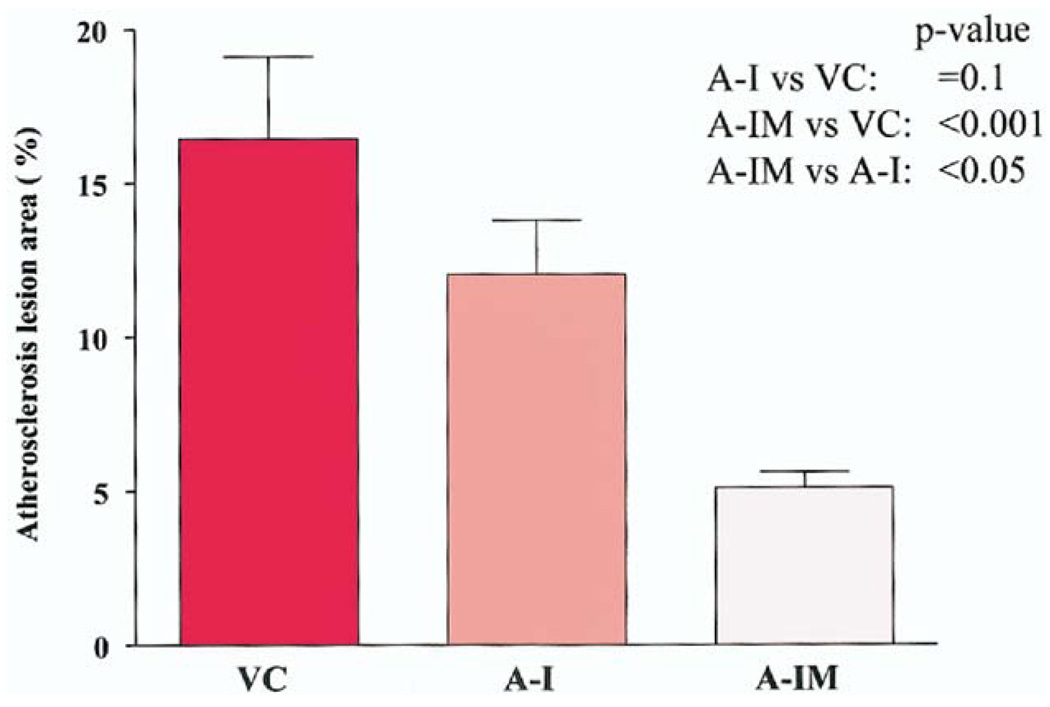
The mean lesion area in mice expressing wild-type apoA-I was 12.0 ± 1.7 (n = 11), versus 16.4 ± 2.6 (n = 12) in mice receiving the empty vector, reflecting a 25% reduction (p = 0.1) (Fig. 4). In contrast, the mean lesion area in the mice expressing apoA-IMilano was 5.6 ± 0.6 (n = 15), reflecting a 65% reduction compared with empty vector control (p < 0.001) and 52% reduction compared with mice expressing wildtype apoA-I (p < 0.05).
Figure 4.
Average percent lesion area (mean ± SD) in en fasse aortas after oil red O staining in vector control group (VC; n = 12), wild-type apolipoprotein A-I (apoA-I) gene recipients (A-I; n = 11), and apolipoprotein A-I Milano (apoA-IMilano) gene recipients (A-IM; n = 15) are shown.
Effect on innominate artery and aortic sinus atherosclerosis
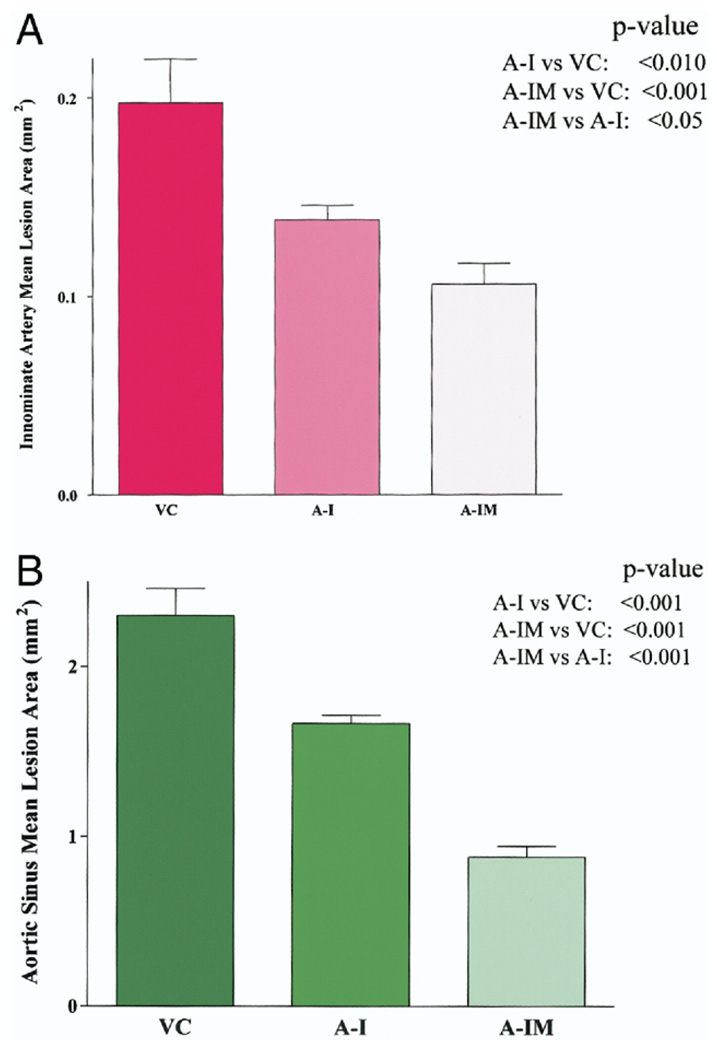
The mean innominate artery lesion area (mm2) in mice expressing wild-type apoA-I was 0.138 ± 0.007, versus 0.197 ± 0.022 in mice receiving the empty vector, reflecting a 29% reduction (p < 0.01) (Fig. 5A). In contrast, the mean lesion area in mice expressing apoA-IMilano was 0.098 ± 0.010, reflecting a 50% reduction compared with empty vector control (p < 0.001) and a 29% reduction compared with mice expressing wild-type apoA-I (p < 0.05). Similarly, the mice transplanted with bone marrow expressing apoA-IMilano had significantly less aortic sinus plaque compared with mice transplanted with wild-type apoA-I expressing bone marrow (Fig. 5B).
Figure 5.
Average percent lesion area (mean ± SD) in innominate artery (A) and aortic sinus (B) in vector control group (VC; n = 5) , wild-type apolipoprotein A-I (apoA-I) gene recipients (A-I; n = 5), and apolipoprotein A-I Milano (apoA-IMilano) gene recipients (A-IM; n = 5) are shown. Mice expressing apoA-IMilano had significantly smaller lesion areas compared with vector control mice and recipients of wild-type apoA-I.
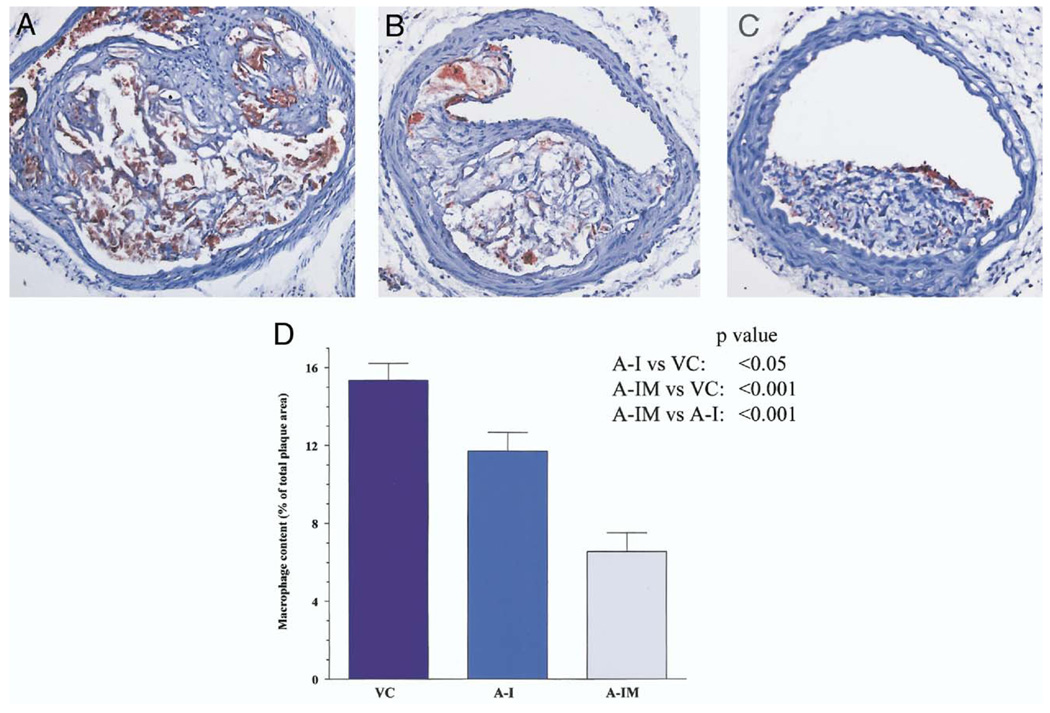
The reduction in lesion size was associated with reduced macrophage immunoreactivity (Fig. 6). The immunoreactivity was reduced by 23% in mice expressing wild-type apoA-I as compared with vector control mice (15.3 ± 0.8% vs. 11.7 ± 0.9%, p < 0.05). Similarly, a 58% reduction in the macrophage immunoreactivity was observed in mice expressing apoA-IMilano compared with vector control mice (15.3 ± 0.8% vs. 6.5 ± 0.9%, p < 0.001). Thus, the macrophage immunoreactive area was 44% less in mice expressing apoA-IMilano compared with those expressing wild-type apoA-I (6.5 ± 0.9% vs. 11.7 ± 0.9%, p < 0.001).
Figure 6.
Representative macrophage immunoreactivity in the innominate artery lesions of mice are shown (A to C) and are presented as quantitative data (D). The macrophage immunoreactive area was significantly lower in recipients of apolipoprotein A-I Milano (A-IM) compared with vector controls (VC) or recipients of wild-type apolipoprotein A-I (A-I).
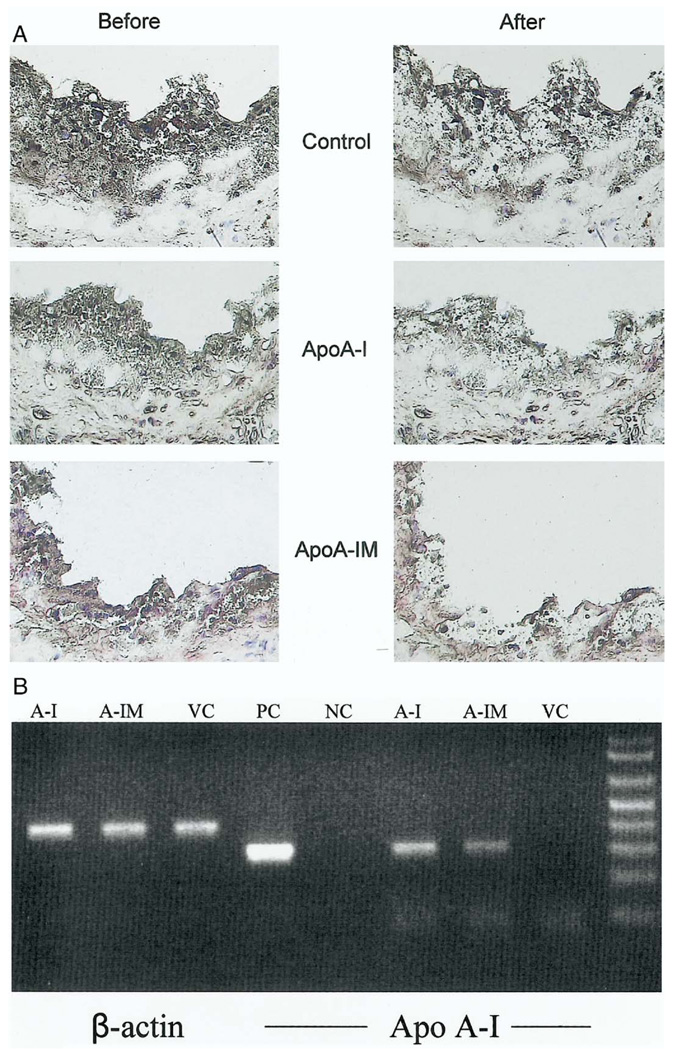
Local expression of transgene
Figure 7A shows representative sections before and after laser dissection. The macrophage-rich dissected segments from 5 mice were pooled and RT-PCR was performed using primers specific for apoA-I. As shown in Figure 7B, the groups of mice that received bone marrow transduced with a retrovirus expressing either apoA-I or apoA-IM show a 301-bp PCR fragment corresponding to apoA-I. In contrast, the control group containing recipient double-knockout mice that were transplanted with bone marrow cells transduced with a control vector did not show a PCR-amplified fragment. All dissected tissues were positive for the expression of 410-bp PCR product corresponding to the beta-actin gene.
Figure 7.
(A) Representative frozen sections of aortic sinus plaques before and after laser microdissection. The serial sections from control, apolipoprotein A-I (apoA-I), and apoA-IM recipient mice were selected (5 mice per group) and the macrophage-rich areas were dissected. (B) Total RNA was extracted from the dissected segments from recipient mice transplanted with bone marrow transduced empty vector (VC), wild-type apoA-I (A-I), or apolipoprotein A-I Milano (A-IM) genes. Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed using primers specific for beta-actin (lanes 1 to 3, 410 bp) or apoA-I (lanes 6 to 8, 301 bp). Lane 4 is a positive control (PC) for apoA-I derived from packaging cell lines; lane 5 is negative control (NC) from PCR in the absence of primers.
Cholesterol efflux
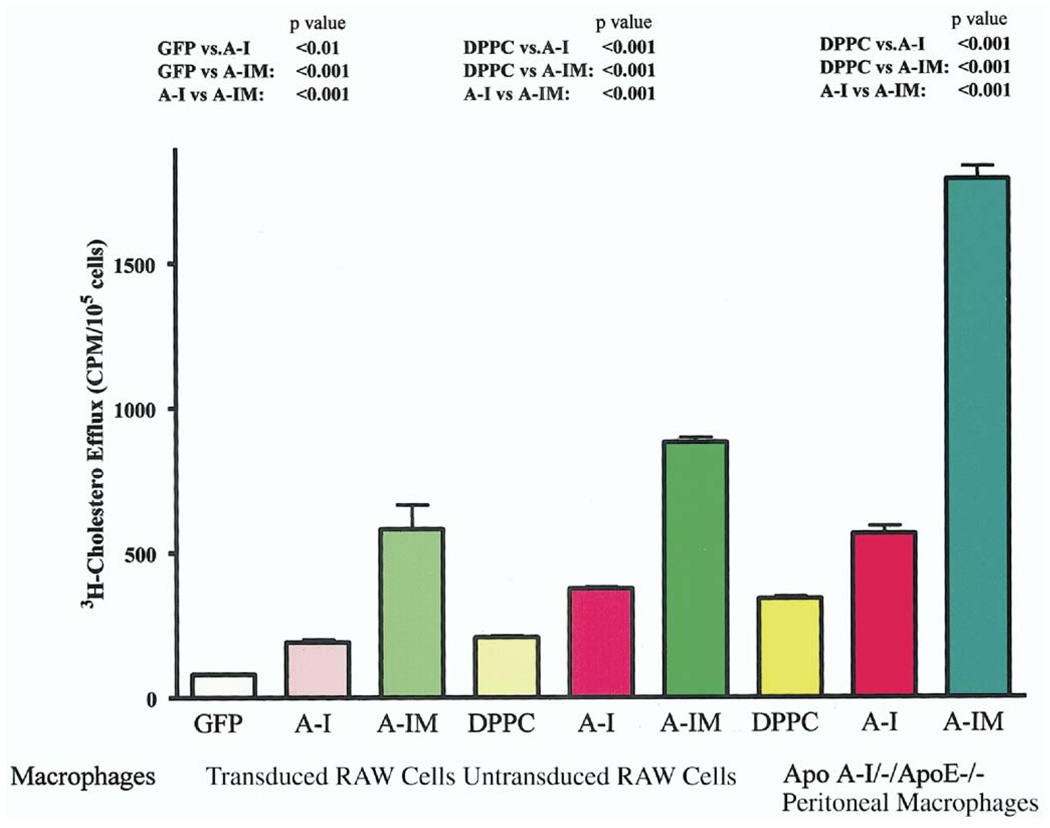
One of the important mechanisms for the atheroprotective effects of apoA-I and apoA-IMilano is their ability to promote cholesterol efflux from macrophages in arterial lesions. It has been previously reported that macrophage expression of apoA-I reduced atherosclerosis by increasing cholesterol efflux (17). Therefore, we evaluated the relative effect of apoA-I and apoA-IMilano on macrophage cholesterol efflux. At 4 h, we observed a significant increase in the cholesterol efflux by RAW cells expressing apoA-IMilano (p < 0.001) or apoA-I (p < 0.01) compared with control cells expressing GFP (Fig. 8). The cholesterol efflux was significantly higher in cells expressing apoA-IMilano compared with cells expressing wild-type apoA-I (p < 0.001). This was further substantiated when untransduced RAW cells or peritoneal macrophages derived from apoA-I/apoE double-knockout mice were exposed to equivalent quantities of wild-type apoA-I or apoA-IMilano complexed with phospholipid DPPC (Fig. 8). Similar to the transduced cells, exogenous apoA-IMilano was found to be significantly more effective than wild-type apoA-I in promoting macrophage cholesterol efflux (p < 0.001).
Figure 8.
Cholesterol efflux from cholesterol-loaded macrophages. Transduced RAW cells expressing genes for green fluorescent protein (GFP), apolipoprotein A-I (A-I), or apolipoprotein A-I Milano (A-IM) were used for cholesterol efflux in the absence of exogenous extracellular acceptors. In addition, nontransduced RAW cells were loaded with 3H-cholesterol, and then cholesterol efflux was measured 4 h after loading in the presence of dipalmitoyl phosphatidylcholine (DPPC) alone or DPPC with 200 µg/ml of either apoA-I (A-I) or apoA-IMilano (A-IM). A similar experiment was performed with the peritoneal macrophages that were isolated from apoA-I– /–/apoE–/– double-knockout mice. Each bar shows the mean ± SD of a triplicate determination. The experiments were repeated twice with similar results.
DISCUSSION
Previous studies have shown that macrophage-specific expression of apoA-I through bone marrow transplantation reduces atherosclerosis in apoE null mice (15,18). We have previously shown that apoA-IMilano prevented progression of aortic atherosclerosis and reduced the lipid and macrophage content of plaques in apoE knockout mice (10), possibly through tissue cholesterol mobilization (11). These observations were recently supported by a small clinical trial in which weekly infusion of recombinant apoA-IMilano was shown to induce rapid coronary atheroma regression within 5 weeks (14). However, the relative antiatherogenic effects of wild-type apoA-I and apoA-IMilano remain unclear. To compare the relative atheroprotective effects of wild-type apoA-I and apoA-IMilano, we used apoA-I/ apoE double-knockout mice for two major reasons. First, the absence of endogenous apoA-I prevents confounding that could have occurred as a result of interaction between exogenous human apoA-I and endogenous mouse protein (e.g., generation of high-molecular-weight forms of apoA-I) (19). Second, the double-knockout mice allowed us to use a single anti–apoA-I antibody to detect expression levels of the 2 human proteins in vitro and in vivo, thus avoiding the variability that could have arisen if we would have used 2 different antibodies with potentially different affinities.
Using this animal model, we found that reconstitution of the double-knockout mice with bone marrow cells expressing apoA-IMilano is significantly more effective than apoA-I in reducing both aortic and innominate artery atherosclerosis. We observed a 65% reduction in aortic atherosclerosis in mice expressing apoA-IMilano compared with the vector control group, which is substantially greater than the 25% reduction observed with wild-type apoA-I. The magnitude of reduction in atherosclerosis with wildtype apoA-I observed in our study is comparable with the 30% lesion reduction by apoA-I reported previously (18). Consistent with the results in the en fasse aorta, we also found a greater reduction in innominate artery and aortic sinus lesions with apoA-IMilano compared with wild-type apoA-I. In addition, we observed that the reduction of atherosclerotic lesions was associated with a 58% decrease in macrophage immunoreactivity in mice expressing apoA-IMilano compared with a 23% decrease observed with wild-type apoA-I, reflecting a further 44% reduction in the macrophage immunoreactivity in the mice expressing apoA-IMilano compared with mice expressing wild-type apoA-I. Notably, the antiatherogenic activity of apoA-IMilano and apoA-I was observed despite low levels of systemic apoA-I or apoA-IMilano (approximately 100 ng/dl) and without any changes in the circulating lipoprotein profile, consistent with previous reports (15,18). However, laser microdissection RT-PCR studies have shown that the transgenes mRNA are expressed in macrophage-rich areas of the plaque, suggesting that local expression of transgenes could in part contribute to the observed effects of apoA-I and apoA-IMilano. Collectively, these data support and extend our earlier observations, in which intravenous injection of apoA-IMilano into apoE knockout mice was found to rapidly mobilize tissue cholesterol and reduce plaque lipid and macrophage content of aortic lesions (11).
Previous studies have shown that the antiatherogenic activity of macrophage-specific apoA-I expression is likely mediated by increased cholesterol efflux and decreased foam cell formation (17,18). Our in vivo data support these findings and show that apoA-IMilano is significantly more effective than apoA-I in reducing lipid-rich atherosclerotic lesions. Our in vitro cholesterol efflux data provide one potential explanation for the differential atheroprotective effects of apoA-IMilano and wild-type apoA-I. Our results show that expression of apoA-I or apoA-IMilano by macrophages promotes cholesterol efflux; however, apoA-IMilano was found to be significantly more effective than apoA-I in stimulating cholesterol efflux. This increased cholesterol efflux-promoting activity of apoA-IMilano versus wild-type apoA-I was also observed in untransduced macrophage cell lines and peritoneal macrophages exposed to exogenous protein phospholipid complexes. These data are consistent with previous reports comparing the cholesterol efflux promoting activity of apoA-IMilano and apoA-I. Franceschini et al. (20) compared the capacity of serum from carriers of apoA-IMilano mutation and from control subjects to extract cholesterol from Fu5AH cells. They reported that serum from carriers of apoA-IMilano was more efficient than serum from normal subjects in promoting cholesterol efflux. This increased efficiency of apoA-IMilano may be related to the protease-sensitive flexible C-terminal domain of apoA-IMilano that is not shared by wild-type apoA-I (21). In addition to this lipid mobilization activity, apoA-IMilano has been found to be more effective than wild-type apoA-I in preventing LDL oxidation (22), providing further evidence in favor of the superior atheroprotective activity of apoA-IMilano compared with wild-type apoA-I.
Study limitations
Although these results were obtained in controlled experimental conditions associated with severe dyslipidemia, it is not possible to predict precisely whether these relative differences in efficacy would be maintained in human disease, in which less severe dyslipidemia is more common. Similarly, whether gene therapy using intravenous or intramuscular injection of the vectors rather than bone marrow transplantation would show similar differences remains unclear, and our laboratory is continuing work in that direction.
Summary
We provide experimental proof of the idea that despite comparable and low levels of circulating transgene expression, gene therapy using macrophage-specific expression of apoA-IMilano is significantly more effective than that using wild-type apoA-I in reducing atherosclerosis and plaque inflammation in hyperlipidemic apo A-I/apoE double knockout mice, possibly because of its increased cholesterol-efflux-promoting effect.
Acknowledgments
This work was supported by grants from the National Institutes of Health (ROI HL50566), The Eisner Foundation, the Entertainment Industry Foundation, the Milken Family Foundation, United Hostesses, and The Heart Foundation. Dr. Sharifi is an established investigator of the American Heart Association. Dr. Shah is a scientific advisor to Pfizer, Inc.
Abbreviations and Acronyms
- apoA-I
apolipoprotein A-I
- apoA-IMilano
apolipoprotein A-I Milano
- DPPC
dipalmitoyl phosphatidylcholine
- ELISA
enzyme-linked immunosorbent assay
- GFP
green fluorescent protein
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- PCR
polymerase chain reaction
- RT
reverse transcriptase
- SR-A
scavenger receptor A
REFERENCES
- 1.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 2.Paszty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawashiri MA, Zhang Y, Pure E, Rader DJ. Combined effects of cholesterol reduction and apolipoprotein A-I expression on atherosclerosis in LDL receptor deficient mice. Atherosclerosis. 2002;165:15–22. doi: 10.1016/s0021-9150(02)00103-x. [DOI] [PubMed] [Google Scholar]
- 5.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 6.Moore RE, Navab M, Millar JS, et al. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ Res. 2005;97:763–771. doi: 10.1161/01.RES.0000185320.82962.F7. [DOI] [PubMed] [Google Scholar]
- 7.Weisgraber KH, Rall SC, Jr, Bersot TP, Mahley RW, Franceschini G, Sirtori CR. Apolipoprotein A-IMilano. Detection of normal A-I in affected subjects and evidence for a cysteine for arginine substitution in the variant A-I. J Biol Chem. 1983;258:2508–2513. [PubMed] [Google Scholar]
- 8.Franceschini G, Vecchio G, Gianfranceschi G, Magani D, Sirtori CR. Apolipoprotein AIMilano. Accelerated binding and dissociation from lipids of a human apolipoprotein variant. J Biol Chem. 1985;260:16321–16325. [PubMed] [Google Scholar]
- 9.Ameli S, Hultgardh-Nilsson A, Cercek B, et al. Recombinant apolipoprotein A-I Milano reduces intimal thickening after balloon injury in hypercholesterolemic rabbits. Circulation. 1994;90:1935–1941. doi: 10.1161/01.cir.90.4.1935. [DOI] [PubMed] [Google Scholar]
- 10.Shah PK, Nilsson J, Kaul S, et al. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 1998;97:780–785. doi: 10.1161/01.cir.97.8.780. [DOI] [PubMed] [Google Scholar]
- 11.Shah PK, Yano J, Reyes O, et al. High-dose recombinant apolipoprotein A-IMilano mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein E–deficient mice; potential implications for acute plaque stabilization. Circulation. 2001;03:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 12.Chiesa G, Monteggia E, Marchesi M, et al. Recombinant apolipoprotein A-I(Milano) infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ Res. 2002;90:974–980. doi: 10.1161/01.res.0000018422.31717.ee. [DOI] [PubMed] [Google Scholar]
- 13.Kaul S, Coin B, Hedayiti A, et al. Rapid reversal of endothelial dysfunction in hypercholesterolemic apolipoprotein E-null mice by recombinant apolipoprotein A-I(Milano)-phospholipid complex. J Am Coll Cardiol. 2004;44:1311–1319. doi: 10.1016/j.jacc.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant apoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 15.Ishiguro H, Yoshida H, Major AS, et al. Retrovirus-mediated expression of apolipoprotein A-I in the macrophage protects against atherosclerosis in vivo. J Biol Chem. 2001;276:36742–36748. doi: 10.1074/jbc.M106027200. [DOI] [PubMed] [Google Scholar]
- 16.Sharifi BG, Wu K, Wang L, Ong JM, Zhou X, Shah PK. AAV serotype-dependent apolipoprotein A-I Milano gene expression. Atherosclerosis. 2005;181:261–269. doi: 10.1016/j.atherosclerosis.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Major AS, Dove DE, Ishiguro H, et al. Increased cholesterol efflux in apolipoprotein AI (ApoAI)-producing macrophages as a mechanism for reduced atherosclerosis in ApoAI(−/−) mice. Arterioscler Thromb Vasc Biol. 2001;21:1790–1795. doi: 10.1161/hq1101.097798. [DOI] [PubMed] [Google Scholar]
- 18.Su YR, Ishiguro H, Major AS, et al. Macrophage apolipoprotein A-I expression protects against atherosclerosis in ApoE-deficient mice and up-regulates ABC transporters. Mol Ther. 2003;8:576–583. doi: 10.1016/s1525-0016(03)00214-4. [DOI] [PubMed] [Google Scholar]
- 19.Hedrick CC, Hassan K, Hough GP, et al. Short-term feeding of atherogenic diet to mice results in reduction of HDL and paraoxonase that may be mediated by an immune mechanism. Arterioscler Thromb Vasc Biol. 2000;20:1946–1952. doi: 10.1161/01.atv.20.8.1946. [DOI] [PubMed] [Google Scholar]
- 20.Franceschini G, Calabresi L, Chiesa G, et al. Increased cholesterol efflux potential of sera from apoA-IMilano carriers and transgenic mice. Arterioscler Thromb Vasc Biol. 1999;19:1257–1262. doi: 10.1161/01.atv.19.5.1257. [DOI] [PubMed] [Google Scholar]
- 21.Calabresi L, Tedeschi G, Treu C, et al. Limited proteolysis of a disulfide-linked apoA-I dimer in reconstituted HDL. J Lipid Res. 2001;42:935–942. [PubMed] [Google Scholar]
- 22.Bielicki JK, Oda MN. Apolipoprotein A-I(Milano) and apolipoprotein A-I(Paris) exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry. 2002;41:2089–2096. doi: 10.1021/bi011716p. [DOI] [PubMed] [Google Scholar]