Abstract
Chemotherapy-induced nausea has been associated with a time-related decrease in cardiac parasympathetic activity. We tested the hypothesis that a time-related decrease in cardiac parasympathetic activity would also be associated with nausea and other motion sickness symptoms during illusory self-motion (vection). Fifty-nine participants (aged 18–34 years: 25 male) were exposed to a rotating optokinetic drum to induce vection. Symptoms of motion sickness and an estimate of cardiac parasympathetic activity (respiratory sinus arrhythmia; RSA) were obtained at baseline and throughout a drum-rotation period. As expected, motion sickness symptoms increased and RSA decreased over time during drum rotation.Moreover, greater decreases in RSA over time correlated with greater motion sickness severity. These results suggest that a time-related decrease in cardiac parasympathetic activity may be an important correlate of nausea and motion sickness across different evocative contexts.
Descriptors: Individual differences, Motion sickness, Nausea, Parasympathetic, Respiratory sinus arrhythmia, Time
Motion sickness may be elicited by real or apparent motion. Given the same motion stimulus, however, there are individual differences in the occurrence and severity of motion sickness symptoms. A number of studies indicate that the development of motion sickness may be mediated, in part, by increased sympathetic and decreased parasympathetic nervous system activity: The report of motion sickness, for example, has been associated with increases in skin conductance and plasma catecholamines (Hu, Grant, Stern, & Koch, 1991; Koch et al., 1990; Kohl, 1993) and decreases in heart period variability (Doweck et al., 1997; Hu et al., 1991; Uijtdehaage, Stern, & Koch, 1992, 1993). Furthermore, effective interventions for motion sickness inhibit sympathetic activation and enhance or maintain parasympathetic activity during motion exposure (Hasler et al., 1995; Kohl & Homick, 1983; Uijtdehaage et al., 1992, 1993).
Similar to motion exposure, symptom development in other potentially nauseogenic contexts has been associated with autonomic reactivity. Morrow, Andrews, Hickok, and Stern (2000), for instance, recently demonstrated that a consistent, time-related decrease in heart period variability was associated with the report of nausea in ovarian cancer patients following the administration of cytotoxic agents. These findings were consistent with those of prior studies indicating reduced heart period variability during motion sickness (Doweck et al., 1997; Hu et al., 1991; Uijtdehaage et al., 1992, 1993); however, no prior motion sickness studies evaluated whether time-related changes in heart period variability correlated with motion sickness symptoms. Indeed, studies that evaluate autonomic reactivity in relation to motion sickness typically average several (e.g., minute-by-minute) autonomic estimates prior to and during a motion exposure period (e.g., Hu et al., 1991; Uijtdehaage et al., 1992, 1993). In view of the findings of Morrow et al., this type of averaging may obscure important information regarding shorter term, time-related autonomic changes. For example, an average heart period variability estimate from a motion exposure period may not distinguish between a case in which there is an overall low level of heart period variability and a case in which there is a rapid, time-related decline in heart period variability—each of which may be differentially related to experienced symptoms. Thus, a measure of the temporal change in autonomic activity may better account for individual differences in the report of motion sickness symptoms, as compared to more static “snapshots” of autonomic activity that are derived from averaging several observations over time.
Here, we examined whether time-related changes in cardiac parasympathetic activity (as estimated by respiratory sinus arrhythmia [RSA]; Berntson et al., 1997) relate to motion sickness severity during illusory self-motion (vection). Similar to a procedure for evaluating cardiovascular recovery from behavioral stressors (Christenfeld, Glynn, & Gerin, 2000), individual regression analyses were used to derive an RSAslope index, which reflected the degree of change in RSA over time for each participant. If a time-related withdrawal of cardiac parasympathetic activity is common to both chemotherapy-induced nausea and motion sickness, then it follows that a greater time-related decline in RSA (as estimated by a more negative RSAslope value) during vection may be related to greater motion sickness severity. In contrast,motion sickness symptoms may not correlate to the same degree with average measures of RSA that do not take into account time-related adjustments in cardiac parasympathetic activity.
Method
Participants
Thirty-four female and 25 male undergraduates (age range: 18–34 years) participated for course credit. Data regarding autonomic and gastric myoelectrical reactivity to laboratory stressors were reported earlier for these participants (Gianaros, Quigley, Mordkoff, & Stern, 2001). Exclusionary criteria were (a) a history of cardiovascular, gastrointestinal, or respiratory illness, (b) a body mass index above or below 35%of recommended body weight, (c) an exercise regimen exceeding 20 hr per week, (d) resting blood pressure above 150/90mmHg, or (e) recent use of prescription medication that could affect gastrointestinal or autonomic activity. Three participants had missing subjective motion sickness data because of experimenter error (n = 1) or because drum rotation was terminated by the participant prior to any symptom assessment (n = 2), and 7 participants had missing physiological data because of signal acquisition failure.
Procedure and Motion Sickness Induction
Participants were asked to refrain from consuming beverages containing caffeine, abstain from smoking, and fast for a minimum of 3 hr prior to testing. Upon arrival at the laboratory, each participant provided her or his informed consent and completed two laboratory stress tasks during which electrogastrographic and impedance cardiographic measures were obtained. 1 Following an 8-min recovery from the second task, impedance band electrodes were removed, and the participant was escorted to another laboratory that housed a rotating optokinetic drum. The participant was then seated on a stationary stool within the optokinetic drum, which is a cylinder 91.5 cm in height and 76 cm in diameter and is lined with alternating 3.8 cm (5.7°) black and 6.2 cm (9.3°) white vertical stripes on its interior. Following a 6-min baseline period, the drum rotated around the participant at 60°/s for 16 min or until the participant requested to terminate the drum rotation period. Following 2 min of drum rotation, each participant was asked whether she or he felt as if the drum was spinning around her or him, or whether she or he was spinning within the drum (i.e., experiencing vection); all reported vection.
Symptom Assessment
At the end of the baseline, and during the 4th, 7th, 10th, 13th, and 16th minutes of drum rotation, motion sickness symptoms were assessed via a two-way intercom using the Pensacola Diagnostic Index (PDI; Graybiel, Wood, Miller, & Cramer, 1968). A PDI score reflects the sum of the ratings of the following motion sickness symptoms: dizziness (1 = moderate or severe), headache (1 = moderate or severe), warmth (1 = moderate or severe), sweating (2 = slight, 4 = moderate, 8 = severe), drowsiness (2 = slight, 4 = moderate, 8 = severe), salivation (2 = slight, 4 = moderate, 8 = severe), and nausea (1 = stomach awareness, 2 = stomach discomfort, 4 = slight nausea, 8 = moderate/severe nausea, 16 = vomiting).
Physiological Recording
Electrocardiographic (ECG) signals were obtained from two disposable Ag/AgCl electrodes that were positioned in a modified Lead II configuration. Respiration was recorded from a piezoelectric respiration transducer (Pneumotrace Model 1132®; UFI, Morro Bay, California) that was attached around the participant’s torso. The ECG and respiratory leads were connected to a UFI Simple Scope® (Model SC2000; UFI), which digitized (12 bit) the ECG signal at 1 kHz and the respiration signal at 100 Hz, and stored each signal for off-line processing.
Physiological Data Reduction
Interbeat intervals (IBIs) were derived from sequential R spikes in the ECG. Prior to the calculation of RSA, IBIs were visually inspected and edited for artifacts (<1%) using the algorithm described by Berntson, Quigley, Jang, and Boysen (1990). An estimate of RSA was calculated for each minute of the baseline and rotation epochs. Specifically, an autoregressive algorithm (Colombo, Mazzuero, Soffiatino, Ardizzoia, & Minuco, 1998) was used to derive the spectral power (in msec2/Hz) in the respiratory frequency bandwidth (0.12–0.40 Hz) for each 60-s IBI time series. Because of distributional violations, spectral power estimates were natural-log transformed. The mean respiratory rate (in breaths per minute; bpm) was also calculated from the respiratory signal for each minute of the baseline and rotation periods.
Statistical Analyses
Prior to evaluating the relationship between motion sickness severity and temporal changes in RSA, we first evaluated changes in PDI symptoms, RSA, and respiratory rate across the baseline and drum rotation periods. For these analyses, RSA and respiratory rate data were averaged over the 6-min baseline and over consecutive 3-min epochs beginning at the second minute of drum rotation (i.e.,min 2–4, 5–7, 8–10). This was done to yield equivalent RSA and respiration estimates that corresponded to periods of symptom assessment. Changes over time in motion sickness symptoms, RSA, and respiration rate were then analyzed using multivariate analyses of variance (MANOVAs) with repeated measures for time, where Time 1 represented the baseline period, and Times 2 through 4 represented the rotation observations from the 4th, 7th, and 10th minutes, respectively; because 14 participants requested to terminate drum rotation after 10min, MANOVAs were performed on the observations obtained until the 10th minute. For these multivariate analyses, the Wilks’ Lambda test statistic was used. Statistically significant (α≤.05) effects of time were then followed with polynomial trend analyses to determine the profile (linear or quadratic) of the response change.
After evaluating the overall changes in motion sickness symptoms, RSA, and respiration rate, we then evaluated the relationship between the change in RSA over time and motion sickness severity. The change in RSA over time for each participant was assessed using individual regression analyses. Specifically, 1-min RSA estimates were regressed on each minute of observation for each participant, with the first RSA point (intercept) representing the average of the baseline values. These regressions yielded an unstandardized regression coefficient (RSAslope) that represented the degree of change in RSA per minute. Inspection of RSA values and residuals over time for each participant indicated that a linear regression function was appropriate for calculating RSAslope values (see Figure 1 for individual illustrations). A two-level hierarchical regression analysis was then used to assess the between-subject relationship between the change in RSA over time (as indexed by the RSAslope) and the maximum PDI score obtained during drum rotation, after covarying baseline symptoms in the first level.
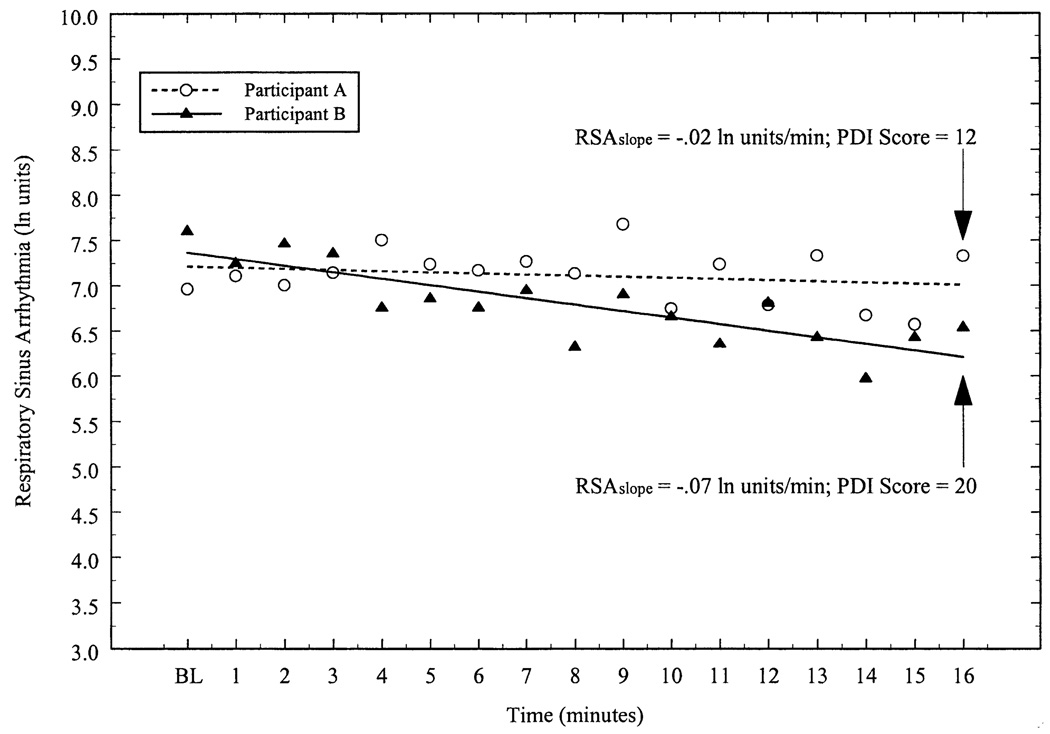
Figure 1.
Respiratory sinus arrhythmia (RSA) as a function of time for two participants. BL represents the average RSA from a 6-min baseline period; min 1–16 correspond to optokinetic drum rotation. Individual regression analyses were used to derive an unstandardized regression coefficient (RSAslope) that represented the change in RSA per minute. These regression coefficients were then correlated to motion sickness scores obtained from the Pensacola Diagnostic Index (PDI). The PDI scores for the participants shown here were reported at the 16th minute of drum rotation. In the present study, RSAslope values were inversely related to motion sickness scores, indicating that a greater degree of cardiac parasympathetic withdrawal over time was associated with increased motion sickness severity.
Maximum PDI values were chosen because they provide an index of symptom severity and are used most commonly in motion sickness studies (e.g., Hu et al., 1991; Uijtdehaage et al., 1992, 1993). For 71% of the participants, the maximum PDI score was the final score obtained; for 17% of the participants, the maximum PDI score was obtained 3 min prior to the final score; for 6% of the participants, the maximum PDI score was obtained 6min prior to the final score; and for the remaining 6% of the participants, the maximum PDI score was obtained 9 min prior to the final score. Overall, the average maximum (M = 9.60, SD = 5.54) and the average final (M = 8.65, SD = 5.94) PDI scores were comparable in the present sample.
Results
Changes in Motion Sickness, RSA, and Respiration over Time
Table 1 shows motion sickness symptoms, RSA values, and respiratory rates for the baseline and drum rotation periods. As expected, a significant change in motion sickness symptoms was observed across the time periods, F(3,55) = 40.23, p<.001, Wilks’ Lambda =.31. Significant linear, F(1,57) = 101.124, p<.001, and quadratic trends, F(1,57) = 12.71, p<.005, in the PDI scores indicated an overall increase in motion sickness symptoms from baseline to rotation, and a moderate change in symptoms from baseline to the fourth minute of rotation, respectively. Overall, these results indicated that exposure to the rotating optokinetic drum was effective in provoking motion sickness at the group level, and that motion sickness symptoms increased progressively over time.
Table 1.
Average Motion Sickness Score, RSA, and Respiratory Rate by Time Period
| Time period | ||||
|---|---|---|---|---|
| Measure | Baseline | Rotation min 4 | Rotation min 7 | Rotation min 10 |
| PDI | 1.28 (0.20) | 4.64 (0.44) | 6.10 (0.58) | 7.16 (0.60) |
| RSA | 6.64 (0.11) | 6.45 (0.14) | 6.23 (0.16) | 6.28 (0.13) |
| Corrected RSA | .25 (.05) | .12 (.04) | −.10 (.05) | −.06 (.04) |
| Resp. rate | 15.72 (0.43) | 16.14 (0.49) | 15.56 (0.47) | 15.01 (0.42) |
Note. PDI: Pensacola Diagnostic Index (arbitrary units); RSA: Respiratory sinus arrhythmia (ln units); Corrected RSA: Respiratory-rate-adjusted RSA values; Resp. Rate5Respiration rate (breaths per minute). Values in parentheses indicate standard error of the mean.
A significant change in RSA was also observed across the baseline and drum rotation periods, F(3,49) = 9.15, p<.001, Wilks’ Lambda = .64. A significant linear trend indicated an overall decline in RSA from baseline to drum rotation, F(1,51) = 24.17, p<.001; however, a quadratic component in RSA over the time periods, F(1,51) = 4.45, p<.05, reflected a slight increase (0.05 ln units) in RSA from the 7th to 10th minutes of drum rotation.
Respiratory rate varied significantly across the baseline and drum rotation periods, F(3,49) = 4.22, p<.05, Wilks’ Lambda = .78. Similar to the RSA findings, trend analyses revealed both a linear, F(1,51) = 4.40, p<.05, and quadratic, F(1,51) = 4.52, p<.05, change in respiration rate over time. These trends indicated a slight increase (0.42 bpm) in respiratory rate from baseline to the 4th minute of drum rotation, and then a gradual decrease in respiratory rate across the remaining time periods compared to baseline, respectively.
Given that changes in respiratory rate could have accounted for the observed changes in RSA (Grossman, Karemaker, & Wieling, 1991), RSA estimates were corrected for respiratory rate on a within-participant basis. Specifically, 1-min estimates of RSA were regressed upon the corresponding respiratory rates for each subject, and the residual RSA values were retained. Similar to the results for the uncorrected RSA estimates, a MANOVA on the respiration-corrected, residual RSA values revealed a main effect of time, F(3,49) = 7.52, p<.001, along with significant linear, F(1,51) = 22.13, p<.001, and quadratic, F(1,51) = 4.14, p<.05, trends (see Table 1). Because these findings paralleled those of the uncorrected RSA values, it is not likely that the observed changes in RSA were substantially altered by changes in respiratory timing.
Relationship between Temporal Changes in RSA and Motion Sickness Symptoms
The first level of a hierarchical regression analysis revealed a positive relationship between baseline and maximum PDI scores, r2 = .10, F(1,50) = 5.78, p<.05. After controlling for baseline symptoms, RSAslope scores accounted for a significant proportion of the remaining variance in maximum PDI scores in the second level of the hierarchical regression analysis, ΔR2 = .07, F(1,49) = 4.41, p<.05.2 Specifically, a negative correlation between RSAslope scores and maximum PDI scores (zero-order r = −.29, p<.05) indicated that greater decreases in RSA over time were related to greater reported symptom severity. Additional analyses showed that baseline (intercept) RSA estimates did not correlate with RSAslope values, r = .04, p>.80, or maximum PDI scores, r = .003, p>.90, indicating that initial RSA values were unrelated to the degree of change in RSA over time or to motion sickness severity. In the present sample, the average observed RSAslope was −.05 ln units/minute (95% confidence interval of the average RSAslope = −.07 to −.03). These data show that RSA decreased over time and that a greater decrease in RSA over time was related to increased symptom severity.
As a comparison to the RSAslope analyses, we also evaluated relationships between maximum PDI scores and (a) the average drum rotation level of RSA, and (b) the overall change in RSA from baseline to rotation (ΔRSA = average drum rotation RSA − average baseline RSA). These measures were used as comparisons because they do not specifically take into account the time-related change in cardiac parasympathetic activity that the RSAslope value indexes. In contrast to the RSAslope results, neither the average drum rotation level of RSA (zero order r = −.05; ΔR2 = .009, F(1,49)< 1, p = .48), nor the overall change in RSA from baseline to rotation (zero order r = −.12; ΔR2 = .02, F(1,49) = 1.33, p = .30) accounted for a significant proportion of the variance in PDI scores after adjusting for basal symptoms in the first step of a hierarchical regression analysis. Moreover, additional hierarchical regression analyses showed that the RSAslope index continued to account for a unique percentage of the variance in motion sickness severity when the average drum rotation level of RSA, ΔR2 = .08, F(1,49) = 4.23, p<.05, and the overall change in RSA from baseline to rotation, ΔR2 = .07, F(1,49) = 3.60, p = .06, were covaried in the first level.3
Discussion
Consistent with prior studies (Hu et al., 1991; Uijtdehaage et al., 1992, 1993), we observed that exposure to a provocative optokinetic stimulus elicited an increase in motion sickness symptoms and a corresponding decrease in cardiac parasympathetic activity, as estimated by changes in RSA that were statistically independent of corresponding changes in respiratory rate. In addition, whereas greater decreases in cardiac parasympathetic activity over time were related to more severe reports of motion sickness, neither the average level of RSA during drum rotation nor the average change in RSA from baseline to rotation correlated with motion sickness symptoms. Moreover, when these two average measures were controlled statistically, time-related changes in RSA continued to account for a unique percentage of the variance in motion sickness severity. These findings (a) underscore the importance of evaluating time-related changes in variables that are related to the report of motion sickness symptoms, and (b) suggest that averaging autonomic estimates over time may mask relationships between dynamic, temporal changes in physiological activity and motion sickness symptoms.
Although a relationship between time-related changes in RSA and motion sickness severity was observed in the present study, the percentage of the variance in motion sickness symptoms that was accounted for by using only RSAslope values was relatively small (9%). If it is assumed that the motion sickness syndrome is mediated by shifts away from basal efferent and afferent autonomic activity to and from multiple target organs (e.g., as proposed by Stern & Koch, 1996) and that RSA does not necessarily reflect parasympathetic activity at target organs other than the heart (e.g., the stomach; Jennings & McKnight, 1994), then we would expect that individual differences in motion sickness symptoms may be better accounted for by examining the time-related change in other physiological processes that are also thought to relate to the motion sickness syndrome or by obtaining estimates of autonomic function at multiple target organs. The present results do, however, suggest that respiratory rate per se may not be a critical variable for evaluating individual differences in motion sickness symptoms as (a) changes in respiratory rate over time did not show a relationship with motion sickness severity, and (b) statistical adjustment for time-related changes in respiratory rate did not affect the relationship between motion sickness severity and temporal changes in RSA (see Footnote 3). However, although prior motion sickness studies (e.g., Cowings, Suter, Toscano, Kamiya, & Naifeh, 1986) have also failed to observe relationships between changes in respiratory rate and the report of motion sickness, it is plausible that other respiratory parameters, which have not been evaluated to date, may play an important role in the development of motion sickness. Consistent with this possibility, preliminary evidence indicates that the simultaneous manipulation of both breathing frequency and depth may affect the development of motion sickness symptoms during motion exposure (Jokerst, Gatto, Fazio, Stern, & Koch, 1999); thus, future work evaluating the role of respiration in motion sickness is warranted.
To summarize, the present findings indicating that a greater temporal decline in cardiac parasympathetic activity correlated with motion sickness severity accord with a recent report by Morrow et al. (2000), which showed that chemotherapy-induced nausea was also related to temporal declines in heart period variability among ovarian cancer patients. Taken together, the present results and those of Morrow et al. suggest that considering temporal changes in autonomic activity that relate to the experience of motion sickness and nausea may help to identify and consequently treat those individuals who are at greater risk for experiencing these syndromes. At present, however, inferences regarding a causal relationship between time-related autonomic changes and symptom development are unsupported. Indeed, neither the present results nor those of Morrow et al. exclude the possibility that changes in autonomic activity over time may reflect other (e.g., subjective or emotional) processes that are concomitant to the development of the nausea and motion sickness syndromes or the possibility that time-related autonomic changes may also reflect a bidirectional association between symptom development and changes in autonomic activation. Given the above issues, future use of autonomic blockades or other experimental manipulations may be useful for evaluating the importance of changes in autonomic activation over time in the mediation of symptom development. Further, it may prove to be useful for future research to examine the extent to which time-related autonomic changes predict more specific symptoms of nausea and motion sickness (e.g., dizziness, loss of appetite, stomach discomfort) and whether time-related autonomic adjustments correlate with symptoms in other clinical (e.g., postoperative recovery) or naturalistic (e.g., pregnancy) conditions.
Acknowledgments
Research support was provided by an Individual NIMH NRSA (1F31MH12418-01) to Peter J. Gianaros and by training grant NIH HL07560 awarded to the University of Pittsburgh. We thank J. Richard Jennings and Natasha Tokowicz for their helpful comments.
Footnotes
Impedance cardiographic measures were not obtained during the drum rotation session. Electrogastrographic measures were obtained, but are omitted here for two reasons. First, quantification of electrogastrographic signals using fast Fourier transforms (FFTs) requires a minimum of 4min of data to achieve sufficient spectral resolution. Because of this constraint, a maximum of only four estimates of electrogastrographic activity were available from the 16-min drum rotation period (e.g., one from min 1–4, one from min 5–8, etc.), and these four points of data were insufficient to calculate stable individual regression coefficients, as were calculated from the minute-by-minute RSA estimates. Therefore, we were unable to adequately assess the time-related change in electrogastrographic measures. Second, electrogastrographic measures are ambiguous with regard to their underlying autonomic origins (Gianaros et al., 2001), and those that were available in the present study showed no relationships with any measure of RSA.
Exclusion of two participants with extreme values improved the zero-order correlation between PDI scores and the RSAslope measure (r = −.38), and the percentage of variance accounted for by the RSAslope measure in the second level of the hierarchical regression analysis, ΔR2 = .13, F(1,47) = 7.80, p<.01.
We also evaluated the extent to which the RSAslope index accounted for variance in motion sickness severity that was unique from time-related changes in respiratory rate. To this end, we first regressed 1-min respiration rate estimates on each minute of observation for each participant, as was done to calculate the RSAslope index, and derived a slope (Respiratory–Rateslope) that reflected the change in respiratory rate over time. Next, we conducted a hierarchical regression analysis relating motion sickness severity scores to the Respiratory–Rateslope index. The results showed that the Respiratory–Rateslope index did not correlate with motion sickness symptoms, r = −.05, p = .73. Further, when the Respiratory–Rateslope index was used as a covariate in a subsequent hierarchical regression model, the RSAslope index continued to account for a significant percentage of the variance in motion sickness symptoms, ΔR2 = .10, p = .024. Thus, given that (a) the changes in respiration rate were not appreciable (e.g., the greatest mean increase observed was 0.42 bpm; see Table 1), (b) within-participant corrections for respiration rate did not alter the pattern of results for RSA changes over time, (c) no relationship was observed between changes in respiration rate over time and subjective symptoms in the present sample, and (d) the RSAslope measure continued to correlate with motion sickness severity after time-related, respiratory-rate corrections, it is unlikely that changes in respiratory rate mediated the relationship between time-related, RSA changes and motion sickness severity.
REFERENCES
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Jang JF, Boyson ST. An approach to artifact identification: Application to heart period data. Psychophysiology. 1990;27:586–598. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Christenfeld N, Glynn LM, Gerin W. On the reliable assessment of cardiovascular recovery: An application of curve-fitting techniques. Psychophysiology. 2000;37:543–550. [PubMed] [Google Scholar]
- Colombo R, Mazzuero G, Soffiatino F, Ardizzoia M, Minuco G. A comprehensive PC solution to heart rate variability analysis in mental stress. Proceedings of 1989 IEEE Computers in Cardiology. 1990:457–478. [Google Scholar]
- Cowings PS, Suter S, Toscano WB, Kamiya J, Naifeh K. General autonomic components of motion sickness. Psychophysiology. 1986;23:542–551. doi: 10.1111/j.1469-8986.1986.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Doweck I, Gordon CR, Shlitner A, Spitzer O, Gonen A, Binah O, Melamed Y, Shupak A. Alterations in R-R variability associated with experimental motion sickness. Journal of the Autonomic Nervous System. 1997;67:31–37. doi: 10.1016/s0165-1838(97)00090-8. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Quigley KS, Mordkoff JT, Stern RM. Gastric myoelectrical and autonomic cardiac reactivity to behavioral stressors. Psychophysiology. 2001;38:642–652. [PMC free article] [PubMed] [Google Scholar]
- Graybiel A, Wood CD, Miller EF, Cramer DB. Diagnostic criteria for grading the severity of acute motion sickness. Aerospace Medicine. 1968;39:453–455. [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Hasler WL, Kim MS, Chey WD, Stevenson V, Stein B, Owyang C. Central cholinergic and alpha-adrenergic mediation of gastric slow wave dysrhythmias evoked during motion sickness. American Journal of Physiology. 1995;268:G539–G547. doi: 10.1152/ajpgi.1995.268.4.G539. [DOI] [PubMed] [Google Scholar]
- Hu S, Grant WF, Stern RM, Koch KL. Motion sickness severity and physiological correlates during repeated exposures to a rotating optokinetic drum. Aviation Space and Environmental Medicine. 1991;62:308–314. [PubMed] [Google Scholar]
- Jennings JR, McKnight JD. Inferring vagal tone from heart rate variability. Psychosomatic Medicine. 1994;56:194–196. doi: 10.1097/00006842-199405000-00003. [DOI] [PubMed] [Google Scholar]
- Jokerst MD, Gatto M, Fazio R, Stern RM, Koch KL. Slow deep breathing prevents the development of tachygastria and symptoms of motion sickness. Aviation, Space, and Environmental Medicine. 1999;70:1189–1192. [PubMed] [Google Scholar]
- Koch KL, Stern RM, Vasey MW, Seaton JF, Demers LM, Harrison TS. Neuroendocrine and gastric myoelectrical responses to illusory self-motion in humans. American Journal of Physiology. 1990;258:E304–E310. doi: 10.1152/ajpendo.1990.258.2.E304. [DOI] [PubMed] [Google Scholar]
- Kohl RL. Autonomic function and plasma catecholamines following stressful sensory stimuli. Aviation, Space, and Environmental Medicine. 1993;64:921–927. [PubMed] [Google Scholar]
- Kohl RL, Homick JL. Motion sickness: A modulatory role for the central cholinergic nervous system. Neurosciene and Biobehavioral Reviews. 1983;7:73–85. doi: 10.1016/0149-7634(83)90008-8. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Andrews PLR, Hickok JT, Stern RM. Parasympathetic changes following cancer chemotherapy: Implications for the development of nausea. Psychophysiology. 2000;37:378–384. [PubMed] [Google Scholar]
- Stern RM, Koch KL. Motion sickness and differential susceptibility. Current Directions in Psychological Science. 1996;5:115–120. [Google Scholar]
- Uijtdehaage SHJ, Stern RM, Koch KL. Effects of eating on vection-induced motion sickness, cardiac parasympathetic tone, and gastric myoelectric activity. Psychophysiology. 1992;29:193–201. doi: 10.1111/j.1469-8986.1992.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Uijtdehaage SHJ, Stern RM, Koch KL. Effects of scopolamine on autonomic profiles underlying motion-sickness susceptibility. Aviation Space and Environmental Medicine. 1993;64:1–8. [PubMed] [Google Scholar]