Abstract
Group A Streptococcus (GAS) is a human-specific pathogen that is highly prevalent throughout the world. The vast majority of GAS infections lead to a mild disease involving the epithelial surfaces of either the throat or skin. The concept of distinct sets of ‘throat’ and ‘skin’ strains of GAS has long been conceived. From an ecological standpoint, the epithelium of the throat and skin are important because it is where the organism is most successful in reproducing and transmitting to new hosts. This article examines key features of the epidemiology, population biology and molecular pathogenesis that underlie the tissue site preferences for infection exhibited by GAS, with an emphasis on work from our laboratory on skin tropisms. Recombinational replacement with orthologous gene forms, following interspecies transfer, appears to be an important genetic step leading up to the exploitation of new niches by GAS.
Keywords: epithelium, impetigo, pharyngitis, Streptococcus, tropisms
Clinical importance of group A streptococcal diseases
Humans are almost exclusively the biological host of Streptococcus pyogenes, commonly known as β-hemolytic group A streptococci (GAS). It is estimated that these Gram-positive cocci are responsible for more than 600 million cases of throat infection (pharyngitis and tonsillitis) worldwide per year, and more than 100 million cases of skin infection (primarily nonbullous impetigo) in children living in resource-limited countries [1]. Streptococcal pharyngitis and impetigo are superficial, self-limiting infections that typically cause a mild illness which resolves within 2 weeks, coincident with the rise of specific host immune defenses [2]. Most morbidity and mortality due to GAS arises from invasive and autoimmune disease, each of which is relatively infrequent in occurrence when compared with the highly prevalent infections at the throat and skin epithelium. The throat and skin are the primary reservoirs for GAS, and human-to-human transmission is mainly via respiratory droplets or direct skin contact [2].
Carriage at the throat, in which the organism persists in a quiescent commensal-like state and does not cause clinical symptoms, is an important facet of GAS ecology. It is not unusual for throat carriage rates to exceed 20% in populations of school-aged children [3,4]. A subclinical pharyngeal infection can also occur, in which the host mounts a specific immune response, yet lacks obvious symptoms of illness. Antibiotics (β-lactams) are the recommended treatment for GAS pharyngitis and impetigo, and although resistant clones do exist (e.g., resistant to macrolides), treatment failures have not yet emerged as a significant public health problem. Currently, there is no licensed vaccine available to prevent GAS disease.
Genotypic markers of throat & skin strains
The GAS cell surface bears M proteins that form short hair-like fibrils of approximately 60 nm and contain antigenic targets of the major serological typing scheme [5]. Determinants of serotype lie at the distal fibril tips (amino termini). Over the past decade, serological typing has been largely replaced by nucleotide (nt) sequence typing, based on sequence identity within the 5′ end of the emm gene encoding the type-specific determinants [6]. To date, over 200 emm types have been identified [201]. Antibody directed to the M type-specific antigenic epitope mediates opsonophagocytosis of the organism, and provides protective immunity.
Decades of epidemiologic studies have shown that some M protein serotypes of GAS have a strong tendency to cause throat infection (pharyngitis), but not superficial skin infection (impetigo). Similarly, other M types are often recovered from impetigo, but rarely from cases of pharyngitis [2,7]. This observation gave rise to the concept of distinct throat and skin M types. The existence of distinct throat- and skin-tropic strains implies that there must also exist some degree of specialization within this species, and that there are intrinsic biological properties of the organism that confer tissue site preference. Clues may lie in the structure of the emm genes that encode the M proteins.
Many strains of GAS have one or two additional emm-like gene paralogs lying immediately upstream or downstream from the emm locus that encodes the type-specific antigen [8,9]. Together, the emm and emm-like genes comprise four major lineages based on extensive nt sequence differences within the portion encoding the cell wall-spanning domain [10,11]. Several arrangements of the emm chromosomal region are observed, based on the number of emm and emm-like genes and their lineage. These arrangements are referred to as ‘emm patterns’, of which three major groupings are recognized: A–C, D and E. Importantly, for nearly all GAS strains examined, a given emm type is restricted to a single emm pattern grouping [12]. Thus, based on knowledge of emm type, it is reasonable to infer the emm pattern of a given strain.
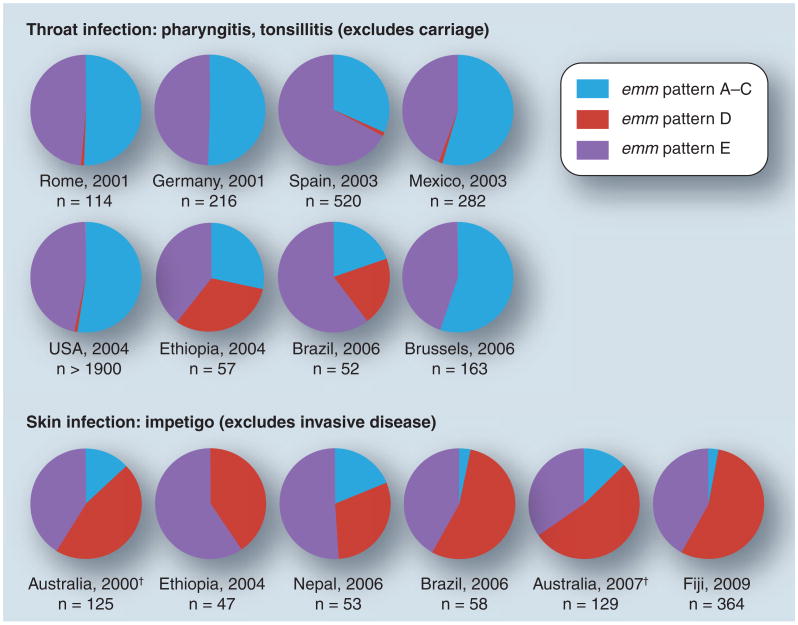
The biological importance of emm pattern genotypes is that they are strong markers for preferred tissue site of infection among GAS strains [13,14]. Several population-based collections of GAS isolates recovered from cases of either throat or skin infection [15–25] were assessed for emm pattern genotype (Figure 1). The combined surveillance studies yield emm typing data on over 4000 isolates collected from the six continents; emm pattern was inferred based on the established relationships with emm type (Figure 1) [12]. For each study evaluated, the designated skin isolates were obtained from cases of impetigo, but not from other types of skin lesions that can be difficult to discern in non-endemic regions, such as wound infections that are often initiated by throat-derived strains. In addition, the throat isolates were obtained from patients with pharyngitis and/or tonsillitis, and not from subjects with asymptomatic carriage.
Figure 1. Population-based surveys of group A Streptococcus infection.
Group A streptococci isolated from patients with either superficial throat or skin infections, in several locales throughout the world by numerous investigators, had been characterized for emm type [15–25]; based on the emm type, the emm pattern group is inferred [12]. The percentage of isolates in each emm pattern group is depicted for the eight pharyngitis and six impetigo collections of group A streptococci.
†Aboriginal populations of tropical Australia.
Population analysis of the more than 4000 GAS isolates reveals a strong trend in which organisms corresponding to the emm pattern A–C group have a strong predilection to cause infection at the throat, and emm pattern D strains have a strong tendency to cause impetigo (Figure 1). For the eight pharyngitis and six impetigo studies, differences in the distribution of emm pattern A–C versus D strains are highly significant (t = 0.003 and t = 0.002, respectively; paired t-test). The emm pattern A–C strains represent throat specialists, whereas the pattern D strains correspond to skin specialists. The emm pattern E strains readily infect both tissues and are considered generalists, accounting for approximately 50% of GAS isolates worldwide. In summary, the population surveillance data provide strong support that emm pattern genotype is a highly valid biomarker for preferred tissue site of infection by GAS, although it is not perfect and there are exceptions.
Population genetics of throat & skin specialists
Not only are the throat and skin specialists physically separated by their distinct ecological niches, but there is also geographic separation as evidenced by the prevalence of throat versus skin infection in different parts of the world. Upper respiratory tract infections predominate in temperate regions, whereas impetigo is mostly observed in communities having a warm and tropical climate and is often endemic (Figure 1) [2,26]. There are also seasonal differences in some locales, with pharyngitis peaking in winter months and impetigo emerging during the summer [2,26]. It appears that climate-related risk factors (e.g., warm humid climate; time spent indoors) for GAS infection are a driving force in promoting adaptation to narrow ecological niches among the specialist strains. Spatial separation, due to both geographic partitioning and innate tissue site preference for infection, might impose a physical barrier to lateral gene exchange between throat and skin specialist strains.
This raises the question of whether emm pattern A–C and D strains are at the beginning stages of forming new species due to reproductive isolation. Several analyses on core housekeeping genes provide strong support for extensive genetic recombination across the three emm pattern groupings and within the GAS population as a whole [14,27–31]. The extensive recombination follows frequent horizontal gene transfer events that occur between different strains. Consequently, ancestor–descendent relationships among GAS strains are not obvious, and genetic signatures for early stages of speciation within the GAS population are lacking. Based on housekeeping genes, there is no evidence that pattern E strains are intermediate to pattern A–C and D strains.
Genes linked to markers for preferred tissue site of infection
Against a background of random associations among core housekeeping genes, other genes that exhibit linkage disequilibrium with emm pattern are strong candidates for having a direct role in causing infection of either the throat or skin. For example, if there is a particular set of accessory genes that is essential for causing superficial throat or skin infection, then the corresponding group of specialist strains should have the full complement of adaptive genes.
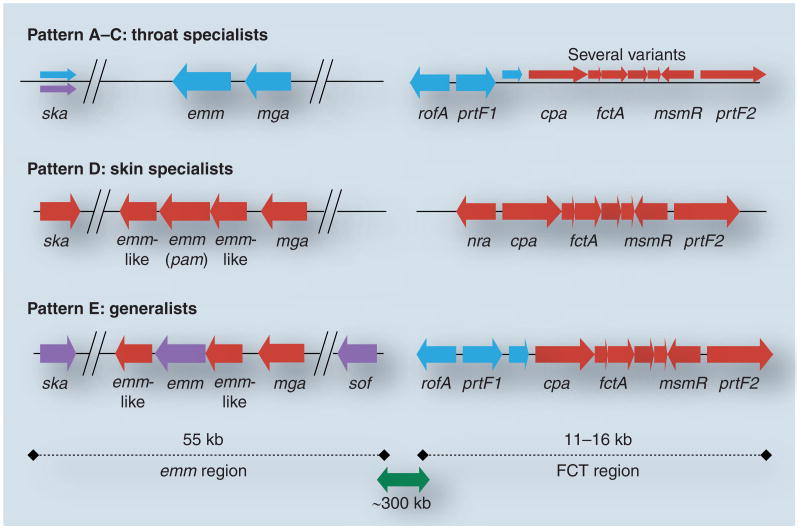
By screening a genetically diverse collection of approximately 100 GAS isolates, several GAS genes that display strong linkage with the emm pattern groupings have been identified [32–34]. Included are genes having a differential presence or absence among strains (cpa, prtF1, sof ), and loci having alleles that form discrete phylogenetic lineages (mga, rofA/nra, ska). Furthermore, most of the linked genes identified to date are clustered within two genomic regions: the emm region itself or the FCT region [35], positioned approximately 300 kB from emm and containing pilus biosynthesis genes (Figure 2). The pili contain the immunodeterminants of T type, which provides the basis for another serotyping scheme of GAS [36]. Additional emm pattern-linked genes located distal to the emm and FCT regions have been uncovered by comparative genomic hybridization studies [Bessen DE et al., Unpublished Data].
Figure 2. Map of the emm and FCT regions of the group A Streptococcus genome.
The most common combinations of genes present within the emm and FCT regions of over 100 group A Streptococcus strains are depicted in accordance to emm pattern grouping, based on findings reported in [32–34,42]. Shown are emm pattern A–C (blue), D (red) and E (purple). For pattern D and E strains, the upstream emm-like genes is also known as mrp and the downstream emm-like gene is also known as enn.
The mga and rofA/nra loci encode transcription regulators of gene expression [37,38]. Alleles at the mga locus comprise two discrete phylogenetic lineages having approximately 28% nt sequence divergence, and are referred to as the mga-1 and mga-2 lineages [32]. The nra and rofA lineages of alleles occupy the same locus within the FCT region, and display approximately 35% nt sequence divergence. All GAS isolates have either an mga-1 or mga-2 lineage allele, but not both. Likewise, all isolates also have either a rofA or nra allele. The products of each allelic lineage also display a high level of divergence in amino acid sequence.
The mga-1 alleles are present in nearly all emm pattern A–C isolates, whereas mga-2 alleles are present in 100% of the emm pattern D and E strains examined [32]. Several other loci display strong linkage with emm pattern and lie within the surrounding region of approximately 55 kB (Figure 2). The sof gene encodes serum opacity factor (SOF), a multifunctional extracellullar protein that modulates high-density lipoprotein [39], and binds fibrinogen and fibronectin (Fn) [40,41], and is largely restricted to emm pattern E strains, present in 100% of them [34]. In addition, SOF provides the basis for a third GAS serological typing scheme. The pam gene encodes a specialized plasminogen (Plg)-binding M protein (PAM) that is restricted to emm pattern D strains [42]. The ska gene encodes the Plg activator streptokinase, and has three main lineages of alleles exhibiting approximately 40% overall sequence divergence [33]. The cluster 2b ska alleles are largely restricted to pam-positive pattern D strains [33].
Given its genome map position is immediately adjacent to the emm gene, it is perhaps not surprising that mga lineage alleles display linkage disequilibrium with emm pattern group (Figure 2). Strong linkage of emm pattern to mga, sof, pam and ska might be explained by physical proximity, yet there is also evidence for genetic recombination within the region of the genome surrounding the emm locus [13,43,44]. Even though emm and sof loci lie only approximately 16-kB apart, a given M type is found in association with a wide array of SOF types, and vice versa, indicative of a history of genetic recombination [45]. Since physical linkage is broken by recombination, the strong genetic linkage that is observed between emm pattern group and neighboring genes might otherwise be preserved via a functional necessity arising from a phenotype that enhances fitness.
Mga functions as a positive regulator of emm gene expression in numerous strains examined [46–48], representing all three emm pattern groups. Several genes that lie outside of the emm region are also under direct transcriptional control of Mga. Comparison of the Mga regulons of mga-1- versus mga-2-positive strains fails to reveal differences among genes likely to be under the direct control of Mga [38,48], except for Mga-regulated genes that are emm pattern restricted (e.g., sof ) in their distribution among strains.
The rofA/nra locus of the FCT region encodes a transcriptional regulator that controls the expression of pilus structural genes [37,49–51]. Nearly all emm pattern A–C and E strains have rofA, whereas most emm pattern D strains harbor nra [32]. The association of rofA versus nra with emm pattern group differs from the distribution of mga alleles, in that the pattern E generalists tend to share rofA with pattern A–C strains, but share mga-2 with pattern D strains. The effect of RofA and Nra on gene transcription varies from strain to strain [50,51]. For example, Nra can function as either an activator or repressor of pilus gene transcription. Similar to Mga, RofA/Nra is a global regulator of GAS gene transcription, affecting the expression of numerous genes lying outside of the FCT region [52].
T type is found in association with numerous M- and SOF-type combinations [53], suggestive of past recombination events involving genetic crossover somewhere between the FCT and emm chromosomal regions. Yet, several loci within the FCT region display strong linkage disequilibrium with emm pattern. FCT region-encoded colonization factors (e.g., cpa and prtF1) may facilitate tissue-specific infection, as evidenced by their strong linkage with emm pattern genotypes [34]. Cpa is a collagen-binding protein, and is also an accessory protein of the pilus [36,54]. The cpa gene is detected by PCR in more than 90% of emm pattern D strains, but in only 25% of the emm pattern A–C strains examined [34]. PrtF1 is a Fn-binding protein that has a strain distribution opposite to that of Cpa, being present in 75% of pattern A–C strains, but in only 20% of pattern D strains [34]. The differential distribution of Cpa and PrtF1 among throat versus skin specialists is supportive of a role for each adhesin in mediating tissue-specific adherence. A high proportion (93%) of pattern E strains harbor prtF1; however, only 55% have cpa [34]. Therefore, if Cpa is a determinant for infection of the skin, then many pattern E strains appear to utilize an alternative molecular strategy.
A striking feature of the FCT region is that its structure is highly homogenous among the emm pattern D strains but, by contrast, the emm pattern A–C throat specialists exhibit a wide array of gene combinations sharing little in common across the group (Figure 2) [34]. The emm pattern E generalists are intermediate in this regard. The highly heterogeneous structure of the FCT regions of throat specialist strains is consistent with the idea that there may be multiple genotypes giving rise to a similar phenotype.
GAS adherence in cell & organ cultures
Adhesins can potentially contribute to bacterial tropisms for host species, tissue and/or cells. The body of knowledge on GAS adherence to epithelial cells is very extensive and only a few of the many important published studies are mentioned below. A key characteristic expected for a tissue-specific GAS adhesin is differential binding to throat- versus skin-derived cells or tissue of human origin. Furthermore, a tissue-specific adhesin is expected to be distributed among the GAS population in accordance with GAS strain tropisms. Human host-derived cells and tissues used to study GAS adherence include tonsils, respiratory tract-derived cell lines, human transgenic cell lines, skin tissue sections, primary keratinocytes and keratinocytes induced to undergo differentiation in culture. However, solid proof for an adhesin conferring tissue tropisms for superficial throat versus skin infection has not yet clearly emerged.
The pilus accessory protein Cpa, derived from an M-type 49 (M49) GAS strain (emm pattern E), selectively binds type I collagen [54], which is abundant in the dermal layer of skin and present in other tissues as well. Localization of Cpa to the pilus tip in an M-type 3/T-type 3 (M3T3) strain may allow it to extend beyond the polysaccharide capsule and become accessible for mediating adherence [55]. A critical role for pili in adherence to human palatine tonsil tissue is demonstrated for the emm pattern A–C strain SF370 (M1T1) [56], whose pili genes display extensive sequence divergence with most other GAS strains [34]. Pili-mediated adherence to tonsillar tissue is not blocked following pre-incubation of GAS with either fibronectin or collagen. The pili of the M1T1 strain also promote adherence to primary skin-derived keratinocytes, yet have no effect on GAS adherence to cell lines originating from the respiratory tract (HEp-2, A549); in another study using an M49 strain, a cpa mutant also failed to show differences in adherence to HEp-2 cells [57]. However, the M1 protein of the M1T1 strain enhances adherence to tonsils but decreases GAS binding to keratinocytes, suggestive of a complex set of competing interactions among adhesins [56]. M1 strains are usually found in association with pharyngitis, although in a surveillance study in Nepal, M1 isolates were recovered from nearly 20% of the impetigo cases [17]. Using the same M1T1 strain and a pharyngeal-derived cell line (Detroit-562), the role of pili in GAS adherence was confirmed by another group of investigators, and a role for GAS pili in biofilm formation was also demonstrated [58].
The GAS population displays numerous combinations of genes encoding the structurally distinct Fn-binding proteins PrtF1, PrtF2, SOF, SfbX, FbaA and FBP54 [59–63]. The Fn-binding proteins appear to exhibit differences in cell surface expression and binding properties. Using an in situ adherence assay with histological sections of human skin, PrtF1 directs GAS adherence to Langerhans cells, in contrast to GAS bound to keratinocytes via M6 protein, suggestive of cell-specific tropisms [64]. Other factors, such as integrin expression and the multimeric tissue form of Fn, can influence GAS adherence to mammalian cells [65,66]. The degree to which the individual Fn-binding proteins of GAS differ in their binding affinities for specific cell types remains to be fully explored.
In addition to adherence to human tissue, GAS must acquire nutrients in order to reproduce within the host and, at the same time, circumvent host defenses. Conceivably, any process that contributes to pathogenesis might be specific for either the throat or skin.
Animal models for GAS infection of the upper respiratory tract
A major challenge to studying GAS tissue tropisms and tissue-specific aspects of molecular pathogenesis at the epithelium lies in the shortcomings of experimental animal models to accurately represent diseases caused by this human-specific pathogen. Also, the extensive genetic diversity among strains often give rise to strain-dependent phenotypes in the various models.
The molecular pathogenesis of GAS pharyngitis is summarized in a recent review [67]. The GAS disease model in cynomolgus macaques probably provides the best demonstration of tonsillitis, following intranasal (IN) inoculation of live GAS [68,69]. Numerous studies have used nonhuman primate models for pharyngeal colonization and/or infection by GAS; however, high inoculating doses of bacteria are consistently employed, ranging from more than 3 × 105 to 108 CFU [68,70–73]. Nonetheless, transcription of several GAS genes that are upregulated in organisms recovered from pharyngitis patients are also expressed at elevated levels during infection in the macaque model [71].
Whether or not the high nonphysiological doses of GAS employed in nonhuman primate models for upper respiratory tract infection are essential to cause disease remains unclear. High doses might be necessary if innate host defenses need to be overcome and/or there is a lack of high-affinity binding sites for GAS adhesins in nonhuman species. Transgenic mice expressing human-derived genes are valuable tools for studying GAS infections [74–78], but none have yet to be applied to superficial infections at the throat or skin epithelium.
Delivery of GAS to mice or rats by an IN route has been widely used to study colonization at the mucosal epithelium [79–82], but evidence for progression to pharyngitis in the mouse has been difficult to obtain. However, GAS are observed in association with nasal-associated lymphoid tissue, a human tonsil homologue [83]; there is also evidence that nasal-associated lymphoid tissue may be an entry point for GAS into deeper tissue. In fact, mice receiving GAS via the IN route often succumb to a disseminated systemic infection. The doses of GAS used for IN inoculation of mice are typically quite high, often exceeding 106 or 107 CFU. Somewhat lower inoculum doses of GAS delivered IN to immunodeficient mice (BALB/c nu/+) lead to a high rate of mortality [84], consistent with the idea that naturally acquired cross-protective immunity may limit GAS infection in mice.
Animal models for superficial GAS infection of the skin
There are numerous models for GAS infection that involve the skin; however, when bacteria are delivered by injection, the epithelial barrier is bypassed and infection is no longer considered to be superficial. GAS causes not only impetigo – a superficial skin infection often limited to the epidermis – but can also cause wound infections that penetrate into deeper tissue and sometimes lead to abscess formation. Impetigo and wound infections represent two distinct pathological processes.
Several animal models for GAS infection leading to skin involvement deliver high doses of bacteria by injection under the skin. It has been reported that macroscopic, impetigo-like lesions are induced in hamsters via intradermal injection of GAS [85]. However, these injections are better described as subcutaneous; subdermal abscesses form that gradually penetrate through the epidermis and rupture on the skin surface, releasing a purulent exudate. Injection of GAS into the hind flanks of hairless mice is used to study skin ulcers and tissue necrosis that originates in deeper tissue and involves the epidermis after the infection has progressed to a later stage [86–88]. Coinjection of bacteria with a foreign substance (e.g., Cytodex™ beads) is often employed to further enhance the inflammatory response. In the mouse skin air sac model, GAS are injected subcutaneously; this model can be used to study bacterial dissemination from a focal infection site to distal organs [89]. In another mouse skin infection model, GAS are applied topically following the complete removal of the epidermis by tape stripping; unusually high nonphysiologic inoculum doses (107 CFU) of GAS are employed here as well [90].
Mouse skin is structurally distinct from human skin. Mouse epidermis is typically three cell layers thick, and the dermis is loaded with mast cells; except in hairless mice, it has a very high density of hair follicles. In sharp contrast, most anatomic areas of human skin epidermis have three morphologically distinct viable layers (basal, spinous and granulosum), each several cell layers thick. Since humans are the only natural host for GAS, a model that accurately reflects the key events during human infection is highly desirable.
A humanized mouse model for superficial skin infection (hu-skin SCID mouse) was developed in which SCID mice are engrafted with human neonatal foreskin [47,51,91–95]. SCID mice fail to reject xenotransplants because they lack functional B and T lymphocytes. For infection, freshly cultured GAS is topically applied to grafts slightly damaged by gentle scratching of the skin surface; inoculation is followed by occlusion of the area with a bandage. These treatments are part of an attempt to mimic the risk factors for impetigo in humans, which include both a nick in the skin and a warm, humid environment.
The humanized mouse model for GAS impetigo has high sensitivity, whereby inoculum doses as low as approximately 300 CFU of a virulent GAS strain can give rise to a purulent exudate at 7 days. This low inoculum dose probably approaches physiologic levels for naturally occurring infection in the human host. The hu-skin SCID mouse model also displays high specificity and can discriminate the GAS strain tropisms observed in human populations, in that emm pattern D skin specialist strains are significantly more virulent than emm pattern A–C throat specialist strains, based on blinded gross pathology scores. The primary outcome measure in the humanized mouse model is the net change in the number of CFU between inoculation and tissue biopsy. This measure is evolutionarily relevant because successful transmission to a new host depends on the ability to generate new progeny. Virulent strains of GAS undergo reproduction and net growth at the skin, resulting in an increase in CFU that can exceed 10,000-fold, a hallmark of evolutionary success.
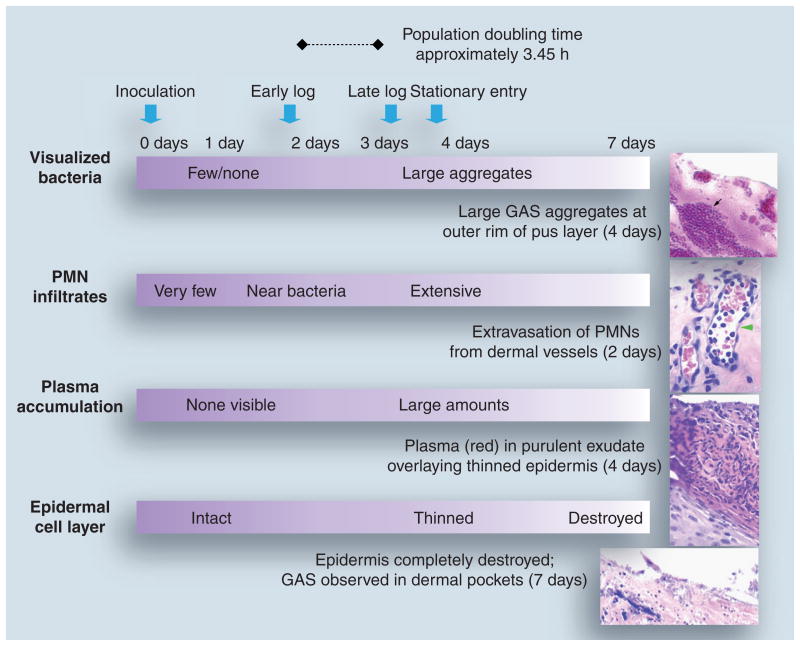
The humanized mouse model provides a striking similarity to the histopathology observed during natural GAS impetigo in humans [96–101]. Infection of the wounded graft with an emm pattern D skin specialist strain typically leads to loss of the stratum corneum over large sections of skin by 48 h postinoculation (Figure 3). At 48 h, polymorphonuclear leukocytes (PMNs) of mouse origin are observed in the dermal vessels, within the epidermis and on the external surface of the skin. Single cocci and short chains are adherent to the outer epidermal cell layer, with nearby PMNs often present. As the GAS infection progresses further, there are dramatic changes. By 4 days postinoculation, there can be a thick coat of PMNs over some sections of skin. As the PMNs move from the vasculature, a small amount of plasma extravasates and gradually accumulates. Together, the PMNs and plasma constitute the purulent exudate, or pus, which can be seen on gross examination. Under high magnification, one can observe massive aggregates of cocci concentrated at the outer rim of the pus layer. Presumably, M protein prevents phagocytosis of the organism, but steric effects due to the aggregates might play a role as well. Importantly, the bacteria are in an environment rich in at least some key nutrients that may facilitate reproductive growth. The GAS at the outer rim of the pus layer also seem well positioned for transmission to a new host. In addition, sometimes GAS can survive within PMNs, which may provide a vehicle for dissemination [102]. From the standpoint of evolutionary success, this is what matters the most to a microbial pathogen: reproduction and transmission to the next host.
Figure 3. Growth dynamics of group A Streptococcus during skin infection in the humanized mouse.
Pathological alterations throughout the time course of GAS infection of skin grafts in the humanized mouse are described. The time scale (top) designates the day postinoculation of skin with GAS. The growth stage of infecting GAS, determined based on the number of colony-forming units recovered from the skin at different time points, is also described. Panels on the right represent hematoxylin- and eosin-stained tissue sections obtained from GAS-infected skin biopsies.
Black arrow: GAS aggregates; green arrowhead: dermal capillary containing PMNs.
GAS: Group A Streptococcus; PMN: Polymorphonuclear leukocyte.
Tissue sections reproduced with permission © American Society of Microbiology.
The dynamics of reproductive growth of GAS in the humanized mouse has been studied using a constant inoculum dose of approximately 2000 CFU of the virulent emm pattern D strain Alab49 [Bessen DE et al., Unpublished Data]. A growth curve at the skin can be constructed using biopsy times postinoculation of 2, 3 and 6–7 days (Figure 3). The average number of bacterial population doublings at these time points is 2.2, 9.1 and 13.5, respectively. The saturation value for GAS on the human skin graft, irrespective of inoculum dose, is approximately 2 × 107 CFU, equivalent to approximately 13.3 population doublings assuming an inoculation dosage of approximately 2000 CFU. The occluding bandage keeps the skin lesion moist, preventing crust formation and healing.
Most exponential phase growth of GAS at the skin occurs between 2 and 3 days postinoculation. The average population doubling time between the 48 and 72 h time points is 3.45 h. In sharp contrast, the doubling time of GAS grown in enriched broth (e.g., Todd-Hewitt with yeast extract) is approximately 40 min during log phase. Using the estimated doubling time of 3.45 h during exponential growth at the skin, saturation of the GAS population is expected to be attained by approximately 86 h postinoculation, after which time the population enters stationary phase. During log phase at the skin, the bacterial growth rate may be slowed by a lack of certain key nutrients, or replication may be countered by a loss of new progeny via innate host defenses.
Role of skin-specific gene candidates in pathogenesis
A set of criteria can be used to provide evidence that a particular gene is a determinant of tissue-specific infection. The first requirement is a strong association between the tissue-specific gene and emm pattern genotype, which, in turn, correlates with the host tissue site of isolation as established by population-based surveys of GAS infection (Figure 1). Several genes that are strongly linked to the emm pattern markers for preferred tissue site of infection have been identified (Figure 2). The second requirement for a tissue-specific determinant of infection is that inactivation of the candidate gene leads to an alteration in virulence using a relevant animal model of disease.
The humanized mouse model for superficial GAS skin infection was used to measure the effect of mutations in genes displaying linkage disequilibrium with the emm pattern D genotype. The virulent emm pattern D strain Alab49 (M53) was the target of mutagenesis. Alab49 harbors mga-2 and nra lineage alleles, as well as the pam form of emm, the ska-2b lineage of the streptokinase gene and the FCT region genes cpa and prtF2. The sof gene that is present in all pattern E strains is missing from Alab49; also absent from Alab49 is the prtF1 gene found in most pattern A–C strains and in many pattern E strains. Differences in virulence at the skin having statistical significance for mutants versus the parental wild-type (wt) Alab49 strain are summarized in Table 1. To prepare the inoculum, GAS is grown to either log or stationary phase in enriched broth; the physiological state of GAS during human-to-human transmission is unknown, but presumably consists of a heterologous mix.
Table 1.
Alab49 mutants having a statistically significant decrease in virulence relative to wild-type, when tested in the humanized mouse model for superficial skin infection.
| Mutant | Function of gene product | Control of gene transcription | Cell association | Virulence at skin (log inoculum)† | Virulence at skin (stat inoculum)† |
|---|---|---|---|---|---|
| Δmga | Transcriptional regulator | Autoregulated (activated)‡ | Cytoplasm | Decreased | Decreased |
| Δnra | Transcriptional regulator | Autoregulated (activated)‡ | Cytoplasm | Decreased | Decreased |
| Δpam (Δemm53) | Plg-binding M protein | Activated by mga | Surface | N.D. | Decreased (partially) |
| Δska | Plg activator (streptokinase) | No effect by mga | Secreted | N.D. | Decreased (partially) |
| ΔspeB | Cysteine protease | Activated by mga (indirect) | Secreted | Decreased | Decreased |
| ΔfctA | Pilus backbone subunit | Activated by nra | Surface | No effect | No effect |
| Δcpa | Collagen-binding protein (pilus) | Activated by nra | Surface | Decreased | No effect |
| ΔprtF2 | Fn-binding protein | Activated by msmR | Surface | Decreased | No effect |
Virulence at the skin was measured for group A Streptococcus inoculum cultures grown to either log or stationary phase in enriched broth.
Assumed but not completely proven for strain Alab49; Mga and Nra function as autoregulators in other strains [37].
Fn: Fibronectin; N.D.: Not determined.
Data show that inactivation of genes encoding the transcriptional regulator Mga leads to a significant and near-complete loss in virulence of strain Alab49 in the humanized mouse (Table 1) [47]. Mga functions as a positive regulator of several Alab49 genes, including pam/emm53 (Plg-binding M53-type protein), speB (secreted cysteine proteinase), sclA (collagen-like surface protein), scpA (C5a peptidase) and fbaA (Fn-binding protein). Mga-regulated gene products are potential determinants of tissue tropisms.
Inactivation of either the pam/emm53 or ska genes in Alab49 leads to a statistically significant but partial attenuation of virulence in the humanized mouse (Table 1) [93]. In addition, both mutants display a complete loss in bacterial-bound plasmin activity. PAM is a specialized multifunctional M protein containing a high affinity binding site for Plg [103], and is restricted to emm pattern D strains [42]. PAM and streptokinase work synergistically; Plg binds to the bacterial cell surface via PAM and, in combination with streptokinase, the bound Plg is activated to form plasmin, a broad-spectrum protease. The plasmin may act to degrade fibrin, which would normally contribute to scab formation. Thus, plasmin may prevent the impetiginous skin lesion from drying out and healing by allowing inflammatory cells and plasma to accumulate over a longer time frame and, in turn, expand the window of opportunity for GAS transmission to new hosts. Like most other emm pattern D strains, Alab49 harbors a ska-2b lineage allele [33]. The product of Ska-2b differs from the other streptokinase forms in that fluid-phase plasmin activity is essentially absent [104; Bessen DE et al., Unpublished Data]
Plasminogen-binding M protein has a measurable function during skin infection in the humanized mouse, despite the finding that binding of mouse Plg is approximately 20-fold lower than that of human Plg [42,93]. Furthermore, activation of fluid-phase Plg by at least some forms of streptokinase is specific for the mammalian species from which the bacterium was isolated [105]. The modest partial attenuation observed for the Δpam and Δska mutants in the hu-skin SCID mouse may reflect a human species restriction that severely limits the amount of mouse-derived plasmin that can be formed. Plg is primarily synthesized by the liver; however, Plg mRNA is detectable in numerous extrahepatic tissues [106–108]. It remains to be established whether the human-derived cells of the skin graft synthesize Plg during GAS infection. In addition to its role in bacterial-bound plasmin activity, PAM/M53 has an antiphagocytic function that may prevent GAS uptake by infiltrating PMNs. However, as observed for other strains having multiple emm- and emm-like genes, selective loss of the type-specific emm gene does not necessarily result in complete killing via opsonophagocytosis [93,109,110].
Transcription of pam/emm53 is positively regulated by Mga. Transcript levels for pam/emm53 are decreased more than 100-fold in the Δmga mutant relative to Alab49 wt; by contrast, ska transcript levels are largely unaffected by Mga [47]. The magnitude of the loss of virulence observed for the Δmga mutant appears to be far greater than that experienced by the Δpam/emm53 mutant, which is only slightly attenuated [93]. Thus, additional Mga-regulated genes probably contribute to skin infection by Alab49.
Expression of the cysteine protease SpeB is regulated by Mga, most likely through an indirect circuit since speB transcript levels are decreased by only approximately threefold in the Alab49 Δmga mutant [47]. All GAS strains harbor speB, but there is wide variation in the level of SpeB activity. With the exception of numerous M1 strains [111], SpeB activity is generally quite low for the emm pattern A–C group of throat specialists [92]. By contrast, SpeB activity is high for most strains of the emm pattern D group of skin specialists. Inactivation of speB leads to a complete loss in Alab49 virulence in the humanized mouse model for impetigo (Table 1) [92,94]. The combination of a strong association between the SpeB phenotype and emm pattern genotype, and the absolute requirement of SpeB for skin infection, makes SpeB another compelling candidate for being a key determinant of tissue tropism. Addition of exogenous SpeB to an aviru-lent emm pattern A–C strain leads to a relative increase in the number of organisms recovered from skin grafts, although the magnitude of the enhancing effect of added SpeB on the throat specialist strain is less than that observed for a ΔspeB mutant of a pattern D strain. This latter finding is consistent with the idea that additional emm pattern D-specific factors are required for virulence [92].
Molecular interactions between the extracellular virulence factors reveal even greater complexity, as evidenced by the post-transcriptional control of Ska activity via SpeB. The digestive activity of SpeB peaks during the stationary phase of bacterial growth, and Ska is among its many degradation targets [93]. The broad-spectrum SpeB proteinase also interacts with the human host to release biologically active kinins from kininogen [112], degrade chemokines [113], cleave Fn [114] and activate IL-1β [115], in addition to several other activities.
Inactivation of nra results in a significant loss in virulence of strain Alab49 in the humanized mouse model (Table 1) [51]. In Alab49, nra encodes a positive regulator of pilus gene transcription and is localized to the FCT region of the genome (Figure 2). Several of the nra-regulated genes display strong linkage with emm pattern, most notably cpa and fctA, which are present in nearly all pattern D strains, but in only approximately half of pattern A–C strains [34]. Inoculation of the Δcpa mutant, following growth to mid-log phase in nutrient-rich broth, leads to a significant decrease in virulence relative to Alab49 wt; however, attenuation of the Δcpa mutant is not observed following its growth to stationary phase (Table 1) [94]. The lack of Cpa dependence on GAS virulence following stationary phase growth in broth might be explained by a redundant function conferred by an unidentified virulence factor that is highly expressed during stationary phase only. One can reasonably speculate that the growth-phase dependency of the GAS inoculum on virulence signifies a critical role of Cpa during the initial stages of infection.
Cpa is an accessory protein of the GAS pilus, and immunoblots show that pilus polymers are produced in the Δcpa mutant, albeit at a reduced level when compared with Alab49 wt [94]. By contrast, inactivation of fctA, encoding the major pilus backbone subunit, results in a (near) complete loss of pilus polymers. Yet, the ΔfctA mutant retains full virulence in the humanized mouse following growth to either log or stationary phase (Table 1) [94,95]. Conceivably, the lack of an FctA virulence effect may be due to the topical mode of delivery of the GAS inoculum, circumventing a pilus-dependent step normally encountered during person-to-person transmission. The prtF2 gene also resides within the FCT region but is not under Nra control. The ΔprtF2 mutant of Alab49 behaves similarly to the Δcpa mutant in the humanized mouse, showing attenuation following growth in culture broth to log phase, but no change in virulence following growth to stationary phase (Table 1). An explanation may lie in the finding that PrtF2, like Ska, is degraded by SpeB, whose activity peaks during stationary growth. Similar to fctA, the prtF2 gene is present in over 90% of emm pattern D strains but in only approximately half of pattern A–C strains [34].
Orthologous gene replacements & altered virulence
A third criterion set forth for establishing that a gene is a determinant of tissue-specific infection requires that substitution of an impetigo-associated gene for a pharyngitis-associated gene leads to altered virulence, and vice versa. Three of the emm pattern D-linked genes that are essential for maximum virulence of strain Alab49 in the humanized mouse model – mga, nra and ska-2b – are present in the GAS population as discrete lineages of alleles.
A rofA lineage allele derived from an emm pattern A–C throat strain, plus its upstream region containing cis-binding sites for autoregulation, was introduced into the Alab49 skin strain in place of the nra gene [95]. The rofA chimeric construct displays a significant increase in the levels of pilus gene transcription and pilus protein production. Thus, despite its extensive sequence divergence, RofA restores Nra function in regulating pilus gene transcription, a finding that underscores the evolutionary flexibility of transcriptional regulatory networks [116]. The rofA chimera also displays an increased growth rate in the humanized mouse model for impetigo, as evidenced by the number of bacterial population doublings on a time course that is approximately 24 h ahead of the Alab49 wt strain. Perhaps throat strains are more heavily piliated than skin strains, to help counter the flow of mucus over the oropharyngeal epithelium.
The hypervirulence observed for the rofA chimeric construct points to an increased fitness of the organism during infection of an individual host. Short-term fitness is readily captured in the humanized mouse model for impetigo. However, properties underlying short-term fitness do not necessarily coincide with long-term fitness attributes. At the level of the host population, transmission success is paramount. If an organism is hypervirulent, infection may run its course more quickly and, thereby, shorten the time period for infectiousness and transmission to new hosts. The relationship between hypervirulence and longer-term fitness is not always clear cut, and survival of the pathogen in the human host population may depend on properly balanced pathogenicity [117]. Precisely how the RofA-associated hypervirulence might relate to long-term fitness, and explain tissue site preferences for GAS infection, will require further exploration.
Hypervirulence in the humanized mouse model for infection is also evident for a chimeric construct involving the ska locus, wherein the ska-2b lineage allele of Alab49 is replaced with the divergent ska-1 allele [Bessen DE et al., Unpublished Data]. Bacterial-bound plasmin activity is equivalent for the chimera and wt Alab49 strain, indicating that, like Ska-2b, the Ska-1 form can activate bacterial-bound Plg. However, fluid-phase plasmin activity, which is lacking in the Ska-2b-containing parent strain, is a newly acquired phenotype of the ska-1 chimera, and may underlie the hypervirulence phenotype observed for the Alab49-based construct.
The existence of discrete lineages of alleles at the mga, rofA/nra and ska loci within the GAS population is highly suggestive of a genetic origin in another bacterial species. A possible donor species for both the rofA and ska-2a lineage alleles is the close genetic relative of GAS, Streptococcus dysgalactiae subspecies equisimilis, also known as group C and G streptococci (GCS/GGS) [32,33]. GCS/GGS typically act as commensals colonizing the oropharynx of the human host and, on occasion, they are recovered in association with disease. All GCS/GGS strains examined harbor both rofA and ska-2a genes. Neither the mga-1 nor mga-2 gene is detected in GCS/GGS, or in the numerous related streptococcal species screened.
The presence of rofA and ska-2a in 100% of GCS/GGS strains is consistent with the idea that interspecies gene transfer from a GCS/GGS donor to a GAS recipient resulted in recombinational replacement of the GAS gene with an orthologous form [32,33]. Experimental orthologous gene replacement of nra with rofA, and ska-2b with ska-1, preserves key functions associated with the original GAS phenotype, yet also introduces novel phenotypes. Thus, orthologous gene replacement may constitute an important step in bacterial evolution, by providing the raw material for new adaptations, such as exploitation of an ecological niche.
Conclusion
The history of horizontal movement of GAS core genes provides a background of random associations against which one can measure functionally driven linkage between adaptive genes and disease phenotypes. Tissue site preference for infection strongly correlates with emm pattern group. Accessory genes that display linkage disequilibrium with the emm pattern marker for throat specialist, skin specialist and generalist strains are strong candidates for conferring tissue-specific tropisms. Several of the tissue-specific candidate genes are required for virulence in a humanized mouse for skin infection. Exchange of impetigo-associated genes with pharyngitis-associated genes can lead to hypervirulence at the skin. The throat specialist-linked gene encoding the transcriptional regulator RofA was likely acquired by GAS from a commensal donor species. The finding of hypervirulence in a skin strain construct harboring RofA, combined with preservation of the transcriptional regulatory network, provides evidence that orthologous gene replacements can lead to new adaptations while maintaining critical functions.
Future perspective
The strong association of certain GAS strains with particular diseases points to an underlying genetic organization of GAS that might explain the molecular basis for disease-specific pathogenic mechanisms, such as throat and skin tropisms. A major challenge faced in trying to relate genotype to phenotype is the enormous range of genetic diversity among GAS strains. Complicating this further is the prospect that some phenotypes may arise via any one of multiple genetic pathways that converge to yield similar biological properties.
To reach the next level of understanding will likely require a high-throughput systems biology approach that simultaneously incorporates a diverse population of GAS strains, so that biologically relevant groups of strains can be compared and contrasted. The complete set of microbial factors that give rise to the tissue tropism phenotype, and their well-orchestrated interactions, need to be identified and fully characterized. Further improvements in experimental models for GAS disease are also essential. Transgenic animals harboring a human-derived gene are valuable in that the impact of singular molecular interactions can be assessed. Human-derived organs are more complex, but have the potential to capture many more of the key interactions between pathogen and host.
Executive summary.
Clinical importance of group A streptococcal diseases
Group A Streptococcus (GAS) is a human-specific pathogen having high disease prevalence worldwide.
The vast majority of GAS infections involve the epithelium of the throat or skin.
The throat and skin are the primary ecological reservoirs for GAS.
Genotypic markers of throat & skin strains
M protein provides the basis for a serotyping scheme; there are over 200 emm types.
The concept of distinct throat and skin strains arose from the strong association of certain M types with one disease or the other (pharyngitis versus impetigo).
emm types can be classified by emm pattern genotype, based on the chromosomal arrangement of emm and emm-like genes.
The phenotype of tissue site preference for infection correlates strongly with emm pattern genotype, which in turn is used to define groups of throat specialist, skin specialist and generalist strains.
Population genetics of throat & skin specialists
The GAS population genetic structure is characterized by extensive recombination.
There is no evidence for early stages of speciation among throat and skin specialist strains.
Genes linked to markers for preferred tissue site of infection
Against a background of random associations between core housekeeping genes, noncore accessory genes that display strong linkage to emm pattern are good candidates for being determinants of tissue tropisms.
Tissue tropism candidates include transcriptional regulators, and secreted and surface proteins, such as pili.
GAS adherence in cell & organ cultures
Many GAS adhesins are encoded by accessory genes having a differential distribution among strains.
Pili mediate GAS adherence to human tonsil tissue and primary keratinocytes.
Solid proof for an adhesin conferring cell-specific tropisms representing throat versus skin infection has not yet clearly emerged.
Animal models for GAS infection of the upper respiratory tract
Since GAS is a human-specific pathogen, animal models tend to fall short in providing a complete and accurate representation of GAS disease.
A nonhuman primate model provides an excellent demonstration of GAS tonsillitis.
Animal models for throat colonization and/or infection employ high nonphysiological doses of GAS for inoculation.
Animal models for superficial GAS infection of the skin
Many models deliver GAS by injection, and do not reflect superficial infection.
Human skin engrafted on SCID mice provides a model for superficial skin infection by GAS that has high specificity and high sensitivity, and shows pathological changes similar to those observed in patients with impetigo.
Role of skin-specific gene candidates in pathogenesis
Inactivation of the major gene candidates for skin-specific infection results in a decrease in virulence in the humanized mouse model for impetigo.
Orthologous gene replacements & altered virulence
Substitution of impetigo-associated genes with pharyngitis-associated genes leads to hypervirulence in the humanized mouse model for impetigo.
Several candidate genes for tissue tropism were acquired by GAS via lateral transfer from a donor commensal species.
Acquisition of a divergent orthologous gene from a related species results in the preservation of many key functions.
Orthologous gene replacement may provide the raw material for important new adaptations, such as exploitation of a new ecological niche.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Work was generously supported by funding from the NIH (GM-60793, AI-053826, AI-065572) and the American Heart Association. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Debra E Bessen, Email: debra_bessen@nymc.edu, Department of Microbiology & Immunology, New York Medical College, Valhalla, NY 10573, USA, Tel.: +1 914 594 4193, Fax: +1 914 594 4176.
Sergio Lizano, Email: sergio-lizano@idexx.com, IDEXX Laboratories, Westbrook, ME 04092, USA.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Bisno AL, Stevens DL. Streptococcus pyogenes. In: Mandell GL, Douglas RG, Dolin R, editors. Principles and Practice of Infectious Diseases. Churchill Livingstone; Philadelphia, PA, USA: 2005. [Google Scholar]
- 3.Martin JM, Green M, Barbadora KA, Wald ER. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics. 2004;114:1212–1219. doi: 10.1542/peds.2004-0133. [DOI] [PubMed] [Google Scholar]
- 4.Tanz RR, Shulman ST. Chronic pharyngeal carriage of group A streptococci. Pediatr Infect Dis J. 2007;26:175–176. doi: 10.1097/01.inf.0000255328.19808.be. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham MW. Pathogenesis of group A streptococcal infections [Review] Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wannamaker LW. Differences between streptococcal infections of the throat and of the skin. N Engl J Med. 1970;282:23–31. doi: 10.1056/NEJM197001012820106. [DOI] [PubMed] [Google Scholar]
- 8.Haanes EJ, Cleary PP. Identification of a divergent M protein gene and an M protein related gene family in serotype 49 Streptococcus pyogenes. J Bacteriol. 1989;171:6397–6408. doi: 10.1128/jb.171.12.6397-6408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podbielski A. Three different types of organization of the vir regulon in group A streptococci. Mol Gen Genet. 1993;237:287–300. doi: 10.1007/BF00282810. [DOI] [PubMed] [Google Scholar]
- 10.Hollingshead SK, Readdy T, Arnold J, Bessen DE. Molecular evolution of a multi-gene family in group A streptococci. Mol Biol Evol. 1994;11:208–219. doi: 10.1093/oxfordjournals.molbev.a040103. [DOI] [PubMed] [Google Scholar]
- 11.Hollingshead SK, Readdy TL, Yung DL, Bessen DE. Structural heterogeneity of the emm gene cluster in group A streptococci. Mol Microbiol. 1993;8:707–717. doi: 10.1111/j.1365-2958.1993.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 12.McGregor KF, Spratt BG, Kalia A, et al. Multi-locus sequence typing of Streptococcus pyogenes representing most known emm-types and distinctions among sub-population genetic structures. J Bacteriol. 2004;186:4285–4294. doi: 10.1128/JB.186.13.4285-4294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessen DE, McGregor KF, Whatmore AM. Relationships between emm and multilocus sequence types within a global collection of Streptococcus pyogenes. BMC Microbiol. 2008;8:59. doi: 10.1186/1471-2180-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Bessen DE. Population biology of the human restricted pathogen, Streptococcus pyogenes. Infect Genet Evol. 2009;9:581–593. doi: 10.1016/j.meegid.2009.03.00. Recent review of the population genetics of group A streptococci (GAS) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bessen DE, Carapetis JR, Beall B, et al. Contrasting molecular epidemiology of group A streptococci causing tropical and non-tropical infections of the skin and throat. J Infect Dis. 2000;182:1109–1116. doi: 10.1086/315842. [DOI] [PubMed] [Google Scholar]
- 16.Dicuonzo G, Gherardi G, Lorino G, et al. Group A streptococcal genotypes from pediatric throat isolates in Rome, Italy. J Clin Microbiol. 2001;39:1687–1690. doi: 10.1128/JCM.39.5.1687-1690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakota V, Fry AM, Lietman TM, Facklam RR, Li ZY, Beall B. Genetically diverse group A streptococci from children in far-western Nepal share high genetic relatedness with isolates from other countries. J Clin Microbiol. 2006;44:2160–2166. doi: 10.1128/JCM.02456-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulman S. Group A streptococcal pharyngitis serotype surveillance in North America, 2000–2002. Clin Infect Dis. 2004;39:325–332. doi: 10.1086/421949. [DOI] [PubMed] [Google Scholar]
- 19.Smeesters PR, Vergison A, Campos D, de Aguiar E, Deyi VY, Van Melderen L. Differences between Belgian and Brazilian group A Streptococcus epidemiologic landscape. PLoS ONE. 2006;1:e10. doi: 10.1371/journal.pone.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tewodros W, Kronvall G. M protein gene (emm type) analysis of group A β-hemolytic streptococci from Ethiopia reveals unique patterns. J Clin Microbiol. 2005;43:4369–4376. doi: 10.1128/JCM.43.9.4369-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberti S, Garcia-Rey C, Dominguez MA, et al. Survey of emm gene sequences from pharyngeal Streptococcus pyogenes isolates collected in Spain and their relationship with erythromycin susceptibility. J Clin Microbiol. 2003;41:2385–2390. doi: 10.1128/JCM.41.6.2385-2390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espinosa LE, Li ZY, Barreto DG, et al. M protein gene type distribution among group A streptococcal clinical isolates recovered in Mexico City, Mexico, from 1991 to 2000, and Durango, Mexico, from 1998 to 1999: overlap with type distribution within the United States. J Clin Microbiol. 2003;41:373–378. doi: 10.1128/JCM.41.1.373-378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandt CM, Spellerberg B, Honscha M, Truong ND, Hoevener B, Lutticken R. Typing of Streptococcus pyogenes strains isolated from throat infections in the region of Aachen, Germany. Infection. 2001;29:163–165. doi: 10.1007/s15010-001-1106-x. [DOI] [PubMed] [Google Scholar]
- 24.McDonald MI, Towers RJ, Fagan P, Carapetis JR, Currie BJ. Molecular typing of Streptococcus pyogenes from remote Aboriginal communities where rheumatic fever is common and pyoderma is the predominant streptococcal infection. Epidemiol Infect. 2007;135:1398–1405. doi: 10.1017/S0950268807008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steer AC, Magor G, Jenney AW, et al. emm and C-repeat region molecular typing of β-hemolytic streptococci in a tropical country: implications for vaccine development. J Clin Microbiol. 2009;47:2502–2509. doi: 10.1128/JCM.00312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carapetis J, Currie B, Kaplan E. Epidemiology and prevention of group A streptococcal infections: acute respiratory tract infections, skin infections, and their sequelae at the close of the twentieth century. Clin Infect Dis. 1999;28:205–210. doi: 10.1086/515114. [DOI] [PubMed] [Google Scholar]
- 27.Kalia A, Spratt BG, Enright MC, Bessen DE. Influence of recombination and niche separation on the population genetic structure of the pathogenStreptococcus pyogenes. Infect Immun. 2002;70:1971–1983. doi: 10.1128/IAI.70.4.1971-1983.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feil EJ, Holmes EC, Bessen DE, et al. Recombination within natural populations of pathogenic bacteria: short-term empirical estimates and long-term phylogenetic consequences. Proc Natl Acad Sci USA. 2001;98:182–187. doi: 10.1073/pnas.98.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vos M, Didelot X. A comparison of homologous recombination rates in bacteria and archaea. ISME J. 2009;3:199–208. doi: 10.1038/ismej.2008.93. [DOI] [PubMed] [Google Scholar]
- 30.Turner KM, Hanage WP, Fraser C, Connor TR, Spratt BG. Assessing the reliability of eBURST using simulated populations with known ancestry. BMC Microbiol. 2007;7:30. doi: 10.1186/1471-2180-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanage WP, Fraser C, Spratt BG. The impact of homologous recombination on the generation of diversity in bacteria. J Theor Biol. 2006;239:210–219. doi: 10.1016/j.jtbi.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Bessen DE, Manoharan A, Luo F, Wertz JE, Robinson DA. Evolution of transcription regulatory genes is linked to niche specialization in the bacterial pathogenStreptococcus pyogenes. J Bacteriol. 2005;187:4163–4172. doi: 10.1128/JB.187.12.4163-4172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalia A, Bessen DE. Natural selection and evolution of streptococcal virulence genes involved in tissue-specific adaptations. J Bacteriol. 2004;186:110–121. doi: 10.1128/JB.186.1.110-121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kratovac Z, Manoharan A, Luo F, Lizano S, Bessen DE. Population genetics and linkage analysis of loci within the FCT region of Streptococcus pyogenes. J Bacteriol. 2007;189:1299–1310. doi: 10.1128/JB.01301-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bessen DE, Kalia A. Genomic localization of a T-serotype locus to a recombinatorial zone encoding extracellular matrix-binding proteins in Streptococcus pyogenes. Infect Immun. 2002;70:1159–1167. doi: 10.1128/IAI.70.3.1159-1167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Mora M, Bensi G, Capo S, et al. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc Natl Acad Sci USA. 2005;102:15641–15646. doi: 10.1073/pnas.0507808102. First report describing the structure and function of pili in GAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪.Kreikemeyer B, McIver KS, Podbielski A. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen–host interactions. Trends Microbiol. 2003;11:224–232. doi: 10.1016/s0966-842x(03)00098-2. Review of global transcriptional regulators of GAS, including Mga and RofA-like proteins. [DOI] [PubMed] [Google Scholar]
- 38.Hondorp ER, McIver KS. The Mga virulence regulon: infection where the grass is greener. Mol Microbiol. 2007;66:1056–1065. doi: 10.1111/j.1365-2958.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- 39.Rosales C, Gillard BK, Courtney HS, Blanco-Vaca F, Pownall HJ. Apolipoprotein modulation of streptococcal serum opacity factor activity against human plasma high-density lipoproteins. Biochemistry. 2009;48:8070–8076. doi: 10.1021/bi901087z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Courtney HS, Hasty DL, Dale JB. Anti-phagocytic mechanisms of Streptococcus pyogenes: binding of fibrinogen to M-related protein. Mol Microbiol. 2006;59:936–947. doi: 10.1111/j.1365-2958.2005.04977.x. [DOI] [PubMed] [Google Scholar]
- 41.Courtney HS, Hasty DL, Li Y, Chiang HC, Thacker JL, Dale JB. Serum opacity factor is a major fibronectin-binding protein and a virulence determinant of M type 2 Streptococcus pyogenes. Mol Microbiol. 1999;32:89–98. doi: 10.1046/j.1365-2958.1999.01328.x. [DOI] [PubMed] [Google Scholar]
- 42.Svensson MD, Sjöbring U, Bessen DE. Selective distribution of a high-affinity plasminogen binding site among group A streptococci associated with impetigo. Infect Immun. 1999;67:3915–3920. doi: 10.1128/iai.67.8.3915-3920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whatmore AM, Kehoe MA. Horizontal gene transfer in the evolution of group A streptococcal emm-like genes: gene mosaics and variation in Vir regulons. Mol Microbiol. 1994;11:363–374. doi: 10.1111/j.1365-2958.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 44.Sriprakash KS, Hartas J. Lateral genetic transfers between group A and G streptococci for M-like genes are ongoing. Microb Pathog. 1996;20:275–285. doi: 10.1006/mpat.1996.0026. [DOI] [PubMed] [Google Scholar]
- 45.Beall B, Gherardi G, Lovgren M, Forwick B, Facklam R, Tyrrell G. emm and sof gene sequence variation in relation to serological typing of opacity factor positive group A streptococci. Microbiology. 2000;146:1195–1209. doi: 10.1099/00221287-146-5-1195. [DOI] [PubMed] [Google Scholar]
- 46.Caparon MG, Scott JR. Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc Natl Acad Sci USA. 1987;84:8677–8681. doi: 10.1073/pnas.84.23.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo F, Lizano S, Banik S, Zhang H, Bessen DE. Role of Mga in group A streptococcal infection at the skin epithelium. Microb Pathog. 2008;45:217–224. doi: 10.1016/j.micpath.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribardo DA, McIver KS. Defining the mga regulon: comparative transcriptome analysis reveals both direct and indirect regulation by Mga in the group A Streptococcus. Mol Microbiol. 2006;62:491–508. doi: 10.1111/j.1365-2958.2006.05381.x. [DOI] [PubMed] [Google Scholar]
- 49.Podbielski A, Woischnik M, Leonard BAB, Schmidt KH. Characterization of nra, a global negative regulator gene in group A streptococci. Mol Microbiol. 1999;31:1051–1064. doi: 10.1046/j.1365-2958.1999.01241.x. [DOI] [PubMed] [Google Scholar]
- 50.Kreikemeyer B, Beckert S, Braun-Kiewnick A, Podbielski A. Group A streptococcal RofA-type global regulators exhibit a strain-specific genomic presence and regulation pattern. Microbiology. 2002;148:1501–1511. doi: 10.1099/00221287-148-5-1501. [DOI] [PubMed] [Google Scholar]
- 51.Luo F, Lizano S, Bessen DE. Heterogeneity in the polarity of Nra regulatory effects on streptococcal pilus gene transcription and virulence. Infect Immun. 2008;76:2490–2497. doi: 10.1128/IAI.01567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakata M, Koller T, Moritz K, et al. Mode of expression and functional characterization of FCT-3 pilus region encoded proteins in the Streptococcus pyogenes serotype M49. Infect Immun. 2009;77(1):32–44. doi: 10.1128/IAI.00772-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson DR, Kaplan EL, VanGheem A, Facklam RR, Beall B. Characterization of group A streptococci (Streptococcus pyogenes): correlation of M-protein and emm-gene type with T-protein agglutination pattern and serum opacity factor. J Med Microbiol. 2006;55:157–164. doi: 10.1099/jmm.0.46224-0. [DOI] [PubMed] [Google Scholar]
- 54.Kreikemeyer B, Nakata M, Oehmcke S, Gschwendtner C, Normann J, Podbielski A. Streptococcus pyogenes collagen type I-binding Cpa surface protein – expression profile, binding characteristics, biological functions, potential clinical impact. J Biol Chem. 2005;280:33228–33239. doi: 10.1074/jbc.M502896200. [DOI] [PubMed] [Google Scholar]
- 55.Quigley BR, Zahner D, Hatkoff M, Thanassi DG, Scott JR. Linkage of T3 and Cpa pilins in the Streptococcus pyogenes M3 pilus. Mol Microbiol. 2009;72:1379–1394. doi: 10.1111/j.1365-2958.2009.06727.x. [DOI] [PubMed] [Google Scholar]
- 56▪.Abbot EL, Smith WD, Siou GP, et al. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell Microbiol. 2007;9:1822–1833. doi: 10.1111/j.1462-5822.2007.00918.x. Role of pili in adherence to human-derived tissue and cells of skin and respiratory tract origin. [DOI] [PubMed] [Google Scholar]
- 57.Kreikemeyer B, Talay S, Chhatwal G. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol Microbiol. 1995;17:137–145. doi: 10.1111/j.1365-2958.1995.mmi_17010137.x. [DOI] [PubMed] [Google Scholar]
- 58.Manetti AG, Zingaretti C, Falugi F, et al. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Mol Microbiol. 2007;64:968–983. doi: 10.1111/j.1365-2958.2007.05704.x. [DOI] [PubMed] [Google Scholar]
- 59.Kreikemeyer B, Oehmcke S, Nakata M, Hoffrogge R, Podbielski A. Streptococcus pyogenes fibronectin-binding protein F2 – expression profile, binding characteristics, impact on eukaryotic cell interactions. J Biol Chem. 2004;279:15850–15859. doi: 10.1074/jbc.M313613200. [DOI] [PubMed] [Google Scholar]
- 60.Gillen CM, Courtney HS, Schulze K, et al. Opacity factor activity and epithelial cell binding by the serum opacity factor protein of Streptococcus pyogenes are functionally discrete. J Biol Chem. 2008;283:6359–6366. doi: 10.1074/jbc.M706739200. [DOI] [PubMed] [Google Scholar]
- 61.Courtney HS, Hasty DL, Dale JB. Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann Med. 2002;34:77–87. doi: 10.1080/07853890252953464. [DOI] [PubMed] [Google Scholar]
- 62.Timmer AM, Kristian SA, Datta V, et al. Serum opacity factor promotes group A streptococcal epithelial cell invasion and virulence. Mol Microbiol. 2006;62:15–25. doi: 10.1111/j.1365-2958.2006.05337.x. [DOI] [PubMed] [Google Scholar]
- 63.Terao Y, Kawabata S, Kunitomo E, Murakami J, Nakagawa I, Hamada S. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol Microbiol. 2001;42:75–86. doi: 10.1046/j.1365-2958.2001.02579.x. [DOI] [PubMed] [Google Scholar]
- 64.Okada N, Pentland AP, Falk P, Caparon MG. M protein and protein F act as important determinants of cell-specific tropism of Streptococcus pyogenes in skin tissue. J Clin Invest. 1994;94:965–977. doi: 10.1172/JCI117463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okada N, Watarai M, Ozeri V, Hanski E, Caparon M, Sasakawa C. A matrix form of fibronectin mediates enhanced binding of Streptococcus pyogenes to host tissue. J Biol Chem. 1997;272:26978–26984. doi: 10.1074/jbc.272.43.26978. [DOI] [PubMed] [Google Scholar]
- 66.Courtney HS, Ofek I, Penfound T, et al. Relationship between expression of the family of M proteins and lipoteichoic acid to hydrophobicity and biofilm formation in Streptococcus pyogenes. PLoS ONE. 2009;4:e4166. doi: 10.1371/journal.pone.0004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olsen RJ, Shelburne SA, Musser JM. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell Microbiol. 2009;11:1–12. doi: 10.1111/j.1462-5822.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 68.Sumby P, Tart AH, Musser JM. A non-human primate model of acute group a Streptococcus pharyngitis. Methods Mol Biol. 2008;431:255–267. doi: 10.1007/978-1-60327-032-8_20. [DOI] [PubMed] [Google Scholar]
- 69▪.Virtaneva K, Porcella SF, Graham MR, et al. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc Natl Acad Sci USA. 2005;102:9014–9019. doi: 10.1073/pnas.0503671102. Characterization of temporal changes in the GAS transcriptome in an experimental model for pharyngitis in macaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson RF, Rothbard S, Swift HF. Type-specific protection and immunity following intranasal inoculation of monkeys with group A hemolytic streptococci. J Exp Med. 1946;84:127–142. [PubMed] [Google Scholar]
- 71.Virtaneva K, Graham MR, Porcella SF, et al. Group A streptococcus gene expression in humans and cynomolgus macaques with acute pharyngitis. Infect Immun. 2003;71:2199–2207. doi: 10.1128/IAI.71.4.2199-2207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gryllos I, Cywes C, Shearer MH, Cary M, Kennedy RC, Wessels MR. Regulation of capsule gene expression by group A Streptococcus during pharyngeal colonization and invasive infection. Mol Microbiol. 2001;42:61–74. doi: 10.1046/j.1365-2958.2001.02635.x. [DOI] [PubMed] [Google Scholar]
- 73.Ashbaugh CD, Moser TJ, Shearer MH, White GL, Kennedy RC, Wessels MR. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal pharyngeal infection. Cell Microbiol. 2000;2:283–292. doi: 10.1046/j.1462-5822.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 74.Sriskandan S, Unnikrishnan M, Krausz T, et al. Enhanced susceptibility to superantigen-associated streptococcal sepsis in human leukocyte antigen-DQ transgenic mice. J Infect Dis. 2001;184:166–173. doi: 10.1086/322018. [DOI] [PubMed] [Google Scholar]
- 75.Sun HM, Ringdahl U, Homeister JW, et al. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305:1283–1286. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- 76.Nooh MM, El-Gengehi N, Kansal R, David CS, Kotb M. HLA transgenic mice provide evidence for a direct and dominant role of HLA class II variation in modulating the severity of streptococcal sepsis. J Immunol. 2007;178:3076–3083. doi: 10.4049/jimmunol.178.5.3076. [DOI] [PubMed] [Google Scholar]
- 77.Matsui H, Sekiya Y, Nakamura M, et al. CD46 transgenic mouse model of necrotizing fasciitis caused by Streptococcus pyogenes infection. Infect Immun. 2009;77:4806–4814. doi: 10.1128/IAI.00577-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanderson-Smith ML, Dinkla K, Cole JN, et al. M protein-mediated plasminogen binding is essential for the virulence of an invasive Streptococcus pyogenes isolate. FASEB J. 2008;22:2715–2722. doi: 10.1096/fj.07-105643. [DOI] [PubMed] [Google Scholar]
- 79.Bessen D, Fischetti VA. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988;56:2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wessels MR, Bronze MS. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc Natl Acad Sci USA. 1994;91:12238–12242. doi: 10.1073/pnas.91.25.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hollingshead SK, Simecka JW, Michalek SM. Role of M protein in pharyngeal colonization by group A streptococci in rats. Infect Immun. 1993;61:2277–2283. doi: 10.1128/iai.61.6.2277-2283.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dale JB, Baird RW, Courtney HS, Hasty DL, Bronze MS. Passive protection of mice against group A streptococcal pharyngeal infection by lipoteichoic acid. J Infect Dis. 1994;169:319–323. doi: 10.1093/infdis/169.2.319. [DOI] [PubMed] [Google Scholar]
- 83.Park HS, Francis KP, Yu J, Cleary PP. Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group AStreptococcus. J Immunol. 2003;171:2532–2537. doi: 10.4049/jimmunol.171.5.2532. [DOI] [PubMed] [Google Scholar]
- 84.Bessen D, Fischetti VA. Passive acquired mucosal immunity to group A streptococci by secretory immunoglobulin A. J Exp Med. 1988;167:1945–1950. doi: 10.1084/jem.167.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dajani AS, Wannamaker LW. Experimental infection of the skin in the hamster simulating human impetigo I Natural history of the infection. J Infect Dis. 1970;122:196–204. doi: 10.1093/infdis/122.3.196. [DOI] [PubMed] [Google Scholar]
- 86.Bunce C, Wheeler L, Reed G, Musser J, Barg N. Murine model of cutaneous infection with Gram-positive cocci. Infect Immun. 1992;60:2636–2640. doi: 10.1128/iai.60.7.2636-2640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Datta V, Myskowski SM, Kwinn LA, et al. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol Microbiol. 2005;56:681–695. doi: 10.1111/j.1365-2958.2005.04583.x. [DOI] [PubMed] [Google Scholar]
- 88.Engleberg NC, Heath A, Vardaman K, DiRita VJ. Contribution of CsrR-regulated virulence factors to the progress and outcome of murine skin infections by Streptococcus pyogenes. Infect Immun. 2004;72:623–628. doi: 10.1128/IAI.72.2.623-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith TC, Sledjeski DD, Boyle MD. Streptococcus pyogenes infection in mouse skin leads to a time-dependent up-regulation of protein H expression. Infect Immun. 2003;71:6079–6082. doi: 10.1128/IAI.71.10.6079-6082.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kugelberg E, Norstrom T, Petersen T, Duvold T, Andersson D, Hughes D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob Agents Chemother. 2005;49:3435–3441. doi: 10.1128/AAC.49.8.3435-3441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scaramuzzino DA, McNiff JM, Bessen DE. Humanized in vivo model for streptococcal impetigo. Infect Immun. 2000;68:2880–2887. doi: 10.1128/iai.68.5.2880-2887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92▪.Svensson MD, Scaramuzzino DA, Sjobring U, Olsen A, Frank C, Bessen DE. Role for a secreted cysteine proteinase in the establishment of host tissue tropism by group A streptococci. Mol Microbiol. 2000;38:242–253. doi: 10.1046/j.1365-2958.2000.02144.x. Association of the SpeB cysteine proteinase with skin strains and its requirement for virulence at the skin. [DOI] [PubMed] [Google Scholar]
- 93.Svensson MD, Sjobring U, Luo F, Bessen DE. Roles of the plasminogen activator streptokinase and plasminogen-associated M protein in an experimental model for streptococcal impetigo. Microbiology. 2002;148:3933–3945. doi: 10.1099/00221287-148-12-3933. [DOI] [PubMed] [Google Scholar]
- 94▪.Lizano S, Luo F, Bessen DE. Role of streptococcal T-antigens in superficial skin infection. J Bacteriol. 2007;189:1426–1434. doi: 10.1128/JB.01179-06. Role of pili and other surface proteins encoded by the FCT region in skin infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95▪.Lizano S, Luo F, Tengra FK, Bessen DE. Impact of orthologous gene replacement on the circuitry governing pilus gene transcription in streptococci. PLoS ONE. 2008;3:e3450. doi: 10.1371/journal.pone.0003450. Replacement of the transcriptional regulator Nra with the RofA ortholog results in hypervirulence, but the transcriptional network is preserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hay R, Adriaans B. Bacterial infections. In: Champion R, Burton J, Burns D, Breathnach S, editors. Textbook of Dermatology. Blackwell Science; Oxford, UK: 1998. p. 1097. [Google Scholar]
- 97.Lever W, Schaumberg-Lever G. Histopathology of the Skin. J.B. Lippincott Co; Philadelphia, USA: 1990. [Google Scholar]
- 98.Leyden J, Gately L. Staphylococcal and streptococcal infections. In: Sanders C, Nesbitt L, editors. The Skin and Infection. Williams and Wilkins; Baltimore, USA: 1995. pp. 27–31. [Google Scholar]
- 99.Montgomery H. Dermatopathology. Harper & Row; New York, USA: 1967. [Google Scholar]
- 100.Murphy G. Dermatopathology: a practical guide to common disorders. Saunders WB: 1995. p. 505. [Google Scholar]
- 101.Rapini R. Bacterial infections. In: Barnhill, editor. Textbook of Dermatopathology. 1998. pp. 379–397. [Google Scholar]
- 102.Medina E, Goldmann O, Toppel AW, Chhatwal GS. Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J Infect Dis. 2003;187:597–603. doi: 10.1086/373998. [DOI] [PubMed] [Google Scholar]
- 103.Berge A, Sjöbring U. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J Biol Chem. 1993;268:25417–25424. [PubMed] [Google Scholar]
- 104.McArthur JD, McKay FC, Ramachandran V, et al. Allelic variants of streptokinase from Streptococcus pyogenes display functional differences in plasminogen activation. FASEB J. 2008;22:3146–3153. doi: 10.1096/fj.08-109348. [DOI] [PubMed] [Google Scholar]
- 105.Schroeder B, Boyle MD, Sheerin BR, Asbury AC, Lottenberg R. Species specificity of plasminogen activation and acquisition of surface-associated proteolytic activity by group C streptococci grown in plasma. Infect Immun. 1999;67:6487–6495. doi: 10.1128/iai.67.12.6487-6495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Twining SS, Wilson PM, Ngamkitidechakul C. Extrahepatic synthesis of plasminogen in the human cornea is up-regulated by interleukins-1α and -1β. Biochem J. 1999;339(Pt 3):705–712. [PMC free article] [PubMed] [Google Scholar]
- 107.Ramharack R, Spahr MA, Kreick JS, Sekerke CS. Expression of apolipoprotein[a] and plasminogen mRNAs in cynomolgus monkey liver and extrahepatic tissues. J Lipid Res. 1996;37:2029–2040. [PubMed] [Google Scholar]
- 108.Zhang L, Seiffert D, Fowler BJ, et al. Plasminogen has a broad extrahepatic distributions. Thromb Haemost. 2002;87:493–501. [PubMed] [Google Scholar]
- 109.Kotarsky H, Thern A, Lindahl G, Sjobring U. Strain-specific restriction of the antiphagocytic property of group A streptococcal M proteins. Infect Immun. 2000;68:107–112. doi: 10.1128/iai.68.1.107-112.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McLellan DG, Chiang EY, Courtney HS, et al. Spa contributes to the virulence of type 18 group A streptococci. Infect Immun. 2001;69:2943–2949. doi: 10.1128/IAI.69.5.2943-2949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kansal RG, McGeer A, Low DE, Norrby-Teglund A, Kotb M. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect Immun. 2000;68:6362–6369. doi: 10.1128/iai.68.11.6362-6369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herwald H, Collin M, Muller-Esterl W, Bjorck L. Streptococcal cysteine proteinase releases kinins: a virulence mechanism. J Exp Med. 1996;184:665–673. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Egesten A, Olin AI, Linge HM, et al. SpeB of Streptococcus pyogenes differentially modulates antibacterial and receptor activating properties of human chemokines. PLoS ONE. 2009;4:e4769. doi: 10.1371/journal.pone.0004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kapur V, Topouzis S, Majesky MW, et al. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 115.Kapur V, Majesky MW, Li LL, Black RA, Musser JM. Cleavage of interleukin 1 β (IL-1 β) precursor to produce active IL-1 β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Perez JC, Groisman EA. Evolution of transcriptional regulatory circuits in bacteria. Cell. 2009;138:233–244. doi: 10.1016/j.cell.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Foreman-Wykert AK, Miller JF. Hypervirulence and pathogen fitness. Trends Microbiol. 2003;11:105–108. doi: 10.1016/s0966-842x(03)00007-6. [DOI] [PubMed] [Google Scholar]
Website
- 201.CDC Streptococcus laboratory. www.cdc.gov/ncidod/biotech/strep/strepindex.htm.