Abstract
Alcohol abuse is associated with immunosuppressive and infectious sequelae. Particularly, alcoholics are more susceptible to pulmonary infections. In this report, gene transcriptional profiles of primary human airway epithelial cells, exposed to varying doses of alcohol (0, 50 and 100 mM), were obtained. Comparison of gene transcription levels between 0 mM and 50 mM alcohol treatments resulted in 2 genes being up-regulated and 16 genes down-regulated by at least two-fold. Moreover, 0 mM and 100 mM alcohol exposure led to the up-regulation of 14 genes and down-regulation of 157 genes. Among the up-regulated genes, glucocorticoid-induced leucine zipper (GILZ) responded to alcohol in a dose-dependent manner. Moreover, GILZ protein levels also correlated with this transcriptional pattern. Lentiviral expression of GILZ siRNA in human airway epithelial cells diminished the alcohol-induced upregulation, confirming that GILZ is indeed an alcohol-responsive gene. Gene-silencing of GILZ in A549 cells resulted in secretion of significantly higher amounts of inflammatory cytokines in response to IL-1β stimulation. The GILZ-silenced cells were more resistant to alcohol-mediated suppression of cytokine secretion. Further data demonstrated that the glucocorticoid receptor is involved in the regulation of GILZ by alcohol. Because GILZ is a key glucocorticoid-responsive factor mediating the anti-inflammatory and immunosuppressive actions of steroids, we propose that similar signaling pathways may play a role in the anti-inflammatory and immunosuppressive effects of alcohol.
Keywords: Alcohol, Airway Epithelia, Lung, Microarray, Transcriptome, GILZ, Glucocorticoids, Glucocorticoid Receptor
Introduction
According to the National Epidemiologic Survey on Alcohol and Related Conditions, greater than one third of the population in the United States aged 18 and older consumes alcohol. Ethanol, the active component in all alcoholic beverages, produces a wide variety of behavioral and physiological effects in humans (1, 2). Alcohol has long been known as an inflammation-inhibitory and immunosuppressive agent. Abuse of this substance is clearly linked to organ and tissue damage (3), host susceptibility to infectious diseases (4, 5) and specific defects in innate and adaptive immunity (6). However, the molecular mechanisms for these effects of alcohol have not been completely defined.
Glucocorticoid hormones are potent modulators of immune responses and inflammatory processes. Glucocorticoids (GCs) are the most commonly used anti-inflammatory and immunosuppressive drugs in the treatment of rheumatic and other inflammatory diseases. Cellular effects of GCs are mediated through the glucocorticoid receptor (GR) which regulates the expression of GC-responsive genes (7, 8). One of the prominent GC-responsive genes is the glucocorticoid-induced leucine zipper (GILZ) (9, 10). GILZ controls T-cell activation and development, and inhibits the activities of the key inflammatory signaling mediators NF-κb and AP-1 (9, 11, 12). When stimulated with GCs, macrophages synthesize significant amounts of GILZ which subsequently attenuates macrophage inflammatory responses (13). Moreover, GILZ gene expression is down-regulated in macrophages from inflammatory lesions of delayed-type hypersensitivity reactions, and persists in tumor-infiltrating macrophages from Burkitt lymphoma (13). GILZ mediates the anti-inflammatory effects of GCs via suppressing the activation of NF-κb in human airway epithelial cells (14) and redirects the maturation of dendritic cells preventing antigen-specific T lymphocyte responses (15). These data strongly suggest that GILZ plays a key role in inflammatory inhibition and immunosuppression. GILZ has several isoforms due to alternative RNA splicing: GILZ-1 (137 amino acids), GILZ-2 (201 amino acids), GILZ-3 (43 amino acids) and GILZ-4 (80 amino acids) (16). These isoforms were found to have striking functional differences in various types of cells. In the present study, we report that human airway epithelial cells respond to alcohol by modulating their transcriptome profiles. Among the identified alcohol-responsive genes, GILZ with a molecular weight of ~17 KDa, which is correlated to the GILZ1 in the previous publications (14, 16), is pronouncedly upregulated. Further experimental data suggest that GILZ upregulation may serve as a key factor for alcohol to modulate inflammatory responses by sharing the same signaling pathway with GCs.
Materials and methods
Cell culture
Primary human bronchial airway epithelia (NHBE) cells (Lot #7F3477; Lonza Walkersville, Inc. Walkersville, MA), A549 cells, human bronchial epithelial cell line (BEAS-2B), and HUVEC-C cells (ATCC) were cultured according to the recommended protocols by manufacturers. The air-liquid interface culture protocol was modified from the previous publication (17). Briefly, 5 × 105 NHBE cells were seeded on collagen-coated Millicell-PCF membrane inserts (Millipore, Billerica, MA). After the cells were cultured in submersion for 5 days, the apical medium was aspirated off and the cells were grown at the air-liquid interface for two more weeks. This air-liquid culture condition closely mimics the in vivo airways and the culture was previously shown to be fully differentiated, pseudostratified airway epithelia (18).
Ethanol exposure and drug treatments
The air-liquid interface cultures were exposed to alcohol by adding ethanol (200 Proof; AAPER Alcohol and Chemical Co., Shelbyville, KY) at varying doses to the basal medium. All other submerged cultures were treated with alcohol by replacing with an ethanol-containing culture media. The alcohol exposure time and concentration are indicated in the individual experiment. All the cultures were kept in 37 °C, 5% CO2 incubators that had been pre-saturated with the specified ethanol concentration. Whenever indicated, dexamethasone (Sigma-Aldrich) at 1 μM or Mifepristone (RU-486) at 10 μM (Sigma-Aldrich) was applied.
Microarray and data analysis
Total RNAs were extracted from three different lots of NHBE cells that had been cultured at the air-liquid interface and treated with different concentrations of ethanol for 24 hours by using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Procedures for cDNA synthesis, labeling, and hybridization were carried out as described at www.affymetrix.com/support/technical/manual/expression_manual.affx (Affymetrix, Red-wood City, CA). All experiments were performed using human genome U133 plus 2.0 Genechips as described at http://www.affymetrix.com/support/technical/byproduct.affx?product=hg-u133-plus. The CEL data file from each array was imported into Genespring GX 7.3 (Agilent Technologies, Santa Clara, CA) and preprocessed using the robust multichip average (RMA) method, and per gene normalization was applied using the median values of the no ethanol control samples. The detection call metrics from the CHP files were used to filter out transcripts that were found absent in all nine samples. In addition, transcripts that were not within a standard deviation of 1.4 and exhibiting less than 1.5 fold change were also removed from further analysis. To identify up-regulated and down-regulated transcripts, genes were filtered using the volcano plot option with the limit set at p < 0.01 and at least a 2.0-fold change. The parametric test, with variance not equal, was applied without multiple test correction. The gene profile data have been deposited to Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; Access Number - GSE12253).
Western blot assay
Western blot assays were performed according to the published procedure with modifications (19). Cells were washed twice with ice cold PBS followed by lysis in NP-40 Lysis Buffer [50 mM Tris/HCl, pH 8.0, 0.5% NP-40, 150 mM NaCl, 0.1 mM EDTA, 10 mM NaF, 1 mM PMSF and protease inhibitors cocktail (Roche, Basel, Switzerland)]. Samples were passed three times sequentially through 25G and 27G needles and centrifuged at 14,000 rpm for 20 minutes at 4 °C. The supernatants were collected and the protein concentrations were determined using a BCA Assay (Pierce, Rockford, IL). An equal amount of protein, as indicated, was loaded per sample for electrophoresis. The resolved proteins were transferred onto a nitrocellulose membrane and the primary antibody: 1) rabbit anti-GILZ (FL-134) (1:200; Santa Cruz, Santa Crutz, CA) or rabbit anti-Actin (I-19) (1:1000; Santa Cruz) was used. The secondary antibody was goat anti-Rabbit-IgG conjugated HRP (1:20,000; Bio-Rad). As needed for re-probing, the membranes were stripped at 50 °C for 30 minutes in Stripping Buffer (62.5 mM Tris/HCl, pH 6.8, 2% SDS and 100 mM 2-mercaptoethanol). For quantification, autoradiograms with an unsaturated exposure were scanned using Alpha Imager EC (Alpha Innotech, San Leandro, CA), analyzed with NIH ImageJ software (National Institute of Health, Bethesda, MD). Signal intensity was adjusted for background of each blot. Data are presented as ratio of signal intensity of the test protein to that of β-actin loading control.
Small interference RNA for gene silencing
The short hairpin structure for GILZ gene silencing was constructed as follows. For GILZ silence (siGILZ), the two complementary oligos were: 5′- AACAGCTTCACCTGACAACGA-CTTCCTGTCATCGTTGTCAGGTGAAGCTGTTTTTTTT-3′ and 5′-TCGAAAAAAAAACAGCTTCACCTGACAACGATGACAGGAAGTCGT TGTCAGGTGAAGCTGTTGGCC-3′. For the scrambled RNA control (siCNTL), the two complementary oligos were 5′-CATAACGAGCGGAAGAACGCTTCCTGTCACGTTCTTCCGCTCGTTATGTTTTTT-3′ and 5′-TCGAAAAAAACATAACGAGCGGAAGAACGTGACAGGAAGCGTTCTTCCGCTCGTTATGGGCC-3′. The ApaI and XhoI restriction enzyme sites were engineered at the ends of each oligo. The hairpin loops are shown in bold. The complementary oligo pairs were annealed and ligated into a pSILENCER-1.0 vector (Ambion Inc., Austin, Texas) at the ApaI and XhoI sites. The expression cassettes containing the mouse U6 promoter and siGILZ or siCNTL were cloned into the HIV-based lentiviral vector system to generate Lenti-U6-siGILZ-CMV-EGFP and Lenti-U6-siCNTL-CMV-EGFP transgene plasmids. Each of the transgene plasmids has two separate expression cassettes: the U6-siGILZ or U6-siCNTL cassette and the CMV-EGFP cassette. For lentiviral vector production, HEK 293T cells were transfected with triple plasmids: 1) the envelope plasmid pLTR-G, 2) the packaging plasmid pCD/NL-BH*ΔΔΔ, and one of the transgene plasmids, by calcium phosphate precipitation method (20). Forty-eight hours later, the viral vector-containing culture media were collected and vectors were concentrated by ultracentrifugation.
Translocation of EGFP-GRα and YFP-GRβ
The EGFP-GRα fusion expression vector was constructed from the cDNA of normal human bronchial epithelial cells. GRα was amplified by RT-PCR using the forward primer containing an Xho1 site (5′-CCGCTCGAGCATGGACTCCAAAGAATCATT-3′) and the reverse primer containing a BamH1 site (5′-CCGG GATCCTCACTTTTGATGAAACAGAAG-3′) in a high fidelity PCR supermix (Invitrogen). The resulting PCR product was digested with Xho1 and BamH1 and cloned into the expression vector pEGFP-C2 (Clontech, Mountain View, CA). The YFP-GRβ was graciously provided by Dr. Cidlowski at NIH (21). Due to the ease and high efficiency of transfection by calcium phosphate precipitation, HEK-293T cells were selected to study the effects of dexamethasone and alcohol on translocation of the two fusion-constructs. Because of the emission spectrum overlap, the yellow fluorescent protein (YFP) fusion protein can be observed using the same filter combination as EGFP.
Cytokine measurement by Bio-plex assay and ELISA
Human distal lung epithelial A549 cells were transduced, respectively, with Lenti-pU6-siGILZ-pCMV-EGFP or Lenti-pU6-siCNTL-pCMV-EGFP at an MOI of 5. The cells were then FACS-sorted to a pure population and referred to as A549-siGILZ and A549-siCNTL. GILZ gene silencing was confirmed by Western blotting. A549-siGILZ and A549-siCNTL were plated at a density of 5 × 105 cells per well in 6-well dish. And these cells were stimulated with 10 ng/ml of Interleukin-1β (IL-1β) (Calbiochem) for 3 hours. For the next 24 hours, the cells were continually exposed to IL-1β in the presence and absence of 50 mM of alcohol. Then, 300 μl of media from each well were collected and centrifuged at 14,000 rpm for 20 minutes at 4 °C. The supernatants were analyzed for inflammatory cytokine levels by using the human 8-Plex Cytokine Assay (Bio-Rad) according to the protocol recommended by the manufacturer. The examined 8 human cytokines were IL-2, IL-4, IL-6, IL-8, IL-10, GM-CSF, IFN-γ, and TNF-α. For ELISA measurement of IL-6, we used the commercial human IL-6 Quantikine kit (R&D Systems, Minneapolis, MN) and the recommended protocol.
Statistical analysis
For statistical analysis in Figure 4B, Student’s t-tests were conducted as two-tail pairwise comparisons between the groups. Results are expressed as means ± S.D. A probability level equal to or smaller than 0.05 was considered significant.
Figure 4.
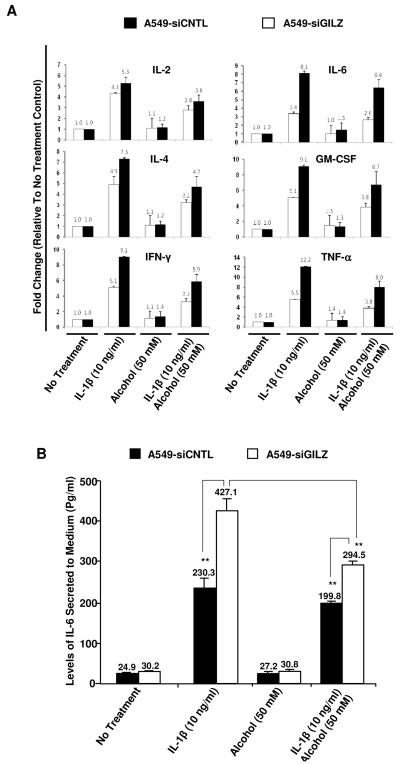
Inflammatory cytokine secretion by the GILZ-silenced and the mock control cells. Panel A: A549 cells, stably expressing siRNA against GILZ (A549-siGILZ) or a scrambled control sequence (A549-siCNTL), were established by transduction with the lentiviral vectors described in Figure 3A. The cells were stimulated by adding IL-1β (10 ng/ml) to the media 3 hours prior to alcohol treatment for 24 hours. Cytokine concentration in each collected medium was assessed by cytokine Bioplex assay and compared to the no treatment control to obtain the fold changes. Open bars: A549-siGILZ; Solid bars: A549-siCNTL. Panel B: IL-6 secretion by A549-siGILZ or A549-siCNTL cells, treated similarly with IL-1β and alcohol, was measured by ELISA. The absolute concentration of IL-6 in the media is expressed as picogram per milliliter. Asterisks indicate statistically significant differences by Student’s t-test (p<0.01, n=3).
Results
Ethanol modulates the gene transcriptional profile of human airway epithelial cells
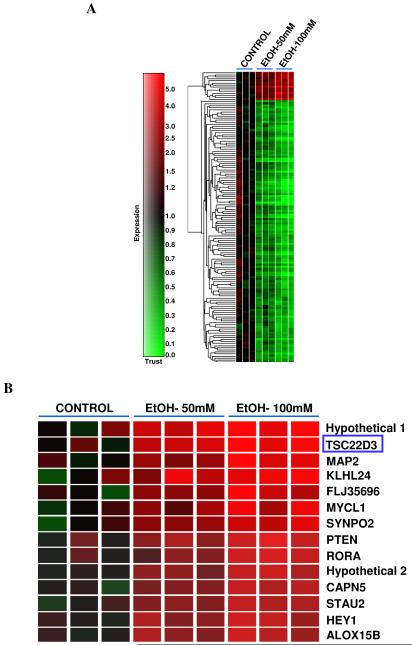
In order to understand the cellular responses of human airway epithelia to alcohol, we first identified those genes whose expressions were significantly altered by alcohol exposure. Well-differentiated primary human bronchial epithelial cells (NHBE), cultured at an air-liquid interface, were treated with varied doses of alcohol (0, 50 and 100 mM) for 24 hours. The samples were then subjected to whole-genome high-density microarray analyses. The obtained data have been deposited to Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; Access Number - GSE12253). Comparison between 0 mM and 50 mM alcohol treatments resulted in only 2 genes being significantly upregulated and 16 genes significantly down-regulated by the two-fold criterion in our system (Fig. 1A & B). The two upregulated genes were 1) TSC22 domain Family-member 3 or glucocorticoid-induced leucine zipper (GILZ) and, 2) a putative gene (human hypothetical protein 644242) with unknown function. Treatment with 100 mM alcohol upregulated 14 genes and down-regulated 157 genes by at least two-fold. These upregulated genes are specifically displayed (Fig. 1B). The profile of genes that showed a dose-dependent increase by at least 1.5-fold with 50 mM and 100 mM alcohol treatments is shown in Table 1.
Figure 1.
Alcohol exposure alters the gene transcription profile of primary human airway epithelial cells by microarray analyses. Panel A: Primary human airway epithelial cells were treated with alcohol (0, 50, 100 mM) for 24 hours. All the genes whose expressions were significantly altered by at least two-fold were clustered and are displayed. The data have been submitted to GEO (http://www.ncbi.nlm.nih.gov/geo/; Access Number – GSE12253). Each group had triplicate samples. Panel B: Upregulated genes by alcohol (100 mM) are displayed. Framed is TSC22D3 or GILZ gene whose transcription responded to alcohol in a dose-dependent manner.
TABLE 1. DOSE-DEPENDENT GENE UPREGULATION BY ALCOHOL.
| Gene Symbol |
Gene Description | Fold Increase in Gene Transcription |
|
|---|---|---|---|
| Alcohol Conc. 0 mM vs 50 mM |
Alcohol Conc. 0 mM vs 100 mM |
||
| TSC22D3 | TSC22 domain family, member 3 | 2.075 | 3.037 |
| – | Homo sapiens, clone IMAGE:4403366 | 2.055 | 3.042 |
| ALOX15B | Arachidonate 15-lipoxygenase, 2nd type | 1.632 | 2.004 |
| SYNPO2 | Synaptopodin 2 | 1.615 | 2.316 |
| HEY1 | Hair/enhancer-of-split related with YRPW motif 1 | 1.615 | 2.054 |
| FLJ35696 | FLJ35696 protein | 1.608 | 2.396 |
| MAP2 | Microtubule-associated protein 2 | 1.586 | 2.707 |
| PTEN | Phosphatase and tensin homolog | 1.555 | 2.278 |
| STAU2 | Staufen, RNA binding protein, homolog 2 (Drosophila) |
1.530 | 2.072 |
Ethanol upregulates GILZ protein expression
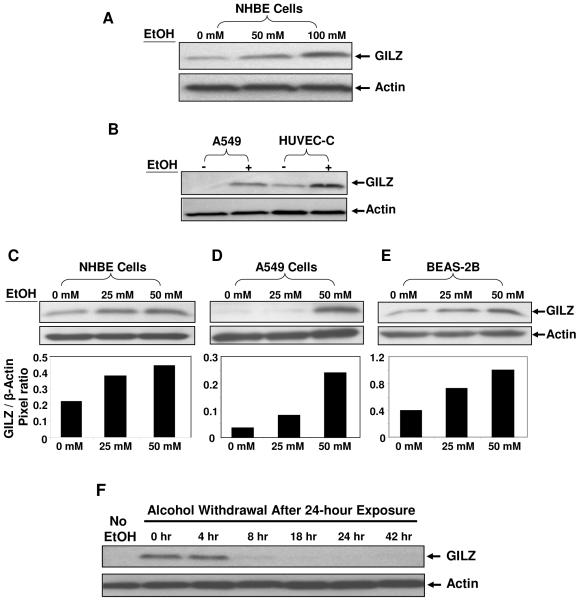
Among the ethanol-upregulated genes, GILZ was elevated by greater than 2-fold at 50 mM and 3-fold at 100 mM alcohol. The alcohol dose-dependent expression pattern of GILZ suggested that GILZ was an alcohol responsive gene and worthy of further investigation. To test whether the levels of GILZ mRNA correlated with the levels of GILZ protein, we performed immunoblot assays using a specific antibody against GILZ. The results showed a clear dose-dependent effect of alcohol on the upregulation of GILZ protein with a molecular weight of ~17 kDa (Fig. 2A). Based on the size of the protein, we judged the detected GILZ to be GILZ1 as documented in the previous publications (14, 16). In contrast, the expression level of the internal actin control remained constant.
Figure 2.
Up-regulation of GILZ protein by alcohol. Panel A: Immunoblots of GILZ and actin of primary human airway epithelial cells (NHBE) treated with alcohol at 0, 50 or 100 mM, respectively, for 24 hours. GILZ expression at the protein level is elevated in response to alcohol doses. The same membrane was stripped and re-probed with the specific antibody against actin, which was used as a loading control. Panel B: Immunoblots of GILZ and actin expression in A549 (human distal lung epithelial cell line) and HUVEC-C: (human umbilical vein endothelial cells) treated with or without 100 mM alcohol for 24 hours. Panel C-E: GILZ expression in lung epithelial cells exposed to physiologically relevant levels of alcohol. BEAS-2B: human bronchial epithelial cell line. Bar graphs below indicate the corresponding pixel ratios of GILZ over actin by densitometry. Panel F: Reversal of GILZ expression by alcohol withdrawal. A549 cells were treated with 100 mM alcohol for 24 hours followed by alcohol withdrawal. Post alcohol withdrawal, cell samples were collected at the indicated time points.
To test if alcohol could induce GILZ upregulation in other cell types, human distal lung epithelial A549 cells and human umbilical vein endothelial cells (HUVEC) were examined. Prior to immunoblotting, all the cells were cultured in the presence or absence of 100 mM alcohol for 24 hours. The results showed that GILZ was similarly upregulated (Fig. 2B) in these cells as well. Thus, alcohol induces GILZ expression not only in primary and immortalized pulmonary epithelial cells but also in primary vascular endothelial cells.
To determine whether GILZ responds to physiologically relevant alcohol concentration, we selected low levels of alcohol (0, 25, 50 mM) to treat various pulmonary epithelial cells: NHBE, A549, and BEAS-2B (human bronchial epithelial cell line), for 24 hours. GILZ expression was similarly assessed by immunoblot assays followed by densitometry analyses. As shown, the level of GILZ protein was increased by alcohol in the three tested cells (Fig. 2C-E), indicating a clear upregulation of GILZ by physiologically encountered concentrations of alcohol.
If alcohol is the causative agent for the induction of GILZ, removal of alcohol should reverse the effect. A549 cells were treated with 100 mM alcohol for 24 hours followed by culturing in alcohol-free media. Cell samples were collected at various time points as indicated (0, 4, 8, 18, 24 and 42 hours). GILZ expression was measured by immunoblot. As shown, A549 cells expressed little detectable GILZ without alcohol stimulation (Fig. 2F). After a 24-hour exposure to 100 mM alcohol, the cells expressed a significant level of GILZ. However, approximately 8 hours after alcohol withdrawal, GILZ expression decreased to pre-alcohol treatment levels. These data suggest that GILZ responsiveness to alcohol is reversible after alcohol withdrawal. Moreover, these data also suggest that alcohol’s effect on GILZ is not caused by ethanol-induced cytotoxicity.
GILZ gene silencing abolishes ethanol-upregulation of GILZ and magnifies cellular response to inflammatory stimulation
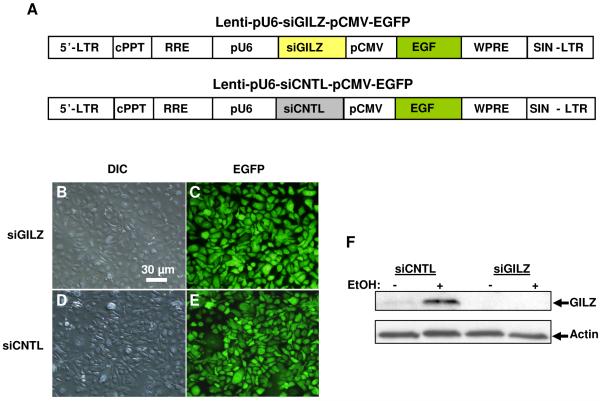
To achieve specific gene silencing of GILZ, we employed the small RNA interference approach by construction of the following two lentiviral vectors (Fig. 3A): 1) Lenti-pU6-siGILZ-pCMV-EGFP and 2) Lenti-pU6-siCNTL-pCMV-EGFP. In both vectors, the mouse U6 promoter drives the expression of either a small interfering RNA for GILZ (siGILZ) or a small scrambled RNA sequence as a control (siCNTL). Additionally, in a separate expression cassette, the CMV promoter drives the expression of EGFP, which serves as an indicator for vector transduction. NHBE cells cultured in submersion were transduced with each of the viral vectors at an MOI of 10. As seen (Fig. 3B-E), all the cells expressed EGFP, suggesting that cells were effectively transduced by the vectors and potentially express the corresponding siRNAs. Immunoblot assays were performed to assess the efficacy of GILZ knockdown and the ethanol effect on GILZ in the cells. Total cellular proteins (15 μg per lane) were loaded and probed with the specific antibody against GILZ or actin. GILZ expression in the cells transduced with Lenti-pU6-siGILZ-pCMV-EGFP was not detectable by immunoblot and alcohol exposure failed to upregulate GILZ expression in these cells (Fig. 3F). In contrast, A549 cells receiving the control vector responded to alcohol normally by upregulation of GILZ expression. These data strongly suggest that GILZ is a true alcohol-responsive gene.
Figure 3.
Lentiviral vectors for knockdown of GILZ expression. Panel A: Structures of lentiviral vectors expressing the small interfering RNA for GILZ (Lenti-pU6-siGILZ-pCMV-EGFP) or small scrambled RNA for the control (Lenti-pU6-siCNTL-pCMV-EGFP). 5′-LTR: 5′-long terminal repeat; cPPT: central polypurine tract; RRE: Rev-responsive element; pU6: mouse U6 promoter; pCMV: CMV viral promoter; EGFP: enhanced green fluorescence protein; WPRE: Woodchuck hepatitis virus posttranscriptional regulatory element; SIN-LTR: self-inactivating long terminal repeat. Panels B-E: Micrographs of primary human airway epithelial cells transduced with either siGILZ or siCNTL vector. B & D: Images by Differential Interference Contrast (DIC) microscopy; C & E: Images by Fluorescence microscopy. Panel F: Immunoblots to examine the GILZ expression levels in the vector-transduced primary human airway epithelial cells in the presence or absence of 100 mM alcohol.
To delineate the role of GILZ in cellular responses to inflammatory stimuli and alcohol-induced immunosuppression, two cell lines derived from A549 cells with either permanent GILZ-knockdown (A549-siGILZ) or mock siRNA control (A549-siCNTL), were generated. These cells were stimulated by adding IL-1β (10 ng/ml) 3 hours prior to treatment with or without 50 mM alcohol for 24 hours. The secretion of eight inflammatory cytokines (IL-2, IL-4, IL-6, GM-CSF, IFN-γ, TNF-α, IL-8 and IL-10) were screened by Bioplex cytokine assays. Without IL-1β stimulation, both cells produced appreciable basal levels of IL-6 and IL-8. However, the other measured cytokines were produced at a minimal level and near the threshold of detection (Table 2). With IL-1β stimulation, six of eight cytokines (IL-2, IL-4, IL-6, GM-CSF, IFN- γ and TNF-α) were increased by 4.3, 4.9, 3.4, 5.1, 5.1 and 5.5 fold, respectively, in the control A549-siCNTL cells (Fig. 4A). In contrast to control cells, these 6 cytokines showed a significantly greater fold increase in GILZ-silenced A549 cells after IL-1β stimulation (5.3, 7.3, 8.1, 9.1, 9.1 and 12.2 fold for each cytokine, respectively). Secretion of IL-8 and IL-10 was not affected by IL-1β stimulation in these cells (data not shown). Ethanol, applied 3 hours after IL-1β stimulation, significantly reduced the stimulated secretion of IL-2, IL-4, IL-6, GM-CSF, IFN-γ and TNF-α secreted by control A549 cells. In contrast ethanol treatment had a far lesser suppressive affect on the stimulated secretion of these cytokines in GILZ-knockdown cells (Fig. 4A). Among these cytokines, the cytokine producing highest amounts and most affected by ethanol was IL-6. Based in this we confirmed our Bio-plex findings by assaying IL-6 by ELISA. As shown in Figure 4B, A549-siGILZ responded to IL-1β stimulation by increasing IL-6 secretion in a much greater magnitude than the control A549-siCNTL cells. Again, GILZ-silenced cells were significantly more resistant to the suppressive effects of ethanol.
TABLE 2. BASE-LEVEL SECRETION OF EIGHT CYTOKINES BY BIO-PLEX ASSAYS*.
| Cytokine | A549-siCNTL (pg/ml) |
A549-siGILZ (pg/ml) |
|---|---|---|
| IL-2 | 18.3 +/− 7.4 | 9.5 +/− 0.7 |
| TNF-α | 79.5 +/− 2.1 | 26.5 +/− 0.7 |
| IL-4 | 24.3 +/− 11.6 | 11.5 +/− 0.7 |
| IL-6 | 2657.5 +/− 1358.4 | 823.3 +/− 100.0 |
| IL-8 | 14298.0 +/− 929.1 | 12341.8 +/− 144.0 |
| GM-CSF | 48.8 +/− 24.4 | 22.8 +/− 0.3 |
| IFN-γ | 38.5 +/− 20.5 | 15.0 +/− 0.0 |
| IL-10 | 64.3 +/− 1.7 | 66.0 +/− 5.6 |
A549-siCNTL and A549-siGILZ cells were cultured for 27 hours without any treatments. The detected cytokine levels represent the base level secretion by the cells. The data were from two parallel experiments.
Glucocorticoid receptor (GR) is involved in ethanol-induced GILZ upregulation
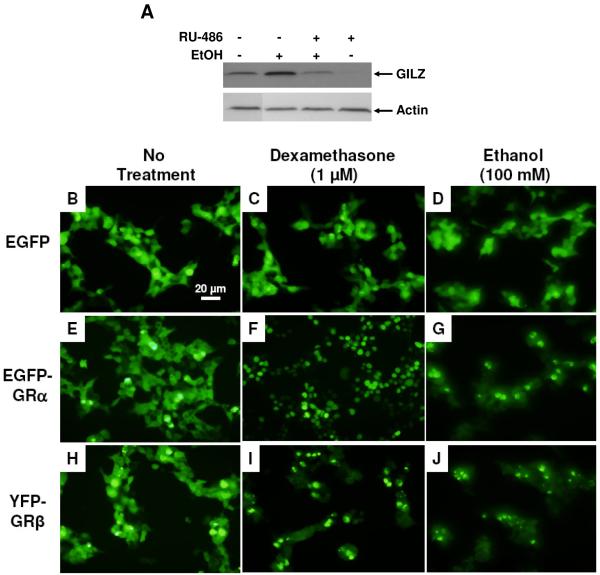
Because GILZ is known to be upregulated by glucocorticoids (GCs) via binding to GR, we hypothesized that alcohol might be acting through the same signaling pathway as GCs. To test this hypothesis, we first utilized the GR-specific antagonist RU486 or mifepristone. Primary human airway epithelial cells were cultured in 100 mM alcohol medium in presence or absence of RU486 (10 μM). Primary human airway epithelial cells had a basal expression of GILZ, and alcohol again stimulated GILZ expression in these cells (Fig. 5A). RU486 was able to substantially suppress the alcohol-stimulated GILZ expression. This incomplete suppression was previously observed in RU486-inhibition of GILZ expression induced with dexamethasone (14, 22) due to the dual activities of RU486 as a potent GC antagonist and a partial GC agonist. These results suggest that GR might be involved in the alcohol-induced GILZ expression. Because GR is a ligand-dependent transcription factor, it must be translocated to the cell nucleus in order to regulate expression of glucocorticoid-targeted genes. Thus, GR localization can be utilized to judge whether alcohol stimulates GILZ expression through GR. Towards this end, we constructed an EGFP-GRα fusion protein expression vector, in which the EGFP gene was fused, in-frame, with GRα at its N-terminus. In addition, a vector expressing the YFP-GRβ fusion protein was obtained to assess GRβ localization (20). HEK-293T cells were transfected with each of the following three vectors: 1) pEGFP, 2) pEGFP-GRα and 3) pYFP-GRβ. Twenty-four hours after transfection, the cells were cultured in control medium (Fig. 5B, E & H) or supplemented with 1 μM dexamethasone (Fig. 5C, F & I), or 100 mM alcohol (Fig. 5D, G & J). After dexamethasone stimulation, EGFP-GRα was completely translocated into the cell nuclei (Fig. 5F) and YFP-GRβ partially into cell nuclei (Fig. 5I). Notably, similar to dexamethasone alcohol exposure resulted in nuclear translocation of GRα and GRβ, suggesting the involvement of GR in alcohol induction of GILZ expression.
Figure 5.
Alcohol-stimulation of GILZ expression through the glucocorticoid receptor. Panel A: Effect of RU486 on GILZ expression induced by alcohol in primary human airway epithelial (NHBE) cells. NHBE cells were exposed to alcohol (100 mM) or RU486 (10 μM) or a combination of both. Immunoblot assays were performed to evaluate GILZ and actin expression levels. Panels B-J: Effect of dexamethasone or alcohol on translocation of glucocorticoid receptors. HEK-293T cells were transfected with one of the following three vectors: 1) pEGFP, 2) pEGFP-GRα and 3) pYFP-GRβ. The cultures were fed with either no-drug control media (B, E & H) or media containing 1 μM dexamethasone (C, F & I) or 100 mM alcohol (D, G & J), respectively. Dexamethasone and alcohol exposure elicits the translocation of the glucocorticoid receptor to the cell nuclei. Scale bar – 20 μm.
Discussion
Alcohol produces a broad spectrum of effects in the human body. The molecular mechanisms underlying these effects are not fully understood. The current report documents that cells specifically alter their gene transcriptional profile in response to alcohol exposure, suggesting that gene regulation might be a mechanism for alcohol to act on cells. Accumulated data demonstrates that moderate alcohol intake has been associated with reductions in many adverse health conditions including coronary artery disease, diabetes, hypertension, congestive heart failure, stroke, arthritis, and dementia (2, 23). Moderate consumption of alcohol is also found to benefit overall survival and quality of life in the elderly (24). However, abuse of this substance leads to detrimental consequences, including immuno-suppression and infections. In this report, we have shown that GILZ is one of the genes significantly up-regulated by alcohol. Such an up-regulation recapitulates the GILZ response to GCs. Clinically, GCs are the most commonly prescribed drugs for management of inflammation. Our finding that alcohol conveys similar effects as GCs, although at a lower potency than dexamethasone, predicts that moderate alcohol intake may mediate its beneficial effects through this signaling pathway. Interestingly, previous clinical data demonstrate that alcohol decreases the risk of certain autoimmune inflammatory disorder such as rheumatoid arthritis (25, 26) and has been associated with improvement in asthma symptoms (27).
It is well-documented that alcohol abuse predisposes individuals to infections by bacteria, fungi, and viruses (4-6), and consequently alcohol-abusing patients suffer higher mortality from bacterial pneumonia compared with control patient populations (28). Microbial infections elicit many inflammatory pathways in the lung, which is an essential response required to mobilize and orchestrate both innate and adaptive host defenses. Depending on how the host is exposed to alcohol, pulmonary inflammatory responses could vary (29). Our data and previous publications show that acute ethanol exposure inhibits the production of inflammatory cytokines (30-33). However, chronic ethanol exposure has varied effects on inflammatory cytokines. In rats chronic ethanol ingestion decreases TNF-α production by alveolar macrophages (34), and down-regulates GM-CSF receptor expression on the cell surface to suppress inflammation (35). In contrast, chronic ethanol abuse results in enhanced macrophage production of TNF-α from both human PBMCs and mouse Kupffer cells (36, 37). Moreover, alcohol abusers can have elevated inflammatory cytokine responses (29). These multifaceted effects of alcohol on lung inflammatory responses indicate a complex regulation of the process. Our data demonstrated that short-term alcohol induces reversible GILZ upregulation in pulmonary epithelial cells, which may be of relevance to lung inflammatory responses. The effect of chronic alcohol exposure on GILZ expression remains to be determined. Nevertheless, GILZ is a transforming growth factor (TGF) β1-stimulated protein. Chronic alcohol ingestion in rats increases TGF-β1 expression in the lung (38). Given that TGF-β1 is an upstream regulator gene, it is possible that GILZ might be upregulated through this mechanism. Thus, further research is required to determine if GILZ upregulation is a direct effect of alcohol or through an autocrine/paracrine signaling pathway. GILZ overexpression in transgenic mice inhibits Th1 CD4+ T-cells responses and enhances Th2 responses (39). In airway epithelial cells, GILZ suppresses activation of NF-κb promoter, which attenuates expression of the downstream luciferase gene in response to cytokine induction (14). Therefore, alcohol-induced GILZ upregulation may contribute to the overall modulation of airway inflammatory responses when infections occur.
Our microarray data revealed that many genes are significantly up-regulated or down-regulated by alcohol exposure. In addition to GILZ, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), also showed a dose-dependent upregulation by alcohol (Table 1). PTEN is a major negative regulator of the PI3-kinase pathway and antagonizes PI3 kinase activity (40). This signaling pathway is responsible for cell proliferation and survival. Moreover, T cells with PTEN deficiency contribute to autoimmune diseases (41). Interestingly, a recent publication documented that GILZ is not only regulated by GC-GR pathway, but also regulated by the PI3-kinase/AKT pathway (42). Therefore, PTEN may be another upstream gene that controls GILZ expression in this system. Even though the current report focuses on GILZ, we are aware that the potential importance of other candidate genes. Future investigations are warranted to understand the interplay of the genes and to map the associated pathways and signaling networks.
In this report, we used the cytokine Bioplex assay to screen for basal and IL-1β stimulated cytokine responses in the A549-derived cell lines. These cells produced IL-6 and IL-8 at the low nanogram-per-milliliter level and the other measured cytokines (IL-2, IL-4, GM-CSF, IFN-γ and TNF-α) at the low picogram-per-milliliter level. As IL-6 showed the greatest induction, we used conventional ELISA to selectively confirm this result. In the course of this study, we noticed that the two methods had varied detection sensitivities. The Bioplex method appeared to be much more sensitive than ELISA, probably due to the differences of the two systems in antigen trapping, signal reading and standard calibration.
In summary, through large-scale microarray analyses, we analyzed the gene expression profiles of human pulmonary epithelial cells exposed to varied doses of alcohol. GILZ, a key GC-responsive gene, responds to alcohol in a dose-dependent manner. These data suggest, for the first time, that alcohol and GCs share the same signaling pathway to modulate cellular inflammatory and immune propensities.
Acknowledgments
Grant Support: This work was supported in part or full by NIH grants 5R21AA16118 to Wang, P60AA09803 to Nelson and general funding from Louisiana Gene Therapy Consortium.
Abbreviations
- GILZ
Glucocorticoid-induced Leucine Zipper
- GC
Glucocorticoids
- GR
Glucocorticoid receptor
- RU486
Mifepristone
- NHBE
Primary human bronchial epithelia cells
- HUVEC
Human umbilical vein endothelial cells
REFERENCES
- 1.Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Sci. Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. 2007;116:1306–1317. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- 3.Zakhari S, Li TK. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz M, Ewig S, Marcos MA, Martinez JA, Arancibia F, Mensa J, Torres A. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am. J. Respir. Crit. Care Med. 1999;160:397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- 5.Zisman DA, Strieter RM, Kunkel SL, Tsai WC, Wilkowski JM, Bucknell KA, Standiford TJ. Ethanol feeding impairs innate immunity and alters the expression of Th1- and Th2-phenotype cytokines in murine Klebsiella pneumonia. Alcohol Clin. Exp. Res. 1998;22:621–627. doi: 10.1111/j.1530-0277.1998.tb04303.x. [DOI] [PubMed] [Google Scholar]
- 6.Nelson S, Kolls JK. Alcohol, host defence and society. Nat. Rev. Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- 7.Miesfeld R, Okret S, Wikstrom AC, Wrange O, Gustafsson JA, Yamamoto KR. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature. 1984;312:779–781. doi: 10.1038/312779a0. [DOI] [PubMed] [Google Scholar]
- 8.Payvar F, Wrange O, Carlstedt-Duke J, Okret S, Gustafsson JA, Yamamoto KR. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc. Natl. Acad. Sci. U S A. 1981;78:6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayroldi E, Migliorati G, Bruscoli S, Marchetti C, Zollo O, Cannarile L, D’Adamio F, Riccardi C. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood. 2001;98:743–753. doi: 10.1182/blood.v98.3.743. [DOI] [PubMed] [Google Scholar]
- 10.D’Adamio F, Zollo O, Moraca R, Ayroldi E, Bruscoli S, Bartoli A, Cannarile L, Migliorati G, Riccardi C. A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity. 1997;7:803–812. doi: 10.1016/s1074-7613(00)80398-2. [DOI] [PubMed] [Google Scholar]
- 11.Mittelstadt PR, Ashwell JD. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. J. Biol. Chem. 2001;276:29603–29610. doi: 10.1074/jbc.M101522200. [DOI] [PubMed] [Google Scholar]
- 12.Riccardi C, Bruscoli S, Ayroldi E, Agostini M, Migliorati G. GILZ, a glucocorticoid hormone induced gene, modulates T lymphocytes activation and death through interaction with NF-kB. Adv. Exp. Med. Biol. 2001;495:31–39. doi: 10.1007/978-1-4615-0685-0_5. [DOI] [PubMed] [Google Scholar]
- 13.Berrebi D, Bruscoli S, Cohen N, Foussat A, Migliorati G, Bouchet-Delbos L, Maillot MC, Portier A, Couderc J, Galanaud P, Peuchmaur M, Riccardi C, Emilie D. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood. 2003;101:729–738. doi: 10.1182/blood-2002-02-0538. [DOI] [PubMed] [Google Scholar]
- 14.Eddleston J, Herschbach J, Wagelie-Steffen AL, Christiansen SC, Zuraw BL. The anti-inflammatory effect of glucocorticoids is mediated by glucocorticoid-induced leucine zipper in epithelial cells. J. Allergy Clin. Immunol. 2007;119:115–122. doi: 10.1016/j.jaci.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Cohen N, Mouly E, Hamdi H, Maillot MC, Pallardy M, Godot V, Capel F, Balian A, Naveau S, Galanaud P, Lemoine FM, Emilie D. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood. 2006;107:2037–2044. doi: 10.1182/blood-2005-07-2760. [DOI] [PubMed] [Google Scholar]
- 16.Soundararajan R, Wang J, Melters D, Pearce D. Differential activities of glucocorticoid-induced leucine zipper protein isoforms. J. Biol. Chem. 2007;282:36303–36313. doi: 10.1074/jbc.M707287200. [DOI] [PubMed] [Google Scholar]
- 17.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol. Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA, Spees JL, Bertucci D, Peister A, Weiss DJ, Valentine VG, Prockop DJ, Kolls JK. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc. Natl. Acad. Sci. U S A. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U S A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 2009;4(4):495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 21.Schaaf MJ, Cidlowski JA. Molecular determinants of glucocorticoid receptor mobility in living cells: the importance of ligand affinity. Mol. Cell. Biol. 2003;23:1922–1934. doi: 10.1128/MCB.23.6.1922-1934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz M, Eggert M, Baniahmad A, Dostert A, Heinzel T, Renkawitz R. RU486-induced glucocorticoid receptor agonism is controlled by the receptor N terminus and by corepressor binding. J. Biol. Chem. 2002;277:26238–26243. doi: 10.1074/jbc.M203268200. [DOI] [PubMed] [Google Scholar]
- 23.O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J. Am. Coll. Cardiol. 2007;50:1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 24.Byles J, Young A, Furuya H, Parkinson L. A drink to healthy aging: The association between older women’s use of alcohol and their health-related quality of life. J. Am. Geriatr. Soc. 2006;54:1341–1347. doi: 10.1111/j.1532-5415.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson IM, Verdrengh M, Brisslert M, Lindblad S, Bokarewa M, Islander U, Carlsten H, Ohlsson C, Nandakumar KS, Holmdahl R, Tarkowski A. Ethanol prevents development of destructive arthritis. Proc. Natl. Acad. Sci. U S A. 2007;104:258–263. doi: 10.1073/pnas.0608620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr. Opin. Rheumatol. 2009;21:279–283. doi: 10.1097/BOR.0b013e32832a2e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41:293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc. Am. Thorac. Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- 29.Goral J, Karavitis J, Kovacs EJ. Exposure-dependent effects of ethanol on the innate immune system. Alcohol. 2008;42:237–247. doi: 10.1016/j.alcohol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boé DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol Clin. Exp. Res. 2003;27(11):1838–1845. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- 31.Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin. Exp. Res. 1996;20(5):900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- 32.Nelson S, Bagby G, Summer WR. Alcohol suppresses lipopolysaccharide-induced tumor necrosis factor activity in serum and lung. Life Sci. 1989;44(10):673–676. doi: 10.1016/0024-3205(89)90472-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhao XJ, Marrero L, Song K, Oliver P, Chin SY, Simon H, Schurr JR, Zhang Z, Thoppil D, Lee S, Nelson S, Kolls JK. Acute alcohol inhibits TNF-alpha processing in human monocytes by inhibiting TNF/TNF-alpha-converting enzyme interactions in the cell membrane. J. Immunol. 2003;170(6):2923–2931. doi: 10.4049/jimmunol.170.6.2923. [DOI] [PubMed] [Google Scholar]
- 34.D’Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol modulates alveolar macrophage tumor necrosis factor-alpha, superoxide anion, and nitric oxide secretion in the rat. Alcohol Clin. Exp. Res. 1996;20(1):156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 35.Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J. Immunol. 2005;175(10):6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- 36.McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin. Liver Dis. 1999;19(2):205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- 37.Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J. Immunol. 2008;181(5):3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am. J. Respir. Crit. Care Med. 2004;170(2):188–194. doi: 10.1164/rccm.200304-478OC. [DOI] [PubMed] [Google Scholar]
- 39.Cannarile L, Fallarino F, Agostini M, Cuzzocrea S, Mazzon E, Vacca C, Genovese T, Migliorati G, Ayroldi E, Riccardi C. Increased GILZ expression in transgenic mice up-regulates Th-2 lymphokines. Blood. 2006;107:1039–1047. doi: 10.1182/blood-2005-05-2183. [DOI] [PubMed] [Google Scholar]
- 40.Chalhoub N, Baker SJ. PTEN and the PI3-Kinase Pathway in Cancer. Annu. Rev. Pathol. 2008;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckler JL, Liu X, Turka LA. Regulation of T-cell responses by PTEN. Immunol. Rev. 2008;224:239–248. doi: 10.1111/j.1600-065X.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grugan KD, Ma C, Singhal S, Krett NL, Rosen ST. Dual regulation of glucocorticoid-induced leucine zipper (GILZ) by the glucocorticoid receptor and the PI3-kinase/AKT pathways in multiple myeloma. J. Steroid Biochem. Mol. Biol. 2008;110:244–254. doi: 10.1016/j.jsbmb.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]