Abstract
Silks are biodegradable, biocompatible, self-assemblying proteins that can also be tailored via genetic engineering to contain specific chemical features, offering utility for drug and gene delivery. Silkworm silk has been used in biomedical sutures for decades and has recently achieved Food and Drug Administration approval for expanded biomaterials device utility. With the diversity and control of size, structure and chemistry, modified or recombinant silk proteins can be designed and utilized in various biomedical application, such as for the delivery of bioactive molecules. This review focuses on the biosynthesis and applications of silk-based multi-block copolymer systems and related silk protein drug delivery systems. The utility of these systems for the delivery of small molecule drugs, proteins and genes are reviewed.
Keywords: Silk, Recombinant protein, Gene delivery, Drug delivery, Biomaterials, Bioengineering
1. Introduction
1.1. Silkworm and spider silk proteins
Silk proteins are produced by a variety of insects and spiders, and form fibrous materials in nature, such as spider orb webs and silkworm cocoons [1]. The functions of silk proteins in nature range from development (cocoons), prey capture (spider webs), to safety lines (spider dragline) [2-4]. Several properties of silk-based materials, such as mechanical properties, solubility, and biodegradability, can be controlled by manipulating the secondary structure [5,6]. Silk proteins have therefore been used as medical sutures because of their mechanical properties and biocompatibility [1], and has also been explored as a biomaterial for cell culture and tissue engineering [2,7]. The biological properties and functions of silkworm and spider silks, which are the most studied silk proteins, are explained separately in the following sections.
1.1.1 Silkworm silk fibroins
Silkworm silk has been used as biomedical sutures for decades, and in textile production for clothing for centuries, because silkworms are easier to domesticate and obtain silk in comparison to spider silks. The silk fibroin from the cocoon of silkworm Bombyx mori, the most studied silkworm silk proteins, contain two major components, light (~25 kDa) and heavy chain (~325 kDa) fibroins. The core sequence in the heavy chain include alanine-glycine repeats. In silkworm cocoons, these two fibroins are encased in a sericin coat, glue-like proteins, to form the composite fibers of the cocoon. Recently, methods to extract and regenerate silk fibroin have been developed [7-13], and several silk-based biomaterials, such as silk porous scaffolds, silk films, hydrogels, and electrospun nanofibers, can be processed from silk solutions (see section 4).
1.1.2 Spider silk fibroins
The most widely studied spider silk in terms of structure and function is dragline silk from the spider Nephila clavipes [14-17]. The dragline silk is secreted as a mixture of two proteins from specialized columnar epithelial cells of the major ampullate gland of orb-weaver spinning spiders [14,18]. The molecular weights of these proteins ranges from 70 to 700 kDa depending on source. Partial cDNA clones encoding the two types of dragline silks have been isolated and analyzed from two species of orb-web weaving spiders, Araneus diadematus (ADF-3 and ADF4) and N. clavipes (MaSpI and MaSpII) [19-21]. These silk proteins are characterized as block copolymers, composed of large hydrophobic blocks with highly conserved repetitive sequences consisting of short side-chain amino acids, such as glycine and alanine, with intervening small hydrophilic blocks with more complex sequences that consist amino acids with bulkier side-chain and charged amino acids [22,23]. The hydrophobic blocks form beta-sheets, or physically cross-linked crystalline domains in silk fibers. The impressive tensile strength of silk fibers is due to the ordered hydrophobic and less ordered hydrophilic regions, in combination with chain orientation achieved during spinning [24,25].
1.2. Recombinant silk proteins
In the last decade, remarkable progress has been made in understanding silk genetics, structures and biophysics [19,20,26]. Cloning and expression of native and synthetic silks has been achieved in a variety of host systems using synthetic oligonucleotide versions of consensus repeats or variants of these repeats garnered from sequence data from native genes [21,27,28]. Silk proteins modified by genetic engineering can also be designed to display new features alongside native properties, (Table 1) [3,29-36].
Table 1.
Recombinant polymers containing silk sequences for biomedical materials
| Silk Domains | Functional Domains | Refs |
|---|---|---|
|
B. mori silkworm silk [GAGAGS] |
Elastin [GVGXP], X=any amino acid except proline |
29-31 |
|
| ||
| B. mori silkworm silk | [GERGDLGPQGIAGQRGVV(GER)3GAS]8GPPGPCCGGG [TGRGDSPAS]8 |
42 |
|
| ||
|
Anaphe silkworm silk [AAG]6 [AG]9 |
ASTGRGDSPAAS, ASTGRGDSPAAS | 47 |
|
| ||
|
A. diadematus major dragline silk proteins ADF-3 and ADF-4 |
ADF-3 and ADF-4 block copolymers | 5 |
|
| ||
| N. clavipes Spidroin I | Hydrophilic/hydrophobic sequences of Spidroin I | 36 |
|
| ||
| 15mer of N. clavipes Spidroin I | RGD | 33 |
|
|
||
| [GRGGLGGQGAGAAAAA | Silaffin (R5) | 34 |
|
|
||
| GGAGQGGYGGLGSQG]15 | Dentin matrix protein 1 (CDMP1) | 35 |
|
| ||
| 6mer of N. clavipes Spidroin I | Polylysine (15, 30, and 45mer) | 48 |
|
|
||
| [GRGGLGGQGAGAAAAA GGAGQGGYGGLGSQG]6 |
Several RGD and polylysine (30mer) | 49 |
1.2.1 Silkworm variants
Silkworm silk from B. mori silkworm (silk-like repeats of GAGAGS) and elastin block (GVGVP) copolymers, silk-elastin-like proteins (SELP) constructed by recombinant DNA techniques, have been utilized as gene and drug delivery systems, by forming hydrogels to release adenovirus containing reporter genes [29-31,37-41]. Enhanced gene expression was reported in target cells, up to 10 fold, when compared to viral injection without the SELP, demonstrating utility for head and neck solid tumors [40,41]. Several reviews on the preparation and application of SELPs are available [30,37,38].
To increase cell-adhesive ability of silk fibroin for practical use as biomaterials, partial collagen and fibronectin sequences were inserted into silk fibroin from B. mori silkworm [42]. The recombinant silk proteins were produced by transgenic B. mori silkworm [42]. Films made from the recombinant silk proteins, especially the recombinant silk with [TGRGDSPAS]8, had six-fold higher activity than the original silk fibroin [42].
Silk fibroin from wild silkworm Anaphe has a much simpler amino acid composition, 60 mol% Ala and 30% Gly, in comparison to B. mori silkworm silk fibroin [43-46]. Anaphe silk fibroin may be a suitable candidate to design silkworm silk fibroin-mimetic recombinant proteins. Fusion proteins of silk fibroin from Anaphe and cell-binding motifs have been designed and synthesized using E. coli, to generate biomaterials with high cell adhesive ability when compared with collagen and Anaphe silk protein [47].
1.2.2 Spider variants
To control self-assembly of beta-sheet structures in silk, a spider silk sequence was modified to contain methionines adjacent to the polyalanine (beta sheet forming domain) sequence [32]. Modified spider silk, which was 15mer of [SGRGGLGGQGAGAAAAAGGAGQGGYGGLGSQGT] consensus derived from the spidroin I sequence of N. clavipes (15 × 2632.23 Da), was bioengineered to include arginyl-glycyl-aspartic acid (RGD) cell-binding domains to enhance cell adhesion [33]. Furthermore, an R5 peptide derived from the silaffin protein of the diatom Cylindrotheca fusiformis, which forms reproducible nanostructures and silica precipitation from silicic acid using species specific peptides known as silaffins, was added into the silk 15mer-RGD fusion proteins to generate nanocomposites for bone regeneration [34]. Dentin matrix protein 1 (CDMP1) and the silk 15mer were combined via genetic engineering to exploit the self-assembly and material properties of silk proteins with controlled hydroxyapatite formation from CDMP1 [35]. Also, hydrophilic [SQGGYGGLGSQGSGRGGLGGQT] and hydrophobic [SGAGAAAAAGGAGT] blocks (consensus sequence derived from the spidroin I of N. clavipes) were combined and cloned with different hydrophilic/hydrophobic block ratios [36]. The structure and morphology of these amphiphilic block copolymers was determined and depended on the number of hydrophobic blocks [36]. For new gene delivery systems, silk-based amphiphilic block copolymers with poly(l-lysine) [48] and RGD motifs have been developed to enhance the transfection efficiency via integrin-mediated endocytosis [49]. These designs can be extended to further control targeting, size, stability and related needs for gene delivery. The recombinant silk-like polymers mentioned herein have demonstrated utility as advanced highly tailored or designed biomaterials with different features – from composite material systems to gene delivery.
1.3. Advantages of silk proteins as biomaterials for drug delivery
Delivery of bioactive molecules and drugs in slow, sustained, controlled release formats is desirable for many applications. In addition, such delivery system would be advantageous if they were biodegradable, biocompatible, and mechanically durable and could be prepared and processed under ambient aqueous conditions to avoid loss of bioactivity of the drugs to be delivered. Silks can help address these needs, due to the self-assembly, mechanical toughness, processing flexibility, biodegradability and biocompatibility, therein presenting considerable utility for a number of human therapeutic interventions. Table 2 lists silk protein-based drug or gene delivery systems. Comprehensive studies, both in-vivo and in-vitro, have demonstrated that silk is biocompatible and less inflammatory than other common biodegradable polymers such as poly(lactide) and collagen [49,50]. Another important attribute of silks is their processability into different material format, such as films, hydrogels, nanofibers, nanoparticles, and three-dimensional porous scaffold [8,9,51-57]. The ability to regulate the structure and morphology of silk proteins in an all-aqueous process render this family of structural proteins important candidates for drug delivery applications. The degradation rate can be adjusted by controlling the crystalline state (beta-sheet content) during processing, to regulate the release profile of bioactive molecules.
Table 2.
Silk protein-based drug/gene delivery systems
| Silk protein | Additional component into silk |
System | Bioactive molecules, drug/gene |
Refs | |
|---|---|---|---|---|---|
|
A. diadematus ADF-3, ADF-4 |
- | Microcapsule | - | 62 | |
|
|
|||||
| Reconstituted | Modified ADF-4 | - | Microsphere | - | 63 |
|
|
|||||
| spider silk | 6mer N. clavipes Spidroin I | Polylysine | Polyioncomplexe | Plasmid DNA | 48 |
|
|
|||||
| 6mer N. clavipes Spidroin I | Polylysine and RGD | Polyioncomplexe | Plasmid DNA | 49 | |
|
| |||||
| A. mylitta silk | - | Matrice | Bovine serum albumin (BSA), FITC-Insulin |
120,121 | |
|
|
|||||
| Silkworm silk | B. mori silk | Elastin | Hydrogel | Adenoviral vector, pDNA, mitotoxin, pantarin, theophylline, vitamin B12, cytochrome c |
29-31, 37-41 |
|
|
|||||
| B. mori silk | - | Scaffold, film | BMP-2 | 8 | |
|
|
|||||
| B. mori silk | - | Scaffold | Horseradish peroxidase (HRP) | 115 | |
|
|
|||||
| B. mori silk | - | Scaffold | Adenosine | 116,117 | |
|
|
|||||
| B. mori silk | - | Nerve conduits (tube) |
Nerve growth factor (NGF) | 118 | |
|
|
|||||
| B. mori silk | - | Scaffold | Insulin-like growth factor I (IGF-I) | 119 | |
|
|
|||||
| B. mori silk | - | Film | Adenosine | 122,123 | |
|
|
|||||
| B. mori silk | - | Film | Glucose oxidase, lipase, HRP | 124 | |
|
|
|||||
| B. mori silk | - | Electrospun nanofibers |
BMP-2 and/or hydroxyapatite | 127 | |
|
|
|||||
| B. mori silk | - | Nanoparticle | Curcumin (dye) | 128 | |
|
|
|||||
|
B. mori silk, A. mylitta silk |
- | Nanoparticle | VEGF | 129 | |
|
|
|||||
| B. mori sericin | - | Nanoparticle | Poloxamer | 130 | |
|
|
|||||
| B. mori silk | - | Microparticle | - | 131 | |
|
|
|||||
| B. mori silk | - | Microparticle | HRP | 132 | |
|
|
|||||
| B. mori silk | - | Microparticle | BMP-2, IGF | 133 | |
|
|
|||||
| B. mori silk | - | Microparticle, nanoparticle |
Tetramethylrhodamine conjugated BSA, tetramethylrhodamine conjugated dextran, rhodamine B |
134 | |
|
|
|||||
| B. mori silk | - | Microparticle | BMP-2, BMP-9, BMP-14 | 135 | |
|
|
|||||
| B. mori silk | poly(lactide-co- glycolic acid) |
Coating | HRP, tetramethylrhodamine- conjugated BSA |
112 | |
|
|
|||||
| B. mori silk | - | Coating | Rhodamine B, azoalbumin | 136 | |
|
|
|||||
| B. mori silk | - | Coating | Heparin, clopidogrel, paclitaxel | 137 | |
|
|
|||||
| B. mori silk | - | Coating | Adenosine | 138 | |
Spider silk-based block copolymers have been designed via genetic engineering and used for the delivery of bioactive molecules, such as genes and drugs (Table 1). In particular, recombinant silk proteins containing ligand molecules, selective delivery to target cells have been generated. In the case of drug delivery for cancer treatments, silk proteins containing tumor-homing peptides can be designed for specialized delivery using nanoparticles or polyplexes targeting tumor cells (Table 3). Other target delivery systems without ligands or tumor-homing peptides for direct introduction to tumor cells via injectable hydrogels or implant materials to release drugs or genes can also be considered.
Table 3.
Possible cancer treatments using silk-based delivery systems
| Route | Systems | Possible types of cancer |
|---|---|---|
| Nanoparticles | Myeloid leukemia | |
|
|
||
| Colon | ||
|
|
||
| Intravenous | Nanoparticles to contain tumor-homing peptides |
Breast |
|
|
||
| Smaller nanoparticles to penetrate Blood Brain Barrier |
Brain | |
|
| ||
| Direct injection or insertion | Injectable hydrogels or implants to release gene complexes or drugs |
Ovary Pancreas Prostate |
2. Biosynthesis of recombinant silk-like polymers
The synthesis of recombinant silk-like polymers can be broadly defined in two major steps: (1) design, construction, and cloning of the genes, and (2) expression and purification of the protein polymers (Figure 1).
Figure 1.
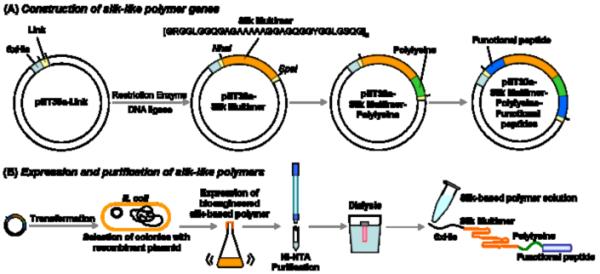
Schematic illustration of the construction of silk-like polymer encoding genes (A) and expression and purification of these silk-like polymers (B). Link: Link sequence reported in Ref [22]. In the construction of silk-like polymer encoding genes (A), restriction enzyme(s), calf intestinal alkaline phosphatase (CIP), and DNA ligase treatments are necessary. For gene delivery, the polylysine sequence is essential to form polyion complexes between the genes and the silk-like polymers. Functional peptides can be chosen based on the application with the silk-like polymers. (B) electroporation or chemical transformation can be used to transform pDNA into E. coli. Expression of the polymer is induced by addition of isopropyl β-D-1-thiogalactopyranoside (IPTG).
2.1. Construction of silk-like polymer genes
Synthetic genes encoding dragline silk from N. clavipes have been successfully constructed, cloned, and expressed [22,58]. The synthesis of these recombinant genes is based on the repetitive sequences found in native dragline silk genes [20]. The methods for construction of these repeats were previously reported, using smaller oligonucleotide repeats and subsequent multimerization [29]. Thus, the size and sequence of the protein generated can be controlled by the primary sequence synthesized as oligonucleotides (the building blocks). The use of synthetic gene technology to control silk protein size allows for the study on relationships between sequence length and structure-function, along with the study of novel compositions [29]. Partial cDNA sequences from N. clavipes encoding the proteins which are repetitive in nature, such as dragline protein MaSp1 sequence [GRGGLGGQGAGAAAAAGGAGQGGYGGLGSQG], have been cloned and used to construct synthetic genes [58]. Plasmid vectors such as pPT358, pPT317 and pET30a have been used to place the synthetic genes under the control of either bacteriophage T5 or T7 promoters and also to add [His]6 at the N-terminus of the recombinant protein to simplify purification by metal affinity chromatography [37,58,59]. Based on these recombinant DNA techniques, many block copolymers containing silk-like sequences have been synthesized by [29-36,47-49]. These variants include modified spider silks bioengineered to include RGD cell-binding domains to enhance cell adhesion, inclusion of molecular triggers to control of self-assembly, chimeric silk proteins for controlled mineralization, and silk block copolymers (Table 1) [29-36,47-49].
2.2. Expression and purification of silk-like polymers
Expression of the encoded proteins in E. coli has been successful [22,33,48,49,58]. E. coli BL21, JM109, SG13009 pREP4, and RY-3041 have been used as host systems, and yields of proteins were inversely correlated with the size of the synthetic genes. In general, expression levels obtained from synthetic silk genes are low, with yields of purified protein from 1 to 10 mg/L, representing usually less than 5% of the total protein in the cell, depending upon the size of the protein. However, the exception to these low yields appears in yeast, with 300 to 1000 mg/L in Pichia Pastoris encoding spider silks [60,61].
After protein expression, cells are harvested by centrifugation and the cell pellets are resuspended and lysed in denaturing buffer such as urea, guanidinium chloride, or guanidinium thiocyanate [5,33,48,49]. Purification of the expressed proteins has been performed by metal affinity chromatography, such as with nickel-nitrilotriacetic acid (Ni-NTA) and [His]6 systems. His-tag purification of the proteins is performed by addition of Ni-NTA agarose resin to the supernatant (batch purification) under denaturing conditions. After washing the column with denaturing buffer at pH 6.3, the proteins are eluted with denaturing buffer at pH 4.5 (without imidazole). Purified samples are extensively dialyzed against acetic buffer or NH4HCO3 solution, and then Milli-Q water [5].
3. Applications of recombinant spider silks to drug delivery
3.1. Reconstituted spider silk proteins
The reconstituted dragline silk proteins from the spider Araneus diadematus have been used to prepare microcapsules for drug delivery using self-assembly of the proteins at an emulsion interface [62]. These microcapsules were suggested to be useful to encapsulate small active ingredients, provided that the active ingredient does not impede the adsorption of the silk and/or that the encapsulation process does not alter the ingredient [62]. Microspheres of bioengineered spider silks, which were derived from ADF4 from A. diadematus, were formed by several methods such as dialysis and micromixing [63]. As a result of their material strength, biocompatibility, and the possibility of functionalization via recombinant protein techniques, spider silk microspheres may offer potential for the development of targeted drug delivery systems.
3.2. Spider silk-polycation block copolymers
In previous studies, a silk-based block copolymer was generated by combining spider silk consensus repeats with poly(l-lysine) for gene delivery [48]. Poly(l-lysine) is a cationic polymer that interacts with DNA through electrostatic interactions to assemble into polyelectrolyte complexes, is degraded in cells and has been used as an alternative to recombinant viruses for the delivery of pDNA into cells [64-66]. The silk-based block copolymers formed ion complexes with pDNA (Figure 2), and the sizes were controllable based on the polymer/DNA ratio or molecular weight of poly(l-lysine) bioengineered into the designs [48]. The sizes of pDNA complexes prepared at copolymer/pDNA ratio of 10 ranged from 310 to 590 nm. Also, the pDNA complexes of silk-based block copolymers with less than 30 lysines showed no cytotoxicity toward human embryonic kidney (HEK) cells. This study demonstrated the feasibility of bioengineering highly designed silk-based pDNA complexes for gene delivery systems; however, the transfection efficiency was too low to be useful for gene vectors.
Figure 2.
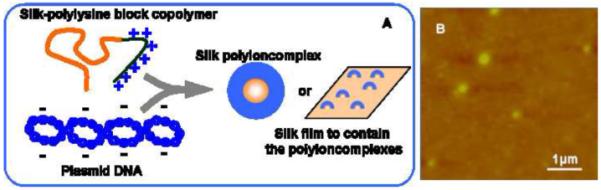
(A) Schematic of silk-based pDNA complexes and silk films containing the complexes. Silk-based polyioncomplexes are formed between negatively charged pDNA and positively charged polylysine sequence of silk-polylysine block copolymer. Silk-based polyioncomplexes and films to contain the complexes are prepared for pDNA delivery. (B) AFM height image of pDNA complexes of the recombinant silk. Reproduced with permission from Numata et al., Biomaterials; published by Elsevier, 2009.
3.3. Spider silk-polycation-functional peptide multiblock copolymers
Silk-based block copolymers are potentially useful candidates for nonviral gene vector, because various functional peptides such as cell binding motifs (RGD), cell penetrating peptides [67-69], signal peptides of virus [70], and/or tumor-homing peptides [71-75] can be added as ligands through recombinant DNA techniques, an important advantage of recombinant silk proteins over liposomes and synthetic polymers as gene delivery systems as shown in Figure 3. Silk-based block copolymers can contain several modules based on the molecular design for specific target delivery, and also regulate sizes, drug release rates, and zeta potentials of complexes of the copolymer and bioactive molecules. Several functional peptides are briefly reviewed, since they may be potentially useful for target gene/drug delivery systems (Table 4).
Figure 3.
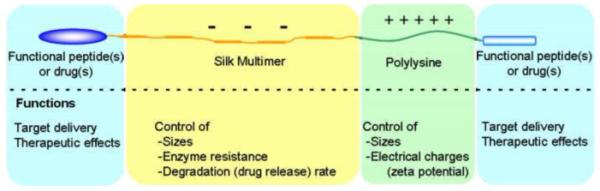
Schematic presentation of primary structure of silk-based block copolymer and functions of each module of the copolymer. Functional peptides can be added at the both ends of molecules for targeted delivery or therapeutic effects. Molecular weight and the secondary structures of silk multimer sequences can control sizes, enzyme resistance, degradation rates, and drug/gene release rates. Polylysine sequences affect sizes as well as surface charge (zeta potential) of the silk-based complexes.
Table 4.
Useful functional peptides for gene/drug delivery to cells
| Functional peptide | Amino acid sequence | Targeted tissue or function | Refs | |
|---|---|---|---|---|
| RGD cell-binding motif | RGD | Integrin receptor | 49,76-79 | |
|
| ||||
| CLT1 | CGLIIQKNEC | Clotted plasma | 75 | |
|
|
||||
| CLT2 | CNAGESSKNC | |||
|
|
||||
| F3 (HMGN2) | KDEPQERSARLSAKPAPPKPEPKP KKAPAKK |
Endothelial cells in tumor blood vessels and nucleolin |
72,73 | |
|
|
||||
| Tumor-homing peptides |
CGKRK | CGKRK | Neovasculature in tumor and heparan sulfates |
71,139 |
|
|
||||
| CDTRL | CDTRL | Neovasculature in tumor | 139 | |
|
|
||||
| CSRPRRSEC | CSRPRRSEC | Neovasculature in dysplastic skin | ||
|
|
||||
| Lyp-1 | CGNKRTRGC | Lymphatic vesselsa | 71,74 | |
|
|
||||
| AGR | CAGRRSAYC | Lymphatics of prostate cancesa | 74 | |
|
| ||||
| LSD | CLSDGKRKC | Melanoma lymphatica | 74 | |
|
| ||||
| REA | CREAGRKAC | Lymphatics of prostate cancesa | 74 | |
|
|
||||
| Tat | GRKKRRQRRRPPQG | HIV-1 Tat | 69 | |
|
|
||||
| Tp10 | AGYLLGKINLKALAALAKKIL | Transportan | 69 | |
|
|
||||
| pVEC | LLIILRRRIRKQAHAHSK | Murine sequence of the cell adhesion molecule vascular endothelial cadherin |
67 | |
|
|
||||
| Cell penetrating | ppTG1 | GLFKALLKLLKSLWKLLLKA | To bind nucleic acids and destabilize liposomes |
68,96 |
|
|
||||
| or | ppTG20 | GLFRALLRLLRSLWRLLLRA | 68 | |
|
|
||||
| cell membrane | K8 | YKAK(8)WK or K(8) | DNA carrier for gene therapy | 140 |
|
|
||||
| destabilizing peptides |
JTS1 | GLFEALLELLESLWELLLEA | Cell-membrane destabilizing peptide |
68,141,142 |
|
|
||||
| INF1 | GLFEAIAGFIENGWEGMIDGGGC | Originated from influenza virus | 141,142 | |
|
|
||||
| GALA | WEAALAEALAEALAEHLAEALA EALEALAAGGSC |
Destabilizing cell membrane and mediating transfection |
143 | |
|
|
||||
| KALA | WEAKLAKALAKALAKHLAKAL AKALKACEA |
Destabilizing cell membrane and mediating transfection | 68,144 | |
|
|
||||
| Virus-derived peptides |
gp64ss | MVSAIVLYVL LAAAAHSAFA | the N-terminal signal sequence derived from the baculovirus major envelope glycoprotein gp64 |
70 |
|
|
||||
| Capsid | - | Promoting cytosolic entry processes |
93 | |
MDA-MB-435 was used for breast cancer cells.
In the case of gene delivery, transfection of silk-based polymers that contain ligand molecules is expected via receptor-mediated endocytosis (Figure 4). The RGD sequence, arginyl-glycyl-aspartic acid, is known to selectively recognize and bind αvβ3 and αvβ5 integrins that are expressed on cell surfaces of certain cell types such as endothelial cells, osteoclasts, macrophages, and platelets [76-79]. The integrins are considered to be a class of transmembrane glycoproteins that interact with the extracellular matrix, and are exploited for cell-binding and entry by receptor-mediated endocytosis, a representative pathway for gene delivery [79]. RGD sequences are therefore a useful candidate as a ligand for gene vectors. Based on the properties of RGD, the biocompatible, biodegradable, and non-toxic cationic polymers with the addition of RGD were exploited to enhance transfection efficiency of silk-based gene vectors via the integrin-mediated endocytosis [49]. Globular complexes of these recombinant silks modified to contain RGD cell-binding motifs with pDNA were prepared for in-vitro gene delivery to HeLa and HEK cells. The pDNA complexes of recombinant silk protein with 30-lysine residues and 11 RGD sequences prepared at the ratio of number of amines/phosphates from pDNA (N/P) of 2, which were positively charged particles of approximately 186 nm in diameter, showed the highest efficiency to HeLa cells of the recombinant silks examined because of integrin-mediated transfection with 11 RGD sequences. However, recombinant silk proteins with few RGD sequences showed low transfection, suggesting that a few RGDs are not enough to be recognized by integrin receptors at the cell surface membrane. The position of RGD motif, at the N- or C-terminus of the recombinant silks, did not influence the transfection efficiency of the pDNA complexes.
Figure 4.
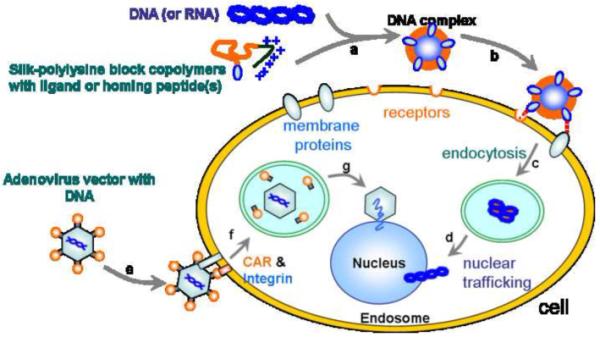
Model of receptor-mediated transfection via silk-based cationic block copolymers with ligands or functional peptides. (a) Formation of ion complexes between gene(s) and silk-polylysine block copolymers. (b) Binding of the complex to the cell via specific receptors or membrane proteins such as integrins. (c) Internalization via endocytosis and degradation of the polymers in lysosomes. (d) Trafficking of genes to the nucleus to initiate gene expression after the degradation of the complex. (e) Binding of adenovirus vector to the cell via the coxsackievirus and adenovirus receptor (CAR). (f) Internalization via the receptor-mediated endocytosis, involving interactions between integrins and RGDs in the adenoviral penton capsid protein. (g) Dismantling of capsid and acidification endosome, and subsequent docking at nuclear pore complexes and passage of DNA through nuclear pores via interaction of naked capsid with microtubules and dynein motors.
Tumor vasculature, which is important in cardiovascular diseases and cancer, is a target for anticancer therapy, however, most carriers for gene or drugs are relatively poor at specific delivery to specific cells. Selective targeting to tumor cells has been reported using several tumor-homing peptides (Table 4). Peptides include antibodies that recognize tumor-specific vascular signatures, identified by in-vivo screening of phage libraries, revealed extensive heterogeneity in tumor blood vessels and lymphatics [80-82]. The functions of tumor-homing peptides are summarized in other reviews [83].
Cell-penetrating peptides (CPPs) can be defined as short peptides that are able to efficiently penetrate cellular lipid bilayers or destabilize cellular membrane. Therefore, CPPs are useful candidates for new nonviral vectors of oligonucleotides, shRNA, double strand and plasmid DNA. Recent data of the CPP internalization mechanisms suggest that endocytosis contributes to the uptake, clathrin-dependent endocytosis and macropinocytosis have been suggested to mediate CPP translation [69,84-87]. There are also reports claiming that cellular uptake is independent of endocytotic pathways and occurs through transient pore formation [88-91]. Additionally, it has been suggested that CPPs simultaneously utilize different mechanisms of endocytosis and uptake occurs by an additional rapid translocation process [92]. Various CPPs have been reported, and some of them are listed in Table 4. One of the highest transfection efficiencies of pDNA complexes with cell-penetrating peptides was reported to be approximately 45-fold higher in comparison to the pDNA complex of polyethyleneimine at low DNA concentration (125 ng/mL) [68]. CPPs have been previously reviewed [83,93-95]. Recombinant silk proteins containing CPP (ppTG1) and their pDNA complexes were prepared for in-vitro gene transfection to HEK cells [96]. The recombinant silk protein containing a dimer of ppTG1 showed higher transfection efficiency, almost identical to lipofectamine 2000, in comparison with the other silk-based gene vectors [96]. Thus, significant improvements are anticipated upon addition of such peptides as an approach to enhance transfection efficiency.
Several virus-derived peptides, such as signal peptides and capsid proteins, have been studied for gene delivery [70,97,98]. Adenovirus infection is mediated predominantly by the penton and fiber capsid proteins. These proteins interact with cellular factors to coordinate key events that permit the passage of viral particles into the cell and subsequently to the nuclear periphery for gene transfer (Figure 4). Key to infection efficiency is the outer protein coat or capsid [99]. One of the earliest targeted systems, which utilized polylysine for DNA transport, produced high levels of receptor binding and internalization but relatively low transgene expression [100]. It was also suggested that the viral capsid itself promotes cytosolic entry in the absence of viral gene expression [101-103]. These functions of signal peptides and capsid proteins can be taken into consideration in the development of gene and drug transport systems with silks for improved delivery.
4. Applications of silkworm silk to drug delivery
4.1. Preparation of silkworm silk-based biomaterials
Silkworm silk has been used as biomedical sutures because of its biocompatibility and mechanical strength. However, virgin silk with the associated contaminant sericin proteins is a potential allergen causing a Type I allergic, due to upregulated IgEs in response to the sericins [104-109]. Once the sericins are properly removed, there is minimal response from the core fibroin structural proteins, as described earlier. The degradation product of silk fibroin proteins with beta-sheet structures from the action of proteases, such as alpha-chymotrypsin, has recently been reported, and no cytotoxicity was observed to neuron cells in-vitro [110]. Removal of sericin from virgin silkworm silk is therefore necessary to prepare non-allergic response and non-cytotoxic silk-based materials. Methods to extract and regenerate silk fibroin have been developed [7-13]: cocoons of B. mori silkworm silk are boiled for 30-60 min in an aqueous solution of 0.02 M Na2CO3 and then rinsed with MilliQ water to extract the sericin proteins. The extracted silk is then dissolved into 9.3M LiBr solution at 60°C, yielding a 20 wt% silk solution. The silk solution is dialyzed in MilliQ water using dialysis membrane with MWCO of less than 3,500 for more than 48 h. According to this basic method, approximately 7-8 wt% silk solutions are obtained. Also, silk sponges, which are obtained from silk solution after lyophilization, can be dissolved in hexafluoro-2-propanol (HFIP). Several silk-based biomaterials can be processed from silk solutions (Figure 5). For instance, aqueous-derived and HFIP-derived silk porous scaffolds have been prepared using salt leaching, gas forming, or freeze drying method [10-13]. Silk films are prepared by cast or layer-by-layer deposition of silk aqueous or HFIP solution with various concentrations [51,111,112]. Hydrogels of silk fibroin are formed via sol-gel transitions by sonication, vortexing, or the presence of acid and/or ions [53,54,113]. Nanofibers of silk fibroin can be prepared by electrospinning [55]. For silk-based materials, methanol can be used to induce beta-sheet structure in the materials, which makes them water-insoluble and slower-biodegradable [51]. Alternatively, water annealing has also been developed for such transitions, avoiding the use of any organic solvents [51].
Figure 5.
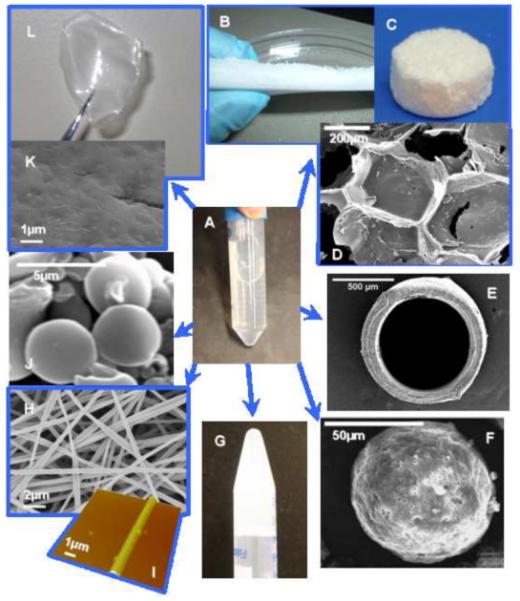
Silk-based biomaterials processed from silk solution (A). (B and C) Silk scaffolds, (D) SEM image of porous structure of scaffold, (E) SEM image of silk tube, (F) SEM image of polymeric microsphere coated with silk layers [112], (G) silk hydrogel, (H) SEM image of silk electrospun fibers, (I) AFM image of single electrospun fibers of silk, (J) SEM image of silk-based microspheres, (K) SEM image of the surface of silk films, (L) Silk film. Reproduced with permission from Wang et al., Biomaterials; published by Elsevier, 2007.
4.2. Implants, tubes, and scaffolds
Silk-based 3D scaffolds are attractive biomaterials for bone tissue regeneration because of their biocompatibility and mechanical properties [2,114]. The 3D silk fibroin scaffolds loaded with bone morphogenetic protein-2 (BMP-2) were successfully developed for sustained release of BMP-2 in order to induce human bone marrow stromal cells to undergo osteogenic differentiation when the seeded scaffolds were cultured in-vitro and in-vivo with osteogenic stimulants for 4 weeks [8]. Horseradish peroxidase (HRP) enzyme gradients were also immobilized on silk 3D scaffolds to prepare new functional scaffolds including regional patterning of the gradients to control cell and tissue outcomes [115]. Recently, adenosine release via silk-based implants to the brain has been studied for refractory epilepsy treatments [116,117]. Silk-based implants to release adenosine demonstrated therapeutic ability, including the sustained release of adenosine over a period of two weeks via slow degradation of silk, biocompatibility, and the delivery of predetermined dose of adenosine [116]. Nerve growth factor (NGF)-loaded silk fibroin nerve conduits have been studied to guide the sprouting of axons and to physically protect the axonal cone for peripheral nerve repair [118]. NGF release from the differently prepared silk fibroin-nerve conduits was prolonged over 3 weeks, while the total amount of NGF released depended on the drying procedures used in the preparation of the nerve conduits, such as air drying or freeze drying [118]. Silk fibroin scaffolds containing insulin-like growth factor I (IGF-I) were prepared for controlled IGF-I release in the context of cartilage repair [119]. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells (hMSCs) was observed, starting after 2 weeks and more strongly after 3 weeks [119]. Tropical tasar silkworm Antheraea mylitta silk-based 3D matrices were also evaluated for in-vitro drug release and for the study of cell-surface interactions [120,121]. The silk-based matrices contained two different model compounds, bovine serum albumin (66 kDa) and FITC-inulin (3.9 kDa), to characterize release profiles [120]. Silk fibroin protein blended calcium alginate beads resulted in prolonged drug release without initial burst for 35 days as compared to calcium alginate beads without silk fibroin [120]. Additionally, silk-based micromolded matrices supported a significant enhancement in cell attachment, spreading, mitochondrial activity, and proliferation with feline fibroblasts in comparison to polystyrene plates as controls [121]. These studies indicate the potential use of slow degrading silk fibroin 3D scaffolds and tubes loaded with bioactive molecules such as BMP, HRP, and adenosine for drug-releasing biomaterials.
4.3. Films
Silk films have been used with covalent decoration of functional peptides as implants for bone formation and drug delivery. For bone regeneration, BMP-2, RGD, and parathyroid hormone (PTH) can be directly immobilized on silk films using carbodiimide chemistry [8,10]. Differentiation of human bone marrow-derived stem cells cultured with the decorated silk films was induced by immobilized BMP-2. Also, the utility of silk films to promote long-term adenosine release from adenosine kinase deficient embryonic stem cells has been investigated [122,123]. These studies demonstrated that silk fibroin constitutes a suitable material for the directed differentiation of embryonic stem cells and for cell-mediated therapeutic release of adenosine. Silk films decorated with bioactive molecules could therefore be used for local drug delivery via direct implantation.
Silk films have also been used to promote stabilization of entrained molecules such as enzymes or therapeutic proteins [124]. Glucose oxidase, lipase, and HRP were entrapped in silk films over 10 months and significant activity if the enzymes were retained, even when stored at 37°C. Silk films result in stabilization of enzymes without the need for cryoprotectants, emulsifiers, covalent immobilization or other techniques. Further, the stabilization of enzymes in silk films is amenable to environmental distribution without refrigeration, and offers potential use in-vivo such as the delivery of bioactive molecules.
4.4. Nanofibers
Scaffolds for tissue engineering may mimic the structure and biological function of the extracellular matrix. The natural extracellular matrix is a composite material with fibrous collagens embedded in proteoglycans. The collagen fibers are organized in a 3D porous network that form hierarchical structures from nanometer length scale multi-fibrils to macroscopic tissue architectures [125,126]. The structures generated by electrospinning contain nanoscale fibers with microscale interconnected pores, resembling the topographic features of the extracellular matrix. Therefore, silk fibroin fiber scaffolds formed by electrospinning have potential as scaffolds. Silk fibroin fiber scaffolds containing BMP-2 and/or nanoparticles of hydroxyapatite (HAP) prepared via electrospinning have been studied for in-vitro bone formation from hMSCs [127]. The bioactivity of BMP-2 was retained after the aqueous-based electrospinning process, and the nanofibrous electrospun scaffolds with co-processed BMP-2 supported high calcium deposition and enhanced transcript levels of bone-specific markers, indicating that the electrospun scaffolds were an efficient delivery system for HAP nanoparticles and BMP-2.
4.5. Nanoparticles
Drug delivery systems via silkworm silk-based nanoparticles have been investigated [128-130]. Biologically derived silk fibroin-based nanoparticles (<100 nm) for local and sustained therapeutic curcumin delivery to cancer cells were fabricated by blending with noncovalent interactions to encapsulate curcumin in various proportions with pure silk fibroin or silk fibroin with chitosan [128]. Silk nanoparticles from silk fibroin solutions of domesticated B. mori and tropical tasar silkworm A. mylitta were stable, spherical, negatively charged, 150-170 nm in average diameter and showed no toxicity [129]. The silk nanoparticles were observed in the cytosol of murine squamous cell carcinoma cells, and the growth factor release from the nanoparticles showed significantly sustained release over 3 weeks, implying potential application as a growth factor delivery system [129]. The silk-based nanoparticles containing curcumin showed a higher efficiency against breast cancer cells and have potential to treat in-vivo breast tumors by local, sustained, and long-term therapeutic delivery. Silk sericin-poloxamer nanoparticles loaded with both hydrophilic and hydrophobic drugs were reported to be stable in aqueous solution, small size (100-110 nm) and rapidly taken up by cells [130].
4.6. Microspheres
Silk fibroin microspheres were processed using spray-drying, however, the sizes of the microspheres were above 100 μm, which is suboptimal for drug delivery [131]. Other methods to prepare silk microspheres include lipid vesicles as templates to efficiently load bioactive molecules for local controlled releases was reported recently [132]. The lipid is subsequently removed by methanol or NaCl, resulting in silk microspheres consisting beta-sheet structure and approximately 2 μm in diameter [132]. The silk microspheres loaded with HRP, used as a model drug, demonstrated controlled and sustained release of active enzyme over 10-15 days. Growth factor delivery via the silk microspheres in alginate gels was also reported to be more efficient in delivering BMP-2 than insulin-like growth factors, probably due to the sustained release of the growth factor [133]. Additionally, growth factors successfully formed linear concentration gradients in scaffolds to control osteogenic and chondrogenic differentiation of hMSCs during culture. This silk microsphere/polymeric scaffold system is an option for the delivery of multiple growth factors with spatial control for in-vitro and in-vivo 3D cultures. In more recent studies, a new mode to generate micro- and nanoparticles from silk, based on blending with polyvinyl alcohol was reported [134]. This method simplifies the overall process compared with lipid templating and provides high yield and good control over the feature sizes, from 300 nm to 20 μm, depending on the ratio of polyvinyl alcohol/silk used [134]. Silk fibroin microparticles containing BMP-2, BMP-9, and BMP-14 were prepared by dropwise addition of ethanol, and exhibited mean diameters of 2.7 ± 0.3 μm, encapsulation efficiencies of 67.9-97.7% depending on the type and amount of BMP loaded, and slow release of BMPs for 14 days [135].
4.7. Coatings
There is a critical need in medicine to develop simple and versatile methods to assemble robust, biocompatible, and functional biomaterial coatings that direct cell outcomes. Coatings of silk fibroin have been studied to provide interfaces for biomaterials [111,112,136,137]. The driving force of self-assembly to form coatings is hydrophobic and some electrostatic interactions. The flexibility of silk-based coatings has been investigated using an aqueous stepwise deposition process with B. mori silk solution, which can control the structure and stability of the silk fibroin in layer-by-layer films [111]. The thickness of one layer was reported to be around 10 nm when deposited from a 1 mg/mL silk aqueous solution. The secondary structure of silk fibroin in the coatings was regulated to control the biodegradation rate, which indicates that release of drugs from these coatings can be controlled via layer thickness, numbers of layers and secondary structure of the layers. The silk coatings have also been formed on poly(lactide-co-glycolic acid) (PLGA) and alginate microspheres for protein delivery [112]. The silk coatings on PLGA microspheres was reported to be ~1 μm and discontinuous, while those on alginate microspheres was ~10 μm thick and continuous. These coatings provide mechanically stable shells as well as a diffusion barrier to the encapsulated protein drugs [112]. Nanolayer coatings of silk fibroin to contain model compounds of small molecule drugs and therapeutically relevant proteins, such as rhodamine B and azoalbumin, have been prepared using the stepwise deposition method [136]. Multilayered silk-based coatings have been developed and used as drug carriers and delivery systems to evaluate vascular cell responses to heparin, paclitaxel, and clopidogrel [137]. Cell attachment and viability with human aortic endothelial cells and human coronary artery smooth muscle cells on the drug-incorporated silk coatings demonstrated that paclitaxel and clopidogrel inhibited smooth muscle cell proliferation and retarded endothelial cell proliferation [137]. The silk multilayers with heparin promoted human aortic endothelial cell proliferation while inhibiting human coronary artery smooth muscle cell proliferation, which was a desired outcome for the prevention of restenosis [137]. Solid adenosine powder coated with silk fibroin were investigated for local and sustained delivery of the anticonvulsant adenosine from encapsulated reservoirs [138]. Reservoir coating thickness was varied through manipulation of the silk coating solution concentration and the number of coatings applied. An increase in either coating thickness or crystallinity delayed adenosine burst, decreased average daily release rate, and increased the duration of release [138]. These reports demonstrate that the silk coating is an effective system for drug-eluting coatings, such as for stem applications, based on its useful micromechanical properties and biological outcomes.
5. Future perspectives
Silk-based biomaterials to deliver bioactive molecules, such as small drugs, proteins, and genes, are described in this review. The remarkable mechanical properties, versatile processing in an aqueous environment, biocompatibility, and controlled degradation suggest silks (both native as well as recombinant) are attractive biomaterials for controlled and sustained release, stabilization and delivery of bioactive molecules. Silk solutions can be morphed into a variety of biomaterial formats, including films, 3D porous scaffolds, hydrogels, micro- and nano-spheres, nanofibers, and coatings. The degradation rate of these biomaterials can be also controlled during processing, by the secondary structures. In addition to these useful properties, silk proteins derived from recombinant DNA technology can be bioengineered for highly tailored chemistries, greatly expanding the suite of options for targeted delivery. Targeted-delivery function is a significant factors in drug delivery, hence, these silk proteins can be prepared with functional sequences to hone to specific cells, tissues or organs, as a useful strategy for silk-based delivery systems with bioactive molecules. When combined with the novel features of the silk proteins themselves, including self-assembly, robust mechanical properties, water-based processing, controlled biodegradation and biocompatibility, silks offer a unique and versatile delivery platform for small molecules, large proteins, DNA and RNA. Hybrid or composite silk-based materials containing other biopolymers, have not been extensively studied, yet should provide applicable mechanical, thermal, and biological properties for not only drug/gene delivery but also for tissue engineering, medical imaging, and regenerative medicine.
Acknowledgements
The authors thank Carmen Preda (M.Sc.), Dean L. Glettig, Dr. Michael Lovett, Eleanor Pritchard, and Dr. Aneta J. Mieszawska for providing the images in Figure 5. This work has been supported by grants from the NIH (Tissue Engineering Resource Center), the NSF and the AFOSR (D.K.). K.N. was supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kaplan DL, Adams WW, Farmer B, Viney C, editors. Silk polymers: materials science and biotechnology; ACS Symp Ser 544; 1994. [Google Scholar]
- [2].Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- [3].Wong Po Foo C, Kaplan DL. Genetic engineering of fibrous proteins: spider dragline silk and collagen. Adv. Drug. Deliv. Rev. 2002;54:1131–1143. doi: 10.1016/s0169-409x(02)00061-3. [DOI] [PubMed] [Google Scholar]
- [4].Shewry PR, Tatham AS, Bailey AJ, editors. Elastomeric proteins: structures, biomechanical properties, and biological roles. Cambridge University Press; 2004. pp. 136–174. [Google Scholar]
- [5].Huemmerich D, Helsen CW, Quedzuweit S, Oschmann J, Rudolph R, Scheibel T. Primary structure elements of spider dragline silks and their contribution to protein solubility. Biochemistry. 2004;43:13604–13612. doi: 10.1021/bi048983q. [DOI] [PubMed] [Google Scholar]
- [6].Li M, Ogiso M, Minoura N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials. 2003;24:357–365. doi: 10.1016/s0142-9612(02)00326-5. [DOI] [PubMed] [Google Scholar]
- [7].Wang Y, Kim H-J, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- [8].Karageorgiou V, Tomkins M, Fajardo R, Meinel L, Snyder B, Wade K, Chen J, Vunjak-Novakovic G, Kaplan DL. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J. Biomed. Mater. Res. A. 2006;78:324–334. doi: 10.1002/jbm.a.30728. [DOI] [PubMed] [Google Scholar]
- [9].Tamada Y. New process to form a silk fibroin porous 3-D structure. Biomacromolecules. 2005;6:3100–3106. doi: 10.1021/bm050431f. [DOI] [PubMed] [Google Scholar]
- [10].Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J. Biomed. Mater. Res. 2001;54:139–148. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [11].Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- [12].Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- [13].Makaya K, Terada S, Ohgo K, Asakura T. Comparative study of silk fibroin porous scaffolds derived from salt/water and sucrose/hexafluoroisopropanol in cartilage formation. J. Biosci. Bioeng. 2009;108:68–75. doi: 10.1016/j.jbiosc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [14].Vollrath F, Knight DP. Liquid crystalline spinning of spider silk. Nature. 2001;410:541–548. doi: 10.1038/35069000. [DOI] [PubMed] [Google Scholar]
- [15].Jin H-J, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- [16].Vollrath F. Strength and structure of spider’s silks. J. Biotechnol. 2000;74:67–83. doi: 10.1016/s1389-0352(00)00006-4. [DOI] [PubMed] [Google Scholar]
- [17].Lazaris A, Arcidiacono S. Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science. 2002;295:472–476. doi: 10.1126/science.1065780. [DOI] [PubMed] [Google Scholar]
- [18].Denny M. The physical properties of spider’s silk and their role in the design of orb-webs. J. Exp. Biol. 1976;65:483–506. [Google Scholar]
- [19].Guerette PA, Ginzinger DG, Weber BH, Gosline JM. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science. 1996;272:112–115. doi: 10.1126/science.272.5258.112. [DOI] [PubMed] [Google Scholar]
- [20].Xu M, Lewis RV. Structure of a protein superfiber: spider dragline silk. Proc. Natl. Acad. Sci. U S A. 1990;87:7120–7124. doi: 10.1073/pnas.87.18.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hinman MB, Lewis RV. Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber. J. Biol. Chem. 1992;267:19320–19324. [PubMed] [Google Scholar]
- [22].Winkler S, Wilson D, Kaplan DL. Controlling beta-sheet assembly in genetically engineered silk by enzymatic phosphorylation/dephosphorylation. Biochemistry. 2000;39:12739–12746. doi: 10.1021/bi001335w. [DOI] [PubMed] [Google Scholar]
- [23].Bini E, Knight DP, Kaplan DL. Mapping domain structures in silks from insects and spiders related to protein assembly. J. Mol. Biol. 2004;335:27–40. doi: 10.1016/j.jmb.2003.10.043. [DOI] [PubMed] [Google Scholar]
- [24].Simmons A, Ray E, Jelinski LW. Solid-state 13C NMR of Nephila clavipes dragline silk establishes structure and identity of crystalline regions. Macromolecules. 1994;27:5235–5237. [Google Scholar]
- [25].Simmons AH, Michal CA, Jelinski LW. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science. 1996;271:84–87. doi: 10.1126/science.271.5245.84. [DOI] [PubMed] [Google Scholar]
- [26].Hayashi CY, Lewis RV. Molecular architecture and evolution of a modular spider silk protein gene. Science. 2000;287:1477–1479. doi: 10.1126/science.287.5457.1477. [DOI] [PubMed] [Google Scholar]
- [27].Arcidiacono S, Mello C, Kaplan D, Cheley S, Bayley H. Purification and characterization of recombinant spider silk expressed in Escherichia coli. Appl. Microbiol. Biotechnol. 1998;49:31–38. doi: 10.1007/s002530051133. [DOI] [PubMed] [Google Scholar]
- [28].Hayashi CY, Lewis RV. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 1998;275:773–784. doi: 10.1006/jmbi.1997.1478. [DOI] [PubMed] [Google Scholar]
- [29].Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic engineering of structural protein polymers. Biotechnol. Prog. 1990;6:198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- [30].Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv. Drug. Deliv. Rev. 2002;54:1075–1091. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- [31].Megeed Z, Haider M, Li D, O’Malley BW, Jr, Cappello J, Ghandehari H. In vitro and in vivo evaluation of recombinant silk-elastin-like hydrogels for cancer gene therapy. J. Control. Release. 2004;94:433–445. doi: 10.1016/j.jconrel.2003.10.027. [DOI] [PubMed] [Google Scholar]
- [32].Szela S, Avtges P, Valluzzi R, Winkler S, Wilson D, Kirschner D, Kaplan DL. Reduction-oxidation control of beta-sheet assembly in genetically engineered silk. Biomacromolecules. 2000;1:534–542. doi: 10.1021/bm0055697. [DOI] [PubMed] [Google Scholar]
- [33].Bini E, Wong Po Foo C, Huang J, Karageorgiou V, Kitchel B, Kaplan DL. RGD-functionalized bioengineered spider dragline silk biomaterial. Biomacromolecules. 2006;7:3139–3145. doi: 10.1021/bm0607877. [DOI] [PubMed] [Google Scholar]
- [34].Wong Po Foo C, Patwardhan SV, Belton DJ, Kitchel B, Anastasiades D, Huang J, Naik RR, Perry CC, Kaplan DL. Novel nanocomposites from spider silk-silica fusion (chimeric) proteins. Proc. Natl. Acad. Sci. U S A. 2006;103:9428–9433. doi: 10.1073/pnas.0601096103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang J, Wong C, George A, Kaplan DL. The effect of genetically engineered spider silk-dentin matrix protein 1 chimeric protein on hydroxyapatite nucleation. Biomaterials. 2007;28:2358–2367. doi: 10.1016/j.biomaterials.2006.11.021. [DOI] [PubMed] [Google Scholar]
- [36].Rabotyagova OS, Cebe P, Kaplan DL. Self-assembly of genetically engineered spider silk block copolymers. Biomacromolecules. 2009;10:229–236. doi: 10.1021/bm800930x. [DOI] [PubMed] [Google Scholar]
- [37].Nagarsekar A, Crissman J, Crissman M, Ferrari F, Cappello J, Ghandehari H. Genetic synthesis and characterization of pH- and temperature-sensitive silk-elastinlike protein block copolymers. J. Biomed. Mater. Res. 2002;62:195–203. doi: 10.1002/jbm.10272. [DOI] [PubMed] [Google Scholar]
- [38].Haider M, Megeed Z, Ghandehari H. Genetically engineered polymers: status and prospects for controlled release. J Control Release. 2004;95:1–26. doi: 10.1016/j.jconrel.2003.11.011. [DOI] [PubMed] [Google Scholar]
- [39].Haider M, Leung V, Ferrari F, Crissman J, Powell J, Cappello J, Ghandehari H. Molecular engineering of silk-elastinlike polymers for matrix-mediated gene delivery: biosynthesis and characterization. Mol. Pharm. 2005;2:139–150. doi: 10.1021/mp049906s. [DOI] [PubMed] [Google Scholar]
- [40].Greish K, Araki K, Li D, O’Malley BW, Jr, Dandu R, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike protein polymer hydrogels for localized adenoviral gene therapy of head and neck tumors. Biomacromolecules. 2009;10:2183–2188. doi: 10.1021/bm900356j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: From molecular definition to controlled gene expression. J. Control. Release. 2009;140:256–261. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yanagisawa S, Zhu Z, Kobayashi I, Uchino K, Tamada Y, Tamura T, Asakura T. Improving cell-adhesive properties of recombinant Bombyx mori silk by incorporation of collagen or fibronectin derived peptides produced by transgenic silkworms. Biomacromolecules. 2007;8:3487–3492. doi: 10.1021/bm700646f. [DOI] [PubMed] [Google Scholar]
- [43].Akai H, Nagashima T. Fine-structural characteristics of Anaphe cocoon filament. Int. J. Wild Silkmoth Silk. 1999;4:13–16. [Google Scholar]
- [44].Akai H, Nagashima T, Mugenyi G. Anaphe in Africa: Are they social insect? Int. J. Wild Silkmoth Silk. 1999;4:7–12. [Google Scholar]
- [45].Lucas F, Shaw JT, Smith SGC. Comparative studies of fibroins. I. The amino acid composition of various fibroins and its significance in relation to their crystal structure and taxonomy. J. Mol. Biol. 1960;2:339–349. doi: 10.1016/s0022-2836(60)80045-9. [DOI] [PubMed] [Google Scholar]
- [46].Warwicker JO. Comparative studies of fibroins. II. The crystal structures of various fibroins. J. Mol. Biol. 1960;2:350–362. doi: 10.1016/s0022-2836(60)80046-0. [DOI] [PubMed] [Google Scholar]
- [47].Tanaka C, Asakura T. Synthesis and characterization of cell-adhesive silk-like proteins constructed from the sequences of Anaphe silk fibroin and fibronectin. Biomacromolecules. 2009;10:923–928. doi: 10.1021/bm801439t. [DOI] [PubMed] [Google Scholar]
- [48].Numata K, Subramanian B, Currie HA, Kaplan DL. Bioengineered silk protein-based gene delivery systems. Biomaterials. 2009;30:5775–5784. doi: 10.1016/j.biomaterials.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Numata K, Hamasaki J, Subramanian B, Kaplan DL. Gene delivery mediated by recombinant silk proteins containing cationic and cell binding motifs. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Meinel L, Karageorgiou V, Hofmann S, Fajardo R, Snyder B, Li C, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J. Biomed. Mater. Res. A. 2004;71:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- [51].Jin H-J, Park J, Karageorgiou V, Kim U-J, Valluzzi R, Cebe P, Kaplan DL. Water-stable silk films with reduced beta-sheet content. Adv. Funct. Mater. 2005;15:1241–1247. [Google Scholar]
- [52].Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786–92. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- [53].Matsumoto A, Chen J, Collette AL, Kim UJ, Altman GH, Cebe P, Kaplan DL. Mechanisms of silk fibroin sol-gel transitions. J. Phys. Chem. B. 2006;110:21630–21638. doi: 10.1021/jp056350v. [DOI] [PubMed] [Google Scholar]
- [54].Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3:1233–1239. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- [56].Zhang X, Reagan MR, Kaplan DL. Electrospun silk biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2009;61:988–1006. doi: 10.1016/j.addr.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- [58].Prince JT, McGrath KP, DiGirolamo CM, Kaplan DL. Construction, cloning, and expression of synthetic genes encoding spider dragline silk. Biochemistry. 1995;34:10879–10885. doi: 10.1021/bi00034a022. [DOI] [PubMed] [Google Scholar]
- [59].Bogush VG, Sokolova OS, Davydova LI, Klinov DV, Sidoruk KV, Esipova NG, Neretina TV, Orchanskyi IA, Makeev VY, Tumanyan VG, Shaitan KV, Debabov VG, Kirpichnikov MP. A novel model system for design of biomaterials based on recombinant analogs of spider silk proteins. J. Neuroimmune. Pharmacol. 2009;4:17–27. doi: 10.1007/s11481-008-9129-z. [DOI] [PubMed] [Google Scholar]
- [60].Cregg JM, Barringer KJ, Hessler AY, Madden KR. Pichia pastoris as a host system for transformations. Mol. Cell. Biol. 1985;5:3376–3385. doi: 10.1128/mcb.5.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cregg JM, Vedvick TS, Raschke WC. Recent advances in the expression of foreign genes in Pichia pastoris. Biotechnology (N Y) 1993;11:905–910. doi: 10.1038/nbt0893-905. [DOI] [PubMed] [Google Scholar]
- [62].Hermanson KD, Huemmerich D, Scheibel T, Bausch AR. Engineered microcapsules fabricated from reconstituted spider silk. Adv. Mater. 2007;19:1810–1815. [Google Scholar]
- [63].Lammel A, Schwab M, Slotta U, Winter G, Scheibel T. Processing conditions for the formation of spider silk microspheres. ChemSusChem. 2008;1:413–416. doi: 10.1002/cssc.200800030. [DOI] [PubMed] [Google Scholar]
- [64].Zauner W, Ogris M, Wagner E. Polylysine-based transfection systems utilizing receptor-mediated delivery. Adv. Drug. Deliv. Rev. 1998;30:97–113. doi: 10.1016/s0169-409x(97)00110-5. [DOI] [PubMed] [Google Scholar]
- [65].Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferring-PEI components, extended circulation in blood and potential for systemic gene delivery. Gene. Ther. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- [66].Oupicky D, Konak C, Dash PR, Seymour LW, Ulbrick K. Effect of albumin and polyanion on the structure of DNA complexes with polycation containing hydrophilic nonionic block. Bioconjug. Chem. 1999;10:764–772. doi: 10.1021/bc990007+. [DOI] [PubMed] [Google Scholar]
- [67].Elmquist A, Lindgren M, Bartfai T, Langel U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp. Cell Res. 2001;269:237–244. doi: 10.1006/excr.2001.5316. [DOI] [PubMed] [Google Scholar]
- [68].Rittner K, Benavente A, Bompard-Sorlet A, Heitz F, Divita G, Brasseur R, Jacobs E. New basic membrane-destabilizing peptides for plasmid-based gene delivery in vitro and in vivo. Mol. Ther. 2002;5:104–114. doi: 10.1006/mthe.2002.0523. [DOI] [PubMed] [Google Scholar]
- [69].Järver P, Langel K, El-Andaloussi S, Langel U. Applications of cell-penetrating peptides in regulation of gene expression. Biochem. Soc. Trans. 2007;35:770–774. doi: 10.1042/BST0350770. [DOI] [PubMed] [Google Scholar]
- [70].Mäkelä AR, Matilainen H, White DJ, Ruoslahti E, Oker-Blom C. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J. Virol. 2006;80:6603–6611. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 2002;8:751–755. doi: 10.1038/nm720. [DOI] [PubMed] [Google Scholar]
- [72].Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7444–7449. doi: 10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Laakkonen P, Akerman ME, Biliran H, Yang M, Ferrer F, Karpanen T, Hoffman RM, Ruoslahti E. Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9381–9386. doi: 10.1073/pnas.0403317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pilch J, Brown DM, Komatsu M, Järvinen TA, Yang M, Peters D, Hoffman RM, Ruoslahti E. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2800–2804. doi: 10.1073/pnas.0511219103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Oba M, Fukushima S, Kanayama N, Aoyagi K, Nishiyama N, Koyama H, Kataoka K. Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing alphavbeta3 and alphavbeta5 integrins. Bioconjug. Chem. 2007;18:1415–1423. doi: 10.1021/bc0700133. [DOI] [PubMed] [Google Scholar]
- [77].Kim WJ, Yockman JW, Lee M, Jeong JH, Kim Y-H, Kim SW. Soluble Flt-1 gene delivery using PEI-g-PEG-RGD conjugate for anti-angiogenesis. J. Controlled Release. 2005;106:224–234. doi: 10.1016/j.jconrel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- [78].Connelly JT, García AJ, Levenston ME. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- [79].Renigunta A, Krasteva G, König P, Rose F, Klepetko W, Grimminger F, Seeger W, Hänze J. DNA transfer into human lung cells is improved with Tat-RGD peptide by caveoli-mediated endocytosis. Bioconjug. Chem. 2006;17:327–334. doi: 10.1021/bc050263o. [DOI] [PubMed] [Google Scholar]
- [80].Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- [81].Pasqualini R, Ruoslahti E. Tissue targeting with phage peptide libraries. Mol. Psychiatry. 1996;1:423. [PubMed] [Google Scholar]
- [82].Ruoslahti E. Drug targeting to specific vascular sites. Drug Discov. Today. 2002;7:1138–1143. doi: 10.1016/s1359-6446(02)02501-1. [DOI] [PubMed] [Google Scholar]
- [83].Vivès E, Schmidt J, Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim. Biophys. Acta. 2008;1786:126–138. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- [84].Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- [85].Ferrari A, Pellegrini V, Arcangeli C, Fittipaldi A, Giacca M, Beltram F. Caveolae-mediated internalization of extracellular HIV-1 tat fusion proteins visualized in real time. Mol. Ther. 2003;8:284–294. doi: 10.1016/s1525-0016(03)00122-9. [DOI] [PubMed] [Google Scholar]
- [86].Holm T, Johansson H, Lundberg P, Pooga M, Lindgren M, Langel U. Studying the uptake of cell-penetrating peptides. Nat. Protoc. 2006;1:1001–1005. doi: 10.1038/nprot.2006.174. [DOI] [PubMed] [Google Scholar]
- [87].Lundberg P, Langel U. Uptake mechanisms of cell-penetrating peptides derived from the Alzheimer’s disease associated gamma-secretase complex. Int. J. Pept. Res. Ther. 2006;12:105–114. [Google Scholar]
- [88].Deshayes S, Gerbal-Chaloin S, Morris MC, Aldrian-Herrada G, Charnet P, Divita G, Heitz F. On the mechanism of non-endosomial peptide-mediated cellular delivery of nucleic acids. Biochim. Biophys. Acta. 2004;1667:141–147. doi: 10.1016/j.bbamem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- [89].Deshayes S, Heitz A, Morris MC, Charnet P, Divita G, Heitz F. Insight into the mechanism of internalization of the cell-penetrating carrier peptide Pep-1 through conformational analysis. Biochemistry. 2004;43:1449–1457. doi: 10.1021/bi035682s. [DOI] [PubMed] [Google Scholar]
- [90].El-Andaloussi S, Johansson HJ, Lundberg P, Langel U. Induction of splice correction by cell-penetrating peptide nucleic acids. J. Gene Med. 2006;8:1262–1273. doi: 10.1002/jgm.950. [DOI] [PubMed] [Google Scholar]
- [91].Abes S, Moulton H, Turner J, Clair P, Richard JP, Iversen P, Gait MJ, Lebleu B. Peptide-based delivery of nucleic acids: design, mechanism of uptake and applications to splice-correcting oligonucleotides. Biochem. Soc. Trans. 2007;35(Pt 1):53–55. doi: 10.1042/BST0350053. [DOI] [PubMed] [Google Scholar]
- [92].Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- [93].Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell. Mol. Life Sci. 2005;62:1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Endoh T, Ohtsuki T. Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Adv. Drug Deliv. Rev. 2009;61:704–709. doi: 10.1016/j.addr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- [96].Numata K, Kaplan DL. Silk-based gene delivery vehicles to contain membrane-destabilizing peptides: control of transfection by their secondary structure. in prepration. [Google Scholar]
- [97].Contreras JL, Smyth CA, Curiel DT, Eckhoff DE. Nonhuman primate models in type 1 diabetes research. ILAR J. 2004;45:334–342. doi: 10.1093/ilar.45.3.334. [DOI] [PubMed] [Google Scholar]
- [98].Medina-Kauwe LK. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Deliv. Rev. 2003;55:1485–1496. doi: 10.1016/j.addr.2003.07.010. [DOI] [PubMed] [Google Scholar]
- [99].Michael SI, Curiel DT. Strategies to achieve targeted gene delivery via the receptor-mediated endocytosis pathway. Gene Ther. 1994;1:223–232. [PubMed] [Google Scholar]
- [100].Wagner E, Zenke M, Cotten M, Beug H, Birnstiel ML. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc. Natl. Acad. Sci. USA. 1990;87:3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cotten M, Wagner E, Zatloukal K, Phillips S, Curiel DT, Birnstiel ML. High-efficiency receptor-mediated delivery of small and large (48 kilobase) gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc. Natl. Acad. Sci. USA. 1992;89:6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Curiel DT, Agarwal S, Wagner E, Cotten M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc. Natl. Acad. Sci. USA. 1991;88:8850–8854. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wagner E, Zatloukal K, Cotten M, Kirlappos H, Mechtler K, Curiel DT, Birnstiel ML. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc. Natl. Acad. Sci. USA. 1992;89:6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hollander DH. Interstitial cystitis and silk allergy. Med Hypotheses. 1994;43:155–156. doi: 10.1016/0306-9877(94)90142-2. [DOI] [PubMed] [Google Scholar]
- [105].Wen CM, Ye ST, Zhou LX, Yu Y. Silk-induced asthma in children: a report of 64 cases. Ann. Allergy. 1990;65:375–378. [PubMed] [Google Scholar]
- [106].Kurosaki S, Otsuka H, Kunitomo M, Koyama M, Pawankar R, Matumoto K. Fibroin allergy. IgE mediated hypersensitivity to silk suture materials. Nippon Ika Daigaku Zasshi. 1999;66:41–44. doi: 10.1272/jnms.66.41. [DOI] [PubMed] [Google Scholar]
- [107].Rossitch E, Jr, Bullard DE, Oakes WJ. Delayed foreign-body reaction to silk sutures in pediatric neurosurgical patients. Childs Nerv. Syst. 1987;3:375–378. doi: 10.1007/BF00270712. [DOI] [PubMed] [Google Scholar]
- [108].Dewair M, Baur X, Ziegler K. Use of immunoblot technique for detection of human IgE and IgG antibodies to individual silk proteins. J. Allergy Clin. Immunol. 1985;76:537–542. doi: 10.1016/0091-6749(85)90772-9. [DOI] [PubMed] [Google Scholar]
- [109].Zaoming W, Codina R, Fernández-Caldas E, Lockey RF. Partial characterization of the silk allergens in mulberry silk extract. J. Investig. Allergol. Clin. Immunol. 1996;6:237–241. [PubMed] [Google Scholar]
- [110].Numata K, Cebe P, Kaplan DL. Mechanism of Enzymatic Degradation of Beta Sheet Crystals. Biomaterials. 2010;31:2926–2933. doi: 10.1016/j.biomaterials.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang X, Kim HJ, Xu P, Matsumoto A, Kaplan DL. Biomaterial coatings by stepwise deposition of silk fibroin. Langmuir. 2005;21:11335–11341. doi: 10.1021/la051862m. [DOI] [PubMed] [Google Scholar]
- [112].Wang X, Wenk E, Hu X, Castro GR, Meinel L, Wang X, Li C, Merkle H, Kaplan DL. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials. 2007;28:4161–4169. doi: 10.1016/j.biomaterials.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yucel T, Cebe P, Kaplan DL. Vortex-induced injectable silk fibroin hydrogels. Biophys. J. 2009;97:2044–2050. doi: 10.1016/j.bpj.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Santin M, Motta A, Freddi G, Cannas M. In vitro evaluation of the inflammatory potential of the silk fibroin. J. Biomed. Mater. Res. 1999;46:382–389. doi: 10.1002/(sici)1097-4636(19990905)46:3<382::aid-jbm11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [115].Vepari CP, Kaplan DL. Covalently immobilized enzyme gradients within three-dimensional porous scaffolds. Biotechnol. Bioeng. 2006;93:1130–1137. doi: 10.1002/bit.20833. DL. [DOI] [PubMed] [Google Scholar]
- [116].Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: therapeutic potential for epilepsy. Biomaterials. 2008;29:3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Li T, Ren G, Kaplan DL, Boison D. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy Res. 2009;84:238–241. doi: 10.1016/j.eplepsyres.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Uebersax L, Mattotti M, Papaloizos M, Merkle HP, Gander B, Meinel L. Silk fibroin matrices for the controlled release of nerve growth factor (NGF) Biomaterials. 2007;28:4449–4460. doi: 10.1016/j.biomaterials.2007.06.034. [DOI] [PubMed] [Google Scholar]
- [119].Uebersax L, Merkle HP, Meinel L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J. Control. Release. 2008;127:12–21. doi: 10.1016/j.jconrel.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [120].Mandal BB, Kundu SC. Calcium alginate beads embedded in silk fibroin as 3D dual drug releasing scaffolds. Biomaterials. 2009;30:5170–5177. doi: 10.1016/j.biomaterials.2009.05.072. [DOI] [PubMed] [Google Scholar]
- [121].Mandal BB, Das T, Kundu SC. Non-bioengineered silk gland fibroin protein micromolded matrices to study cell-surface interactions. Biomedical Microdevices. 2009;11:467–476. doi: 10.1007/s10544-008-9252-x. [DOI] [PubMed] [Google Scholar]
- [122].Uebersax L, Fedele DE, Schumacher C, Kaplan DL, Merkle HP, Boison D, Meinel L. The support of adenosine release from adenosine kinase deficient ES cells by silk substrates. Biomaterials. 2006;27:4599–4607. doi: 10.1016/j.biomaterials.2006.04.025. [DOI] [PubMed] [Google Scholar]
- [123].Szybala C, Pritchard EM, Lusardi TA, Li T, Wilz A, Kaplan DL, Boison D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp. Neurol. 2009;219:126–135. doi: 10.1016/j.expneurol.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto FG, Kaplan DL. Stabilization of enzymes in silk films. Biomacromolecules. 2009;10:1032–1042. doi: 10.1021/bm800956n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem. J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nara M, Tasumi M, Tanokura M, Hiraoki T, Yazawa M, Tsutsumi A. Infrared studies of interaction between metal ions and Ca(2+)-binding proteins. Marker bands for identifying the types of coordination of the side-chain COO- groups to metal ions in pike parvalbumin (pI = 4.10) FEBS Lett. 1994;349:84–88. doi: 10.1016/0014-5793(94)00645-8. [DOI] [PubMed] [Google Scholar]
- [127].Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- [128].Gupta V, Aseh A, Rios CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomedicine. 2009;4:115–22. doi: 10.2147/ijn.s5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kundu J, Chung YI, Kim YH, Tae G, Kundu SC. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010;388:242–250. doi: 10.1016/j.ijpharm.2009.12.052. [DOI] [PubMed] [Google Scholar]
- [130].Mandal BB, Kundu SC. Self assembled silk sericin/poloxamer nanoparticles as nanocarriers of hydrophobic and hydrophilic drugs for targeted delivery. Nanotechnology. 2009;20:355101–355111. doi: 10.1088/0957-4484/20/35/355101. [DOI] [PubMed] [Google Scholar]
- [131].Hino T, Tanimoto M, Shimabayashi S. Change in secondary structure of silk fibroin during preparation of its microspheres by spray-drying and exposure to humid atmosphere. J. Colloid. Interface Sci. 2003;266:68–73. doi: 10.1016/s0021-9797(03)00584-8. [DOI] [PubMed] [Google Scholar]
- [132].Wang X, Wenk E, Matsumoto A, Meinel L, Li C, Kaplan DL. Silk microspheres for encapsulation and controlled release. J. Control. Release. 2007;117:360–370. doi: 10.1016/j.jconrel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- [133].Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wang X, Yucel T, Lu Q, Hu X, Kaplan DL. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials. 2010;31:1025–1035. doi: 10.1016/j.biomaterials.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bessa PC, Balmayor ER, Azevedo HS, Nürnberger S, Casal M, van Griensven M, Reis RL, Redl H. Silk fibroin microparticles as carriers for delivery of human recombinant BMPs, Physical characterization and drug release. J. Tissue Eng. Regen. Med. (online) doi: 10.1002/term.245. [DOI] [PubMed] [Google Scholar]
- [136].Wang X, Hu X, Daley A, Rabotyagova O, Cebe P, Kaplan DL. Nanolayer biomaterial coatings of silk fibroin for controlled release. J. Control. Release. 2007;121:190–199. doi: 10.1016/j.jconrel.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang X, Zhang X, Castellot J, Herman I, Iafrati M, Kaplan DL. Controlled release from multilayer silk biomaterial coatings to modulate vascular cell responses. Biomaterials. 2008;29:894–903. doi: 10.1016/j.biomaterials.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Pritchard EM, Szybala C, Boison D, Kaplan DL. Silk fibroin encapsulated powder reservoirs for sustained release of adenosine. J. Control. release (online) doi: 10.1016/j.jconrel.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hoffman JA, Giraudo E, Singh M, Zhang L, Inoue M, Porkka K, Hanahan D, Ruoslahti E. Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell. 2003;4:383–391. doi: 10.1016/s1535-6108(03)00273-3. [DOI] [PubMed] [Google Scholar]
- [140].Gottschalk S, Sparrow JT, Hauer J, Mims MP, Leland FE, Woo SL, Smith LC. A novel DNA-peptide complex for efficient gene transfer and expression in mammalian cells. Gene Ther. 1996;3:448–457. [PubMed] [Google Scholar]
- [141].Lear JD, DeGrado WF. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J. Biol. Chem. 1987;262:6500–6505. [PubMed] [Google Scholar]
- [142].Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J. Biol. Chem. 1994;269:12918–12924. [PubMed] [Google Scholar]
- [143].Subbarao NK, Parente RA, Szoka FC, Jr, Nadasdi L, Pongracz K. pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry. 1987;26:2964–2972. doi: 10.1021/bi00385a002. [DOI] [PubMed] [Google Scholar]
- [144].Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC., Jr Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36:3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]


