Abstract
Both the metabolic syndrome (MS) and type 2 diabetes mellitus (T2DM) confer an increased risk of coronary heart disease and cardiovascular disease (CVD). As MS and T2DM become more prevalent, there will be an associated rise in the number of individuals with or at risk for CVD and its related disorders. One major underlying cause of CVD in patients with MS or T2DM is a characteristic form of atherogenic dyslipidemia. This article reviews the evidence that demonstrates that individuals with MS or T2DM are at increased risk for CVD and highlights atherogenic dyslipidemia as an important risk factor for the development of CVD in these individuals. In an accompanying article, current pharmacotherapies available for the management of atherogenic dyslipidemia in individuals with MS or T2DM are discussed.
It is estimated that over 1 billion persons worldwide are overweight, more than 300 million of whom are clinically obese.1 In the United States, >60% of adults are overweight or obese, and the number of obese children and adolescents is dramatically increasing.2 Given its high and increasing prevalence, obesity is considered to be at pandemic levels. This has been attributed to an increasing worldwide adoption of energy-dense diets and sedentary lifestyles, probably as a consequence of urbanization and economic globalization. 1 Unfortunately, most health care systems are based on treating diseases caused by specific agents after they occur. What is really needed for the pandemic of obesity, the metabolic syndrome (MS), and type 2 diabetes mellitus (T2DM) is prevention based on changes in lifestyle. However, neither governments nor private insurers have typically provided funds for these approaches.
Obesity is an established risk factor for T2DM and a central component of MS. Not surprisingly, therefore, the increasing prevalence of obesity is being paralleled by similar increases in the number of persons with T2DM or MS. The global burden of diabetes, currently estimated at more than 171 million individuals (>2.8% of the world’s population), is predicted to increase to 366 million (4.4% of the world’s population) by 2030. 3 In the United States, approximately 20.8 million individuals (7.0% of the population) were estimated to have diabetes in 2005.4
MS is a defined cluster of cardiometabolic abnormalities that increases an individual’s risk of T2DM, coronaty heart disease (CHD), and cardiovascular disease (CVD). The core components of MS are glucose intolerance or diabetes, obesity, hypertension, and dyslipidemia—specifically hyper-triglyceridemia and low levels of high-density lipoprotein cholesterol (HDL-C). Its precise definition varies slightly between guidelines issued by expert groups such as the World Health Organization (WHO), the European Group for the Study of Insulin Resistance, the National Cholesterol Education Program Third Adult Treatment Panel (NCEP ATP III) and, more recently, the International Diabetes Federation (IDF) and the American Heart Association/National Heart, Lung, and Blood Institute. However, all definitions include obesity, particularly abdominal obesity, as a risk factor, with the IDF guidelines identifying obesity as a prerequisite (Table I). The NCEP ATP III guidelines define MS as the presence of ≥3 of the following abnormalities: waist circumference ≥102 cm (~40 in) in men or ≥88 cm (~35 in) in women, HDL-C <40 mg/dL in men and <50 mg/dL in women, triglycerides ≥150 mg/dL, elevated blood pressure (BP) (systolic BP ≥130 mm Hg or diastolic BP ≥85 mm Hg), and fasting blood glucose ≥110 mg/dL. It must be noted that the threshold for fasting glucose was reduced to ≥100 mg/dL due to a modification in the American Diabetes Association (ADA) criteria for impaired fasting glucose.
Table I.
Definitions of the Metabolic Syndrome
| OrgaNization/GRoup | Waist Circumference/BMI | Triglyceride Level | HDL-C Level | Blood pressure Value | Fasting Glucose Level |
|---|---|---|---|---|---|
| American Heart Association/ National Heart, Lung, and Blood Institute |
Men: 0≥102 cm (40 in) women: ≥88 cm (35 in) |
≥150 mg/dl(1.7 mmol/L) | Men: <40 mg/dL(1.03 mmol/L) Women: <50 mg/dL(1.29 mmol/L) |
≥130/85mm Hg or previous hypertension treament |
≥100 mg/dL |
| European Group for the Study of lnsulin Resistance |
Men: ≥94 cm (37 in) women: ≥80 cm (31 in) |
≥178 mg/dL(2.0 mmol/L) | <40 mg/dL(1.03 mmol/L) | ≥140/90 mm Hg | ≥1l0 mg/dL(6.1 mmol/L) |
| International Diabetes Federation |
Men: ≥94 cm Women: ≥80 cm |
≥150 mg/dL(l.7 mmol/L) | Men: <40 mg/dL(1.03 mmol/L) Women: <50 mg/dL (1.29 mmol/L) |
≥130/85 mm Hg or previous hypertension treament |
≥100 mg/dL(5.6 mmol/L) |
| National cholesterol Education Program Adult Treatment Panel III |
Men: ≥102 cm (40 in) Women: ≥88 cm (35 in) |
≥150 mg/dL(1.7 mmol/L) | Men: <40 mg/dL(1.03 mmol/L) Women: <50 mg/dL (1.29 mmol/L) |
≥130/85 mm Hg | ≥ll0 mg/dL(6.1 mmol/L) |
| World Health Organization | Waist to hip ratio Men: >0.90 Women: >0.85 or BMI > 30 kg/m2 |
≥150 mg/dL (1.7 mmol/L) | Men: <35 mg/dL (0.90 mmol/L) Women: <39 mg/dL(1.00 mmol/L) |
≥140/90 mm Hg | ≥1l0 mg/dL (6.1 mmol/L) |
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol.
The prevalence of MS in US adults is estimated to be approximately 22%5 to 34%6 using the NCEP ATP III definition and 39% when the IDF criteria are applied.6 In other countries, the IDF guidelines also identify a greater (approximately 80% increase) prevalence of MS than do the NCEP ATP III criteria.7,8 Over the past decade, the prevalence has increased significantly in US adults and adolescents, mainly because of rising rates of obesity—a phenomenon that seems likely to continue given current socioeconomic trends. An inevitable consequence of the obesity-driven increase in MS and diabetes will be a corresponding Increase in CVD incidence. Consequently, there is an urgent need for improved therapeutic strategies to manage CVD in patients with MS or T2DM.
Risk of CVD in Individuals With MS or T2DM
There is an abundance of evidence showing that individuals with MS or T2DM are at increased risk for CVD.
The Metabolic Syndrome
Several epidemiologic studies have identified an increased risk of CVD in individuals with MS,9–15 although MS does not necessarily predict an elevated CHD risk beyond the sum of its components 12,16 or provide better predictive power than the Framingham risk score.15 For example, in multivariate analyses, diabetes, BP, and HDL-C were all identified as significant predictors of CHD, but the presence of MS was not.12,16
Nevertheless, the concept of MS is useful for physicians in identifying patients at high risk for CVD. In a population-based cohort of 1209 Finnish men aged 42 to 60 years without CVD or diabetes at baseline, the risk of death from CHD or CVD over a 12-year follow-up period was significantly higher in individuals with MS.9 After adjustment for other major CVD risk factors (but not the components of MS), men with NCEP ATP III–defined MS had a relative risk (RR) of death from CHD of 2.9 (95% confidence interval [CI], 1.2–7.2) to 4.2 (95% CI, 1.6–10.8). Using WHO criteria for MS, the RR of death from CHD was 2.9 (95% CI, 1.2–6.8) to 3.3 (95% CI, 1.4–7.7). In addition, men with WHO-defined MS had an RR of death from CVD of 2.6 (95% CI, 1.4–5.1) to 3.0 (95% CI, 1.5–5.7).
In a subgroup analysis of 4483 individuals aged 35 to 70 years who participated in the Botnia study of T2DM in Finland and Sweden, the risk of CHD or stroke over a median follow-up of 6.9 years was 3-fold higher in individuals with WHO-defined MS (P<.001).10 The incidence of cardiovascular mortality was 12.0% and 2.2% in individuals with and without MS, respectively (P<.001). In a meta-analysis of 11 prospective cohort studies in European populations totaling almost 12,000 nondiabetic individuals aged 30 to 89 years, those with WHO-defined MS were found to have a hazard ratio (HR) of 2.26 (95% CI, 1.61–3.17) for death from CVD during a median follow-up of 8.8 years.11
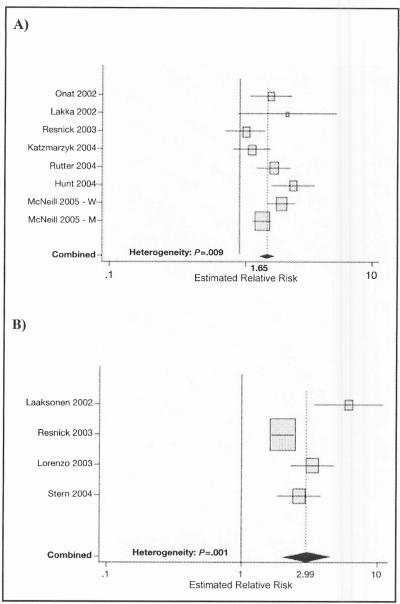
Epidemiologic studies in ethnically diverse populations in the United States have also identified an elevated risk of CVD in individuals with MS.12–15 In a subgroup analysis of 2815 individuals aged 25 to 64 years, mostly Mexican Americans, from the San Antonio Heart Study, MS at baseline was a significant predictor of cardiovascular mortality over a mean follow-up of 12.7 years. 14 In the Atherosclerosis Risk in Communities population-based study of more than 12,000 individuals without baseline diabetes or CVD, the adjusted HRs for CHD over a mean follow-up of 11 years for men and women with NCEP ATP III–defined MS were 1.46 (95% CI, 1.23–1.74) and 2.05 (95% CI, 1.59–2.64), respectively.15 Similarly, in a meta-analysis of worldwide data from studies published between 1998 and 2005, random effects estimates of the combined RRs of the MS as defined by NCEP ATP III criteria were 1.27 (95% CI, 0.90–1.78) for allcause mortality, 1.65 (95% CI, 1.38–1.99) for CVD, and 2.99 (95% CI, 1.96–4.57) for diabetes; for the smaller number of studies that used the most exact WHO definition, the fixed effects estimates were 1.37 (95% CI, 1.09–1.74) for all-cause mortality and 1.93 (95% CI, 1.39–2.67) for CVD (Figure lA).17
Figure 1.
Estimates of relative risk for cardiovascular disease (CVD) and diabetes reported in prospective studies in samples from the general population using definitions of the metabolic syndrome (MS) developed by the National Cholesterol Education Program (NCEP) (adapted from Ford, 200517) (A) Associations between MS (using the NCEP definition) and CVD. (B) Associations between MS (using the NCEP definition) and diabetes.
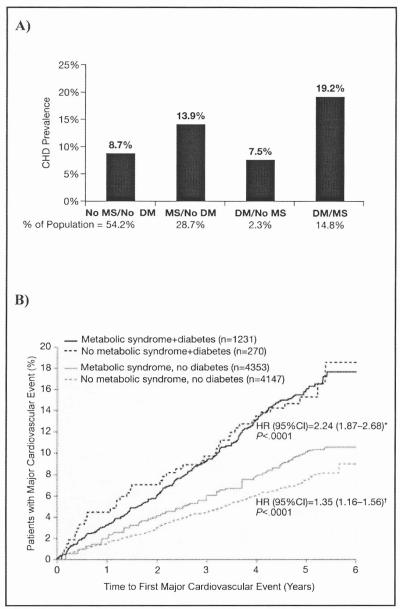
Given that MS, as defined by NCEP ATP III criteria, has been found to identify individuals with a high risk of T2DM (Figure 1B), 18,19 it is interesting to examine the cardiovascular risk when these 2 conditions are co-prevalent. Data from the Third National Health and Nutrition Examination Survey (NHANES III) in adults aged 50 years or older indicated that the prevalence of CHD was greatest in individuals with MS and diabetes.12 CHD prevalence was 19.2% in individuals with both MS and diabetes, 13.9% in those with MS but not diabetes, and only 7.5% in those with diabetes but not MS (in whom CHD prevalence was not incrementally increased vs those with neither condition) (Figure 2A). However, in a recent subanalysis of the Treating to New Targets (TNT) study of high-dose vs usual-dose atorvastatin therapy in patients with clinically evident CHD (55% of whom had MS), patients with diabetes with and without MS had similar CVD event rates over the 4.9-year follow-up period (irrespective of treatment assignment). Both patient groups had higher CVD event rates than those with MS but not diabetes and those without either the MS or diabetes (Figure 2B).20 The anomalous findings from these 2 studies are most likely due to the fact that TNT study participants had a prior major CVD event and were followed prospectively, while NHANES III was a crosssectional study that included individuals with and without a history of CVD. In addition, the proportion of individuals with diabetes and without MS in NHANES III was very small, probably representing the small number of patients with T2DM who are not insulin-resistant.
Figure 2.
(A) Age-adjusted prevalence of coronary heart disease (CHD) in the US population older than 50 years according the presence of the metabolic syndrome (MS) and diabetes mellitus (DM) (adapted from Alexander et al, 200312). (B) Prevalence of patients with major cardiovascular disease events in all patients in the Treating to New Targets study by MS and DM status (adapted from Deedwania et al, 200620). *Hazard ratio for patients with MS and diabetes vs patients without MS or diabetes. †Hazard ratio for patients with MS without diabetes vs patients without MS or diabetes.
The link between MS and increased CVD risk has also been established by post hoc analysis of data from largescale clinical intervention trials.19,21 In the West of Scotland Coronary Prevention Study, individuals with NCEP ATP III–defined MS at baseline had an increased risk of a coronary event vs those without MS during a mean follow-up of 4.9 years (HR, 1.76; 95% CI, 1.44–2.15).19 Similarly, results from the Scandinavian Simvastatin Survival Study and Air Force/Texas Coronary Atherosclerosis Prevention Study showed that placebo-treated patients with NCEP ATP III–defined MS had age-adjusted HRs of 1.5 (95% CI, 1.2–1.8) and 1.4 (95% CI, 1.04–1.9), respectively, for major coronary events during a median follow-up of 5.4 years.21 Finally, as noted above, significantly more participants in the TNT study who had MS had cardiovascular events, irrespective of treatment, compared with those who did not have MS.20
Overall, MS, as defined by the NCEP ATP III or WHO criteria, appears to almost double the risk of CVD,17 although it does not appear to predict cardiovascular mortality independent of its individual components.16 In this regard, it is worth noting that in a recent appraisal of MS, the ADA, in conjunction with the European Association for the Study of Diabetes, issued a joint statement raising concerns over the value of using MS as a CVD risk marker and recommended that clinicians should evaluate and treat all CVD risk factors without regard to whether a patient meets the criteria for diagnosis of MS.
Type 2 Diabetes Mellitus
Epidemiologic studies and clinical trials have demonstrated that diabetic individuals are at increased risk for CVD.22–25 In the Multiple Risk Factor Intervention Trial, a cohort study of approximately 350,000 men aged 35 to 57 years without diabetes and approximately 5000 men of the same ages with diabetes, the incidence of cardiovascular mortality over a mean follow-up of 12 years was 11.7% and 2.6% in diabetic and nondiabetic individuals, respectively.23 Similarly, the 5-year Helsinki Heart Study found that the incidence of myocardial infarction (MI) and cardiac death in diabetic individuals was more than twice that in individuals without diabetes (7.4% and 3.3%, respectively; P<.02),25 while a study in approximately 13,000 Danish individuals who were followed prospectively for 20 years showed that those with diabetes had a 2- to 3-fold increased risk of MI or stroke compared with those without diabetes.24
Data from a Finnish population based cohort of 2432 diabetic and nondiabetic individuals suggested that T2DM conferred as great a risk of CVD death as existing CHD.22 In this 7-year study, the CVD death rates per 100 person-years in diabetic individuals with no history of MI at baseline and nondiabetic individuals with previous MI at baseline were 2.6 and 2.5, respectively. However, several other studies have since indicated that the risk of CVD death in diabetic individuals may not be equivalent to that of individuals with prior MI.26,27 For example, in a 10-year study of 5934 men aged 52 to 74 years, the CVD death rate per 100 person-years was 2.12 for those with diabetes only, as compared with 3.35 in men with prior MI only and 7.78 in those with both diabetes and CHD.28 These differences may be related to the severity of associated risk factors within the study populations as well as the duration of diabetes.
Overall, it is generally accepted that T2DM increases the risk of CVD by 2- to 4-fold and that the risk is increased in the presence of prior CVD. The elevated risk of CVD in patients with MS or T2DM, together with obesity-driven increases in the prevalence of these conditions, is likely to give rise to an increased incidence of cardiovascular events in coming years. To combat this, preventive strategies targeting CVD risk factors in patients with MS or T2DM are becoming increasingly important.
It is critical to remember that because of the clustering of several major risk factors for CVD in persons with MS or T2DM, multiple preventive strategies may be required, often simultaneously. Of importance, lifestyle modifications, including reductions in dietary cholesterol, reductions in saturated and trans fatty acids, and increased physical activity, remain central to any therapeutic program. It is clear from the Finnish Diabetes Prevention Study and the Diabetes Prevention Program that modest weight loss (~5% over 2–3 years) and moderate increases in exercise can significantly reduce the incidence of diabetes in individuals with glucose intolerance.29,30 In addition, the Steno-2 study demonstrated dramatic reductions in CVD events in patients with T2DM who were treated with intensive lifestyle modification and pharmacologic agents.31
Yet, while acknowledging that the elevated cardiovascular risk in patients with MS and T2DM is multifactorial and therefore requires a multipronged approach to therapeutic intervention, one risk factor in particular—atherogenic dyslipidemia—may be particularly difficult to manage.
Atherogenic Dyslipidemia: An Important Risk Factor for CVD in Patients With MS or T2DM
Individuals with MS or T2DM exhibit a characteristic pattern of abnormalities in serum lipids: low levels of HDL-C and elevated triglycerides. This dyslipidemia is also characterized by elevations of apolipoprotein B (apoB) and a shift of the low-density lipoprotein (LDL) pool toward small, dense LDL (sdLDL) particles that are cholesteryl esterdepleted (Table II).32 Central abnormalities of dyslipidemia are increases in apoB-carrying lipoproteins and decreases in apolipoprotein A-I–carrying lipoproteins. It is believed that this complex dyslipidemia, which is termed atherogenic dyslipidemia, diabetic dyslipidemia, or dyslipidemia of insulin resistance, reflects underlying insulin resistance and plays a key role in the increased cardiovascular risk in patients with T2DM.
Table II.
Lipid Abnormalities in Type 2 Diabetes Mellitus (T2DM) and the Metabolic Syndrome(MS)
| Lipid | T2DM | MS |
|---|---|---|
| HDL-C | ↓ ↓ | ↓ |
| LDL-C | ↔ | ↔ |
| sdLDL | ↑ ↑ | ↑ |
| Triglycerides | ↑ ↑ | ↑ |
| apoB | ↑ ↑ | ↑ |
Abbreviations: apoB, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; sdLDL, small, dense low-density lipoprotein particles; ↑, increased; ↑↑,markedly increased; ↔, unchanged; ↓ decreased; ↓↓, markedly decreased. Data are from Brunzell and Ayyobi.32
The altered lipid levels associated with atherogenic dyslipidemia are individual and collective risk factors for CVD. Epidemiologic and clinical studies have clearly established that a low level of HDL-C is a strong independent risk factor for CHD (Figure 3).33 The magnitude of this association has been estimated as a 2% to 3% decrease in risk of CVD for every 1-mg/dL increase in HDL-C. In the UK Prospective Diabetes Study, a low level of HDL-C was found to be a significant risk factor for future CVD in diabetic patients.34 Despite some controversy, elevated levels of triglycerides—both fasting and nonfasting—also appear to be an independent risk factor for CHD.35–38 Evidence from epidemiologic studies suggests that the co-occurrence of low HDL-C and elevated triglyceride levels is a strong risk factor for CHD,39,40 while post hoc analyses of several studies41–43 have shown that patients With low HDL-C and elevated triglycerides have the highest rate of major coronary events. Whether an increased level of sdLDLs represents an independent risk factor remains somewhat controversial, but it is clearly associated with an increase in CHD.44 Based on in vitro and tissue culture experiments, sdLDL particles may be more atherogenic than large, less dense LDL-C particles due to a greater susceptibility to oxidation prior to macrophage uptake and a higher affinity for arterial wall proteoglycans. There is also evidence that smaller apoB lipoproteins can cross the endothelial cell layer more readily than larger members of this atherogenic class. However, all apoB-carrying lipoproteins, with the possible exception of nascent chylomicrons, are atherogenic.
Figure 3.
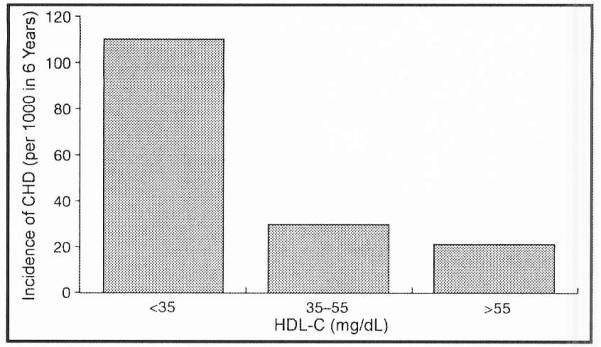
Incidence of coronary heart disease (CHD) per 1000 persons over a period of 6 years according to the high-density lipoprotein cholesterol (HDL-C) level (adapted from Assmann et al, 199645.
Conclusions
Patients with MS or T2DM are at an increased risk for CVD. A major underlying cause of this CVD risk is a characteristic form of atherogenic dyslipidemia. Treatment of this atherogenic dyslipidemia may help reduce the CVD risk that is associated with the increased prevalence of MS and T2DM. Current strategies available for the pharmacologic management of the atherogenic dyslipidemia found in these patients are discussed in an accompanying article. Novel strategies in clinical development will also be highlighted.
Footnotes
Disclosure: Paul MacCallum, PhD, a medical writer at Envision Pharma, was a paid consultant for development of this manuscript, funded by Pfizer Inc.
References
- 1.World Health Organization [Accessed September 16, 2005];Obesity and Overweight: World Health Organization global strategy on diet, physical activity and health fact sheet. 2003 http://www.who.int.
- 2.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331:166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association [Accessed September 16, 2005];National Fact Sheet. 2005 Available at http://www.diabetesorg/diabetes-statisticsjsp. [Google Scholar]
- 5.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 7.Athyros VG, Ganotakis ES, Elisaf M, et al. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin. 2005;21:1157–1159. doi: 10.1185/030079905x53333. [DOI] [PubMed] [Google Scholar]
- 8.Harzallah F, Alberti H, Ben Khalifa F. The metabolic syndrome in an Arab population: a first look at the new International Diabetes Federation criteria. Diabet Med. 2006;23:441–444. doi: 10.1111/j.1464-5491.2006.01866.x. [DOI] [PubMed] [Google Scholar]
- 9.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middlee-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 10.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 11.Hu G, Qiao Q, Tuomilehto J, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–1076. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]
- 12.Alexander CM, Landsman PB, Teutsch SM, et al. NCEP-defined metabolic syndrome. diabetes. and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Hunt KJ, Resendez RG, Williams K, et al. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 15.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom J, Vallhagen E, Riserus U, et al. Risk associated with the metabolic syndrome versus the sum of its individual components. Diabetes Care. 2006;29:1673–1674. doi: 10.2337/dc06-0664. [DOI] [PubMed] [Google Scholar]
- 17.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzo C, Okoloise M, Williams K, et al. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26:3153–3159. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 19.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronaty Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 20.Deedwania P, Barter P, Carmena R, et al. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–928. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 21.Girman CJ, Rhodes T, Mercuri M, et al. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Am J Cardiol. 2004;93:136–141. doi: 10.1016/j.amjcard.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in non-diabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 23.Stamler J, Vaccaro O, Neaton JD, et al. Diabetes. other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 24.A1mdal T, Scharling H, Jensen JS, et al. The independent effect of type 2 diabetes mellinus on ischemic heart disease, stroke, and death: a population–based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 25.Koskinen P, Manttari M, Manninen V, et al. Coronary heart disease incidence in NIDDM patients in the Helsinki Heart Study. Diabetes Care. 1992;15:820–825. doi: 10.2337/diacare.15.7.820. [DOI] [PubMed] [Google Scholar]
- 26.Lee CD, Folsom AR, Pankow JS, et al. Cardiovascular events in diabetic and non-diabetic adults with or without history of myocardial infarction. Circulation. 2004;109:855–860. doi: 10.1161/01.CIR.0000116389.61864.DE. [DOI] [PubMed] [Google Scholar]
- 27.Evans JM, Wang J, Morris AD. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: cross sectional and cohort studies. BMJ. 2002;324:939–942. doi: 10.1136/bmj.324.7343.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wannamethee SG, Shaper AG, Lennon L. Cardiovascular disease incidence and mortality in older men with diabetes and in men with coronary heart disease. Healrt. 2004;90:1398–1403. doi: 10.1136/hrt.2003.026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laaksonen DE, Lindstrom J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 31.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 32.Brunzell JD, Ayyobi AF. Dyslipidemia in the metabolic syndrome and type 2 diabetes mellitus. Am J Med. 2003;115(suppl 8A):24S–28S. doi: 10.1016/j.amjmed.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Chapman MJ. Therapeutic elevation of HDL-cholesterol to prevent atherosclerosis and coronary heart disease. Pharmacol Ther. 2006;III:893–908. doi: 10.1016/j.pharmthera.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eberly LE, Stamler J, Neaton JD. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163:1077–1083. doi: 10.1001/archinte.163.9.1077. [DOI] [PubMed] [Google Scholar]
- 36.Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 37.Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 38.Tirosh A, Rudich A, Shochat T, et al. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med. 2007;147:377–385. doi: 10.7326/0003-4819-147-6-200709180-00007. [DOI] [PubMed] [Google Scholar]
- 39.Jeppesen J, Hein HO, Suadicani P, et al. Relation of high TG-Iow HOL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. An 8-year follow-up in the Copenhagen Male Study. Arterioscler Thromb Vasc Biol. 1997;17:1114–1120. doi: 10.1161/01.atv.17.6.1114. [DOI] [PubMed] [Google Scholar]
- 40.Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). Prospective Cardiovascular Munster study. Am J Cardiol. 1992;70:733–737. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- 41.Ballantyne CM, Olsson AG, Cook TJ, et al. Influence of low high–density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046–3051. doi: 10.1161/hc5001.100624. [DOI] [PubMed] [Google Scholar]
- 42.Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 43.The Bezafibrate Infarction Prevention (BIP) Study Group Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 44.Sacks FM, Campos H. Clinical review 163: cardiovascular endocrinology: low-density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab. 2003;88:4525–4532. doi: 10.1210/jc.2003-030636. [DOI] [PubMed] [Google Scholar]
- 45.Assmann G, Schulte H, von Eckardstein A, et al. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(suppl):S11–S20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]