Abstract
The mechanism of FGF23 action in calcium/phosphorus metabolism of patients with chronic kidney disease (CKD) was studied using a mathematical model and clinical data in a public domain. We have previously built a physiological model that describes interactions of PTH, calcitriol, and FGF23 in mineral metabolism encompassing organs such as bone, intestine, kidney, and parathyroid glands. Since an elevated FGF23 level in serum is a characteristic symptom of CKD patients, we evaluate herein potential metabolic alterations in response to administration of a neutralizing antibody against FGF23. Using the parameters identified from available clinical data, we observed that a transient decrease in the FGF23 level elevated the serum concentrations of PTH, calcitriol, and phosphorus. The model also predicted that the administration reduced a urinary output of phosphorous. This model-based prediction indicated that the therapeutic reduction of FGF23 by the neutralizing antibody did not reduce phosphorus burden of CKD patients and decreased the urinary phosphorous excretion. Thus, the high FGF23 level in CKD patients was predicted to be a failure of FGF23-mediated phosphorous excretion. The results herein indicate that it is necessary to understand the mechanism in CKD in which the level of FGF23 is elevated without effectively regulating phosphorus.
Keywords: FGF23, PTH, phosphate, calcium, chronic kidney disease
Introduction
Fibroblast growth factor-23 (FGF23) has recently been demonstrated to represent a critical circulating hormone involved in phosphorus metabolism.1 Since phosphorus metabolism is tightly linked to calcium homeostasis in organs such as bone and kidney, signaling interactions should exist between FGF23 and regulators of calcium metabolism including parathyroid hormone (PTH) and the active form of vitamin D (1,25-dihydroxyvitamin D; calcitriol).2 Thus, a traditional, physiological model with PTH and calcitriol needs to be rebuilt in accordance with the emerging role of FGF23 and its interacting molecules. To understand probable interactions among FGF23, PTH and calcitriol, we previously developed a minimum physiological model of calcium/phosphorus metabolism and investigated potential influences of FGF23 on the observable state variables such as the serum concentrations of PTH, calcitriol, calcium (Ca), and phosphorous (P), as well as the urinary excretion of Ca and P.3 In this study, we extended the model and evaluated the mechanism of FGF23-mediated regulation in chronic kidney diseases (CKD).
The FGF23 gene was identified by its mutations associated with autosomal dominant hypophosphatemic rickets (ADHR), which is an inherited phosphate wasting disorder.4 Thereafter, a variety of disorders resulting from gain or loss of FGF23 bioactivity have been reported.5 These disorders, which are caused by mutations in the genes that directly or indirectly interact with FGF23, include hyperphosphatemic familial tumoral calcinosis (HFTC), hereditary hypophosphatemic rickets with hypercalciuria (HHRH), autosomal recessive hypophosphatemic rickets (ARHR), and X-linked dominant hypophosphatemic rickets (XLH, HYP). CKD patients who need dialysis have very high levels of FGF23 in serum that are linked with increased rates of death.6 Little is known about functional significance of the elevated FGF123 level in CKD.
In this study, we examined whether the therapeutic reduction of FGF23 by the neutralizing antibody would modulate phosphorus balance of CKD patients. To this end, we first evaluated the levels of physiological variables such as the levels of PTH, calcitriol, FGF23, Ca, and P in serum as well as urinary outputs of Ca and P using clinical data. Since a glomerular filtration rate (GFR) is a good indicator of severity of CKD, data were processed as a function of GFR. We then employed the previously developed mathematical model for mineral metabolism, and conducted numerical simulations in response to the modulation of FGF23 by neutralizing antibody.
Materials and Methods
Clinical data
Clinical data such as the serum concentrations of FGF23, PTH, calcitriol, Ca, and P, as well as urinary outputs of Ca and P were obtained from published articles as previously described.7 We analyzed data in the following literature: data for healthy individuals;8,9 data for CKD;10–15 data for Tumor Induced Osteomalacia;8 data for Fibrous Dysplasia;16 and data for XLH.17 In brief, the individual points in literature were retrieved with the DIGITIZEIT software. To establish correlations between two of the chosen physiological variables, one variable was treated as an independent (driving) variable, sorted in an ascending order and then smoothed out with a sliding average of 3–5 consecutive values.18,19 Values larger than 1.5 times the interquartile range between 1st and 3rd quartiles (e.g. 500 pg/ml or larger for PTH) were considered outliers. The smoothed data sets were then partitioned into frequency classes with the help of the SPSS (version 16.0) software. For each class, means and their standard errors were computed and plotted.
Estimation of the relationship of the FGF23 level to other physiological variables
The FGF23 concentrations, reported in literature, considerably varied among available datasets, presumably caused by differential baseline levels or sensitivity variations among individual assays. To predict a quantitative relationship among the FGF23 level and other physiological variables, the reported FGF23 level was linearly modified:
| (1) |
in which [FGF23] = reported FGF23 level, [FGF23]AB = linearly modified FGF23 level, and A and B = two correction factors. Note that these correction factors are constant and they were chosen independently for each of the physiological variables such as the serum level of PTH and the urinary output of P. The “+” and “−” values of the factor B indicate positive and negative correlations to the FGF23 level, respectively. We applied the described modification in analyzing clinical data since the observed FGF23 variation was larger than others. Without this procedure, it was difficult to estimate a quantitative relationship of its concentrations to other variables.
Mathematical model and prediction of effects of FGF23 antibody
We previously developed a pair of metabolism models of calcium and phosphorus with and without including the predicted action of FGF23.3,20 In this study we considered an additional state variable, GFRf, as a multiplicative term pertaining to the calcium and phosphorus renal thresholds and the kidney production of calcitriol:
| (2) |
in which GFR0 and GFR = glomerular filtration rates in the control state and at any given degree of renal failure, respectively, and a factor k (>0) was chosen so as to fit the clinical data as described previously.7
To predict the effects of intravenous administration of a neutralizing antibody against FGF23, we numerically examined 5 different dosages for i.v. administration at 0.003, 0.01, 0.03, 0.1 and 0.3 mg/kg (dosage levels 1–5). These dosages corresponded to a clinical trial study being proposed for a dose-escalation study of KRN23 (Kyowa Hakko Kirin Pharma Inc.). A primary outcome measure of this Phase I clinical trial is a change in a serum phosphate level, and a single dose by intravenous or subcutaneous administration is planned. The initial target is X-linked hypophosphatemia but no clinical data regarding efficacy and side effects are available. To simulate a probable injection procedure, we assumed a form of a single, smoothed-out pulse. The rise in the antibody concentration was modeled using a Gaussian type diffusion profile with a period dependent on the distribution volume and cardiac output.
Results
Glomerular filtration rate (GFR) as an indicator in CKD patients
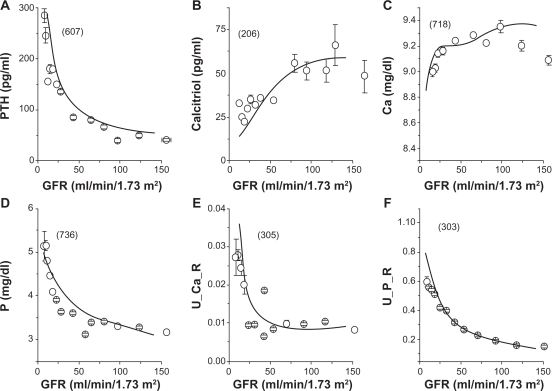
Since GFR serves as one of the best indicators of kidney function and a stage of kidney disease, we plotted physiological variables of CKD patients as a function of GFR in ml/min/1.73 m2. Figure 1 illustrated the levels of PTH (pg/ml), calcitriol (pg/ml), Ca (mg/dl), and P (mg/dl) in serum as well as urinary outputs of Ca and P expressed as a fraction of the glomerular loads. The numbers in the brackets in Figure 1 were the numbers of patients. The average and SEM values were obtained in each of the sampling bins. As GFR was normal above 90, the levels of PTH and P in serum as well as the fractions of urinary Ca and P outputs were lowered. On the contrary, the level of calcitriol in serum was higher as GFR increased.
Figure 1.
The glomerular filtration rate (GFR) and the selected physiological states in CKD patients. The numbers in brackets are the numbers of patients. For a description of data gathering and processing see text. A) PTH concentration in serum. B) Calcitriol concentration in serum. C) Ca concentration in serum. D) P concentration in serum. E) Urinary output of Ca expressed as a fraction of the glomerular Ca load (U_Ca_R). F) Urinary output of P expressed as a fraction of the glomerular P load (U_P_R).
Estimation of FGF23 levels in serum in CKD patients
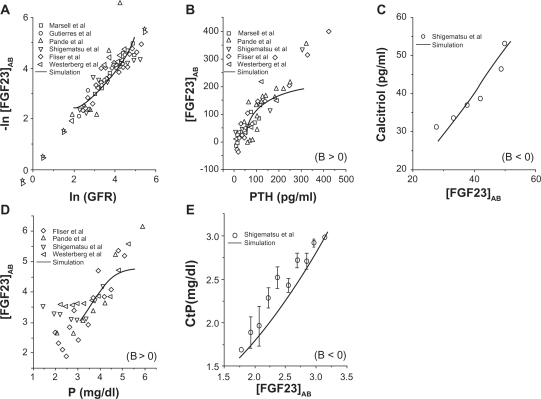
The relationships of the linearly modified FGF23 concentration in serum, [FGF23]AB, to the selected physiological variables in CKD patients were illustrated in Figure 2. First, a strong correlation was observed between loge(GFR) and a negative form of loge[FGF23]AB, indicating that the FGF23 level was sharply elevated in CKD patients with reduction in GFR. Second, an increase in [FGF23]AB was correlated to the levels of PTH, calcitriol, P in serum, and the renal threshold for P. Note that a positive correlation (i.e. B > 0) was observed for the levels of PTH and P in serum, while a negative correlation (i.e. B < 0) for the serum level of calcitriol and the renal threshold for P. Note that a majority of data points had the PTH level above 50 pg/ml, indicating a poor balance of mineral metabolism in CKD patients.
Figure 2.
Evaluation of FGF23 concentrations in serum in CKD patients. To accommodate variations among data sources, the modified FGF23 concentration in a form of [FGF23]AB = {[FGF23]−A}/B is displayed, where A and B are constants. Note that the relationship is a positive correlation with B > 0, while a negative correlation with B < 0. A) Logarithmic relationship between GFR and [FGF23]AB. B) Positive correlation between PTH and [FGF23]AB. C) Negative correlation between [FGF23]AB and calcitriol. D) Positive correlation between P in serum and [FGF23]AB. E) Negative correlation between [FGF23]AB and the renal threshold for P (CtP).
Linkage of FGF23 and P levels in serum
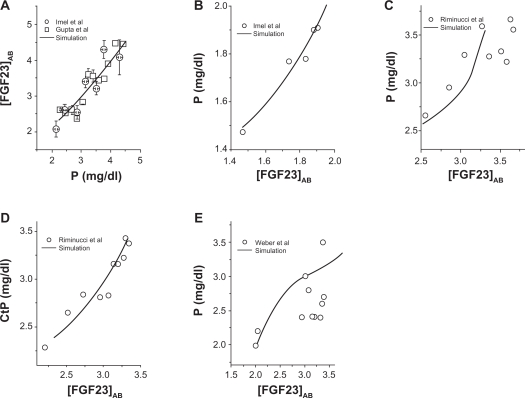
Since a primary known role of FGF23 is regulation of the serum level of P, we evaluated the dependence of P on the modified FGF23 level in healthy populations, and patients with Tumor Induced Osteomalacia, Fibrous Dysplasia, and XLH (Fig. 3). In all groups, a positive correction was observed between the level of P and the modified level of FGF23 in serum. Note that CKD data in Figure 2D showed the elevated P level up to 6 mg/dl, while the higher bound of the P level was ∼2 mg/dl (Tumor Induced Osteomalacia), 3.5 ∼4 mg/dl (Fibrous Dysplasia and XLH), and 4.5 mg/dl (healthy populations).
Figure 3.
Linkage of the FGF23 concentration in serum ([FGF23]AB) and the P concentration ([P]) in serum. A) [P] in healthy populations. B) [P] in patients with tumor induced osteomalacia. (C) and (D) [P] and the renal threshold for P in patients with Fibrous Dysplasia. E) [P] in patients with XLH.
Predicted effects of the antibody specific to FGF23
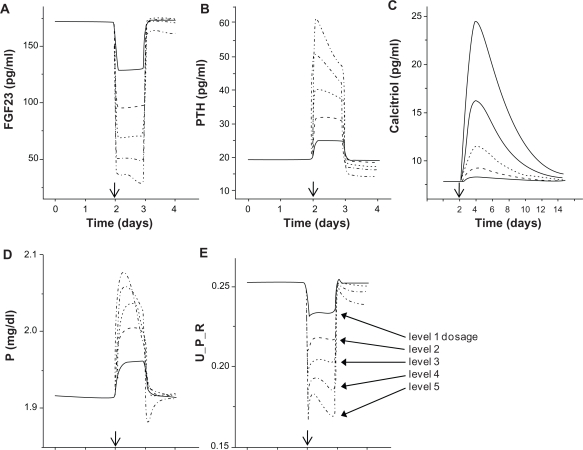
Although the observed increase of FGF23 in CKD is apparently a physiological response to hyperphosphatemia, the use of FGF23 antibody is suggested for transplanted hypophosphatemic patients of CKD with a high level of FGF23.21 In response to intravenous administration of the antibody specific to FGF23, we evaluated the predicted changes in the serum levels of PTH, calcitriol, and P as well as a normalized urinary output of P. The prediction is also useful for a patient with a primary increase in FGF23 secretion such as in XLH. The initial conditions were: FGF23 = 170 pg/ml, PTH = 20 pg/ml, calcitriol = 8 pg/ml, and P = 1.92 mg/dl. Five different dosages were employed as indicated in the Materials and Methods section (dosage levels 1–5).
The results showed that antibody injection immediately reduced the level of FGF23 in a dosage dependent manner, but the level was restored to its original concentration in 1 hr except for the case with the highest antibody concentration (Fig. 4). In concert with this alteration in the FGF23 concentration, the levels of PTH, calcitriol, and P in serum were elevated. Interestingly, the transient responses presented their own characteristics. The PTH level was elevated for 1 hr and then stabilized at the level below the original concentration. The level of calcitriol had a longer response time than that of PTH, and it took 10–14 hrs to reach to the original level. The serum concentration of P exhibited an overshoot for the injection with the highest antibody concentration. In response to the lowered level of FGF23, the urinary output of P, expressed as a fraction of the P glomerular load, was transiently decreased for 1 hr. No increase of the urinary output of P was observed.
Figure 4.
Predicted effects of administration of the neutralizing antibody specific to FGF23. The administration was given on day 2 (indicated by the arrow), and five different dosages (levels 1 to 5) were employed. A) FGF23 concentration in serum. B) PTH concentration in serum. C) Calcitriol concentration in serum. D) P concentration in serum. E) Urinary output of P expressed as a fraction of the P glomerular load (U_P_R).
Discussion
Analysis of clinical data from patients with CKD clearly showed that GFR was a good overall indicator of examining physiological state variables such as the serum concentrations of PTH, calcitriol, FGF23, Ca, and P as well as urinary outputs of Ca and P. In a severe CKD state with GFR < 30 ml/min/1.73 m2, the serum levels of PTH and P were significantly elevated while the levels of calcitriol and Ca were lower than healthy individuals. These clinical data also revealed a negative correlation between loge(GFR) and loge(FGF23), indicating that CKD patients with low GFR presented an exponential increase of the FGF23 level in serum. Since CKD patients are hyperphosphatemic with a high level of P in serum, dietary restriction with low phosphorus is recommended. Furthermore, administrations of sevelamer carbonate22 and lanthanum carbonate23 have been clinically examined to reduce phosphorus burden. However, without using a quantitative physiological model it is difficult to evaluate the role of FGF23 in CKD.
In this study, model-based evaluations of the role of FGF23 in CKD were conducted using numerical simulations in response to neutralizing antibody against FGF23. Antibodies specific to FGF23 were originally developed to investigate the effects of FGF23 inhibition on the serum levels of P and calcitriol in normal mice as well as Hyp mice.24,25 In Hyp mice (hypophosphatemic mice, phenotypically similar to XLH), injections of FGF23 antibody ameliorated the rachitic bone phenotypes such as impaired longitudinal elongation, defective mineralization, and abnormal cartilage development, suggesting a novel therapeutic potential of the FGF23 antibodies for XLH. Although the serum level of P substantially differs in CKD and XLH, these two metabolic diseases exhibit a high serum level of FGF23. However, application of FGF23 antibody to CKD in our mathematical model did not result in removing phosphorous burden.
The present results from the computational simulations herein do not support the notion that the elevated level of FGF23 in serum in CKD is a direct cause of hyperphosphotemia. On the contrary, these simulations indicate that the high FGF23 level is not a cause but an outcome of an ineffective FGF23-mediated phosphorus removal. Our current working hypothesis is, therefore, that an exponential increase in the level of FGF23 in CKD is induced by the reduction in GFR for restoring normal phosphorus homeostasis. Although the increase in FGF23 in serum is linked with high rates of death, the results from this study suggest that a removal of FGF23 by administration of neutralizing antibody to FGF23 is not a proper therapeutic strategy for CKD. The exact mechanism of FGF23 regulation in CKD is, however, not known, and further basic and clinical investigations are necessary.
There are limitations in the mathematical model, since many molecular pathways in mineral metabolism are not completely determined. FGF23 is known to affect, for instance, multiple forms of sodium-phosphate co-transporters in kidney through interactions with Klotho and cognate FGF receptors,26 but the current mathematical model does not include individual molecular components as state variables. Furthermore, a phosphorous sensing mechanism, presumably by conducted osteocytes in bone, or a molecular communication link of intestine to kidney is yet to be clarified.
In summary, we presented computational analysis using a multiscale, physiological model and clinical data to understand calcium/phosphorus metabolism. The present model in this study extended the previous ones including the action of FGF23 and GFR as an additional state variable and clinical data from patients with CKD.3,20 Through further refinements of molecular interactions encompassing a multi-organ system, we expect that model-based simulations will be useful to predict efficacy of potential therapeutic strategies prior to clinical trials.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.White KE, Evans WE, O’Riordan JLH, et al. Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nature Genetics. 2000;26:345–8. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Gupta A, Quarles LD. Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr Opin Nephrol Hypertens. 2007;16:329–35. doi: 10.1097/MNH.0b013e3281ca6ffd. [DOI] [PubMed] [Google Scholar]
- 3.Yokota H, Raposo JF, Chen A, Jiang C, Ferreira HG. Evaluation of the role of FGF23 in mineral metabolism. Gene Regulation Systems Biol. 2009;3:131–42. doi: 10.4137/grsb.s2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu X, White KE. Fibroblast growth factor 23 and its receptors. Therapeutic Apheresis and Dialysis. 2005;9:308–12. doi: 10.1111/j.1744-9987.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.White KE, Larsson TL, Econs MJ. The role of specific genes implicatd as circulating factors involved in normal and disordered phosphate homeostasis: Frizzled related proteins-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocrine Rev. 2006;27:221–41. doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]
- 6.Seiler S, Heine GH, Fliser D. Clinical relevance of FGF-23 in chronic kidney disease. Kidney Int. 2009;76:S34–42. doi: 10.1038/ki.2009.405. [DOI] [PubMed] [Google Scholar]
- 7.Pires A, Adragao T, Pais MJ, Vinhas J, Ferreira HG. Inferring disease mechanisms from epidemiological data in chronic kidney disease: calcium and phosphorus metabolism. Nephron Clin Pract. 2009;112:c137–47. doi: 10.1159/000214208. [DOI] [PubMed] [Google Scholar]
- 8.Imel EA, Peacock M, Pitukcheewanont P, et al. Sensitivity of fibroblast growth factor 23 measurements in Tumor-Induced Osteomalacia. J Clin Endocrin Metab. 2006;91:2055–61. doi: 10.1210/jc.2005-2105. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrin Metab. 2004;89:4489–92. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 10.Marsell R, Grundberg E, Krajisnik T, et al. Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Euro J Endo. 2008;158:125–29. doi: 10.1530/EJE-07-0534. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nehrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 12.Pande S, Ritter CS, Rothstein M, et al. FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron Phys. 2006;104:23–32. doi: 10.1159/000093277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44:250–6. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Flier D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the mild to moderate kidney disease (MMKD) study. J Am Soc Nephrol. 2007;18:2601–8. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 15.Westerberg P, Kinde T, Wikstrom B, Ljunggren O, Stridsberg M, Larsson TE. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol Dial Transplant. 2007;22:3202–7. doi: 10.1093/ndt/gfm347. [DOI] [PubMed] [Google Scholar]
- 16.Riminucci M, Collins MT, Fedarko NS, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Inv. 2003;112:683–92. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–34. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 18.Hald A. Statistical theory with engineering applications Chapter 17. John Wiley & sons; 1960. p. 510. [Google Scholar]
- 19.Weiss N. Elementary Statistics. 5 ed. Boston: Addison Wesley; 2002. Descriptive measures; pp. 120–1. [Google Scholar]
- 20.Raposo JF, Sobrinho LG, Ferreira HG. A minimal mathematical model of calcium homeostasis. J Clin Endo Metab. 2002;87:4330–40. doi: 10.1210/jc.2002-011870. [DOI] [PubMed] [Google Scholar]
- 21.Komaba H, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010;77:292–8. doi: 10.1038/ki.2009.466. [DOI] [PubMed] [Google Scholar]
- 22.Ketteler M, Rix M, Fan S, et al. Efficacy and tolerability of sevelamer carbonate in hyperphosphatemic patients who have chronic kidney disease and are not on dialysis. Clin J Am Soc Nephrol. 2008;3:1125–30. doi: 10.2215/CJN.05161107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sprague SM, Asbboud H, Qiu P, Dauphin M, Zhang P, Finn W. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin J Am Soc Nephrol. 2008;4:178–85. doi: 10.2215/CJN.02830608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki Y, Tamada T, Kasai N, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23:1509–18. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 25.Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of Anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–88. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Inv. 2007;117:2684–91. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]