Abstract
A major cause of azole resistance in Candida albicans is overexpression of CDR1, CDR2, and/or MDR1, which encode plasma membrane efflux pumps. To analyze the catalytic properties of these pumps, we used ACT1- and GAL1-regulated expression plasmids to overexpress CDR1, CDR2, or MDR1 in a C. albicans cdr1 cdr2 mdr1-null mutant. When the genes of interest were expressed, the resulting transformants were more resistant to multiple azole antifungals, and accumulated less [3H]fluconazole intracellularly, than empty-vector controls. Next, we used a GAL1-regulated dominant negative sec4 allele to cause cytoplasmic accumulation of post-Golgi secretory vesicles (PGVs), and we found that PGVs isolated from CDR1-, CDR2-, or MDR1-overexpressing cells accumulated much more [3H]fluconazole than did PGVs from empty-vector controls. The Kms (expressed in micromolar concentrations) and Vmaxs (expressed in picomoles per milligram of protein per minute), respectively, for [3H]fluconazole transport were 0.8 and 0.91 for Cdr1p, 4.3 and 0.52 for Cdr2p, and 3.5 and 0.59 for Mdr1p. [3H]fluconazole transport by Cdr1p and Cdr2p required ATP and was unaffected by carbonyl cyanide 3-chlorophenylhydrazone (CCCP), whereas [3H]fluconazole transport by Mdr1p did not require ATP and was inhibited by CCCP. [3H]fluconazole uptake by all 3 pumps was inhibited by all other azoles tested, with 50% inhibitory concentrations (IC50s; expressed as proportions of the [3H]fluconazole concentration) of 0.2 to 5.6 for Cdr1p, 0.3 to 3.1 for Cdr2p, and 0.3 to 3.1 for Mdr1p. The methods used in this study may also be useful for studying other plasma membrane transporters in C. albicans and other medically important fungi.
Candida albicans is a major cause of serious infections in immunocompromised patients. Azole antifungals are widely used to treat C. albicans infections, but resistance to this class of drugs has been reported frequently (40). The ability of C. albicans to pump azoles out of the cell is an important drug resistance mechanism, and several groups have shown that the most important azole efflux pumps in the plasma membrane of C. albicans are the ATP-binding cassette (ABC) transporters Cdr1p and Cdr2p and the major facilitator superfamily (MFS) transporter Mdr1p (40). A great deal is now known about transcriptional regulation of the genes encoding these pumps (i.e., CDR1, CDR2, and MDR1), but less is known about these pumps' catalytic properties (3–8, 10, 12, 13, 27, 28, 30, 47, 56–58). One reason for this is that the inaccessibility of the cytoplasmic face of the plasma membrane precludes direct examination of these transporters' abilities to pump azoles out of intact cells. To circumvent this problem, several groups have studied the ability of C. albicans or of Saccharomyces cerevisiae cells expressing the C. albicans genes of interest to pump fluorescent marker compounds out of the cell. These studies have provided important insights into the energetics and kinetics of these pumps, but the fluorescent compounds used in most of these studies are unrelated structurally or functionally to the azole antifungals (14, 19, 23, 36, 38, 50, 52). Moreover, the fact that C. albicans translates the codon CTG as leucine rather than serine complicates the interpretation of results obtained by heterologous expression of CTG-containing C. albicans genes in S. cerevisiae or other convenient hosts (17, 21, 39, 45, 48, 50).
In the 1990s, a new method was developed for studying plasma membrane efflux pumps (32, 41). The general strategy was to overexpress the transporter of interest in a temperature-sensitive S. cerevisiae sec6-4 mutant and to use post-Golgi secretory vesicles (PGVs) isolated from spheroplasts to study the transporter's catalytic properties. Since the membranes of PGVs and whole cells are oriented in opposite directions, transporters that pump substrates out of whole cells pump the same substrates into the lumens of PGVs. Thus, isolated PGVs are especially useful for studying the properties of plasma membrane efflux pumps, and this approach has been used to characterize the transport properties of multiple membrane transporters from yeast, fungi, and mammalian cells (9, 22, 32, 41, 42, 44). Of particular relevance to the present study is the work of Cannon et al. (2), who expressed C. albicans CDR1 in S. cerevisiae sec6-4 mutants and showed that PGVs isolated from these cells transported [3H]fluconazole into to their lumens in a time-dependent manner. This study established the feasibility of using PGVs to study azole transport by a C. albicans membrane transporter, but this approach has not been used to study the catalytic properties of C. albicans Cdr1p in detail or to study azole transport by other C. albicans efflux pumps.
In an earlier study from this laboratory, Mao et al. (29) showed that GAL1-regulated overexpression of a dominant negative allele [sec4(S28N)] of the essential post-Golgi secretion pathway gene SEC4 in C. albicans inhibited the growth and secretion of soluble aspartyl proteases from cells and caused PGVs to accumulate in the cytoplasm. These results suggested that it should be possible to generate PGVs with Cdr1p, Cdr2p, or Mdr1p in their membranes by overexpressing the C. albicans sec4(S28N) allele in C. albicans cells that also overexpress CDR1, CDR2, or MDR1 and to use these vesicles to study the catalytic properties of Cdr1p, Cdr2p, and Mdr1p. Therefore, in the present study, we (i) examined the abilities of recombinant Cdr1p, Cdr2p, and Mdr1p to transport fluconazole across the plasma membranes of intact C. albicans cells, (ii) developed a method for isolating functional PGVs from C. albicans, and (iii) used the resulting PGVs to study the catalytic properties of Cdr1p, Cdr2p, and Mdr1p.
MATERIALS AND METHODS
Strains and media.
Candida albicans SC5314 was from W. Fonzi (Georgetown University), and C. albicans DSY1050 (Δcdr1::hisG/Δcdr1::hisG Δcdr2::hisG/Δcdr2::hisG Δmdr1::hisG-URA3-hisG/Δmdr1::hisG) was from D. Sanglard (University of Lausanne, Lausanne, Switzerland). C. albicans DSY1050F is a ura3 derivative of C. albicans DSY1050; it was obtained by (i) selecting for growth of C. albicans DSY1050 on 5-fluorootic acid (FOA) and (ii) testing stable uridine auxotrophs for growth in minimal medium lacking uridine when they were transformed with plasmids encoding C. albicans URA3.
C. albicans was grown in YP medium (1% yeast extract, 2% peptone) or in minimal YNB medium (0.67% yeast nitrogen base without amino acids) containing 2% glucose, 2% galactose, or 2% raffinose. C. albicans transformed with plasmids conferring hygromycin B resistance were grown either in YP medium with 600 μg hygromycin B per ml and either 2% glucose, 2% galactose, or 2% raffinose or in YNB medium buffered to pH 7.0 with 0.15 M HEPES-NaOH plus 1,000 μg hygromycin B per ml and either 2% glucose, 2% galactose, or 2% raffinose. Plasmids were amplified in Escherichia coli DH5α in Luria-Bertani medium with 100 μg ampicillin per ml.
Plasmids and transformation methods.
PCR with oligonucleotides CDR1-5 and CDR1-3, CDR2-5 and CDR2-3, and MDR1-5 and MDR1-3 (Table 1), respectively, was used to amplify the CDR1, CDR2, and MDR1 open reading frames (ORFs) from C. albicans SC5314 genomic DNA and to fuse a 3× Flag epitope tag to each recombinant protein's C terminus. The resulting PCR products were ligated into the PacI and SacII restriction sites in the multicopy plasmid pYM70, which contains C. albicans ARS2, a synthetic hygromycin B resistance marker, and the C. albicans ACT1 promoter (GenBank accession number GU937092). Plasmid pYM71 is identical to pYM70 except that the ACT1 promoter was replaced by the C. albicans GAL1 promoter, which was amplified from C. albicans SC5314 genomic DNA by PCR with oligonucleotides GAL1pt-5 and GAL1pt-3 (Table 1). The accuracy of all plasmid constructions was verified by DNA sequencing. Plasmid pS28N is a multicopy plasmid that contains C. albicans URA3 and the dominant negative C. albicans sec4(S28N) allele under the control of the C. albicans GAL1 promoter (29).
Table 1.
Oligonucleotide sequences used to construct the pYM71 plasmid and to clone CDR1, CDR2, and MDR1 from C. albicans
| Name | Sequence (5′ → 3′) |
|---|---|
| GAL1pt-5 | GGTGGTGGTCTAGAGGGAAGATCTGATATTGACGAAG |
| GAL1pt-3 | GGTGGTGGTTAATTAAGGTATAACTCTTTCTTATAAAAATCGG |
| CDR1-5 | CGGACTTTAATTAAATGTCAGATTCTAAGATGTCGTCGCAAG |
| CDR1-3 | GCCTGACCGCGGTTATTTATCATCATCATCTTTATAATCAATATCATGATCTTTATAATCACCATCATGATCTTTATAATCTTTCTTATTTTTTTTCTCTCTGTTACCCTTTGG |
| CDR2-5 | CGGACTTTAATTAAATGAGTACTGCAAACACGTCTTTGTC |
| CDR2-3 | GCCTGACCGCGGTTATTTATCATCATCATCTTTATAATCAATATCATGATCTTTATAATCACCATCATGATCTTTATAATCTTTTTTCATCTTCTTTTCTCTATTACCTTTTGG |
| MDR1-5 | CGGACTTTAATTAAATGCATTACAGATTTTTGAGAGATAGTTTTG |
| MDR1-3 | GCCTGACCGCGGTTATTTATCATCATCATCTTTATAATCAATATCATGATCTTTATAATCACCATCATGATCTTTATAATCATTAGCATACTTAGATCTTGATCTCAACTT |
C. albicans DSY1050F was transformed by the lithium acetate method (54), and the resulting transformants were selected and expanded on appropriate media.
Antifungal susceptibility testing.
The NCCLS (now CLSI) M27-A broth microdilution method (34) was used to test C. albicans strains for antifungal susceptibility. The strains of interest were grown on YP medium plus glucose or on YP medium plus galactose, after which the cells were diluted to an optical density at 600 nm (OD600) of 5 × 10−5 in RPMI 1640 medium containing either glucose or galactose, 80 μg/ml uridine, 0.165 M morpholinepropanesulfonic acid (MOPS) (pH 7.0), and graded concentrations of fluconazole, voriconazole, posaconazole, miconazole, itraconazole, clotrimazole, or caspofungin. The presence or absence of visible growth was scored after incubation at 35°C for 48 h.
[3H]fluconazole accumulation by C. albicans cells.
Intracellular [3H]fluconazole was quantified as described by Sanglard et al. (46), with modifications. pACT1-CDR1-, pACT1-CDR2-, pACT1-MDR1-, or pACT1-transformed C. albicans cells were incubated in YP-glucose plus hygromycin B, and pGAL1-CDR1-, pGAL-CDR2-, pGAL1-MDR1-, or pGAL1-transformed C. albicans cells were incubated in YP-raffinose plus hygromycin B. The cells were harvested by centrifugation, washed in YNB-glucose or YNB-raffinose, and resuspended to an OD600 of 30 in the same medium containing [3H]fluconazole (final concentration, 0.05 μM; specific activity, 20 Ci/mmol; Amersham Biosciences). The cells were then shaken at 30°C; aliquots were removed at intervals and added to cold stop solution (the same medium plus 25 μM unlabeled fluconazole); the cells were collected on 0.45-μm-pore-size nitrocellulose filters; the filters were washed twice with cold stop solution; and [3H]fluconazole was quantified by liquid scintillation counting. Each result was expressed as the mean of duplicate measurements from three independent experiments.
Isolation and properties of post-Golgi secretory vesicles.
C. albicans DSY1050F transformed with pACT1-CDR1, pACT1-CDR2, pACT1-MDR1, or pACT1 was transformed again with pS28N, and the resulting transformants were expanded in YNB-glucose plus hygromycin B. To induce PGV accumulation, cells were washed in YNB-galactose and were then grown for 7 h in YNB-galactose plus hygromycin B at 30°C. Cell growth was stopped by the addition of NaN3 (10 mM), and the cells were washed in 10 mM Tris-HCl (pH 7.5)-5 mM NaN3, collected by centrifugation, and stored at −80°C.
Post-Golgi secretory vesicles were isolated as described by Ruetz and Gros (42), with modifications. The frozen cells were resuspended in 100 mM Tris-SO4 (pH 9.4) at 25°C, collected by centrifugation, and converted to spheroplasts with Zymolyase 20T (25 mg/g [wet weight] of cells, 1 h, 30°C) in SM buffer (1.4 M sorbitol, 20 mM HEPES-KOH [pH 7.0]) supplemented with 10 mM NaN3, 2 mM EDTA, and 40 mM β-mercaptoethanol. The spheroplasts were washed twice in SM buffer with 10 mM NaN3 and were then incubated on ice in SM buffer (5 ml/g of cells) supplemented with 1 mM CaCl2, 5 mM MnSO4, and concanavalin A (1.5 mg/g of cells) for 15 min. The spheroplasts were collected by centrifugation and washed twice with cold SM buffer, after which they were incubated in hypotonic lysis buffer (0.6 M sorbitol, 20 mM HEPES-KOH [pH 7.0], 2 mM EDTA, protease inhibitor cocktail for use with fungal and yeast extracts [diluted 1:100; Sigma]) for 10 min on ice. The spheroplasts were further disrupted by Dounce homogenization (30 strokes), and the lysate was centrifuged (10,000 × g, 10 min, 4°C). The supernatant was centrifuged to remove additional cell debris (13,000 × g, 10 min, 4°C). The supernatant was then centrifuged (100,000 × g, 45 min, 2°C) to pellet the PGVs, which were resuspended in gluconate or nitrate vesicle buffer (50 mM sucrose, 10 mM Tris-HEPES [pH 7.5], and either 100 mM potassium gluconate or 100 mM potassium nitrate) with 5 mM EGTA. The resuspended PGV samples were centrifuged (100,000 × g, 45 min, 2°C). The final pellet was resuspended in gluconate or nitrate vesicle buffer. Total protein concentrations in PGV samples were determined with the Bradford reagent using bovine serum albumin as a reference.
PGV samples were adjusted to 0.5 mg of total protein/ml in gluconate or nitrate buffer. [3H]fluconazole uptake was initiated by adding PGV samples to prewarmed (37°C) gluconate or nitrate buffer supplemented with ATP (2.5 mM), creatine phosphate (10 mM), creatine phosphokinase (3 μg/ml), and [3H]fluconazole (0.05 μM). [3H]fluconazole transport was interrupted by the addition of an ice-cold stop solution (200 mM sucrose, 10 mM Tris-HCI [pH 7.5], 25 μM fluconazole), and the PGVs were collected as described previously. After two additional washes with stop solution, the radioactivity level was determined by liquid scintillation counting.
To minimize the nonspecific effects of other transporters, organelles, and/or membranes, [3H]fluconazole uptake by PGVs from empty-vector controls was subtracted from uptake by PGVs containing Cdr1p, Cdr2p, or Mdr1p. The kinetic constants Km and Vmax were calculated by the Lineweaver-Burk method, using PGVs that were exposed to graded concentrations of [3H]fluconazole for 10 s. The abilities of other compounds to inhibit [3H]fluconazole uptake were assessed by adding PGVs to a reaction mixture containing the compound of interest at a concentration 50-fold higher than that of [3H]fluconazole. PGVs were collected at 10 s and 30 s (data not shown) and were processed as described above. Any compound that did not inhibit [3H]fluconazole uptake (mean uptake, ≥85% of control values; P, ≥0.05 versus control) was considered to have a 50% inhibitory concentration (IC50) of ≥50. The IC50s of all other compounds were determined by retesting over a range of concentrations (0.125-fold to 64-fold relative to the concentration of [3H]fluconazole in the medium [0.05 μM]), using an equation for a sigmoid plot {Y = min + (max − min)/[1 + 10exp(X − logIC50)], where X is log(inhibitor), Y is pmol/mg total protein, min is the minimum [3H]fluconazole pmol/mg total protein, and max is the maximum [3H]fluconazole pmol/mg total protein}. The IC50 of each compound is the amount required to inhibit fluconazole uptake by 50%, expressed as the ratio of the concentration of that compound to the concentration of [3H]fluconazole.
Electron microscopy.
Whole C. albicans cells and PGV samples were examined by transmission electron microscopy as described by Walworth and Novick (55).
Western blotting.
The presence of the recombinant proteins of interest in whole C. albicans cells or in isolated PGVs was assessed by Western blotting (16). Lysates of whole cells or isolated PGVs were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and probed with a mouse monoclonal anti-Flag primary antibody (Sigma) and a goat anti-mouse secondary antibody. The bands of interest were detected with ECL Western blotting reagents (GE Healthcare).
RESULTS
Effects of expressing CDR1, CDR2, and MDR1 in C. albicans.
The cdr1 cdr2 mdr1-null mutant C. albicans DSY1050F had a much lower fluconazole MIC than did its wild-type parent, C. albicans SC5314. When C. albicans DSY1050F was transformed with pACT1-CDR1, pACT1-CDR2, or pACT1-MDR1, the fluconazole MICs increased at least 4-fold, and similar increases were observed in the MICs of several other antifungal azoles. In contrast, transformation of C. albicans DSY1050F with pACT1 alone had no effect on susceptibility to any azole antifungal, and transformation of C. albicans DSY1050F with pACT1-CDR1, pACT1-CDR2, or pACT1-MDR1 had no effect on susceptibility to the echinocandin antifungal caspofungin (Table 2).
Table 2.
Effects of CDR1, CDR2, or MDR1 overexpression on antifungal MICs for DSY1050F cells
| Antifungal | MIC (μg/ml) for DSY1050F cells transformed with the following plasmid: |
||||
|---|---|---|---|---|---|
| None | pACT | pACT1-CDR1 | pACT1-CDR2 | pACT1-MDR1 | |
| Fluconazole | 0.032 | 0.032 | 0.25 | 0.187 | 0.125 |
| Voriconazole | 0.016 | 0.016 | 0.187 | 0.032 | 0.062 |
| Posaconazole | 0.032 | 0.032 | 0.187 | 0.062 | 0.125 |
| Miconazole | 0.004 | 0.004 | 0.032 | 0.016 | 0.008 |
| Itraconazole | 0.125 | 0.125 | 0.50 | 0.187 | 0.250 |
| Clotrimazole | 0.008 | 0.008 | 0.062 | 0.016 | 0.032 |
| Caspofungin A | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 |
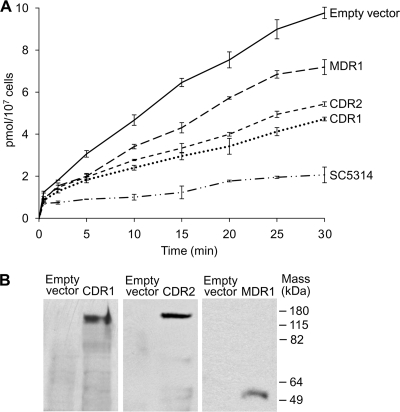
Whether these changes in the fluconazole MIC were associated with differences in intracellular fluconazole levels was assessed by quantifying intracellular [3H]fluconazole levels at intervals after the cells of interest were incubated in 0.05 μM [3H]fluconazole. We found that (i) C. albicans DSY1050F cells transformed with pACT1 accumulated substantially more intracellular [3H]fluconazole than did C. albicans SC5314 cells and (ii) C. albicans DSY1050F cells transformed with pACT1-CDR1, pACT1-CDR2, or pACT1-MDR1 accumulated less intracellular [3H]fluconazole than did pACT1-transformed controls and more intracellular [3H]fluconazole than did C. albicans SC5314 (Fig. 1A). Furthermore, immunoreactive proteins of the sizes expected for Flag-tagged Cdr1p, Cdr2p, and Mdr1p, respectively, were found in whole-cell lysates of the pACT1-CDR1, pACT1-CDR2, and pACT1-MDR1 transformants but not in lysates of pACT1-transformed controls (Fig. 1B).
Fig. 1.
Effect of ACT1-regulated overexpression of CDR1, CDR2, or MDR1. (A) Intracellular [3H]fluconazole levels after incubation in 0.05 μM [3H]fluconazole for the times shown were highest in C. albicans DSY1050F cells transformed with pACT1 alone (empty vector), lower in C. albicans DSY1050F cells transformed with pACT1-CDR1 (CDR1), pACT1-CDR2 (CDR2), or pACT1-MDR1 (MDR1), and lowest in wild-type C. albicans SC5314. Data are means ± SD for 3 experiments. (B) Immunoreactive proteins of the sizes expected for Flag-tagged Cdr1p, Cdr2p, and Mdr1p, respectively, were demonstrated in Western blots of lysates of the pACT1-CDR1, pACT1-CDR2, and pACT-MDR1 transformants probed with anti-Flag antibodies but not in Western blots of lysates of pACT1-transformed controls (empty vector) (100 μg total protein per lane).
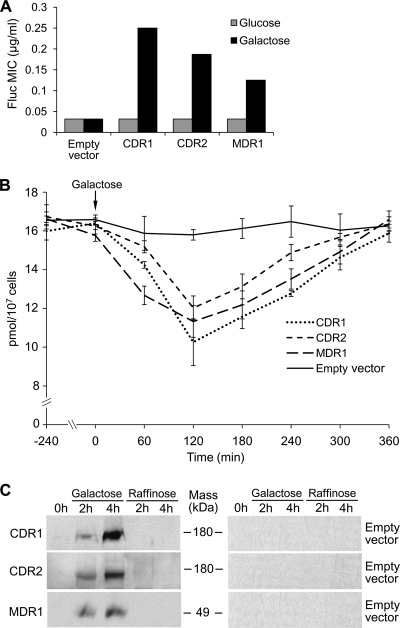
Since overexpression of CDR1, CDR2, and MDR1 could increase fluconazole MICs and decrease intracellular [3H]fluconazole levels by decreasing fluconazole uptake and/or by increasing fluconazole efflux, we also examined the effects of expressing CDR1, CDR2, and MDR1 under the control of the regulatable C. albicans GAL1 promoter. The fluconazole MICs were substantially higher when C. albicans transformed with pGAL1-CDR1, pGAL1-CDR2, or pGAL1-MDR1 (but not pGAL1 alone) was incubated in inducing medium (galactose) than when these transformants were incubated in repressing medium (glucose) (Fig. 2A). Next, the pGAL1-CDR1, pGAL1-CDR2, and pGAL1-MDR1 transformants were incubated in YNB medium with the noninducing and nonrepressing sugar raffinose (2%) and 0.05 μM [3H]fluconazole until steady-state intracellular [3H]fluconazole levels were achieved, and intracellular [3H]fluconazole was quantified after either 2% galactose or 2% raffinose was added to the cell suspensions. Intracellular [3H]fluconazole levels fell substantially by 2 h and then returned to baseline levels by 6 h after 2% galactose was added to the pGAL1-CDR1, pGAL1-CDR2-, or pGAL1-MDR1 transformants, but not after 2% galactose was added to controls transformed with pGAL1 (Fig. 2B). In contrast, intracellular [3H]fluconazole levels did not change when 2% raffinose was added to pGAL1-CDR1, pGAL1-CDR2, pGAL1-MDR1, or pGAL1 transformants (data not shown). To determine if intracellular [3H]fluconazole increased to baseline levels by 6 h because of galactose depletion, we added 2% galactose to suspensions of pGAL1-CDR1, pGAL1-CDR2-, or pGAL1-MDR1 transformants at 0, 1, 2, 3, and 4 h. Intracellular [3H]fluconazole levels in these transformants again fell sharply by 1 to 2 h and remained low through 6 h (data not shown). Lastly, Flag-tagged forms of Cdr1p, Cdr2p, and Mdr1p, respectively, were demonstrated by Western blotting of whole-cell lysates of pGAL-CDR1, pGAL-CDR2, and pGAL-MDR1 transformants 2 to 4 h after these cells were exposed to galactose, but not by Western blotting of raffinose-exposed or pGAL1-transformed controls (Fig. 2C).
Fig. 2.
Effect of GAL1-regulated overexpression of CDR1, CDR2, or MDR1. (A) Fluconazole MICs were higher when C. albicans DSY1050F cells transformed with pGAL1-CDR1 (CDR1), pGAL1-CDR2 (CDR2), or pGAL1-MDR1 (MDR1) were incubated in galactose than when they were incubated in glucose, whereas the fluconazole MICs of pGAL-transformed controls (empty vector) were the same in galactose and glucose. (B) Intracellular [3H]fluconazole levels fell when 2% galactose was added to pGAL1-CDR1-, pGAL1-CDR2-, or pGAL1-MDR1-transformed C. albicans DSY1050F cells that had been incubated in YNB medium with 2% raffinose and 0.05 μM [3H]fluconazole until steady-state intracellular [3H]fluconazole levels were attained (16 h), but not when 2% galactose was added to pGAL1-transformed controls. Data are means ± SD for 3 experiments. (C) Immunoreactive proteins of the sizes expected for Flag-tagged Cdr1p, Cdr2p, and Mdr1p, respectively, were demonstrated in Western blots probed with anti-Flag monoclonal antibodies 2 h and 4 h after the pGAL1-CDR1, pGAL1-CDR2, and pGAL1-MDR1 transformants were exposed to galactose, but not in Western blots for controls exposed to raffinose or for pGAL1-transformed controls exposed to galactose or raffinose (100 μg total protein per lane).
Isolation and properties of post-Golgi secretory vesicles.
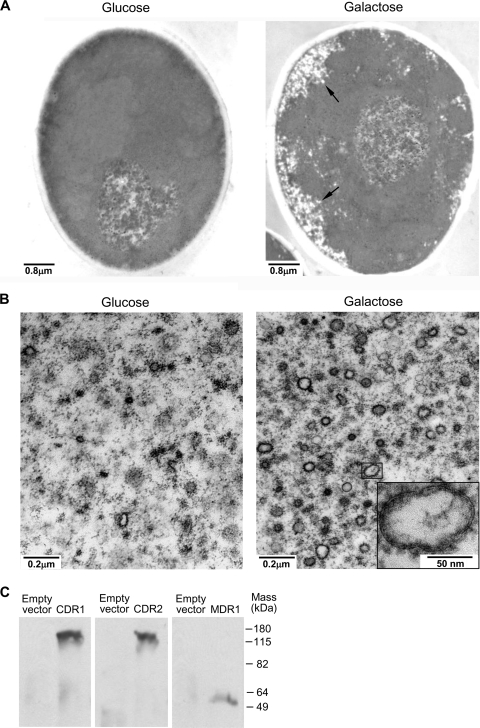
The results summarized above constituted strong direct evidence that Cdr1p, Cdr2p, and Mdr1p pumped fluconazole out of C. albicans cells, but the inaccessibility of the cytoplasmic face of the plasma membrane precluded detailed characterization of these transporters' catalytic properties. Since the membranes of PGVs and those of whole cells are oriented in opposite directions, and since PGVs isolated from temperature-sensitive S. cerevisiae sec6-4 mutants have been used to study multiple eukaryotic plasma membrane efflux pumps (9, 22, 42, 43), we reasoned that it should be possible to use PGVs from pACT1-CDR1-, pACT1-CDR2-, or pACT1-MDR1-transformed C. albicans DSY1050F cells to characterize Cdr1p, Cdr2p, or Mdr1p, respectively. In earlier studies, GAL1-regulated overexpression of a dominant negative allele of the post-Golgi secretion pathway gene SEC4 in wild-type C. albicans caused PGVs to accumulate in the cytoplasm (29) and interfered with the targeting of fluorescently labeled Cdr1p to the plasma membrane (25). Therefore, we introduced the GAL1-regulated plasmid (pS28N) that Mao et al. (29) and Lee et al. (26) had used to overexpress the dominant negative sec4(S28N) allele into C. albicans DSY1050F cells that had previously been transformed with either pACT1-CDR1, pACT1-CDR2, pACT1-MDR1, or pACT1 alone. When the resulting transformants were shifted from glucose (repressing) to galactose (inducing) medium, PGVs accumulated intracellularly (Fig. 3A), and subcellular fractions prepared by differential centrifugation of lysed spheroplasts of galactose-incubated pS28N transformants contained many more intact PGVs than did the corresponding fractions from glucose-incubated controls (Fig. 3B). Furthermore, abundant amounts of Flag-tagged Cdr1p, Cdr2p, and Mdr1p, respectively, were demonstrated by Western blotting of PGV-containing fractions from galactose-incubated pACT1-CDR1-, pACT1-CDR2-, or pACT1-MDR1-transformed cells, but not by Western blotting of the corresponding fractions from pACT1-transformed controls (Fig. 3C).
Fig. 3.
Isolation of C. albicans post-Golgi vesicles (PGVs). (A) Transmission electron microscopy showed that membrane-bound PGVs accumulated in the cytoplasm when pACT1- and pS28N-transformed C. albicans DSY1050F cells were incubated for 7 h in 2% galactose, but not when they were incubated in 2% glucose. (B) In addition, there were many more intact PGVs in 100,000 × g pellets prepared from lysed spheroplasts of pACT1- and pS28N-transformed C. albicans DSY1050F cells incubated in galactose than in glucose-incubated controls. (C) Lastly, immunoreactive proteins of the sizes expected for Cdr1p, Cdr2p, and Mdr1p, respectively, were demonstrated by probing Western blots of the 100,000 × g pellets from C. albicans DSY1050F cells transformed with pS28N and either pACT1-CDR1 (CDR1), pACT1-CDR2 (CDR2), or pACT1-MDR1 (MDR1) with anti-Flag monoclonal antibodies, but not by probing Western blots of the 100,000 × g pellets prepared from pACT1-transformed controls (empty vector) (10 μg total protein per lane).
[3H]fluconazole transport by C. albicans PGVs.
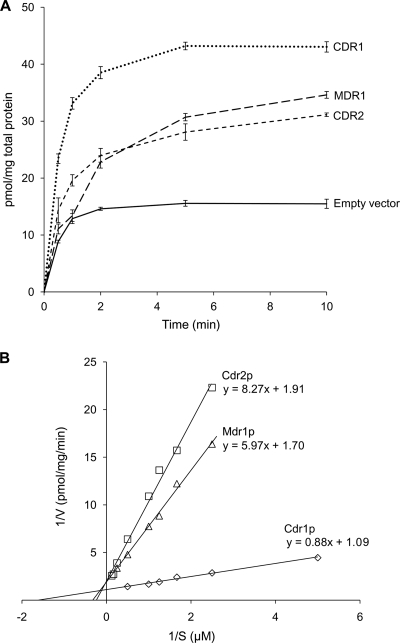
Having established that intact PGVs containing Cdr1p, Cdr2p, or Mdr1p can be isolated from C. albicans transformants, we next tested the PGV fractions for their abilities to accumulate [3H]fluconazole. PGVs isolated from CDR1-, CDR2-, or MDR1-overexpressing C. albicans cells accumulated substantially more [3H]fluconazole than did PGVs from pACT1-transformed controls (Fig. 4A). [3H]fluconazole uptake by Cdr1p-, Cdr2p-, and Mdr1p-containing PGVs conformed to Michaelis-Menten kinetics (Fig. 4B), and the Km and Vmax values, respectively, were 0.80 ± 0.20 μM and 0.91 ± 0.15 pmol/mg protein/min for Cdr1p, 4.3 ± 1.0 μM and 0.52 ± 0.10 pmol/mg protein/min for Cdr2p, and 3.5 ± 1.2 μM and 0.59 ± 0.06 pmol/mg protein/min for Mdr1p (means ± standard deviations [SD] for 3 experiments).
Fig. 4.
[3H]fluconazole accumulation by PGVs. (A) PGVs isolated from C. albicans DSY1050F cells transformed with pACT1-CDR1 (CDR1), pACT1-CDR2 (CDR2), or pACT1-MDR1 (MDR1) accumulated substantially more [3H]fluconazole after incubation in 0.05 μM [3H]fluconazole for the times shown than did PGVs from pACT1-transformed controls (empty vector). Data are means ± SD for 3 experiments. (B) Lineweaver-Burk plots of initial rates of [3H]fluconazole uptake by PGVs containing Cdr1p, Cdr2p, or Mdr1p.
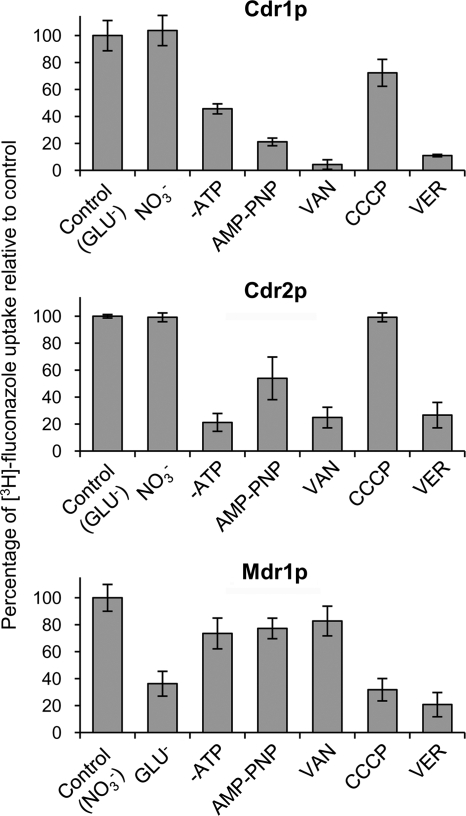
The level of [3H]fluconazole accumulation by PGVs from CDR1- or CDR2-overexpressing C. albicans was markedly lower in the absence of ATP than in its presence, and accumulation was inhibited by the ATPase inhibitor orthovanadate and by the nonhydrolyzable ATP analog 5′-adenylyl-β-γ-imidodiphosphate (AMP-PNP). In contrast, the absence of ATP and the presence of orthovanadate or AMP-PMP had little effect on [3H]fluconazole accumulation by PGVs from MDR1-overexpressing transformants (Fig. 5). Whether [3H]fluconazole transport required a membrane potential and/or proton motive force was assessed by measuring [3H]fluconazole accumulation by PGVs in buffers containing the permeable anion nitrate or the nonpermeable anion gluconate and also in the presence or absence of the proton ionophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP). The level of [3H]fluconazole accumulation by PGVs from MDR1-overexpressing transformants was markedly lower in gluconate-containing buffer than in nitrate-containing buffer, and accumulation was also inhibited by CCCP. In contrast, the levels of [3H]fluconazole accumulation by PGVs from CDR1- or CDR2-overexpressing transformants were similar in gluconate- and nitrate-containing buffers and were only slightly affected by CCCP (Fig. 5). Lastly, verapamil (a general modulator of P glycoproteins) markedly inhibited [3H]fluconazole transport by PGVs containing Cdr1p, Cdr2p, or Mdr1p (Fig. 5).
Fig. 5.
Energy dependence of [3H]fluconazole transport. PGVs isolated from C. albicans DSY1050F transformed with pACT1-CDR1 (Cdr1p) or pACT1-CDR2 (Cdr2p) accumulated much more [3H]fluconazole after 10 s in gluconate buffer with ATP (GLU−) than in gluconate buffer without ATP (−ATP) or in gluconate buffer with ATP plus the nonhydrolyzable ATP analog AMP-PNP, the ATP inhibitor sodium orthovanadate (VAN), or the P-glycoprotein inhibitor verapamil (VER). In contrast, the proton ionophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP) had little effect on [3H]fluconazole transport by Cdr1p or Cdr2p. PGVs isolated from C. albicans DSY1050F cells transformed with pACT1-MDR1 (Mdr1p) accumulated much more [3H]fluconazole after 10 s in nitrate buffer (NO3−) than in gluconate buffer, and they accumulated much less [3H]fluconazole in nitrate buffer plus CCCP or in the presence of verapamil. In contrast, the absence of ATP or the presence of AMP-PNP or VAN had little effect on [3H]fluconazole transport by Mdr1p. Data are means ± SD for 3 experiments.
Effects of alternative compounds on [3H]fluconazole transport.
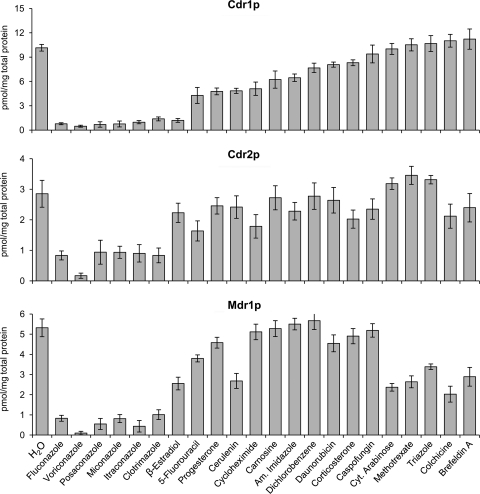
Since the results summarized above established that Cdr1p-, Cdr2p-, and Mdr1p-mediated [3H]fluconazole transport could be quantified using isolated C. albicans PGVs, we next examined the abilities of 22 unlabeled compounds to inhibit Cdr1p-, Cdr2p-, and Mdr1p-mediated [3H]fluconazole uptake by PGVs. These potential inhibitors included several antifungal azoles, compounds that have been reported to interact with azole transport in whole-cell assays, known substrates of other eukaryotic plasma membrane efflux pumps, and selected related compounds. All of the antifungal azoles we tested substantially inhibited [3H]fluconazole uptake by PGVs isolated from CDR1-, CDR2-, and MDR1-overexpressing cells (Fig. 6), with IC50s ranging from 0.2 to 0.3 for voriconazole to 2.0 to 5.6 for clotrimazole (Table 3). Among the other compounds we examined, inhibition of [3H]fluconazole transport with IC50s of <10 was observed only for β-estradiol, cycloheximide, and 5-fluorouracil with Cdr1p and for methotrexate with Mdr1p. No compound other than azoles inhibited [3H]fluconazole transport by Cdr2p with an IC50 of <10 (Fig. 6; Table 3).
Fig. 6.
Inhibitors of [3H]fluconazole transport by Cdr1p, Cdr2p, and Mdr1p. [3H]fluconazole uptake by PGVs isolated from C. albicans DSY1050F cells transformed with pACT1-CDR1 (Cdr1p), pACT1-CDR2 (Cdr2p), or pACT1-MDR1 (Mdr1p) was quantified after incubation for 10 s in the presence of 50-fold molar excesses of the compounds listed. Data are means ± SD for 3 experiments. Am. Imidazole, 1-3-aminopropyl-imidazole; Cyt. Arabinose, cytosine β-d-arabinofuranoside.
Table 3.
IC50s for recombinant Cdr1p, Cdr2p, and Mdr1p
| Substratea | IC50b for: |
||
|---|---|---|---|
| Cdr1p | Cdr2p | Mdr1p | |
| Fluconazole | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.7 |
| Voriconazole | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.2 |
| Posaconazole | 0.8 ± 0.3 | 0.6 ± 0.3 | 0.7 ± 0.4 |
| Miconazole | 0.7 ± 0.3 | 0.9 ± 0.4 | 3.1 ± 1.1 |
| Itraconazole | 4.4 ± 1.0 | 2.4 ± 0.6 | 0.8 ± 0.4 |
| Clotrimazole | 5.6 ± 1.3 | 3.1 ± 1.0 | 2.0 ± 0.7 |
| β-Estradiol | 1.9 ± 0.3 | 21 ± 7.5 | 22 ± 10 |
| 5-Fluorouracil | 9.6 ± 2.3 | 10 ± 4.9 | 15 ± 9.0 |
| Progesterone | 21 ± 10 | 32 ± 12 | ≥50 |
| Cerulenin | 33 ± 11 | 30 ± 9.1 | 17 ± 6.2 |
| Cycloheximide | 7.6 ± 2.8 | 27 ± 9.6 | ≥50 |
| Carnosine | 12 ± 4.5 | ≥50 | ≥50 |
| Am. imidazole | 13 ± 3.6 | ≥50 | ≥50 |
| Dichlorobenzene | 16 ± 3.0 | ≥50 | ≥50 |
| Daunorubicin | 23 ± 4.9 | ≥50 | ≥50 |
| Corticosterone | 14 ± 3.8 | 17 ± 6.8 | ≥50 |
| Caspofungin | ≥50 | ≥50 | ≥50 |
| Cyt. arabinose | ≥50 | ≥50 | 20 ± 4.2 |
| Methotrexate | ≥50 | ≥50 | 6.7 ± 2.2 |
| Triazole | ≥50 | ≥50 | 16 ± 3.0 |
| Colchicine | ≥50 | ≥50 | 11 ± 4.2 |
| Brefeldin A | ≥50 | ≥50 | 11 ± 3.7 |
Am. imidazole, 1-3-aminopropyl-imidazole; cyt. arabinose, cytosine β-d-arabinofuranoside.
Expressed as the ratio of the concentration of each compound to the concentration of fluconazole. Data are means ± SD for 3 experiments.
DISCUSSION
The goals of this study were to determine directly if Cdr1p, Cdr2p, and Mdr1p transport fluconazole out of C. albicans cells, to develop a method for studying the catalytic properties of C. albicans plasma membrane transport proteins, and to use this method to characterize Cdr1p, Cdr2p, and Mdr1p. The principal new findings were that (i) ACT1- or GAL1-regulated overexpression of CDR1, CDR2, or MDR1 increased the fluconazole MIC and decreased intracellular [3H]fluconazole levels in the C. albicans cdr1 cdr2 mdr1-null mutant DSY1050F; (ii) PGVs isolated from C. albicans cells overexpressing both the dominant negative sec4(S28N) allele and either CDR1, CDR2, or MDR1 actively transported [3H]fluconazole into their lumens and thus could be used to study the transport properties of Cdr1p, Cdr2p, and Mdr1p; and (iii) studies of CDR1-, CDR2-, and MDR1-overexpressing whole C. albicans cells and of PGVs isolated from these cells indicate that Cdr1p, Cdr2p, and Mdr1p transport multiple antifungal azoles across the plasma membrane.
Although a large body of evidence shows that overexpression of CDR1, CDR2, and MDR1 is a major cause of fluconazole resistance in C. albicans, direct genetic and biochemical evidence that Cdr1p, Cdr2p, and Mdr1p pump fluconazole out of C. albicans cells is limited. For example, it has been shown that intracellular [3H]fluconazole levels decreased when CDR1 (20, 33) and MDR1 (37) were overexpressed in S. cerevisiae and also that fluconazole MICs increased when CDR1, CDR2 (36, 53), and MDR1 (31) were overexpressed in C. albicans. The levels to which CDR1, CDR2, and MDR1 were expressed in C. albicans in the present study did not approach the expression levels that others achieved in S. cerevisiae or C. albicans; this may have been due to our use of episomal rather than integrating vectors and our use of the ACT1 and GAL1 promoters instead of fluconazole-inducible promoters. Nevertheless, we were able to show that ACT1- and GAL1-regulated overexpression of CDR1, CDR2, or MDR1 caused both increased fluconazole MICs and decreased intracellular [3H]fluconazole concentrations. To our knowledge, this is the first direct demonstration that Cdr1p, Cdr2p, and Mdr1p pump fluconazole out of C. albicans cells. One reason that it was possible to show that ACT1- or GAL1-regulated expression of CDR1, CDR2, or MDR1 increased fluconazole MICs and decreased intracellular [3H]fluconazole levels was that the C. albicans cdr1 cdr2 mdr1-null mutant used in our experiments had a much lower fluconazole MIC and much higher intracellular [3H]fluconazole levels than did its wild-type parent.
When the methods of Mao et al. (29) and Ruetz and Gros (42) were adapted to isolate PGVs from CDR1-, CDR2-, and MDR1-overexpressing C. albicans cells, transmission electron microscopy showed that (i) PGVs accumulated intracellularly when the GAL1-regulated dominant negative sec4(S28N) allele was overexpressed and (ii) subcellular fractions prepared from these cells were enriched for intact PGVs. Moreover, Western blotting with anti-Flag antibodies showed that PGVs from CDR1-, CDR2-, and MDR1-overexpressing C. albicans contained abundant amounts of each recombinant protein of interest, whereas PGVs from empty-vector controls did not. Most importantly, PGVs from CDR1-, CDR2-, and MDR1-overexpressing C. albicans cells accumulated substantially more [3H]fluconazole than did PGVs from empty-vector controls. We concluded from these results that PGVs from CDR1-, CDR2-, and MDR1-overexpressing C. albicans specifically transported [3H]fluconazole across their membranes and thus could be used to examine directly these pumps' catalytic constants, energy requirements, and inhibitor profiles.
Membrane fractions from CDR1-, CDR2-, and MDR1-overexpressing S. cerevisiae transformants have previously been used to determine the respective apparent Km and Vmax values for the ATPase activities of Cdr1p, Cdr2p, and Mdr1p (23), but so far as we are aware, none of these pumps' Km and Vmax values for azoles had been determined prior to the present study. We also found that [3H]fluconazole transport by Cdr1p and Cdr2p (but not by Mdr1p) required ATP and was inhibited by ATP inhibitors and also that [3H]fluconazole transport by Mdr1p (but not by Cdr1p or Cdr2p) required a transmembrane proton gradient and was blocked by CCCP. These results were not surprising, because (i) Cdr1p and Cdr2p are members of the ABC transporter superfamily, (ii) Mdr1p is a member of the MFS transporter family (40), and (iii) Cdr1p- and Cdr2p-mediated transport across the plasma membranes of whole S. cerevisiae cells was known to be ATP dependent (11, 21, 49). However, we found that orthovanadate had only a slight inhibitory effect on [3H]fluconazole uptake by PGVs from MDR1-overexpressing C. albicans cells, whereas orthovanadate increased intracellular [3H]fluconazole levels in MDR1-overexpressing whole S. cerevisiae cells in an earlier study (20). Two possible explanations for these differing results are that (i) other vanadate-sensitive transporters may also pump [3H]fluconazole out of whole S. cerevisiae cells or (ii) a step required to process and/or transport Mdr1p to the plasma membrane of S. cerevisiae may be inhibited by vanadate.
One striking finding was that [3H]fluconazole transport into PGVs by Cdr1p, Cdr2p, and Mdr1p was markedly inhibited by all of the azole antifungals tested. These results suggest that all three pumps of interest transport multiple azoles through the plasma membrane, and this conclusion was supported by the observation that C. albicans DSY1050F cells expressing CDR1, CDR2, or MDR1 had higher MICs for all azoles tested than empty-vector controls. Our results were consistent with those of previous studies showing that overexpression of CDR1 or CDR2 in S. cerevisiae increased the MICs of multiple antifungal azoles (14, 15, 17, 18, 23, 33, 35, 36, 45, 50, 52). However, previous studies indicated that Mdr1p is more selective than Cdr1p or Cdr2p (16, 20, 23, 37). For example, Lamping et al. (23) showed that the fluconazole MICs for MDR1-overexpressing S. cerevisiae were markedly higher than those for controls, whereas the itraconazole MICs for the same transformants were not. We found that itraconazole markedly inhibited [3H]fluconazole uptake by Mdr1p-containing PGVs but also that MDR1 overexpression in whole C. albicans cells resulted in only a 2-fold increase in the itraconazole MIC. Since compounds can inhibit the transport of a labeled substrate by ABC transporters without themselves being transport substrates, and since overexpression of MDR1 increased the MIC of itraconazole less than the MICs of other azoles, our results do not necessarily imply that itraconazole is transported by Mdr1p. The apparent differences in Mdr1p-mediated itraconazole transport between our study and earlier studies of S. cerevisiae may have been due to the different promoters used to drive MDR1 expression, the use of plasmids to express MDR1 in our study versus integrating vectors in earlier studies, and/or the presence of two CUG codons in the MDR1 ORF. For all of these reasons, definitive conclusions about the ability or inability of Mdr1p to transport itraconazole through the plasma membrane will require studies of labeled itraconazole, which was not available for these studies.
Two other compounds also influenced [3H]fluconazole uptake by the efflux pumps of interest. First, β-estradiol markedly inhibited [3H]fluconazole transport by Cdr1p (IC50, 1.9) but not that by Cdr2p or Mdr1p. These findings support the observation by Krishnamurthy et al. (21) that overexpression of CDR1 in S. cerevisiae decreased intracellular β-[3H]estradiol levels. Since C. albicans occupies at least one niche with high estrogen levels (i.e., the vaginal mucosa), and since estrogens have profound effects on the growth, morphology, and gene expression of C. albicans (1, 5, 21, 24, 59), one would expect C. albicans to have mechanisms for regulating intracellular estrogen levels. Since Cdr1p may play a role in this process, we plan in future studies (i) to test the abilities of PGVs from CDR1-overexpressing C. albicans to accumulate β-[3H]estradiol and (ii) to compare the effects of estrogens on C. albicans null mutants and wild-type controls. Second, methotrexate inhibited [3H]fluconazole uptake by Mdr1p (IC50, 6.7) but not that by Cdr1p or Cdr2p. Earlier studies showed that overexpression of MDR1 in S. cerevisiae reduced intracellular levels of [3H]methotrexate and also that exposure of these cells to unlabeled fluconazole slightly inhibited intracellular [3H]methotrexate accumulation (20); our results support a role for Mdr1p in pumping methotrexate out of C. albicans cells.
We also found that several compounds that others have reported as potential substrates for Cdr1p, Cdr2p, or Mdr1p did not inhibit [3H]fluconazole transport by these transporters. For example, overexpression of CDR1 or CDR2 in S. cerevisiae increased brefeldin A MICs (36, 45), but brefeldin A did not reduce [3H]fluconazole uptake by PGVs from CDR1- or CDR2-overexpressing C. albicans. Similarly, overexpression of MDR1 in S. cerevisiae increased cycloheximide MICs (20, 38), but cycloheximide did not inhibit [3H]fluconazole transport by PGVs isolated from MDR1-overexpressing C. albicans. Lastly, earlier studies have reported conflicting results for the effects of CDR2 overexpression on resistance to the antifungal echinocandin caspofungin. Schuetzer-Muehlbauer et al. (48) reported that overexpression of CDR2 in S. cerevisiae increased caspofungin MICs measured on solid media but not those in liquid media. In contrast, Niimi et al. (35) found that overexpression of CDR2 in S. cerevisiae did not significantly increase caspofungin MICs, and Silver et al. (51) found no significant differences between the caspofungin MICs of C. albicans clinical strains that did or did not overexpress CDR2. In our studies, caspofungin did not inhibit the uptake of [3H]fluconazole by PGVs isolated from CDR1-, CDR2-, or MDR1-overexpressing C. albicans, and overexpression of CDR1, CDR2, or MDR1 in C. albicans DSY1050F had no effect on caspofungin MICs. Since membrane transporters can have different binding sites for different transport substrates (50), the observations that brefeldin A, cycloheximide, and caspofungin did not inhibit [3H]fluconazole uptake by PGVs isolated from CDR1-, CDR2-, or MDR1-overexpressing C. albicans cells do not rule out the possibility that these compounds are transported out of the cell by the transporters of interest. Definitive conclusions that Cdr1p, Cdr2p, or Mdr1p can or cannot pump brefeldin A, cycloheximide, or caspofungin out of the cell would require studies of the radiolabeled compounds of interest, which were not available for this study.
In summary, we have shown that ACT1- and GAL1-regulated overexpression of C. albicans CDR1, CDR2 and MDR1 in a C. albicans cdr1 cdr2 mdr1-null mutant resulted in increased fluconazole MICs and decreased intracellular [3H]fluconazole concentrations, thereby providing strong direct evidence that Cdr1p, Cdr2p, and Mdr1p transport fluconazole out of C. albicans cells. We also developed a new method for isolating C. albicans PGVs with abundant amounts of catalytically active Cdr1p, Cdr2p, or Mdr1p in their membranes, and we used these PGVs to analyze the energy requirements, kinetic constants, and inhibitor profiles of [3H]fluconazole transport by recombinant Cdr1p, Cdr2p, and Mdr1p. In addition to providing new information about the catalytic properties of three important C. albicans drug efflux pumps, the methods and approaches used in this study should be useful for studying other plasma membrane transporters from C. albicans and other medically important fungi.
ACKNOWLEDGMENT
This work was supported by NIH grant R01 AI-64085.
Footnotes
Published ahead of print on 26 March 2010.
REFERENCES
- 1.Banerjee D., Martin N., Nandi S., Shukla S., Dominguez A., Mukhopadhyay G., Prasad R. 2007. A genome-wide steroid response study of the major human fungal pathogen Candida albicans. Mycopathologia 164:1–17 [DOI] [PubMed] [Google Scholar]
- 2.Cannon R. D., Fischer F. J., Niimi K., Niimi M., Arisawa M. 1998. Drug pumping mechanisms in Candida albicans. Nippon Ishinkin Gakkai Zasshi 39:73–78 [DOI] [PubMed] [Google Scholar]
- 3.Chen C. G., Yang Y. L., Shih H. I., Su C. L., Lo H. J. 2004. CaNdt80 is involved in drug resistance in Candida albicans by regulating CDR1. Antimicrob. Agents Chemother. 48:4505–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C. G., Yang Y. L., Tseng K. Y., Shih H. I., Liou C. H., Lin C. C., Lo H. J. 2009. Rep1p negatively regulating MDR1 efflux pump involved in drug resistance in Candida albicans. Fungal Genet. Biol. 46:714–720 [DOI] [PubMed] [Google Scholar]
- 5.Cheng G., Yeater K. M., Hoyer L. L. 2006. Cellular and molecular biology of Candida albicans estrogen response. Eukaryot. Cell 5:180–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coste A., Turner V., Ischer F., Morschhäuser J., Forche A., Selmecki A., Berman J., Bille J., Sanglard D. 2006. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 172:2139–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coste A. T., Crittin J., Bauser C., Rohde B., Sanglard D. 2009. Functional analysis of cis- and trans-acting elements of the Candida albicans CDR2 promoter with a novel promoter reporter system. Eukaryot. Cell 8:1250–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coste A. T., Karababa M., Ischer F., Bille J., Sanglard D. 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 3:1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coury L. A., Mathai J. C., Prasad G. V., Brodsky J. L., Agre P., Zeidel M. L. 1998. Reconstitution of water channel function of aquaporins 1 and 2 by expression in yeast secretory vesicles. Am. J. Physiol. 274:F34–F42 [DOI] [PubMed] [Google Scholar]
- 10.de Micheli M., Bille J., Schueller C., Sanglard D. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43:1197–1214 [DOI] [PubMed] [Google Scholar]
- 11.Dogra S., Krishnamurthy S., Gupta V., Dixit B. L., Gupta C. M., Sanglard D., Prasad R. 1999. Asymmetric distribution of phosphatidylethanolamine in C. albicans: possible mediation by CDR1, a multidrug transporter belonging to ATP binding cassette (ABC) superfamily. Yeast 15:111–121 [DOI] [PubMed] [Google Scholar]
- 12.Dunkel N., Blass J., Rogers P. D., Morschhäuser J. 2008. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 69:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaur N. A., Manoharlal R., Saini P., Prasad T., Mukhopadhyay G., Hoefer M., Morschhäuser J., Prasad R. 2005. Expression of the CDR1 efflux pump in clinical Candida albicans isolates is controlled by a negative regulatory element. Biochem. Biophys. Res. Commun. 332:206–214 [DOI] [PubMed] [Google Scholar]
- 14.Gauthier C., Weber S., Alarco A. M., Alqawi O., Daoud R., Georges E., Raymond M. 2003. Functional similarities and differences between Candida albicans Cdr1p and Cdr2p transporters. Antimicrob. Agents Chemother. 47:1543–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haque A., Rai V., Bahal B. S., Shukla S., Lattif A. A., Mukhopadhyay G., Prasad R. 2007. Allelic variants of ABC drug transporter Cdr1p in clinical isolates of Candida albicans. Biochem. Biophys. Res. Commun. 352:491–497 [DOI] [PubMed] [Google Scholar]
- 16.Hiller D., Sanglard D., Morschhäuser J. 2006. Overexpression of the MDR1 gene is sufficient to confer increased resistance to toxic compounds in Candida albicans. Antimicrob. Agents Chemother. 50:1365–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes A. R., Lin Y. H., Niimi K., Lamping E., Keniya M., Niimi M., Tanabe K., Monk B. C., Cannon R. D. 2008. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob. Agents Chemother. 52:3851–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes A. R., Tsao S., Ong S. W., Lamping E., Niimi K., Monk B. C., Niimi M., Kaneko A., Holland B. R., Schmid J., Cannon R. D. 2006. Heterozygosity and functional allelic variation in the Candida albicans efflux pump genes CDR1 and CDR2. Mol. Microbiol. 62:170–186 [DOI] [PubMed] [Google Scholar]
- 19.Ivnitski-Steele I., Holmes A. R., Lamping E., Monk B. C., Cannon R. D., Sklar L. A. 2009. Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter Mdr1p. Anal. Biochem. 394:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohli A., Gupta V., Krishnamurthy S., Hasnain S. E., Prasad R. 2001. Specificity of drug transport mediated by CaMDR1: a major facilitator of Candida albicans. J. Biosci. 26:333–339 [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy S., Gupta V., Snehlata P., Prasad R. 1998. Characterization of human steroid hormone transport mediated by Cdr1p, a multidrug transporter of Candida albicans, belonging to the ATP binding cassette super family. FEMS Microbiol. Lett. 158:69–74 [DOI] [PubMed] [Google Scholar]
- 22.Laizé V., Rousselet G., Verbavatz J. M., Berthonaud V., Gobin R., Roudier N., Abrami L., Ripoche P., Tacnet F. 1995. Functional expression of the human CHIP28 water channel in a yeast secretory mutant. FEBS Lett. 373:269–274 [DOI] [PubMed] [Google Scholar]
- 23.Lamping E., Monk B. C., Niimi K., Holmes A. R., Tsao S., Tanabe K., Niimi M., Uehara Y., Cannon R. D. 2007. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot. Cell 6:1150–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen B., Anderson S., Brockman A., Essmann M., Schmidt M. 2006. Key physiological differences in Candida albicans CDR1 induction by steroid hormones and antifungal drugs. Yeast 23:795–802 [DOI] [PubMed] [Google Scholar]
- 25.Lee S. A., Khalique Z., Gale C. A., Wong B. 2005. Intracellular trafficking of fluorescently tagged proteins associated with pathogenesis in Candida albicans. Med. Mycol. 43:423–430 [DOI] [PubMed] [Google Scholar]
- 26.Lee S. A., Mao Y., Zhang Z., Wong B. 2001. Overexpression of a dominant-negative allele of YPT1 inhibits growth and aspartyl protease secretion in Candida albicans. Microbiology 147:1961–1970 [DOI] [PubMed] [Google Scholar]
- 27.Liu T. T., Lee R. E., Barker K. S., Lee R. E., Wei L., Homayouni R., Rogers P. D. 2005. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 49:2226–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manoharlal R., Gaur N. A., Panwar S. L., Morschhäuser J., Prasad R. 2008. Transcriptional activation and increased mRNA stability contribute to overexpression of CDR1 in azole-resistant Candida albicans. Antimicrob. Agents Chemother. 52:1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao Y., Kalb V. F., Wong B. 1999. Overexpression of a dominant-negative allele of SEC4 inhibits growth and protein secretion in Candida albicans. J. Bacteriol. 181:7235–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morschhäuser J. 2002. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta 1587:240–248 [DOI] [PubMed] [Google Scholar]
- 31.Morschhäuser J., Barker K. S., Liu T. T., Blaß-Warmuth J., Homayouni R., Rogers P. D. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 3:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamoto R. K., Rao R., Slayman C. W. 1991. Expression of the yeast plasma membrane [H+]ATPase in secretory vesicles. A new strategy for directed mutagenesis. J. Biol. Chem. 266:7940–7949 [PubMed] [Google Scholar]
- 33.Nakamura K., Niimi M., Niimi K., Holmes A. R., Yates J. E., Decottignies A., Monk B. C., Goffeau A., Cannon R. D. 2001. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 45:3366–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Committee for Clinical Laboratory Standards 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 35.Niimi K., Maki K., Ikeda F., Holmes A. R., Lamping E., Niimi M., Monk B. C., Cannon R. D. 2006. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob. Agents Chemother. 50:1148–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niimi M., Niimi K., Takano Y., Holmes A. R., Fischer F. J., Uehara Y., Cannon R. D. 2004. Regulated overexpression of CDR1 in Candida albicans confers multidrug resistance. J. Antimicrob. Chemother. 54:999–1006 [DOI] [PubMed] [Google Scholar]
- 37.Pasrija R., Banerjee D., Prasad R. 2007. Structure and function analysis of CaMdr1p, a major facilitator superfamily antifungal efflux transporter protein of Candida albicans: identification of amino acid residues critical for drug/H+ transport. Eukaryot. Cell 6:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasrija R., Panwar S. L., Prasad R. 2008. Multidrug transporters CaCdr1p and CaMdr1p of Candida albicans display different lipid specificities: both ergosterol and sphingolipids are essential for targeting of CaCdr1p to membrane rafts. Antimicrob. Agents Chemother. 52:694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad R., de Wergifosse P., Goffeau A., Balzi E. 1995. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320–329 [DOI] [PubMed] [Google Scholar]
- 40.Prasad R., Kapoor K. 2005. Multidrug resistance in yeast Candida. Int. Rev. Cytol. 242:215–248 [DOI] [PubMed] [Google Scholar]
- 41.Rao R., Slayman C. W. 1992. Mutagenesis of the yeast plasma membrane H(+)-ATPase. A novel expression system. Biophys. J. 62:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruetz S., Gros P. 1994. Functional expression of P-glycoproteins in secretory vesicles. J. Biol. Chem. 269:12277–12284 [PubMed] [Google Scholar]
- 43.Ruetz S., Gros P. 1994. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell 77:1071–1081 [DOI] [PubMed] [Google Scholar]
- 44.Ruetz S., Raymond M., Gros P. 1993. Functional expression of P-glycoprotein encoded by the mouse mdr3 gene in yeast cells. Proc. Natl. Acad. Sci. U. S. A. 90:11588–11592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanglard D., Ischer F., Monod M., Bille J. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405–416 [DOI] [PubMed] [Google Scholar]
- 46.Sanglard D., Kuchler K., Ischer F., Pagani J. L., Monod M., Bille J. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanglard D., Odds F. C. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73–85 [DOI] [PubMed] [Google Scholar]
- 48.Schuetzer-Muehlbauer M., Willinger B., Egner R., Ecker G., Kuchler K. 2003. Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int. J. Antimicrob. Agents 22:291–300 [DOI] [PubMed] [Google Scholar]
- 49.Shukla S., Rai V., Banerjee D., Prasad R. 2006. Characterization of Cdr1p, a major multidrug efflux protein of Candida albicans: purified protein is amenable to intrinsic fluorescence analysis. Biochemistry 45:2425–2435 [DOI] [PubMed] [Google Scholar]
- 50.Shukla S., Saini P., Smriti, Jha S., Ambudkar S. V., Prasad R. 2003. Functional characterization of Candida albicans ABC transporter Cdr1p. Eukaryot. Cell 2:1361–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silver P. M., Oliver B. G., White T. C. 2008. Characterization of caspofungin susceptibilities by broth and agar in Candida albicans clinical isolates with characterized mechanisms of azole resistance. Med. Mycol. 46:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smriti, Krishnamurthy S., Dixit B. L., Gupta C. M., Milewski S., Prasad R. 2002. ABC transporters Cdr1p, Cdr2p and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast 19:303–318 [DOI] [PubMed] [Google Scholar]
- 53.Tsao S., Rahkhoodaee F., Raymond M. 2009. Relative contributions of the Candida albicans ABC transporters Cdr1p and Cdr2p to clinical azole resistance. Antimicrob. Agents Chemother. 53:1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walther A., Wendland J. 2003. An improved transformation protocol for the human fungal pathogen Candida albicans. Curr. Genet. 42:339–343 [DOI] [PubMed] [Google Scholar]
- 55.Walworth N. C., Novick P. J. 1987. Purification and characterization of constitutive secretory vesicles from yeast. J. Cell Biol. 105:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White T. C., Marr K. A., Bowden R. A. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng Y. B., Qian Y. S., Ma L., Gu H. N. 2007. Genome-wide expression profiling of the response to terbinafine in Candida albicans using a cDNA microarray analysis. Chin. Med. J. (Engl.) 120:807–813 [PubMed] [Google Scholar]
- 58.Znaidi S., De Deken X., Weber S., Rigby T., Nantel A., Raymond M. 2007. The zinc cluster transcription factor Tac1p regulates PDR16 expression in Candida albicans. Mol. Microbiol. 66:440–452 [DOI] [PubMed] [Google Scholar]
- 59.Znaidi S., Weber S., Al-Abdin O. Z., Bomme P., Saidane S., Drouin S., Lemieux S., de Deken X., Robert F., Raymond M. 2008. Genomewide location analysis of Candida albicans Upc2p, a regulator of sterol metabolism and azole drug resistance. Eukaryot. Cell 7:836–847 [DOI] [PMC free article] [PubMed] [Google Scholar]