Abstract
Summary: Since 1971, the CDC, EPA, and Council of State and Territorial Epidemiologists (CSTE) have maintained the collaborative national Waterborne Disease and Outbreak Surveillance System (WBDOSS) to document waterborne disease outbreaks (WBDOs) reported by local, state, and territorial health departments. WBDOs were recently reclassified to better characterize water system deficiencies and risk factors; data were analyzed for trends in outbreak occurrence, etiologies, and deficiencies during 1971 to 2006. A total of 833 WBDOs, 577,991 cases of illness, and 106 deaths were reported during 1971 to 2006. Trends of public health significance include (i) a decrease in the number of reported outbreaks over time and in the annual proportion of outbreaks reported in public water systems, (ii) an increase in the annual proportion of outbreaks reported in individual water systems and in the proportion of outbreaks associated with premise plumbing deficiencies in public water systems, (iii) no change in the annual proportion of outbreaks associated with distribution system deficiencies or the use of untreated and improperly treated groundwater in public water systems, and (iv) the increasing importance of Legionella since its inclusion in WBDOSS in 2001. Data from WBDOSS have helped inform public health and regulatory responses. Additional resources for waterborne disease surveillance and outbreak detection are essential to improve our ability to monitor, detect, and prevent waterborne disease in the United States.
INTRODUCTION
Statistical data on the occurrence and causes of waterborne disease outbreaks (WBDOs) in the United States have been collected and reported since 1920 (10, 12, 14, 46). Since 1971, the Centers for Disease Control and Prevention (CDC), the U.S. Environmental Protection Agency (EPA), and the Council of State and Territorial Epidemiologists (CSTE) have maintained the collaborative Waterborne Disease and Outbreak Surveillance System (WBDOSS) and periodically reported these data (47). In this article, we review WBDOs associated with water intended for drinking, water not intended for drinking, and water of unknown intent reported to the surveillance system during 1971 to 2006. We have included all reported outbreaks and cases associated with exposure to drinking water—through ingestion, inhalation, or dermal contact—and all types of illness acquired from exposure to drinking water, including acute gastrointestinal illness (e.g., cryptosporidiosis and giardiasis), acute respiratory illness (e.g., legionellosis), skin infections (e.g., Pseudomonas), neurological illness (e.g., primary amebic meningoencephalitis), and inflammation of the liver (e.g., hepatitis A). Excluded from this review are all reported outbreaks and cases associated with recreational water use.
Waterborne Disease and Outbreak Surveillance
Public health departments in U.S. states, territories, localities, and Freely Associated States (i.e., the Republic of the Marshall Islands, the Federated States of Micronesia, and the Republic of Palau) have primary responsibility for detecting and investigating outbreaks and cases of disease. Outbreaks associated with exposures to drinking, recreational, and other types of water are voluntarily reported to the CDC.
For an outbreak to be defined as a WBDO, two or more persons must have experienced a similar illness after exposure to water and be epidemiologically linked by time and by location of exposure to water. Furthermore, the epidemiologic evidence must implicate water as the probable source of illness. For inclusion in the WBDOSS, CDC and EPA classify reported outbreaks according to the strength of the evidence implicating water as the vehicle of transmission (Table 1). Epidemiologic data are weighted more heavily than water quality data. Outbreaks without water quality data are included, but reports that lack epidemiologic data are not.
TABLE 1.
Classification of investigations of waterborne disease and outbreaks based on strength of evidence implicating water as a vehicle of transmission
| Class | Epidemiologic data | Water quality data |
|---|---|---|
| I | Adequate; data provided about exposed and unexposed persons, with relative risk or odds ratio of ≥2 or P value of ≤0.05 | Provided and adequate; laboratory data or historical information (e.g., reports of a chlorinator malfunction, a water main break, no detectable free-chlorine residual, or the presence of coliforms in water) |
| II | Adequate | Not provided or inadequate (e.g., laboratory testing of water not conducted and no historical information) |
| III | Provided but limited | Provided and adequate |
| Epidemiologic data provided that did not meet the criteria for class I, or claim made that ill persons had no exposures in common besides water but no data provided | ||
| IV | Provided but limited | Not provided or inadequate |
The primary unit of analysis for this surveillance system is the outbreak, but single cases of selected waterborne diseases are also reported when waterborne transmission is certain (e.g., single cases of chemical poisoning when water quality data indicate water contamination by the chemical). Although single cases are analyzed separately from outbreaks for WBDOSS surveillance reports, they are included in the outbreak analyses for this review. WBDOs caused by Legionella and resulting in legionellosis (i.e., Legionnaires' disease) are also included, even though they have been reported to WBDOSS only since 2001. Single cases of legionellosis are captured in a separate surveillance system at CDC (3) and are not included in this review.
When an infectious etiologic agent is identified, the genus is reported along with any other details that are available, including the species, serotype, serogroup, serovar, genotype, and subtype. In this review, the absence of species names or other descriptors indicates that only the genus name was reported. It is not a requirement that infectious etiologic agents be identified or fully characterized to be included in WBDOSS. WBDOs with no reported etiologic agent (e.g., specimens not collected or specimens analyzed but no agent ascertained) were labeled as having an undetermined etiology and included in all analyses.
During 1971 to 2006, multiple versions of a standard form were used to collect information about WBDOs. The initial form was designed for food-borne disease outbreak reporting (HSM 4.245-NCDC) and was replaced with a WBDO-specific form in 1975 (CDC 4.461, renamed CDC 52.12 in 1981). The CDC 52.12 form was developed to solicit data on characteristics of the outbreak (e.g., cases, time, and location), results from epidemiologic investigations and studies, results from clinical specimens and water sample testing, and other potential contributing factors (e.g., environmental concerns, disinfection deficiencies, and filtration problems). Formal investigative reports were frequently submitted with the reporting forms to provide supplemental information for many WBDOs and were the only information available for many of the WBDOs in the early years of the surveillance system. CDC maintains files of these paper-based outbreak forms and reports and also has an internal electronic database housing these data. In 2009, waterborne disease outbreak reporting was transitioned from paper-based reporting to electronic reporting through the National Outbreak Reporting System (NORS), a state-accessible electronic system maintained at CDC that also includes food-borne, person-to-person, and animal-to-person outbreaks. NORS will provide enhanced and timelier data collection for public health agencies and waterborne disease researchers.
METHODS OF OUTBREAK EVALUATION
Outbreak Characterization and Classification
Recently, we conducted an extensive evaluation of all WBDOs associated with drinking water, water not intended for drinking, and water of unknown intent in the WBDOSS database. Because specific guidelines for inclusion of an outbreak in the surveillance system were first applied to WBDOs occurring in 1989 (15) and have been revised since then, the earlier WBDOs were reevaluated to ensure the adequacy and consistency of the epidemiologic information needed for inclusion in the surveillance system based on current requirements. In addition, definitions and other parameters have been expanded in recent years to better reflect the changing epidemiology of waterborne disease (23, 47), and it was necessary to apply the changed definitions to earlier WBDOs (Table 2). For example, only one etiologic agent was documented for WBDOs reported during 1971 to 2002, whereas WBDOs reported after 2002 are documented as having more than one etiologic agent if multiple pathogens or chemicals are identified and if each agent is identified in ≥5% of all positive clinical specimens (e.g., stool samples). All WBDOs have been reevaluated for additional etiologic agents using the current definition. Outbreaks that have multiple etiologic agents of the same agent type (i.e., bacterial, chemical, parasitic, or viral), are included in analyses for that agent type, whereas outbreaks that have etiologic agents of more than one agent type are excluded from analyses of single-agent types and are analyzed separately as mixed-agent outbreaks. A similar approach has been applied to outbreaks with more than one illness type (e.g., acute gastrointestinal illness and hepatitis). Only the predominant illness type was documented during 1971 to 2002; outbreaks that occurred during this time period have now been reclassified as mixed illness when illness symptoms clustered into more than one illness type and were reported by ≥50% of patients. Mixed-illness outbreaks are analyzed separately from single-illness outbreaks. Likewise, outbreaks have been further evaluated for the involvement of more than one water source (e.g., both groundwater and surface water), and mixed-source outbreaks have been separated from single-source outbreaks for analyses.
TABLE 2.
Timeline of WBDOSS definitions and inclusion criteria for drinking water
| Reporting period | WBDOSS definitions and inclusion criteria |
|---|---|
| 1971-1972 | WBDOSS initiated: “outbreak” defined as two or more cases epidemiologically linked to consumption of water from municipal, semipublic or individual water systems; individual water system defined as wells or springs used exclusively by single residences in areas without municipal systems |
| 1974 | Inclusion of single cases of chemical poisoning when drinking water was demonstrated to be contaminated by a chemical |
| 1976 | Individual water systems redefined as wells or springs used by a single residence or several residences or by persons traveling outside populated areas |
| 1979 | Drinking water systems redefined as community systems, noncommunity systems, and individual systems |
| 1989-1990 | Total no. of cases redefined to exclude secondary cases |
| 1991-1992 | Specific exclusion of outbreaks due to contamination of water or ice at point of use |
| 1995-1996 | Estimated case count used instead of actual case count when the study population was randomly sampled or the estimated count was calculated using the attack rate |
| 1999-2000 | Inclusion of outbreaks associated with occupational water; inclusion of water not intended for drinking and bottled water in individual water systems |
| 2001-2002 | Inclusion of outbreaks of Legionnaires' disease |
| 2003-2004 | Introduction of expanded deficiency classifications that capture point-of-use outbreaks except contamination of ice; removal of water not intended for drinking and bottled water outbreaks from individual water system classification; revision to definition of etiologic agent (multiple etiologies listed when each agent individually represents ≥5% of positive specimens); “unidentified” is now used instead of “AGI” to identify acute gastrointestinal illness of unknown etiology; illness types listed when ≥50% of patients reported a symptom in that category |
| 2005-2006 | Deficiency classifications expanded to include a deficiency whereby current treatment is not expected to remove a chemical contaminant; single cases excluded from analyses of outbreaks |
WBDOs are assigned water system deficiencies that summarize the contributing factors and events associated with individual outbreaks. Substantial modifications to the water system deficiency classifications were assigned to WBDOs reported for 2003 to 2006 to provide better data for public health prevention and response efforts (23, 47). The revised deficiency classifications have now been applied to WBDOs reported for 1971 to 2002, providing greater detail concerning the points of contamination and the circumstances responsible for contamination. Outbreaks associated with more than one deficiency are no longer restricted to the primary deficiency when multiple deficiencies have been identified; all deficiencies associated with an outbreak are now included. WBDOs with no reported deficiencies (e.g., deficiencies not investigated, deficiencies investigated but not ascertained, and known deficiencies not reported) have been labeled as having unknown deficiencies. For analysis purposes, these unknown deficiencies are included in the denominators unless otherwise noted. In the new deficiency classification, a clear distinction is made between contamination occurring at or in the source water, water treatment facility, or water distribution system versus contamination at the point of use (e.g., contaminated taps, hoses, or containers) and other points not under the jurisdiction of a public water utility (e.g., contamination of premise plumbing). This distinction allows for separate analyses of outbreaks associated with contamination of the water mains and storage facilities within the distribution system (e.g., infrastructure before the water meter or the customer's property line) and outbreaks associated with contamination of premise plumbing (e.g., pipes and storage facilities after the customer's property line or within buildings). Contamination of distribution water mains can occur due to broken or leaky water pipes, cross-connections, back siphonage, or corrosion. Premise plumbing deficiencies not only include similar sources of contamination but also include contamination associated with premise water treatment and with devices and equipment using or distributing water (such as drink mix machines). WBDOs due to Legionella colonization of premise plumbing systems (e.g., in heated water systems) are analyzed separately from other premise plumbing WBDOs.
The classification of drinking water systems in WBDOSS was revised in 1979 to reflect definitions of the Safe Drinking Water Act (SDWA), and appropriate changes were made for outbreaks reported during 1971 to 1978 (9). Drinking water systems are either public or nonpublic. Public water systems, which may be publicly or privately owned, are defined and regulated by EPA under this act. Public water systems are further classified as community or noncommunity systems. A community water system is a public system that serves at least 15 service connections used by year-round residents or that regularly serves at least 25 year-round residents. A noncommunity system is a public system that is not a community system but makes water available for public use (e.g., schools, restaurants, campgrounds, highway rest stations, and parks with their own water systems). An individual system does not meet the EPA definition of a public water system. It typically serves a single family or farm and might regularly provide water for as many as 24 persons or 14 connections. Individual systems are not subject to EPA regulations but may be regulated at the state level. Approximately 15 million households in the United States rely on individual water systems that are privately owned (19, 36). In past WBDOSS reports and surveillance summaries, WBDOs associated with commercially bottled water, bulk water purchases, and water not intended for drinking were included with WBDOs associated with individual water systems for analysis purposes. Commercially bottled water is subject to regulation by the Food and Drug Administration (FDA) and not the EPA. However, the FDA regulations generally follow EPA regulations. Since 2003, outbreaks associated with commercially bottled water, bulk water purchases, and water not intended for drinking have been classified and analyzed as their own water system categories; WBDOs involving mixed water system types have also been identified and analyzed as such. All outbreaks reported to the WBDOSS during 1971 to 2006 were reevaluated for consistent application of current water system classifications.
Nonrecreational outbreaks associated with water not intended for drinking (e.g., lakes, springs, and creeks used by campers and boaters; irrigation water; and other nonpotable sources with or without taps) and outbreaks associated with water of unknown intent are analyzed separately from outbreaks associated with drinking water. WBDOs occurring aboard cruise ships operating from U.S. ports are not included in the WBDOSS (47).
Statistical Analyses
WBDOSS data were analyzed using SAS 9.1 (SAS Institute Inc., Cary, NC). Trends in counts over time were investigated using Poisson regression. Trends in proportions were analyzed using logistic regression. Exact logistic regression was used when data were too sparse or skewed to assume large sample approximations. The chi-square test for equality of proportions was used to assess seasonal differences. Statistical significance was defined as a P value of <0.0500.
OUTBREAK CHARACTERIZATION AND TRENDS
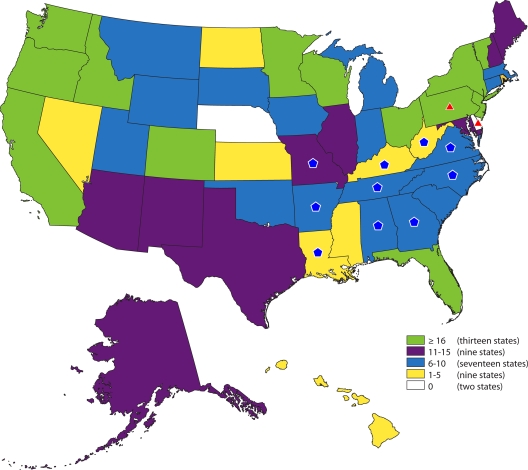
A total of 833 outbreaks associated with drinking water, water not intended for drinking, and water of unknown intent; 577,991 cases of illness; and 106 deaths reported during 1971 to 2006 met the criteria for inclusion in WBDOSS and are included in this review. WBDOs were reported in 48 states, Puerto Rico, the U.S. Virgin Islands, the Northern Mariana Islands, Palau, and the Republic of the Marshall Islands (Fig. 1). Included in the count of outbreaks are 15 single-case events where acute symptoms occurred shortly after water consumption and high levels of chemicals were identified in the water. Almost all of the reported outbreaks (n = 780 [93.6%], including 15 single-case chemical events), cases (n = 577,094, 99.8%), and deaths (n = 93, 87.7%) were associated with the contamination of drinking water. Forty-seven outbreaks (5.6%), 861 cases (0.1%), and 13 deaths (12.3%) were associated with water not intended for drinking; six outbreaks (0.7%) and 36 cases (<0.1%) were associated with water of unknown intent. The outbreaks reported for each of these three categories of water are analyzed separately in separate sections. The primary focus of the review is on drinking water outbreaks.
FIG. 1.
Drinking water-associated disease outbreaks (n = 780) reported to WBDOSS by state, 1971-2006, including 15 single cases. Thirteen outbreaks that occurred in Puerto Rico (n = 8), the Virgin Islands (n = 2), the Northern Mariana Islands (n = 1), Palau (n = 1), and the Republic of the Marshall Islands (n = 1) are not shown in the state outbreak counts but are included in the total outbreak count. Two multistate outbreaks (Alabama, Arkansas, Georgia, Kentucky, Louisiana, Missouri, North Carolina, Tennessee, Virginia, and West Virginia; Pennsylvania and Delaware), which are highlighted on the map using blue pentagons and red triangles, respectively, did not contribute to individual state counts. Numbers are dependent on reporting and surveillance activities in individual states and do not necessarily indicate that more outbreaks occurred in a given state.
All Outbreaks
Etiologic agents.
An etiology was identified in 467 (56.1%) of the 833 outbreaks reported during the 36-year period (Table 3). The most frequently identified etiologic agents were parasites (n = 153, 18.4%) and non-Legionella bacteria (n = 113, 13.6%). Fewer outbreaks were caused by chemicals (n = 90, 10.8%) or viruses (n = 66, 7.9%). Although WBDOs of legionellosis have been reported only since 2001, Legionella was identified as the etiology of 38 outbreaks. The largest outbreak reported since 1920 occurred in Milwaukee in 1993 and was associated with drinking water; Cryptosporidium hominis was eventually identified as the etiologic agent (32, 35, 48). Investigators estimated that 403,000 illnesses (24), 4,400 hospitalizations (8), and 50 deaths (16) were associated with this single outbreak. Deaths associated with other outbreaks were primarily due to bacterial pathogens: Legionella spp. (25 deaths), Salmonella enterica serovar Typhimurium (7 deaths), Vibrio cholerae O1 (6 deaths), Escherichia coli O157:H7 (6 deaths), and Shigella spp. (2 deaths). Six deaths were attributed to chemicals: arsenic (two deaths), fluoride (two deaths), ethylene glycol (one death), and nitrate (one death). Two deaths were attributed to primary amebic meningoencephalitis caused by Naegleria fowleri (4, 25) after exposure to drinking water; the exposure route was thought to be inhalation rather than ingestion. One death was attributed to norovirus. One death occurred in an outbreak of undetermined etiology.
TABLE 3.
Etiology of waterborne outbreaks and cases, 1971 to 2006
| Etiologic agent | No. (%) associated with: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Drinking water |
Water not intended for drinking |
Water of unknown intent |
All water types |
|||||
| Outbreaks | Cases | Outbreaks | Cases | Outbreaks | Cases | Outbreaks | Cases | |
| Non-Legionella bacteria | 105 (13.5) | 22,446 (3.9) | 7 (14.9) | 172 (20.0) | 1 (16.7) | 14 (38.9) | 113 (13.6) | 22,632 (3.9) |
| Legionellaa | 24 (3.1) | 126 (0.0) | 9 (19.1) | 241 (28.0) | 5 (83.3) | 22 (61.1) | 38 (4.6) | 389 (0.1) |
| Chemicals | 90b (11.5) | 3,901 (0.7) | 0 | 0 | 0 | 0 | 90 (10.8) | 3,901 (0.7) |
| Parasites | 143 (18.3) | 449,959c (78.0) | 10 (21.3) | 126 (14.6) | 0 | 0 | 153 (18.4) | 450,085 (77.9) |
| Viruses | 64 (8.2) | 16,728 (2.9) | 2 (4.3) | 47 (5.5) | 0 | 0 | 66 (7.9) | 16,775 (2.9) |
| Mixedd | 6 (0.8) | 1,755 (0.3) | 1 (2.1) | 2 (0.2) | 0 | 0 | 7 (0.8) | 1,757 (0.3) |
| Undetermined | 348 (44.6) | 82,179 (14.2) | 18 (38.3) | 273 (31.7) | 0 | 0 | 366 (43.9) | 82,452 (14.3) |
| Total | 780 (100) | 577,094 (100) | 47 (100) | 861 (100) | 6 (100) | 36 (100) | 833 (100) | 577,991 (100) |
Outbreaks associated with Legionella were reported only during 2001 to 2006.
Includes 15 events in which a single ill person was identified.
Includes 403,000 cases from a single cryptosporidiosis outbreak in Milwaukee, WI.
More than one infectious agent type (i.e., bacteria, chemicals, parasites, and viruses).
Water system deficiencies.
A total of 854 deficiencies were identified in the 833 WBDOs. Single deficiencies were identified in 815 (97.8%) of the 833 outbreaks, and two or more deficiencies were identified in 18 (2.2%) of the outbreaks. Included in this total were 38 outbreaks with unknown deficiencies. These deficiencies are described in greater detail in subsequent sections. The deficiency classifications in Table 5 make a clear distinction between outbreaks associated with contamination at points under the jurisdiction of a public water utility and those associated with contamination at points not under the jurisdiction of a public water utility. Legionellosis outbreaks are considered in separate categories according to the types of water involved (i.e., drinking water, water not intended for drinking, and water of unknown intent). WBDOs associated with Legionella are analyzed separately because they were not reported to WBDOSS prior to 2001 and they share characteristics that are distinct from other types of outbreaks (e.g., Legionella colonization of premise plumbing systems or cooling towers and inhalation as the route of water exposure). In this review, analyses of water system deficiency trends and causes of premise plumbing outbreaks exclude legionellosis outbreaks.
TABLE 5.
Deficiencies (n = 801) identified in drinking water outbreaks (n = 780), 1971 to 2006
| Deficiency | No.a |
|---|---|
| Contamination at points under the jurisdiction of a public water utilityb | |
| Untreated surface water | 21 |
| Untreated groundwater | 243 |
| Treatment deficiency (e.g., inadequate disinfection, no filtration) | 312 |
| Current treatment not expected to remove a chemical contaminant | |
| Surface water | 0 |
| Ground water | 1 |
| Distribution system deficiency (e.g., cross-connection, contamination of water mains or storage facility) | 79 |
| Contamination of plumbing and pipes not under the jurisdiction of a public water utilityc | |
| Legionella spp. in drinking water system (reported only during 2001 to 2006) | 24 |
| Plumbing system deficiency after the water meter or property line (e.g., cross-connection, corrosion products) | 55 |
| Deficiency in building/home-specific water treatment | 1 |
| Deficiency or contamination of equipment using or distributing water (e.g., drink mix machines) | 9 |
| Contamination of water at point of usec | |
| Container, bottle, or pitcher | 5 |
| Tap or hose | 4 |
| Commercially bottled water | 2 |
| Unknown source | 1 |
| Contamination during shipping, hauling, or storage | |
| Tap water stored in containers | 1 |
| Commercially bottled water | 1 |
| Contamination or treatment deficiency during commercial bottling | 5 |
| Insufficient information about deficiency | |
| Tap water | 33 |
| Commercially bottled water | 4 |
More than one deficiency was identified during the investigation of some outbreaks.
Contamination of water and deficiencies occurring in the drinking water system at or in the water source, treatment facility, or distribution system of pipes and storage facilities. For a community water system, the distribution system refers to the pipes and storage infrastructure prior to the water meter or property line; for noncommunity and individual water systems, the distribution system refers to the pipes and storage infrastructure prior to entry into a house or other building (47).
Contamination of drinking water and deficiencies occurring in plumbing and pipes that are not part of the distribution system. For community systems, the contamination occurred after the water meter or service connection; for noncommunity and individual water systems, contamination occurred in a service line leading to a house or building or in the plumbing inside a house or other building (47).
Outbreaks Associated with Drinking Water
Most (n = 680, 87.2%) of the 780 outbreaks associated with drinking water occurred in public water supplies, either in community (n = 338, 43.3%) or noncommunity (n = 342, 43.8%) water systems; 82 outbreaks (10.5%) occurred in individual water systems (Table 4). Eleven outbreaks (1.4%) were associated with the use of commercially bottled water, and one (0.1%) was associated with the purchase of bulk water used for drinking.
TABLE 4.
Waterborne outbreaks and cases associated with drinking water by water system type, 1971 to 2006
| Water type | No. (%) of: |
|
|---|---|---|
| Outbreaks | Cases of illness | |
| Community systems | 338 (43.3) | 518,091b (89.8) |
| Noncommunity systems | 342 (43.8) | 55,601 (9.6) |
| Individual systems | 82 (10.5) | 1,176 (0.2) |
| Bottled water | 11 (1.4) | 505 (<0.1) |
| Bulk water purchase | 1 (0.1) | 103 (<0.1) |
| Mixed systemsa | 3 (0.4) | 1,565 (0.3) |
| System not identified | 3 (0.4) | 53 (<0.1) |
| Total | 780 (100) | 577,094 (100) |
One outbreak of 20 cases (community and individual systems), one outbreak of 95 cases (individual system and bottled water), and one outbreak of 1,450 cases (noncommunity and individual systems).
Includes 403,000 cases from a single cryptosporidiosis outbreak in Milwaukee, WI.
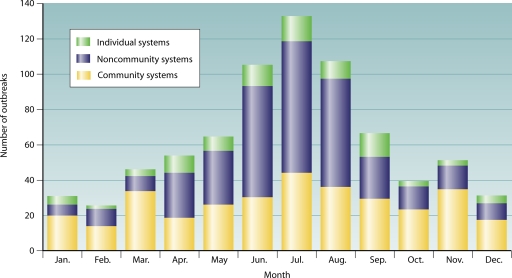
Outbreaks occurred throughout the year (Fig. 2) in all system types. Outbreaks in community, noncommunity, and individual water systems showed seasonal trends (community, P = 0.0002; noncommunity, P < 0.0001; and individual, P = 0.0006) and were reported most frequently during the summer from June to August, although there was no statistical difference between summer and fall among outbreaks in community systems. Nine outbreaks were excluded from analyses of seasonal effect: an outbreak associated with bulk water purchase, three outbreaks in mixed water systems, three outbreaks with unknown water system types, and two outbreaks with unknown months of onset. Too few outbreaks were associated with commercially bottled water to evaluate a seasonal distribution.
FIG. 2.
Number of outbreaks associated with drinking water by system type and month (n = 762), 1971 to 2006, excluding outbreaks associated with commercially bottled water (n = 11), bulk water purchase (n = 1), mixed water systems (n = 3), and unknown water systems (n = 3).
Illnesses.
During 1971 to 2006, 685 (87.8%) of the drinking water outbreaks were associated with acute gastrointestinal illness. The next most common illness type was hepatitis (n = 29, 3.7%), followed by acute respiratory illness (n = 24, 3.1% [all due to Legionella reported to WBDOSS since 2001]), skin conditions (n = 5, 0.6%), and neurological illness (n = 1, 0.1% [due to N. fowleri]). Ten (1.3%) of the drinking water outbreaks did not have illness types identified. The remaining outbreaks were associated with mixed illness types (n = 15, 1.9%), methemoglobinemia (n = 5, 0.6%), miscarriages (n = 2, 0.3%), or other illness types that were not easily categorized (n = 4, 0.5%) (i.e., change in hair color, metabolic acidosis and shock, fatigue and dizziness, and burning in the mouth).
Etiologic agents.
An etiology was determined for 432 (55.4%) of the 780 outbreaks associated with drinking water (Fig. 3), and no etiology was reported for 348 (44.6%) outbreaks. The most frequently identified etiologic agent types were parasites (n = 143, 18.3%) and non-Legionella bacteria (n = 105, 13.5%). Fewer outbreaks were caused by chemicals (n = 90, 11.5%) and viruses (n = 64, 8.2%). Legionella was identified as the etiology of 24 outbreaks reported during 2001 to 2006. Outbreaks involving multiple pathogens of the same agent type (e.g., Campylobacter jejuni and Shigella in a single bacterial outbreak) occurred in bacterial and parasitic WBDOs. None of the reported outbreaks involved multiple viral pathogens. Six outbreaks (0.8%) involved mixed agents (e.g., bacteria and parasites in the same outbreak). Three of the mixed-agent outbreaks involved non-Legionella bacteria and parasites (C. jejuni, Campylobacter lari, Cryptosporidium, and Helicobacter canadensis; C. jejuni, Entamoeba, and Giardia; and Salmonella and Giardia). The etiologic agents for the fourth mixed-agent outbreak were noroviruses G1 and G2 and C. jejuni. The etiologic agents for the fifth mixed-agent outbreak were C. jejuni, norovirus, and Giardia intestinalis. The final mixed-agent outbreak involved cases of self-limited acute gastroenteritis of undetermined etiology followed by the development of hepatitis A cases.
FIG. 3.
Percentages of etiologic agents in outbreaks associated with drinking water (n = 780), 1971 to 2006.
(i) Parasites.
G. intestinalis was identified as the sole pathogen in 123 (86.0%) of the 143 drinking water outbreaks of parasitic etiology. These Giardia outbreaks caused 28,127 cases (6.3% of the 449,959 cases attributed to parasites). Cryptosporidium was identified as the sole pathogen in 13 parasitic drinking water outbreaks (9.1%), causing 421,301 cases (93.6%), 403,000 of which were attributed to a single outbreak of Cryptosporidium hominis in Milwaukee, WI (32, 35, 48). Two outbreaks and 63 cases were attributed to Entamoeba histolytica and occurred in Missouri and Palau. Cyclospora caused a single outbreak of 21 cases in Illinois (17). N. fowleri caused a single outbreak of two cases in Arizona (4). In three outbreaks, more than one parasite was identified: Cryptosporidium and Giardia were identified in two outbreaks, and Giardia and E. histolytica were identified in one outbreak.
(ii) Non-Legionella bacteria.
Shigella was identified as the sole pathogen in 44 of the 105 nonlegionellosis bacterial drinking water outbreaks (41.9%) and in 9,177 of the 22,446 nonlegionellosis bacterial cases (40.9%); 34 (77.3%) of the Shigella outbreaks were caused by S. sonnei. Salmonella was identified as the sole pathogen in 20 outbreaks (19.0%) and 3,588 cases (16.0%), including 5 outbreaks of S. Typhi which occurred in Washington (n = 2), Florida, Maryland, and the U.S. Virgin Islands. Campylobacter was identified as the sole pathogen in 19 outbreaks (18.1%) and 5,565 cases (24.8%); 17 (89.5%) of the Campylobacter outbreaks were caused by C. jejuni. Escherichia coli was identified as the sole pathogen in 12 outbreaks (11.4%) and 2,901 cases (12.9%); 10 (83.3%) of the E. coli outbreaks were caused by E. coli O157:H7. Two additional sole-pathogen nonlegionellosis bacterial outbreaks were caused by Pleisiomonas shigelloides (60 cases), and Yersinia enterocolitica (2 cases). Non-O1 V. cholerae caused an outbreak of 11 cases in the Commonwealth of the Northern Mariana Islands, and V. cholerae O1 caused an outbreak of 103 cases in the Republic of the Marshall Islands. Multiple non-Legionella bacterial pathogens were identified in six outbreaks, all of which included C. jejuni. The bacteria that accompanied C. jejuni included Shigella, P. shigelloides, Y. enterocolitica, E. coli O157:H7, E. coli O145 and E. coli O157:H7, and other Campylobacter isolates that were not identified to the species level.
(iii) Viruses.
Almost all of the 64 viral drinking water outbreaks were attributed to norovirus (n = 34, 53.1%) or hepatitis A virus (n = 29, 45.3%). Most of the 16,728 viral cases were attributed to norovirus (n = 14,133, 84.5%); 834 cases (5.0%) were attributed to hepatitis A outbreaks. Rotavirus caused one outbreak (1.6%) and 1,761 cases (10.5%). There were no reported viral outbreaks involving multiple viral pathogens.
(iv) Chemicals.
Copper was the sole chemical in 27 (30.0%) of the 90 chemical outbreaks and in 369 (9.5%) of the 3,901 chemical cases. Fluoride was identified as the sole chemical in 11 outbreaks (12.2%) and 969 cases (24.8%). Nitrate was identified as the sole chemical in 8 outbreaks (8.9%) and cases 16 (0.4%). Thirty-eight additional single-chemical outbreaks occurred, causing 486 cases. Multiple chemicals were identified in six outbreaks, three of which included copper accompanied by fluoride, nitrate, or other minerals. The three remaining multiple-chemical outbreaks involved exposure to chlordane, heptachlor, toxaphene, and kerosene; ethyl benzene, toluene, and xylene; and bromate and other by-products of disinfection.
Water system types.
Although the annual number of reported drinking water outbreaks decreased considerably after 1980 (P < 0.0001), outbreaks continued to occur (Fig. 4). When the outbreaks were analyzed by water system type, the annual proportion of all drinking water outbreaks reported in public water systems (community and noncommunity) was found to decrease during 1971 to 2006 (P < 0.0001). In contrast, the annual proportion of drinking water outbreaks associated with individual water systems increased (P = 0.0002). Trends in the outbreaks associated with commercially bottled water, bulk water purchases, mixed water systems, and unknown water systems were not assessed because so few of these outbreaks were reported.
FIG. 4.
Number of outbreaks associated with drinking water by water system type and year (n = 780), 1971 to 2006. “Other” includes outbreaks associated with bottled water (n = 11), mixed water system types (n = 3), unknown systems (n = 3), and bulk water purchase (n = 1).
Magnitude of outbreaks and strength of evidence classification.
During 1971 to 2006, no change was observed in the mean annual number of cases per outbreak for community water systems (excluding the Milwaukee outbreak), noncommunity systems, or individual systems. For outbreaks in community systems, the average size of an outbreak during 1971 to 2006 was 340.5 cases per WBDO, excluding the Milwaukee outbreak. The average size of an outbreak in a noncommunity water system during 1971 to 2006 was 162.6 cases per WBDO. The average size of an outbreak in an individual water system during 1971 to 2006 was 14.3 cases per WBDO.
The mean strength of evidence class designation for outbreaks associated with drinking water increased during 1989 (when such classification began) through 2006 (P < 0.0001). The mean class designation for outbreaks in 1989 was 1.3 (class 1, adequate epidemiologic and water quality data). In contrast, the mean class designation in 2006 was 2.4, with a peak in 2004 of 2.9 (class 2, adequate epidemiologic data but insufficient water quality data; class 3, limited epidemiologic data but adequate water quality data).
Water system deficiencies.
In the 780 WBDOs associated with drinking water systems, investigators identified a single deficiency in 725 WBDOs (92.9%) and two or more deficiencies in 18 WBDOs (2.3%). In the remaining 37 WBDOs (4.7%), no deficiencies were reported; consequently, these outbreaks were categorized as having unknown deficiencies. The 801 deficiencies for the 780 WBDOs (Table 5) included the 37 WBDOs categorized as having unknown deficiencies. Also included in this total were the 24 outbreaks associated with Legionella in the water system. Although Legionella grows in biofilms in premise plumbing, Legionella outbreaks are considered separately from other premise plumbing deficiencies and are described in a subsequent section of this review.
During the 36-year period, the majority (n = 422, 52.7%) of the 801 deficiencies involved the use of contaminated groundwater, which had received either no treatment (n = 243, 30. 3%) or inadequate or interrupted treatment (n = 179, 22.3%) (Fig. 5). The next most common category of deficiencies (n = 148, 18.5%) involved the use of contaminated surface water, which had received either no treatment (n = 21, 2.6%) or inadequate or interrupted treatment (n = 127, 15.9%). Contamination within the distribution system and in premise plumbing, not including legionellosis outbreaks, was also important, accounting for 79 (9.9%) and 65 (8.1%) of the deficiencies, respectively.
FIG. 5.
Percentages of deficiencies (n = 801) in 780 outbreaks associated with drinking water, 1971 to 2006. The untreated groundwater deficiency category includes one outbreak involving treated groundwater where the treatment provided was not expected to remove the chemical contaminants.
Commercially Bottled Water, Bulk Water, and Point-of-Use Contamination
Commercially bottled water.
Eleven outbreaks reported during 1971 to 2006 were associated with contaminated commercially bottled water. Four (36.4%) of these outbreaks were due to inadequate treatment or contamination during the bottling process, two (18.2%) were due to contamination at the point of use, and one (9.1%) was due to contamination during shipping or storage. Insufficient information was available to characterize deficiencies for the other four commercially bottled water outbreaks (36.4%).
All commercially bottled water outbreaks were associated with acute gastrointestinal illness. Chemical contaminants were identified in four outbreaks (36.4%); in one of these WBDOs, investigators found illness associated with high levels of bromate, a by-product of ozone disinfection. Bacterial contaminants were identified in two outbreaks (18.2%). In 1994, non-O1 V. cholerae was responsible for an outbreak of 11 cases when contamination occurred at a plant providing bottled water for a small community in the Northern Mariana Islands (20). In 2000, S. sonnei was identified in an outbreak of 58 cases at a New Jersey school, which was associated with contamination of commercially bottled water during on-site storage. No etiologic agents were identified in the remaining five outbreaks (45.5%).
One mixed-system outbreak reported in 2000 was associated with both individual groundwater systems and commercially bottled water (22). Some of the bottles of water were marketed as water for infants, and others were marketed as spring water taken from the same geographic area as the individual wells. Investigators identified 95 cases of acute gastrointestinal illness attributed to S. Bareilly in 10 states. In an epidemiologic investigation that involved 46 of these cases, the median age was 6 years and many of the patients were infants. This outbreak was due to a combination of factors, including contamination during the bottling process, use of untreated groundwater, deficiencies in treatment of groundwater, and deficiencies in building-specific water treatment.
Bulk water.
In 2000, a single outbreak in the Republic of the Marshall Islands resulted in 103 acute gastrointestinal illness cases caused by V. cholerae O1 (2). This outbreak involved bulk water that had been contaminated during hauling or storage. Residents of Ebeye Island traveled by ferry to a neighboring island to purchase water, which they transported back to Ebeye Island in loosely sealed containers from which water was later removed by opening the vessels.
Point of use.
Twelve outbreaks were caused by contamination of water at its point of use. In addition to the two previously described commercially bottled water outbreaks involving point-of-use contamination, 10 outbreaks were associated with water contaminated in containers, pitchers, bottles, hoses, and hose bibs. All of these outbreaks resulted in acute gastrointestinal illness. In four outbreaks (40.0%), no etiologic agents were identified. In the remaining six outbreaks, water was contaminated by Shigella, Cryptosporidium parvum, G. intestinalis, norovirus (n = 2), or a chemical detergent.
Individual Water Systems
During the 36-year period, 82 outbreaks were associated with contaminated water from individual systems.
Illnesses.
The most common illnesses in individual systems were acute gastrointestinal illness (n = 58, 70.7%) and hepatitis (n = 10, 12.2%). The remaining illnesses were classified as other (n = 5, 6.1%), unknown (n = 5, 6.1%), or mixed (n = 4, 4.9%).
Etiologic agents.
An etiology was determined for 55 (67.1%) of the 82 outbreaks associated with individual water systems. Non-Legionella bacteria (n = 18, 22.0%) were more frequently identified than chemicals (n = 14, 17.1%), parasites (n = 12, 14.6%), or viruses (n = 10, 12.2%). One outbreak was of mixed-agent etiology (self-limited acute gastroenteritis of undetermined etiology followed by hepatitis A). None of the reported outbreaks in individual water systems were associated with Legionella.
(i) Parasites.
Ten (83.3%) of the 12 parasitic outbreaks had G. intestinalis as the sole pathogen. Cryptosporidium was identified as the sole pathogen in one outbreak (8.3%). One multiple-parasite outbreak (8.3%) involved both Giardia and C. parvum.
(ii) Non-Legionella bacteria.
The etiologies of the 18 nonlegionellosis bacterial outbreaks included E. coli O157:H7 (n = 4, 22.2%), S. Typhi (n = 3, 16.7%), S. sonnei (n = 3, 16.7%), C. jejuni (N = 2, 11.1%), Shigella flexneri (n = 2, 11.1%), C. jejuni and other Campylobacter isolates not identified to the species level (n = 1, 5.6%), Shigella isolates not identified to the species level (n = 1, 5.6%), Y. enterocolitica (n = 1, 5.6%), and both C. jejuni and Shigella in one nonlegionellosis multiple-bacterium outbreak (5.6%).
(iii) Viruses.
Hepatitis A virus was identified in all 10 viral outbreaks that occurred in individual water systems.
(iv) Chemicals.
Nitrate was the sole chemical identified in 7 of the 14 chemical outbreaks (50.0%), and copper was the sole chemical in 2 outbreaks (14.3%). Arsenic, gasoline, phenol, and selenium each caused one sole-chemical outbreak (7.1%). One chemical outbreak (7.1%) involved both nitrate and copper.
Locations.
The majority of outbreaks in individual systems occurred in private residences (n = 58, 70.7%) and in farms, rural, or agricultural settings (n = 9, 11.0%).
Water system deficiencies.
In the 82 WBDOs associated with individual water systems, investigators reported 80 deficiencies in 78 (95.1%) of these WBDOs. In the remaining four individual water system WBDOs (4.9%), no deficiencies were reported; consequently, these outbreaks were categorized as having unknown deficiencies. The 84 deficiencies for the 82 WBDOs included the 4 WBDOs categorized as having unknown deficiencies.
The majority (n = 70, 83.3%) of the 84 deficiencies were due to the use of contaminated untreated groundwater (n = 61, 72.6%) or deficiencies in treatment of contaminated groundwater (n = 9, 10.7%). The next most common deficiency category involved the use of contaminated, untreated surface water (n = 5, 6.0%); no treatment deficiencies associated with the use of surface water were reported. The remaining deficiencies included contamination in the distribution system (n = 4, 4.8%), contamination of premise plumbing (n = 1, 1.2%), and unknown deficiencies (n = 4, 4.8%).
Public Water Systems
During the 36-year period, 680 outbreaks were associated with contaminated water from public water systems: 338 outbreaks in community systems (49.7%) and 342 outbreaks in noncommunity systems (50.3%).
Illnesses.
Of the 338 community water system outbreaks, 282 (83.4%) were associated with acute gastrointestinal illness. Other illness types included acute respiratory illness caused by Legionella (n = 20, 5.9%), hepatitis A (n = 10, 3.0%), other illness (caused by copper, ethylene glycol [n = 2], nitrate, nitrite, and sodium hydroxide) (n = 6, 1.8%), skin conditions (n = 4, 1.2%), and neurological illness caused by N. fowleri (n = 1, 0.3%). Four (1.2%) of the community outbreaks did not have an illness type identified. The remaining community outbreaks were associated with mixed illness types (n = 11, 3.3%).
Of the 342 noncommunity water system outbreaks, 328 (95.9%) were associated with acute gastrointestinal illness. The next most common illness type was hepatitis (n = 9, 2.6%), followed by acute respiratory illness caused by Legionella (n = 4, 1.2%); one noncommunity outbreak (0.3%) had an unknown illness type.
Etiologic agents: community systems.
An etiology was determined for 244 (72.2%) of the 338 outbreaks associated with community water systems. Parasites (n = 99, 29.3%) were more frequently identified than chemicals (n = 60, 17.8%), non-Legionella bacteria (n = 41, 12.1%), and viruses (n = 21, 6.2%). Three community outbreaks (0.9%) were of mixed etiology: C. jejuni, Entamoeba, and Giardia; C. jejuni, C. lari, Helicobacter canadensis, and Cryptosporidium; and Salmonella and Giardia. Legionella was associated with 20 (5.9%) of the reported community outbreaks.
(i) Parasites.
G. intestinalis was identified as the sole pathogen in most (n = 84, 84.8%) of the 99 parasitic outbreaks in community systems. C. parvum (n = 9) or Cryptosporidium not identified to the species level (n = 1) was identified in 10 sole-pathogen outbreaks (10.1%). Single outbreaks of E. histolytica (37), Cyclospora (17), and N. fowleri (4, 25) were also reported. Two outbreaks (2.0%) involving multiple parasites were identified; both involved G. intestinalis, one with E. histolytica and one with C. parvum.
(ii) Non-Legionella bacteria.
Most of the 41 nonlegionellosis bacterial outbreaks in community systems were due to Shigella (n = 14, 34.1%), followed by Salmonella (n = 11, 26.8%), Campylobacter (n = 11, 26.8%), and E. coli (n = 5, 12.2%). Of the 14 outbreaks due to Shigella, S. sonnei was identified in 10 outbreaks (71.4%) and S. flexneri in two outbreaks (14.3%). Of the 11 outbreaks due to Salmonella, S. Typhimurium was identified in 3 outbreaks (27.3%), S. Enteritidis in one outbreak (9.1%), and S. Typhi in one outbreak (9.1%). The single outbreak of 60 cases of typhoid fever occurred in 1985 at a public housing facility in the U.S. Virgin Islands (34). Of the 11 outbreaks due to Campylobacter, C. jejuni was identified in 9 outbreaks (81.8%). Of the five toxigenic E. coli outbreaks, E. coli O157:H7 was identified in four outbreaks (80.0%).
(iii) Viruses.
Of the 21 viral outbreaks in community water systems, hepatitis A virus caused 10 outbreaks (47.6%), norovirus 10 outbreaks (47.6%), and rotavirus 1 outbreak (4.8%).
(iv) Chemicals.
Of the 60 outbreaks due to chemical contamination in community systems, 23 (38.3%) were caused by copper as the sole chemical, 6 (10.0%) were caused by fluoride, and 6 (10.0%) were caused by sodium hydroxide. The remaining 25 outbreaks were caused by other chemical contaminants, including two outbreaks (3.3%) that were caused by multiple chemicals: chlordane, heptachlor, toxaphene, and kerosene; and fluoride and copper.
Etiologic agents: noncommunity systems.
In 221 (64.6%) of the 342 outbreaks associated with noncommunity water systems an etiology was not determined. Non-Legionella bacteria (n = 42, 12.3%) were more frequently identified than viruses (n = 33, 9.7%), parasites (31, 9.1%), or chemicals (n = 10, 2.9%). One outbreak (0.3%) was of mixed-agent etiology (noroviruses G1 and G2 and C. jejuni). Legionella was associated with 4 noncommunity outbreaks (1.2%).
(i) Parasites.
G. intestinalis caused most (n = 28, 90.3%) of the 31 parasitic outbreaks in noncommunity systems. C. parvum was identified in two outbreaks (6.5%), and E. histolytica was identified in one outbreak (3.2%).
(ii) Non-Legionella bacteria.
Shigella was identified as the sole pathogen in 23 (54.8%) of the 42 nonlegionellosis bacterial outbreaks associated with noncommunity systems, with S. sonnei identified in 20 (87.0%) of these outbreaks. In six outbreaks (14.3%), C. jejuni was identified as the sole pathogen. Salmonella caused five sole-pathogen outbreaks (11.9%), with S. Javiana, S. Typhimurium, S. Weltev, and S. Typhi each identified in single outbreaks (one outbreak had no identified Salmonella serotype). The single outbreak of 210 cases of typhoid fever due to S. Typhi occurred in 1973 at a migrant labor camp in Dade County, FL, and was associated with a contaminated well (6). E. coli O157:H7 was identified in two sole-pathogen outbreaks (4.8%), and E. coli O6:H16 was identified in one outbreak (2.4%). P. shigelloides caused one sole-pathogen outbreak (2.4%). Four (9.5%) outbreaks involving multiple bacteria were identified: E. coli O157:H7 and C. jejuni; E. coli O157:H7, C. jejuni, and E. coli O145; C. jejuni and P. shigelloides; and C. jejuni and Y. enterocolitica.
(iii) Viruses.
Of the 33 viral outbreaks in noncommunity systems, norovirus caused 24 outbreaks (72.7%) and hepatitis A virus caused 9 outbreaks (27.3%).
(iv) Chemicals.
Fluoride caused 50.0% (n = 5) of the 10 outbreaks associated with chemical contamination in noncommunity systems. Other agents associated with chemical outbreaks include copper (n = 2, 20.0%), arsenic (n = 1, 10.0%), and oil (n = 1, 10.0%). One outbreak (10.0%) involving multiple chemicals was associated with both copper and other minerals.
Locations.
The majority of community outbreaks (n = 231, 68.3%) occurred in residential settings (e.g., community municipalities, apartments, subdivisions, mobile homes, and private residences). Other community outbreak locations included hospitals and nursing homes (n = 19, 5.6%); hotels, motels, lodges, and resorts (n = 18, 5.3%); restaurants and cafeterias (n = 16, n = 4.7%); and school, college, and university settings (n = 16, 4.7%). Other locations (n = 38) accounted for the remaining 11.2% of community outbreaks.
Most noncommunity outbreaks occurred in camps or recreational settings, e.g., camps, cabins, parks, and recreational areas (n = 151, 44.2%). Other noncommunity outbreak locations included hotels, motels, lodges, and resorts (n = 62, 18.1%); restaurants and cafeterias (n = 59, 17.3%); school, college, and university settings (n = 20, 5.8%); and other locations (n = 50, 14.6%).
Water system deficiencies.
In the 338 WBDOs associated with community water systems, investigators reported 323 deficiencies in 320 (94.7%) of these WBDOs. In the remaining 18 community water system WBDOs (5.3%), no deficiencies were reported; consequently, these outbreaks were categorized as having unknown deficiencies. The 341 deficiencies for the 338 WBDOs included the 18 WBDOs categorized as having unknown deficiencies. Of the 341 deficiencies, 103 (30.2%) were due to the use of contaminated, untreated surface water (n = 3, 0.9%) or to deficiencies in treatment of contaminated surface water (n = 100, 29.3%). The next most common category of deficiencies (n = 88, 25.8%) involved the use of contaminated untreated groundwater (n = 34, 10.0%) or deficiencies in treatment of contaminated groundwater (n = 54, 15.8%). Additionally, a substantial percentage of the outbreaks in community systems were due to premise plumbing deficiencies (n = 55, 16.1%) and contamination of the distribution system (n = 49, 14.4%). Not included among the premise plumbing outbreaks were 20 outbreaks associated with Legionella (5.9%); all were reported in community systems during 2001 to 2006. Of the remaining deficiencies, 18 (5.3%) were unknown, four (1.2%) involved treatment deficiencies in mixed groundwater and surface water systems, three (0.9%) involved point-of-use contamination, and one (0.3%) involved the use of a treatment method not expected to remove a chemical contaminant in a groundwater system.
In the 342 WBDOs associated with noncommunity water systems, investigators reported 343 deficiencies in 331 (96.8%) of these WBDOs. In the remaining 11 noncommunity water system WBDOs (3.2%), no deficiencies were reported; consequently, these outbreaks were categorized as having unknown deficiencies. The 354 deficiencies for the 342 WBDOs included the 11 WBDOs categorized as having unknown deficiencies. The majority (n = 261, 73.7%) of the 354 deficiencies in noncommunity systems were due to the use of contaminated untreated groundwater (n = 145, 41.0%) or to deficiencies in treatment of contaminated groundwater (n = 116, 32.8%). The next most common category of deficiencies (n = 40, 11.3%) involved the use of contaminated untreated surface water (n = 13, 3.7%) or deficiencies in treatment of contaminated surface water (n = 27, 7.6%). WBDOs in noncommunity systems were also caused by water distribution system contamination (n = 25, 7.1%) and premise plumbing deficiencies (n = 8, 2.3%). Not included among the premise plumbing outbreaks were four outbreaks (1.1%) associated with Legionella; all were reported in noncommunity systems during 2001 to 2006. Of the remaining deficiencies, 11 (3.1%) were unknown, 4 (1.1%) involved point-of-use contamination, and 1 (0.3%) involved a treatment deficiency in a mixed groundwater and surface water system.
Water System Deficiency Trends: Outbreaks in Public Water Systems
Although both the number of reported outbreaks and the number of associated deficiencies decreased during 1971 to 2006, the annual proportions of the different types of water system deficiencies showed various patterns. Because these patterns have public health implications, trends in the annual proportions of deficiencies were evaluated; these trends in deficiencies are summarized in Fig. 6 in 12-year periods. The trend analyses involving water system deficiencies exclude legionellosis outbreaks from both the numerators and denominators. However, outbreaks with unknown deficiencies are included because this category is an important indicator of surveillance functionality (investigation, ascertainment, and reporting) and because the proportion of unknown deficiencies also varies over time, affecting the proportions of the other categories of deficiencies.
FIG. 6.
Percentages of outbreak deficiencies (n = 671) in public water systems (n = 656) by time period, 1971 to 2006, excluding outbreaks associated with Legionella (n = 24). “Other” includes outbreaks associated with treatment deficiencies in mixed water sources (n = 5) and point of use contamination (n = 7).
Untreated drinking water.
During the 36-year period, a trend analysis found a statistically significant decrease in the annual proportion of reported deficiencies in public water systems associated with contaminated, untreated surface water (P = 0.0179). In contrast, there was no statistically significant change in the annual proportion of reported deficiencies in public water system outbreaks (either in community or noncommunity systems) associated with contaminated, untreated groundwater.
Water treatment deficiencies.
During the 36-year period, a trend analysis found a statistically significant decrease in the annual proportion of reported deficiencies that were associated with the inadequate or interrupted treatment of water by public water systems (P = 0.0495). This decrease in water treatment deficiencies was observed in community systems (P = 0.0177) but not in noncommunity systems.
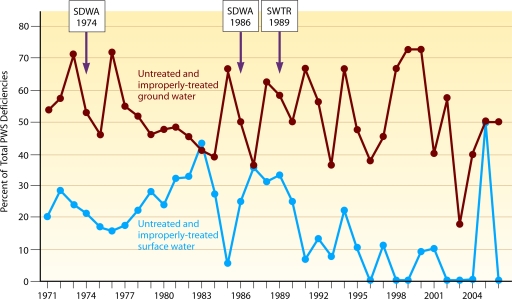
Further evaluation showed differences in the importance of water treatment in WBDOs by type of water source (Fig. 7). The annual proportion of treatment deficiencies involving surface water in public systems decreased over time (P = 0.0043). These deficiencies included inadequate disinfection of unfiltered surface water (e.g., contamination overwhelmed the chlorine dosage), interrupted disinfection (e.g., breakdown in equipment), inadequate filtration and/or pretreatment, and inadequate control of the addition of chemicals other than disinfectants (e.g., fluoride or sodium hydroxide). In contrast, no statistically significant change over time was observed in the annual proportion of treatment deficiencies involving groundwater in public systems. Inadequate or interrupted disinfection was the primary treatment deficiency for groundwater systems and was identified in 88.4% of the groundwater outbreaks reported in public systems. Although filtration is not generally required for most groundwaters, a few public water system WBDOs (3.4%) were also attributed to inadequate filtration of groundwater. The remaining groundwater outbreaks (8.2%) in public systems were caused either by a malfunction of equipment for the addition of chemicals other than a disinfectant or by an unspecified treatment deficiency.
FIG. 7.
Percentages of outbreak deficiencies in public water systems associated with untreated and improperly treated source water by year, 1971 to 2006, excluding outbreaks associated with Legionella and treatment deficiencies in mixed water sources. SDWA, Safe Drinking Water Act; SWTR, Surface Water Treatment Rule.
Untreated and inadequately treated water.
When deficiencies of untreated and improperly treated source water in public water system outbreaks were considered together (Fig. 7), the annual proportion of deficiencies associated with untreated and improperly treated surface water decreased over the 36-year period (P = 0.0004). However, the annual proportion of deficiencies associated with untreated and improperly treated groundwater did not change over time.
To explore the changing trends more closely, the 36-year surveillance period was divided in half and the trends in deficiencies associated with public water systems during each 18-year period were reexamined. No changes in the annual proportion of deficiencies associated with untreated and improperly treated source water (either surface water or groundwater) were observed during 1971 to 1988. Similarly, no change in the annual proportion of deficiencies associated with untreated and improperly treated groundwater were observed during 1989 to 2006, but a decrease in the annual proportion of those deficiencies associated with surface water was seen after 1989 (P = 0.0077).
Distribution system and premise plumbing deficiencies.
There was no statistically significant change in the annual proportion of distribution system deficiencies in public water systems during 1971 to 2006 in either community or noncommunity water system outbreaks. In contrast, there was an increase in the annual proportion of premise plumbing deficiencies (excluding legionellosis outbreaks) in public water systems during this time period (P = 0.0024) (Fig. 6).
Deficiencies under the jurisdiction of a public water utility or regulated by the government.
Deficiencies in public water systems involving untreated source water, treatment, or the distribution system are under the jurisdiction of a public water utility or a commercial water supplier (in the case of noncommunity water systems) and are therefore regulated by state and federal governments. During 1971 to 2006, 572 (83.3%) of the 695 deficiencies identified in public water system outbreaks met these criteria. Although each of these deficiencies was analyzed separately as noted previously, a combined analysis provides an indication of the effectiveness of regulations and efforts of water utilities to improve drinking water quality. The annual proportions of these deficiencies, when considered together, decreased over time during the 36-year period (P < 0.0001). In contrast, the annual proportion of deficiencies observed in premise plumbing increased, as described previously. Point-of-use deficiencies are also outside the jurisdiction of a water utility. However, trend analysis for these deficiencies was not possible, because only seven deficiencies were reported and six of them occurred since 1993.
Unknown deficiencies.
During the 36-year period, 29 outbreaks in public water systems (4.2%) had no deficiencies identified. The annual proportion of these unknown deficiencies increased over time (P = 0.0135).
Etiology Trends: Outbreaks in Drinking Water
Illnesses.
During 1971 to 2000, 630 (90.5%) of the 696 drinking water outbreaks were associated with acute gastrointestinal illness and none with acute respiratory illness. However, during 2001 to 2006, when legionellosis outbreaks were reported, 55 (65.5%) of the 84 drinking water outbreaks were associated with acute gastrointestinal illness and 24 (28.6%) with acute respiratory illness. From 1971 to 2000, the annual proportion of acute gastrointestinal illness outbreaks remained unchanged over time. In contrast, during 1971 to 2000, the annual proportion of hepatitis outbreaks decreased over time (P = 0.0052); only one hepatitis outbreak was reported during 2001 to 2006. The annual proportion of drinking water outbreaks with unknown illnesses also decreased over time during 1971 to 2000 (P = 0.0197); no drinking water outbreaks with unknown illnesses were reported during 2001 to 2006. Trend analyses for skin conditions (n = 5) and neurological conditions (n = 1) were not performed because so few outbreaks associated with these illnesses were reported.
Etiologic agents.
The annual proportion of nonlegionellosis bacterial, chemical, parasitic, and viral outbreaks in drinking water did not change over the 36-year period. Only the annual proportion of drinking water outbreaks with unidentified etiologic agents changed, decreasing over time during 1971 to 2000 (P = 0.0134) and during 2001 to 2006 (P = 0.0132) with the inclusion of legionellosis outbreaks.
Etiologic agents by water system type.
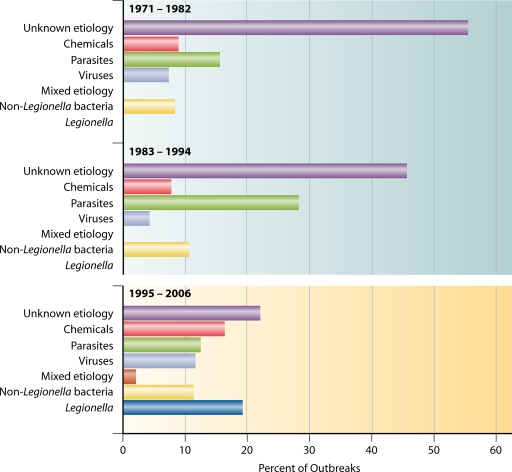
The proportions of etiologic agents associated with outbreaks in public water systems are shown collectively for each of the three 12-year periods in Fig. 8; this analysis included legionellosis outbreaks and illustrates the importance of legionellosis outbreaks during the most recent period, the only period in which they were reported. The following trend analyses considered the annual proportions of etiologic agents by water system type and excluded legionellosis outbreaks from both the numerators and denominators.
FIG. 8.
Percentages of etiologies in outbreaks associated with public water systems (n = 680) by time period, 1971 to 2006. Not included are two outbreaks involving both a public water system and an individual water system. Outbreaks associated with Legionella were reported only during 2001 to 2006.
In community water systems, the annual proportion of chemical outbreaks increased over time (P = 0.0018). No statistically significant trends were observed for nonlegionellosis bacterial, parasitic, and viral outbreaks. The annual proportion of outbreaks with unknown etiologies decreased over time (P = 0.0001).
In noncommunity water systems, the annual proportion of viral outbreaks increased over time (P = 0.0100). However, no statistically significant trends were observed for non-Legionella bacterial, chemical, and parasitic outbreaks. The annual proportion of outbreaks with unknown etiologies decreased over time (P = 0.0052).
In individual water systems, the annual proportion of chemical outbreaks decreased (P = 0.0229) over time. However, no statistically significant trends were observed for non-Legionella bacterial, parasitic, or viral outbreaks. In contrast with the overall trend toward more complete identification of etiologic agents in outbreaks reported in public systems, the proportion of unknown agents in individual systems increased (P = 0.0081).
Etiologic agents by source water type.
Over the 36-year period, no statistically significant trends in etiologic agents by source water type were observed among drinking water outbreaks except for those caused by parasitic diseases in the second half of the surveillance period (1989 to 2006). The annual proportion of parasitic outbreaks in drinking water associated with either groundwater or surface water did not change during 1971 to 1988. However, the annual proportion of parasitic outbreaks associated with groundwater increased (P = 0.0271) during 1989 to 2006, while the annual proportion of parasitic outbreaks associated with surface water decreased (P = 0.0060). Similar patterns were seen when only parasitic outbreaks associated with public water systems (community and noncommunity) were considered, although the decrease in the annual proportion of outbreaks associated with surface water after 1989 was borderline statistically significant (P = 0.0560). These trend analyses excluded legionellosis outbreaks from both the numerators and denominators.
Outbreaks Associated with Legionella in Drinking Water
During 2001 to 2006, Legionella was responsible for 24 (28.6%) of the 84 drinking water outbreaks, 126 (2.8%) of the 4,460 drinking water-related cases, and 12 (80.0%) of the 15 drinking water-related deaths. All outbreaks of acute respiratory illness (1971 to 2006) were caused by Legionella. Most of the legionellosis outbreaks were associated with L. pneumophila (n = 17, 70.8%). L. micdadei was identified in one outbreak (4.2%) and two cases. L. anisa was a copathogen in one outbreak (4.2%) with L. pneumophila.
The growth of Legionella in premise plumbing contributed to 24 (26.7%) of the 90 deficiencies reported in drinking water WBDOs during 2001 to 2006. Most legionellosis outbreaks associated with drinking water occurred in hospitals, health care facilities, and nursing homes (n = 16, 66.7%). Of these, 14 (87.5%) occurred in community water systems and 2 (12.5%) in noncommunity water systems. Four outbreaks (16.7%) occurred in hotels, motels, lodges, and inns; half of these were in community water systems and half in noncommunity water systems. Three (12.5%) outbreaks occurred in apartments and condominiums; all were associated with community water systems. The remaining legionellosis outbreak occurred in a gym with a community water supply.
Outbreaks in Water Not Intended for Drinking
During 1971 to 2006, 47 outbreaks and 861 cases involving water not intended for drinking were reported, comprising 5.6% of all reported outbreaks and 0.1% of all cases (0.5% without inclusion of the Milwaukee outbreak in 1993). Twenty-six outbreaks (55.3%) and 354 cases (41.1%) associated with water not intended for drinking were reported during the 13-year period from 1971 to 1983, and 21 outbreaks (44.7%) and 507 cases (58.9%) were reported during the 11-year period from 1996 to 2006; there were 12 years in the middle of the 36-year surveillance period during which no outbreaks associated with water not intended for drinking were reported, making trend analysis invalid.
Etiologic agents were identified in 29 (61.7%) of these outbreaks (Table 3). Parasitic and bacterial etiologies were the most frequently reported causes of these outbreaks, with G. intestinalis (n = 9), Legionella (n = 9), and E. coli O157:H7 (n = 3) the most frequently identified single etiologic agents. One outbreak among individuals who had consumed wastewater at a wastewater treatment plant involved mixed agents (Giardia and an unidentified agent) and caused two cases of acute gastrointestinal illness. Most of the outbreaks associated with water not intended for drinking (n = 36, 76.6%) involved acute gastrointestinal illness; nine outbreaks (19.1%) involved acute respiratory illness (all caused by Legionella), one (2.1%) involved hepatitis, and one (2.1%) involved dermatitis associated with Pseudomonas aeruginosa. All of the 13 deaths associated with these outbreaks were attributed to Legionella.
The outbreaks occurred primarily among hikers, campers, and other persons who drank untreated water from streams and lakes (n = 17, 36.2%). Four outbreaks (8.5%) caused legionellosis among hospital or nursing home patients exposed to cooling tower aerosols, four outbreaks (8.5%) occurred in factories and industrial facilities, and three outbreaks (6.4%) occurred at wastewater treatment plants as a result of drinking partially treated or untreated wastewater. The remaining outbreaks occurred in multiple different settings.
Outbreaks in Water of Unknown Intent
Six reported outbreaks and 36 cases involving water not intended for drinking were reported since 2003. No deaths were reported. Five (83.3%) of the outbreaks were associated with legionellosis (Table 3); two occurred at hotels, one at a nursing home, one at a city garage, and one at an apartment building. One outbreak (16.7%) that occurred at a sports complex was associated with acute gastrointestinal illness caused by E. coli O157:H7.
IMPLICATIONS OF OUTBREAK EVALUATION
Analysis of WBDOs reported during the past 36 years reveals several clear trends. The following discussion focuses on these trends and highlights emergent issues that need renewed emphasis and further research to help prevent waterborne disease and outbreaks associated with drinking water.
Decreased Reports of Drinking Water Outbreaks
The number of WBDOs associated with drinking water decreased during the 36-year review period. Possible explanations for this decrease include the impact of national drinking water regulations, changes in water system management and practices, and improvements to drinking water infrastructure, especially water treatment facilities. Also important is the cooperative partnership of public water systems and organizations, including EPA, the American Water Works Association, and the Association of State Drinking Water Administrators, known as the Partnership for Safe Water, with the objective of improving water plant performance (1). Although a significant decrease has occurred since peak reporting in 1980, a number of drinking water outbreaks are still reported each year. Continued cooperation and vigilance by water utility operators, regulators, and public health officials are required to maintain improvements in public health protection and ensure the safety of drinking water for the future. An effective program should include a focus on water system operation with monitoring of treatment effectiveness and water quality as well as the implementation of regulations and the periodic evaluation of their effectiveness.
Decreased Importance of Outbreaks in Public Systems and Increased Importance of Outbreaks in Individual Systems
A significant decrease in the annual proportion of reported outbreaks in public drinking water systems was observed; however, an increase was observed in the annual proportion of outbreaks associated with individual water systems. Although springs, lakes, and ponds may be used as water sources, privately owned individual systems primarily use well water. EPA regulations that protect public drinking water systems do not apply to individual water systems. The installation of these systems (e.g., location and construction of the well and any needed water treatment and water quality monitoring) may be regulated by state or local authorities, but the individual owners are usually responsible for ensuring that their water is safe once the system is in operation. To safeguard the quality of well water, homeowners should seek information on needed protective measures, including disinfection and other treatment options, and implement the recommended source protection, operation, and maintenance guidelines. Periodic water quality analyses should also be conducted. Additional educational activities should focus on the importance of routine water testing and system maintenance and should be targeted to well owners, users, drillers, and local and state drinking water personnel. EPA recommendations for protecting private wells are available at http://www.epa.gov/safewater/pwells1.html. CDC information about private wells is available at http://www.cdc.gov/healthywater/drinking/private/wells/index.html.
Decreased Importance of Surface Water Outbreaks and Continued Importance of Groundwater Outbreaks
There was a decrease in the annual proportion of deficiencies associated with untreated and improperly treated surface water as a cause of outbreaks in public drinking water systems. During 1986 to 2006, a decreased number of outbreaks in public systems using surface water were reported as the number of these systems was increasing from 9,527 (38) to 14,544 (43) systems. The decrease in the proportion of deficiencies associated with untreated or improperly treated surface water occurred in the second half of the surveillance period (1989 to 2006) after the promulgation of the Surface Water Treatment Rule (SWTR) in 1989 and subsequent amendments (39, 40, 41). The 1989 SWTR was designed to reduce waterborne risks from Giardia, Legionella, viruses, and heterotrophic bacteria; treatment requirements specified for the inactivation/removal of the more disinfection-resistant Giardia (99.9%) and viruses (99.99%) ensure that the bacteria are inactivated or removed. Amendments to the 1989 SWTR provided for more stringent treatment requirements to further reduce risks, including those posed by Cryptosporidium. To ensure that conventional and direct filtration systems provide for a 99% removal of Cryptosporidium, treatment requirements include the disinfection of all surface water sources and the filtration of surface water sources, except for those that meet strict requirements regarding watershed activities and raw water quality, and more stringent turbidity standards and monitoring requirements. The last outbreak in a public system that used unfiltered, disinfected surface water was reported in 1997. Giardia was the cause of the outbreak.
The annual proportion of deficiencies associated with untreated and improperly treated groundwater, however, did not change over time. The number of reported outbreaks in public systems using contaminated groundwater decreased slightly during 1986 to 2006, but the number of these systems also decreased, from 182,749 (38) to 142,100 (43). This suggests that improvements and interventions with groundwater systems were not as successful as those with surface water systems and that greater attention is needed to address and reduce the risk for outbreaks in groundwater systems. These outbreaks are a reminder not only of the potential for contamination of wells and springs but also that treatment, if provided, should be adequate to cope with source water contamination and should be operated without interruption.
Groundwater contamination can have a broad public health impact. Approximately 45% of the U.S. population is supplied by groundwater, through either public or individual water systems (19, 43). Outbreaks have occurred when improperly protected groundwater sources have been contaminated by surface water intrusion or sewage discharges and when wells in limestone, rock, or other unconfined aquifers have become contaminated. Even properly developed wells in confined aquifers may become contaminated. A recent study of groundwater aquifers serving public systems in Madison, WI, found the occurrence of human-pathogenic viruses in well water from a deep (65-m) confined aquifer. Twenty-three percent of samples were positive for enterovirus, including coxsackievirus and echovirus (5).
EPA's Ground Water Rule (42) was promulgated in 2006 to provide for increased protection against pathogens in public groundwater systems. Instead of requiring disinfection for all groundwater systems, the rule establishes a risk-targeted approach to identify those systems that may be most susceptible to fecal contamination and specifies corrective actions to reduce the risk of waterborne illness. The risk-targeted strategy includes regular sanitary surveys to identify significant deficiencies and source water monitoring to test for the presence of indicators of fecal contamination. Measures to protect public health include requirements to correct deficiencies, eliminate the source of contamination, provide an alternative source of water, or provide treatment that reliably achieves a reduction of at least 99.99% of viruses. When treatment is provided, compliance monitoring must be maintained to ensure that 99.99% removal is maintained. Implementation of the rule began on 1 December 2009, and a reduction in groundwater outbreaks should be seen in the future, analogous to what was seen after implementation of the SWTR for surface water systems.
Legionella and the Changing Illness Profile of Drinking Water Outbreaks
An important finding for outbreaks associated with drinking water is the change in illness profile of reported outbreaks since the addition of legionellosis outbreaks to the WBDOSS in 2001. Prior to 2001, acute gastrointestinal illness was the dominant illness type among reported outbreaks, and no outbreaks associated with acute respiratory illness were reported. However, from 2001 to 2006, while acute gastrointestinal illness remained the dominant illness type, 24 acute respiratory illness outbreaks were reported, all associated with Legionella, comprising 29% of all reported drinking water outbreaks during that 6-year period. Even though these WBDOs have been included in the surveillance system for only 6 years, Legionella (n = 38 outbreaks) is the third most common etiologic agent among all of the outbreaks associated with drinking water, water not intended for drinking, and water of unknown intent reported during the entire 36-period, surpassed only by Giardia (n = 135) and Shigella (n = 45). These outbreaks were all caused by the amplification and dissemination of Legionella through premise plumbing, pipes, and storage infrastructure not normally under the jurisdiction of a public water utility. Two-thirds of the legionellosis outbreaks associated with drinking water occurred in health care settings, demonstrating the ability of Legionella to colonize the biofilms frequently found inside the large, complex plumbing systems of hospitals. These data underscore the importance of maintaining a high index of suspicion for legionellosis associated with health care settings.
Legionellosis was first identified in 1976 (13), and outbreaks of legionellosis occurred prior to 2001 (18, 27, 33). CDC is currently conducting a review of legionellosis outbreaks that occurred prior to 2001 and plans to add these outbreaks to WBDOSS to provide a more complete representation of the role of Legionella in waterborne outbreaks. The expanded legionellosis outbreak review will likely increase the number of identified outbreaks and further emphasize the importance of Legionella as a waterborne pathogen.
The expanded review will also assess outbreak settings and deficiencies associated with these outbreaks to provide a better understanding of the location of outbreaks and the survival and inactivation of Legionella in parts of the water system not addressed by federal regulations.
Increased Importance of Outbreaks Due to Premise Plumbing Deficiencies; Distribution System Outbreaks Remained Constant over Time
The surveillance data indicate an increasing trend in the annual proportion of reported outbreaks associated with premise plumbing deficiencies with and without consideration of legionellosis WBDOs, which have been reported only since 2001 and are associated primarily with premise plumbing. No such trend is seen for outbreaks associated with distribution system deficiencies. Premise plumbing deficiencies occur outside the jurisdiction of a water utility at points not federally regulated and include plumbing deficiencies (e.g., cross-connections), deficiencies in building-specific water treatment, and deficiencies in or contamination of equipment using or distributing water (e.g., drink mix machines) in addition to Legionella. Point-of-use deficiencies are not included with premise plumbing deficiencies but comprise 1.5% of all drinking water deficiencies. Although the total number of outbreaks and their associated deficiencies has declined since 1980, the shifting epidemiology among reported WBDOs toward outbreaks associated with deficiencies occurring after the water meter or property line, including deficiencies inside buildings, highlights the need to focus greater attention on premise plumbing and point-of-use issues.
Among public water system WBDOs, the annual proportion of deficiencies associated with the distribution system has not significantly changed during the 36-year period. Previous reviews (10, 11) indicated a greater influence of distribution systems deficiencies, particularly in more recent years of WBDOSS reporting. The newly reclassified categories of deficiencies implemented in 2003 and expanded to the entire 36-year period for this review (23, 47) have allowed for separation of deficiencies under the jurisdiction of federal regulations and water utility management. This analysis has elucidated more clearly the growing role of Legionella and premise plumbing deficiencies in reported drinking water outbreaks. These distinctions will help inform regulatory processes and have important implications for future research and prevention efforts. Although distribution system deficiencies that are under the jurisdiction of federal regulations and water utility management represent a relatively small proportion of all deficiencies in drinking water outbreaks (i.e., approximately 10% overall and ranging from 0% to 31% in any given year), their continuing presence suggests that additional efforts are needed to reduce this risk. Recent epidemiologic studies in Sweden and Norway suggest that distribution system contamination can be a source of endemic illness and that factors contributing to contamination include leaking water mains and low water pressure (30, 31). LeChevallier et al. (21) demonstrated the occurrence of transient low-pressure events in distribution systems and cautioned that during these events, contaminants may enter the water system through pipeline leaks. The EPA's recent Drinking Water Infrastructure Needs Survey and Assessment (44) found that transmission and distribution system projects represent the largest category of infrastructure need by public drinking water utilities. Revisions to the Total Coliform Rule (TCR) should improve distribution system operations and monitoring to reduce health risks. In addition, the research agenda established through the Research and Information Collection Partnership (RICP) should develop a better understanding of seven priority distribution system issues identified during the TCR Federal Advisory Committee in 2008 to 2009: cross connections and backflow, storage facilities, mains construction and repair, pressure and intrusion, biofilm, nitrification, and contaminant accumulation (45).
Locations of Outbreaks: Recreational and Residential Foci
The primary outbreak locations in noncommunity systems are different from those locations common among community and individual systems and suggest that both recreational areas and residential settings need increased attention to prevent waterborne disease. Nearly half of noncommunity outbreaks occurred in camps, cabins, or other recreational areas, underscoring the importance of regular monitoring and water testing in these types of settings, which tend to be seasonal and have intermittent water use. Other important locations for noncommunity outbreaks included restaurants and cafeterias; hotels; and educational settings, including schools, colleges, and universities.
More than two-thirds of outbreaks in community systems during 1971 to 2006 occurred in residential settings (e.g., apartments, condominiums, and private residences), emphasizing the need for continued vigilance to protect community water supplies, the importance of multiagency and stakeholder coordination, and the need for rapid and effective communication of boil-water advisories to residents and the community when public water system breaches occur in residential areas. Health care settings, such as hospitals and nursing homes, were the second most common outbreak location in community systems, highlighting the need for continued vigilance to ensure provision of safe water to locations that serve populations that are more vulnerable, such as hospitalized patients or nursing home residents with preexisting medical conditions.
Seventy-one percent of outbreaks in individual systems occurred in private residences, suggesting that greater outreach, awareness, and assistance are needed to help protect water quality in private wells.
Sporadic Outbreaks Associated with Water Not Intended for Drinking and Water of Unknown Intent
Relatively few outbreaks associated with water not intended for drinking and water of unknown intent were reported during the 36-year period, and their sporadic reports offer no information about trends. A review of the most recent outbreaks (47) noted the continued occurrence of outbreaks of legionellosis in health care settings associated with aerosols from cooling towers and outbreaks associated with consumption of water not intended for drinking during camping and backcountry travel (i.e., travel in wilderness environments) in the United States.
Surveillance Data Quality Issues
The quality of the surveillance data is variable and needs improvement for better consistency across data fields and individual outbreaks. Some positive data quality indicators include the decreasing annual proportion of outbreaks associated with unknown etiologies; this might be a function of newly developed and improved laboratory diagnostic tools to identify etiologic agents in clinical specimens and in water samples (e.g., detection of norovirus), resulting in more frequent identification of etiologic agents in recent years. The proportion of illnesses labeled as unknown has also decreased; improved epidemiologic investigation is one possible explanation for this trend.
Data quality indicators of concern include the consistency in the mean number of cases per outbreak per year among outbreaks associated with drinking water. The average size of reported outbreaks has not changed over time, suggesting that detection of smaller outbreaks, which are presumably more common than larger outbreaks, might not be improving. Furthermore, the proportion of outbreaks with unknown deficiencies and the mean class designation for strength of evidence (with class 1 indicating strong epidemiologic, clinical, and environmental evidence implicating water and class 4 indicating less comprehensive evidence [47]) (Table 1) have increased over time. This suggests a trend toward less complete information, particularly environmental information, and lower strength of evidence for outbreaks over time. This decrease in environmental investigation data might be related to decreasing local health department regulation, licensing, or inspection of public and private water systems during the last 2 decades (28, 29), potentially leading to decreased ability to investigate waterborne disease outbreaks. These trends indicate the need for improved surveillance and investigation, in particular for local health departments to maintain the capacity for appropriate environmental and risk factor investigation to identify the antecedent causes of outbreaks. Such investigations will provide information to help public health practitioners, regulatory agencies, and water utilities improve management practices, develop appropriate policy and regulation, and implement interventions and preventive measures to reduce the incidence of waterborne disease. A first step toward improving surveillance data quality is to provide greater emphasis on and more resources for outbreak detection and investigation, particularly environmental investigation, at local, state, territorial, and national levels. It is important to remember that the information available in WBDOSS has been voluntarily provided to CDC and EPA by state, local, and territorial health departments. Not all outbreaks have been recognized, investigated, or reported, and reliable estimates of the number of unrecognized WBDOs are not available. Additional recommendations for improving monitoring, detection, and prevention of waterborne disease in the United States are listed in Table 6.
TABLE 6.
Recommendations to prevent waterborne disease and improve surveillance
| Recommendation |
|---|
| Continued focus on water system operations, management, water quality, and effective regulations to sustain gains made in the provision of safe drinking water |
| Greater attention to address and prevent waterborne disease transmission in unregulated drinking water systems |
| Greater attention to address and reduce the risk for outbreaks in groundwater systems |
| Improved understanding of the biology, ecology, and inactivation of Legionella in biofilm and premise plumbing |
| Improved recognition and focus on premise plumbing and point-of-use issues |
| Additional efforts to reduce the risk of outbreaks associated with drinking water distribution systems |
| Improved prevention efforts in residential and recreational settings (e.g., campgrounds and parks) to reduce waterborne disease outbreaks |
| Research to better characterize the burden of waterborne disease in the United States so that prevention actions can be prioritized and interventions to reduce waterborne disease can be developed |
| Additional resources for outbreak detection and investigation of waterborne disease and outbreaks—including epidemiologic, environmental, and laboratory support—at local, state, territorial, and national levels |
CONCLUSIONS
In this review, we characterize the causes of WBDOs, focusing on changes that have occurred during the past 36 years in the etiologic agents, risk factors, and major deficiencies identified during WBDO investigations. The outbreak surveillance data captured by WBDOSS do not include reports of endemic or nonoutbreak waterborne disease. Thus, the statistics in this report represent only a portion of the burden of illness associated with contaminated drinking water and thereby limit the interpretations that can be made in terms of illness burden. Also, the epidemiologic trends and water quality concerns observed in outbreaks might not necessarily reflect or correspond with trends associated with endemic waterborne illness. The annual number of endemic acute gastrointestinal illness cases associated with consumption of public drinking water in the United States has been estimated to range from 4.3 to 11.7 million cases (7) and from 5.5 to 32.8 million cases (26). These estimates, however, describe only a portion of the annual incidence of endemic waterborne disease cases; they do not include the number of cases of waterborne disease other than acute gastrointestinal illness or the number of cases associated with nonpublic drinking water systems, commercially bottled water, water not intended for drinking, water of unknown intent, and recreational water. The two estimates have wide confidence intervals, indicating that further research is needed to characterize national estimates of endemic waterborne disease in the United States.
Developing a comprehensive, pathogen-based waterborne disease burden estimate is important to help public health agencies prioritize needs, support programmatic efforts that focus on waterborne disease surveillance and prevention, and develop interventions to reduce the burden of waterborne disease in the United States.
Although WBDOSS surveillance statistics do not reflect endemic waterborne illness, analysis of WBDOSS data has identified important trends and contributing factors that cause outbreaks, which in turn has helped shape research, regulations, and improvements in drinking water quality. In recent years, these analyses have uncovered new areas of concern (e.g., individual systems, premise plumbing, and Legionella). Improvements in the WBDOSS should help lead to more timely recognition and reporting of WBDOs and identification of water system problems and deficiencies. Maintaining the WBDOSS is essential to inform and guide prevention efforts and allocate needed resources at national, state, territorial, and local levels.
Acknowledgments
This review was written during the illness and loss of our colleague, Dr. Rebecca Calderon, who passed away in December 2008, and we dedicate this review to her memory. Rebecca served in many leadership roles during her nearly 20-year career at EPA, with a final posting as the Director of the Human Studies Division at EPA's National Health and Environmental Effects Research Laboratory. She was well known for her enthusiasm, dedication, and commitment to the prevention of waterborne disease, and since 1993, she was a member of the CDC-EPA collaboration efforts to analyze and report data on waterborne disease outbreaks. We acknowledge her significant contributions to public health as well as her extensive contributions to waterborne disease outbreak surveillance and her championing of waterborne disease prevention in general. Those of us who worked closely with Rebecca will miss her greatly in the future as a colleague, mentor, and friend.
We thank the following persons for contributions to this report: state and territorial waterborne disease surveillance coordinators, environmental health personnel, and drinking water administrators; state epidemiologists; Lauri Hicks, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases; Sharlene Persaud, Eric Dziuban, Elizabeth Medlin, Melanie Strahm, and Brittany Eddy, Division of Parasitic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, CDC; and Susan Shaw, Office of Ground Water and Drinking Water, EPA.
Biography
 Gunther F. Craun, M.P.H., has been active for more than 40 years in identifying waterborne risks and ways to reduce them. In addition to performing research and writing, he has chaired or participated in advisory panels and workshops held by various agencies and national and international organizations. From 1970 to 1991, he held several positions with the U.S. Environmental Protection Agency, including Coordinator of the Environmental Epidemiology Program in the Health Effects Research Laboratory. During this time, he conducted epidemiological and engineering research and helped initiate and maintain the waterborne disease outbreak surveillance system described in this article. In 1991, he established a firm that provides research and consulting services on water-related health issues. Mr. Craun received the degree of master of science in environmental engineering from Virginia Polytechnic Institute and State University and the degrees of master of public health and master of science in epidemiology from Harvard University.
Gunther F. Craun, M.P.H., has been active for more than 40 years in identifying waterborne risks and ways to reduce them. In addition to performing research and writing, he has chaired or participated in advisory panels and workshops held by various agencies and national and international organizations. From 1970 to 1991, he held several positions with the U.S. Environmental Protection Agency, including Coordinator of the Environmental Epidemiology Program in the Health Effects Research Laboratory. During this time, he conducted epidemiological and engineering research and helped initiate and maintain the waterborne disease outbreak surveillance system described in this article. In 1991, he established a firm that provides research and consulting services on water-related health issues. Mr. Craun received the degree of master of science in environmental engineering from Virginia Polytechnic Institute and State University and the degrees of master of public health and master of science in epidemiology from Harvard University.
 Joan M. Brunkard, Ph.D., is an epidemiologist with the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. She received a B.S. degree in the Department of Environmental Science, Policy and Management from the University of California, Berkeley, in 1998 and a Ph.D. degree in the Department of Environmental Studies from the University of California, Santa Cruz, in 2006; her doctoral research focused on dengue fever seroprevalence and risk factors on the United States-Mexico border. Dr. Brunkard has worked for more than 20 years on environmental and public health issues in the United States, Africa, and Latin America in academic, corporate, government, and nongovernment settings. She joined CDC in 2006 as an Epidemic Intelligence Service (EIS) officer, assigned to New Orleans, LA, following Hurricane Katrina. Her research interests include the ecology of infectious diseases, drinking water, climate change science and policy, and the epidemiology of waterborne and mosquito-borne diseases.
Joan M. Brunkard, Ph.D., is an epidemiologist with the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. She received a B.S. degree in the Department of Environmental Science, Policy and Management from the University of California, Berkeley, in 1998 and a Ph.D. degree in the Department of Environmental Studies from the University of California, Santa Cruz, in 2006; her doctoral research focused on dengue fever seroprevalence and risk factors on the United States-Mexico border. Dr. Brunkard has worked for more than 20 years on environmental and public health issues in the United States, Africa, and Latin America in academic, corporate, government, and nongovernment settings. She joined CDC in 2006 as an Epidemic Intelligence Service (EIS) officer, assigned to New Orleans, LA, following Hurricane Katrina. Her research interests include the ecology of infectious diseases, drinking water, climate change science and policy, and the epidemiology of waterborne and mosquito-borne diseases.
 Jonathan Yoder, M.S.W., M.P.H., is an epidemiologist with the Centers for Disease Control and Prevention in the Waterborne Disease Prevention Branch. He coordinates the Waterborne Disease and Outbreak Surveillance System at CDC. This system is the primary source of information on waterborne disease outbreaks in the United States. Duties include assisting states and territories with outbreak investigations, analysis of waterborne disease outbreak data, and answering inquiries from concerned citizens. He has previously worked with the Illinois Department of Public Health, assisting state and local health departments with infectious disease outbreaks, and in India, supporting polio eradication efforts. Mr. Yoder obtained his graduate degrees from the University of South Florida.
Jonathan Yoder, M.S.W., M.P.H., is an epidemiologist with the Centers for Disease Control and Prevention in the Waterborne Disease Prevention Branch. He coordinates the Waterborne Disease and Outbreak Surveillance System at CDC. This system is the primary source of information on waterborne disease outbreaks in the United States. Duties include assisting states and territories with outbreak investigations, analysis of waterborne disease outbreak data, and answering inquiries from concerned citizens. He has previously worked with the Illinois Department of Public Health, assisting state and local health departments with infectious disease outbreaks, and in India, supporting polio eradication efforts. Mr. Yoder obtained his graduate degrees from the University of South Florida.
 Virginia A. Roberts, M.S.P.H., is an epidemiologist with the Atlanta Research and Education Foundation, Inc., working at the Centers for Disease Control and Prevention in the Waterborne Disease Prevention Branch. She received a joint master of science in public health degree in 2007 from Emory University's Rollins School of Public Health in the areas of epidemiology and environmental and occupational health. She is the surveillance epidemiologist for the Waterborne Disease and Outbreak Surveillance System.
Virginia A. Roberts, M.S.P.H., is an epidemiologist with the Atlanta Research and Education Foundation, Inc., working at the Centers for Disease Control and Prevention in the Waterborne Disease Prevention Branch. She received a joint master of science in public health degree in 2007 from Emory University's Rollins School of Public Health in the areas of epidemiology and environmental and occupational health. She is the surveillance epidemiologist for the Waterborne Disease and Outbreak Surveillance System.
 Joe Carpenter, P.E., is an environmental engineer in the Division of Healthcare Quality Promotion at the Centers for Disease Control and Prevention in Atlanta, GA. He previously worked at the U.S. Environmental Protection Agency, Region IV, in Atlanta. He received an M.S. in environmental engineering from Clemson University in 1975 and an M.B.A. from Georgia State University in 1989. He is a licensed professional engineer and provides outbreak support for waterborne epidemic investigations.
Joe Carpenter, P.E., is an environmental engineer in the Division of Healthcare Quality Promotion at the Centers for Disease Control and Prevention in Atlanta, GA. He previously worked at the U.S. Environmental Protection Agency, Region IV, in Atlanta. He received an M.S. in environmental engineering from Clemson University in 1975 and an M.B.A. from Georgia State University in 1989. He is a licensed professional engineer and provides outbreak support for waterborne epidemic investigations.
 Timothy J. Wade, Ph.D., M.P.H. is an epidemiologist with the U.S. Environmental Protection Agency in the Office of Research and Development at the Human Studies Division in Chapel Hill, NC. He received a master of public health degree in epidemiology and biostatistics in 1998 from the University of California, Berkeley, and a Ph.D. degree in epidemiology in 2002, also from the University of California at Berkeley. He joined the U.S. EPA ORD in 2003 as an R-authority postdoc and was awarded a full-time position in 2005. His research focuses on quantifying and measuring the health effects of waterborne contaminants. Dr. Wade holds an adjunct appointment in the Epidemiology Division at the University of North Carolina.
Timothy J. Wade, Ph.D., M.P.H. is an epidemiologist with the U.S. Environmental Protection Agency in the Office of Research and Development at the Human Studies Division in Chapel Hill, NC. He received a master of public health degree in epidemiology and biostatistics in 1998 from the University of California, Berkeley, and a Ph.D. degree in epidemiology in 2002, also from the University of California at Berkeley. He joined the U.S. EPA ORD in 2003 as an R-authority postdoc and was awarded a full-time position in 2005. His research focuses on quantifying and measuring the health effects of waterborne contaminants. Dr. Wade holds an adjunct appointment in the Epidemiology Division at the University of North Carolina.
 Jacquelin M. Roberts, M.S., is a statistician with the Centers for Disease Control and Prevention in the Center for Global Health, Division of Parasitic Diseases and Malaria. She received her master's degree in decision science/statistics from Georgia State University and has provided statistical support to the division since 1983.
Jacquelin M. Roberts, M.S., is a statistician with the Centers for Disease Control and Prevention in the Center for Global Health, Division of Parasitic Diseases and Malaria. She received her master's degree in decision science/statistics from Georgia State University and has provided statistical support to the division since 1983.
 Michael J. Beach, Ph.D., received his Ph.D. in biochemistry from Purdue University. He joined the Centers for Disease Control and Prevention (CDC) as a research chemist in 1989 and served in CDC's Epidemic Intelligence Service from 1995 to 1997. He subsequently created CDC's Healthy Swimming Program and has served as Team Leader for the Water and Environment Activity and Associate Director for Healthy Water in the National Center for Zoonotic, Vector-borne, and Enteric Diseases. He is now heading the Waterborne Disease Prevention Branch and serving as Associate Director for Healthy Water in CDC's new National Center for Emerging and Zoonotic Infectious Diseases. He is a strong advocate for reducing the global burden of disease associated with water, sanitation, and hygiene. His domestic interests include collecting and solidifying data that will better characterize the magnitude and burden of waterborne disease in the United States and increase recognition of waterborne disease transmission in the United States.
Michael J. Beach, Ph.D., received his Ph.D. in biochemistry from Purdue University. He joined the Centers for Disease Control and Prevention (CDC) as a research chemist in 1989 and served in CDC's Epidemic Intelligence Service from 1995 to 1997. He subsequently created CDC's Healthy Swimming Program and has served as Team Leader for the Water and Environment Activity and Associate Director for Healthy Water in the National Center for Zoonotic, Vector-borne, and Enteric Diseases. He is now heading the Waterborne Disease Prevention Branch and serving as Associate Director for Healthy Water in CDC's new National Center for Emerging and Zoonotic Infectious Diseases. He is a strong advocate for reducing the global burden of disease associated with water, sanitation, and hygiene. His domestic interests include collecting and solidifying data that will better characterize the magnitude and burden of waterborne disease in the United States and increase recognition of waterborne disease transmission in the United States.
 Sharon L. Roy, M.D., M.P.H., is a medical epidemiologist with the Centers for Disease Control and Prevention in the Waterborne Disease Prevention Branch. She coordinates a program focused on water/sanitation/hygiene (WASH)-related disease and infrastructure sustainability in underserved communities around the world, with an emphasis on WASH-related neglected tropical diseases. Dr. Roy completed a master's of public health and a preventive medicine residency at Johns Hopkins University. She is board certified in family medicine and general preventive medicine, and prior to working for CDC, she practiced medicine in Canada, Australia, and Saudi Arabia. She came to CDC as an Epidemic Intelligence Service (EIS) officer in 2001 and has worked on domestic and international WASH issues for the agency since 2004.
Sharon L. Roy, M.D., M.P.H., is a medical epidemiologist with the Centers for Disease Control and Prevention in the Waterborne Disease Prevention Branch. She coordinates a program focused on water/sanitation/hygiene (WASH)-related disease and infrastructure sustainability in underserved communities around the world, with an emphasis on WASH-related neglected tropical diseases. Dr. Roy completed a master's of public health and a preventive medicine residency at Johns Hopkins University. She is board certified in family medicine and general preventive medicine, and prior to working for CDC, she practiced medicine in Canada, Australia, and Saudi Arabia. She came to CDC as an Epidemic Intelligence Service (EIS) officer in 2001 and has worked on domestic and international WASH issues for the agency since 2004.
REFERENCES
- 1.American Water Works Association. 1995. Partnership for safe water. http://www.awwa.org. Accessed 16 February 2010.
- 2.Beatty, M. E., T. Jack, S. Sivapalasingam, S. S. Yao, I. Paul, B. Bibb, K. D. Greene, K. Kubota, E. D. Mintz, and J. T. Brooks. 2004. An outbreak of Vibrio cholerae O1 infections on Ebeye Island, Republic of the Marshall Islands, associated with use of an adequately chlorinated water source. Clin. Infect. Dis. 38:1-9. [DOI] [PubMed] [Google Scholar]
- 3.Benin, A. L., R. F. Benson, and R. E. Besser. 2002. Trends in Legionnaires disease, 1980-1998: declining mortality and new patterns of diagnosis. Clin. Infect. Dis. 35:1039-1046. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn, B. G., G. F. Craun, J. S. Yoder, V. Hill, R. L. Calderon, N. Chen, S. H. Lee, D. A. Levy, and M. J. Beach. 2004. Surveillance for waterborne-disease outbreaks associated with drinking water—United States, 2001-2002. MMWR Surveill. Summ. 53(SS-8):23-45. [PubMed] [Google Scholar]
- 5.Borchardt, M. A., K. R. Bradbury, M. B. Gotkow, J. A. Cherry, and B. L. Parker. 2007. Human enteric viruses in groundwater from a confined bedrock aquifer. Environ. Sci. Technol. 41:6606-6612. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control. 1973. Epidemiologic notes and reports typhoid fever—Florida. MMWR Morb. Mortal. Weekly Rep. 22:77-78. [Google Scholar]
- 7.Colford, J. M., S. L. Roy, M. J. Beach, A. Hightower, S. E. Shaw, and T. J. Wade. 2006. A review of household drinking water intervention trials and an approach to the estimation of endemic waterborne gastroenteritis in the United States. J. Water Health 4(Suppl. 2):71-88. [DOI] [PubMed] [Google Scholar]
- 8.Corso, P. S., M. H. Kramer, K. A. Blair, D. B. Addiss, J. P. Davis, and A. C. Haddix. 2003. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg. Infect. Dis. 9:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craun, G. F. 1979. Waterborne disease outbreaks in the United States. J. Environ. Health 41:259-265. [Google Scholar]
- 10.Craun, G. F., and L. J. McCabe. 1973. Review of the causes of waterborne-disease outbreaks. J. Am. Water Works Assoc. 65:74-84. [Google Scholar]
- 11.Craun, G. F., and R. L. Calderon. 2001. Waterborne disease outbreaks caused by distribution system deficiencies. J. Am. Water Works Assoc. 93:64-75. [Google Scholar]
- 12.Eliassen, R., and R. H. Cummings. 1948. Analysis of waterborne outbreaks, 1938-45. J. Am. Water Works Assoc. 40:509-528. [Google Scholar]
- 13.Fraser, D. W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, C. C. Shepard, and P. S. Brachman. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189-1197. [DOI] [PubMed] [Google Scholar]
- 14.Gorman, A. E., and A. Wolman. 1939. Water-borne outbreaks in the United States and Canada and their significance. J. Am. Water Works Assoc. 31:225-275. [Google Scholar]
- 15.Herwaldt, B. L., G. F. Craun, S. L. Stokes, and D. D. Juranek. 1991. Waterborne-disease outbreaks, 1989-1990. MMWR Surveill. Summ. 40(SS-3):1-21. [PubMed] [Google Scholar]
- 16.Hoxie, N. J., J. P. Davis, J. M. Vergeront, R. D. Nashold, and K. A. Blair. 1997. Cryptosporidiosis-associated mortality following a massive waterborne outbreak in Milwaukee, Wisconsin. Am. J. Pub. Health 87:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, P., J. T. Weber, D. M. Sosin, P. M. Griffin, E. G. Long, J. J. Murphy, F. Kocka, C. Peters, and C. Kallick. 1995. The first reported outbreak of diarrheal illness associated with Cyclospora in the United States. Ann. Intern. Med. 123:409-414. [DOI] [PubMed] [Google Scholar]
- 18.Keller, D. W., R. Hajjeh, A. DeMaria, B. S. Fields, J. M. Pruckler, R. S. Benson, P. E. Kludt, S. M. Lett., L. A. Mermel, C. Giorgio, and R. F. Breiman. 1996. Community outbreak of Legionnaire's disease: an investigation confirming the potential for cooling towers to transmit Legionella species. Clin. Infect. Dis. 22:257-261. [DOI] [PubMed] [Google Scholar]
- 19.Kenny, J. F., N. L. Barber, S. S. Hutson, K. S. Linsey, J. K. Lovelace, and Maupin, M. A. 2009. Estimated use of water in the United States in 2005: U.S. Geological Survey circular 1344, 52 p. http://pubs.usgs.gov/circ/1344/. Accessed 19 February 2010.
- 20.Kramer, M. H., B. L. Herwadlt, G. F. Craun, R. L. Calderon, and D. D. Juranek. 1996. Surveillance for waterborne-disease outbreaks—United States, 1993-1994. MMWR Surveill. Summ. 45(SS-1):1-33. [PubMed] [Google Scholar]
- 21.LeChevallier, M. W., R. W. Gullick, M. R. Karim, M. Friedman, and J. E. Funk. 2003. The potential for health risks from intrusion of contaminants into the distribution system from pressure transients. J. Water Health 1:3-14. [PubMed] [Google Scholar]
- 22.Lee, S. H., D. A. Levy, G. F. Craun, M. J. Beach, and R. L. Calderon. 2002. Surveillance for waterborne-disease outbreaks—United States, 1999-2000. MMWR Surveill. Summ. 51(SS-8):1-47. [PubMed] [Google Scholar]
- 23.Liang, J. L., E. J. Dziuban, G. F. Craun, V. Hill, M. R. Moore, R. J. Gelting, R. L. Calderon, M. B. Beach, and S. L. Roy. 2006. Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking—United States, 2003-2004. MMWR Surveill. Summ. 55(SS-12):31-65. [PubMed] [Google Scholar]
- 24.MacKenzie, W. R., N. J. Hoxie, M. E. Proctor, M. S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. G. Addiss, K. R. Fox, J. B. Rose, and J. P. Davis. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Eng.J. Med. 331:161-167. [DOI] [PubMed] [Google Scholar]
- 25.Marciano-Cabral, F., R. MacLean, A. Mensah, and L. LaPat-Polasko. 2003. Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl. Environ. Microbiol. 69:5864-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messner, M., S. Shaw, S. Regli, K. Rotert, V. Blank, and J. Soller. 2006. An approach for developing a national estimate of waterborne disease due to drinking water and a national estimate model application. J. Water Health 4(Suppl. 2):201-240. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell, E., M. O'Mahony, J. M. Watson, D. Lynch, C. Joseph, C. Quigley, R. Aston, G. N. Constable, R. J. Farrand, S. Maxwell, D. N. Hutchinson, J. Craske, and Lee, J. V. 1990. Two outbreaks of Legionnaires' disease in Bolton Health District. Epidemiol. Infect. 104:159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Association of County and City Health Officials. 1990. National profile of local health departments. http://www.naccho.org/topics/infrastructure/research/upload/NationalPrifileofLocalHealthDepartment.pdf. Accessed 16 February 2010.
- 29.National Association of County and City Health Officials. 2008. National profile of local health departments. http://www.naccho.org/topics/infrastructure/profile/resources/2008reports/upload/NACCHO_2008_ProfileReport_post-to-website-2.pdf. Accessed 16 February 2010.
- 30.Nygard, K., Y. Andersson, J. A. Rottingen, A. Svensson, J. Lindback, T. Kistemann, and J. Giesecke. 2004. Association between environmental risk factors and Campylobacter infections in Sweden. Epidemiol. Infect. 132:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nygard, K., E. Wahl, T. Krogh, O. A. Tveit, E. Bohleng, A. Tverdal, and P. Aavitsland. 2007. Breaks and maintenance work in the water distribution systems and gastrointestinal illness: a cohort study. Int. J. Epidemiol. 36:873-880. [DOI] [PubMed] [Google Scholar]
- 32.Peng, M. M., L. Xiao, A. R. Freeman, M. J. Arrowood, A. A. Escalante, A. C. Weltman, C. S. Ong, W. R. MacKenzie, A. A. Lal, and C. B. Beard. 1997. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct transmission cycles. Emerg. Infect. Dis. 3:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shands, K. N., J. L. Ho, R. D. Meyer, G. W. Gorman, P. H. Edelstein, G. F. Mallison, S. M. Finegold, and D. W. Fraser. 1985. Potable water as a source of Legionnaires' disease. JAMA 253:1412-1416. [PubMed] [Google Scholar]
- 34.St. Louis, M. E. 1988. Water-related disease outbreaks, 1985. MMWR Surveill. Summ. 37(SS-2):15-24. [PubMed] [Google Scholar]
- 35.Sulaiman, I. M., A. A. Lal, and L. Xiao. 2001. A population genetic study of the Cryptosporidium parvum human genotype parasites. J. Eukaryot. Microbiol. Suppl:24S-27S. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Census Bureau. 2008. American housing survey for the United States: 2007. http://www.census.gov/prod/2008pubs/h150-07.pdf. Accessed 16 February 2010.
- 37.U.S. Department of Health and Human Services. 1985. Water-related disease outbreaks surveillance. Annual summary 1984. CDC Publication no. cdc 99-2510. U.S. Department of Health and Human Services, Washington, DC.
- 38.U.S. Environmental Protection Agency. 1987. National primary drinking water regulations: filtration, disinfection; turbidity, Giradia lambia, viruses, Legionella and heterotrophic bacteria. Proposed rule 40 CFR parts 141 and 142. Fed. Regist. 52:212:42718. [Google Scholar]
- 39.U.S. Environmental Protection Agency. 1989. Drinking water; national primary drinking water regulations; filtration, disinfection; turbidity, Giardia lamblia, viruses, Legionella, and heterotrophic bacteria; final rule. 40 CFR parts 141 and 142. Fed. Regist. 54:27486-27541. [Google Scholar]
- 40.U.S. Environmental Protection Agency. 1996. National primary drinking water regulations: monitoring requirements for public drinking water supplies: Cryptosporidium, Giardia, viruses, disinfection byproducts, water treatment plant data and other information requirements; final rule. 40 CFR Part 141. Fed. Regist. 61:24353-24388. [Google Scholar]
- 41.U.S. Environmental Protection Agency. 1998. National primary drinking water regulations: interim enhanced surface water treatment; final rule. 40 CFR parts 9, 141, and 142. Fed. Regist. 63:69478-69521. [Google Scholar]
- 42.U.S. Environmental Protection Agency. 2006. National primary drinking water regulations: ground water rule; final rule. 40 CFR parts 9, 141, and 142. Fed. Regist. 71:65574-65660. [Google Scholar]
- 43.U.S. Environmental Protection Agency. 2006. FY2006 drinking water factoids. http://www.epa.gov/safewater/databases/pdfs/data_factoids_2006.pdf. Accessed 25 February 2010.
- 44.U.S. Environmental Protection Agency. 2007. Drinking water infrastructure needs survey and assessment. Fourth report to congress (EPA 816-R-09-001). http://www.epa.gov/safewater/needssurvey/pdfs/2007/report_needssurvey_2007.pdf. Accessed 16 February 2010.
- 45.U.S. Environmental Protection Agency. 2010. Total coliform rule revisions. http://www.epa.gov/safewater/disinfection/tcr/regulation_revisions.html. Accessed 25 February 2010.
- 46.Weibel, S. R., F. R. Dixon, R. B. Weidner, and L. J. McCabe. 1964. Waterborne disease outbreaks 1946-60. J. Am. Water Works Assoc. 56:947-958. [Google Scholar]
- 47.Yoder, J. S., V. Roberts, G. F. Craun, V. Hill, L. Hicks, N. T. Alexander, V. Radke, R. L. Calderon, M. Hlavsa, M. J. Beach, and S. L. Roy. 2008. Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking—United States, 2005-2006. MMWR Surveill. Summ. 57(SS-9):39-70. [PubMed] [Google Scholar]
- 48.Zhou, L., A. Singh, J. Jiang, and L. Xiao. 2003. Molecular surveillance of Cryptosporidium spp. in raw wastewater in Milwaukee: implications for understanding outbreak occurrence and transmission dynamics. J. Clin. Microbiol. 41:5254-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]