Abstract
Summary: Macrolides have diverse biological activities and an ability to modulate inflammation and immunity in eukaryotes without affecting homeostatic immunity. These properties have led to their long-term use in treating neutrophil-dominated inflammation in diffuse panbronchiolitis, bronchiectasis, rhinosinusitis, and cystic fibrosis. These immunomodulatory activities appear to be polymodal, but evidence suggests that many of these effects are due to inhibition of extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation and nuclear factor kappa B (NF-κB) activation. Macrolides accumulate within cells, suggesting that they may associate with receptors or carriers responsible for the regulation of cell cycle and immunity. A concern is that long-term use of macrolides increases the emergence of antimicrobial resistance. Nonantimicrobial macrolides are now in development as potential immunomodulatory therapies.
INTRODUCTION
The term “macrolide” is used to describe drugs with a macrocyclic lactone ring of 12 or more elements (183). This class of compounds includes a variety of bioactive agents, including antibiotics, antifungal drugs, prokinetics, and immunosuppressants. The 14-, 15-, and 16-membered macrolides are a widely used family of antibiotics. They have excellent tissue penetration and antimicrobial activity, mainly against Gram-positive cocci and atypical pathogens (27). Macrolide concentrations are at least 10-fold higher in the epithelial lung fluid than in serum. Erythromycin A, a 14-membered macrolide, was isolated more than 50 years ago from cultures of Streptomyces and was the first macrolide introduced into clinical practice (183, 325). In this review, macrolide antibiotics are called “macrolides.”
The nonantimicrobial properties of macrolides were suspected as far back as the 1960s (110), but their dramatic clinical effectiveness in treating diffuse panbronchiolitis (DPB) has served to extend their use to a number of chronic inflammatory diseases (71, 157, 202). DPB is a chronic debilitating disorder of unknown etiology primarily afflicting East Asians and resulting in refractory airway infection and life-threatening chronic respiratory failure. By helping to resolve unregulated and destructive inflammation, macrolides increased the 10-year survival rate from <40% in 1970 to 1979 to >90% after the widespread use of chronic erythromycin therapy (157). The characteristics of the clinical response to macrolide therapy are summarized as follows (71, 157, 202, 258): (i) it takes up to 3 months of therapy for macrolides to show a significant effect; (ii) doses that are much lower than the MIC (i.e., low-dose macrolide therapy) are effective; (iii) the effect is seen even when patients are infected with macrolide-resistant bacteria, such as Pseudomonas; (iv) clinical improvement is recognized even when bacteria persist in posttreatment sputum; and (v) these actions are seen only for treatment with 14- and 15-membered macrolides (e.g., erythromycin, clarithromycin, roxithromycin, and azithromycin), not with the 16-membered macrolide antibiotics (120). The effects of macrolides in patients with chronic inflammatory airway disease appear to be independent of antimicrobial properties. Immunomodulation, which differs from immunosuppression or anti-inflammation, is a nonlinear resetting of the inflammatory response by modifying or regulating one or more functions of the immune system (238). We use the term “immunomodulation” to describe the downregulation of a hyperimmunity or hyperinflammation without impairing the normal immune or inflammatory response to defend against infection.
This review summarizes what is known about the immunomodulatory activities of macrolides on the host and bacteria. Clinical applications are briefly discussed.
NONANTIMICROBIAL EFFECTS
Effects on Airway Secretion
The volume and biophysical properties of mucus or phlegm (airway secretions that would be called sputum if expectorated) play an important role in the regulation of mucociliary clearance. Hypersecretion is a feature of chronic airway inflammation and can cause airflow limitation, impairment of mucociliary transport, and recurrent respiratory infection. Macrolides can decrease mucus hypersecretion both in vitro (214) and in vivo (241, 290). Clarithromycin has been shown to improve the transportability of secretions in human subjects (241, 290). This mucoregulatory effect is seen even when hypersecretion is not induced by bacteria. Improved mucus transport may be associated with changes in the biophysical properties of secretions as well as with reduced inflammation.
Ion transport.
Tamaoki and coworkers (289) studied the effects of macrolides on the airway bioelectric current measured in an Ussing chamber. Erythromycin and clarithromycin decreased short-circuit current (ISC), transepithelial potential difference (PD), and cell conductance in a dose-dependent manner, and these effects were not altered by a Na channel blocker but were abolished by a Cl channel blocker. Using a patch-clamp whole-cell technique, Ikeda and colleagues (104) showed that roxithromycin and erythromycin inhibit the acetylcholine-evoked Cl current in acinar cells isolated from the guinea pig nasal gland. This effect was thought to be due to inhibition of the Ca2+-activated Cl channel. Likewise, erythromycin was shown to inhibit gamma interferon (IFN-γ)-induced outwardly rectifying chloride channel (ORCC) activation in cultured BEAS-2B cells (a human bronchial epithelial cell line) (76). The in vivo effects of macrolides on Cl channel activity were investigated in the rabbit tracheal mucosa. Intravenous administration of clarithromycin reduced the Cl diffusion potential difference in a dose-dependent fashion (288). These findings suggest that macrolides may reduce water and, possibly, mucin secretion through inhibition of the airway epithelial Cl channel.
However, there appears to be no significant effect of macrolides on chloride transport in persons with cystic fibrosis (CF). Barker and associates (23) investigated the effects of macrolides on airway epithelial ion transport in CF mice (both knockout and DeltaF508 homozygous mice) and human subjects. There was no effect of macrolides on PD across normal or CF nasal epithelium in either mice or humans, consistent with clinical reports (63).
There is a significant association between the increase of human calcium-activated chloride channel 1 (hCLCA1) mRNA and MUC5AC expression in asthmatics (321), and CLCA proteins may regulate mucin gene expression in humans (222). Modulation of this channel may be a promising treatment for mucus overproduction (204). Macrolides can affect ion channels by stabilizing Ca2+ homeostasis (151, 346).
In summary, although macrolides may have an effect on airway chloride ion transport, this seems to be an acute and dose-related effect and is probably not of clinical relevance.
Mucus secretion.
Goswami et al. (83) studied the effect of erythromycin on mucus glycoconjugate secretion, using cultured human airway explants and also using an endometrial adenocarcinoma cell line. Following treatment with erythromycin, both cell types had decreased baseline mucus secretion. However, the mechanism of this effect was not explored.
Airway mucin is synthesized by epithelial goblet cells and by mucous cells of the submucosal glands. MUC5AC and MUC5B are the major gel-forming mucins in the human airway (281, 316, 317). Shimizu et al. (254) documented that erythromycin and clarithromycin inhibit tumor necrosis factor alpha (TNF-α)-induced mucus secretion in a dose- and time-dependent manner in NCI-H292 cells (a human mucoepidermal cell line) and in nasal epithelial cells. This effect is accompanied by a reduced expression of MUC5AC mRNA. Immunohistochemistry showed that clarithromycin and roxithromycin inhibit mucus production induced by ovalbumin (OVA) instillation in OVA-sensitized rats or by lipopolysaccharide (LPS) exposure in rats (216, 254). Similar results were obtained after Pseudomonas aeruginosa infection in mice (125). In this investigation, clarithromycin inhibited mucus production and extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation in the lungs of Pseudomonas aeruginosa-infected mice.
The P. aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL), a quorum sensing signal molecule, also stimulates the production of MUC5AC in NCI-H292 cells, through ERK1/2 and IκB phosphorylation (105). This induction was suppressed by azithromycin, and 3-oxo-C12-HSL-induced MUC5AC production was blocked by the ERK pathway inhibitor PD98059. Similarly, stimulation of cells with LPS or transforming growth factor alpha (TGF-α) induces phosphorylation of IκBα (285). Macrolides inhibited LPS-induced MUC5AC gene expression and attenuated TGF-α-induced and LPS-induced phosphorylation of IκBα.
In summary, macrolides can inhibit mucus hypersecretion but do not appear to affect normal physiologic secretion. Macrolides are able to modulate mucin gene expression, most likely at the level of mitogen-activated protein kinase (MAPK) pathways or transcription factors, suggesting that this effect is part of a general modulation of immunity and inflammation.
Effects on Inflammation
Cytokine production.
Cytokines and chemokines are key regulators of the inflammatory response, with both proinflammatory (e.g., TNF-α, granulocyte-macrophage colony-stimulating factor [GM-CSF], interleukin-1 [IL-1], IL-6, IL-8, and IFN-γ) and anti-inflammatory (e.g., IL-10) effects. Macrolides appear to decrease the production of proinflammatory cytokines that are detrimental to the host (48, 240).
In an earlier clinical study, erythromycin (600 mg daily for 3 months) reduced IL-8 and neutrophil elastase in bronchoalveolar lavage (BAL) fluid from subjects with chronic airway diseases, including those infected with P. aeruginosa (213). In subjects with DPB, treatment with erythromycin or roxithromycin for up to 24 months decreased IL-1β, IL-8, and neutrophils in BAL fluid (244). Other studies have shown that macrolides suppress proinflammatory cytokines in human airway washings and in serum (17, 133, 135, 198, 283).
IL-8 is one of the cysteine-X-cysteine (CXC) chemokines and is a potent neutrophil chemoattractant. Erythromycin inhibits release of IL-8, epithelial cell-derived neutrophil attractant (ENA-78), and macrophage inflammatory protein 1 (MIP-1) from macrophages and leukocytes (248). Clarithromycin can suppress LPS-induced IL-8 production in human peripheral monocytes and the human monocytic leukemia cell line THP-1 (140). Oishi and associates (213) reported that erythromycin inhibited IL-8 production from human neutrophils stimulated with formalin-killed P. aeruginosa. Erythromycin and clarithromycin also showed a concentration-dependent suppression of IL-8 release by eosinophils isolated from atopic subjects (149).
Physiological concentrations of erythromycin and clarithromycin inhibit IL-8 mRNA and protein in bronchial epithelial cells from healthy subjects and those with chronic inflammatory airway diseases (283). Erythromycin at a concentration of 10 μg/ml inhibited IL-6, IL-8, and soluble intercellular adhesion molecule 1 (sICAM-1) secretion from human bronchial epithelial cells (BEC) stimulated by endotoxin (135). Erythromycin decreased production of TNF-α and IL-6 in human whole blood stimulated with heat-killed Streptococcus pneumoniae (249). Similar effects have been observed using roxithromycin, which suppressed the production of IL-6, IL-8, and GM-CSF in the human epithelial cell line Bet-1A stimulated with IL-1α (133). Media conditioned with TNF-α-stimulated A549 human airway epithelial cells prolonged neutrophil survival in culture, an effect that was abolished by pretreatment of A549 cells with macrolides, which inhibited TNF-α-induced GM-CSF expression at both the protein and mRNA levels (333). While azithromycin stimulated TNF-α secretion from murine CF airway epithelial cells (75), azithromycin at 10 ng/ml in primary BEC from human lung transplant recipients inhibited release of IL-8 and GM-CSF after stimulation with LPS (201). This result may be due to the time of measurement, as it is characteristic of immunomodulation to both stimulate and suppress cytokine suppression, depending on the length of exposure.
As immunomodulatory drugs rather than immunosuppressive drugs, macrolides do not show simple time-dependent suppression of proinflammatory cytokines. Shinkai and associates (255) confirmed the multiphase response of IL-8 release from normal human BEC stimulated with LPS in the presence of macrolides. Addition of 10 μg/ml clarithromycin immediately decreased IL-8 production, then potentiated the LPS-evoked response (2.5-fold), and subsequently normalized IL-8 to the LPS-untreated control level. This effect was preceded by phosphorylation of ERK1/2, a p44/42 MAPK (255, 257). These findings are similar to those reported by Culić et al. for human neutrophils (49). The reason for this nonlinear response may be related to interaction with cross talk of intracellular signal transduction or oscillation-damping feedback (3, 315). ERK mediates normal human bronchial epithelial (NHBE) cell proliferation, and clarithromycin affects the transition from G1 phase to S phase of the cell cycle and delays growth of NHBE cells (256). This effect is consistent with a previous in vivo study in which the oral administration of roxithromycin to healthy BALB/c mice increased levels of IL-1 and IL-2 activity for the initial 14 days, followed by decreases to the control level after 42 days of therapy (153). It has been proposed that macrolides first activate leukocytes and then suppress cytokine production in the presence of inflammatory priming (49). Acute neutrophil activation by a macrolide can facilitate the killing of microorganisms, while the suppression of chronic inflammation may limit airway damage (221). These studies suggest that macrolides initially increase the production of proinflammatory cytokines but that this then rapidly normalizes via immunomodulation.
Azithromycin slows the rate of lung function decline in CF patients. Cigana and colleagues (41) used three CF (IB3-1, 16HBE14o-AS3, and 2CFSMEo−) and two isogenic non-CF (C38 and 16HBE14o-S1) airway epithelial cell lines to investigate whether azithromycin reduced TNF-α mRNA and protein levels. Azithromycin reduced TNF-α mRNA levels and decreased TNF-α secretion, to approximately the levels in the isogenic non-CF cells. NF-κB and specificity protein 1 (Sp1) DNA-binding activities were also significantly decreased after azithromycin treatment. Similar to these findings, azithromycin significantly reduced lung TNF-α levels, and this was associated with inhibition of neutrophil recruitment to the lung in a murine model stimulated with Pseudomonas beads (304).
In addition to effects on neutrophils, macrolides probably inhibit eosinophil recruitment. Macrolides inhibit secretion of the eosinophil-chemotactic cytokines RANTES and eotaxin (245). Erythromycin significantly decreased TNF-α-induced eotaxin mRNA as well as eotaxin release from human lung fibroblasts. Roxithromycin at 5 mg/kg of body weight inhibited formation of IL-5 by mouse spleen cells (152). These doses are comparable to those in humans, and it was speculated that oral administration of roxithromycin can inhibit the function of Th2-type lymphocytes.
Human studies demonstrated that macrolides can reverse the serum IFN-γ/IL-4 (Th1/Th2) ratio (229, 324). Roxithromycin dose dependently and significantly inhibited both IL-4 and IL-5 secretion by T cells prepared from both healthy and allergic rhinitis donors without affecting IL-2 and IFN-γ levels (15). However, there are also contradictory data suggesting a shift from Th1 to Th2 cytokine production after the use of macrolides. Park et al. (219) quantified changes in Th1 and Th2 cytokines in BAL fluid from patients with DPB after long-term treatment with erythromycin. After the treatment, IL-2 and IFN-γ levels in the BAL fluid were significantly decreased and the IL-4, IL-5, and IL-13 levels were significantly increased. In the ovalbumin-sensitized rat, a model of Th2 stimulation, BAL fluid and lung tissues were examined for mRNAs for cytokines (171). In these experiments, erythromycin did not decrease Th2-related cytokines or mRNAs. Clarithromycin and other macrolides were reported to inhibit Th1 cytokines, such as IL-2 and TNF-α, more markedly than Th2-type cytokines produced by mitogen-stimulated human T lymphocytes in vitro (197).
More recent studies have evaluated the effect of macrolides on T-cell regulation by dendritic cells (DCs). Azithromycin and clarithromycin significantly upregulated the expression of CD80, a costimulatory molecule for T-cell activation, from murine bone marrow-derived DCs (270). Azithromycin, but not clarithromycin, increased the production of IL-10, and clarithromycin, but not azithromycin, inhibited the production of IL-6 by DCs. Moreover, azithromycin increased IL-10 and clarithromycin significantly decreased IL-2 when naive splenic T cells were cocultured with DCs stimulated with LPS and exposed to macrolides. These data suggest that macrolides modulate the functions of DCs and that these effects may be different among macrolides.
In summary, there are clear and compelling data demonstrating that the 14- and 15-membered (but not the 16-membered) macrolide antibiotics can decrease the hypersecretion of proinflammatory cytokines and chemokines in cell culture, in animal models of disease, and in persons with chronic inflammatory pulmonary diseases. This effect appears to be nonlinear, and thus truly immunomodulatory, with an initial and short-lived increase in inflammation followed by a sustained decrease of cytokine production and secretion to normal, noninflamed levels, conceptualized as a “resetting” of the circuits. As discussed later, many of these effects appear to be medicated through inhibition of ERK1/2 or downstream transcription factors.
Adhesion molecule expression.
Adhesion molecules are necessary for neutrophils and other inflammatory cells to migrate into the airway in response to inflammatory signals. Mac-1 (CD11b/CD18) expression on peripheral neutrophils from patients with DPB is higher than that on cells from healthy volunteers (159). Roxithromycin treatment caused a significant decrease in the expression of Mac-1, which was associated with decreased neutrophil numbers in BAL fluid. There were no changes in lymphocyte function-associated antigen 1 (LFA-1) expression (159). Downregulation of the integrin CD11b/CD18 has also been reported with erythromycin (173). Erythromycin inhibited LPS-induced neutrophil recruitment to the middle ear of rats, in part by downregulating L-selectin and Mac-1 expression on peripheral blood neutrophils (61).
Roxithromycin at therapeutic concentrations inhibited neutrophil adhesion to epithelial cells and decreased the expression of ICAM-1 on IFN-γ-treated epithelial cells (133). It was reported that soluble ICAM-1 derived from cultured epithelial cells was also reduced by erythromycin treatment (135). A subsequent study has shown that 14-membered macrolides directly inhibit vascular adhesion molecule 1 (VCAM-1) mRNA induction and leukocyte migration into the lung in a mouse model of bleomycin-induced lung injury (169).
In summary, macrolides have been shown to decrease stimulated expression of adhesion molecules, which may contribute to resolution of airway neutrophilic inflammation.
Inflammatory cells. (i) Chemotaxis.
Neutrophilic inflammation is one of the key cellular features of DPB. In DPB, long-term erythromycin therapy significantly reduces the number of neutrophils and neutrophil chemotactic activity in BAL fluid (119, 211), and this is associated with a profound decrease in IL-8 (244). Macrolides also inhibit neutrophil migration after acute lung injury in animals, induced by intratracheal instillation of LPS (119), Pseudomonas beads (304), or bleomycin (20, 134).
However, not all studies have demonstrated inhibition of neutrophil chemotaxis. The concentration of macrolide needed in vitro to inhibit chemotaxis of peripheral blood neutrophils from healthy volunteers was much greater than therapeutic doses (293). Anderson (6) demonstrated that erythromycin increased neutrophil migration in response to a leukocyte attractant in vitro. Chemotaxis of neutrophils from 8 CF subjects was not affected by 4 weeks of oral erythromycin treatment (34).
It is likely that the effects of macrolides on neutrophil chemotaxis are due to decreased production of chemoattractants and decreased expression of adhesion molecules (97, 285).
(ii) Chemical mediators.
Neutrophil elastase stimulates degranulation of mucin granules and release of mucin glycoprotein by goblet cells and submucosal glands. This protease also regulates the expression of IL-8, ICAM-1, and MUC5AC mRNAs (252, 317). Macrolides have been thought to have anti-elastolytic properties in the inflamed airway (98). Erythromycin can act as an alternate substrate inhibitor of neutrophil elastase, and flurythromycin can inactivate this enzyme in vitro (82). In asthmatics treated with clarithromycin, there was a decrease in sputum neutrophil elastase and matrix metalloproteinase 9 (MMP-9) (260).
Macrolides may modulate the lipoxygenase pathway of arachidonic acid metabolism. Leukotriene B4 (LTB4), a metabolite of arachidonic acid, is an important chemotactic factor for neutrophils and is elevated in patients with chronic airway disease. Erythromycin and roxithromycin reduced LTB4 in BAL fluid or epithelial lining fluid (ELF) in subjects with DPB. This was associated with decreased neutrophil numbers and neutrophil chemotactic activity (203, 212).
Endothelin-1, a potent bronchoconstrictor and vasoconstrictor, is also a mediator of airway inflammation (68). Bronchial smooth muscle cells have specific binding sites for endothelin-1, and BEC of asthmatic patients release large amounts of active endothelin-1 (314). Erythromycin and clarithromycin suppress endothelin-1 expression and release by human BEC, but this was not seen with josamysin, a 16-membered macrolide, or with tacrolimus, a very large macrolide (284).
Erythromycin dose dependently attenuates the contractile response of isolated human bronchial strips to electrical field stimulation, suggesting that macrolides may block bronchoconstriction by inhibiting neurotransmitter release or the cholinergic response in airway smooth muscle (286).
(iii) ROS.
Reactive oxygen species (ROS) are recognized as mediators of cell and tissue injury (46). Macrolides are able to inhibit the production of ROS from neutrophils (161), and this effect is incubation time dependent (1, 166). Macrolide inhibition of stimulated neutrophil or eosinophil superoxide generation has been reported in many studies (6, 7, 47, 173, 225). It has been suggested that this is due in part to cell membrane stabilization. Macrolides attenuate the membrane-destabilizing effect of bioactive phospholipids, such as lysophosphatidylcholine, platelet-activating factor (PAF), and lyso-PAF, and this is accompanied by a dose-related inhibition of superoxide production (7). Interference with phospholipase D/phosphatidic acid phosphohydrolase may also decrease superoxide generation by phagocytes (1, 225).
Nitric oxide (NO) is a second messenger that is produced by NO synthase (NOS) early in the inflammatory response. Erythromycin stimulates endothelial NOS (eNOS) production by a protein kinase A-dependent mechanism (189). This is believed to inhibit leukocyte adhesion to endothelial cells. On the other hand, inducible NOS (iNOS) in pulmonary alveolar macrophages is suppressed by erythromycin (291). The same group reported that clarithromycin inhibited in vitro iNOS mRNA expression induced by endotoxin and IFN-γ (148).
(iv) Apoptosis.
Neutrophil recruitment and activation are usually followed by neutrophil clearance and resolution of inflammation. In chronic inflammatory lung disease, neutrophils continue to be recruited to the airway, die by necrosis, and release their granule contents, increasing lung damage. Macrolides may induce apoptosis of activated neutrophils (12, 106). Erythromycin increased cyclic AMP (cAMP) in neutrophils in vitro, and it accelerated apoptosis at 24 h (12). Azithromycin has also been reported to promote apoptosis of neutrophils, but this effect was not seen in the presence of Streptococcus pneumoniae (146). However, in other studies, no direct effects of macrolides on neutrophil apoptosis and survival were observed, although shortening of neutrophil survival was mediated indirectly through inhibition of GM-CSF release from epithelial cells or IL-8 production in activated neutrophils (307, 333).
Roxithromycin induces apoptosis of anti-CD3-activated Jurkat T cells in vitro by enhancing Fas-Fas ligand and caspase-3 but not caspase-8 (118). Similarly, apoptosis of unstimulated peripheral lymphocytes from healthy subjects is induced by macrolides through the Fas-Fas ligand pathway (107). Clarithromycin and azithromycin can also increase the phagocytosis of apoptotic epithelial cells and neutrophils by alveolar macrophages (92, 331).
Although macrolides may promote neutrophil apoptosis as a mechanism to promote the resolution of inflammation, these data have been difficult to confirm in vivo and may be of limited clinical relevance.
(v) Cell differentiation.
Macrolides promote differentiation of the human monocytoid cell line THP-1 into macrophages (271). Azithromycin may also alter the macrophage phenotype. Azithromycin shifted a mouse macrophage cell line (J774) from the classical activated phenotype (M1) to the alternatively activated phenotype (M2), reducing secretion of proinflammatory cytokines and increasing the anti-inflammatory cytokine IL-10 (200). A similar downregulation of inflammatory cytokines by azithromycin was reported for M1-polarized CF alveolar macrophages (186).
Airway epithelial cells. (i) Epithelial barrier.
Data suggest that macrolides may help to stabilize the epithelial cell membrane. In the inflamed airway, phospholipases A2 cleave arachidonic acid from the cell wall, and subsequent metabolism of arachidonic acid produces proinflammatory mediators. Feldman et al. have shown that macrolides can stabilize cell walls, protecting them against activated phospholipases (67). Furthermore, neutrophils potentiated the ciliary dysmotility and epithelial damage caused by proinflammatory phospholipids; this was ameliorated by pretreating the leukocytes with macrolides (67).
Junctional complexes between epithelial cells are a physical and chemical barrier. Inhalation of PAF causes ciliary dysmotility and disrupts the tight junction barrier in the rabbit trachea (205). Oral administration of roxithromycin significantly ameliorated PAF-induced ciliary dysfunction and the increase in epithelial permeability. Azithromycin, but not penicillin or erythromycin, increased the transepithelial electrical resistance (barrier) of human airway epithelial cells cultured on filter supports (16). In this model, azithromycin induced processing of the tight junction proteins claudin-1, claudin-4, occludin, and junctional adhesion molecule A.
Epithelial β-defensins are components of innate immunity in the airway. Erythromycin increased bactericidal activity of the airway surface liquid taken from cultured human primary tracheal cells, bronchial BEAS-2B cells, and pneumocyte II-like A549 cells (109). Erythromycin significantly increased the production of human β-defensin-1 and β-defensin-2 mRNA and protein. On the other hand, macrolides appeared to decrease β-defensin-2 levels, but not β-defensin-1 levels, in BAL fluid from DPB patients (90).
Thus, although macrolides appear to beneficially affect the epithelial barrier and ciliary function, this may be secondary to the decrease in airway inflammation.
(ii) CFTR expression.
Cystic fibrosis transmembrane ion conductance regulator (CFTR) is a member of the ATP-binding cassette (ABC) transporter superfamily whose function is the transport of a wide variety of substrates. Multidrug-resistant (MDR) protein and a P-glycoprotein also belong to the ABC transporter family and share sequence homology. It was reported that a CF patient who was treated with chemotherapy for fibrosarcoma had a dramatic improvement in lung function and that P. aeruginosa was no longer isolated from this patient (162). An increase in MDR mRNA in the patient's nasal epithelial cells was found, whereas no MDR mRNA was detected in a CF subject who had not received chemotherapy. It was speculated that the upregulated MDR protein complemented deficient CFTR function, leading to clinical improvement. Erythromycin is known to upregulate P-glycoprotein expression in epithelial cells (73), suggesting that this may be a mechanism by which macrolides improve pulmonary function in CF (4).
However, Equi and collaborators (63) demonstrated that neither nasal epithelial MDR nor CFTR mRNA levels in adult subjects with CF were changed after 2 weeks of azithromycin treatment, during which time there was a significant improvement in forced exhaled volume in the first second of exhalation (FEV1). In addition, data show that neither clarithromycin nor azithromycin changes the nasal potential difference, sodium absorption, or chloride secretion (23), and azithromycin has no effect on MRP promoter transcriptional activity (42).
Although macrolides are clearly beneficial in persons with CF, as discussed below, the data do not support the hypothesis that this is due to either expression or function of the CFTR protein.
(iii) TLRs.
The Toll-like receptors (TLRs) are an evolutionarily conserved family of receptors that function in innate immunity by recognizing some invariant regions in bacterial molecules. TLR2 recognizes peptidoglycan and bacterial lipoproteins, and TLR4 and TLR5 recognize LPS and flagellin, respectively. LPS can promote mucus secretion through TLR4, even in neutropenic animals (287). Epithelial TLR signaling stimulated by LPS or flagellin is decreased by macrolides, and this appears to be mediated through the MAPK-NF-κB pathway (255, 257).
Macrolides have been shown to affect TLR expression. Peripheral blood mononuclear cells stimulated with LPS have increased TLR4 mRNA levels. This was suppressed by clarithromycin and was accompanied by decreased LPS-induced IL-8 production (218). In contrast, Yasutomi et al. (337) demonstrated that erythromycin did not affect mRNA levels of TLR4 in monocyte-derived dendritic cells but that TLR2 mRNA was upregulated. In TLR2-transfected HeLa cells infected with S. pneumoniae, erythromycin did not influence the activation of TLR2 (196).
Because the macrolides are antimicrobial drugs and many of the innate immunity TLRs recognize specific bacterial products as an initial inflammatory response, it is tempting to speculate that macrolides may act in part by decreasing TLR expression or function. However, the limited data available do not support this as a primary mode of action for the macrolides' immunomodulatory properties.
Other cells. (i) Fibroblasts.
Roxithromycin was shown to inhibit fibroblast proliferation in nasal polyps from patients treated for 1 month before polypectomy (210). Similar effects were reported in another study, along with inhibition of IL-8 levels in nasal lavage fluid, which was thought to drive polyp growth (330). Clarithromycin dose dependently reduced the expression of IL-8 and NF-κB in human adenoidal fibroblasts (279), and erythromycin significantly decreased TNF-α-induced eotaxin mRNA as well as eotaxin release from human lung fibroblasts (245). EM703, a novel derivative of erythromycin A (discussed below), inhibited fibroblast proliferation and collagen production in the murine lung fibroblast cell line MLg2908 treated with TGF-β (170).
Vascular endothelial growth factor (VEGF) plays a role in tumor growth as well as in inflammation of the respiratory tract by enhancing neovascularization and vasopermeability. Clarithromycin and roxithromycin have been reported to inhibit VEGF production from fibroblasts stimulated by hypoxia or TNF-α (182).
MMPs are secreted by a wide variety of cells, including fibroblasts, and are important in the migration of inflammatory cells through the basement membrane (60). Roxithromycin, but not josamycin, suppressed the production of MMP-2 and -9 in nasal polyp fibroblasts induced by TNF-α in vitro, accompanied by suppression of MMP mRNA expression through the inhibition of NF-κB and activator protein 1 (AP-1) activation (124). The inhibition of MMPs by macrolides may reduce the extracellular spread of inflammation.
Kohyama and coworkers (150) documented that clarithromycin, but not ampicillin, minocycline, or azithromycin, had a concentration-dependent inhibitory effect on migration of human fetal lung fibroblasts (HFL-1 cells) stimulated with thromboxane A2, whereas there was no effect on HFL-1 cell-mediated gel contraction, another function of fibroblasts at the wound area.
Modulation of fibroblast function by macrolides appears to occur in vivo, perhaps as a result of generalized immunomodulation. Inhibition of fibroblast migration by macrolides may regulate the wound healing response following tissue injury.
(ii) Vascular endothelial cells.
Angiogenesis, the proliferation and migration of endothelial cells resulting in the formation of new blood vessels, is associated with lesion formation in chronic inflammation as well as with progressive growth of solid tumors (69). Roxithromycin inhibited endothelial cell migration in vitro (339). The 14-membered macrolides appear to reduce tumor angiogenesis (340). Likewise, roxithromycin inhibits TNF-α-induced VEGF production, which is associated with suppression of NF-κB and AP-1 (217). The same group demonstrated an anticancer effect secondary to inhibition of angiogenesis in the mouse dorsal air sac model (10). These inhibitory effects on angiogenesis may be beneficial to prevent the neovascularization associated with airway remodeling seen in chronic inflammatory disorders such as CF and bronchiectasis.
The macrolide effects modulating cellular functions and related references are summarized in Table 1.
TABLE 1.
Immunomodulatory effects of macrolides on cellular functions
| Target cells and actions | Reference(s) |
|---|---|
| Airway epithelial cells | |
| Inhibition of: | |
| Chloride secretion | 76, 104, 151, 288, 289, 346 |
| Mucus secretion | 83, 125, 216, 254 |
| Adhesion molecules | 133, 135, 169 |
| Proinflammatory cytokines | 41, 135, 255, 257, 283, 333 |
| Inflammatory mediators | 284, 286 |
| Enhancement of: | |
| Tight junction or cell barrier | 16, 67, 205 |
| Defensin | 109 |
| Neutrophils | |
| Inhibition of: | |
| Chemotaxis | 20, 119, 134, 304 |
| Adhesion molecules | 61, 159, 173, 198 |
| Proinflammatory cytokines | 49, 213, 248, 307 |
| Elastase | 82, 260 |
| Reactive oxygen species | 1, 6, 7, 161, 166, 173, 225 |
| Promotion of: | |
| Chemotaxis | 6 |
| Apoptosis | 12, 106, 146 |
| Eosinophils | |
| Inhibition of: | |
| Proinflammatory cytokines | 149 |
| Reactive oxygen species | 47 |
| Lymphocytes | |
| Inhibition of proinflammatory | |
| cytokines | 15 |
| Enhancement of: | |
| Apoptosis | 107, 118 |
| Regulation by dendritic cells | 270 |
| Monocytes | |
| Inhibition of proinflammatory | |
| cytokines | 140, 197 |
| Enhancement of differentiation | 271 |
| Macrophages | |
| Inhibition of: | |
| Proinflammatory cytokines | 186, 200, 248 |
| Inducible NO | 148, 291 |
| Fibroblasts | |
| Inhibition of: | |
| Proliferation | 170, 210, 330 |
| Collagen | 103, 341 |
| Matrix protease | 124 |
| Proinflammatory cytokines | 182, 245, 279, 330 |
| Vascular endothelial cells | |
| Inhibition of: | |
| Angiogenesis | 10, 217, 339, 340 |
| Adhesion molecules | 169 |
Effects on Cell Signaling
There have been so many different reported effects of macrolides on cell signaling pathways that attempts to put these actions together as one mechanism is difficult. In fact, all macrolides seem not to have similar ranges or degrees of activity. Proposed mechanisms and their influence on cell signaling are discussed below.
Intracellular calcium.
Intracellular Ca2+ plays a fundamental role in signal transduction and regulates many enzymes, such as proteases, phospholipases, and endonucleases (44). Moreover, alteration of intracellular Ca2+ homeostasis is an early event in the development of irreversible cell injury (209). In airway epithelium, intracellular Ca2+ mobilization induced by proinflammatory mediators influences various cell functions, including ion transport and mucus secretion (43, 141). Experiments on epithelial cell lines suggest that Ca2+ signaling is involved in activation of the ERK1/2-NF-κB pathway and in ATP release as well as TLR-mediated responses by P. aeruginosa or flagellin (70, 184, 231, 277). Furthermore, intracellular Ca2+ agonists such as bradykinin and ATP can also increase cytokine expression and secretion in airway epithelia (199, 234, 235).
We and others (126, 127, 151, 289) have shown that macrolides inhibit purinoceptor-mediated intracellular Ca2+ oscillations and Ca2+ influx in primary cultured airway epithelial cells, thereby reducing Cl secretion, perhaps through suppression of Ca2+-activated Cl channels. Similarly, Zhao et al. (346) have shown that erythromycin inhibits the ATP-mediated Ca2+ increase by suppressing Ca2+ influx from the extracellular space in A549 cells. Although molecular mechanisms of macrolide action on Ca2+ dynamics remain unclear, erythromycin does not affect verapamil-sensitive, voltage-dependent Ca2+ channels (151, 346). The effect of macrolides on the calcium response has been observed in other cells. Ca2+ influx into neutrophils and the oxidative burst were inhibited by erythromycin but not clarithromycin, suggesting that effects of macrolides on calcium mobilization are not completely homogenous (189). Recently, roxithromycin was shown to suppress histamine release and prostaglandin D2 production from human β-defensin-2-stimulated mast cells, and this was accompanied by inhibition of the intracellular Ca2+ increase (130).
Calcium signaling after activation of apical G-protein-coupled receptors by proinflammatory mediators is amplified in CF airway epithelia (234, 235). Data suggest that the increased Ca2+ signal in CF cells may be due to the endoplasmic reticulum Ca2+ store increase or to inositol 1,4,5-triphosphate receptor (IP3R) dysfunction (9, 234). Macrolides may normalize Ca2+ responses and stabilize intracellular Ca2+ levels.
MAPKs.
MAPKs form a signaling network that responds to diverse extracellular and intracellular stimuli, controlling a vast array of physiological processes. Three well-characterized subfamilies of MAPKs have been identified: extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK (116, 344). These MAPK signaling pathways interact and play an important role in the regulation of inflammatory cytokine gene expression and cell proliferation, differentiation, and apoptosis (93, 345). Activation of p38 MAPK can induce the production of inflammatory cytokines such as TNF-α, IL-1β, GM-CSF, and RANTES (87, 185). ERK1/2 and JNK can mediate proinflammatory cytokine levels (309), and MAPKs can regulate IL-8 promoter activity by NF-κB-dependent and -independent processes (168). ERK and other MAPK pathways are involved in the pathogenesis of hyperimmune reactions and inflammatory diseases (28, 174, 185) and appear to be major targets of macrolide immunomodulation.
ERK and p38 MAPK increase IL-8 expression in human bronchial cells (168, 185, 228). ERK can also activate NF-κB in human airway epithelial cells (28, 39). Shinkai and associates (255) focused on the ERK pathway and evaluated effects of macrolides on IL-8 and GM-CSF release from NHBE cells in culture. Clarithromycin exposure decreased ERK activity in the initial 30 to 90 min, the level was increased at 2 h to 3 days compared with that of the control, and it normalized to the unstimulated baseline level by 5 days. It was hypothesized that this was due to interactions of intracellular signal transduction or negative feedback mechanisms (3, 315). These data are consistent with the well-defined time lag after macrolide administration to effective modulation of inflammation.
Inhibition of DNA-binding activity by macrolides may be due in part to suppression of upstream cell signaling. Clarithromycin increased IL-8 release at 24 h, an effect that was abolished by an ERK inhibitor but potentiated by a p38 MAPK inhibitor, suggesting that p38 MAPK may downregulate IL-8 release by NHBE cells (255). A similar increase in ERK activation was seen with flagellin-induced IL-8 secretion via the ERK pathway (257). Enhancement of the ERK pathway by the p38 MAPK inhibitor was postulated for a leukemia cell line (30), and thus cross talk between ERK1/2 and p38 MAPK may be involved in the immune response (181).
The effects of macrolides on MAPK signaling are not limited to cytokine production. Clarithromycin or an ERK inhibitor decreased MUC5AC gene expression and ERK1/2 phosphorylation in P. aeruginosa-infected mouse lung homogenates, suggesting that clarithromycin inhibits MUC5AC glycoprotein production through ERK inhibition (125). Azithromycin inhibited 3-oxo-C12-HSL-induced MUC5AC production through inhibition of ERK phosphorylation in NCI-H292 cells (105). ERK1/2 activation was also inhibited in neutrophils treated with azithromycin (304). Erythromycin inhibited IL-1β-induced p38 MAPK phosphorylation in rheumatoid synovial cells in vitro (72).
The preponderance of published data strongly suggest that a primary immunomodulatory mechanism of action is through the macrolide effects on MAPK activation, in particular, via the ERK1/2 pathway. This makes an exceptionally compelling story, as it explains most of the observed immunomodulatory effects of these drugs and, to a great extent, predicts that MAPK-independent immune effects are less likely to occur as a primary effect. These effects are so reproducible that it has been suggested that modulation of ERK1/2 can be used as a drug screen for the immunomodulatory effects of novel macrolides.
Transcription factors.
Transcription factors, including NF-κB and AP-1, regulate gene transcription in inflammatory responses (282, 285). These transcription factors bind upstream of promoters and regulate a number of actions, including generation of cytokines, chemical mediators, and oxidative metabolites. Aoki and Kao (11) investigated the effects of erythromycin at the level of transcriptional regulation of cytokine gene expression in Jurkat T cells. Erythromycin significantly inhibited NF-κB activation induced by phorbol 12-myristate 13-acetate (PMA) and calcium ionophore and suppressed IL-8 but not IL-2 expression. Erythromycin did not inhibit transcriptional activation of IL-2 and DNA-binding activity of nuclear factor of activated T cells (NFAT). This suggests the inhibition of IL-8, NF-κB, and DNA-binding activities by erythromycin. Similar findings have been reported for airway epithelial cell, fibroblast, monocyte, and leukemia cell lines (2, 55, 102, 140, 191).
Desaki and collaborators (55) reported that erythromycin and clarithromycin inhibited PMA-induced activation of NF-κB and AP-1 in the human bronchial epithelial cell line BET-1A, as well as the release of IL-8, whereas the macrolides showed no effect on the activation of cAMP-responsive element binding protein (CREB), suggesting that the effect on transcription regulation may be specific. Furthermore, erythromycin did not affect the phosphorylation of inhibitor of NF-κB (IκB), which is a crucial step for transactivation of NF-κB, indicating that erythromycin may act at the level of nuclear translocation of NF-κB or at the stage of DNA binding within nuclei (54). In a CF airway cell line, azithromycin inhibited NF-κB and Sp1 DNA-binding activities, reducing TNF-α secretion (41).
Macrolides also inhibit mRNA expression of mediators and cytokines such as IL-1, endothelin-1, iNOS, and MUC5AC (191, 216, 284, 291). The mechanism for this is likely to involve suppression of NF-κB and AP-1 (2, 11, 55, 140).
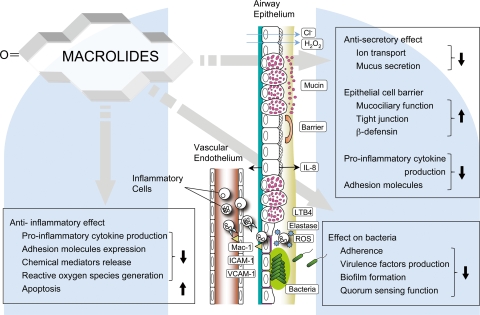
There are extensive data documenting the immunomodulatory effects of macrolides on transcription factors such as NF-κB. Interactions between MAPK signaling and transcription factor expression and function can explain most of the immunomodulatory effects observed. Figure 1 shows putative intracellular mechanisms by which macrolides can exert their immunomodulatory actions, and Fig. 2 gives a schematic presentation of potential immunomodulatory sites in the inflamed airway.
FIG. 1.
Intracellular signal transduction pathways that have been proposed to be involved in macrolide immunomodulation. There are three major pathways that are influenced by macrolides. Receptor tyrosine kinases (RTKs) are receptors for many polypeptide growth factors and cytokines. RTKs such as epidermal growth factor receptor (EGFR) stimulate MEKK, and this activates the MAPK cascade. MAPK phosphorylates and activates transcription factors inducing proinflammatory genes (345). Macrolides inhibit MAPK activation, in particular, ERK1/2 activation. TLRs recognize bacterial molecules. For example, in response to LPS stimulation, TLR4 and adaptor molecules (not shown) activate the IRAK family and TAK1. TAK1 then stimulates two distinct pathways, the IKK complex and the MAPK pathway. The latter leads to the induction of AP-1, while the former activates NF-κB through the degradation of IκB proteins and the subsequent translocation of NF-κB (194). G-protein-coupled receptor (GPCR)- or RTK-mediated activation of phospholipase C (PLC) produces inositol triphosphate (IP3). IP3 is a ligand for the intracellular IP3R channel of the endoplasmic reticulum's internal Ca2+ stores. Activation of PLC also leads to the production of diacylglycerol (DAG), which in turn activates protein kinase C (PKC). PKC and Ca2+/calmodulin signaling are then activated (44). Macrolides inhibit intracellular Ca2+ increase. Abbreviations: AP-1, activator protein 1; CaMK, calmodulin kinase; DAG, diacylglycerol; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; GFR, cytokine receptor/growth factor receptor; GPCR, G-protein-coupled receptor; IKK, IκB kinase; IP3R, inositol triphosphate receptor; IRAK, IL-1 receptor-associated kinase; MEK, MAPK/ERK kinase; PKC, protein kinase C; TAK1, transforming growth factor-activated protein kinase 1; TLR, Toll-like receptor. Blue arrows, major pathways influenced by macrolides; dashed arrows, subpathways or cross-talk pathways; red lines with “ML,” inhibition by macrolides; white bent arrow, cell response.
FIG. 2.
Beneficial effects of macrolides in the inflamed airway. In a chronically inflamed airway, there is epithelial cell damage, infiltration of inflammatory cells, goblet cell hyperplasia, hypersecretion, mucociliary dysfunction, and recurrent airway infection. Macrolides have been reported to attenuate inflammation and cellular damage in a variety of ways, as represented in this diagram. Downward-facing arrows, inhibition; upward-facing arrows, enhancement.
EFFECTS ON BACTERIA
DPB and CF are associated with P. aeruginosa infection, which causes progressive pulmonary damage. In a mouse model of endogenous P. aeruginosa bacteremia, low-dose erythromycin improved survival, from 20% for control mice to 80% for treated mice (89). A nonbactericidal effect on this organism has been postulated as one mode of action (96).
Inhibition of Adherence
Adherence of organisms to epithelium is essential for establishing a chronic bacterial infection. Baumann et al. studied the effect of roxithromycin by using a model of P. aeruginosa adherence to buccal epithelial cells from 11 CF children (25). Increased adherence was found in CF patients, and 3 months of azithromycin therapy decreased the adherence to normal control levels. Low-dose erythromycin also altered P. aeruginosa morphology and reduced adherence to human type IV basement membrane collagen in vitro (305). Yamasaki et al. (332) showed that exposure of P. aeruginosa to a sub-MIC level of erythromycin reduced the number of pili and, hence, adherence. Erythromycin suppresses the production of P. aeruginosa hemagglutinins, including the adhesins lectins as well as extracellular protease, hemolysin, and homoserine lactone autoinducers (266). Azithromycin at concentrations below the MIC inhibited flagellin expression and the motility of P. aeruginosa (132). One study using strain PAO1 and 13 strains (8 nonmucoid and 5 mucoid types) isolated from sputa of CF patients demonstrated that the decrease in adherence observed after azithromycin treatment is a strain-dependent event, although azithromycin at sub-MIC levels reduced bacterial adherence to bronchial mucins for many nonmucoid strains (36). The increased binding to CF cells may be related to mutant CFTR, in that cationic liposome-mediated CFTR gene transfer ex vivo significantly reduced adherence (52), but there was no effect on P. aeruginosa adherence to epithelial cells from CF patients after 2 weeks of azithromycin treatment (63).
Inhibition of Virulence Factors
Tanaka and associates (292) showed in vitro that erythromycin inhibits the nasal epithelial damage caused by neutrophils in combination with filtrates of P. aeruginosa. However, preincubation of neutrophils alone with erythromycin was without effect, suggesting that erythromycin might reduce P. aeruginosa's production of factors that damage epithelia or stimulate neutrophil-mediated cytotoxicity. At sub-MIC levels, macrolides inhibit the synthesis of exotoxin A, elastase, phospholipase C, DNase, lecithinase, gelatinase, lipase, and pyocyanin, all of which are virulence factors associated with P. aeruginosa infection (89, 143, 193, 195, 266). Tateda and coworkers (297) showed that a 48-hour exposure to macrolides at sub-MIC levels decreased the viability of both mucoid and nonmucoid P. aeruginosa strains. It was speculated that the time-dependent suppression of bacterial proteins, including GroEL, a major stress protein required for survival, might be lethal (298). Because of this, long-term macrolide therapy may shift the host-pathogen interaction from infection to a relatively benign colonization state. Pretreatment P. aeruginosa isolates from subjects randomized to receive azithromycin in a U.S. trial were analyzed, and the results suggested an in vitro effect of azithromycin, which decreased the production of phospholipase C by the isolates; this correlated with the pulmonary function (FEV1) improvement seen with azithromycin therapy (208).
Inhibition of Biofilms
Chronic infection is a major cause of reactive inflammation and tissue destruction in DPB and CF. Strains of P. aeruginosa involved in these disorders develop a mucoid phenotype, synthesizing alginate, a component of bacterial biofilms, when environmental conditions are unfavorable for survival (144). The mucoid phenotype is associated with a change in morphology from flagellar motility to twitching motility, a loss of pili and flagella, and exopolysaccharide alginate production by guanosine diphospho-d-mannose dehydrogenase (GMD). Within mature biofilms, the bacteria are resistant to attack by antibacterial agents and phagocytic cells. Alginate can also act as an antigen and induce antigen-antibody reactions in the airways, producing circulating immune complexes in the host (145). Immune complex deposition in the airway, with resultant neutrophil chemotaxis, leads to lung damage (95). Sub-MIC levels of macrolides can inhibit alginate production (100, 336). Macrolides decreased the number of viable bacteria in a murine model of biofilm-associated respiratory P. aeruginosa infection (335). Decreased biofilm formation may be due to inhibition of the GMD production cycle (145, 188) or to prevention of pilus-dependent twitching motility (328). Long-term treatment with clarithromycin can decrease serum immune complexes in patients with DPB (144). Because macrolides can disrupt biofilms and revert bacterial morphology to a planktonic state, combining macrolides with antipseudomonal antibiotics may be more effective than using antibiotics alone for bacterial killing (145).
Inhibition of Quorum Sensing
Cell-cell communication among bacteria is mediated by chemical signaling molecules termed autoinducers, which increase in concentration as a function of cell density. Chemical communication involves producing, releasing, detecting, and responding to these small autoinducers, and this process is referred to as quorum sensing (187). In Gram-negative bacteria, including P. aeruginosa, the quorum sensing system comprises the genes encoding transcriptional activators (R genes) and autoinducer syntheses (I genes). The I genes (i.e., lasI and rhlI) are engaged in the biosynthesis of the corresponding autoinducer signal molecules, i.e., 3-oxo-C12-HSL and N-butyryl-l-homoserine lactone (C4-HSL), which regulate the production of virulence determinants and the formation of biofilms (295). Sputum samples from CF patients chronically infected with P. aeruginosa contain mRNA transcripts for the quorum sensing genes (268) and the autoinducers 3-oxo-C12-HSL and C4-HSL (261), indicating that quorum sensing signaling profiles consistent with a biofilm mode of growth are operative in CF isolates.
Azithromycin at a concentration of 2 μg/ml suppressed transcription of lasI by 80% and that of rhlI by 50% in P. aeruginosa strain PAO1 (294). Additionally, the production of 3-oxo-C12-HSL and C4-HSL was decreased to 6% and 28% of the control levels, respectively, suggesting that azithromycin interferes with the synthesis of autoinducers, leading to a reduction of virulence factor production. Azithromycin decreased bacterial loads, neutrophil infiltration, and lung pathology in P. aeruginosa-infected CF mice, in part by blocking quorum sensing (94). Similarly, erythromycin, clarithromycin, and roxithromycin, but not oleandomycin or josamycin, suppressed lasI gene expression (295).
The quorum sensing molecule 3-oxo-C12-HSL is also a potent stimulator of host cells and can induce IL-8 production from human bronchial epithelial cells in vitro (56, 264) and produce inflammation in vivo (265). This molecule can induce macrophages and neutrophil apoptosis (296).
In summary, the effects of macrolides on biofilm disease include (i) inhibition of alginate production; (ii) suppression of the antibody reaction to alginate, thereby decreasing immune complex formation; and (iii) inhibition of lasI and rhlI expression and deactivation of the autoinducer 3-oxo-C12-HSL in quorum sensing systems in P. aeruginosa. This activity may improve the resolution of the airway biofilm diseases CF and DPB (145).
SUMMARY OF MECHANISMS OF IMMUNOMODULATION
A wealth of supportive and detailed data support the observation that in mammalian cells, macrolides influence intracellular MAPK, especially ERK1/2, and the NF-κB pathway downstream of ERK. Because these pathways are involved in many cellular functions, including inflammatory cytokine production, cell proliferation, and mucin secretion, effects on ERK1/2 and NF-κB can explain most of the reported immunomodulatory effects of the macrolides (Table 2). The specific proteins or receptors targeted by macrolides that affect MAPK/NF-κB signaling have not been identified. The putative binding molecule(s) may have multiple mechanisms of action. Unpublished data suggest that portions of the macrolide molecule not involved in either antimicrobial actions or motilin receptor binding are responsible for immunomodulation. This information has been used to develop nonantimicrobial, immunomodulatory macrolides, as discussed below.
TABLE 2.
Inhibition of ERK1/2 and NF-κB pathways by macrolides
| Pathway (references) | Response | Stimulator or cell type |
|---|---|---|
| ERK1/2 (105, 125, 255, 256, 257, 304) | Inhibition of mucus secretion, MUC5AC production, and MUC5AC gene expression | 3-Oxo-C12-HSL, P. aeruginosa infection, NCI-H292 cells |
| Inhibition of proinflammatory cytokine secretion (IL-8, GM-CSF) | Flagellin, NHBE cells | |
| Inhibition of cell proliferation | NHBE cells | |
| Inhibition of chemotaxis | Neutrophils | |
| NF-κB (11, 41, 54, 55, 102, 105, 124, 140, 191, 216, 279, 285) | Inhibition of mucus secretion, MUC5AC production, and MUC5AC gene expression | TNF-α, LPS, TGF-α, 3-oxo-C12-HSL, NCI-H292 cells |
| Inhibition of proinflammatory cytokine secretion (TNF-α, IL-1, IL-6, IL-8) | CF cells, 16HBE cells, BET-1A cells, T cells, monocytes, fibroblasts | |
| Inhibition of MMPs | Fibroblasts | |
| Inhibition of iNOS | Macrophages |
CLINICAL EFFECTS
Asthma
The immunomodulatory effects of macrolides were first reported for the treatment of patients with asthma in the 1960s, when troleandomycin (TAO), a 14-membered macrolide, was shown to decrease the amount of oral corticosteroid needed for asthma control in steroid-dependent asthmatics (110). This “steroid-sparing” effect was initially thought to be due to the action of TAO on corticosteroid metabolism via the cytochrome P450 system. TAO can reduce the clearance of methylprednisolone (276), but the clinical reduction in the dose of methylprednisolone achieved after TAO is far greater than would be anticipated from its effects on corticosteroid metabolism alone (342). A number of patients with steroid-dependent asthma were able to discontinue prednisolone entirely while on TAO (122, 237, 262, 318), suggesting that direct effects on the inflammatory response were involved. Similar properties were not seen with other antibiotics. In contrast, in a double-blind placebo-controlled study enrolling 75 adult asthmatics, there was no significant reduction in steroid use for 2 years, but the group receiving TAO had more side effects, such as reductions in bone density (207). Routine use of TAO for the therapy of asthma was largely limited by hepatic side effects (122, 207). A Cochrane review analyzed available data from 90 patients with steroid-dependent asthma in three randomized controlled studies of TAO (64). In this report, there was no treatment effect for TAO to reduce the steroid dose (standardized mean difference [SMD], −0.29; 95% confidence interval [95% CI], −0.75 to 0.17) and no significant improvement in lung function (SMD, 0.06; 95% CI, −0.8 to 0.9).
Gotfried and associates (84) evaluated add-on therapy of asthma, using clarithromycin administered at 500 mg twice daily, in a 6-week randomized controlled pilot study of 21 subjects with corticosteroid-dependent asthma. In the clarithromycin group, there were increases in forced vital capacity (FVC; P = 0.04) and FEV1 (P = 0.07), as well as decreases in nocturnal dyspnea (P = 0.05) and chest discomfort (P = 0.02), compared with baseline values. In addition, subjects were able to reduce their prednisone dosage by a mean of 30% from baseline. Favorable effects of macrolide therapy were also observed in patients who were not taking oral corticosteroids. Miyatake et al. (192) evaluated the effects of 10 weeks of treatment with erythromycin (200 mg given three times daily) in a group of 11 atopic and 12 nonatopic asthmatics not receiving steroid therapy. Erythromycin decreased the degree of bronchial hyperresponsiveness in both groups, as measured by a significant increase in the provocative concentration required to produce a 20% fall in FEV1 from the baseline (PC20) in response to a histamine challenge. They also reported that there was no significant difference in serum theophylline concentrations before and after erythromycin treatment. A similar study was performed on children with asthma, using roxithromycin (253). The PC20 significantly increased with a dose of 150 mg roxithromycin daily after 4 and 8 weeks of treatment. No significant changes in serum theophylline concentrations and serum cortisol levels were observed during this study. Kamoi et al. (123) evaluated the effects of roxithromycin on the generation of free radicals by neutrophils and on bronchial hyperresponsiveness in 10 asthmatics over the course of a 3-month treatment and observed significant reductions in oxygen radicals and airway hyperreactivity compared with those in untreated controls. In a randomized controlled crossover clinical study, clarithromycin, given at 200 mg twice a day (b.i.d.), or placebo was administered for 8 weeks to 17 subjects with mild to moderate asthma (5). There were significant decreases in sputum eosinophils and eosinophilic cationic protein as well as in bronchial hyperresponsiveness compared to those for the placebo group. These results were consistent with a study to evaluate the effects of clarithromycin on bronchial hyperresponsiveness (154). Subjects with stable asthma who were using budesonide at 400 mg daily were randomized to three study arms. Group A (22 subjects) received clarithromycin at 500 mg daily for 8 weeks, group B (20 subjects) received clarithromycin at 750 mg daily, and group C (21 subjects) received placebo. Bronchial hyperresponsiveness was quantified by measurement of the provocative dose required to produce a 20% fall in FEV1 (PD20) in response to methacholine provocation. Compared to the baseline, there was a significant increase in the median PD20 in groups A and B but not in the placebo group. The median values (interquartile ranges) for the three groups before and after treatment were as follows: group A, 0.3 (0.1 to 1) and 1.3 (0.6 to 2) mg (P < 0.001); group B, 0.4 (0.1 to 0.9) and 2 (2 to 2) mg (P < 0.001); and group C, 0.4 (0.1 to 0.9) and 0.3 (0.1 to 0.6) mg (not significant). The effectiveness of azithromycin was demonstrated in another study in which 11 subjects with mild asthma received 250 mg azithromycin orally twice weekly for 8 weeks and had significantly increased PC20 values in response to methacholine, without changes in FEV1 (59). These macrolides had little or no effect on CYP3A4, one of the cytochrome P450 enzymes (224).
Asthma is a complex, multifactorial disease. Atypical organisms such as Chlamydia pneumoniae and Mycoplasma pneumoniae are thought by some to be associated with chronic asthma and asthma exacerbations (32, 155). Black and colleagues (31) conducted a randomized clinical trial of roxithromycin given at 150 mg twice daily for 6 weeks in 232 asthmatic subjects who were antibody positive for C. pneumoniae. There was no statistically significant change in the mean morning peak expiratory flow (PEF) or symptom score, but there was a significant improvement in evening PEF. These effects disappeared over a 6-month follow-up period after stopping the treatment. The prevalence of M. pneumoniae or C. pneumoniae in subjects with chronic asthma has been investigated by PCR, using BAL fluids (155). Clarithromycin given at 500 mg twice daily for 6 weeks improved the FEV1 from 2.50 ± 0.16 to 2.69 ± 0.19 liters (P = 0.05) in PCR-positive subjects, but there was no significant effect in PCR-negative subjects. A Cochrane review updated in 2007, evaluating 7 studies including 416 subjects with chronic asthma, did not show a clinically significant benefit of using macrolides in the management of chronic asthma (236). This may suggest differences in disease duration and severity, the relatively short treatment duration in most studies, and the small number of subjects. A more recent clinical trial was performed with children who required medium to high doses of inhaled corticosteroids (ICS) with salmeterol to achieve asthma control (269). After a budesonide-stable period of 6 weeks, 55 children were randomized to receive once-nightly azithromycin, montelukast, or placebo plus the established controlling dose of budesonide (minimum of 400 mg twice daily) and salmeterol twice daily. The primary outcome was the time from randomization to loss of asthma control. There were no differences in time to loss of control (median for azithromycin, 8.4 weeks [95% confidence limit, 4.3 to 17.3 weeks]; median for montelukast, 13.9 weeks [95% confidence limit, 4.7 to 20.6 weeks]; and median for placebo, 19.1 weeks [95% confidence limit, >11.7 weeks]), but the study may not have been powered adequately.
In contrast, a placebo-controlled study to evaluate an 8-week course of azithromycin in 16 asthmatic children showed decreased bronchial hyperresponsiveness to hypertonic saline inhalation, and there was a reduction in airway neutrophil infiltration in the azithromycin-treated children but not in the placebo group (227). A randomized clinical trial with 45 adults with severely refractory asthma showed that 8 weeks of clarithromycin at 500 mg twice daily reduced concentrations of sputum IL-8, neutrophil elastase, and MMP-9 and improved clinical outcomes, including quality-of-life scores and wheezing frequencies, compared with those for the placebo group (260). There was no change in predicted FEV1%, airway hyperresponsiveness to hypertonic saline, or the asthma control score. The authors found that the improvements in inflammation were most marked in those with refractory noneosinophilic asthma. Asthmatics who benefit from macrolide therapy appear to have cases of more-severe, steroid-resistant asthma, and this may be related to airway neutrophilic inflammation.
Non-CF Bronchiectasis
The effectiveness of macrolides in treating DPB has led to trials in other lung disorders characterized by productive cough, airway infection, and chronic inflammation. A randomized controlled trial of low-dose clarithromycin was reported for treatment of chronic inflammatory airway diseases, including bronchiectasis and chronic bronchitis (290). In the group that received clarithromycin at 100 mg twice daily for 8 weeks, sputum production decreased from 51 to 24 g/day and the percent solids composition increased from 2.4% to 3.0%, with no effect seen in the placebo group. Furthermore, the elasticity of the sputum increased, but its viscosity was unchanged. Similarly, in 11 patients with bronchiectasis, erythromycin given at 500 mg twice daily for 8 weeks decreased the 24-h sputum volume and improved pulmonary function compared with those of the placebo group (306). There was no change in sputum bacteria, leukocytes, IL-1α, IL-8, TNF-α, or LTB4. Tagaya and coworkers reported reduced sputum volume with a short course of clarithromycin at 400 mg daily for 7 days (278). Thirty-eight percent of the subjects with chronic bronchitis or bronchiectasis had decreased sputum compared to the baseline volume; amoxicillin and cefaclor treatment had no effect on sputum volume.
A small open-label trial of azithromycin at 500 mg twice per week for 6 months in subjects with bronchiectasis also suggested a clinical benefit, as the patients had fewer exacerbations (50). In a randomized controlled trial, roxithromycin was given for 12 weeks to 13 children with airway hyperresponsiveness and bronchiectasis. The subjects taking roxithromycin had decreased sputum purulence and leukocyte counts, and the PC20 in response to methacholine increased significantly compared with that for the placebo group (147).
Low-dose roxithromycin decreased sputum IL-8, neutrophil elastase, and C5a and reduced neutrophil recruitment into the lung in subjects with bronchiectasis (203). BAL fluid IL-8 and neutrophil percentages, but not TNF-α or IL-10 percentages, decreased compared with those for controls in 17 children with stable bronchiectasis after 3 months of clarithromycin given daily at 15 mg/kg (329).
COPD
Chronic obstructive pulmonary disease (COPD) is an epidemic disease predicted to become the third leading cause of death in the world by 2020. There are no pharmacological therapies today that will prevent disease progression or reduce mortality once COPD is established (24). Frequent exacerbations in COPD patients lead to a faster decline in lung function (57) and to increased airway inflammation (29). Many proinflammatory cytokines, chemokines, adhesion molecules, proteinases, and reactive oxygen species are involved in COPD pathogenesis (24).
The results of studies using macrolides to treat COPD have been mixed. Compared to placebo (n = 36), 3 months of oral clarithromycin given to subjects with stable COPD (n = 31) did not significantly improve health status or decrease sputum bacteria or the exacerbation rate (22). In contrast, Suzuki et al. (275) studied 109 Japanese subjects with COPD who were treated with low-dose erythromycin in an open-label study, and this was associated with decreased exacerbations and hospitalizations. These subjects were randomly assigned to prophylactic erythromycin therapy or no active treatment and were observed for 12 months. The frequency of upper respiratory tract infection was 76% for controls and 13% for the erythromycin group, and the exacerbation rate was 56% for the controls and 11% (6/55 patients) for the erythromycin group (relative risk [RR], 4.71; 95% CI, 1.53 to 14.5; P < 0.007). In an in vitro study by the same group, this effect was thought to be due to inhibiting human rhinovirus (RV) adherence by suppressing ICAM-1, an RV receptor, blocking RV RNA entry into the endosomes and decreasing proinflammatory cytokine production (274). Jang et al. (114) also showed that clarithromycin can decrease RV titers in infected A549 pulmonary epithelial cells. A retrospective multicenter analysis of 123 Japanese patients observed over a mean of 42.9 months indicated that macrolide therapy reduced the frequencies of COPD exacerbations and hospitalizations due to exacerbations (334). The frequency of exacerbations was 4.4% for the macrolide group (n = 45) and 15.4% for those not receiving chronic macrolide therapy (P = 0.01). Seemungal et al. (250) conducted a 12-month randomized clinical trial of erythromycin, given at 250 mg b.i.d. (n = 53), or placebo (n = 56) in subjects with moderate to severe COPD. There were 206 moderate to severe exacerbations, and 125 occurred in the placebo arm. The odds ratio for exacerbations for the erythromycin- compared with placebo-treated subjects was 0.65 (95% confidence interval, 0.48 to 0.86; P = 0.003), and subjects receiving erythromycin had shorter exacerbations.
Alveolar macrophages isolated from patients with COPD who had been given azithromycin had enhanced phagocytosis of apoptotic bronchial epithelial cells, and this was associated with increased expression of the mannose receptor (91).
Chronic Rhinosinusitis
Chronic low-dose macrolides were first used as immune modulators for chronic sinusitis in Japan (139, 142, 280), but this has also been studied in other countries (88, 241). In a 2-week randomized controlled study of clarithromycin given at 500 mg twice daily, there was a decreased nasal secretion volume (500 mg versus 28 mg; P = 0.01) and increased secretion mucociliary transportability (by 30%; P = 0.005) compared with those for controls. Clarithromycin also normalized the rheology and cohesivity of nasal mucus (88). Another study showed that clarithromycin treatment for 4 weeks increased spinnability (cohesivity) and decreased the ratio of viscosity to elasticity (tangent delta) of nasal mucus in 18 subjects with chronic sinusitis (233).
Hashiba and Baba (86) investigated the clinical efficacy of clarithromycin, given at 400 mg daily for 12 weeks, in 45 subjects with chronic sinusitis. Subjects taking clarithromycin had improved rhinoscopic findings and fewer symptoms of nasal obstruction, and the benefits correlated with the duration of therapy. There was a decrease in the size of nasal polyps associated with chronic sinusitis in 52% of patients after 8 weeks of roxithromycin treatment, given at 150 mg daily, but no correlation with allergy or eosinophilic infiltration (101). In another study, there was a decrease in nasal lavage fluid IL-8 for subjects with chronic rhinosinusitis whose nasal polyps were smaller after 12 weeks of clarithromycin treatment at 400 mg per day (P < 0.005) (330). It was speculated that IL-8 may drive the development of nasal polyps in some persons with rhinosinusitis. It has also been shown that macrolides can decrease IL-8 production by nasal epithelial cells in vitro (272).
In a prospective, single-center, open-label study, 25 subjects with stable chronic sinusitis and persistent maxillary sinus inflammation were treated with clarithromycin at 500 mg twice daily for 14 days (176). There were improvements in clinical signs and symptoms which lasted for up to 2 weeks after completing therapy, including significant decreases in edema scores and nasal biopsy specimen elastase, IL-6, IL-8, and TNF-α levels compared with those measured pretreatment.
Macrolide therapy also benefits patients with chronic persistent rhinosinusitis after sinus surgery. Cervin and colleagues (38) conducted a prospective open-label study of 17 subjects who did not respond to sinus surgery, antibiotics, and systemic steroids. Subjects were treated with erythromycin at 250 mg twice daily or with clarithromycin at 250 mg once daily. After 3 months, 12 of the 17 participants subjectively improved, and these responders were reassessed after 12 months of treatment. At 12 months, there were significant improvements in saccharin transit time (testing mucociliary clearance) (P < 0.05), nasal endoscopic scoring (P < 0.01), and symptoms of nasal congestion, sticky secretions, and runny nose by visual analog scale scoring (P < 0.01).
A randomized clinical trial was conducted with 64 subjects with chronic rhinosinusitis who were given 3 months of roxithromycin at 150 mg daily. There were significant improvements in SinoNasal Outcome Test 20 (SNOT20) scores, nasal endoscopy results, saccharin transit times, and IL-8 levels in lavage fluid compared with those for the placebo group (P < 0.05), and a correlation was noted between improved outcome measures and low initial IgE levels (319). This is consistent with the inhibitory effect of macrolides on neutrophilic inflammation (272, 330) and with studies suggesting that symptomatic improvement after macrolide therapy correlates with low levels of IgE and with low eosinophil counts in peripheral blood, nasal smears, and the nasal mucosa (101, 273).
CF
CF is a genetic disease that is clinically and bacteriologically similar to DPB. Because of these common features, an open pilot study of erythromycin given for 4 weeks to CF patients was performed and showed that sputum IL-8 concentrations for 6 subjects with CF were decreased (65). Jaffe and colleagues (113) then evaluated long-term (mean duration, 0.6 years) azithromycin therapy in 7 children with CF and infection with P. aeruginosa. These children had median increases in FVC of 11.3% and in FEV1 of 11% (P < 0.03).
Wolter and colleagues (326) reported the results of a prospective placebo-controlled study of 60 adults with CF who were given daily azithromycin, at 250 mg, or placebo for 3 months. FEV1 was maintained in the azithromycin group, while there was a significant decline in the placebo group. Systemic inflammation, as measured by C-reactive protein levels, decreased only in the azithromycin group. Quality of life improved over time in patients on azithromycin and remained unchanged in those on placebo. Similarly, Equi et al. (62) conducted a placebo-controlled crossover trial with 41 children who received either azithromycin or placebo for 6 months. Twenty-two subjects were homozygous for the ΔF508 mutation. After 2 months of washout, the treatments were crossed over. Equi et al. found a 5.4% improvement in FEV1 with azithromycin, but there was no effect on either bacterial density or sputum inflammatory markers. It was suggested that children homozygous for the ΔF508 mutation did better than the others. A larger, multicenter study also demonstrated significant lung function improvements and fewer intravenous antibiotic courses for subjects with CF who were on azithromycin (243). A total of 185 adults and children who had CF and were infected with P. aeruginosa were randomly assigned to receive placebo or 250 mg (for body weights of <40 kg) or 500 mg (for body weights of ≥40 kg) of azithromycin 3 days a week for 168 days. The predicted FEV1% increased 4.4% in the azithromycin group but declined 1.8% in the placebo group (mean difference, 6.2%; P = 0.001). Subjects taking azithromycin had fewer exacerbations than those in the placebo group (hazard ratio [HR], 0.65; 95% CI, 0.44 to 0.95; P = 0.03), and they weighed an average of 0.7 kg more at the end of the study (95% CI, 0.1 to 1.4 kg; P = 0.02 compared with placebo group). A meta-analysis of 4 studies with 296 participants concluded that there is a small but significant treatment effect for azithromycin to improve pulmonary function in persons with CF (267).
In a 1-year randomized controlled study, 82 young CF patients (≥6 years old; mean age, 11.0 years) were randomized to receive azithromycin at 250 or 500 mg, depending on body weight, or oral placebo three times a week for 12 months (45). The relative changes in predicted FEV1% at the end of the study did not differ significantly between the two groups, but the number of pulmonary exacerbations, the time to the first pulmonary exacerbation, and the number of additional courses of antibiotics were significantly reduced in the azithromycin group. Similarly, maintenance azithromycin therapy given over 3 years was assessed retrospectively in 100 patients with CF (303). The predicted FEV1% improved in the first year after initiation of azithromycin but decreased during the second and third years. Another long-term observational cohort study of 45 adult CF patients with chronic P. aeruginosa infection demonstrated a significant decrease in the rate of decline in lung function (85). The negative annual median slope of decline of pretreatment FEV1 was changed from −4.1% of the predicted value to a positive annual slope, with an increase of +0.8%, after 12 months of treatment with 250 mg daily azithromycin, corresponding to a difference of +4.9% (P < 0.001).
CF lung inflammation may represent a primary event due to the genetic defect. CF airway epithelial cells have a spontaneously increased production of IL-8 and TNF-α and an enhanced inflammatory response to bacteria (37, 41). Furthermore, CF airway cells produce inflammatory peptides even in the absence of infection (302), and they respond to P. aeruginosa with a greater-than-normal inflammatory response (247). In the F508del-CF mouse, there is spontaneous and exaggerated LPS-induced macrophage infiltration in the airways, but both spontaneous and induced inflammatory responses are attenuated following azithromycin treatment (163). Azithromycin downregulates proinflammatory cytokine expression in M1-polarized CF alveolar macrophages and wild-type alveolar macrophages (186). However, it is not clear whether this hyperinflammatory process is the direct or indirect result of the CFTR defect (175, 239). The level of phosphorylated ERK1/2, but not p38 MAPK, is markedly elevated in CF bronchial cells and further supports the hypothesis that CFTR, the intracellular Ca2+ response, and NF-κB activation of CF airway epithelial cells are interdependent (112, 175, 277).
DPB
The initial clinical trials of macrolides as immunomodulatory medications for the therapy of chronic inflammatory airway diseases were driven by the dramatic effects reported for use of these drugs to treat DPB. DPB is a progressive inflammatory airway disease, primarily reported from Japan, Korea, and Taiwan, which is characterized by a chronic cough, copious sputum expectoration, dyspnea, and chronic sinusitis (156). The airways of persons with DPB are infected by Haemophilus influenzae, S. pneumoniae, and eventually mucoid P. aeruginosa. The bacterial exacerbations lead to airflow obstruction, respiratory failure, and cor pulmonale. Symptoms usually appear in the fourth or fifth decade of life in nonsmokers, and the 10-year survival rate was <15% for patients infected with P. aeruginosa (71). Low-dose erythromycin was first used to treat DPB in 1982. Work by Kudoh and colleagues (158) demonstrated that low-dose erythromycin therapy significantly improves the long-term survival of DPB patients. Many subsequent trials have shown the effectiveness of erythromycin for the treatment of DPB (71, 99, 144, 202, 258). Erythromycin therapy improves symptoms, pulmonary function, and hypoxemia and increased the 10-year survival rate to >90% (156). The benefit has been demonstrated with other 14- and 15-membered macrolides as well (120, 258), but josamycin, a 16-membered macrolide, has been ineffective (19). A long-term study by Kadota et al. (120) reported the effectiveness of clarithromycin at 200 mg/day for 4 years in patients with DPB. Pulmonary function and oxygenation improved significantly within 3 months of starting therapy, and the effects persisted for the full 4 years.
Neutrophil influx into airways and the production of oxidants and proteolytic enzymes play a crucial role in the pathogenesis of DPB (98). In DPB, macrolides decrease neutrophil chemotactic activity in BAL fluid. Erythromycin therapy decreased the number of neutrophils and neutrophil-derived elastolytic-like activity in BAL fluid (98). There were decreased numbers of neutrophils and neutrophil chemotactic activity in BAL fluid, not only for patients following erythromycin treatment but also for animal models (119, 211). Several studies have shown a reduction in BAL fluid levels of IL-8 and IL-1β for subjects with DPB following macrolide treatment (17, 198, 283). Additionally, there is decreased adhesion molecule macrophage activating complex 1 (Mac-1) in peripheral blood neutrophils after macrolide therapy (198). Macrolides suppress CD8+ CD11b− cytotoxic T cells in BAL fluid from subjects with DPB (131). Macrolide suppression of proinflammatory cytokine production may be the principal mechanism of action in DPB (71, 183, 283).
The working group of the Diffuse Lung Disease Committee of the Ministry of Health and Welfare of Japan prepared clinical guidelines for macrolide therapy for DPB in 2000. The guidelines suggest that a macrolide be started as soon as the diagnosis of DPB is made, and erythromycin at 400 or 600 mg daily is recommended. If this treatment provides insufficient clinical benefits after 6 months of therapy or if undesirable adverse effects are associated with this drug, clarithromycin at 200 or 400 mg daily or roxithromycin at 150 or 300 mg daily is then recommended (206).
Influenza and Sepsis
Systemic infectious diseases such as severe influenza and sepsis can produce a severe systemic inflammatory response syndrome (SIRS). The parenchyma injury is associated with overexpression of cytokines and inflammatory mediators and with upregulation of adhesion molecules in vascular beds (40, 310, 320). Modulation of SIRS has been proposed on the basis of experimental and clinical evidence (79, 220, 230). In murine influenza virus-induced pneumonia, erythromycin dose dependently improves survival, and this is associated with inhibition of inflammatory cell responses and attenuation of NO production in the lung (246). Other studies demonstrated that clarithromycin suppresses inflammatory cytokines such as TNF-α (138) but increases IL-12 and immunoglobulin A in airway fluids (308), reducing mortality in treated mice. Azithromycin can significantly improve survival in mice infected with influenza virus and superinfected with S. pneumoniae (129). Improved survival appears to be mediated by decreased inflammation, manifested as fewer inflammatory cells and proinflammatory cytokines in the lungs. Clarithromycin also inhibits virus production from influenza A virus-infected host cells in vitro (190).
European and North American guidelines recommend a combination therapy consisting of a β-lactam and a macrolide for hospitalized patients with community-acquired pneumonia (CAP) (178, 327). However, there are no randomized controlled trials comparing a single β-lactam with the same drug supplemented with a macrolide, and these recommendations are largely supported by observational studies of selected populations which have shown lower mortality rates for patients treated with β-lactam-macrolide combination therapy than those for patients treated with β-lactam monotherapy (21, 35, 74, 180, 301). However, other studies failed to show a benefit of macrolide-containing combinations (58, 223). The synergy between β-lactams and macrolides against S. pneumoniae is not documented in vitro (172) or in vivo (115), although macrolides inhibit the production of the pneumococcal toxin, pneumolysin (8). Oral azithromycin decreased the rate of lethality in a septic shock model in mice challenged with intraperitoneal or intravenous LPS, accompanied by a reduced serum level of TNF-α, without suppressing neutrophil tissue infiltration (111). Therefore, the reason for the beneficial effect appears to be unrelated to β-lactam resistance.
In humans, the cytokine profile for 23 subjects with CAP treated with clarithromycin at 500 mg twice daily for 7 days was compared with that for subjects treated with amoxicillin at 1 g three times daily for 7 days. In the clarithromycin group, there was a decrease in the proinflammatory cytokine IL-6, an increase in the anti-inflammatory cytokine IL-10, and an increase of IFN-γ (53). At therapeutic concentrations, clarithromycin inhibited IL-8 release from human monocytes stimulated with bacterial extracts of Escherichia coli or P. aeruginosa (140).
Intravenous clarithromycin in the therapeutic range improved survival in a murine model of sepsis caused by susceptible E. coli, multidrug-resistant P. aeruginosa, or pandrug-resistant Klebsiella pneumoniae, and this was associated with decreased serum TNF-α and reactive oxygen species (78, 80, 81). These pathogens were not susceptible to clarithromycin in vitro, and the effects were observed even if clarithromycin was administered after the animals developed sepsis-associated pulmonary edema. TNF-α secretion by monocytes isolated from clarithromycin-treated animals was also decreased ex vivo (26).
A randomized, controlled, multicenter trial was designed to evaluate the efficacy of clarithromycin in patients with sepsis and ventilator-associated pneumonia (VAP) (79). Clarithromycin was administered intravenously at a dose of 1 g once daily for 3 consecutive days in 100 subjects, and another 100 subjects were treated with placebo. Other antimicrobials for treating VAP were selected by the attending physicians. Although 28-day mortality was not affected, clarithromycin shortened the median time for resolution of VAP and time to extubation among the 141 survivors compared with those for placebo (10.0 days versus 15.5 days [P = 0.011] and 16.0 days versus 22.5 days [P = 0.049], respectively). Fourteen subjects died of a cause other than sepsis, and 45 died of sepsis. The odds ratio for death due to a sepsis-related cause among clarithromycin-treated patients was reduced compared with that for placebo-treated patients (3.78 versus 19.00; P = 0.043). A pathogen was identified in 70% of cases, and the eradication rates were similar in both groups.
Restrepo and associates (232) found a survival benefit of macrolide therapy in 30- and 90-day mortality rates for patients with sepsis after pneumonia. In this retrospective cohort study, 104 (43.9%) of 237 subjects with severe sepsis received macrolides. Overall, 30- and 90-day mortality rates were lower for subjects who received macrolides than for the others (11% versus 29% [P = 0.001] and 12% versus 34% [P < 0.001], respectively). The multivariable analysis revealed that the use of macrolides was associated with decreased mortality at 30 days (HR, 0.3; 95% CI, 0.2 to 0.7) and at 90 days (HR, 0.3; 95% CI, 0.2 to 0.6) in patients with severe sepsis and in patients with macrolide-resistant pathogens (HR, 0.1; 95% CI, 0.02 to 0.5).
Posttransplantation BOS
Bronchiolitis obliterans syndrome (BOS) is the leading cause of death after lung transplantation. An open-label pilot study demonstrated that 6 subjects with BOS who were treated with azithromycin at 250 mg three times a week had improved FEV1, with a mean increase of 17% (P ≤ 0.05) after an average of 13.7 weeks (77). Four subsequent clinical trials of BOS showed significant improvements in FEV1 with azithromycin treatment (136, 312, 313, 338), while one showed no demonstrable effect (259). The most recent of these studies treated 14 subjects with azithromycin at 250 mg daily for 5 days and then three times weekly for 3 months (313). There were 6 responders to azithromycin (with an FEV1 increase of >10%), and the mean FEV1 increased from 2.36 to 2.67 liters (P = 0.007). Azithromycin reduced BAL fluid neutrophils and IL-8 mRNA. The authors concluded that in subjects with >15% neutrophils in their BAL fluid, there was a positive predictive value of 85% for a significant FEV1 response to azithromycin. Finally, pulmonary IL-17-induced IL-8 production is thought to be important in BOS, and azithromycin has been shown to inhibit IL-17 through its effect on MAPK signaling (311).
SAFETY OF LONG-TERM MACROLIDE THERAPY
Macrolide Resistance
The antimicrobial activity of macrolides is based on inhibition of bacterial protein biosynthesis after binding of the macrolide, selectively and reversibly, to the 50S ribosomal subunit. More precisely, macrolides prevent peptide bond formation by interacting with 23S rRNA, a major component of the 50S subunit and an important part of the peptidyltransferase center (179). The main binding sites lie within domain V in the 23S rRNA. In addition, it is postulated that macrolides cause dissociation of the peptidyl-tRNA during translocation by blocking the path through which nascent peptide chains exit the ribosome, thus interfering with elongation of the polypeptide chain (300).
There is a concern that the long-term administration of macrolides can promote antimicrobial resistance. Malhotra-Kumar and coworkers (177) conducted a randomized, double-blind, placebo-controlled study, enrolling a total of 224 healthy volunteers: 74 were treated with azithromycin, 74 with clarithromycin, and 76 with placebo. Oral streptococci were used as model organisms to evaluate the development of resistance over 180 days. Both macrolides significantly increased the proportion of macrolide-resistant streptococci, with resistance peaking at day 4 in the azithromycin group (mean increase of 53.4% versus placebo; P < 0.001) and at day 8 in the clarithromycin group (50.0% increase versus placebo; P < 0.001). For CF patients taking maintenance azithromycin therapy, marked increases in Staphylococcus aureus and Haemophilus spp. resistant to macrolides have been reported (226, 303). Staphylococcal resistance to macrolides went from 83% in the first year to 97% in the second year and, finally, to 100% in the third year after starting therapy (303). The long-term use of azithromycin in CF patients also leads to macrolide resistance in the commensal viridans group streptococci, as assessed by molecular characterization (299). Since the incidence of nontuberculous mycobacterial (NTM) pulmonary infections is also increasing among CF patients, it is feared that azithromycin therapy may induce selection or acquisition of macrolide resistance in NTM (167, 215).
There are three principal mechanisms responsible for acquired macrolide resistance (299, 343). The first is due to ribosomal target modification, which is mediated by methylases encoded by the erm(B) gene. The methylation of the adenine at position 2058 of domain V prevents macrolides from binding to the ribosome, resulting in high-level resistance. The second is an active drug efflux mechanism, which is determined by the presence of the membrane-bound efflux protein, encoded by the mef(A) gene. This mechanism confers low-level macrolide resistance. The third is a mutation in the 23S rRNA or ribosomal proteins L4 and L22, leading to conformational changes at the binding site of the macrolides and to a large reduction in effectiveness. The PROTEKT study is a global longitudinal surveillance study of the distribution of mechanisms for macrolide resistance to Streptococcus pneumoniae (66). Year 5 PROTEKT data (2003-2004) showed that the rate of erythromycin resistance in S. pneumoniae isolates from patients with community-acquired respiratory tract infections was 37.2%. The overall distributions of the resistance mechanisms erm(B) and mef(A) were 55.0% and 30.6%, respectively, with a combination of both mechanisms accounting for 12.0% of cases of resistance. Ribosomal mutations accounted for a small minority of cases (2.0%). Long-term use of macrolides for common conditions such as asthma and COPD should be examined comprehensively on the basis of the clinical evidence, and there may be a benefit to developing nonantimicrobial macrolides that retain immunomodulatory properties without inducing resistance (see “EM703”).
Long-Term Macrolide Safety
Kadota and colleagues reported on the safety and effectiveness of once-daily clarithromycin given at a low dose for 4 years to 10 patients with DPB (120). They found that pulmonary function improved in most of these patients within 6 months, and often the improvement was seen within 2 to 3 months. There were also improvements in oxygen saturation at rest and in quality of life within 6 months in nine of these patients. These improvements persisted for at least 4 years, with no reported side effects. The same authors later reported on long-term macrolide antibiotic therapy in the treatment of chronic small-airway disease that clinically mimicked DPB but had a very different histology. Unlike the 80 to 90% response to long-term erythromycin therapy reported for DPB, pulmonary function, total cell counts, and neutrophil percentages in bronchoalveolar lavage fluid did not improve in these patients (121). However, similar to the case for patients with DPB, there were no reported side effects seen with long-term use. In a similar study, Phaff and colleagues from the Netherlands reported on the safety and effectiveness of long-term azithromycin therapy in patients with CF (226). In their CF center, 156 patients were monitored during the study period of January 1999 to March 2004, and by October 2003, 50 patients were taking azithromycin three times a week at a low dose. As the duration of treatment increased, sputum cultures of patients who were using azithromycin were positive for either Staphylococcus or Haemophilus significantly less often than cultures of patients not on azithromycin, although the percentage of resistant cultures in those that were culture positive dramatically increased. Macrolide resistance increased in both those taking azithromycin and those not using the antibiotic.
It has been reported that the concurrent use of CYP3A inhibitors and erythromycin can increase the risk of cardiac death. Postmarketing data surveillance from Japan indicates that although 10 tons of erythromycin are used annually for low-dose, long-term immunomodulatory treatment, there have been only 4 cases of torsade de pointes due to erythromycin among an estimated 34,000 patients per year treated with erythromycin over the last 10 years. Furthermore, these four patients received erythromycin without concomitant treatment with CYP3A inhibitors, and they recovered after discontinuing erythromycin treatment (18).
Thus, it appears that the long-term and low-dose use of macrolides can increase the development of resistance in Gram-positive organisms, apparently without compromising the effectiveness of the medications or increasing the risk of cardiovascular death. The development of nonantimicrobial macrolides that possess immunomodulatory properties would be expected to decrease the risk of macrolide resistance developing in both patients and community isolates. Guidance for chronic use of macrolide therapy is summarized in Table 3.
TABLE 3.
Clinical application of macrolides
| Disease | Recommendation for routine use | Expected efficacy | Outstanding issues or comments |
|---|---|---|---|
| Asthma | Discouraged (few patients) | Steroid-sparing effect | Insufficient evidence, small study population |
| Bronchiectasis | Partially recommended | Decreased sputum, reduced exacerbations | Few data, small number of patients, short duration |
| COPD | Discouraged | Reduced exacerbations | Insufficient evidence, small study population |
| Chronic rhinosinusitis | Partially recommended (for noneosinophilic cases) | Reduced nasal mucus, improved quality of life | Study design, study population |
| Cystic fibrosis | Recommended | Reduced exacerbations, less deterioration in pulmonary functions | Short duration |
| DPB | Recommended | Improved prognosis, potential cure | First-line therapy |
| Influenza/sepsis | Discouraged | Improved prognosis | Insufficient evidence |
| Posttransplantation BOS | Discouraged | Improved prognosis | Insufficient evidence |
OTHER RELATED DRUGS
Ketolides
Ketolides are a class of semisynthetic agents derived from erythromycin A by replacement of the cladinose at C-3 with a keto group. Ketolides bind to ribosomes with a higher affinity than that of macrolides, and they potently inhibit protein synthesis by interacting with the peptidyltransferase site of the bacterial 50S ribosomal subunit (343).
Telithromycin was the first member of this class, and it has been reported to have immunomodulatory properties similar to those of the macrolides. In monocytes from eight volunteers, telithromycin inhibited LPS-induced IL-1α and TNF-α, but not IL-1β, IL-6, or IL-10 (14). In a septic mouse model, telithromycin downregulated production of TNF-α and IL-1β and improved the rates of survival after a lethal dose of E. coli LPS was given (165). Acute airway inflammation in mice after airway nebulization of LPS was attenuated by telithromycin pretreatment, and this was associated with decreased neutrophilia and reduced levels of protein, nitrite, MIP-2, and TNF-α in the BAL fluid (164). In vitro, telithromycin inhibited the production of MIP-2 and TNF-α in response to LPS stimulation in the macrophage cell line RAW 264.7, and it decreased the production of MIP-2 by murine lung epithelial (MLE-12) cells cultured with supernatants of LPS-stimulated RAW 264.7 macrophages. NF-κB activation was inhibited and apoptosis was increased in both cell lines by use of telithromycin (164). Telithromycin, as well as azithromycin and clarithromycin, can inhibit the increased production of MUC5AC mRNA and protein in NCI-H292 cells stimulated with LPS (108). However, telithromycin did not inhibit MUC5AC production induced by human neutrophil peptide-1 (HNP-1), while azithromycin and clarithromycin did inhibit MUC5AC.
Johnston et al. (117) evaluated the effects of telithromycin in 278 patients with acute exacerbations of asthma in a double-blind, randomized, placebo-controlled study. Telithromycin given at 800 mg daily for 10 days decreased asthma symptoms significantly compared with placebo, although there was no significant treatment effect on the morning peak expiratory flow. There was no relationship between the response to asthma treatment and C. pneumoniae or M. pneumoniae infection, as ascertained by serologic analysis, PCR, and culture.
Quinolones
Antimicrobial activity of fluoroquinolones is due to interference with DNA replication by inhibiting bacterial DNA gyrase and topoisomerase IV (51). Although eukaryotic cells do not contain these enzymes, fluoroquinolones accumulating intracellularly could potentially affect topoisomerase type II enzymes, the mammalian counterparts, thereby having the potential to damage mammalian cells (263).
Fluoroquinolones were also proposed as immunomodulators and have been compared with macrolides (51, 220). Fluoroquinolones are able to modify transepithelial Cl secretion, phagocyte functions, T-cell responses, and cytokine production (128, 160, 242). Moxifloxacin and ciprofloxacin dose dependently decreased IFN-γ and IL-4 expression by T cells in vitro (324). Therapeutic concentrations of moxifloxacin significantly inhibited IL-1α and TNF-α secretions from human monocytes stimulated with LPS (13). In mice injected with a lethal dose of LPS, ciprofloxacin and trovafloxacin protected mice from death and decreased TNF-α and IL-6 levels in serum (137). Likewise, moxifloxacin inhibited IL-8 and TNF-α, preventing neutrophilic pneumonitis in immunosuppressed mice (251). Furthermore, moxifloxacin has been shown to suppress ERK1/2 activation and NF-κB nuclear translocation in human monocytes and a lung respiratory cell line (322, 323). Blau et al. (33) showed that moxifloxacin inhibited proinflammatory cytokines and MAPK activation, more potently than ciprofloxacin or azithromycin did, in TNF-α- or IL-β-treated CF airway cells. In differentiated human bronchial cells cultured at an air-liquid interface, moxifloxacin as well as azithromycin was able to inhibit TNF-α-induced IL-8 secretion (347). These data may be of relevance for the use of fluoroquinolones in the treatment of respiratory tract infections in patients with chronic airway inflammatory diseases.
Interestingly, immunomodulatory properties seem to be limited to members of the quinolone family with a cyclopropyl moiety at position N-1 of the quinolone ring (e.g., ciprofloxacin, sparfloxacin, and moxifloxacin) (51). The basic mechanisms for these effects of quinolones have not been evaluated. Further studies, including studies of a quinolone-topoisomerase II interaction with an effect on transcription factors, are required (51).
EM703
EM703 is a novel 12-membered-ring macrolide derivative of erythromycin which was developed by the Kitasato Institute for Life Sciences of Kitasato University in Japan (170, 271). EM703 has neither antimicrobial nor gastrointestinal motilin-binding effects. Similar to erythromycin, EM703 can inhibit PMA-mediated IL-8 release and NF-κB activation in BET-1A cells, a human bronchial epithelial cell line (54). EM703 can inhibit proliferation and collagen production of murine and human lung fibroblasts by interfering with TGF-β/Smad signaling (103, 341). The relationship between these effects and the three-dimensional structure of macrolides is under investigation.
CONCLUSIONS
Macrolides have many diverse biological activities and an ability to modulate inflammation. These properties have led to their use for treatment of DPB, asthma, bronchiectasis, rhinosinusitis, and CF. These actions seem to be polymodal and exerted at different levels of cellular signaling, but among these, modulation of ERK1/2 and transcription factors is prominent, consistent, and clearly unrelated to antimicrobial properties. Macrolides (and quinolones) accumulate within cells, suggesting that they may associate with receptors or carriers responsible for the regulation of immune cell activities.
Acknowledgments
We thank Tsuyoshi Tanabe and Melissa Pearce for manuscript review and helpful discussions.
Biography
 Bruce Kalman Rubin received his B.Sc., Master of Engineering, and M.D. degrees from Tulane University in New Orleans. After medical studies, he went to Oxford University as a Rhodes Scholar to do research in biomedical engineering. He completed an MBA at Wake Forest University in 2004. He is now the Jessie Ball duPont Professor and Chairman of the Department of Pediatrics and Professor of Biomedical Engineering at Virginia Commonwealth University. Dr. Rubin is on the editorial boards of 12 pulmonary journals, has published more than 200 research papers and chapters, and holds 5 patents. He is listed in Who's Who in the United States, The Best Doctors in America, and Castle Connolly Top Doctors. He is a Fellow of the Royal College of Physicians of Canada, the American College of Chest Physicians, and the American Pediatric Association. Dr. Rubin's research is on the regulation of mucus clearance in health and disease and the aerosol delivery of medications.
Bruce Kalman Rubin received his B.Sc., Master of Engineering, and M.D. degrees from Tulane University in New Orleans. After medical studies, he went to Oxford University as a Rhodes Scholar to do research in biomedical engineering. He completed an MBA at Wake Forest University in 2004. He is now the Jessie Ball duPont Professor and Chairman of the Department of Pediatrics and Professor of Biomedical Engineering at Virginia Commonwealth University. Dr. Rubin is on the editorial boards of 12 pulmonary journals, has published more than 200 research papers and chapters, and holds 5 patents. He is listed in Who's Who in the United States, The Best Doctors in America, and Castle Connolly Top Doctors. He is a Fellow of the Royal College of Physicians of Canada, the American College of Chest Physicians, and the American Pediatric Association. Dr. Rubin's research is on the regulation of mucus clearance in health and disease and the aerosol delivery of medications.
 Soichiro Kanoh received his M.D. degree from the National Defense Medical College (NDMC) in Saitama, Japan. After completing training as a chest physician, he studied intracellular calcium signaling and chloride transport in airway epithelium in the laboratory of Jun Tamaoki and received his Ph.D. from the Graduate School of Medicine at NDMC. He was promoted to Assistant Professor in Internal Medicine at NDMC. For the past 2 years, he has been at Virginia Commonwealth University School of Medicine in the laboratory of Professor Rubin. His research focuses on intracellular mechanisms of immunomodulatory drugs and their clinical application to inflammatory respiratory diseases.
Soichiro Kanoh received his M.D. degree from the National Defense Medical College (NDMC) in Saitama, Japan. After completing training as a chest physician, he studied intracellular calcium signaling and chloride transport in airway epithelium in the laboratory of Jun Tamaoki and received his Ph.D. from the Graduate School of Medicine at NDMC. He was promoted to Assistant Professor in Internal Medicine at NDMC. For the past 2 years, he has been at Virginia Commonwealth University School of Medicine in the laboratory of Professor Rubin. His research focuses on intracellular mechanisms of immunomodulatory drugs and their clinical application to inflammatory respiratory diseases.
REFERENCES
- 1.Abdeighaffar, H., D. Vazifeh, and M. T. Labro. 1997. Erythromycin A-derived macrolides modify the functional activities of human neutrophils by altering the phospholipase D-phosphatidate phosphohydrolase transduction pathway. J. Immunol. 159:3995-4005. [PubMed] [Google Scholar]
- 2.Abe, S., H. Nakamura, S. Inoue, H. Takeda, H. Saito, S. Kato, N. Mukaide, K. Matsushima, and H. Tomoike. 2000. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 22:51-60. [DOI] [PubMed] [Google Scholar]
- 3.Adi, S., B. Bin-Abbas, N. Y. Wu, and S. M. Rosenthal. 2002. Early stimulation and late inhibition of extracellular signal-regulated kinase 1/2 phosphorylation by IGF-I: a potential mechanism mediating the switch in IGF-I action on skeletal muscle cell differentiation. Endocrinology 143:511-516. [DOI] [PubMed] [Google Scholar]
- 4.Altschuler, E. L. 1998. Azithromycin, the multidrug-resistant protein, and cystic fibrosis. Lancet 351:1286. [DOI] [PubMed] [Google Scholar]
- 5.Amayasu, H., S. Yoshida, S. Ebana, Y. Yamamoto, T. Nishikawa, T. Shoji, H. Nakagawa, H. Hasegawa, M. Nakabayashi, and Y. Ishizaki. 2000. Clarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthma. Ann. Allergy Asthma Immunol. 84:594-598. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, R. 1989. Erythromycin and roxithromycin potentiate human neutrophil locomotion in vitro by inhibition of leukoattractant activated superoxide generation and autooxidation. J. Infect. Dis. 5:966-972. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, R., A. J. Theron, and C. Feldman. 1996. Membrane-stabilizing, anti-inflammatory interactions of macrolides with human neutrophils. Inflammation 20:693-705. [DOI] [PubMed] [Google Scholar]
- 8.Anderson, R., H. C. Steel, R. Cockeran, A. von Gottberg, L. de Gouveia, K. P. Klugman, T. J. Mitchell, and C. Feldman. 2007. Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro. J. Antimicrob. Chemother. 60:1155-1158. [DOI] [PubMed] [Google Scholar]
- 9.Antigny, F., C. Norez, A. Cantereau, F. Becq, and C. Vandebrouck. 2008. Abnormal spatial diffusion of Ca2+ in F508del-CFTR airway epithelial cells. Respir. Res. 9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki, D., S. Ueno, F. Kubo, T. Oyama, T. Sakuta, K. Matsushita, I. Maruyama, and T. Aikou. 2005. Roxithromycin inhibits angiogenesis of human hepatoma cells in vivo by suppressing VEGF production. Anticancer Res. 25:133-138. [PubMed] [Google Scholar]
- 11.Aoki, Y., and P. N. Kao. 1999. Erythromycin inhibits transcriptional activation of NF-κB, but not NFAT, through calcineurin-independent signalling in T-cells. Antimicrob. Agents Chemother. 43:2678-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoshiba, K., A. Nagai, and K. Konno. 1995. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob. Agents Chemother. 39:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araujo, F. G., T. L. Slifer, and J. S. Remington. 2002. Effect of moxifloxacin on secretion of cytokines by human monocytes stimulated with lipopolysaccharide. Clin. Microbiol. Infect. 8:26-30. [DOI] [PubMed] [Google Scholar]
- 14.Araujo, F. G., T. L. Slifer, and J. S. Remington. 2002. Inhibition of secretion of interleukin-1 and tumor necrosis factor alpha by the ketolide antibiotic telithromycin. Antimicrob. Agents Chemother. 46:3327-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asano, K., K. Kamakazu, T. Hisamitsu, and H. Suzaki. 2001. Modulation of Th2 type cytokine production from human peripheral blood leukocytes by a macrolide antibiotic, roxithromycin, in vitro. Int. Immunopharmacol. 1:1913-1921. [DOI] [PubMed] [Google Scholar]
- 16.Asgrimsson, V., T. Gudjonsson, G. H. Gudmundsson, and O. Baldursson. 2006. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob. Agents Chemother. 50:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashitani, J., H. Mukae, M. Nakazato, T. Ihi, H. Mashimoto, J. Kadota, S. Kohno, and S. Matsukura. 1998. Elevated concentrations of defensins in bronchoalveolar lavage fluid in diffuse panbronchiolitis. Eur. Respir. J. 11:104-111. [DOI] [PubMed] [Google Scholar]
- 18.Azuma, A., and S. Kudoh. 2005. Securing the safety and efficacy of macrolide therapy for chronic small airway diseases. Intern. Med. 44:167-168. [DOI] [PubMed] [Google Scholar]
- 19.Azuma, A., and S. Kudoh. 2005. The use of macrolides for treatment of diffuse panbronchiolitis, p. 147-165. In B. K. Rubin and J. Tamaoki (ed.), Antibiotics as anti-inflammatory and immunomodulatory agents. Birkhäuser Verlag, Basel, Switzerland.
- 20.Azuma, A., T. Furuta, T. Enomoto, Y. Hashimoto, K. Uematsu, N. Nukariya, A. Murata, and S. Kudoh. 1998. Preventive effect of erythromycin on experimental bleomycin-induced acute lung injury in rats. Thorax 53:186-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baddour, L. M., V. L. Yu, K. P. Klugman, C. Feldman, A. Ortqvist, J. Rello, A. J. Morris, C. M. Luna, D. R. Snydman, W. C. Ko, M. B. Chedid, D. S. Hui, A. Andremont, C. C. Chiou, et al. 2004. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am. J. Respir. Crit. Care Med. 170:440-444. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee, D., O. A. Khair, and D. Honeybourne. 2005. The effect of oral clarithromycin on health status and sputum bacteriology in stable COPD. Respir. Med. 99:208-215. [DOI] [PubMed] [Google Scholar]
- 23.Barker, P. M., D. J. Gillie, M. S. Schechter, and B. K. Rubin. 2005. Effect of macrolides on in vivo ion transport across cystic fibrosis nasal epithelium. Am. J. Respir. Crit. Care Med. 17:868-871. [DOI] [PubMed] [Google Scholar]
- 24.Barnes, P. J. 2008. Frontrunners in novel pharmacotherapy of COPD. Curr. Opin. Pharmacol. 8:300-307. [DOI] [PubMed] [Google Scholar]
- 25.Baumann, U., J. J. Fischer, P. Gudowius, M. Lingner, S. Herrmann, B. Tümmler, and H. von der Hardt. 2001. Buccal adherence of Pseudomonas aeruginosa in patients with cystic fibrosis under long-term therapy with azithromycin. Infection 29:7-11. [DOI] [PubMed] [Google Scholar]
- 26.Baziaka, F., E. J. Giamarellos-Bourboulis, M. Raftogiannis, T. Adamis, V. Tziortzioti, L. Sabracos, M. Chrisofos, P. Koutoukas, H. Giamarellou, and E. E. Douzinas. 2008. Immunomodulatory effect of three-day continuous administration of clarithromycin for experimental sepsis due to multidrug-resistant Pseudomonas aeruginosa. J. Chemother. 20:63-68. [DOI] [PubMed] [Google Scholar]
- 27.Bearden, D. T., and K. A. Rodvold. 1999. Penetration of macrolides into pulmonary sites of infection. Infect. Med. 16:480A-484A. [Google Scholar]
- 28.Beinke, S., M. J. Robinson, M. Hugunin, and S. C. Ley. 2004. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IκB kinase-induced proteolysis of NF-κB1 p105. Mol. Cell. Biol. 24:9658-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhowmik, S. T., R. J. Sapsford, and J. A. Wedzicha. 2000. Relation of sputum inflammatory markers to symptoms and physiological changes at COPD exacerbations. Thorax 55:114-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birkenkamp, K. U., L. M. Tuyt, C. Lummen, A. T. Wierenga, W. Kruijer, and E. Vellenga. 2000. The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br. J. Pharmacol. 131:99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black, P. N., F. Blasi, C. R. Jenkins, R. Scicchitano, G. D. Mills, A. R. Rubinfeld, R. E. Ruffin, P. R. Mullins, J. Dangain, B. C. Cooper, D. B. David, and L. Allegra. 2001. Trial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniae. Am. J. Respir. Crit. Care Med. 164:536-541. [DOI] [PubMed] [Google Scholar]
- 32.Black, P. N., R. Scicchitano, C. R. Jenkins, F. Blasi, L. Allegra, J. Wlodarczyk, and B. C. Cooper. 2000. Serological evidence of infection with Chlamydia pneumoniae is related to the severity of asthma. Eur. Respir. J. 15:254-259. [DOI] [PubMed] [Google Scholar]
- 33.Blau, H., K. Klein, I. Shalit, D. Halperin, and I. Fabian. 2007. Moxifloxacin but not ciprofloxacin or azithromycin selectively inhibits IL-8, IL-6, ERK1/2, JNK, and NF-κB activation in a cystic fibrosis epithelial cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L343-L352. [DOI] [PubMed] [Google Scholar]
- 34.Brennan, S., D. Cooper, and P. D. Sly. 2001. Directed neutrophil migration to IL-8 is increased in cystic fibrosis: a study of the effect of erythromycin. Thorax 56:62-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown, R. B., P. Iannini, P. Gross, and M. Kunkel. 2003. Impact of initial antibiotic choice on clinical outcomes in community-acquired pneumonia: analysis of a hospital claims-made database. Chest 123:1503-1511. [DOI] [PubMed] [Google Scholar]
- 36.Carfartan, G., P. Gerardin, D. Turck, and M. O. Husson. 2004. Effect of subinhibitory concentrations of azithromycin on adherence of Pseudomonas aeruginosa to bronchial mucins collected from cystic fibrosis patients. J. Antimicrob. Chemother. 53:686-688. [DOI] [PubMed] [Google Scholar]
- 37.Carrabino, S., D. Carpani, A. Livraghi, M. Di Cicco, D. Costantini, E. Copreni, C. Colombo, and M. Conese. 2006. Dysregulated interleukin-8 secretion and NF-κB activity in human cystic fibrosis nasal epithelial cells. J. Cyst. Fibros. 5:113-119. [DOI] [PubMed] [Google Scholar]
- 38.Cervin, A., O. Kalm, P. Sandkull, and S. Lindberg. 2002. One-year low-dose erythromycin treatment of persistent chronic sinusitis after sinus surgery: clinical outcome and effects on mucociliary parameters and nasal nitric oxide. Otolaryngol. Head Neck Surg. 126:481-489. [DOI] [PubMed] [Google Scholar]
- 39.Chen, B.-C., C.-C. Yu, H.-C. Lei, M.-S. Chang, M.-J. Hsu, C.-L. Huang, M.-C. Chen, J.-R. Sheu, T.-F. Chen, T.-L. Chen, H. Inoue, and C.-H. Lin. 2004. Bradykinin B2 receptor mediates NF-κB activation and cyclooxygenase-2 expression via the Ras/Raf-1/ERK pathway in human airway epithelial cells. J. Immunol. 173:5219-5228. [DOI] [PubMed] [Google Scholar]
- 40.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 36:1801-1802. [DOI] [PubMed] [Google Scholar]
- 41.Cigana, C., B. M. Assael, and P. Melotti. 2007. Azithromycin selectively reduces tumor necrosis factor alpha levels in cystic fibrosis airway epithelial cells. Antimicrob. Agents Chemother. 51:975-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cigana, C., E. Nicolis, M. Pasetto, B. M. Assael, and P. Melotti. 2007. Effects of azithromycin on the expression of ATP binding cassette transporters in epithelial cells from the airways of cystic fibrosis patients. J. Chemother. 19:643-649. [DOI] [PubMed] [Google Scholar]
- 43.Clancy, J. P., J. D. McCann, M. Li, and M. J. Welsh. 1990. Calcium-dependent regulation of airway epithelial chloride channels. Am. J. Physiol. 258:L25-L32. [DOI] [PubMed] [Google Scholar]
- 44.Clapham, D. E. 2007. Calcium signaling. Cell 131:1047-1058. [DOI] [PubMed] [Google Scholar]
- 45.Clement, A., A. Tamalet, E. Leroux, S. Ravilly, B. Fauroux, and J.-P. Jais. 2006. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax 61:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cochrane, C. G. 1991. Cellular injury by oxidants. Am. J. Med. 91:23S-30S. [DOI] [PubMed] [Google Scholar]
- 47.Cui, C. H., K. Honda, N. Saito, Y. Yamada, S. Sannohe, S. Ueki, K. Hamada, K. Yamaguchi, Y. Kobayashi, T. Adachi, H. Kayaba, and J. Chihara. 2001. Effect of roxithromycin on eotaxin-primed reactive oxygen species from eosinophils. Int. Arch. Allergy Immunol. 125(Suppl. 1):38-41. [DOI] [PubMed] [Google Scholar]
- 48.Culić, O., V. Eraković, and M. J. Parnham. 2001. Anti-inflammatory effects of macrolide antibiotics. Eur. J. Pharmacol. 429:209-229. [DOI] [PubMed] [Google Scholar]
- 49.Culić, O., V. Eraković, I. Cepelak, K. Barisić, K. Brajsa, Z. Ferencić, R. Galović, I. Glojnarić, Z. Manojlović, V. Munić, R. Novak-Mircetić, V. Pavicić-Beljak, M. Sucić, M. Veljaca, T. Zanić-Grubisić, and M. J. Parnham. 2002. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur. J. Pharmacol. 450:277-289. [DOI] [PubMed] [Google Scholar]
- 50.Cymbala, A. A., L. C. Edmonds, M. A. Bauer, P. J. Jederlinic, J. J. May, J. M. Victory, and G. W. Amsden. 2005. The disease modifying effects of twice-weekly oral azithromycin in patients with bronchiectasis. Treat. Respir. Med. 4:117-122. [DOI] [PubMed] [Google Scholar]
- 51.Dalhoff, A., and I. Shalit. 2003. Immunomodulatory effects of quinolones. Lancet Infect. Dis. 3:359-371. [DOI] [PubMed] [Google Scholar]
- 52.Davies, J. C., M. Stern, A. Dewar, N. J. Caplen, F. M. Munkonge, T. Pitt, F. Sorgi, L. Huang, A. Bush, D. M. Geddes, and E. W. Alton. 1997. CFTR gene transfer reduces the binding of Pseudomonas aeruginosa to cystic fibrosis respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 16:657-663. [DOI] [PubMed] [Google Scholar]
- 53.Demarini, G., D. Esposti, P. Marthyn, A. Lapidari, F. Fraschini, and F. Scaglione. 2004. Effect of multiple dose of clarithromycin and amoxicillin on IL-6. IFNγ and IL-10 plasuma levels in patients with community acquired pneumonia. J. Chemother. 16:82-85. [DOI] [PubMed] [Google Scholar]
- 54.Desaki, M., H. Okazaki, T. Sunazuka, S. Omura, K. Yamamoto, and H. Takizawa. 2004. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: possible role in the signaling pathway that regulates nuclear factor-kappaB activation. Antimicrob. Agents Chemother. 48:1581-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desaki, M., H. Takizawa, T. Ohtoshi, T. Kasama, K. Kobayasgi, T. Sunazuka, S. Omura, K. Yamamoto, and K. Ito. 2000. Erythromycin suppresses nuclear factor-κB and activator protein-1 activation in human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 267:124-128. [DOI] [PubMed] [Google Scholar]
- 56.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Invest. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donaldson, G. C., T. A. R. Seemungal, A. Bhowmik, and J. A. Wedzicha. 2002. The relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57:847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dwyer, R., Å. Örtqvist, E. Aufwerber, B. Henriques Normark, T. J. Marrie, M. A. Mufson, A. Torres, M. A. Woodhead, M. Ålenius, and M. Kalin. 2006. Addition of a macrolide to a β-lactam in bacteremic pneumococcal pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 25:518-521. [DOI] [PubMed] [Google Scholar]
- 59.Ekici, A., M. Ekici, and A. K. Erdemoğlu. 2002. Effect of azithromycin on the severity of bronchial hyperresponsiveness in patients with mild asthma. J. Asthma 39:181-185. [DOI] [PubMed] [Google Scholar]
- 60.Elkington, P. T. G., and J. S. Friedland. 2006. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 61:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enomoto, F., G. Ichikawa, I. Nagaoka, and T. Yamashita. 1998. Effect of erythromycin on otitis media with effusion in experimental rat model. Acta Otolaryngol. 539(Suppl.):57-60. [PubMed] [Google Scholar]
- 62.Equi, A., I. M. Balfour-Lynn, A. Bush, and M. Rosenthal. 2002. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 360:978-984. [DOI] [PubMed] [Google Scholar]
- 63.Equi, A. C., J. C. Davies, H. Painter, S. Hyde, A. Bush, D. M. Geddes, and E. W. F. W. Alton. 2006. Exploring the mechanisms of macrolides in cystic fibrosis. Respir. Med. 100:687-697. [DOI] [PubMed] [Google Scholar]
- 64.Evans, D. J., P. Cullinan, D. M. Geddes, E. H. Walters, S. J. Milan, and P. Jones. 2001. Troleandomycin as an oral corticosteroid steroid sparing agent in stable asthma. Cochrane Database Syst. Rev. 2001:CD002987. [DOI] [PMC free article] [PubMed]
- 65.Everard, M. L., P. Sly, S. Brenan, and G. Ryan. 1997. Macrolide antibiotics in diffuse panbronchiolitis and in cystic fibrosis. Eur. Respir. J. 10:2926. [DOI] [PubMed] [Google Scholar]
- 66.Farrell, D. J., C. Couturier, and W. Hryniewicz. 2008. Distribution and antibacterial susceptibility of macrolide resistance genotypes in Streptococcus pneumoniae: PROTEKT year 5 (2003-2004). Int. J. Antimicrob. Agents 31:245-249. [DOI] [PubMed] [Google Scholar]
- 67.Feldman, C., R. Anderson, A. J. Theron, G. Ramafi, P. J. Cole, and R. Wilson. 1997. Roxithromycin, clarithromycin, and azithromycin attenuate the injurious effects of bioactive phospholipids on human respiratory epithelium in vitro. Inflammation 21:655-665. [DOI] [PubMed] [Google Scholar]
- 68.Finsnes, F., O. H. Skjønsberg, T. Tønnessen, O. Naess, T. Lyberg, and G. Christensen. 1997. Endothelin-1 production and effects of endothelin antagonism during experiment airway inflammation. Am. J. Respir. Crit. Care Med. 155:1404-1412. [DOI] [PubMed] [Google Scholar]
- 69.Folkman, J. 1995. Clinical applications of research on angiogenesis. N. Engl. J. Med. 333:1757-1763. [DOI] [PubMed] [Google Scholar]
- 70.Fu, Z., K. Bettega, S. Carroll, K. R. Buchholz, and T. E. Machen. 2007. Role of Ca2+ in responses of airway epithelia to Pseudomonas aeruginosa, flagellin, ATP, and thapsigargin. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L353-L364. [DOI] [PubMed] [Google Scholar]
- 71.Fujii, T., J. Kadota, K. Kawakami, K. Iida, R. Shirai, M. Kaseda, S. Kawamoto, and S. Kohno. 1995. Long-term effects of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax 50:1246-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fumimori, T., S. Honda, K. Migita, M. Hamada, T. Yoshimuta, J. Honda, T. Fukuda, R. Suzuki, M. Gotoh, K. Eguchi, and H. Aizawa. 2004. Erythromycin suppresses the expression of cyclooxygenase-2 in rheumatoid synovial cells. J. Rheumatol. 31:436-441. [PubMed] [Google Scholar]
- 73.Gant, T. W., C. K. O'Connor, R. Corbitt, U. Thorgeirsson, and S. S. Thorgeirsson. 1995. In vivo induction of liver P-glycoprotein expression by xenobiotics in monkeys. Toxicol. Appl. Pharmacol. 133:269-276. [DOI] [PubMed] [Google Scholar]
- 74.García Vázquez, E., J. Mensa, J. A. Martínez, M. A. Marcos, J. Puig, M. Ortega, and A. Torres. 2005. Lower mortality among patients with community-acquired pneumonia treated with a macrolide plus a beta-lactam agent versus a beta-lactam agent alone. Eur. J. Clin. Microbiol. Infect. Dis. 24:190-195. [DOI] [PubMed] [Google Scholar]
- 75.Gavilanes, X., F. Huaux, M. Meyer, P. Lebecque, E. Marbaix, D. Lison, B. Scholte, P. Wallemacq, and T. Leal. 2009. Azithromycin fails to reduce increased expression of neutrophil-related cytokines in primary-cultured epithelial cells from cystic fibrosis mice. J. Cyst. Fibros. 8:203-210. [DOI] [PubMed] [Google Scholar]
- 76.Ge, N., Y. Nakamura, Y. Nakaya, and S. Sone. 2001. Interferon-gamma activates outwardly rectifying chloride channels in the human bronchial epithelial cell line BEAS-2B. J. Med. Invest. 48:97-101. [PubMed] [Google Scholar]
- 77.Gerhardt, S. G., J. F. McDyer, R. E. Girgis, J. V. Conte, S. C. Yang, and J. B. Orens. 2003. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am. J. Respir. Crit. Care Med. 168:121-125. [DOI] [PubMed] [Google Scholar]
- 78.Giamarellos-Bourboulis, E. J., F. Baziaka, A. Antonopoulou, P. Koutoukas, V. Kousoulas, L. Sabracos, C. Panagou, D. Perrea, and H. Giamarellou. 2005. Clarithromycin co-administered with amikacin attenuates systemic inflammation in experimental sepsis with Escherichia coli. Int. J. Antimicrob. Agents 25:168-172. [DOI] [PubMed] [Google Scholar]
- 79.Giamarellos-Bourboulis, E. J., J. C. Pechère, C. Routsi, D. Plachouras, S. Kollias, M. Raftogiannis, D. Zervakis, F. Baziaka, A. Koronaios, A. Antonopoulou, V. Markaki, P. Koutouka, E. Papadomichelakis, T. Tsaganos, A. Armaganidis, V. Koussoulas, A. Kotanidou, C. Roussos, and H. Giamarellou. 2008. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin. Infect. Dis. 46:1157-1164. [DOI] [PubMed] [Google Scholar]
- 80.Giamarellos-Bourboulis, E. J., T. Adamis, G. Laoutaris, L. Sabracos, V. Koussoulas, M. Mouktaroudi, D. Perrea, P. E. Karayannacos, and H. Giamarellou. 2004. Immunomodulatory clarithromycin treatment of experimental sepsis and acute pyelonephritis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48:93-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giamarellos-Bourboulis, E. J., V. Tziortzioti, P. Koutoukas, F. Baziaka, M. Raftogiannis, A. Antonopoulou, T. Adamis, L. Sabracos, and H. Giamarellou. 2006. Clarithromycin is an effective immunomodulator in experimental pyelonephritis caused by pan-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother. 57:937-944. [DOI] [PubMed] [Google Scholar]
- 82.Gorrini, M., A. Lupi, S. Viglio, F. Pamparana, G. Cetta, P. Iadarola, J. C. Powers, and M. Luisetti. 2001. Inhibition of neutrophil elastase by erythromycin and flurythromycin, two macrolide antibiotics. Am. J. Respir. Cell Mol. Biol. 25:492-499. [DOI] [PubMed] [Google Scholar]
- 83.Goswami, S. K., S. Kivity, and Z. Marom. 1990. Erythromycin inhibits respiratory glycoconjugate secretion from human airways in vitro. Am. Rev. Respir. Dis. 141:72-78. [DOI] [PubMed] [Google Scholar]
- 84.Gotfried, M. H., R. Jung, C. R. Messick, I. Rubinstein, K. W. Garey, K. A. Rodvold, and L. H. Danziger. 2004. Effects of six week clarithromycin therapy in corticosteroid-dependent asthma: a randomized double-blind, placebo-controlled pilot study. Curr. Ther. Res. 65:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hansen, C. R., T. Presslera, C. Koch, and N. Høiby. 2005. Long-term azithromycin treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection: an observational cohort study. J. Cyst. Fibros. 4:35-40. [DOI] [PubMed] [Google Scholar]
- 86.Hashiba, M., and S. Baba. 1996. Efficacy of long-term administration of clarithromycin in the treatment of intractable chronic sinusitis. Acta Otolaryngol. 525(Suppl.):73-78. [PubMed] [Google Scholar]
- 87.Hashimoto, S., K. Matsumoto, Y. Gon, S. Maruoka, K. Kujime, S. Hayashi, I. Takeshita, and T. Horie. 2000. p38 MAP kinase regulates TNFα-, IL-1α- and PAF-induced RANTES and GM-CSF production by human bronchial epithelial cells. Clin. Exp. Allergy 30:48-55. [DOI] [PubMed] [Google Scholar]
- 88.Hatipoglu, U., and I. Rubinstein. 2005. Treatment of chronic rhinosinusitis with low-dose, long-term macrolide antibiotics: an evolving paradigm. Curr. Allergy Asthma Rep. 5:491-494. [DOI] [PubMed] [Google Scholar]
- 89.Hirakata, Y., M. Kaku, K. Tomono, K. Tateda, N. Furuya, T. Matsumoto, R. Araki, and K. Yamaguchi. 1992. Efficacy of erythromycin lactobionate for treating Pseudomonas aeruginosa bacteremia in mice. Antimicrob. Agents Chemother. 36:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hiratsuka, T., H. Mukae, H. Iiboshi, J. Ashitani, K. Nabeshima, T. Minematsu, N. Chino, T. Ihi, S. Kohno, and M. Nakazato. 2003. Increased concentrations of human beta-defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Thorax 58:425-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hodge, S., G. Hodge, H. Jersmann, G. Matthews, J. Ahern, M. Holmes, and P. N. Reynolds. 2008. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 178:139-148. [DOI] [PubMed] [Google Scholar]
- 92.Hodge, S., G. Hodge, S. Brozyna, H. Jersmann, M. Holmes, and P. N. Reynolds. 2006. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 28:486-495. [DOI] [PubMed] [Google Scholar]
- 93.Hoffmann, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72:847-855. [PubMed] [Google Scholar]
- 94.Hoffmann, N., B. Lee, M. Hentzer, T. B. Rasmussen, Z. Song, H. K. Johansen, M. Givskov, and N. Høiby. 2007. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates P. aeruginosa lung infection in Cftr−/− mice. Antimicrob. Agents Chemother. 51:3677-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Høiby, N., G. Döring, and P. O. Schiøtz. 1986. The role of immune complexes in pathogenesis of bacterial infections. Annu. Rev. Microbiol. 40:29-53. [DOI] [PubMed] [Google Scholar]
- 96.Howe, R. A., and R. C. Spencer. 1997. Macrolides for the treatment of Pseudomonas aeruginosa infections? J. Antimicrob. Chemother. 40:153-155. [DOI] [PubMed] [Google Scholar]
- 97.Ianaro, A., A. Ialenti, P. Maffia, L. Sautebin, L. Rombolà, R. Carnuccio, T. Iuvone, F. D'Acquisto, and M. Di Rosa. 2000. Anti-inflammatory activity of macrolide antibiotics. J. Pharmacol. Exp. Ther. 292:156-163. [PubMed] [Google Scholar]
- 98.Ichikawa, Y., H. Ninomiya, H. Koga, M. Tanaka, M. Kinoshita, N. Tokunaga, T. Yano, and K. Oizumi. 1992. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am. Rev. Respir. Dis. 146:196-203. [DOI] [PubMed] [Google Scholar]
- 99.Ichikawa, Y., M. Hotta, S. Sumita, K. Fujimoto, and K. Oizumi. 1995. Reversible airway lesions in diffuse panbronchiolitis: detection by high-resolution computed tomography. Chest 107:120-125. [DOI] [PubMed] [Google Scholar]
- 100.Ichimiya, T., K. Takeoka, K. Hiramatsu, K. Hirai, T. Yamasaki, and M. Nasu. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186-191. [DOI] [PubMed] [Google Scholar]
- 101.Ichimura, K., Y. Shimazaki, T. Ishibashi, and R. Higo. 1996. Effect of new macrolide roxithromycin upon nasal polyps associated with chronic sinusitis. Auris Nasus Larynx 23:48-56. [DOI] [PubMed] [Google Scholar]
- 102.Ichiyama, T., M. Nishikawa, T. Yoshitomi, S. Hasegawa, T. Matsubara, T. Hayashi, and S. Furukawa. 2001. Clarithromycin inhibits NF-kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob. Agents Chemother. 45:44-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ikeda, H., T. Sunazuka, H. Suzuki, Y. Hamasaki, S. Yamazaki, S. Omura, and A. Hatamochi. 2007. EM703, the new derivative of erythromycin, inhibits transcription of type I collagen in normal and scleroderma fibroblasts. J. Dermatol. Sci. 49:195-205. [DOI] [PubMed] [Google Scholar]
- 104.Ikeda, K., D. Wu, and T. Takasaka. 1995. Inhibition of acetylcholine-evoked Cl− currents by 14-membered macrolide antibiotics in isolated acinar cells of the guinea pig nasal gland. Am. J. Respir. Cell Mol. Biol. 13:449-454. [DOI] [PubMed] [Google Scholar]
- 105.Imamura, Y., K. Yanagihara, Y. Mizuta, M. Seki, H. Ohno, Y. Higashiyama, Y. Miyazaki, K. Tsukamoto, Y. Hirakata, K. Tomono, J. Kadota, and S. Kohno. 2004. Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone in NCI-H292 cells. Antimicrob. Agents Chemother. 48:3457-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inamura, K., N. Ohta, S. Fukase, N. Kasajima, and M. Aoyagi. 2000. The effects of erythromycin on human peripheral neutrophil apoptosis. Rhinology 38:124-129. [PubMed] [Google Scholar]
- 107.Ishimatsu, Y., J. Kadota, T. Iwashita, T. Nagata, H. Ishii, C. Shikuwa, H. Kaida, H. Mukae, and S. Kohno. 2004. Macrolide antibiotics induce apoptosis of human peripheral lymphocytes in vitro. Int. J. Antimicrob. Agents 24:49-55. [DOI] [PubMed] [Google Scholar]
- 108.Ishimoto, H., H. Mukae, N. Sakamoto, M. Amenomori, T. Kitazaki, Y. Imamura, H. Fujita, H. Ishii, S. Nakayama, K. Yanagihara, and S. Kohno. 2009. Different effects of telithromycin on MUC5AC production induced by human neutrophil peptide-1 or lipopolysaccharide in NCI-H292 cells compared with azithromycin and clarithromycin. J. Antimicrob. Chemother. 63:109-114. [DOI] [PubMed] [Google Scholar]
- 109.Ishizawa, K., T. Suzuki, M. Yamaya, Y. X. Jia, S. Kobayashi, S. Ida, H. Kubo, K. Sekizawa, and H. Sasaki. 2005. Erythromycin increases bactericidal activity of surface liquid in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L565-L573. [DOI] [PubMed] [Google Scholar]
- 110.Itkin, I. H., and M. L. Menzel. 1970. The use of macrolide antibiotic substances in the treatment of asthma. J. Allergy 45:146-162. [DOI] [PubMed] [Google Scholar]
- 111.Ivetić Tkalcević, V., B. Bosnjak, B. Hrvacić, M. Bosnar, N. Marjanović, Z. Ferencić, K. Situm, O. Culić, M. J. Parnham, and V. Eraković. 2006. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur. J. Pharmacol. 539:131-138. [DOI] [PubMed] [Google Scholar]
- 112.Jacquot, J., O. Tabary, P. Le Rouzic, and A. Clement. 2008. Airway epithelial cell inflammatory signalling in cystic fibrosis. Int. J. Biochem. Cell Biol. 40:1703-1715. [DOI] [PubMed] [Google Scholar]
- 113.Jaffe, A., J. Francis, M. Rosenthal, and A. Bush. 1998. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet 351:420. [DOI] [PubMed] [Google Scholar]
- 114.Jang, Y. J., H.-J. Kwon, and B. J. Lee. 2006. Effects of clarithromycin on rhinovirus-16 infection in A549 cells. Eur. Respir. J. 27:12-19. [DOI] [PubMed] [Google Scholar]
- 115.Johansen, H. K., T. G. Jensen, R. B. Dessau, B. Lundgren, and N. Frimodt-Moller. 2000. Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo. J. Antimicrob. Chemother. 46:973-980. [DOI] [PubMed] [Google Scholar]
- 116.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 117.Johnston, S. L., F. Blasi, P. N. Black, R. J. Martin, D. J. Farrell, R. B. Nieman, et al. 2006. The effect of telithromycin in acute exacerbations of asthma. N. Engl. J. Med. 354:1589-1600. [DOI] [PubMed] [Google Scholar]
- 118.Jun, Y. T., H. J. Kim, M. J. Song, J. H. Lim, D. G. Lee, K. J. Han, S. M. Choi, J. H. Yoo, W. S. Shin, and J. H. Choi. 2003. In vitro effects of ciprofloxacin and roxithromycin on apoptosis of Jurkat T lymphocytes. Antimicrob. Agents Chemother. 47:1161-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kadota, J., O. Sakito, S. Kohno, H. Sawa, H. Mukae, H. Oda, K. Kawakami, K. Fukushima, K. Hiratani, and K. Hara. 1993. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 147:153-159. [DOI] [PubMed] [Google Scholar]
- 120.Kadota, J., H. Mukae, H. Ishii, T. Nagata, H. Kaida, K. Tomono, and S. Kohno. 2003. Long-term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitis. Respir. Med. 97:844-850. [DOI] [PubMed] [Google Scholar]
- 121.Kadota, J., H. Mukae, S. Mizunoe, K. Kishi, I. Tokimatsu, H. Nagai, K. Tomono, S. Kohno, and M. Nasu. 2005. Long-term macrolide antibiotic therapy in the treatment of chronic small airway disease clinically mimicking diffuse panbronchiolitis. Intern. Med. 44:200-206. [DOI] [PubMed] [Google Scholar]
- 122.Kamada, A. K., M. R. Hill, D. N. Iklé, A. M. Brenner, and S. J. Szefler. 1993. Efficacy and safety of low-dose troleandomycin therapy in children with severe, steroid-requiring asthma. J. Allergy Clin. Immunol. 91:873-882. [DOI] [PubMed] [Google Scholar]
- 123.Kamoi, H., N. Kurihara, H. Fujiwara, K. Hirata, and T. Takeda. 1995. The macrolide antibacterial roxithromycin reduces bronchial hyperresponsiveness and superoxide anion production by polymorphonuclear leukocytes in patients with asthma. J. Asthma 32:191-197. [DOI] [PubMed] [Google Scholar]
- 124.Kanai, K., K. Asano, T. Hisamitsu, and H. Suzaki. 2004. Suppression of matrix metalloproteinase production from nasal fibroblasts by macrolide antibiotics in vitro. Eur. Respir. J. 23:671-678. [DOI] [PubMed] [Google Scholar]
- 125.Kaneko, Y., K. Yanagihara, M. Seki, M. Kuroki, Y. Miyazaki, Y. Hirakata, H. Mukae, K. Tomono, J. Kadota, and S. Kohno. 2003. Clarithromycin inhibits overproduction of muc5ac core protein in murine model of diffuse panbronchiolitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L847-L853. [DOI] [PubMed] [Google Scholar]
- 126.Kanoh, S., M. Kondo, J. Tamaoki, H. Kobayashi, K. Motoyoshi, and A. Nagai. 2001. FK506 inhibits Cl− secretion in airway epithelium via calcineurin-independent mechanism. Eur. J. Pharmacol. 419:121-126. [DOI] [PubMed] [Google Scholar]
- 127.Kanoh, S., M. Kondo, J. Tamaoki, H. Shirakawa, S. Miyazaki, H. Kobayashi, N. Nagata, and A. Nagai. 1999. Effect of FK506 on ATP-induced intracellular calcium oscillations in cow tracheal epithelium. Am. J. Physiol. 276:L891-L899. [DOI] [PubMed] [Google Scholar]
- 128.Kanoh, S., J. Tamaoki, M. Kondo, Y. Nagano, and A. Nagai. 2001. Effects of new quinolones on transepithelial electrical potential difference of tracheal mucosa in vivo. Antimicrob. Agents Chemother. 45:2928-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karlström, Å., K. L. Boyd, B. K. English, and J. A. McCullers. 2009. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J. Infect. Dis. 199:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kase, K., J. Hua, H. Yokoi, K. Ikeda, and I. Nagaoka. 2009. Inhibitory action of roxithromycin on histamine release and prostaglandin D2 production from β-defensin 2-stimulated mast cells. Int. J. Mol. Med. 23:337-340. [DOI] [PubMed] [Google Scholar]
- 131.Kawakami, K., J. Kadota, K. Iida, T. Fujii, R. Shirai, Y. Matsubara, and S. Kohno. 1997. Phenotypic characterization of T cells in bronchoalveolar lavage fluid (BALF) and peripheral blood of patients with diffuse panbronchiolitis; the importance of cytotoxic T cells. Clin. Exp. Immunol. 107:410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kawamura-Sato, K., T. Iinuma, T. Hasegawa, T. Holii, T. Yamashino, and M. Ohta. 2000. Effect of subinhibitory concentrations of macrolides on expression of flagellin in Pseudomonas aeruginosa and Proteus mirabilis. Antimicrob. Agents Chemother. 44:2869-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kawasaki, S., H. Takizawa, T. Ohtoshi, N. Takeuchi, T. Kohyama, H. Nakamura, T. Kasama, K. Kobayashi, K. Nakahara, Y. Morita, and K. Yamamoto. 1998. Roxithromycin inhibits cytokine production by and neutrophil attachment to human bronchial epithelial cells in vitro. Antimicrob. Agents Chemother. 42:1499-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kawashima, M., J. Yatsunami, Y. Fukuno, M. Nagata, M. Tominaga, and S. Hayashi. 2002. Inhibitory effects of 14-membered ring macrolide antibiotics on bleomycin-induced acute lung injury. Lung 180:73-89. [DOI] [PubMed] [Google Scholar]
- 135.Khair, O. A., J. L. Devalia, M. M. Abdelaziz, R. J. Sapsford, and R. J. Davies. 1995. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur. Respir. J. 8:1451-1457. [PubMed] [Google Scholar]
- 136.Khalid, M., A. Al Saghir, S. Saleemi, S. Al Dammas, M. Zeitouni, A. Al Mobeireek, N. Chaudhry, and E. Sahovic. 2005. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur. Respir. J. 25:490-493. [DOI] [PubMed] [Google Scholar]
- 137.Khan, A. A., T. R. Slifer, F. G. Araujo, Y. Suzuki, and J. S. Remington. 2000. Protection against lipopolysaccharide-induced death by fluoroquinolones. Antimicrob. Agents Chemother. 44:3169-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kido, H., Y. Okumura, H. Yamada, D. Mizuno, Y. Higashi, and M. Yano. 2004. Secretory leukoprotease inhibitor and pulmonary surfactant serve as principal defenses against influenza A virus infection in the airway and chemical agents up-regulating their levels may have therapeutic potential. Biol. Chem. 385:1029-1034. [DOI] [PubMed] [Google Scholar]
- 139.Kikuchi, S., H. Suzaki, A. Aoki, O. Ito, and Y. Nomura. 1991. Clinical effect of long-term low dose erythromycin therapy for chronic sinusitis. Pract. Otol. (Kyoto) 84:41-47. [Google Scholar]
- 140.Kikuchi, T., K. Hagiwara, Y. Honda, K. Gomi, T. Kobayashi, H. Takahashi, Y. Tokue, A. Watanabe, and T. Nukiwa. 2002. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J. Antimicrob. Chemother. 49:745-755. [DOI] [PubMed] [Google Scholar]
- 141.Kim, K. C., and B. C. Lee. 1993. Involvement of a signal transduction mechanism in ATP-induced mucin release from cultured airway goblet cells. Am. J. Respir. Cell Mol. Biol. 8:121-125. [DOI] [PubMed] [Google Scholar]
- 142.Kimura, N., K. Nishioka, K. Nishizawa, T. Ogawa, Y. Naitou, and Y. Masuda. 1997. Clinical effect of low-dose, long-term roxithromycin chemotherapy in patients with chronic sinusitis. Acta Med. Okayama 51:33-37. [DOI] [PubMed] [Google Scholar]
- 143.Kita, E., M. Sawaki, D. Oku, A. Hamuro, K. Mikasa, M. Konishi, M. Emoto, S. Takeuchi, N. Narita, and S. Kashiba. 1991. Suppression of virulence factors of Pseudomonas aeruginosa by erythromycin. J. Antimicrob. Chemother. 27:273-284. [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi, H. 1995. Biofilm disease: its clinical manifestation and therapeutic possibilities of macrolides. Am. J. Med. 99(Suppl. 6A):26S-30S. [DOI] [PubMed] [Google Scholar]
- 145.Kobayashi, H., O. Kobayashi, and S. Kawai. 2009. Pathogenesis and clinical manifestations of chronic colonization by Pseudomonas aeruginosa and its biofilms in the airway tract. J. Infect. Chemother. 15:125-142. [DOI] [PubMed] [Google Scholar]
- 146.Koch, C. C., D. J. Esteban, A. C. Chin, M. E. Olson, R. R. Read, H. Ceri, D. W. Morck, and A. G. Buret. 2000. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J. Antimicrob. Chemother. 46:12-26. [DOI] [PubMed] [Google Scholar]
- 147.Koh, Y. Y., M. H. Lee, Y. H. Sun, K. W. Sung, and J. H. Chae. 1997. Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double-blind, placebo-controlled study. Eur. Respir. J. 10:994-999. [DOI] [PubMed] [Google Scholar]
- 148.Kohri, K., J. Tamaoki, M. Kondo, K. Aoshiba, E. Tagaya, and A. Nagai. 2000. Macrolide antibiotics inhibit nitric oxide generation by rat pulmonary alveolar macrophages. Eur. Respir. J. 15:62-67. [DOI] [PubMed] [Google Scholar]
- 149.Kohyama, T., H. Takizawa, S. Kawasaki, N. Akiyama, M. Sato, and K. Ito. 1999. Fourteen-member macrolides inhibit interleukin-8 release by human eosinophils from atopic donors. Antimicrob. Agents Chemother. 43:907-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kohyama, T., Y. Yamauchi, H. Takizawa, S. Itakura, S. Kamitani, J. Kato, and T. Nagase. 2008. Clarithromycin inhibits fibroblast migration. Respir. Med. 102:1769-1776. [DOI] [PubMed] [Google Scholar]
- 151.Kondo, M., S. Kanoh, J. Tamaoki, H. Shirakawa, S. Miyazaki, and A. Nagai. 1998. Erythromycin inhibits ATP-induced intracellular calcium responses in bovine tracheal epithelial cells. Am. J. Respir. Cell Mol. Biol. 19:799-804. [DOI] [PubMed] [Google Scholar]
- 152.Konno, S., M. Adachi, K. Asano, K. Okamoto, and T. Takahashi. 1993. Anti-allergic activity of roxithromycin: inhibition of interleukin-5 production from mouse T lymphocytes. Life Sci. 52:PL25-PL30. [DOI] [PubMed]
- 153.Konno, S., M. Adachi, K. Asano, T. Kawazoe, K. Okamoto, and T. Takahashi. 1992. Influences of roxithromycin on cell-mediated immune responses. Life Sci. 51:PL107-PL112. [DOI] [PubMed] [Google Scholar]
- 154.Kostadima, E., S. Tsiodras, E. I. Alexopoulos, A. G. Kaditis, I. Mavrou, N. Georgatou, and A. Papamichalopoulos. 2004. Clarithromycin reduces the severity of bronchial hyperresponsiveness in patients with asthma. Eur. Respir. J. 23:714-717. [DOI] [PubMed] [Google Scholar]
- 155.Kraft, M., G. H. Cassell, J. Pak, and R. J. Martin. 2002. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest 121:1782-1788. [DOI] [PubMed] [Google Scholar]
- 156.Kudoh, S. 2004. Applying lessons learned in the treatment of diffuse panbronchiolitis to other chronic inflammatory diseases. Am. J. Med. 117(Suppl. 9A):12S-19S. [DOI] [PubMed] [Google Scholar]
- 157.Kudoh, S., A. Azuma, M. Yamamoto, T. Izumi, and M. Ando. 1998. Improvement in survival of patients with diffuse panbronchiolitis treated with low dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829-1832. [DOI] [PubMed] [Google Scholar]
- 158.Kudoh, S., K. Uetake, K. Hagiwara, M. Hirayama, L. H. Hus, H. Kimura, and Y. Sugiyama. 1987. Clinical effects of low-dose erythromycin chemotherapy on difuse panbronchioliris. Jpn. J. Thorac. Dis. 25:632-642. [PubMed] [Google Scholar]
- 159.Kusano, S., J. Kadota, S. Kohno, K. Iida, K. Kawakami, T. Morikawa, and K. Hara. 1995. Effect of roxithromycin on peripheral neutrophil adhesion molecules in patients with chronic respiratory tract disease. Respiration 62:217-222. [DOI] [PubMed] [Google Scholar]
- 160.Labro, M. T. 2000. Interference of antibacterial agents with phagocyte functions: immunomodulation or “immuno-fairy tales”? Clin. Microbiol. Rev. 13:615-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Labro, M. T., J. el Benna, and C. Babin-Chevaye. 1989. Comparison of the in-vitro effect of several macrolides on the oxidative burst of human neutrophils. J. Antimicrob. Chemother. 24:561-572. [DOI] [PubMed] [Google Scholar]
- 162.Lallemand, J. Y., V. Stoven, J. P. Annereau, J. Boucher, S. Blanquwt, J. Barthe, and G. Lenoir. 1997. Induction by antitumoral drugs of proteins that functionally complement CFTR: a novel therapy for cystic fibrosis? Lancet 350:711-712. [DOI] [PubMed] [Google Scholar]
- 163.Legssyer, R., F. Huaux, J. Lebacq, M. Delos, E. Marbaix, P. Lebecque, D. Lison, B. J. Scholte, P. Wallemacq, and T. Leal. 2006. Azithromycin reduces spontaneous and induced inflammation in ΔF508 cystic fibrosis mice. Respir. Res. 7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Leiva, M., A. Ruiz-Bravo, and M. Jimenez-Valera. 2008. Effects of telithromycin in in vitro and in vivo models of lipopolysaccharide-induced airway inflammation. Chest 134:20-29. [DOI] [PubMed] [Google Scholar]
- 165.Leiva, M., A. Ruiz-Bravo, E. Moreno, and M. Jimenez-Valera. 2007. The anti-inflammatory activity of telithromycin in a mouse model of septic shock. Int. J. Antimicrob. Agents 29:364-365. [DOI] [PubMed] [Google Scholar]
- 166.Levert, H., B. Gressier, I. Moutard, C. Brunet, T. Dine, M. Luyckx, M. Cazin, and J. C. Cazin. 1998. Azithromycin impact on neutrophil oxidative metabolism dependent on exposure time. Inflammation 22:191-201. [DOI] [PubMed] [Google Scholar]
- 167.Levy, I., G. Grisaru-Soen, L. Lerner-Geva, E. Kerem, H. Blau, L. Bentur, M. Aviram, J. Rivlin, E. Picard, A. Lavy, Y. Yahav, and G. Rahav. 2008. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg. Infect. Dis. 14:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li, J., S. Kartha, S. Lasvovskaia, A. Tan, R. K. Bhat, J. M. Manaligod, K. Page, A. R. Brasier, and M. B. Hershenson. 2002. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L690-L699. [DOI] [PubMed] [Google Scholar]
- 169.Li, Y., A. Azuma, S. Takahashi, J. Usuki, K. Matsuda, A. Aoyama, and S. Kudoh. 2002. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule 1 messenger RNA induction and leukocyte migrations: role in preventing lung injury and fibrosis in bleomycin-challenged mice. Chest 122:2137-2145. [DOI] [PubMed] [Google Scholar]
- 170.Li, Y. J., A. Azuma, J. Usuki, S. Abe, K. Matsuda, T. Sunazuka, T. Shimizu, Y. Hirata, H. Inagaki, T. Kawada, S. Takahashi, S. Kudoh, and S. Omura. 2006. EM703 improves bleomycin-induced pulmonary fibrosis in mice by the inhibition of TGF-β signaling in lung fibroblasts. Respir. Res. 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin, C. C., S. F. Liaw, K. M. Wu, and C. H. Lin. 2008. Effect of erythromycin on bronchial hyperreactivity and inflammation in ovalbumin-sensitized brown Norway rats. Respir. Physiol. Neurobiol. 161:267-272. [DOI] [PubMed] [Google Scholar]
- 172.Lin, E., R. J. Stanek, and M. A. Mufson. 2003. Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro. Antimicrob. Agents Chemother. 47:1151-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin, H. C., C. H. Wang, C. Y. Liu, C. T. Yu, and H. P. Kuo. 2000. Erythromycin inhibits β2 integrins (CD11b/CD18) expression, interleukin-8 release and intracellular oxidative metabolism in neutrophils. Respir. Med. 94:654-660. [DOI] [PubMed] [Google Scholar]
- 174.Liu, W., Q. Liang, S. Balzar, S. Wenzel, M. Gorska, and R. Alam. 2008. Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. J. Allergy Clin. Immunol. 121:893-902. [DOI] [PubMed] [Google Scholar]
- 175.Machen, T. E. 2006. Innate immune response in CF airway epithelia: hyperinflammatory? Am. J. Physiol. Cell. Physiol. 291:C218-C230. [DOI] [PubMed] [Google Scholar]
- 176.MacLeod, C. M., Q. A. Hamid, L. Cameron, C. Tremblay, and W. Brisco. 2001. Anti-inflammatory activity of clarithromycin in adults with chronically inflamed sinus mucosa. Adv. Ther. 18:75-82. [DOI] [PubMed] [Google Scholar]
- 177.Malhotra-Kumar, S., C. Lammens, S. Coenen, K. van Herck, and H. Goossens. 2007. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet 369:482-490. [DOI] [PubMed] [Google Scholar]
- 178.Mandell, L. A., R. G. Wunderink, A. Anzueto, J. G. Bartlett, G. D. Campbell, N. C. Dean, S. F. Dowell, T. M. File, Jr., D. M. Musher, M. S. Niederman, A. Torres, C. G. Whitney, et al. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl. 2):S27-S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mankin, A. S. 2008. Macrolide myths. Curr. Opin. Microbiol. 11:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martínez, J. A., J. P. Horcajada, M. Almela, F. Marco, A. Soriano, E. García, M. A. Marco, A. Torres, and J. Mensa. 2003. Addition of a macrolide to a β-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 36:389-395. [DOI] [PubMed] [Google Scholar]
- 181.Mathur, R. K., A. Awasthi, P. Wadhone, B. Ramanmurthy, and B. Saha. 2004. Reciprocal SD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat. Med. 10:540-544. [DOI] [PubMed] [Google Scholar]
- 182.Matsune, S., D. Sun, J. Ohori, K. Nishimoto, T. Fukuiwa, M. Ushikai, and Y. Kurono. 2005. Inhibition of vascular endothelial growth factor by macrolides in cultured fibroblasts from nasal polyps. Laryngoscope 115:1953-1956. [DOI] [PubMed] [Google Scholar]
- 183.Mazzei, T., E. Mini, A. Novelli, and P. Perti. 1993. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 31(Suppl. C):1-9. [DOI] [PubMed] [Google Scholar]
- 184.McNamara, N., M. Gallup, A. Sucher, I. Maltseva, D. McKemy, and C. Basbaum. 2006. AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate Erk1/2. Am. J. Respir. Cell Mol. Biol. 34:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Meusel, T. R., and F. Imani. 2003. Viral induction of inflammatory cytokines in human epithelial cells follows a p38 mitogen-activated protein kinase-dependent but NF-κB-independent pathway. J. Immunol. 171:3768-3774. [DOI] [PubMed] [Google Scholar]
- 186.Meyer, M., F. Huaux, X. Gavilanes, S. van den Brule, P. Lebecque, S. Lo Re, D. Lison, B. Scholte, P. Wallemacq, and T. Leal. 2009. Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 41:590-602. [DOI] [PubMed] [Google Scholar]
- 187.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 188.Mitsuya, Y., S. Kawai, and H. Kobayashi. 2000. Influence of macrolides on guanosine diphospho-d-mannose dehydrogenase activity in Pseudomonas biofilm. J. Infect. Chemother. 6:45-50. [DOI] [PubMed] [Google Scholar]
- 189.Mitsuyama, T., K. Hidaka, T. Furono, and N. Hara. 1997. Neutrophil-induced endothelial cell damage: inhibition by a 14-membered ring macrolide through the action of nitric oxide. Int. Arch. Allergy Immunol. 114:111-115. [DOI] [PubMed] [Google Scholar]
- 190.Miyamoto, D., S. Hasegawa, N. Sriwilaijaroen, S. Yingsakmongkon, H. Hiramatsu, T. Takahashi, K. Hidari, C. T. Guo, Y. Sakano, T. Suzuki, and Y. Suzuki. 2008. Clarithromycin inhibits progeny virus production from human influenza virus-infected host cells. Biol. Pharm. Bull. 31:217-222. [DOI] [PubMed] [Google Scholar]
- 191.Miyanohara, T., M. Ushikai, S. Matsune, K. Ueno, S. Katahira, and Y. Kurono. 2000. Effects of clarithromycin on cultured human nasal epithelial cells and fibroblasts. Laryngoscope 110:126-131. [DOI] [PubMed] [Google Scholar]
- 192.Miyatake, H., F. Taki, H. Taniguchi, R. Suzuki, K. Takagi, and T. Satake. 1991. Erythromycin reduces the severity of bronchial hyperresponiveness in asthma. Chest 99:670-673. [DOI] [PubMed] [Google Scholar]
- 193.Mizukane, R., Y. Hirakata, M. Kaku, Y. Ishii, N. Furuya, K. Ishida, H. Koga, S. Kohno, and K. Yamaguchi. 1994. Comparative in vitro exoenzyme-suppressing activities of azithromycin and other macrolide antibiotics against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 38:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mogensen, T. H. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22:240-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Molinari, G., C. A. Guzmán, A. Pesce, and G. C. Schito. 1993. Inhibition of Pseudomonas aeruginosa virulence factors by subinhibitory concentrations of azithromycin and other macrolide antibiotics. J. Antimicrob. Chemother. 31:681-688. [DOI] [PubMed] [Google Scholar]
- 196.Moore, L. J., A. C. Pridmore, S. K. Dower, and R. C. Read. 2003. Penicillin enhances the Toll-like receptor 2-mediated proinflammatory activity of Streptococcus pneumoniae. J. Infect. Dis. 188:1040-1048. [DOI] [PubMed] [Google Scholar]
- 197.Morikawa, K., J. Zhang, M. Nonaka, and S. Morikawa. 2002. Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production. Int. J. Antimicrob. Agents 19:53-59. [DOI] [PubMed] [Google Scholar]
- 198.Mukae, H., J. Kadota, J. Ashitani, H. Taniguchi, H. Mashimoto, S. Kohno, and S. Matsukura. 1997. Elevated levels of soluble adhesion molecules in serum of patients with diffuse panbronchiolitis. Chest 112:1615-1621. [DOI] [PubMed] [Google Scholar]
- 199.Müller, T., H. Bayer, D. Myrtek, D. Ferrari, S. Sorichter, M. W. Ziegenhagen, G. Zissel, J. C. Virchow, Jr., W. Luttmann, J. Norgauer, F. Di Virgilio, and M. Idzko. The P2Y14 receptor of airway epithelial cells: coupling to intracellular Ca2+ and IL-8 secretion. Am. J. Respir. Cell Mol. Biol. 33:601-609. [DOI] [PubMed]
- 200.Murphy, B. S., V. Sundareshan, T. J. Cory, D. Hayes, Jr., M. I. Anstead, and D. J. Feola. 2008. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 61:554-560. [DOI] [PubMed] [Google Scholar]
- 201.Murphy, D. M., I. A. Forrest, P. A. Corris, G. E. Johnson, T. Small, D. Jones, A. J. Fisher, J. J. Egan, T. E. Cawston, J. L. Lordan, and C. Ward. 2008. Azithromycin attenuates effects of lipopolysaccharide on lung allograft bronchial epithelial cells. J. Heart Lung Transplant. 27:1210-1216. [DOI] [PubMed] [Google Scholar]
- 202.Nagai, H., H. Shishido, R. Yoneda, E. Yamaguchi, A. Tamura, and A. Kurashima. 1991. Long-term, low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration 58:145-149. [DOI] [PubMed] [Google Scholar]
- 203.Nakamura, H., S. Fujishima, T. Inoue, Y. Ohkubo, K. Soejima, Y. Waki, M. Mori, T. Urano, F. Sakamaki, S. Tasaka, A. Ishizaka, M. Kanazawa, and K. Yamaguchi. 1999. Clinical and immunomodulatory effects of roxithromycin therapy for chronic respiratory tract infection. Eur. Respir. J. 13:1371-1379. [DOI] [PubMed] [Google Scholar]
- 204.Nakano, T., H. Inoue, S. Fukuyama, K. Matsumoto, M. Matsumura, M. Tsuda, T. Matsumoto, H. Aizawa, and Y. Nakanishi. 2006. Nifulmic acid suppresses interleukin-13-induced asthma phenotypes. Am. J. Respir. Crit. Care Med. 173:1216-1221. [DOI] [PubMed] [Google Scholar]
- 205.Nakano, T., Y. Ohashi, A. Tanaka, Y. Kakinoki, Y. Washio, and Y. Nakai. 1998. Roxithromycin reinforces epithelial defense function in rabbit trachea. Acta Otolaryngol. 538(Suppl.):233-238. [DOI] [PubMed] [Google Scholar]
- 206.Nakata, K., Y. Taguchi, and S. Kudoh. 2000. Annual report on the study of diffuse lung disease in 1999, p. 111. Ministry of Health and Welfare of Japan, Tokyo, Japan. (In Japanese.)
- 207.Nelson, H. S., D. L. Hamilos, P. R. Corsello, N. V. Levesque, A. D. Buchmeier, and B. L. Bucher. 1993. A double-blind study of troleandomycin and methylprednisolone in asthmatic subjects who require daily corticosteroids. Am. Rev. Respir. Dis. 147:398-404. [DOI] [PubMed] [Google Scholar]
- 208.Nguyen, D., M. J. Emond, N. Mayer-Hamblett, L. Saiman, B. C. Marshall, and J. L. Burns. 2007. Clinical response to azithromycin in cystic fibrosis correlates with in vitro effects on Pseudomonas aeruginosa phenotypes. Pediatr. Pulmonol. 42:533-541. [DOI] [PubMed] [Google Scholar]
- 209.Nicotera, P., and S. Orrenius. 1998. The role of calcium in apoptosis. Cell Calcium 23:173-180. [DOI] [PubMed] [Google Scholar]
- 210.Nonaka, M., R. Pawankar, S. Tomiyama, and T. Yagi. 1999. A macrolide antibiotic, roxithromycin, inhibits the growth of nasal polyp fibroblasts. Am. J. Rhinol. 13:267-272. [DOI] [PubMed] [Google Scholar]
- 211.Oda, H., J. Kadota, S. Kohno, and K. Hara. 1994. Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest 106:1116-1123. [DOI] [PubMed] [Google Scholar]
- 212.Oda, H., J. Kadota, S. Kohno, and K. Hara. 1995. Leukotriene B4 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Chest 108:116-122. [DOI] [PubMed] [Google Scholar]
- 213.Oishi, K., F. Sonoda, S. Kobayashi, A. Iwagaki, T. Nagarake, K. Matsushima, and K. Matsumoto. 1994. Role of interleukin-8 (IL-8) and an inhibitory effect on IL-8 release in the airway of patients with chronic airway diseases. Infect. Immun. 62:4145-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Okamoto, K., C. Kishioka, J. S. Kim, C. D. Wegner, J. A. Meulbroek, and B. K. Rubin. 1999. Erythromycin inhibits mucin secretion in the inflamed trachea. Am. J. Respir. Crit. Care Med. 159:A35. [Google Scholar]
- 215.Olivier, K. N., D. J. Weber, R. J. Wallace, Jr., A. R. Faiz, J. H. Lee, Y. Zhang, B. A. Brown-Elliot, A. Handler, R. W. Wilson, M. S. Schechter, L. J. Edwards, S. Chakraborti, M. R. Knowles, et al. 2003. Nontuberculous mycobacteria. I. Multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 167:828-834. [DOI] [PubMed] [Google Scholar]
- 216.Ou, X.-M., Y.-L. Feng, F.-Q. Wen, K. Wang, J. Yang, Z.-P. Deng, D.-S. Liu, and Y.-P. Li. 2008. Macrolides attenuate mucus hypersecretion in rat airways through inactivation of NF-κB. Respirology 13:63-72. [DOI] [PubMed] [Google Scholar]
- 217.Oyama, T., T. Sakuta, K. Matsushita, I. Maruyama, S. Nagaoka, and M. Torii. 2000. Effects of roxithromycin on tumor necrosis factor-alpha-induced vascular endothelial growth factor expression in human periodontal ligament cells in culture. J. Periodontol. 71:1546-1553. [DOI] [PubMed] [Google Scholar]
- 218.Park, J. Y., H. Y. Kim, J. Y. Lee, K. H. Lee, M. K. Jang, J. H. Lee, J. Y. Yoo, D. S. Han, and J. S. Hahm. 2005. Macrolide-affected Toll-like receptor 4 expression from Helicobacter pylori-infected monocytes does not modify interleukin-8 production. FEMS Immunol. Med. Microbiol. 44:171-176. [DOI] [PubMed] [Google Scholar]
- 219.Park, S.-J., Y.-C. Lee, Y.-K. Rhee, and H.-B. Lee. 2004. The effect of long-term treatment with erythromycin on Th1 and Th2 cytokines in diffuse panbronchiolitis. Biochem. Biophys. Res. Commun. 324:114-117. [DOI] [PubMed] [Google Scholar]
- 220.Parnham, M. J. 2005. Immunomodulatory effects of antimicrobials in the therapy of respiratory tract infections. Curr. Opin. Infect. Dis. 18:125-131. [DOI] [PubMed] [Google Scholar]
- 221.Parnham, M. J., O. Culić, V. Eraković, V. Munić, S. Popović-Grle, K. Baristić, M. Bosnar, K. Brajsa, I. Cepelak, S. Cuzić, I. Glojnarić, Z. Manojlović, R. Novak-Mircetić, K. Oresković, V. Pavicić-Beljak, S. Radosević, and M. Sucić. 2005. Modulation of neutrophil and inflammation markers in chronic obstructive disease by short-term azithromycin treatment. Eur. J. Pharmacol. 517:132-143. [DOI] [PubMed] [Google Scholar]
- 222.Patel, A. C., T. J. Brett, and M. J. Holtzman. 2009. The role of CLCA proteins in inflammatory airway disease. Annu. Rev. Physiol. 71:425-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Paul, M., A. D. Nielsen, A. Gafter-Gvili, E. Tacconelli, S. Andreassen, N. Almanasreh, E. Goldberg, R. Cauda, U. Frank, and L. Leibovici. 2007. The need for macrolides in hospitalised community-acquired pneumonia: propensity analysis. Eur. Respir. J. 30:525-531. [DOI] [PubMed] [Google Scholar]
- 224.Periti, P., T. Mazzei, E. Mini, and A. Novelli. 1992. Pharmacokinetic drug interactions of macrolides. Clin. Pharmacokinet. 23:106-131. [DOI] [PubMed] [Google Scholar]
- 225.Perry, D. K., W. L. Hand, D. E. Edmondson, and J. D. Lambeth. 1992. Role of phospholipase D-derived diradylglycerol in the activation of the human neutrophil respiratory burst oxidase: inhibition by phosphatidic acid phosphohydrolase inhibitors. J. Immunol. 149:2749-2758. [PubMed] [Google Scholar]
- 226.Phaff, S. J., H. A. W. M. Tiddens, H. A. Verbrugh, and A. Ott. 2006. Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long-term azithromycin use in cystic fibrosis. J. Antimicrob. Chemother. 57:741-746. [DOI] [PubMed] [Google Scholar]
- 227.Piacentini, G. L., D. G. Peroni, A. Bodini, R. Pigozzi, S. Costella, A. Loiacono, and A. L. Boner. 2007. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy Asthma Proc. 28:194-198. [DOI] [PubMed] [Google Scholar]
- 228.Pilette, C., Y. Ouadrhiri, F. Dimanche, J. P. Vaerman, and Y. Sibille. 2003. Secretory component is cleaved by neutrophil serine proteinases but its epithelial production is increased by neutrophils through NF-κB- and p38 mitogen-activated protein kinase-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 28:485-498. [DOI] [PubMed] [Google Scholar]
- 229.Pukhalsky, A. L., G. V. Shmarina, N. I. Kapranov, S. N. Kokarovtseva, D. Pukhalskaya, and N. J. Kashirskaja. 2004. Anti-inflammatory and immunomodulating effects of clarithromycin in patients with cystic fibrosis lung disease. Mediators Inflamm. 13:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rainsford, K. D. 2006. Influenza (“bird flu”), inflammation and anti-inflammatory/analgesic drugs. Inflammopharmacology 14:2-9. [DOI] [PubMed] [Google Scholar]
- 231.Ratner, A. J., R. Bryan, A. Weber, S. Nguyen, D. Barnes, A. Pitt, S. Gelber, A. Cheung, and A. Prince. 2001. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J. Biol. Chem. 276:19267-19275. [DOI] [PubMed] [Google Scholar]
- 232.Restrepo, M. I., E. M. Mortensen, G. W. Waterer, R. G. Wunderink, J. J. Coalson, and A. Anzueto. 2009. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur. Respir. J. 33:153-159. [DOI] [PubMed] [Google Scholar]
- 233.Rhee, C. S., Y. Majima, S. Arima, H. W. Jung, T. H. Jinn, Y. G. Min, and Y. Sakakura. 2000. Effects of clarithromycin on rheological properties of nasal mucus in patients with chronic sinusitis. Ann. Otol. Rhinol. Laryngol. 109:484-487. [DOI] [PubMed] [Google Scholar]
- 234.Ribeiro, C. M. P., A. M. Paradiso, M. A. Carew, S. B. Shears, and R. C. Boucher. 2005. Cystic fibrosis airway epithelial Ca2+i signaling: the mechanism for the larger agonist-mediated Ca2+i signals in human cystic fibrosis airway epithelia. J. Biol. Chem. 280:10202-10209. [DOI] [PubMed] [Google Scholar]
- 235.Ribeiro, C. M. P., A. M. Paradiso, U. Schwab, J. Perez-Vilar, L. Jones, W. O'Neal, and R. C. Boucher. 2005. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 280:17798-17806. [DOI] [PubMed] [Google Scholar]
- 236.Richeldi, L., G. Ferrara, L. Fabbri, T. J. Lasserson, and P. G. Gibson. 2005. Macrolides for chronic asthma. Cochrane Database Syst. Rev. 2005:CD002997. [DOI] [PubMed] [Google Scholar]
- 237.Rosenberg, S. M., H. Gerhard, M. M. Grunstein, and C. M. Schramm. 1991. Use of TAO without methylprednisolone in the treatment of severe asthma. Chest 100:849-850. [DOI] [PubMed] [Google Scholar]
- 238.Rubin, B. K. 2004. Immunomodulatory properties of macrolides: overview and historical perspective. Am. J. Med. 117(Suppl. 9A):2S-4S. [DOI] [PubMed] [Google Scholar]
- 239.Rubin, B. K. 2007. CFTR is a modulator of airway inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L381-L382. [DOI] [PubMed] [Google Scholar]
- 240.Rubin, B. K., and J. Tamaoki. 2000. Macrolide antibiotics as biological response modifiers. Curr. Opin. Invest. Drugs 1:169-172. [PubMed] [Google Scholar]
- 241.Rubin, B. K., H. Druce, O. E. Ramirez, and R. Palmer. 1997. Effect of clarithromycin on nasal mucus properties in healthy subjects and in patients with purulent rhinitis. Am. J. Respir. Crit. Care Med. 155:2018-2023. [DOI] [PubMed] [Google Scholar]
- 242.Rubin, B. K., M. O. Henke, and A. Dalhoff. 2005. Anti-inflammatory properties of antibiotics other than macrolides, p. 247-267. In B. K. Rubin and J. Tamaoki (ed.), Antibiotics as anti-inflammatory and immunomodulatory agents. Birkhäuser Verlag, Basel, Switzerland.
- 243.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, P. W. Campbell III, et al. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749-1756. [DOI] [PubMed] [Google Scholar]
- 244.Sakito, O., J. Kadota, S. Kohno, K. Abe, R. Shirai, and K. Hara. 1996. Interleukin 1β, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration 63:42-48. [DOI] [PubMed] [Google Scholar]
- 245.Sato, E., D. K. Nelson, S. Koyama, J. C. Hoyt, and R. A. Robbins. 2001. Erythromycin modulates eosinophil chemotactic cytokine production by human lung fibroblasts in vitro. Antimicrob. Agents Chemother. 45:401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sato, K., M. Suga, T. Akaike, S. Fujii, H. Muranaka, T. Doi, H. Maeda, and M. Ando. 1998. Therapeutic effect of erythromycin on influenza virus-induced lung injury in mice. Am. J. Respir. Crit. Care Med. 157:853-857. [DOI] [PubMed] [Google Scholar]
- 247.Scheid, P., L. Kempster, U. Griesenbach, J. C. Davies, A. Dewar, P. P. Weber, W. H. Colledge, M. J. Evans, D. M. Geddes, and E. W. F. W. Alton. 2001. Inflammation in cystic fibrosis airways: relationship to increased bacterial adherence. Eur. Respir. J. 17:27-35. [DOI] [PubMed] [Google Scholar]
- 248.Schultz, M. J., P. Speelman, C. E. Hack, W. A. Buurman, S. J. van Deventer, and T. van der Poll. 2000. Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with Streptococcus pneumoniae. J. Antimicrob. Chemother. 46:235-240. [DOI] [PubMed] [Google Scholar]
- 249.Schultz, M. J., P. Speelman, S. Zaat, S. H. J. van Deventer, and T. van der Poll. 1998. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob. Agents Chemother. 42:1605-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Seemungal, T. A., T. M. Wilkinson, J. R. Hurst, W. R. Perera, R. J. Sapsford, and J. A. Wedzicha. 2008. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am. J. Respir. Crit. Care Med. 178:1139-1147. [DOI] [PubMed] [Google Scholar]
- 251.Shalit, I., L. Horev-Azaria, I. Fabian, H. Blau, N. Kariv, I. Shechtman, H. Alteraz, and Y. Kletter. 2002. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in mice injected with cyclophosphamide. Antimicrob. Agents Chemother. 46:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shao, M. X., and J. A. Nadel. 2005. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-α-converting enzyme. J. Immunol. 175:4009-4016. [DOI] [PubMed] [Google Scholar]
- 253.Shimizu, T., M. Kato, H. Mochizuki, K. Tokuyama, A. Morikawa, and T. Kuroume. 1994. Roxithromycin reduces the degree of bronchial hyperresponsiveness in children with asthma. Chest 106:458-461. [DOI] [PubMed] [Google Scholar]
- 254.Shimizu, T., S. Shimizu, R. Hattori, E. C. Gabazza, and Y. Majima. 2003. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. Am. J. Respir. Crit. Care Med. 168:581-587. [DOI] [PubMed] [Google Scholar]
- 255.Shinkai, M., G. H. Foster, and B. K. Rubin. 2006. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L75-L83. [DOI] [PubMed] [Google Scholar]
- 256.Shinkai, M., J. Tamaoki, H. Kobayashi, S. Kanoh, K. Motoyoshi, T. Kute, and B. K. Rubin. 2006. Clarithromycin delays progression of bronchial epithelial cells from G1 phase to S phase and delays cell growth via extracellular signal-regulated protein kinase suppression. Antimicrob. Agents Chemother. 50:1738-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shinkai, M., Y. S. López-Boado, and B. K. Rubin. 2007. Clarithromycin has an immunomodulatory effect on ERK-mediated inflammation induced by Pseudomonas aeruginosa flagellin. J. Antimicrob. Chemother. 59:1096-1101. [DOI] [PubMed] [Google Scholar]
- 258.Shirai, T., A. Sato, and K. Chida. 1995. Effect of 14-membered ring macrolide therapy on chronic respiratory tract infections and polymorphonuclear leukocyte activity. Intern. Med. 34:469-474. [DOI] [PubMed] [Google Scholar]
- 259.Shitrit, D., D. Bendayan, S. Gidon, M. Saute, I. Bakal, and M. R. Kramer. 2005. Long-term azithromycin use for treatment of bronchiolitis obliterans syndrome in lung transplant recipients. J. Heart Lung Transplant. 24:1440-1443. [DOI] [PubMed] [Google Scholar]
- 260.Simpson, J. L., H. Powell, M. J. Boyle, R. J. Scott, and P. G. Gibson. 2008. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am. J. Respir. Crit. Care Med. 177:148-155. [DOI] [PubMed] [Google Scholar]
- 261.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 262.Siracusa, A., G. Brugnami, T. Fiordi, S. Areni, C. Severini, and A. Marabini. 1993. Troleandomycin in the treatment of difficult asthma. J. Allergy Clin. Immunol. 92:677-682. [DOI] [PubMed] [Google Scholar]
- 263.Sissi, C., and M. Palumbo. 2003. The quinolone family: from antibacterial to anticancer agents. Curr. Med. Chem. Anticancer Agents 3:439-450. [DOI] [PubMed] [Google Scholar]
- 264.Smith, R. S., E. R. Fedyk, T. A. Springer, N. Mukaida, B. H. Iglewski, and R. P. Phipps. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-κB and activator protein-2. J. Immunol. 167:366-374. [DOI] [PubMed] [Google Scholar]
- 265.Smith, R. S., S. G. Harris, R. Phipps, and B. Iglewski. 2002. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 184:1132-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sofer, D., N. Gilboa-Garber, A. Belz, and N. C. Garber. 1999. ‘Subinhibitory’ erythromycin represses production of Pseudomonas aeruginosa lectins, autoinducer and virulence factors. Chemotherapy 45:335-341. [DOI] [PubMed] [Google Scholar]
- 267.Southern, K. W., P. M. Barker, and A. Solis. 2004. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst. Rev. 2004:CD002203. [DOI] [PubMed] [Google Scholar]
- 268.Storey, D. G., E. E. Ujack, H. R. Rabin, and I. Mitchell. 1998. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect. Immun. 66:2521-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Strunk, R. C., L. B. Bacharier, B. R. Phillips, S. J. Szefler, R. S. Zeiger, V. M. Chinchilli, F. D. Martinez, R. F. Lemanske, Jr., L. M. Taussig, D. T. Mauger, W. J. Morgan, C. A. Sorkness, I. M. Paul, T. Guilbert, M. Krawiec, R. Covar, G. Larsen, et al. 2008. Azithromycin or montelukast as inhaled corticosteroid-sparing agents in moderate-to-severe childhood asthma study. J. Allergy Clin. Immunol. 122:1138-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sugiyama, K., R. Shirai, H. Mukae, H. Ishimoto, T. Nagata, N. Sakamoto, H. Ishii, S. Nakayama, K. Yanagihara, Y. Mizuta, and S. Kohno. 2007. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin. Exp. Immunol. 147:540-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sunazuka, T., K. Yoshida, M. Oohori, K. Otoguro, Y. Harigaya, Y. Iwai, K. S. Akagawa, and S. Omura. 2003. Effect of 14-membered macrolide compounds on monocyte to macrophage differentiation. J. Antibiot. (Tokyo) 56:721-724. [DOI] [PubMed] [Google Scholar]
- 272.Suzuki, H., A. Shimomura, K. Ikeda, M. Furukawa, T. Oshima, and T. Takasaka. 1997. Inhibitory effect of macrolides on interleukin-8 secretion from cultured human nasal epithelial cells. Laryngoscope 107:1661-1666. [DOI] [PubMed] [Google Scholar]
- 273.Suzuki, H., K. Ikeda, R. Honma, S. Gotoh, T. Oshima, M. Furukawa, and T. Takasaka. 2000. Prognostic factors of chronic rhinosinusitis under long-term low-dose macrolide therapy. ORL J. Otorhinolaryngol. Relat. Spec. 62:121-127. [DOI] [PubMed] [Google Scholar]
- 274.Suzuki, T., M. Yamaya, K. Sekizawa, M. Hosoda, N. Yamada, S. Ishizuka, A. Yoshino, H. Yasuda, H. Takahashi, H. Nishimura, and H. Sasaki. 2002. Erythromycin inhibits rhinovirus infection in cultured human tracheal epithelial cells. Am. J. Respir. Crit. Care Med. 165:1113-1118. [DOI] [PubMed] [Google Scholar]
- 275.Suzuki, T., M. Yanai, M. Yamaya, T. Satoh-Nakagawa, K. Sekizawa, S. Ishida, and H. Sasaki. 2001. Erythromycin and common cold in COPD. Chest 120:730-733. [DOI] [PubMed] [Google Scholar]
- 276.Szefler, R. S., J. Q. Rose, E. F. Ellis, S. L. Spector, A. W. Green, and W. J. Jusko. 1980. The effect of troleandomycin on methylprednisolone elimination. J. Allergy Clin. Immunol. 66:447-451. [DOI] [PubMed] [Google Scholar]
- 277.Tabary, O., E. Boncoeur, R. de Martin, R. Pepperkok, A. Clément, C. Schultz, and J. Jacquot. 2006. Calcium-dependent regulation of NF-κB activation in cystic fibrosis airway epithelial cells. Cell Signal. 18:652-660. [DOI] [PubMed] [Google Scholar]
- 278.Tagaya, E., J. Tamaoki, M. Kondo, and A. Nagai. 2002. Effect of short course of clarithromycin therapy on sputum production in patients with chronic airway hypersecretion. Chest 122:213-218. [DOI] [PubMed] [Google Scholar]
- 279.Takaki, M., M. Ushikai, K. Deguchi, K. Nishimoto, S. Matsune, and Y. Kurono. 2003. The role of nuclear factor-κB in interleukin-8 expression by human adenoidal fibroblasts. Laryngoscope 113:1378-1385. [DOI] [PubMed] [Google Scholar]
- 280.Takeuchi, K., Y. Majima, and Q. Hamid. 2005. Macrolides and upper airway/sinus disease, p. 193-203. In B. K. Rubin and J. Tamaoki (ed.), Antibiotics as anti-inflammatory and immunomodulatory agents. Birkhäuser Verlag, Basel, Switzerland.
- 281.Takeyama, K., J. V. Fahy, and J. A. Nadel. 2001. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am. J. Respir. Crit. Care Med. 163:511-516. [DOI] [PubMed] [Google Scholar]
- 282.Takizawa, H. 2005. Cytokines, p. 77-86. In B. K. Rubin and J. Tamaoki (ed.), Antibiotics as anti-inflammatory and immunomodulatory agents. Birkhäuser Verlag, Basel, Switzerland.
- 283.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, M. Tanaka, T. Kasama, K. Kobayashi, J. Nakajima, and K. Ito. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 156:266-271. [DOI] [PubMed] [Google Scholar]
- 284.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, J. Nakajima, M. Yanagisawa, and K. Ito. 1998. Erythromycin and clarithromycin attenuate cytokine-induced endothelin-1 expression in human bronchial epithelial cells. Eur. Respir. J. 12:57-63. [DOI] [PubMed] [Google Scholar]
- 285.Tamaoki, J. 2004. The effects of macrolides on inflammatory cells. Chest 125:41S-51S. [DOI] [PubMed] [Google Scholar]
- 286.Tamaoki, J., E. Tagaya, A. Sakai, and K. Konno. 1995. Effects of macrolide antibiotics on neurally-mediated contraction of human isolated bronchus. J. Allergy Clin. Immunol. 95:853-859. [DOI] [PubMed] [Google Scholar]
- 287.Tamaoki, J., E. Tagaya, I. Yamawaki, M. Kondo, N. Sakai, A. Nagai, and K. Konno. 1995. Effect of erythromycin on endotoxin-induced microvascular leakage in the rat trachea and lungs. Am. J. Respir. Crit. Care Med. 151:1582-1588. [DOI] [PubMed] [Google Scholar]
- 288.Tamaoki, J., H. Takemura, E. Tagaya, and K. Konno. 1995. Effect of clarithromycin on transepithelial potential difference in rabbit tracheal mucosa. J. Infect. Chemother. 1:112-115. [Google Scholar]
- 289.Tamaoki, J., K. Isono, N. Sakai, T. Kanemura, and K. Konno. 1992. Erythromycin inhibits Cl secretion across canine tracheal epithelial cells. Eur. Respir. J. 5:234-238. [PubMed] [Google Scholar]
- 290.Tamaoki, J., K. Takeyama, E. Tagaya, and K. Konno. 1995. Effect of clarithromycin on sputum production and its rheological properties in chronic respiratory tract infections. Antimicrob. Agents Chemother. 39:1688-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tamaoki, J., M. Kondo, K. Kohri, K. Aoshiba, E. Tagaya, and A. Nagai. 1999. Macrolide antibiotics protect against immune complex-induced lung injury in rats: role of nitric oxide from alveolar macrophages. J. Immunol. 163:2909-2915. [PubMed] [Google Scholar]
- 292.Tanaka, E., K. Kanthakumar, D. R. Cundell, K. W. Tsang, G. W. Taylor, F. Kuze, P. J. Cole, and R. Wilson. 1994. The effect of erythromycin on Pseudomonas aeruginosa and neutrophil mediated epithelial damage. J. Antimicrob. Chemother. 33:765-775. [DOI] [PubMed] [Google Scholar]
- 293.Tarre, D., M. Broggini, V. Botta, C. Sampietro, R. Busarello, and C. Garberi. 1991. In vitro and ex vivo effects of recent and new macrolide antibiotics on chemotaxis of human polymorphonuclear leukocytes. J. Chemother. 3:236-239. [DOI] [PubMed] [Google Scholar]
- 294.Tateda, K., R. Comte, J. C. Pechere, T. Köhler, K. Yamaguchi, and C. Van Delden. 2001. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1930-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Tateda, K., T. J. Standiford, J. C. Pechere, and K. Yamaguchi. 2004. Regulatory effects of macrolides on bacterial virulence: potential role as quorum-sensing inhibitors. Curr. Pharm. Des. 10:3055-3065. [DOI] [PubMed] [Google Scholar]
- 296.Tateda, K., Y. Ishii, M. Horikawa, T. Matsumoto, S. Miyairi, J. C. Pechere, T. J. Standiford, M. Ishiguro, and K. Yamaguchi. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tateda, K., Y. Ishii, T. Matsumoto, N. Furuya, M. Nagashima, T. Matsunaga, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1996. Direct evidence for antipseudomonal activity of macrolides: exposure-dependent bactericidal activity and inhibition of protein synthesis by erythromycin, clarithromycin, and azithromycin. Antimicrob. Agents Chemother. 40:2271-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tateda, K., Y. Ishii, T. Matsumoto, T. Kobayashi, S. Miyazaki, and K. Yamaguchi. 2000. Potential of macrolide antibiotics to inhibit protein synthesis of Pseudomonas aeruginosa: suppression of virulence factors and stress response. J. Infect. Chemother. 6:1-7. [DOI] [PubMed] [Google Scholar]
- 299.Tazumi, A., Y. Maeda, C. E. Goldsmith, W. A. Coulter, C. Mason, B. C. Millar, M. McCalmont, J. Rendall, J. S. Elborn, M. Matsuda, and J. E. Moore. 2009. Molecular characterization of macrolide resistance determinants [erm(B) and mef(A)] in Streptococcus pneumoniae and viridans group streptococci (VGS) isolated from adult patients with cystic fibrosis (CF). J. Antimicrob. Chemother. 64:501-506. [DOI] [PubMed] [Google Scholar]
- 300.Tenson, T., M. Lovmar, and M. Ehrenberg. 2003. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 30:1005-1014. [DOI] [PubMed] [Google Scholar]
- 301.Tessmer, A., T. Welte, P. Martus, M. Schnoor, R. Marre, and N. Suttorp. 2009. Impact of intravenous β-lactam/macrolide versus β-lactam monotherapy on mortality in hospitalized patients with community-acquired pneumonia. J. Antimicrob. Chemother. 63:1025-1033. [DOI] [PubMed] [Google Scholar]
- 302.Tirouvanziam, R., S. de Bentzmann, C. Hubeau, J. Hinnrasky, J. Jacquot, B. Peault, and E. Puchelle. 2000. Inflammation and infection in naive human cystic fibrosis airway grafts. Am. J. Respir. Cell Mol. Biol. 23:121-127. [DOI] [PubMed] [Google Scholar]
- 303.Tramper-Stranders, G. A., T. F. Wolfs, A. Fleer, J. L. Kimpen, and C. K. van der Ent. 2007. Maintenance azithromycin treatment in pediatric patients with cystic fibrosis: long-term outcomes related to macrolide resistance and pulmonary function. Pediatr. Infect. Dis. J. 26:8-12. [DOI] [PubMed] [Google Scholar]
- 304.Tsai, W. C., M. L. Rodriguez, K. S. Young, J. C. Deng, V. J. Thannickal, K. Tateda, M. B. Hershenson, and T. J. Standiford. 2004. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am. J. Respir. Crit. Care Med. 170:1331-1339. [DOI] [PubMed] [Google Scholar]
- 305.Tsang, K. W., P. Ng, P. L. Ho, S. Chan, G. Tipoe, R. Leung, J. Sun, J. C. Ho, M. S. Ip, and W. K. Lam. 2003. Effects of erythromycin on Pseudomonas aeruginosa adherence to collagen and morphology in vitro. Eur. Respir. J. 21:401-406. [DOI] [PubMed] [Google Scholar]
- 306.Tsang, K. W. T., P.-L. Ho, K.-N. Chan, M. S. M. Ip, W.-K. Lam, C.-S. Ho, K. Y. Yuen, G. C. Ooi, R. Amitani, and E. Tanaka. 1999. A pilot study of low-dose erythromycin in bronchiectasis. Eur. Respir. J. 13:361-364. [DOI] [PubMed] [Google Scholar]
- 307.Tsuchihashi, Y., K. Oishi, H. Yoshimine, S. Suzuki, A. Kumatori, T. Sunazuka, S. Omura, K. Matsushima, and T. Nagatake. 2002. Fourteen-member macrolides suppress interleukin-8 production but do not promote apoptosis of activated neutrophils. Antimicrob. Agents Chemother. 46:1101-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tsurita, M., M. Kurokawa, M. Imakita, Y. Fukuda, Y. Watanabe, and K. Shiraki. 2001. Early augmentation of interleukin (IL)-12 level in the airway of mice administered orally with clarithromycin or intranasally with IL-12 results in alleviation of influenza infection. J. Pharmacol. Exp. Ther. 298:362-368. [PubMed] [Google Scholar]
- 309.Tuyt, L. M., W. H. Dokter, K. Birkenkamp, S. B. Koopmans, C. Lummen, W. Kruijer, and E. Vellenga. 1999. Extracellular-regulated kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in NF-κB-dependent IL-6 expression in human monocytes. J. Immunol. 162:4893-4902. [PubMed] [Google Scholar]
- 310.Van Amersfoort, E. S., T. J. Van Berkel, and J. Kuiper, Jr. 2003. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 16:379-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Vanaudenaerde, B. M., W. A. Wuyts, N. Geudens, L. J. Dupont, K. Schoofs, S. Smeets, D. E. Van Raemdonck, and G. M. Verleden. 2007. Macrolides inhibit IL17-induced IL8 and 8-isoprostane release from human airway smooth muscle cells. Am. J. Transplant. 7:76-82. [DOI] [PubMed] [Google Scholar]
- 312.Verleden, G. M., and L. J. Dupont. 2004. Azithromycin therapy for patients with bronchiolitis obliterans syndrome after lung transplantation. Transplantation 77:1465-1467. [DOI] [PubMed] [Google Scholar]
- 313.Verleden, G. M., B. M. Vanaudenaerde, L. J. Dupont, and D. E. Van Raemdonck. 2006. Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 174:566-570. [DOI] [PubMed] [Google Scholar]
- 314.Vittori, I., M. Marini, A. Fasoli, R. De Franchis, and S. Mattoli. 1992. Increased expression of endothelin in bronchial epithelial cells of asthmatic patients and effects of corticosteroids. Am. Rev. Respir. Dis. 146:1320-1325. [DOI] [PubMed] [Google Scholar]
- 315.Vogel, S., T. Kubin, D. von der Ahe, E. Deindl, W. Schaper, and R. Zimmermann. 2006. MEK hyperphosphorylation coincides with cell cycle shut down of cultured smooth muscle cells. J. Cell. Physiol. 206:25-35. [DOI] [PubMed] [Google Scholar]
- 316.Voynow, J. A., and B. K. Rubin. 2009. Mucins, mucus, and sputum. Chest 135:505-512. [DOI] [PubMed] [Google Scholar]
- 317.Voynow, J. A., L. R. Young, Y. Wang, T. Horger, M. C. Rose, and B. M. Fischer. 1999. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory cells. Am. J. Physiol. 276:L835-L843. [DOI] [PubMed] [Google Scholar]
- 318.Wald, J. A., B. F. Friedman, and R. S. Farr. 1986. An improved protocol for the use of troleandomycin (TAO) in the treatment of steroid-requiring asthma. J. Allergy Clin. Immunol. 78:36-43. [DOI] [PubMed] [Google Scholar]
- 319.Wallwork, B., W. Coman, A. Mackay-Sim, L. Greiff, and A. Cervin. 2006. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 116:189-193. [DOI] [PubMed] [Google Scholar]
- 320.Wang, H., and S. Ma. 2008. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am. J. Emerg. Med. 26:711-715. [DOI] [PubMed] [Google Scholar]
- 321.Wang, K., F.-Q. Wen, Y.-L. Feng, X.-M. Ou, D. Xu, J. Yang, and Z.-P. Deng. 2007. Increased expression of human calcium-activated chloride channel 1 gene is correlated with mucus overproduction in Chinese asthmatic airway. Cell Biol. Int. 31:1388-1395. [DOI] [PubMed] [Google Scholar]
- 322.Weiss, T., I. Shalit, H. Blau, S. Werber, D. Halperin, A. Levitov, and I. Fabian. 2004. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-κB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines. Antimicrob. Agents Chemother. 48:1974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Werber, S., I. Shalit, I. Fabian, G. Steuer, T. Weiss, and H. Blau. 2005. Moxifloxacin inhibits cytokine-induced MAP kinase and NF-κB activation as well as nitric oxide synthesis in a human respiratory epithelial cell line. J. Antimicrob. Chemother. 55:293-300. [DOI] [PubMed] [Google Scholar]
- 324.Williams, A. C., H. F. Galley, A. M. Watt, and N. R. Webster. 2005. Differential effects of three antibiotics on T helper cell cytokine expression. J. Antimicrob. Chemother. 56:502-506. [DOI] [PubMed] [Google Scholar]
- 325.Williams, J. D., and A. M. Sefton. 1993. Comparison of macrolide antibiotics. J. Antimicrob. Chemother. 31(Suppl. C):11-26. [DOI] [PubMed] [Google Scholar]
- 326.Wolter, J., S. Seeney, S. Bell, S. Bowler, P. Masel, and J. McCormack. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Woodhead, M., F. Blasi, S. Ewig, G. Huchon, M. Ieven, A. Ortqvist, T. Schaberg, A. Torres, G. van der Heijden, T. J. Verheij, et al. 2005. Guidelines for the management of adult lower respiratory tract infections. Eur. Respir. J. 26:1138-1180. [DOI] [PubMed] [Google Scholar]
- 328.Wozniak, D. J., and R. Keyser. 2004. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest 125:62S-69S. [DOI] [PubMed] [Google Scholar]
- 329.Yalçin, E., N. Kiper, U. Ozçelik, D. Doğru, P. Firat, A. Sahin, M. Ariyürek, G. Mocan, N. Gürcan, and A. Göçmen. 2006. Effects of clarithromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. J. Clin. Pharm. Ther. 31:49-55. [DOI] [PubMed] [Google Scholar]
- 330.Yamada, T., S. Fujieda, S. Mori, H. Yamamoto, and H. Saito. 2000. Macrolide treatment decreased the size of nasal polyps and IL-8 levels in nasal lavage. Am. J. Rhinol. 14:143-148. [DOI] [PubMed] [Google Scholar]
- 331.Yamaryo, T., K. Oishi, H. Yoshimine, Y. Tsuchihashi, K. Matsushima, and T. Nagatake. 2003. Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob. Agents Chemother. 47:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yamasaki, T., T. Ichimiya, K. Hirai, K. Hiramatsu, and M. Nasu. 1997. Effect of antimicrobial agents on the piliation of Pseudomonas aeruginosa and adherence to mouse tracheal epithelium. J. Chemother. 9:32-37. [DOI] [PubMed] [Google Scholar]
- 333.Yamasawa, H., K. Oshikawa, S. Ohno, and Y. Sugiyama. 2004. Macrolides inhibit epithelial cell-mediated neutrophil survival by modulating granulocyte macrophage colony-stimulating factor release. Am. J. Respir. Cell Mol. Biol. 30:569-575. [DOI] [PubMed] [Google Scholar]
- 334.Yamaya, M., A. Azuma, H. Tanaka, H. Takizawa, K. Chida, Y. Taguchi, K. Mikasa, J. Kadota, and S. Kudoh. 2008. Inhibitory effects of macrolide antibiotics on exacerbations and hospitalization in chronic obstructive pulmonary disease in Japan: a retrospective multicenter analysis. J. Am. Geriatr. Soc. 56:1358-1360. [DOI] [PubMed] [Google Scholar]
- 335.Yanagihara, K., K. Tomono, Y. Imamura, Y. Kaneko, M. Kuroki, T. Sawai, Y. Miyazaki, Y. Hirakata, H. Mukae, J. Kadota, and S. Kohno. 2002. Effect of clarithromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine model. J. Antimicrob. Chemother. 49:867-870. [DOI] [PubMed] [Google Scholar]
- 336.Yasuda, H., Y. Ajiki, T. Koga, H. Kawada, and T. Yokota. 1993. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob. Agents Chemother. 37:1749-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Yasutomi, M., Y. Ohshima, N. Omata, A. Yamada, H. Iwasaki, Y. Urasaki, and M. Mayumi. 2005. Erythromycin differentially inhibits lipopolysaccharide- or poly(I:C)-induced but not peptidoglycan-induced activation of human monocyte-derived dendritic cells. J. Immunol. 175:8069-8076. [DOI] [PubMed] [Google Scholar]
- 338.Yates, B., D. M. Murphy, I. A. Forrest, C. Ward, R. M. Rutherford, A. L. Fisher, J. L. Lordan, J. H. Dark, and P. A. Corris. 2005. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 172:772-775. [DOI] [PubMed] [Google Scholar]
- 339.Yatsunami, J., N. Tsuruta, N. Hara, and S. Hayashi. 1998. Inhibition of tumor angiogenesis by roxithromycin, a 14-membered ring macrolide antibiotic. Cancer Lett. 131:137-143. [DOI] [PubMed] [Google Scholar]
- 340.Yatsunami, J., Y. Fukuno, M. Nagata, M. Tominaga, S. Aoki, N. Tsuruta, M. Kawashima, S. Taniguchi, and S. Hayashi. 1999. Antiangiogenetic and antitumor effects of 14-membered macrolides on mouse B16 melanoma cells. Clin. Exp. Metastasis 17:361-367. [DOI] [PubMed] [Google Scholar]
- 341.Yu, C., A. Azuma, Y. Li, C. Wang, S. Abe, J. Usuki, K. Matsuda, S. Kudoh, T. Sunazuka, and S. Omura. 2008. EM703, a new derivative of erythromycin, inhibits transforming growth factor-β signaling in human lung fibroblasts. Exp. Lung Res. 34:343-354. [DOI] [PubMed] [Google Scholar]
- 342.Zeiger, R. S., M. Schatz, W. Sperling, R. A. Simon, and D. D. Stevenson. 1980. Efficacy of troleandomycin in outpatients with severe, corticosteroid-dependent asthma. J. Allergy Clin. Immunol. 66:438-446. [DOI] [PubMed] [Google Scholar]
- 343.Zhanel, G. G., M. Dueck, D. J. Hoban, L. M. Vercaigne, J. M. Embil, A. S. Gin, and J. A. Karlowsky. 2001. Review of macrolides and ketolides: focus on respiratory tract infections. Drugs 61:443-498. [DOI] [PubMed] [Google Scholar]
- 344.Zhang, W., and H. T. Liu. 2002. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 12:9-18. [DOI] [PubMed] [Google Scholar]
- 345.Zhang, Y. L., and C. Dong. 2005. MAP kinases in immune responses. Cell. Mol. Immunol. 2:20-27. [PubMed] [Google Scholar]
- 346.Zhao, D.-M., H.-H. Xue, K. Chida, T. Suda, Y. Oki, M. Kanai, C. Uchida, A. Ichiyama, and H. Nakamura. 2000. Effect of erythromycin on ATP-induced intracellular calcium response in A549 cells. Am. J. Physiol. 278:L726-L736. [DOI] [PubMed] [Google Scholar]
- 347.Zimmermann, G. S., C. Neurohr, H. Villena-Hermoza, R. Hatz, and J. Behr. 2009. Anti-inflammatory effects of antibacterials on human bronchial epithelial cells. Respir. Res. 10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]