Abstract
Summary: Human T-cell leukemia virus type 1 (HTLV-1), the first human retrovirus to be discovered, is present in diverse regions of the world, where its infection is usually neglected in health care settings and by public health authorities. Since it is usually asymptomatic in the beginning of the infection and disease typically manifests later in life, silent transmission occurs, which is associated with sexual relations, breastfeeding, and blood transfusions. There are no prospects of vaccines, and screening of blood banks and in prenatal care settings is not universal. Therefore, its transmission is active in many areas such as parts of Africa, South and Central America, the Caribbean region, Asia, and Melanesia. It causes serious diseases in humans, including adult T-cell leukemia/lymphoma (ATL) and an incapacitating neurological disease (HTLV-associated myelopathy/tropical spastic paraparesis [HAM/TSP]) besides other afflictions such as uveitis, rheumatic syndromes, and predisposition to helminthic and bacterial infections, among others. These diseases are not curable as yet, and current treatments as well as new perspectives are discussed in the present review.
INTRODUCTION
The discovery of the first human retrovirus proceeded rather independently in Japan and the United States. In 1980, Poiesz et al. (83) identified human T-cell leukemia virus (HTLV) in a T-cell line from a patient with cutaneous T-cell lymphoma. Independently, in 1982, Yoshida et al. (103) identified adult T-cell leukemia virus (ATLV). Soon, HTLV and ATLV were shown to be identical at the sequence level and were named HTLV type 1 (HTLV-1) (27).
After the discovery of HTLV-1, a second human retrovirus, HTLV-2, was described. Prevalent in Central and West Africa; in native Amerindian populations in North, Central, and South America; and among cohorts of intravenous drug users in the United States and Europe, HTLV-2 has a similar genome structure and shares approximately 70% nucleotide sequence homology with HTLV-1 (53). In 2005, two more related viruses, HTLV-3 and HTLV-4, were reported in central Africa (49). However, only HTLV-1 has been convincingly linked to human diseases at present.
HTLV-1 has six reported subtypes (subtypes A to F). Diverse studies have been performed on HTLV-1 subtyping but present a minor role in the epidemiological status of the virus. The great majority of infections are caused by the cosmopolitan subtype A, and there is no report of subtype influence on the pathogenic potential of HTLV-1 (24).
Approximately 20 million people worldwide are estimated to be infected with HTLV-1 (21). Approximately 90% of the infected individuals remain asymptomatic carriers during their lives. In reality, the immunopathogenesis of this retrovirus is intriguing, since its lifelong persistence in CD4+ lymphocytes determines a prolonged interaction between the virus and the immune system, which may result in the broad spectrum of diseases associated with HTLV-1. This may be related to the direct action of the virus on the immune system or a consequence of the response of the immune system to the virus. Since 1986, HTLV-1 screening has been developed and was slowly implemented worldwide (34). In 1993, HTLV-1 screening of blood donors was already performed in all developed countries and in many developing countries where HTLV-1 is endemic. Moreover, hemovigilance studies were performed, where patients who received blood from HTLV-positive donors, transfused before screening was performed or from repeat donors who presented seroconversion to HTLV, were traced and tested for the virus (70).
TRANSMISSION
The most important routes of HTLV-1 transmission were found to be from mother to child and predominantly through breastfeeding, sexual intercourse, and blood contact, including the transfusion of infected cellular products or sharing of needles and syringes (84). The efficiency of the mother-to-child transmission route is estimated to be 20% and has been correlated with individual variables such as HTLV-1 proviral load, the concordance of HLA class I type between mother and child, and the duration of breastfeeding (5). Mother-to-child transmission during the intrauterine period or peripartum has been reported to occur in fewer than 5% of cases (26).
Similarly to other sexually transmitted infections, sexual transmission of HTLV-1 is associated with unprotected sex, multiple sexual partners, lifetime contact with an HTLV-1-infected partner, the presence of genital sores or ulcers, and paying or receiving money for sex (65).
Intravenous exposure to blood is the most efficient mode of HTLV-1 transmission. In the past, this occurred mainly through the transfusion of blood not tested for HTLV-1. Most epidemiological studies of HTLV-1 reported transfusion as an important risk factor for HTLV-1 seropositivity (65). The highest risk is associated with the transfusion of packed red cells (92). Plasma products and cold storage of blood lower the risk of transmission, presumably due to the death of HTLV-1-infected lymphocytes (22). The results from hemovigilance and look-back studies presented additional evidence correlating the transmission of HTLV with cellular blood components (70).
The route of infection has been shown to be related to the development of specific diseases associated with HTLV-1. Adult T-cell leukemia/lymphoma (ATL) has been associated with breastfeeding (26, 98), and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) has been associated with blood transfusion (79). Cases of posttransfusion ATL are exceptional.
GEOGRAPHIC DISTRIBUTION
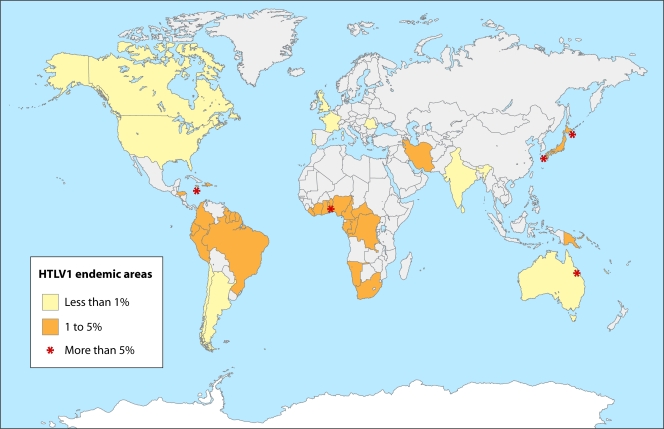
The geographic distribution of the virus has been studied in the almost 30 years since its initial description, with Japan, Africa, the Caribbean islands, and Central and South America emerging as the areas of highest prevalence in the world (84). When interpreting and comparing data from international prevalence studies, caution must be taken in the population selection criteria, because any difference in the diagnostic strategies can interfere with the final result. The estimation of the global prevalence of HTLV-1 is based mainly on the serological screening of healthy blood donors, which might underestimate the prevalence in the population. The geographic distribution of HTLV-1 infection is shown in Fig. 1. The HTLV-1 prevalence rates have been stratified into high (more than 5% of the population tested), middle (5% to 1%), and low (less than 1%) prevalences (84). The geographic distribution of HTLV-1 infection has intriguing aspects. Areas of extremely high prevalence, clusters of HTLV-1, are surrounded by areas of middle or low prevalence. The clusters predominate in a same-latitude trend (Fig. 1). Phylogeny and molecular epidemiology studies have been used to explain this behavior of the infection (24).
FIG. 1.
Geographic distribution of HTLV-1 in countries where the disease is endemic. The stars emphasize high-prevalence areas. The country boundaries shown in the map are not coincidental with the areas of endemicity, reflecting the cluster nature of HTLV infection. (Adapted from reference 84.)
Japan is the most important area where HTLV-1 is endemic. The estimated prevalence in the general population varies from areas in which the virus is not found to selected areas with seroprevalences of up to 37%, such as the southwestern isles of Shikoku, Kyushu, and Okinawa (103). High rates of HTLV-1 infection have been reported for some Caribbean islands in studies of blood donors or segments of the general population. In Jamaica, the prevalence is around 5% (64). In Africa, the seroprevalence increases from the north to the south, varying from 0.6% in Morocco to greater than 5% in several sub-Saharan African countries, for example, Benin, Cameroon, and Guinea-Bissau (23), but more studies of the region are needed. In Europe and North America, the prevalence is low and limited to groups that emigrated from areas of endemicity (64). For blood donors, very low rates were found in France (0.0039%) (17) and the United States (0.025%) (64). In South America, the virus was found in all countries, but more studies of the general population are needed to ascertain the real prevalence of HTLV-1. Medium prevalence was found in blood donors from Chile (0.73%) and Argentina (0.07%) (99). In Brazil, Colombia, and Peru, the prevalences vary considerably according to the area and have been correlated with latitude and altitude in some countries. In Brazil, the highest prevalence was described for the central area and the coast (1.35%), with low prevalences in the north and south (0.08%) (28). In Colombia, a prevalence of 4.3% was reported for the low-altitude areas and a prevalence of 0.73% was reported for the high-altitude areas (50). In Australia, even though the prevalence in blood donors is low, a cluster among Aborigines in the Northern Territory was described, with a prevalence of 14% (4).
SCREENING TESTS AND RESIDUAL RISK OF TRANSFUSION-TRANSMITTED HTLV-1
HTLV-1 screening tests are usually an enzyme-linked immunoassay (EIA) or particle agglutination (PA) assays. EIAs combine testing for HTLV-1 and HTLV-2, while PA assays test only for HTLV-1. Therefore, the chosen technique takes into account the type of retrovirus found in the geographic area. For example, in Japan, where HTLV-2 is not usually found, PA assays are preferred. In Brazil, where both HTLV-1 and HTLV-2 are prevalent, an EIA is used (70). The confirmation is done by testing of the blood with another method that also discriminates between HTLV-1 and HTLV-2. The most commonly used tests are Western blotting (WB), immunofluorescence assay (IFA), or radioimmunoprecipitation assay (RIPA). Indeterminate results of the confirmatory test may occur. The most commonly reported reasons for indeterminate results are the window period, the presence of a viral variant, and an unspecific reaction of the patient's serum to viral antigens (70). PCR, which detects HTLV-1 proviral DNA, has been used for clarifying indeterminate results and also as a confirmatory test. When combined with proviral DNA sequencing or restriction fragment length polymorphism (RFLP) analysis, PCR may also be used for the subtyping of the virus (84). The exclusion of seropositive individuals from the pool of blood donors has resulted in a reduction in the numbers of recipients of contaminated transfusions and in the number of new infections in the overall population (79).
A program to prevent the transmission of HTLV-1 by blood transfusion through the implementation of anti-HTLV-1 screening of donated blood by the PA method was instituted in Japan in 1986 (34). Consecutively, the Centers for Disease Control and Prevention recommended anti-HTLV-1 screening in the United States in 1988 (13). In Canada, the Caribbean, and the French Isles, anti-HTLV-1 screening started in 1989 (15). Continental France started screening in 1991 (17). In Brazil and Australia, blood screening for HTLV-1 was initiated in 1993 (70, 88). In Demark, screening was started in 1994; Portugal and Greece followed soon afterwards (17). In areas of low prevalence, questions remain concerning the cost-benefit of performing blood donor screening for HTLV. In 1995, Sweden decided to screen only the first blood donation for anti-HTLV-1 (97) due to the almost nonexistent local transmission of the virus. Wales and Scotland decided to use minipools (mixture of plasma from blood donors) in 2002 (19).
The risk of HTLV-1 transmission by transfusion varies with the prevalence of this virus in the general population as well as in blood donors. The time interval before seroconversion is also another important variable that interferes with the calculation of the residual risk of transmission. In the case of transfusion-transmitted HTLV-1, the window period usually varies between 41 and 65 days but could be longer (74). The type of blood product is another variable that influences transmission. Plasma and industrial blood products (albumin, immunoglobulin, and antihemophilic factors) do not transmit HTLV-1 (22). Transmission related to packed red cells and platelets varies according to the period of storage, being the greatest in the first 14 days (92).
The residual risk of transfusion-transmitted HTLV-1 in low-prevalence countries is minimal. In the United States, the estimated risk was 1 out of 921,000 transfusions between 1999 and 2004 (90). In France, the estimated risk was 1 out of 345,000 transfusions between 1996 and 1998 (17). in Canada and Australia between 2000 and 2003, the estimated residual risk was 1 in 1,000,000 (88). In these countries, look-back studies of HTLV-1 and -2 had a very low yield, and their public health benefit is questionable. Conversely, in areas where HTLV-1 is endemic, the residual risk is greater, and the importance of look-back studies and continued monitoring through hemovigilance should be considered due to the higher transmission rates and, therefore, seroconversions in repeat blood donors (10, 70).
IMMUNOPATHOGENESIS
After transmission, reverse transcriptase generates proviral DNA from genomic viral RNA, and the provirus is integrated into the host genome by viral integrase. Afterwards, HTLV-1 infection is thought to spread only through dividing cells, with minimal particle production. Therefore, the quantification of provirus reflects the number of HTLV-1-infected cells, which defines the proviral load. In this regard, an increase in numbers of HTLV-1-infected cells using cell division, by actions of accessory viral genes, especially tax, may provide an enhancement of infectivity (94). tax expression induces proliferation, inhibits the apoptosis of HTLV-1-infected cells and, conversely, evokes the host immune response, including cytotoxic T cells, to kill virus-infected cells.
In ATL, the tax gene plays a central role in the proliferation and transformation of HTLV-1-infected cells in vivo (54). Another gene recently described, the HTLV-1 bZIP factor (HBZ), uniformly expressed in ATL cells, seems to have a more important functional role in cellular transformation and leukemogenesis than does tax (55). HBZ transcription seems to be correlated with provirus load and also with the severity of HAM/TSP (86).
In HAM/TSP, a proinflammatory microenvironment is the hallmark of the immunological profile (31). The cytokine pattern in the peripheral blood from HTVL-1-infected individuals has been evaluated by using in vitro short-term leukocyte incubation and was shown to present distinct clinical grades. It was observed that HAM/TSP individuals display a high frequency of tumor necrosis factor alpha-positive (TNF-α+) neutrophils and an increased frequency of CD8+ gamma interferon-positive (IFN-γ+) and CD8+ TNF-α+ cells, confirming the inflammatory microenvironment related to HAM/TSP progression (9).
HTLV-1-ASSOCIATED DISEASES
The association of HTLV-1 infection with ATL, HAM/TSP, and HTLV-associated uveitis (HAU) has been established (84).
HTLV-1 infection causes subclinical immune suppression that can result in an elevated rate of opportunistic coinfections such as tuberculosis and strongyloidiasis, among others (3). Previous studies have described that arthritis, urinary tract disorders, fibromyalgia, and major depression are frequently found in HTLV-1-infected populations (18, 63, 77, 91). Possibly, some of these diseases either share a pathological mechanism with HTLV-1-related diseases or are related to psychological distress due to the infection. Table 1 summarizes the most common clinical entities associated with HTLV-1.
TABLE 1.
Correlation between HTLV-1-associated diseases and the most common reported clinical manifestations
| Common association(s) (reference[s]) | Manifestation in HTLV-associated disease |
|||
|---|---|---|---|---|
| HAM/TSP | ATL | HAU | Asymptomatic carrier | |
| Neurological (2) | Polyneuropathy | HAM/TSP | Polineuropathy | |
| Dermatological (6, 7, 8, 29, 45, 51, 60, 71, 76) | Infective dermatitis, acquired Ichthyosis | Infective dermatitis, crusted scabies | Acquired ichthyosis, dermatophytosis | |
| Ophthalmological (59, 61, 69, 81, 82) | Uveitisa | Uveitis, conjunctivitis sicca | ||
| Urological (77) | Urinary incontinency, sexual disturbances | |||
| Rheumatologic (18, 35, 37, 40, 56, 57, 82) | Polymyositis, thyroiditis, Sjögren's syndrome, artropathy | Sjögren's syndrome | Polymyositis, fibromyalgia | |
| Pulmonary (40) | Pneumopathy | |||
| Psychiatric (91) | Depression | Depression | ||
| Infections (7, 8) | Strongyloides stercoralis, crusted scabies, tuberculosis, leprosy | |||
See reference 58.
Adult T-Cell Leukemia/Lymphoma
ATL is an aggressive lymphoproliferative malignancy of peripheral T cells, with short survival in its acute form and an incidence of less than 5% in HTLV-1-infected people (89). It was initially described in Japan and later in the Caribbean region and South America (98). In Europe and the United States, ATL was diagnosed in immigrants from regions of endemicity. Its occurrence is associated with vertical transmission through breastfeeding (26).
ATL occurs more commonly in adults at least 20 to 30 years after the onset of HTLV-1 infection and is more common in males, and individuals infected in childhood may be at a higher risk of developing ATL (80, 89). In Japan, the fifth decade of life is predominant for the occurrence of ATL (89), and the occurrence of ATL in the fourth decade predominates in Brazil and in Jamaica (84). Possibly, local factors play a role in disease pathogenesis.
Classification.
ATL is a heterogeneous disease clinically divided into four subtypes (89). The diagnostic criteria are described in Table 2. The diagnosis of ATL requires the detection of ATL cells in peripheral blood of patients with the acute, chronic, or smoldering type with leukemic manifestations. Typical ATL cells have convoluted nuclei with homogeneous and condensed chromatin, small or absent nucleoli, and agranular and basophilic cytoplasm. These cells are called flower cells and are considered characteristic of ATL (96). Analysis of CD3, CD4, CD7, CD8, and CD25 is required for an immunophenotypic diagnosis. In the case of the lymphoma subtype, an excisional biopsy of the lymph node is recommended. Samples should be obtained both for histopathological examination concerning malignancy and for molecular analyses in order to evaluate provirus integration into the tissue (96).
TABLE 2.
Adult T-cell leukemia/lymphoma subtypes and diagnosis criteria
| Parametera | Criterion for ATL subtype |
|||
|---|---|---|---|---|
| Smoldering | Chronic | Lymphoma | Acute | |
| Lymphocyte count (109 lymphocytes/liter) | Less than 4 | 4 or mored | Less than 4 | More than 4 |
| % atypical lymphocytes | 5 or more | 5 or more | 1 or less | More than 5 |
| LDH level | 1.5× normal upper limit | 2× normal upper limit | —b | —b |
| Calcium level (mmol/liter) | Less than 2.74 | less than 2.74 | —b | More than 2.74b |
| Presence of: | ||||
| Lymphadenopathy | No | No | Atypical lymphocyte in histological analysis | —b |
| Skin lesions | —c | —b | —b | —b |
| Pulmonary lesions | —c | —b | —b | —b |
| Liver lesions | No | —b | —b | —b |
| Spleen lesions | No | —b | —b | —b |
| CNS lesions | No | No | —b | —b |
| Bone lesions | No | No | —b | —b |
| Ascites | No | No | —b | —b |
| Pleural effusion | No | No | —b | —b |
| Gastrointestinal tract lesions | No | No | —b | —b |
See reference 89.
Not essential.
Not essential; cases of atypical lymphocytes are fewer than 5%; other items have to be completed, and histological analysis of a lesion has to confirm malignancy.
Lymphocytosis, T-cell count of 3.5 × 109 cells/liter or more.
The rate of survival varies depending on the subtype: 4 to 6 months for the acute type, 9 to 10 months for the lymphomatous type, 17 to 24 months for the chronic type, and 34 months to more than 5 years for the smoldering type (89).
Clinical characteristics and diagnosis.
In the most aggressive forms (acute and lymphomatous types), half of the patients show adynamia; lymphoadenomegalia; hepatosplenomegalia; skin, bone, and multiple visceral lesions or pulmonary infiltration; and hypercalcemia (Table 1).
In the lymphomatous form, superficial or deep lymph node chains are involved.
In the smoldering or chronic form, the symptoms are unspecific; there is no tumor mass involvement, and the skin alterations are predominant, with papulae, plaques, tumor, or long-time erythroderma (89). Strongyloidiasis is frequently associated with all forms (96).
The diagnostic criteria for ATL include the detection of antibodies against HTLV-1 in the peripheral blood of a patient with T-cell lymphoma or leukemia, the presence of hypercalcemia, and, in cases of tumor, monoclonal insertion of HTLV-1 proviral DNA into the tumor cells (Table 1). Skin lesions, high leukocyte counts, and CD25+ cells may be present or absent. When the diagnosis of ATL is not done by peripheral blood examination or when a new lesion appears during the monitoring of indolent ATL, biopsy of suspicious lesions should be considered. Frequently involved tissues include lymph nodes, skin, liver, spleen, lung, gastrointestinal tract, bone marrow, bone, and the central nervous system (CNS) (96).
Treatment and prognosis.
Despite advances in the support and development of novel treatment agents, the prognosis for ATL remains poor. The variety of therapeutic approaches tested over the past 2 decades is immense. Patients with aggressive ATL have a poor prognosis because of the multidrug resistance of malignant cells, a large tumor burden with multiorgan failure, hypercalcemia, and/or frequent infectious complications as a result of a profound T-cell immunodeficiency (94). A combination of arsenic trioxide, zidovudine, and alpha interferon achieved an impressive remission rate with moderate toxicity (38). The treatment strategy has to be based on the clinical subclassification of ATL and prognostic factors, including watchful expectant approach, chemotherapy, antiviral therapy, and allogeneic hematopoietic stem cell transplantation (94, 96). The major prognostic factors are advanced performance status, high calcium or lactic dehydrogenase (LDH) levels, age of more than 40 years, and more than three involved lesions. Bone marrow involvement is an independent poor prognostic factor for ATL (96). Previous reports have suggested that high doses of corticosteroids in patients with smoldering ATL without concomitant antiretroviral therapy might increase the risk of acute ATL (30).
HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis
HAM/TSP is a chronic meningomyelitis of the gray and white matter in the spinal cord, with perivascular demyelination and axonal degeneration (16). Patients will develop a slowly progressive spastic paraparesis, without remissions, with high impairment of gait, autonomic dysfunction of bladder and bowel, and profound repercussions on their abilities and quality of life. Women are affected more frequently than men, and the disease onset occurs in adulthood at an average age of 40 years (78).
The lesion in the CNS caused by HTLV-1 is associated with dense infiltrates of mononuclear cells, largely the CD8+ lymphocyte response (3). Thus, HAM/TSP is considered an inflammatory disease in which cellular damage leads to demyelination (3, 31). Early in the disease, leptomeninges, blood vessels, and parenchyma are infiltrated by CD4+ and CD8 lymphocytes, B lymphocytes, and foamy macrophages. In the chronic phase, the cord infiltrate consists predominantly of CD8+ lymphocytes. The entire spinal cord can be affected, although the lower thoracic level is predominantly affected (20).
Clinical characteristics and diagnosis.
The first symptoms are weakness of the lower limbs and lumbar pain, although the initial complaint can be sensory, such as tingling, burning, or pins and needles. In many patients, urinary and sexual problems can be the first symptoms (20, 78). Dizziness is common in the early phase, preceding abnormalities upon a neurological exam, and would be related to a functional disturbance in the vestibule-spinal and motor tracts (25).
The weakness in the lower limbs is associated with moderate to severe spasticity, Babinski's sign, and hyperreflexia. The sensory impairment is mild, the vibratory sense is frequently impaired, and the proprioception is less affected. In the upper limbs, there is also hyperreflexia, usually without muscle weakness. With disease progression, the weakness and the spasticity increase, and the gait becomes worse (66). Urinary and sexual dysfunction are common (77).
Spasticity is a serious problem. It occurs due to a velocity-dependent increase in the muscle tone in response to passive movement and plays a major role in the progression of disability (39). When the spinal cord is deprived of supraspinal control, the motor neuron activity is manifested as muscular spasms. The elevated muscle tone can cause pain, joint contractures, and impaired function. No treatment for spasticity is considered to be effective. Pharmacological management is used to alleviate symptoms (baclofen, diazepam, and tizanidine, etc.). With the evolution of HAM/TSP, the patient goes from a domiciliary walk to a wheelchair (39).
This spinal cord syndrome in HTLV-1 infection affects the autonomic function of the bladder and bowel (14, 63). The patients usually experience frequency, urgency, or urge-incontinence and an elevated postvoid urinary residue (77). The voiding dysfunction, according to the usual level of injury and urodynamic parameters, is an upper motor neuron lesion, with hyperreflexic bladder. There is an overactivity of the detrusor muscle and a dyssynergy of the bladder-sphincter, resulting in an impaired communication between the sacral and the brainstem micturition centers (63, 77).
Urinary tract infections are common and are complicated by lithiasis, vesicoureteral reflux, chronic pyelonephritis, and chronic renal failure. The best bladder management is intermittent cleaning catheterization associated with an anticholinergic drug for the detrusor hyperactivity and an antispastic muscle agent for the external sphincter dysfunction (12, 77).
Constipation is a very common bowel dysfunction. An effective bowel management regimen must take into consideration diet, adequate and timely fluid intake, and pharmacological agents in case of necessity (14).
Neuropathic pain is common in the advanced stage of myelopathy. Dysesthesia is chronic and debilitating pain. It can be affected by the weather, stress, anxiety, or visceral or musculoskeletal stimulus. For many patients, the urinary incontinence or the lumbar pain may be more disabling than the leg weakness (48).
The diagnosis requires the demonstration of HTLV-1 infection and the exclusion of other causes of myelopathy. The accuracy of the diagnosis has improved with neurophysiological tests (1, 25) and with magnetic resonance imaging (MRI) (41). In the early stages of the disease, the neuroimaging of the spinal cord does not define myelopathy, although patients have signals and symptoms of upper motor neuron disease (62). In this phase, functional abnormalities of the spinal cord can be demonstrated through neurophysiological tests (25). The strategies utilized to reach an early and precise diagnosis have epidemiological and therapeutic implications, allowing better counseling and treatment options.
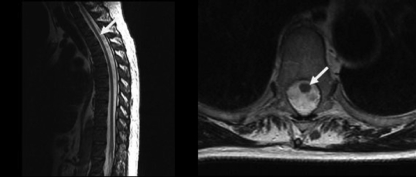
HAM/TSP has a subclinical phase in which the inflammatory lesions are already present but the infected patient presents mild or no neurological abnormalities (16, 25, 46, 62). Vestibular evoked myogenic potentials have been used for testing the vestibule-spinal motor tract and have shown abnormal responses in HTLV-1 carriers with a functional spine otherwise considered asymptomatic (25). In the advanced stage of HAM/TSP, spinal cord atrophy, particularly of the thoracic level, has been reported (Fig. 2).
FIG. 2.
MRIs showing thoracic cord atrophy (white arrow) in a patient with HAM/TSP.
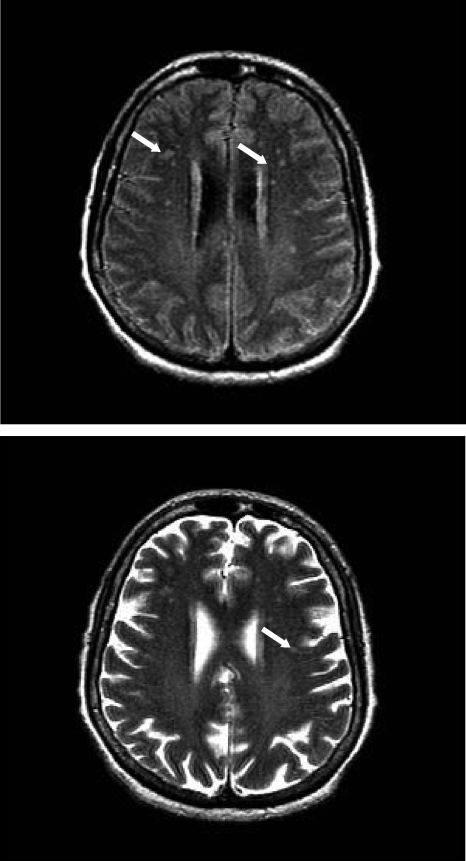
Brain MRI shows periventricular and subcortical white matter alterations. The high signal intensity and the contrast enhancement are located mainly in the posterior columns, posterior horns, or lateral columns at the cervical or thoracic levels (41, 62). The white matter lesions can be indistinguishable from those of other demyelinating diseases (Fig. 3) (62).
FIG. 3.
Brain MRI performed with 1.5-T equipment (sagittal T2 weighted; axial FLAIR, T1- and T2-weighted images). (Top) T1-weighted images following venous paramagnetic contrast at three planes. (Bottom) T2-weighted MRI of the brain reveals punctate nodular discrete hyperintense foci without a mass effect in the periventricular and subcortical white matter. These lesions do not increase.
Examination of the cerebrospinal fluid (CSF) may show mild lymphocytic pleocytosis, moderate increases in levels of protein, oligoclonal bands, and a high local synthesis of HTLV-1 antibodies (33, 47). The HTLV-1 provirus can be found in CSF cells of HAM/TSP patients. The increase in the proviral load in this fluid has been associated with the progression of myelopathy (47).
Neurogenic bladder is common in patients with HAM/TSP, and ultrasound (US) and video-urodynamic studies are the usual methods to define the diagnosis (63). Sonographic evaluation of the urinary system allows the study of the kidneys, the lower urinary tract, and the bladder. Many kidney disturbances can occur due to HAM/TSP neurogenic bladder. US evaluation determines the renal size and parenchyma detail, enlargement of the ureters, hydronephrosis, and kidney stones. Neurogenic bladder leads to incomplete emptying and chronic inflammation of the bladder. Stone formation and acquired diverticula are possible complications (87).
The study of urodynamics is useful for establishing the bladder pattern and for choosing the best therapeutic approach. This evaluation identifies the storage pressures, the residual volume, and voiding dysfunctions, which are important for the planning of treatment strategies. Cystoscopy is a method that complements bladder evaluation, since it allows a direct view of its contents and mucosa aspect (87).
Treatment and prognosis.
The individual immune response and proviral load define the prognosis, since there is no curative treatment. Corticosteroids, IFN-α, and IFN-β1a have shown limited results (36, 67, 73). The combination of two nucleoside analogues (zidovudine and lamivudine) was evaluated in a randomized, double-blind, placebo-controlled study, and no clinical improvement was observed (95). However, in that work, the patients had advanced myelopathy, which could be considered a limitation of the treatment response.
An increase in the proviral load was observed for HAM/TSP patients undergoing corticosteroid treatment (93). Conversely, antiretroviral drugs caused a decrease in the load (95). These observations indicated that the proviral load in the blood may be of value to monitor disease activity in therapeutic trials (72). The proviral load in the CSF may be a more accurate biomarker of HAM/TSP progression than the load in the blood (31). More studies involving randomized controlled therapeutic trials may clarify the value of the proviral load to define disease control.
HTLV-1-Associated Uveitis
Ophthalmological disturbances related to HTLV-1 include vasculitis, exudation or degeneration of the peripheral retina, and keratoconjunctivitis sicca (61, 81), and HAU is an accepted entity associated with this viral infection (61, 69). Middle-aged HTLV-1-infected adults of both genders are the target population. HAU is characterized by a granulomatous or nongranulomatous reaction accompanied by vitreous opacities and retinal vasculitis with rare exudative retinochoroidal alterations in one or both eyes (61). It can occur as the only HTLV-1 manifestation or can be associated with HAM/TSP. Uveitis usually occurs as an isolated ophthalmological alteration (69). Qualitative lachrymal film defects have been shown to be frequent (81). Sicca syndrome associated with HTLV-1 differs from this ocular manifestation in primary or secondary Sjögren's syndrome, because it does not reveal any of the immunological alteration related to a rheumatologic disease.
Clinical characteristics and diagnosis.
Characteristic symptoms have been described as “flying flies” and visual blurring of acute or subacute installation (61, 69). In patients with HAM/TSP, uveitis was more frequent among those with an earlier onset of HAM/TSP and in patients with severe motor disability (58). HAU has clinical characteristics that can vary according to geographic area. In the Caribbean and Brazil, the corneal disease observed for HTLV-1-infected patients was not described for Japanese patients (58, 59, 61, 81).
Treatment and prognosis.
The treatment of uveitis is based on topical and systemic corticosteroids. The response to therapy is satisfactory, as is the visual prognosis, although recurrence is common. Lubricating medication is the most important aid to treat mild and moderate keratoconjunctivitis sicca. Lubricating drops are used to reduce morbidity and to prevent ocular complications such as corneal ulcers.
Concerning prognosis, the immune system of patients with HAU has abnormalities similar to those seen for patients with HAM/TSP, such as elevated proviral load, the overproduction of proinflammatory cytokines, and the presence of HTLV-1 provirus in the spinal fluid (42, 75, 93). A significant elevation in levels of CD4+ HLA-DR+ and CD8+ HLA-DR+ cells and ocular abnormalities were demonstrated for patients with advanced HAM/TSP (81). Moreover, among asymptomatic HTLV-1 carriers, individuals with HAU presented immunological disturbances similar to those for HAM/TSP, and asymptomatic carriers without HAU presented a normal immunological profile (81). These results suggest the HAU can be either an independent inflammatory disease associated with HTLV-1 or, perhaps, a predictor of HAM/TSP.
Dermatological Conditions Associated with HTLV-1
Dermatological lesions are commonly associated with HTLV-1. Their importance is a special topic because cutaneous involvement in an apparently asymptomatic carrier has been considered a premonitory indication of the future development of either ATL or HAM/TSP.
Infective dermatitis (ID) and crusted scabies have been associated with HTLV-1 seropositivity in regions of endemicity and have also been reported for patients with ATL and HAM/TSP (8, 44). Cutaneous lesions in asymptomatic HTLV-1 carriers have also been found (29).
ID.
ID was the first pediatric syndrome associated with HTLV-1 infection (44). It is characterized by a severe and relapsing eczematous disease with lesions involving the scalp, neck, external ear, axillae, and groin (44, 76). Fluid nasal discharge and crusts in the nostrils are common, as is blepharoconjunctivitis. Follicular papules and pustules on affected areas were noticed. Positive cultures for Staphylococcus aureus and beta-hemolytic Streptococcus were found for the nostrils or skin (44, 76). An adequate response to systemic antibiotics and topical steroids is usually obtained, although relapse is common (44). Long-term use of antibiotics has been recommended.
Differential diagnosis with other eczematous diseases is mandatory, since histological characteristics are not distinctive. Emphasis should be given to the differential diagnosis with atopic and seborrheic dermatitis. In atopic dermatitis, the distribution and morphological aspects of the lesions are similar to those seen for ID, in which the infection is more evident, with exudation and fetid odor (43). Conversely, in seborrheic dermatitis, the lesions are erythematous and scaly. It occurs predominantly during childhood (76). Laboratory parameters such as the erythrocyte sedimentation rate, immunoglobulin level, CD4 and CD8 cell counts, and CD4/CD8 ratio, when elevated, must be considered when evaluating a patient with suspected ID, along with positivity for HTLV-1 (43).
ID cases occur mainly in tropical regions where HTLV-1 is endemic, such as Jamaica, and few cases in Japan have been described (76). This HTLV-1-associated dermatological lesion has been correlated with vertical transmission and long-term breastfeeding (44, 76). ID was found to be related to ATL and HAM/TSP (51, 71, 76). In an evaluation of severe and relapsing eczematous conditions observed for children in areas where HTLV-1 is endemic, a serological test for this virus should be considered (76).
CS.
Crusted scabies (CS) is a severe variant of scabies, and the main characteristic is crusted lesions located over exposed areas, with millions of parasites. The link between CS and immunosuppression is well established (85). In Brazil, severe CS (more than 80% of the body area affected) was strongly associated with HTLV-1 infection and, to a lesser degree, with HIV infection (8). In Peru, the association was also shown (7). CS diagnosis can be done by scraping the lesion and obtaining a demonstration of numerous scabies mites. Treatment with oral ivermectin is recommended; relapses may occur. Special treatment protocols are recommended for severe cases (85).
Skin abnormalities related to ATL.
ATL is generally categorized into four forms: acute, chronic, smoldering, and lymphoma types (89, 96). In a Brazilian ATL series of cases, 67% of the individuals had cutaneous lesions (6).
The acute form comprises 55 to 75% of all cases of ATL (89). In chronic ATL, skin lesions can be related to inflammatory reactions, immunosuppression, or direct infiltration of the skin by neoplastic cells. Lesions observed for chronic and smoldering variants of ATL can be similar to those seen for mycosis fungoides patients. (6, 96, 100). Monoclonal integration of provirus DNA is mandatory as diagnostic proof of chronic and smoldering ATL, since the histopathological characteristics of both are similar to those seen for mycosis fungoides (6). Many types of skin lesions have been related to HTLV-1, such as macular, papular, nodular, and tumoral lesions, with erythema, desquamation, and erythroderma. The skin involvement in an otherwise asymptomatic individual carrying HTLV-1 may be an indicator of smoldering ATL (6, 30).
Skin abnormalities in HAM/TSP.
Acquired ichthyosis has been shown to be common in patients with HAM/TSP (60). The involvement of the autonomic nervous system was suggested to explain the pathophysiology, although direct damage of the skin by infected cells is another possibility (45). Keratinocyte activation, with the induction of alternative keratinization pathways probably depending on the cytokine liberation of infected cells situated in dermal infiltrates, was also proposed as a mechanism for acquired ichthyosis (60).
Other dermatological conditions described for HAM/TSP include candidiasis, palmar erythema, dermatophytosis, and folliculitis decalvans (45).
Skin abnormalities in asymptomatic HTLV-1 carriers.
A cross-sectional study of Brazilian candidates for blood donation demonstrated a predominance of skin disease in asymptomatic patients infected with HTLV-1 in comparison to seronegative cases. Diseases associated with HTLV-1 in that study were dermatophytic infections, seborrheic dermatitis, and acquired ichthyosis (29). In a Jamaican cohort study, the presence of atopic and seborrheic dermatitis was also associated with seropositivity for HTLV-1. Both conditions were already associated with hyperreflexia but without a definite HAM/TSP diagnosis (51).
OTHER HTLV-1-ASSOCIATED ABNORMALITIES
Opportunistic Infections
Opportunistic infections have been associated with acute ATL (96). HTLV-1-infected carriers seem to have an increased risk of acquiring strongyloidiasis, indicating a possible subclinical immune deficiency. HTLV-1-infected individuals from areas where Strongyloides stercoralis is highly endemic should probably undergo examination for stool ova and parasites, utilizing serial exams to increase sensitivity. Other infections associated with HTLV-1 in case reports include Norwegian scabies, disseminated molluscum contagiosum, and extrapulmonary histoplasmosis (8).
Rheumatic and Autoimmune Conditions
Inflammatory rheumatic conditions, including rheumatoid arthritis and Sjögren's syndrome, have been reported for HTLV-1-infected individuals, and increased proviral load has been associated with HTLV-1 infection (57, 82, 101).
Other autoimmune conditions associated with HTLV-1 include endemic polymyositis, bronchoalveolar pneumonitis, and autoimmune thyroiditis (35, 37, 40). In most of these reports, HTLV-1 proviral sequences can be readily detected, while viral antigen expression is low or absent in areas with extensive lymphocytic infiltration (68). It was suggested previously that restricted viral gene expression by antigen-presenting cells in the infected tissues triggers a vigorous CD4+ and CD8+ immune response against HTLV Tax or some other gene product (68). HTLV-1 was also associated with fibromyalgia in a case-control study with prevalent HTLV-1 cases (18).
Inclusion Body Myositis and Polymyositis
Muscle inflammation has been associated with HTLV-1 (56). It can be the single manifestation of the disease, or it can be associated with HAM/TSP. Patient complaints are myalgia and proximal muscle weakness. Laboratory tests show an elevated erythrocyte sedimentation rate and creatine kinase level. Muscle biopsy specimens show a myopathic pattern of inflammation.
Polyneuropathy
Peripheral neuropathies in HTLV-1-infected patients with and without HAM/TSP have been described (2). Polyneuropathy is usually asymmetric, sensory motor, axonal, or of a mixed type. The major complaints are hypoesthesia in a stock-and-glove distribution, paresthesias, and burning of the lower limbs. For this reason, HTLV infection should be investigated for individuals with polyneuropathies of unknown origin.
Electroneuromyography of these patients shows abnormalities in peripheral nerves that consist of minimal increases in the distal latencies with moderated slowing of the proximal waves and of the distal conduction velocities along the peroneal and sural nerves.
Psychiatric Disorders
Among asymptomatic HTLV-1 carriers, a high rate of depression was found compared to noninfected individuals. Depression could be related to the psychological impact of the incurable state or could be related to a biological effect of the retroviral infection (91).
PREVENTION
The prognosis for ATL and HAM/TSP is poor, and no vaccine is yet available. For HAM/TSP, a long-lasting, progressive disease, the financial cost for the individual, the family, and the health system is very high. In this sense, public health interventions such as counseling and education of high-risk individuals and populations are of paramount importance (10).
With the implementation of a program to prevent transfusion-transmitted HTLV-1 in Japan in 1986, many countries in areas where the disease is endemic started to implement systematic and permanent screening of all blood donors (79). Screening of blood donor candidates has been shown to be an effective strategy in preventing HTLV-1 transmission. For areas where the disease is not endemic, reports showed that the risk of HTLV-1 infection might be enhanced in some selected donor populations, recommending the implementation of policies for selective donor recruitment. However, more cost-effective strategies for blood donation screening need to be designed and evaluated for developing countries, given the high cost of imported test kits. Blood transfusion still represents a risk of HTLV-1 infection for recipients in most African countries as well as for other less developed areas that lack appropriate public policies and infrastructure of transfusion services (53, 84).
HTLV-1-seropositive individuals should be advised not to donate blood, semen, organs, or milk, where milk banks are available. Prevention of mother-to-child transmission would probably have the most significant impact on the occurrence of HTLV-1 infection and associated diseases.
Prenatal screening for HTLV-1 should be implemented in specific geographical areas, combined with counseling of seropositive mothers regarding transmission through breastfeeding. Avoidance of breastfeeding is fundamental, since it is the major form of vertical transmission of HTLV-1 (11). Due to the risk of malnutrition in developing countries, public health policies should consider this adverse effect in less developed countries and recommend an alternative feeding formula for children at risk of acquiring HTLV-1 infection through mother's milk (11, 52). The practice of cross-feeding should also be contraindicated due to the possibility of a “milk mother” being seropositive as well. In the case of pregnancy, a cesarean section should be recommended, to minimize the risk of perinatal transmission.
Recommendations to prevent sexually transmitted infections should be emphasized, including condom use and avoiding multiple and unknown sexual partners and paying or receiving money for sex. When one of the partners in a stable relationship is negative, the need for condom use should be emphasized.
Counseling and education of intravenous drug users (IDU) to implement harm reduction practices may be effective in preventing HTLV-1 infection in this population group.
Psychological and social problems such as depression, increased anxiety, difficulty in establishing and maintaining relationships, and fear or guilt about pregnancy are common in HTLV-1-infected individuals and should be dealt with accordingly. Access to correct information about HTLV infection is very important. Confusion with HIV is common, even in health care settings, and leads to unnecessary stress on the patient, which is frequently associated with self-destructive thoughts (32).
Counseling should include orientation about the transmission of the virus and the possible source of the infection and an offering of testing to the partner/spouse and children. This is especially true when counseling blood donor candidates, because they are usually young, asymptomatic, and of a reproductive age. In our experience, when counseled about pregnancy and the avoidance of breastfeeding, female donors are usually compliant, and therefore, transmission may be blocked.
CONCLUSION
HTLV-1 should be added to the list of diseases that are preventable with safe sex, thus being a further stimulus to the use of condoms and to the adoption of safe sexual behavior. The development of an effective and safe vaccine as well as preventive measures in blood banks and prenatal care settings in areas of endemicity should be emphasized (21).
Although the incidence of ATL and HAM/TSP is found to be relatively low among individuals with HTLV-1 infection, ranging from 5 to 10% during a person's lifetime, the diseases are generally severe and progressively incapacitating.
Therefore, the prevention of virus transmission is advantageous not only at the individual level but also in the public health setting as well. Despite this, considerable effort is continuously being done by researchers to prove to health officers in countries where the disease is endemic that HTLV-1 infection should be prevented, and it is usually a neglected disease, with the majority of patients worldwide ignoring the source of their illness.
Acknowledgments
We acknowledge the support of Fapemig and CNPq to the Interdisciplinary HTLV Research Group (GIPH).
A.B.F.C.-P. and F.A.P. participate in the REDS II International Donor Safety Study.
Biography
 Denise Utsch Gonçalves, MD, Ph.D., has been a member of the Interdisciplinary HTLV Research Group since 1997. She has a Ph.D. in Infectology and Tropical Medicine from the Federal University of Minas Gerais (2000). She studied the clinical manifestations of disease in deferred blood donors infected with HTLV-1, Minas Gerais, Brazil. She is currently a professor at the Federal University of Minas Gerais, since 2003, with the following areas of concentration: neuro-otology, HTLV-1, and HIV. She has coordinated the sector of vestibular disorders in the Hospital of Clinics, Federal University of Minas Gerais, since 2006. She participated in the committee of the Ministry of Health in 2008, which worked on the construction of the public health policies directed toward individuals infected with HTLV-1.
Denise Utsch Gonçalves, MD, Ph.D., has been a member of the Interdisciplinary HTLV Research Group since 1997. She has a Ph.D. in Infectology and Tropical Medicine from the Federal University of Minas Gerais (2000). She studied the clinical manifestations of disease in deferred blood donors infected with HTLV-1, Minas Gerais, Brazil. She is currently a professor at the Federal University of Minas Gerais, since 2003, with the following areas of concentration: neuro-otology, HTLV-1, and HIV. She has coordinated the sector of vestibular disorders in the Hospital of Clinics, Federal University of Minas Gerais, since 2006. She participated in the committee of the Ministry of Health in 2008, which worked on the construction of the public health policies directed toward individuals infected with HTLV-1.
 Fernando Augusto Proietti, MD, MPH, Sc.D., is an Associate Professor of Collective Health at the School of Medicine, Federal University of Minas Gerais (UFMG), in Brazil. He graduated with a degree in Medicine at UFMG and obtained a doctoral degree in Epidemiology at Johns Hopkins School of Hygiene and Public Health. He has been working for more than 20 years in the field of infectious diseases. He co-coordinates the Interdisciplinary HTLV Research Group and the Observatory on Urban Health in Belo Horizonte, Brazil. Dr. Proietti has authored/co-authored over 70 papers and/or book chapters in peer-reviewed publications. He has been the recipient of the Brazilian National Council for Scientific and Technological Development (CNPq) scholarship for over 10 years, for researching, teaching, and mentoring in the epidemiological field, and participates in the REDS II international blood donor project.
Fernando Augusto Proietti, MD, MPH, Sc.D., is an Associate Professor of Collective Health at the School of Medicine, Federal University of Minas Gerais (UFMG), in Brazil. He graduated with a degree in Medicine at UFMG and obtained a doctoral degree in Epidemiology at Johns Hopkins School of Hygiene and Public Health. He has been working for more than 20 years in the field of infectious diseases. He co-coordinates the Interdisciplinary HTLV Research Group and the Observatory on Urban Health in Belo Horizonte, Brazil. Dr. Proietti has authored/co-authored over 70 papers and/or book chapters in peer-reviewed publications. He has been the recipient of the Brazilian National Council for Scientific and Technological Development (CNPq) scholarship for over 10 years, for researching, teaching, and mentoring in the epidemiological field, and participates in the REDS II international blood donor project.
 João Gabriel Ramos Ribas, MD, graduated in Pharmacy in 1968 and in Medicine in 1973 at the Federal University of Minas Gerais (UFMG). He was trained in Internal Medicine (Medical Residence) and is a physician at the rehabilitation hospital belonging to the Sarah Network in Belo Horizonte, Minas Gerais, Brazil, where he monitors patients with nontraumatic myelopathy, including HAM/TSP. He is a member of the HTLV Interdisciplinary Research Group (GIPH) and has published diverse articles and book chapters on neurological syndromes related to HTLV-1 infection.
João Gabriel Ramos Ribas, MD, graduated in Pharmacy in 1968 and in Medicine in 1973 at the Federal University of Minas Gerais (UFMG). He was trained in Internal Medicine (Medical Residence) and is a physician at the rehabilitation hospital belonging to the Sarah Network in Belo Horizonte, Minas Gerais, Brazil, where he monitors patients with nontraumatic myelopathy, including HAM/TSP. He is a member of the HTLV Interdisciplinary Research Group (GIPH) and has published diverse articles and book chapters on neurological syndromes related to HTLV-1 infection.
 Marcelo Grossi Araújo, MD, MPH, is an assistant professor at the Federal University of Minas Gerais, Faculty of Medicine, Department of Internal Medicine, Minas Gerais, Brazil. He obtained his MD in dermatology, and was a postgraduate student at the Ph.D. level in the program CASA (Applied Sciences in Adult Health), Faculty of Medicine, Federal University of Minas Gerais. Dr. Araújo is a researcher on infectious disease with emphasis on leprosy and HTLV infection. He is a member of the HTLV Interdisciplinary Research Group (GIPH) and of the Brazilian Dermatology Society.
Marcelo Grossi Araújo, MD, MPH, is an assistant professor at the Federal University of Minas Gerais, Faculty of Medicine, Department of Internal Medicine, Minas Gerais, Brazil. He obtained his MD in dermatology, and was a postgraduate student at the Ph.D. level in the program CASA (Applied Sciences in Adult Health), Faculty of Medicine, Federal University of Minas Gerais. Dr. Araújo is a researcher on infectious disease with emphasis on leprosy and HTLV infection. He is a member of the HTLV Interdisciplinary Research Group (GIPH) and of the Brazilian Dermatology Society.
 Sônia Regina Pinheiro is an ophthalmologist who graduated in Medicine at the Medical School of the Federal University of Minas Gerais (Brazil) and has a Ph.D. from the Federal University of São Paulo (Brazil). She was a postdoctoral fellow at the School of Hygiene and Public Health of the Johns Hopkins University. She has a private clinic and has been a member of the Interdisciplinary HTLV Research Group (GIPH) since 1997.
Sônia Regina Pinheiro is an ophthalmologist who graduated in Medicine at the Medical School of the Federal University of Minas Gerais (Brazil) and has a Ph.D. from the Federal University of São Paulo (Brazil). She was a postdoctoral fellow at the School of Hygiene and Public Health of the Johns Hopkins University. She has a private clinic and has been a member of the Interdisciplinary HTLV Research Group (GIPH) since 1997.
 Antônio Carlos Guedes, MD, Ph.D., graduated in Medicine at the Federal University of Minas Gerais (1975), where he also obtained his master's degree in Medicine (Dermatology) in 1980. In 1979 he took a position as a medical resident in dermatology at the Federal University of São Paulo, where he also obtained his Ph.D. in 1996. He is currently Assistant Professor of Medicine at the Federal University of Minas Gerais and a member of the Editorial Board of Brazilian Annals of Dermatology. He is a member of the HTLV Interdisciplinary Research Group (GIPH) and has published diverse articles and book chapters on dermatological aspects of HTLV infection.
Antônio Carlos Guedes, MD, Ph.D., graduated in Medicine at the Federal University of Minas Gerais (1975), where he also obtained his master's degree in Medicine (Dermatology) in 1980. In 1979 he took a position as a medical resident in dermatology at the Federal University of São Paulo, where he also obtained his Ph.D. in 1996. He is currently Assistant Professor of Medicine at the Federal University of Minas Gerais and a member of the Editorial Board of Brazilian Annals of Dermatology. He is a member of the HTLV Interdisciplinary Research Group (GIPH) and has published diverse articles and book chapters on dermatological aspects of HTLV infection.
 Anna Bárbara F. Carneiro-Proietti, MD, Ph.D., is a hematologist and virologist. She did her medical studies in Belo Horizonte, Brazil, where she graduated in Medicine in 1979 and finished medical residence in hematology in 1981. She did a postdoctoral fellowship in hematology at the Johns Hopkins Hospital in Baltimore, MD, and was trained in molecular virology at the School of Hygiene and Public Health, Johns Hopkins University, and at the National Institutes of Health in Bethesda, MD. She finished her Ph.D. in virology in 1997 and is the president of the Hemominas Foundation, the Minas Gerais State Center of Hematology and Blood Transfusion, since 1999, where she previously held the positions of Research Department Director and medical chief of the ambulatory. She participates in the REDS II international project, with studies on transfusion safety. She is the founder of and coordinates the Interdisciplinary HTLV Research Group (GIPH), which congregates more than 20 researchers of five institutions.
Anna Bárbara F. Carneiro-Proietti, MD, Ph.D., is a hematologist and virologist. She did her medical studies in Belo Horizonte, Brazil, where she graduated in Medicine in 1979 and finished medical residence in hematology in 1981. She did a postdoctoral fellowship in hematology at the Johns Hopkins Hospital in Baltimore, MD, and was trained in molecular virology at the School of Hygiene and Public Health, Johns Hopkins University, and at the National Institutes of Health in Bethesda, MD. She finished her Ph.D. in virology in 1997 and is the president of the Hemominas Foundation, the Minas Gerais State Center of Hematology and Blood Transfusion, since 1999, where she previously held the positions of Research Department Director and medical chief of the ambulatory. She participates in the REDS II international project, with studies on transfusion safety. She is the founder of and coordinates the Interdisciplinary HTLV Research Group (GIPH), which congregates more than 20 researchers of five institutions.
REFERENCES
- 1.Andrade, D. O. 2005. Somatosensitive and motor evoked potentials in HTLV-I associated myelopathy. Arq. Neuropsiquiatr. 63:652-655. [DOI] [PubMed] [Google Scholar]
- 2.Araujo, A. Q. C., and M. T. T. Silva. 2006. The HTLV-1 neurological complex. Lancet Neurol. 5:1068-1076. [DOI] [PubMed] [Google Scholar]
- 3.Bangham, C. R. M., and M. Osame. 2005. Cellular immune response to HTLV-1. Oncogene 24:6035-6046. [DOI] [PubMed] [Google Scholar]
- 4.Bastian, I., Y. Hinuma, and R. R. Doherty. 1993. HTLV-I among Northern Territory Aborigines. Med. J. Aust. 159:12-16. [DOI] [PubMed] [Google Scholar]
- 5.Biggar, R. J., J. Ng, N. Kim, M. Hisada, H. C. Li, B. Cranston, B. Hanchard, and E. M. Maloney. 2006. Human leukocyte antigen concordance and the transmission risk via breast-feeding of human T cell lymphotropic virus type I. J. Infect. Dis. 193:277-282. [DOI] [PubMed] [Google Scholar]
- 6.Bittencourt, A. L., and L. Farré. 2008. Leucemia/linfoma de células T do adulto. An. Bras. Dermatol. 83:351-359. [Google Scholar]
- 7.Blas, M., F. Bravo, W. Castillo, W. J. Castillo, R. Ballona, P. Navarro, J. Catacora, R. Cairampoma, and E. Gotuzzo. 2005. Norwegian scabies in Peru: the impact of human T cell lymphotropic virus type I infection. Am. J. Trop. Med. Hyg. 72:855-857. [PubMed] [Google Scholar]
- 8.Brites, C., M. Weyll, C. Pedroso, and R. Badaró. 2002. Severe and Norwegian scabies are strongly associated with retroviral (HIV-1/HTLV-1) infection in Bahia, Brazil. AIDS 16:1292-1293. [DOI] [PubMed] [Google Scholar]
- 9.Brito-Melo, G. E., V. Peruhype-Magalhães, A. Teixeira-Carvalho, E. F. Barbosa-Stancioli, A. B. Carneiro-Proietti, B. Catalan-Soares, J. G. Ribas, Grupo Interdisciplinar de Pesquisas sobre HTLV (GIPH), and O. A. Martins-Filho. 2007. IL-10 produced by CD4+ and CD8+ T cells emerge as a putative immunoregulatory mechanism to counterbalance the monocyte-derived TNF-alpha and guarantee asymptomatic clinical status during chronic HTLV-I infection. Clin. Exp. Immunol. 147:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carneiro-Proietti, A. B., B. C. Catalan-Soares, C. M. Castro-Costa, E. L. Murphy, E. C. Sabino, M. Hisada, B. Galvão-Castro, L. C. Alcantara, C. Remondegui, K. Verdonck, and F. A. Proietti. 2006. HTLV in the Americas: challenges and perspectives. Rev. Panam. Salud Publica 19:44-53. [DOI] [PubMed] [Google Scholar]
- 11.Carneiro-Proietti, A. B. F., B. C. Catalan-Soares, and F. A. Proietti. 2002. Human T-cell lymphotropic viruses (HTLV-I/II) in South America: should it be a public health concern? J. Biomed. Sci. 9:587-595. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Costa, C. M., A. Q. Araújo, M. Menna-Barreto, and A. C. Penalva-de-Oliveira. 2005. Guide of clinical management of HTLV patient: neurological aspects. Arq. Neuropsiquiatr. 63:548-551. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control (CDC). 1990. Human T-lymphotropic virus type I screening in volunteer blood donors—United States, 1989. MMBR Morb. Mortal. Wkly. Rep. 39:915, 921-924. [PubMed] [Google Scholar]
- 14.Chen, D., and S. B. Nussbaum. 2000. The gastrointestinal system and bowel management following spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 11:45-46. [PubMed] [Google Scholar]
- 15.Chiavetta, J. A., M. Escobar, A. Newman, Y. He, P. Driezen, S. Deeks, D. E. Hone, S. F. O'Brien, and G. Sher. 2003. Incidence and estimated rates of residual risk for HIV, hepatitis C, hepatitis B and human T-cell lymphotropic viruses in blood donors in Canada, 1990-2000. CMAJ 69:767-773. [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper, S. A., M. S. Van der Loeff, and G. P. Taylor. 2009. The neurology of HTLV-1 infection. Pract. Neurol. 9:16-26. [DOI] [PubMed] [Google Scholar]
- 17.Couroucé, A. M., J. Pillonel, J. M. Lemarie, and C. Saura. 1998. HTLV testing in blood transfusion. Vox Sang. 74:165-169. [DOI] [PubMed] [Google Scholar]
- 18.Cruz, B., B. Catalan-Soares, and F. A. Proietti. 2006. Higher prevalence of fibromyalgia in patients infected with human T cell lymphotropic virus type I. J. Rheumatol. 33:2300-2303. [PubMed] [Google Scholar]
- 19.Davidson, F., C. Lycett, L. M. Jarvis, D. Kerr, S. Lumley, J. Petrik, and B. C. Dow. 2006. Detection of HTLV-I and II in Scottish blood donor samples and archive donations. Vox Sang. 91:231-236. [DOI] [PubMed] [Google Scholar]
- 20.De Castro-Costa, C. M., A. Q. Araújo, M. M. Barreto, O. M. Takayanagui, M. P. Sohler, E. L. da Silva, S. M. de Paula, R. Ishak, J. G. Ribas, L. C. Rovirosa, H. Carton, E. Gotuzzo, W. W. Hall, S. Montano, E. L. Murphy, J. Oger, C. Remondegui, and G. P. Taylor. 2006. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res. Hum. Retroviruses 22:931-935. [DOI] [PubMed] [Google Scholar]
- 21.de Thé, G., and M. Kazanji. 1996. An HTLV-I/II vaccine: from animal models to clinical trials? J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:191-198. [DOI] [PubMed] [Google Scholar]
- 22.Donegan, E., M. P. Busch, J. A. Galleshaw, G. M. Shaw, and J. W. Mosley. 1990. Transfusion of blood components from a donor with human T-lymphotropic virus type II (HTLV-II) infection. The Transfusion Safety Study Group. Ann. Intern. Med. 113:555-556. [DOI] [PubMed] [Google Scholar]
- 23.Dumas, M., D. Houinato, M. Verdier, T. Zohoun, R. Josse, J. Bonis, I. Zohoun, A. Massougbodji, and F. Denis. 1991. Seroepidemiology of human T-cell lymphotropic virus type I/II in Benin (West Africa). AIDS Res. Hum. Retroviruses 7:447-451. [DOI] [PubMed] [Google Scholar]
- 24.Ehrlich, G. D., J. Andrews, M. P. Sherman, S. J. Greenberg, and B. J. Poiesz. 1992. DNA sequence analysis of the gene encoding the HTLV-I p21e transmembrane protein reveals inter- and intraisolate genetic heterogeneity. Virology 186:619-627. [DOI] [PubMed] [Google Scholar]
- 25.Felipe, L., D. U. Gonçalves, M. A. Santos, F. A. Proietti, J. G. Ribas, A. B. Carneiro-Proietti, and J. R. Lambertucci. 2008. Vestibular-evoked myogenic potential (VEMP) to evaluate cervical myelopathy in human T-cell lymphotropic virus type I infection. Spine 33:1180-1184. [DOI] [PubMed] [Google Scholar]
- 26.Fujito T, and Y. Nagata. 2000. HTLV-I transmission from mother to child. J. Reprod. Immunol. 47:197-206. [DOI] [PubMed] [Google Scholar]
- 27.Gallo, R. C. 2005. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene 24:5626-5930. [DOI] [PubMed] [Google Scholar]
- 28.Galvão-Castro, B., L. Loures, L. G. Rodriques, A. Sereno, O. C. Ferreira, Jr., L. G. Franco, M. Muller, D. A. Sampaio, A. Santana, L. M. Passos, and F. Proietti. 1997. Distribution of human T-lymphotropic virus type I among blood donors: a nationwide Brazilian study. Transfusion 37:242-243. [DOI] [PubMed] [Google Scholar]
- 29.Gonçalves, D. U., A. C. M. Guedes, A. B. F. Carneiro-Proietti, M. L. Martins, F. A. Proietti, J. R. Lambertucci, and the Interdisciplinary HTLV-1/2 Research Group. 2003. Dermatologic lesions in asymptomatic blood donors seropositive for human T cell lymphotropic virus type-1. Am. J. Trop. Med. Hyg. 68:562-565. [DOI] [PubMed] [Google Scholar]
- 30.Gonçalves, D. U., A. C. Guedes, A. B. F. Carneiro-Proietti, S. R. Pinheiro, B. C. Catalan-Soares, F. A. Proietti, and GIPH. 1999. Simultaneous occurrence of HTLV-I associated myelopathy, uveitis and smouldering adult T-cell leukaemia. Int. J. STD AIDS 10:336-337. [DOI] [PubMed] [Google Scholar]
- 31.Gonçalves, D. U., F. A. Proietti, E. F. Barbosa-Stancioli, M. L. Martins, J. G. Ribas, O. A. Martins-Filho, A. Teixeira-Carvalho, V. Peruhype-Magalhães, and A. B. Carneiro-Proietti. 2008. HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) inflammatory network. Inflamm. Allergy Drug Targets 7:98-107. [DOI] [PubMed] [Google Scholar]
- 32.Guiltinan, A. M., E. L. Murphy, J. A. Horton, C. C. Nass, R. L. McEntire, and K. Watanabe. 1998. Psychological distress in blood donors notified of HTLV-I/II infection. Retrovirus Epidemiology Donor Study. Transfusion 38:1056-1062. [DOI] [PubMed] [Google Scholar]
- 33.Ijichi, S., N. Eiraku, M. Osame, S. Izumo, R. Kubota, I. Maruyama, M. Matsumoto, T. Niimura, and S. Sonoda. 1989. Activated T lymphocytes in cerebrospinal fluid of patients with HTLV-I associated myelopathy (HAM/TSP). J. Neuroimmunol. 25:251-254. [DOI] [PubMed] [Google Scholar]
- 34.Inaba, S., H. Sato, K. Okochi, K. Fukada, F. Takakura, K. Tokunaga, H. Kiyokawa, and Y. Maeda. 1989. Prevention of transmission of human T-lymphotropic virus type 1 (HTLV-1) through transfusion, by donor screening with antibody to the virus. One-year experience. Transfusion 29:7-11. [DOI] [PubMed] [Google Scholar]
- 35.Inose, M., I. Higuchi, K. Yoshimine, M. Suehara, S. Izumo, K. Arimura, and M. J. Osame. 1992. Pathological changes in skeletal muscle in HTLV-I-associated myelopathy. Neurol. Sci. 110:73-78. [DOI] [PubMed] [Google Scholar]
- 36.Izumo, S., I. Goto, Y. Itoyama, S. Watanabe, Y. Kuroda, S. Araki, M. Mori, S. Nagataki, S. Matsukura, T. Akamine, M. Nakagawa, I. Yamamoto, and M. Osame. 1996. Interferon-alpha is effective in HTLV-I-associated myelopathy: a multicenter, randomized, doubleblind, controlled trial. Neurology 46:1016-1021. [DOI] [PubMed] [Google Scholar]
- 37.Kawai, H., T. Inui, S. Kashiwagi, T. Tsuchihashi, K. Masuda, A. Kondo, S. Niki, M. Iwasa, and S. Saito. 1992. HTLV-I infection in patients with autoimmune thyroiditis (Hashimoto's thyroiditis). J. Med. Virol. 38:138-141. [DOI] [PubMed] [Google Scholar]
- 38.Kchour, G., M. Tarhini, M. M. Kooshyar, H. El Hajj, E. Wattel, M. Mahmoudi, H. Hatoum, H. Rahimi, M. Maleki, H. Rafatpanah, S. A. Rezaee, M. T. Yazdi, A. Shirdel, H. de Thé, O. Hermine, R. Farid, and A. Bazarbachi. 2009. Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood 113:6528-6532. [DOI] [PubMed] [Google Scholar]
- 39.Khan, R. B., T. E. Bertorini, and M. C. Levin. 2001. HTLV-1 and its neurological complications. Neurologist 7:271-278. [DOI] [PubMed] [Google Scholar]
- 40.Kimura, I. 1992. HABA (HTLV-I associated bronchiolo-alveolar disorder). Nihon Kyobu Shikkan Gakkai Zasshi 30:787-795. (In Japanese.) [PubMed] [Google Scholar]
- 41.Kira, J., K. Fujihara, Y. Itoyama, I. Goto, and K. Hasuo. 1991. Leukoencephalopathy in HTLV-I associated myelopathy/tropical spastic paraparesis: MRI analysis and a two year follow-up study after corticosteroid therapy. J. Neurol. Sci. 106:41-49. [DOI] [PubMed] [Google Scholar]
- 42.Kubota, R., T. Kawanishi, H. Matsubara, A. Manns, and S. Jacobson. 2000. HTLV-I specific IFN-gamma+ CD8+ lymphocytes correlate with the proviral load in peripheral blood of infected individuals. J. Neuroimmunol. 102:208-215. [DOI] [PubMed] [Google Scholar]
- 43.La Grenade, L., A. Manns, V. Fletcher, C. Carberry, B. Hanchard, E. M. Maloney, B. Cranston, N. P. Williams, R. Wilks, E. C. Kang, and W. A. Blattner. 1998. Clinical, pathologic, and immunologic features of human T-lymphotrophic virus type I-associated infective dermatitis in children. Arch. Dermatol. 134:439-444. [DOI] [PubMed] [Google Scholar]
- 44.La Grenade, L., B. Hanchard, V. Fletcher, B. Cranston, and W. Blattner. 1990. Infective dermatitis of Jamaican children: a marker of HTLV-I infection. Lancet 336:1345-1347. [DOI] [PubMed] [Google Scholar]
- 45.Lénzi, M. E. R., T. Cuzzi-Maia, L. A. Oliveira, M. J. Andrada-Serpa, and A. Q. C. Araújo. 2003. Dermatological findings of human T lymphotropic virus type 1 (HTLV-I)-associated myelopathy/tropical spastic paraparesis. Clin. Infect. Dis. 36:507-513. [DOI] [PubMed] [Google Scholar]
- 46.Levin, M. C., M. Krichavsky, R. J. Fox, T. Lehky, S. Jacobson, C. Fox, F. Kleghorn, J. White, N. Young, R. J. Edwards, N. E. Jack, and C. Bartholomew. 1997. Extensive latent retroviral infection in bone marrow of patients with HTLV-I-associated neurologic disease. Blood 89:346-348. [PubMed] [Google Scholar]
- 47.Lézin, A., S. Olindo, S. Oliere, M. Varrin-Doyer, R. Marlin, P. Cabre, D. Smadja, and R. Cesaire. 2005. Human T lymphotropic virus type I (HTLV-I) proviral load in cerebrospinal fluid: a new criterion for the diagnosis of HTLV-I-associated myelopathy/tropical spastic paraparesis? J. Infect. Dis. 191:1830-1834. [DOI] [PubMed] [Google Scholar]
- 48.Lima, M. A., A. C. Leite, M. T. Silva, and A. Q. Araújo. 2007. Other important aspects of human T-lymphotropic virus 1-associated myelopathy. Arch. Neurol. 64:1059-1060. [DOI] [PubMed] [Google Scholar]
- 49.Mahieux, R., and A. Gessain. 2009. The human HTLV-3 and HTLV-4 retroviruses: new members of the HTLV family. Pathol. Biol. (Paris) 57:161. [DOI] [PubMed] [Google Scholar]
- 50.Maloney, E. M., H. Ramirez, A. Levin, and W. A. Blattner. 1989. A survey of the human T-cell lymphotropic virus type I (HTLV-I) in south-western Colombia. Int. J. Cancer 44:419-423. [DOI] [PubMed] [Google Scholar]
- 51.Maloney, E. M., S. Z. Wiktor, P. Palmer, B. Cranston, E. J. Patê, S. Cohn, N. Kim, W. Miley, T. L. Thomas, W. A. Blattner, and B. Hanchard. 2003. A cohort study of health effects of human T-cell lymphotropic virus type I infection in Jamaican children. Pediatrics 112:136-142. [DOI] [PubMed] [Google Scholar]
- 52.Manns, A., R. J. Wilks, E. L. Murphy, G. Haynes, J. P. Figueroa, M. Barnett, B. Hanchard, and W. A. Blattner. 1992. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int. J. Cancer 51:886-891. [DOI] [PubMed] [Google Scholar]
- 53.Manns, A., and W. A. Blattner. 1991. The epidemiology of the human T-cell lymphotrophic virus type I and type II: etiologic role in human disease. Transfusion 31:67-75. [DOI] [PubMed] [Google Scholar]
- 54.Marriott, S., and O. J. Semmes. 2005. Impact of HTLV-1 Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 24:5986-5995. [DOI] [PubMed] [Google Scholar]
- 55.Matsuoka, M., and P. L. Green. 2009. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology 6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsura, E., F. Umehara, H. Nose, I. Higuchi, E. Matsuoka, K. Izumi, R. Kubota, M. Saito, S. Izumo, K. Arimura, and M. Osame. 2008. Inclusion body myositis associated with human T-lymphotropic virus-type I infection: eleven patients from an endemic area in Japan. J. Neuropathol. Exp. Neurol. 67:41-49. [DOI] [PubMed] [Google Scholar]
- 57.McCallum, R. M., D. D. Patel, J. O. Moore, and B. F. Haynes. 1997. Arthritis syndromes associated with human T cell lymphotropic virus type I infection. Med. Clin. N. Am. 81:261-276. [DOI] [PubMed] [Google Scholar]
- 58.Merle, H., D. Smadja, O. Bera, P. Cabre, and J. C. Vernant. 1994. Ophthalmologic manifestations in spinal cord diseases caused by HTLV-I virus. Clinical study of 30 cases. J. Fr. Ophtalmol. 17:403-413. (In French.) [PubMed] [Google Scholar]
- 59.Merle, H., P. Cabre, S. Olindo, S. Merle, and D. Smadja. 2001. Ocular lesions in 200 patients infected by the human T-cell lymphotropic virus type 1 in Martinique (French West Indies). Am. J. Ophthalmol. 131:309-313. [DOI] [PubMed] [Google Scholar]
- 60.Milagres, S. P., J. A. Sanches, Jr., A. C. Milagres, and N. Y. Valente. 2003. Histopathological and immunohistochemical assessment of acquired ichthyosis in patients with human T-cell lymphotropic virus type I-associated myelopathy. Br. J. Dermatol. 149:776-781. [DOI] [PubMed] [Google Scholar]
- 61.Mochizuki, M., T. Watanabe, K. Yamaguchi, K. Yoshimura, S. Nakashima, M. Shirao, S. Araki, K. Takatsuki, S. Mori, and N. Miyata. 1992. Uveitis associated with human T-cell lymphotropic virus type I: seroepidemiologic, clinical and virologic studies. J. Infect. Dis. 166:943-944. [DOI] [PubMed] [Google Scholar]
- 62.Morgan, D. J., M. F. Caskey, C. Abbehusen, J. Oliveira-Filho, C. Araujo, A. F. Porto, S. B. Santos, G. O. Orge, M. J. Joia, A. L. Muniz, I. Siqueira, M. J. Glesby, and E. Carvalho. 2007. Brain magnetic resonance imaging white matter lesions are frequent in HTLV-I carriers and do not discriminate from HAM/TSP. AIDS Res. Hum. Retroviruses 23:1499-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mori, K., M. Noguchi, M. Matsuo, K. Nomata, T. Nakamura, and H. Kanetake. 2004. Natural course of voiding function in patients with human T-cell lymphotrophic virus type 1-associated myelopathy. J. Neurol. Sci. 217:3-6. [DOI] [PubMed] [Google Scholar]
- 64.Murphy, E. L., J. P. Figueroa, W. N. Gibbs, M. Holding-Cobham, B. Cranston, K. Malley, A. J. Bodner, S. S. Alexander, and W. A. Blattner. 1991. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I. Demographic determinants. Am. J. Epidemiol. 133:1114-1124. [DOI] [PubMed] [Google Scholar]
- 65.Murphy, E. L., R. Wilks, B. Hanchard, B. Cranston, J. P. Figueroa, W. N. Gibbs, J. Murphy, and W. A. Blattner. 1996. A case-control study of risk factors for seropositivity to human T-lymphotropic virus type I (HTLV-I) in Jamaica. Int. J. Epidemiol. 25:1083-1089. [DOI] [PubMed] [Google Scholar]
- 66.Nagai, M., and M. Osame. 2003. Human T-cell lymphotropic virus type I, and neurological diseases. J. Neurovirol. 9:228-235. [DOI] [PubMed] [Google Scholar]
- 67.Nakagawa, M. K., Y. Nakahara, M. Maruyama, I. Kawabata, I. Higuchi, H. Kubota, S. Izumo, K. Arimura, and M. Osame. 1996. Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Neurovirol. 2:345-355. [DOI] [PubMed] [Google Scholar]
- 68.Nakamaru, Y., A. Ishizu, H. Ikeda, T. Sugaya, K. Fugo, M. Higuchi, H. Yamazaki, and T. Yoshiki. 2001. Immunological hyperresponsiveness in HTLV-I LTR-env-pX transgenic rats: a prototype animal model for collagen vascular and HTLV-I-related inflammatory diseases. Pathobiology 69:11-18. [DOI] [PubMed] [Google Scholar]
- 69.Nakao, K., N. Ohba, M. Nakagawa, and M. Osame. 1999. Clinical course of HTLV-I associated uveitis. Jpn. J. Ophthalmol. 43:404-409. [DOI] [PubMed] [Google Scholar]
- 70.Namen-Lopes, M. S., M. L. Martins, P. C. Drummond, R. R. Lobato, Interdisciplinary HTLV Research Group (GIPH), and A. B. Carneiro-Proietti. 2009. Lookback study of HTLV-1 and 2 seropositive donors and their recipients in Belo Horizonte, Brazil. Transfus. Med. 19:180-188. [DOI] [PubMed] [Google Scholar]
- 71.Nascimento, M. C., J. Primo, A. Bittencourt, I. Siqueira, M. F. Oliveira, R. Meyer, A. Schriefer, S. B. Santos, and E. M. Carvalho. 2009. Infective dermatitis has similar immunological features to human T lymphotropic virus-type 1 associated myelopathy/tropical spastic paraparesis. Clin. Exp. Immunol. 156:455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nyambi, P. N., Y. Ville, J. Louwagie, I. Bedjabaga, E. Glowaczower, M. Peeters, D. Kerouedan, M. Dazza, B. Larouze, G. van der Groen, and E. Delaporte. 1996. Mother-to-child transmission of human T-cell lymphotropic virus types I and II (HTLV-I/II) in Gabon: a prospective follow-up of 4 years. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:187-192. [DOI] [PubMed] [Google Scholar]
- 73.Oh, U., Y. Yamano, C. A. Mora, J. Ohayon, F. Bagnato, J. A. Butman, J. Dambrosia, T. P. Leist, H. McFarland, and S. Jacobson. 2005. Interferon-beta1a therapy in human T-lymphotropic virus type I-associated neurologic disease. Ann. Neurol. 7:526-534. [DOI] [PubMed] [Google Scholar]
- 74.Okochi, K., and H. Sato. 1986. Transmission of adult T-cell leukemia virus (HTLV-I) through blood transfusion and its prevention. AIDS Res. 2:157-161. [PubMed] [Google Scholar]
- 75.Olindo, S., A. Lézin, P. Cabre, H. Merle, M. Saint-Vil, M. E. Kaptue, A. Signate, R. Césaire, and D. Smadja. 2005. HTLV-1 proviral load in peripheral blood mononuclear cells quantified in 100 HAM/TSP patients: a marker of disease progression. J. Neurol. Sci. 237:53-59. [DOI] [PubMed] [Google Scholar]
- 76.Oliveira, M. F., C. Brites, N. Ferraz, P. Magalhães, F. Almeida, and A. L. Bittencourt. 2005. Infective dermatitis associated with the human T-cell lymphotropic virus type I in Salvador, Bahia, Brazil. Clin. Infect. Dis. 40:e90-e96. doi: 10.1086/430064. [DOI] [PubMed] [Google Scholar]
- 77.Oliveira, P., N. M. de Castro, and E. M. Carvalho. 2007. Urinary and sexual manifestations of patients infected by HTLV-I. Clinics 62:191-196. [DOI] [PubMed] [Google Scholar]
- 78.Orland, J. R., J. Engstrom, J. Fridey, R. A. Sacher, J. W. Smith, C. Nass, G. Garratty, B. Newman, D. Smith, B. Wang, K. Loughlin, E. L. Murphy, and the HTLV Outcomes Study. 2003. Prevalence and clinical features of HTLV neurologic disease in the HTLV Outcomes Study. Neurology 61:1588-1594. [DOI] [PubMed] [Google Scholar]
- 79.Osame, M., R. Janssen, H. Kubota, H. Nishitani, A. Igata, S. Nagataki, M. Mori, I. Goto, H. Shimabukuro, and R. Khabbaz. 1990. Nationwide survey of HTLV-I-associated myelopathy in Japan: association with blood transfusion. Ann. Neurol. 28:50-56. [DOI] [PubMed] [Google Scholar]
- 80.Pawson, R., D. S. Richardson, A. Pagliuca, S. M. Kelsey, S. Hoque, J. Breuer, A. C. Newland, and G. J. Mufti. 1998. Adult T-cell leukemia/lymphoma in London: clinical experience of 21 cases. Leuk. Lymphoma 31:177-185. [DOI] [PubMed] [Google Scholar]
- 81.Pinheiro, S. R., O. A. Martins-Filho, J. G. Ribas, B. C. Catalan-Soares, F. A. Proietti, S. Namen-Lopes, G. E. Brito-Melo, A. B. Carneiro-Proietti, and GIPH (Interdisciplinary HTLV-I/II Research Group). 2006. Immunologic markers, uveitis, and keratoconjunctivitis sicca associated with human T-cell lymphotropic virus type 1. Am. J. Ophthalmol. 142:811-815. [DOI] [PubMed] [Google Scholar]
- 82.Pinheiro, S. R., M. A. Lana-Peixoto, A. B. C. Proietti, F. Oréfice, M. V. Lima-Martins, and F. A. Proietti. 1995. HTLV-I associated uveitis, myelopathy, rheumatoid arthritis and Sjögren's syndrome. Arq. Neuropsiquiatr. 53:777-781. [DOI] [PubMed] [Google Scholar]
- 83.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Proietti, F. A., A. B. F. Carneiro-Proietti, B. C. Catalan-Soares, and E. L. Murphy. 2005. Global epidemiology of HTLV-1 infection and associated diseases. Oncogene 24:6058-6068. [DOI] [PubMed] [Google Scholar]
- 85.Roberts, L. J., S. E. Huffam, S. F. Walton, and B. J. Currie. 2005. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J. Infect. 50:375-381. [DOI] [PubMed] [Google Scholar]
- 86.Saito, M., T. Matsuzaki, Y. Satou, J. Yasunaga, K. Saito, K. Arimura, M. Matsuoka, and Y. Ohara. 2009. In vivo expression of the HBZ gene of HTLV-1 correlates with proviral load, inflammatory markers and disease severity in HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP). Retrovirology 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samson, G., and D. D. Cardenas. 2007. Neurogenic bladder in spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 18:255-274. [DOI] [PubMed] [Google Scholar]
- 88.Seed, C. R., P. Kiely, and A. J. Keller. 2005. Residual risk of transfusion transmitted human immunodeficiency virus, hepatitis B virus, hepatitis C virus and human T lymphotrophic virus. Intern. Med. J. 35:592-598. [DOI] [PubMed] [Google Scholar]
- 89.Shimoyama, M. 1991. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br. J. Haematol. 79:428-437. [DOI] [PubMed] [Google Scholar]
- 90.Stramer, S. L., G. A. Foster, and R. Y. Dodd. 2006. Effectiveness of human T lymphotropic virus (HTLV) recipient tracing (lookback) and the current HTLV-I and II confirmatory algorithm, 1999 to 2004. Transfusion 46:703-707. [DOI] [PubMed] [Google Scholar]
- 91.Stumpf, B. P., A. B. Carneiro-Proietti, F. A. Proietti, F. L. Rocha, and the Interdisciplinary HTLV Research Group. 2008. Higher rate of major depression among blood donor candidates infected with human T-cell lymphotropic virus type 1. Int. J. Psychiatry Med. 38:345-355. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan, M. T., A. E. Williams, C. T. Fang, T. Grandinetti, B. J. Poiesz, and G. D. Ehrlich. 1991. Transmission of human T-lymphotropic virus types I and II by blood transfusion: a retrospective study of recipients of blood components (1983 through 1988). The American Red Cross HTLV-I/II Collaborative Study Group. Arch. Intern. Med. 151:2043-2048. [PubMed] [Google Scholar]
- 93.Takenouchi, N., Y. Yamano, K. Usuku, M. Osame, and S. Izumo. 2003. Usefulness of proviral load measurement for monitoring of disease activity in individual patients with human T-lymphotropic virus type-I-associated myelopathy/tropical spastic paraparesis. J. Neurovirol. 9:29-35. [DOI] [PubMed] [Google Scholar]
- 94.Taylor, G. P., and M. Matsuoka. 2005. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene 24:6047-6057. [DOI] [PubMed] [Google Scholar]
- 95.Taylor, G. P., P. Goon, Y. Furukawa, H. Green, A. Barfield, A. Mosley, H. Nose, A. Babiker, P. Rudge, K. Usuku, M. Osame, C. R. Bangham, and J. N. Weber. 2006. Zidovudine plus lamivudine in human T-lymphotropic virus type-I-associated myelopathy: a randomised trial. Retrovirology 3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsukasaki, K., O. Hermine, A. Bazarbachi, L. Ratner, J. C. Ramos, W. Harrington, Jr., D. O'Mahony, J. E. Janik, A. L. Bittencourt, G. P. Taylor, K. Yamaguchi, A. Utsunomiya, K. Tobinai, and T. Watanabe. 2009. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J. Clin. Oncol. 27:453-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tynell, E., S. Andersson, E. Lithander, M. Arneborn, J. Blomberg, H. B. Hansson, A. Krook, M. Nomberg, K. Ramstedt, A. Shanwell, and A. Bjorkman. 1998. Screening for human T-cell leukaemia/lymphoma virus among blood donors in Sweden: cost effectiveness analysis. BMJ 316:1417-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uchiyama, T., J. Yodoi, K. Sagawa, K. Takatsuki, and H. Uchino. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481-492. [PubMed] [Google Scholar]
- 99.Vasquez, P., G. Sanchez, C. Volante, L. Vera, E. Ramirez, G. Soto, and H. Lee. 1991. Human T-lymphotropic virus type I: new risk for Chilean population. Blood 78:850-851. [PubMed] [Google Scholar]
- 100.Willenze, R., E. S. Jaffe, G. Burg, L. Cerroni, E. Berti, S. H. Swerdlow, E. Ralfkiaer, S. Chimenti, J. L. Diaz-Perez, L. M. Duncan, F. Grange, N. L. Harris, W. Kempf, H. Kerl, M. Kurrer, R. Knobler, N. Pimpinelli, C. Sander, M. Santucci, W. Sterry, M. H. Vermeer, J. Wechsler, S. Whittaker, and C. J. Meijer. 2005. WHO-EORTC classification for cutaneous lymphomas. Blood 105:3768-3785. [DOI] [PubMed] [Google Scholar]
- 101.Yakova, M., A. Lézin, F. Dantin, G. Lagathu, S. Olindo, G. Jean-Baptiste, S. Arfi, and R. Césaire. 2005. Increased proviral load in HTLV-1-infected patients with rheumatoid arthritis or connective tissue disease. Retrovirology 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reference deleted.
- 103.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. U. S. A. 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]