Abstract
Acetyl coenzyme A (CoA) is a central metabolite in carbon and energy metabolism and in the biosynthesis of cellular molecules. A source of cytoplasmic acetyl-CoA is essential for the production of fatty acids and sterols and for protein acetylation, including histone acetylation in the nucleus. In Saccharomyces cerevisiae and Candida albicans acetyl-CoA is produced from acetate by cytoplasmic acetyl-CoA synthetase, while in plants and animals acetyl-CoA is derived from citrate via ATP-citrate lyase. In the filamentous ascomycete Aspergillus nidulans, tandem divergently transcribed genes (aclA and aclB) encode the subunits of ATP-citrate lyase, and we have deleted these genes. Growth is greatly diminished on carbon sources that do not result in cytoplasmic acetyl-CoA, such as glucose and proline, while growth is not affected on carbon sources that result in the production of cytoplasmic acetyl-CoA, such as acetate and ethanol. Addition of acetate restores growth on glucose or proline, and this is dependent on facA, which encodes cytoplasmic acetyl-CoA synthetase, but not on the regulatory gene facB. Transcription of aclA and aclB is repressed by growth on acetate or ethanol. Loss of ATP-citrate lyase results in severe developmental effects, with the production of asexual spores (conidia) being greatly reduced and a complete absence of sexual development. This is in contrast to Sordaria macrospora, in which fruiting body formation is initiated but maturation is defective in an ATP-citrate lyase mutant. Addition of acetate does not repair these defects, indicating a specific requirement for high levels of cytoplasmic acetyl-CoA during differentiation. Complementation in heterokaryons between aclA and aclB deletions for all phenotypes indicates that the tandem gene arrangement is not essential.
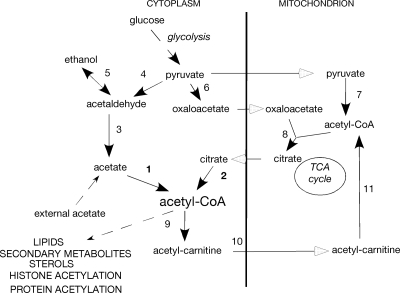
Acetyl coenzyme A (CoA) is a crucial metabolite involved in both intermediary carbon and energy metabolism as well as in biosynthetic pathways. Appropriate levels of this metabolite must be present in the correct cellular compartment for normal growth and development, and the pathways producing acetyl-CoA vary with the carbon source(s) available. When glycolytic carbon sources are present, pyruvate is the major source of acetyl-CoA in the mitochondrion as a result of pyruvate dehydrogenase activity (Fig. 1). Oxaloacetate, also derived from pyruvate via pyruvate carboxylase activity, is combined with acetyl-CoA by citrate synthase to form citrate, allowing the tricarboxylic acid (TCA) cycle to occur in mitochondria (Fig. 1). This results in the formation of biosynthetic intermediates and ATP generation via oxidative phosphorylation under aerobic conditions.
Fig. 1.
Relevant metabolic pathways for acetyl-CoA generation. Enzymes are numbered as follows: 1, acetyl-CoA synthetase (EC 6.2.1.13); 2, ATP-citrate lyase (EC 2.3.3.8); 3, maldehyde dehydrogenase (EC 1.2.1.5); 4, pyruvate decarboxylase (EC 4.1.1.1.1); 5, alcohol dehydrogenase (EC 1.1.1.1); 6, pyruvate carboxylase (EC 6.4.1.1); 7, pyruvate dehydrogenase complex (EC 2.3.1.12); 8, citrate synthase (EC 2.3.3.1); 9, cytoplasmic carnitine acetyltransferase (EC 2.3.1.7); 10, acetyl-carnitine mitochondrial carrier; 11, mitochondrial/peroxisomal carnitine acetyltransferase (EC 2.3.1.7).
Cytoplasmic acetyl-CoA is essential as a building block for the biosynthesis of fatty acids and various secondary metabolites by virtue of its conversion to malonyl-CoA by cytoplasmic acetyl-CoA carboxylase and for sterol biosynthesis via the synthesis of hydroxymethyl glutaryl-CoA in the mevalonate pathway. It is also the substrate for acetylation of lysine residues in many proteins, including histones. The widespread occurrence of protein acetylation has recently been documented for both liver cells and Salmonella enterica to the extent that this posttranslational modification may rival that of phosphorylation or ubiquitination in affecting protein function and stability (55, 61). There are two potential sources of cytoplasmic acetyl-CoA: from citrate via ATP-citrate lyase (ACL), which depends on citrate entering the cytoplasm from the mitochondrion, or from acetate via acetyl-CoA synthetase (ACS) (Fig. 1). Acetate may be obtained from externally supplied acetate in the growth medium, or it may be produced from acetaldehyde derived from either pyruvate via pyruvate decarboxylase or from ethanol via alcohol dehydrogenase. A further potential source of acetyl-CoA is by β-oxidation of fatty acids in peroxisomes (not shown in Fig. 1) and, in this case, acetyl-CoA must exit the peroxisome into the cytoplasm.
There is evidence for the pyruvate-acetaldehyde-acetate pathway being the major source of cytoplasmic acetyl-CoA in Saccharomyces cerevisiae. Deletion of all three structural genes for pyruvate decarboxylase (PDCI, PDCS, and PDC6) results in loss of growth in defined glucose medium, and this can be restored by acetate or ethanol (15, 39). There are multiple genes for aldehyde dehydrogenase, and the situation is more complex. During anaerobic growth on glucose the cytoplasmic (Ald6) and the mitochondrial aldehyde dehydrogenases (Ald4 and Ald5) are the major sources of acetate (40). However, deletion of all three genes does not result in loss of viability or acetate formation, suggesting an unknown compensating pathway. Two ACSs, Acs1 and Acs2, are present in S. cerevisiae, and loss of both enzymes is lethal on all carbon sources, indicating that this activity is essential (47, 52). Acs2 is essential for growth on glucose, and the 5′ region of ACS2 contains binding sites for the global transcriptional activator Abf1, but it is not essential for growth on nonfermentable carbon sources, where Acs1 is active as a result of transcriptional activation by Cat8 acting at a carbon source response element (CSRE) and by Adr1 (11, 24, 43, 45, 47). Mitochondrial localization of Acs1 has been proposed, but some is present in the cytoplasm and the nucleus and Acs2 is nuclear/cytosolic (47). Acs2 has been shown to be a major source of acetyl-CoA for histone acetylation on glucose, while on glycerol and ethanol this function can be replaced by Acs1. Neither peroxisomal β-oxidation nor mitochondrial pyruvate dehydrogenase activity was found to contribute to histone acetylation. The ability of a Salmonella enterica ACS targeted to different cell compartments to complement loss of Acs2 activity showed that cytoplasmic- or nuclear-localized activity was sufficient for growth on glucose but not mitochondrial localized activity. These data are consistent with cytoplasmic pools of acetyl-CoA being interchangeable with the nuclear but not the mitochondrial pool (47). The yeast pathogen Candida albicans also has two ACSs (6). However, the situation is different in that Acs2 is required not only for growth on glucose but also on ethanol, acetate, and lactate, and acs2 is expressed on all of these carbon sources, while Acs1 is not essential. Acs2 is not required for growth on the fatty acid, oleate, or on glycerol, and acs1 is only highly expressed on oleate. No effects on global histone acetylation were detected in that study (6). Two acs genes are predicted in the genome sequences of Saccharomycotina with the exception of Yarrowia lipolytica (see orthogroup 4318 at http://www.broadinstitute.org/regev/orthogroups/). A mutant of Y. lipolytica lacking acetate-inducible ACS activity has been found to be able to grow on glucose and glycerol but not on acetate (28).
ACL is present in only a few prokaryotes or Archaea but is widely present in algae, plants, and animals (reference 14 and references therein). Cytosolic ACL activity is the major source of cytoplasmic acetyl-CoA in plants, where tissue-specific expression is correlated with acetyl-CoA carboxylase expression (14). In mammalian cells, ACL is localized in both the cytoplasm and the nucleus, and small interfering RNA-mediated silencing of ACL expression affects the patterns of histone acetylation and lipid accumulation in adipocytes (56). Silencing of ACS expression has little effect in the absence of added supraphysiological levels of acetate (56). Analysis of sequenced fungal genomes indicates that predicted ACL-encoding genes are present widely in fungi but are notably absent from sequenced members of the Saccharomycotina with the exception of Y. lipolytica (Fig. 2). A survey of a wide variety of (unsequenced) yeast species has found a correlation between those that accumulate high levels of lipids (oleaginous yeasts) and the presence of ACL activity, and these included some members of the Saccharomycotina as well as the Basidiomycota (4). A further correlation has been found between ACL activity and secondary metabolite and lipid production in filamentous fungi (57). ACL activity in Aspergillus niger has been found to be much higher than ACS activity, and the enzyme is inhibited by palmitoyl-CoA, indicating a role in fatty acid synthesis (38).
Fig. 2.
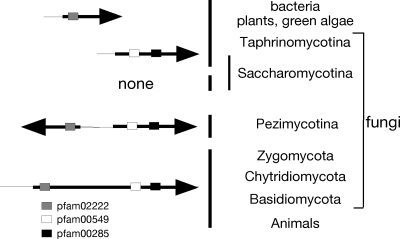
ACL gene arrangements in different phyla. See reference 14 for information on bacteria, algae, plants, and animals. BlastP searches of fungal genomes (http://www.broadinstitute.org/annotation/fungi/index.html) using the predicted A. nidulans ACL-encoding genes AN2435.3 and AN2436.3 showed predicted divergently transcribed genes in the genomes of all species of the Pezimycotina, e.g., Magnaporthe grisea (MGG_06720.5 and MGG_06719.5), Scelerotinia sclerotiorum (SS1G_02378.1 and SS1G_02379.1), and Botrytis cinerea (BC1G_05991.1 and BC1G_05989.1). In the case of Neurospora crassa (NCU06783 and NCU06785), a very short dubious predicted gene (NCU06784) occurs between the genes. Single genes are found in Zygomycota (Rhizopus oryzae [RO3G_14197.1]), Chytridiomycota (Batrachochytrium dendrobatidis [BDEG_06723]), and Basidiomycota (e.g., Ustilago maydis [UM01005.1], Cryptococcus neoformans [CNAG_04640.1], and Coprinus cinerea [CC1G_09654.1]). Analysis of fungal orthogroups (http://www.broadinstitute.org/regev/orthogroups/) showed no ACL homologues in the Saccharomycotina, with the exception of Y. lipolytica (YALI0D24431g and YALI0E34793g). Schizosaccharomyces pombe (Taphrinomycotina) contains two unlinked ACL genes (SPBC1703.07 and SPAC22A12.16). Conserved protein domains (http://www.ncbi.nlm.nih.gov/sites/entrez) are shown: pfam02222 (ATP grasp domain), pfam 00549 (CoA ligase), and pfam00285 (citrate synthase).
The primary sequences of ACL contain conserved domains for ATP and CoA binding as well as sequences related to the oxaloacetate binding and active site motifs of citrate synthase (14). However, ACL has been found to be either heteromeric or homomeric, and analysis of sequenced genomes reveals divergence in the distribution of these different structures in different phyla (Fig. 2) (see reference 14 for a phylogenetic tree). The Arabidopsis thaliana enzyme is a hetero-octamer containing four A-subunits (45 kDa) encoded by three genes and four B-subunits (65 kDa) encoded by two genes, while, in contrast, animal enzymes are homotetramers (14). Both homomeric and heteromeric ACL structures are predicted in fungi. The filamentous ascomycete Sordaria macrospora has been shown to have two adjacent genes for different subunits of ACL separated by 3.4 kb, and these are divergently transcribed (34). ACL purified from Aspergillus nidulans has two dissimilar subunits of 70 and 55 kDa and is a hexamer containing three of each kind of subunit (1). This is supported by the presence of two predicted divergently transcribed genes in the A. nidulans genome sequence (see Fig. 3, below, and Results). This gene arrangement is also predicted for other Pezimycotina species. Two ACL genes encoding dissimilar subunits are also predicted for Y. lipolytica as well as for Schizosaccharomyces pombe, but in these species the genes are unlinked. In contrast, only one gene encoding ACL is predicted in the other fungal phyla (Fig. 2), and ACL from the basidiomycete Rhodotorula gracilis has been shown to be a homotetramer (44). The most parsimonious evolutionary model is that the heteromeric structure is ancestral and a gene fusion in the common ancestor of fungi and animals occurred, resulting in the homomeric enzyme structure. The heteromeric structure was restored by a subsequent genome rearrangement in the ancestor of the ascomycetes that produced two genes, one for each kind of subunit. Subsequent gene loss occurred at least once in the Saccharomycotina.
Fig. 3.
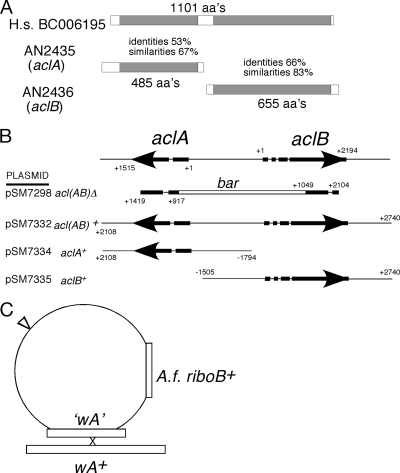
Gene structure and deletion of ACL-encoding genes. (A) Comparison of the predicted products of the aclA and aclB genes with the human ACL (H. sapiens accession number BC006195). (B) Schematic of the deletion constructs. The structures of the divergently transcribed aclA and aclB genes are shown with the positions of introns indicated by gaps. The positions of start codons are indicated by +1 for each gene. The bar gene encoding glufosinate resistance replaced the indicated sequences in plasmid pSM7298, and a linear fragment generated by PCR was used to generate the initial acl(AB) deletion by transformation. pSM7332, pSM7334, and pSM7335 containing both aclA+ and aclB+ genes, aclA+and aclB+, respectively, were constructed with the sequences inserted into the wA targeting vector shown. (C) The wA targeting vector contains the A. fumigatus riboB gene for selecting RiboA+ transformants and contains an internal fragment of the wA coding region (nt +812 to +2966), which allows screening for insertional inactivation of the wA+ gene based on the presence of white conidia. These plasmids were used to generate the acl(AB)+/acl(AB)Δ, aclA+/acl(AB)Δ, and aclB+/acl(AB)Δ transformants by transforming the original acl(AB)Δ strain. In addition, an empty wA targeting vector was used to generate a control acl(AB)Δ strain.
Our interests are in the intracellular distribution of acetyl-CoA during growth on different carbon sources in A. nidulans (19, 20, 46). The extent to which mitochondrial and peroxisomal acetyl-CoA pools are separate from the cytoplasmic pool and the role of ACL is therefore of considerable importance. The pyruvate-acetaldehyde-acetate pathway is unlikely to be a major source of acetyl-CoA. Pyruvate decarboxylase was undetectable in glucose media (37). However, a single gene encoding pyruvate decarboxylase, together with alcC, encoding an alcohol dehydrogenase, has been found to be involved in a fermentation response under anaerobic conditions (22, 26). The utilization of ethanol as a sole carbon source is mediated by AlcA (alcohol dehydrogenase) and AldA (aldehyde dehydrogenase). The transcription of the genes for these enzymes is activated by the AlcR transcription factor, which responds to acetaldehyde as the inducer, and expression is repressed by carbon catabolite repression mediated by CreA, which represses alcR and alcA (16, 27). There is a single cytoplasmic ACS encoded by facA, and loss-of-function mutations in this gene result in loss of growth on acetate or ethanol as sole carbon sources but have no effect on growth on glucose or amino acids, such as proline or glutamate (3, 7, 42). Expression of facA is inducible by acetate mediated by the transcription factor FacB, which has considerable similarity to Cat8 in S. cerevisiae, and it is also repressed by glucose (18, 21, 48, 49). Therefore, FacA is unlikely to be a source of cytoplasmic acetyl-CoA during growth on glucose or noninducing carbon sources. Acetyl-CoA is also produced in peroxisomes by fatty acid β-oxidation, and expression of the genes required is induced by fatty acids mediated by the transcription factors FarA, FarB, and ScfA (19, 20). Acetyl-CoA, produced in peroxisomes from fatty acids or in the cytoplasm from acetate, is transferred to the mitochondria via the acetyl-carnitine shuttle (Fig. 1) (9, 10, 46).
Overall, it is therefore predicted that, in A. nidulans, ACL is important for acetyl-CoA synthesis on some carbon sources but not others. Impaired synthesis of lipid-based signaling molecules as well as secondary metabolites as a result of lowered cytoplasmic acetyl-CoA levels are likely to result in developmental defects. A mutation in one of the ACL structural genes was found to affect maturation of fruiting bodies in Sordaria macrospora (36). We have therefore investigated the effects of deleting the genes encoding ACL on growth and development. Growth is severely compromised in the absence of sources of cytoplasmic acetyl-CoA; asexual sporulation (conidiation) is greatly reduced and sexual development is absent.
MATERIALS AND METHODS
A. nidulans strains, media, transformation, and molecular and genetic techniques.
Media and conditions for growth of A. nidulans were as described previously (20). Minimal media contained the required supplements to allow auxotrophic strains to grow. All strains were derived from the original Glasgow strain and contained the veA1 mutation unless otherwise indicated. The facA303, acuH253, facCΔ, and facB101 mutations have been described elsewhere (3, 46, 49). The acuJΔ deletant was generated by gene replacement using pyrG as the selectable marker (unpublished data). The genotypes of strains described here are presented in Table 1. Standard methods of A. nidulans genetic manipulations were as previously described (50) with the modification that all manipulations of aclΔ strains required the addition of acetate to the medium to allow growth. Preparation of protoplasts and transformation were as described previously (31) with the modification that the acl(AB)Δ strain was grown in 0.1% glucose minimal medium containing 50 mM acetate for the preparation of protoplasts. Recipient strains contained nkuAΔ to promote homologous integration events, and selectable markers were the bar gene (glufosinate resistance) and riboB+ from A. fumigatus (31). DNA from transformants was analyzed by Southern blotting to confirm predicted integration events. Standard methods for DNA manipulations, RNA isolation, nucleic acid blotting, and hybridization have been described previously (19, 41).
Table 1.
Strains used in this study
| Strain no. | Name | Genotypea |
|---|---|---|
| 54 | Wild type | biA1 niiA4 |
| 11036 | TNO2A21 | pyroA4 nkuA::argB+riboB2 |
| 12393 | acl(AB)Δ (original) | pyroA4 nkuA::argB+acl(AB)::bar riboB2 |
| 12410 | acl(AB)Δ | acl(AB)::bar niiA4 |
| 12408 | acl(AB)Δ (empty vector) | wAA. fumigatusriboB+/pyroA4 nkuA::argB+acl(AB)::bar riboB2 |
| 12404 | aclA+/acl(AB)Δ | wAA. fumigatusriboB+aclA+/pyroA4 nkuA::argB+acl(AB)::bar riboB2 |
| 12406 | aclB+/acl(AB)Δ | wAA. fumigatusriboB+aclB+/pyroA4 nkuA::argB+acl(AB)::bar riboB2 |
| 12400 | acl(AB)+/acl(AB)Δ | wAA. fumigatusriboB+acl(AB)+/pyroA4 nkuA::argB+acl(AB)::bar riboB2 |
| 12438 | acl(AB)Δ veA+ | biA1 acl(AB)::bar veA+niiA4 |
| 10593 | facA303 | biA1 pyroA4 facA303 niiA4 |
| 12445 | acuJΔ acl(AB)Δ | biA1 acuJ::pyrG+pyroA4 acl(AB)::bar riboB2 |
| 12436 | acuH253 acl(AB)Δ | pabaA2 acuH253 acl(AB)::bar |
| 12431 | facCΔ acl(AB)Δ | acl(AB)::bar facC::argB+riboB2 |
| 12421 | facB101 acl(AB)Δ | wA3 acl(AB)::bar facB101 riboB2 |
The presence of nkuA::argB+ is not known for all strains. wA represents a disruption of the wA+ locus as described in Fig. 3. All strains with the exception of 12438 contained the veA1 mutation.
DNA sequences and sequence analysis.
Aspergillus spp. sequences were obtained from the genome sequences at the Broad Institute (http://www.broadinstitute.org/annotation/genome/aspergillus_group/MultiHome.html). Other fungal sequences were derived from either specific genome sequences available at the Broad Institute (http://www.broadinstitute.org/annotation/fungi/index.html), NCBI (http://www.ncbi.nlm.nih.gov/), or the Broad Institute orthogroups database (http://www.broadinstitute.org/regev/orthogroups/). Analysis of potential mitochondrial targeting used TargetP (http://www.cbs.dtu.dk/services/TargetP/) and MitoProt (http://ihg2.helmholtz-muenchen.de/ihg/mitoprot.html).
Deletion of acl genes.
A 5.6-kb DNA fragment containing part of the AN2435 (aclA) and AN2436 (aclB) genes and the intergenic region (nucleotide [nt] +1419 of aclA to +2104 of aclB) was cloned by PCR using the primers 5′-ACCAGTGCGCAGTCTCTTCT-3′ and 5′-GTTGAGGCCGAGTTCTTCAC-3′ and was then inserted into EcoRV-cut pBluescript SK(+) (Stratagene) to create pSM7296. The bar gene encoding glufosinate resistance was isolated as an NcoI/SpeI fragment from pMT1612 (31) and inserted into NcoI/NheI-cut pSM7296 to generate pSM7298, in which the entire intergenic region as well as the region to nt +917 of aclA and to nt +1049 of aclB were removed and replaced by the bar gene. A linear PCR fragment using the above primers was used to transform the strain TNO2A21, selecting for glufosinate resistance to give the acl(AB)Δ strain (Fig. 3). A 6.7-kb DNA fragment containing both the aclA and aclB genes was cloned by the PCR using the primers 5′-CTCAATGGCCCTGGAATAAA-3′ and 5′-CCATCTGGACGGCTTATGTT-3′, and this was inserted into EcoRV-cut pBluescript SK(+) to create pSM7333 and also into the wA targeting vector pRG6501 (Fig. 3C) cut with EcoICRI to create pSM7332 containing aclA+ and aclB+ genes. To isolate individual ACL genes, pSM7333 was cut with SalI and NotI, and the fragment was cloned into pRG6501 cut with XhoI and NotI to generate pSM7334 containing aclA+ (corresponding to nt −1794 to +2108 of aclA); alternatively, it was cut with XbaI and XhoI and the fragment was cloned into pRG6501 cut with XbaI and XhoI to generate pSM7335 containing aclB+ (corresponding to nt −1505 to +2740 of aclB). pSM7332, pSM7334, pSM7335, and pRG6501 were transformed into the acl(AB)Δ strain, selecting for RiboB+ transformants on medium containing 50 mM acetate, and screened for white conidiating colonies. In this way the acl(AB)+/acl(AB)Δ, aclA+/acl(AB)Δ, aclB+/acl(AB)Δ, and acl(AB)Δ (empty vector) strains were isolated (Table 1; Fig. 3B).
Conidial counts.
Conidial suspensions in dilute Tween 80 solution (0.0005%) were added to 0.6% C-free minimal medium with 0.6% agar and added as a top layer to plates containing 1% glucose minimal medium containing 10 mM acetate and 10 mM ammonium tartrate as the nitrogen source or with 1 M sucrose added, and plates were incubated for 3 days to generate a lawn of conidiating mycelia. Three to four plugs for each plate were made with a 0.28-cm2 glass tube, and conidia were resuspended by vortexing in 5 ml of dilute Tween 80. Suspensions were diluted and spread on complete medium containing 10 mM acetate, and the number of colonies was counted after 2 days of incubation. The number of viable colonies per cm2 was calculated.
Microscopy.
Images of conidia and cleistothecial development were captured on an Olympus SZX12 microscope.
RESULTS
Deletion of aclA and aclB genes.
The AN2435 and AN2436 genes are predicted to encode ACL subunits with high similarity to the predicted sequences of the ACL subunits of S. macrospora (34) and to the single polypeptide of Homo sapiens (Fig. 3A). Neither polypeptide had predicted mitochondrial or peroxisomal targeting sequences consistent with cytoplasmic localization. The genes were designated aclA and aclB, respectively. Both genes were simultaneously inactivated by gene replacement with the bar gene, which encodes glufosinate resistance, to generate the acl(AB)Δ strain (Fig. 3B). Initially, transformants selected on protoplast medium (1% glucose–1 M sucrose) containing glufosinate required extended incubation because of extremely slow growth. Subsequently, it was found that growth was greatly enhanced by adding 50 mM acetate to the selection plates, and isolated transformants grew strongly on acetate as a sole carbon source (see below). The aclA and aclB genes were cloned together on a single fragment, and each gene was cloned individually (Fig. 3B). These fragments were inserted into a vector designed for targeting to the wA locus (Fig. 3C), transformed into the acl(AB)Δ strain, selecting for RiboB+ transformants, and screened for white conidia. In this way acl(AB)+/acl(AB)Δ, aclA+/acl(AB)Δ, and aclB+/acl(AB)Δ strains were isolated. Complementation of acl(AB)Δ for all phenotypes required the presence of both aclA+ and aclB+.
Effects of deletion of ACL genes on growth on different carbon sources.
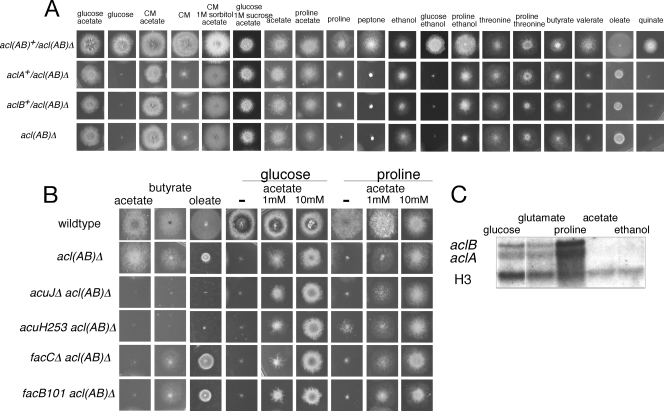
Deletion of the acl genes had major effects on growth in the absence of sources of cytoplasmic acetyl-CoA (Fig. 4A). Growth was greatly affected on glucose medium but restored by acetate. Growth of the acl deletions was only slightly better on rich complete medium, which contains 1% glucose, peptone, yeast extract, and casein hydrolysate, indicating that these supplements do not provide sufficient acetyl-CoA. Growth of the acl deletion strains was poor on carbon sources metabolized via 2-oxoglutarate, such as proline (or alanine, glutamate, or 2-aminobutyric acid [data not shown]), and acetate restored growth. In contrast, growth was normal on acetate or ethanol as sole carbon sources where cytoplasmic acetyl-CoA is produced by ACS. While the presence of glucose did not prevent growth repair by acetate (see below), it completely prevented repair by ethanol, consistent with strong glucose repression of ethanol catabolism (27). Growth on threonine was unaffected, and threonine repaired growth on proline, consistent with catabolism of threonine via acetaldehyde and acetate (16). The deletion strains were able to grow on butyrate and to a much lesser extent on valerate and oleate. These fatty acids are metabolized via β-oxidation in peroxisomes (20), and therefore this result suggests that some acetyl-CoA can enter the cytoplasm from peroxisomes.
Fig. 4.
Deletion of the aclA or aclB gene results in a requirement for cytoplasmic acetyl-CoA. (A) Growth on various carbon sources for 2 to 3 days at 37°C. The following carbon sources (concentrations shown in parenthese) were added to minimal medium with 10 mM ammonium chloride as the nitrogen source: glucose (1%); peptone (0.24%); acetate and proline (50 mM); ethanol (0.5%); quinate (0.25%); butyrate, valerate, and oleate (5 mM); threonine (20 mM). CM, complete medium containing 1% glucose. (B) Repair of acl(AB)Δ in mutant backgrounds affected in acetyl-CoA metabolism. Media were as for panel A, with acetate added at the indicated concentrations (with a dash indicating no added acetate). (C) Northern blot analysis of aclA and aclB expression. RNA was extracted from wild-type mycelia grown for 16 h in 1% glucose medium and then transferred to minimal medium containing 1% glucose, 50 mM glutamate, 50 mM proline, 50 mM acetate, or 0.5% ethanol as the sole carbon source, with 10 mM ammonium chloride as the nitrogen source for 4 h before harvesting and extraction. Ten micrograms of total RNA was run on a 1.2% agarose–formaldehyde gel, blotted, and probed sequentially with 32P-labeled PCR products of aclA (primers 5′-CCAACACTCACAACCCTCCT-3′ and 5′-GTTGAGGCCGAGTTCTTCAC-3′) and aclB (primers 5′-GTTGAGGCCGAGTTCTTCAC-3′ and 5′-ATGCCCTCTTCCACAACAAG-3′), with the EcoRI fragment of the histone H3 gene as a loading control.
The observation that growth of the deletion strains on the aromatic quinate was completely abolished is of particular interest. This result was surprising because quinate is metabolized to acetyl-CoA and succinyl-CoA via the protocatechuate pathway (as characterized in bacteria [32]), which has been analyzed by the isolation of pca mutants unable to grow on either quinate or benzoate (25). The genes for the enzymes of this pathway have not been cloned. The last step in the pathway is catalyzed by β-ketoadipyl-CoA thiolase, producing acetyl-CoA and succinyl-CoA, and mutations in the proposed structural gene, pcaF, have been isolated (25). The likely gene has been identified as AN5698 by a BlastP search of the A. nidulans genome sequence using the Escherichia coli PcaF sequence (38% identity and 53% similarity), and this gene is on linkage group V, in accordance with the mapped location of the pcaF mutation (25). An N-terminal mitochondrial targeting sequence is predicted in the AN5698 sequence by Mitoprot and TargetP. Therefore, it is highly likely that the acetyl-CoA resulting from quinate catabolism is produced in the mitochondrion by β-ketoadipyl-CoA thiolase. The inability of the acl deletants to grow on quinate is consistent with the cytoplasmic and mitochondrial pools of acetyl-CoA being distinct. Identification of all the genes encoding the enzymes of the protocatechuate pathway and the determination of their cellular localization is clearly worthwhile.
Effects of acetate utilization mutations on growth of acl(AB)Δ.
The addition of acetate to glucose or proline medium allowed growth of the acl deletion mutants. It was likely that this resulted from the activity of cytoplasmic ACS encoded by the facA gene. Strains containing acl(AB)Δ were fertile in heterozygous crosses, yielding acl(AB)Δ progeny readily identified by a requirement for acetate and poor conidiation. We were unable to isolate acl(AB)Δ facA double mutants. In crosses to a strain containing the facA303 loss-of-function mutation (3), all acl(AB)Δ progeny were able to grow on acetate as the sole carbon source, i.e., facA+. This clearly indicated that ACS is required for growth repair by acetate. It was surprising that sufficient ACS activity was detected in the presence of glucose, which represses the expression of facA (3, 42). The FacB activator is responsible for acetate induction of facA (18, 42, 48). However, FacB was not required for acetate repair of growth of strains containing acl(AB)Δ, because acl(AB)Δ facB101 double mutants were readily isolated from a cross and acetate was able to repair growth on both glucose and proline media (Fig. 4B). It has been recently shown that there is some FacB-independent expression of ACS activity in A. niger (29). It is not known whether this represents an unregulated constitutive level of expression or whether there is an unknown activation mechanism in the absence of acetate as inducer.
The effects of mutations affecting acetyl-CoA metabolism and cellular distribution via the acetyl-carnitine shuttle were investigated. Double mutant strains of acl(AB)Δ with loss-of-function mutations were isolated from crosses and tested for effects on acetate growth repair on glucose and proline media (Fig. 4B). In this way it was found that loss of FacC, the cytoplasmic carnitine acetyl-CoA transferase required for growth on acetate (Fig. 1, step 9) (46), AcuJ, the peroxisomal/mitochondrial carnitine acetyl-CoA transferase required for growth on both acetate and fatty acids (Fig. 1, step 11) (3, 19, 46), and AcuH, the mitochondrial acetyl-carnitine carrier protein required for growth on both acetate and fatty acids (Fig. 1, step 10) (3, 9, 10), did not affect the ability of acetate to repair growth of the acl(AB)Δ. In fact the effects of acetate were slightly enhanced, suggesting that, in wild-type strains, a low basal level of these activities on glucose or proline media containing acetate allows some transfer of acetyl-CoA into the mitochondrion, thereby reducing cytoplasmic acetyl-CoA levels.
Regulation of the expression of aclA and aclB.
Northern blot analysis showed that both aclA and aclB were expressed when glucose, proline, or glutamate was present as the sole carbon source (Fig. 4C). Expression was not detected when either acetate or ethanol was the carbon source. This suggested that sources of cytoplasmic acetyl-CoA result in repression. These results were consistent with the finding that ACL enzyme activity was 9-fold higher in glucose-grown cells than acetate-grown cells (1) and also microarray data indicating that expression levels of both genes were about 20-fold higher in glucose than ethanol (8).
Developmental effects of loss of ACL.
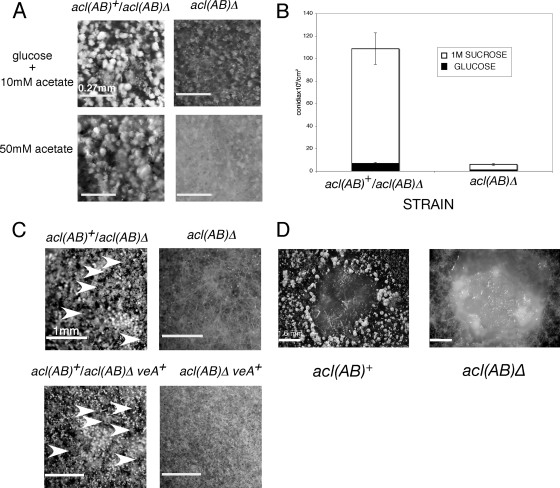
Deletion of the acl genes resulted in obvious reduced production of asexual spores (conidia) in colonies growing on all media, including acetate (Fig. 4A and B). This was partially relieved by high-osmotic medium (1 M sorbitol, 1 M sucrose, and 0.6 M KCl), which is known to enhance conidiation (17). Reduced conidiation was confirmed at the microscopic level (Fig. 5A) and quantitatively by counting viable conidia (Fig. 5B). A. nidulans is homothallic and sexual development can occur by selfing under appropriate conditions, and the presence of the veA+ allele increases the level of sexual development (17). Outcrosses of acl(AB)Δ-containing strains to acl(AB)+strains set up on media containing glucose and acetate were fully fertile. A complete loss of sexual development was found in selfings of acl(AB)Δ strains in both veA1 and veA+ backgrounds, with no nurse cells (Hulle cells) or fruiting bodies (cleistothecia) produced (Fig. 5C). The fatty acid oleate has been found to greatly stimulate the production of cleistothecia, presumably by altering the level of fatty acid-derived oxylipins, which determine the balance between asexual and sexual reproduction (20, 51). acl(AB)Δ was completely insensitive to oleate-induced cleistothecia formation (Fig. 5D).
Fig. 5.
Effects of deletion of aclA and aclB on conidiation and sexual development. (A) Reduced conidiation in the the acl(AB)Δ strain compared to the complemented strain. (B) Viable conidial counts of the acl(AB)Δ strain compared to the complemented strain. One molar sucrose increased the number of conidia for both strains: 7-fold for acl(AB)Δ and 20-fold for the complemented strain. Acetate at 10 mM was present in all media. (C) Loss of sexual development in the acl(AB)Δ strain. White arrowheads indicate mature cleistothecia. Sexual development was not restored in the veA+ background. Selfed crosses were set up on 1% glucose medium containing 10 mM acetate and allowed to develop for 14 days. (D) Sexual development was not restored by oleate-Tween 80. Conidia of each strain were streaked on thick crossing plates containing 1% glucose and 10 mM acetate and incubated for 1 day, and then a drop of 1 M oleate mixed with a drop of Tween 80 was added to the plates. After a further incubation for 1 day, plates were sealed and incubated for 10 days. A dense ring of mature cleistothecia formed around the oleate in the wild-type strain but not in the acl(AB)Δ strain.
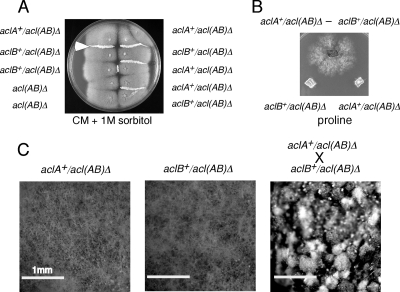
Complementation between aclA and aclB genes in trans.
It has been suggested that divergently transcribed gene pairs are more commonly conserved in fungi than convergently orientated genes and that this may reflect functional relatedness and coexpression via bidirectional promoters (23). This situation is apparent for aclA and aclB in A. nidulans and for ACL genes in other members of the Pezimycotina (Fig. 2). This might indicate a requirement for highly coordinated metabolic and developmental controls. We found that aclA+ and aclB+ genes could complement each other in trans in heterokaryons for conidiation (Fig. 6A), for growth on proline as a carbon source (Fig. 6B), and for sexual development (Fig. 6C). Therefore, aclA+ and aclB+ genes do not have to be adjacent for functional expression. However, it should be noted that each gene contains most of the intergenic upstream sequence, which presumably contains cis-acting sequences that determine appropriate transcriptional regulation.
Fig. 6.
Complementation between aclAΔ and aclBΔ. (A) Complementation for conidiation in heterokaryons. Strains were inoculated adjacent to each other on complete medium containing 1 M sorbitol and 10 mM acetate and incubated for 4 days. Strongly conidiating heterokaryotic mycelia were formed only when aclA+ and aclB+ strains were adjacent (an example is by the white arrow). (B) Complementation for growth on proline in a heterokaryon. An aclB+/acl(AB)Δ niiA4 strain was isolated by crossing and a heterokaryon with aclA+/acl(AB)Δ established by repeated transfers of mycelium to glucose minimal medium containing 10 mM acetate. An agar plug of the heterokaryon together with control agar plugs of aclA+/acl(AB)Δ and aclB+/acl(AB)Δ were transferred to medium containing 50 mM proline as the carbon source. Complementation was observed as strong growth on proline after 4 days of incubation. (C) Complementation for sexual development. A cross between the aclB+/acl(AB)Δ niiA4 strain and aclA+/acl(AB)Δ was set up on 1% glucose minimal medium with sodium nitrate as the sole nitrogen source and 10 mM acetate. Conidia and mature cleistothecia developed after 10 days of incubation, while few conidia and no cleistothecia were produced in the selfed deletion strains.
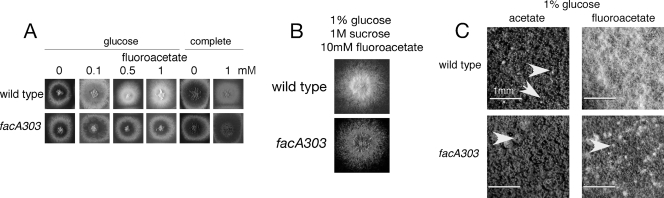
Fluoroacetate inhibition of development.
High levels of fluoroacetate in the absence of a repressing carbon source are toxic to A. nidulans. Loss-of-function mutations in the facA (ACS), facC (cytoplasmic carnitine acetyltransferase), and facB (acetate regulator) genes result in resistance (2, 3). The major mechanism of inhibition is probably due to conversion of fluoroacetate to fluoroacetyl-CoA by FacA and then to fluorocitrate by citrate synthase in the mitochondria, resulting in inhibition of aconitase and hence blocking the TCA cycle. We found that low concentrations of fluoroacetate inhibited development, but not growth, even in the presence of glucose (Fig. 7). Conidiation was inhibited on both glucose minimal medium and complete medium and, furthermore, conidial pigmentation was inhibited. The facA303 mutation resulted in resistance to these effects (Fig. 7A). The effects of fluoroacetate on conidiation were reversed on high-osmotic medium, but conidial pigmentation was still inhibited (Fig. 7B). Loss-of-function mutations in facC or facB did not result in resistance (data not shown), indicating that FacB-independent expression of facA is responsible for the synthesis of cytoplasmic fluoroacetyl-CoA, which results in inhibition of conidiation. Fluoroacetate also resulted in complete inhibition of sexual development, and again the facA303 mutation resulted in resistance (Fig. 7C). Propionyl-CoA, and to a lesser extent acetyl-CoA and butyryl-CoA, has been shown to inhibit ACL activity in A. nidulans (5). Propionate also inhibits the biosynthesis of polyketides, including the conidial pigment, via the formation of propionyl-CoA (59, 60). Therefore, it is likely that the effects of fluoroacetate on asexual and sexual development are mainly due to inhibition of ACL activity by fluoroacetyl-CoA acting in a similar way to propionyl-CoA. The lack of growth inhibition by low levels of fluoroacetate provides support for the hypothesis that higher levels of ACL activity are required for development than for hyphal growth. In addition it is possible that fluoroacetyl-CoA inhibits other enzyme activities that use acetyl-CoA as a substrate and that are involved in development.
Fig. 7.
Inhibition of conidiation and sexual development in the presence of fluoroacetate. (A) Inhibition of conidiation. The indicated amounts of fluoroacetate were added to 1% glucose minimal medium with 10 mM ammonium tartrate as the nitrogen source or to complete medium, and strains were inoculated and grown for 2 days. Fluoroacetate inhibited conidiation and conidial pigment formation in the wild-type strain but not in facA303. (B) Osmotic remediation of the effects of fluoroacetate on conidiation. Colonies were inoculated on glucose medium containing 1 M sucrose and 10 mM fluoroacetate and incubated for 2 days. Conidiation was not inhibited, although conidia lacked pigment in the wild-type strain, but not in the facA303 strain. (C) Inhibition of sexual development by fluoroacetate. Strains were streaked onto 1% glucose minimal medium with 10 mM ammonium tartrate as the nitrogen source, plates were taped to exclude air after 2 days of incubation, and cultures were incubated for an additional 10 days. Either acetate or fluoroacetate was present at 50 mM. Development of cleistothecia (white arrows) and conidia was inhibited by fluoroacetate in the wild type but not the facA303 strain.
DISCUSSION
Deletion of the acl genes results in loss of growth of A. nidulans in the absence of external sources of cytoplasmic acetyl-CoA, strongly suggesting that ACL activity is required to generate cytoplasmic acetyl-CoA. In contrast, S. cerevisiae and C. albicans lack ACL genes, and cytoplasmic ACS activity is essential (6, 47).
A source of cytoplasmic acetyl-CoA is required for fatty acid and sterol biosynthesis. In A. nidulans the gene accA, encoding acetyl-CoA carboxylase, which is necessary for the conversion of acetyl-CoA to malonyl-CoA (and therefore for fatty acid biosynthesis and chain elongation), has been isolated, but attempts to delete the gene were unsuccessful (30). Furthermore, the ACC-specific inhibitor soraphen A inhibited growth even in the presence of added fatty acids, indicating that this enzyme is essential (30). Acetyl-CoA is a substrate for acetoacetyl-CoA thiolase (EC 2.3.1.9) and also for the subsequent synthesis of 3-hydroxy-3-methyl-glutaryl-CoA in the mevalonate-ergosterol biosynthetic pathway. Loss of or reduced acetylation of proteins, including histones, is likely to have significant effects on normal growth (see the introduction). It will be of interest to determine whether ACL is present in the nucleus for histone acetylation, as in mammalian cells (56), and to investigate changes in the patterns of global protein acetylation during growth on different carbon sources.
Our results are in contrast with the properties of an S. macrospora mutant isolated based on a defect in sexual development and found to have a mutation in one of the ACL-encoding genes (34, 36). This mutation did not affect vegetative growth. This could result from media differences or residual ACL activity (about 4% of wild type). A more interesting possibility is that sufficient acetyl-CoA is formed by the pyruvate-acetaldehyde-acetate pathway during growth on glucose. In Neurospora crassa significant levels of ethanol are formed even during aerobic growth on glucose, and expression of the genes for pyruvate decarboxylase and one of the alcohol dehydrogenases is higher in glucose medium compared to carbon-starved conditions (58). It is likely therefore that some of the acetaldehyde formed from pyruvate may be converted to acetate by basal levels of aldehyde dehdrogenase and then to acetyl-CoA by ACS. This pathway may operate in the closely related S. macrospora (35). This could be tested by determining whether the S. macrospora ACL-deficient mutant is affected in growth on carbon sources such as glutamate and proline, where the production of pyruvate by glycolysis cannot occur.
The FacA-dependent acetate repair of the growth of the acl deletants even in the presence of glucose and independent of FacB is consistent with recent observations in A. niger (29). Apparently there is sufficient constitutive ACS present to allow acetyl-CoA formation when acetate is present. This may be a scavenging mechanism when small amounts of acetate are present, although no energy is saved, since one molecule of ATP is consumed by both ACL and ACS reactions. The inability of the acl deletants to grow on quinate, which we propose results in the formation of acetyl-CoA in the mitochondrion, is in accord with a separation of mitochondrial and cytoplasmic acetyl-CoA pools. However, citrate synthesized in the mitochondrion must be able to enter the cytoplasm for ACL activity to occur, and investigations of the mechanisms involved in mitochondrial-cytoplasmic shuttling are needed. The ability of the acl deletion strains to grow to some extent on the fatty acids butyrate and oleate indicates that peroxisomal and cytoplasmic pools of acetyl-CoA are not completely separate. This is in accord with our previous observation that mislocalization of the glyoxylate cycle enzyme malate synthase to the cytoplasm does not affect growth on fatty acids (20). It is of interest that loss of FacC, the cytoplasmic carnitine acetyltransferase, does not prevent growth of the acl(AB)Δ on butyrate or oleate. This shows that some acetyl-CoA can pass through the peroxisomal membrane and does not require conversion to acetyl-carnitine, as previously suggested for S. cerevisiae (53).
Loss of ACL results in greatly reduced production of conidia, even with the provision of acetate as a source of acetyl-CoA. At least one reason for this could be insufficient levels of the siderophores necessary for iron import and intracellular transport required for conidiation (12, 13, 54). The production of both triacetylfusarinine C and ferricrocin requires mevalonate synthesis, and there are also acetyl-CoA-dependent steps in their respective pathways (12). A complete loss of sexual development was found in acl deletion strains, with none of the specialized cell types that occur under inducing conditions being observed. Fatty acid-derived oxylipins are key signaling molecules for development (51). However, it is unlikely that this is the only reason for a requirement for ACL, because oleate did not induce cleistothecial formation in the acl(AB)Δ mutant. Siderophores are important for cleistothecial but not Hulle cell development (13), and the synthesis of polyketide secondary metabolites is important for fruiting body formation in N. crassa and S. macrospora (33). Clearly a number of acetyl-CoA-dependent pathways are essential in A. nidulans. The isolated acl mutant of S. macrospora is not completely deficient in sexual differentiation but only affects later stages (36). As discussed above, this may arise from residual activity in the mutant combined with some acetyl-CoA production from acetate allowing limited development, or it may reflect a real difference in developmental controlling molecules. The phenotype of acl gene deletions would be interesting to determine.
In S. macrospora ACL activity has been found to be present constitutively in medium used for promoting fruiting. However, activity increases at 48 h, corresponding to the initiation of perithecial development, and manipulation of mycelial density to delay the timing of initiation also delayed the increase in activity. This correlated with protein levels and transcription and strongly indicates developmental control of acl gene expression (36). We have demonstrated metabolic regulation of acl gene expression in A. nidulans with strong repression resulting from growth on sources of acetyl-CoA. The molecular basis of this novel control mechanism is entirely unknown and warrants further detailed study. Evidence for one or more developmental controls is provided by the observation that low levels of fluoroacetate inhibit conidiation and sexual development without greatly affecting growth on glucose and that conidiation is reduced in the acl(AB)Δ mutant even during growth on acetate. Divergent transcription of adjacent acl genes is highly conserved in the Pezimycotina. This may reflect a need for strict coordinate regulation by cis-acting bidirectional regulatory sequences to prevent undesirable metabolic effects of unbalanced synthesis of the individual subunits. We have shown that the acl genes do not have to be adjacent to function. Deletion analyses of the intergenic region aimed at determining the extent to which metabolic and developmental regulation during both asexual and sexual reproduction are separable will be of great interest. Our results emphasize the need for fungi to alter their central metabolic pathways during the differentiation of reproductive cell types.
ACKNOWLEDGMENTS
This work was supported by the Australian Research Council.
Assistance by Khanh Nguyen and photography by Quentin Lang are gratefully acknowledged.
Footnotes
Published ahead of print on 21 May 2010.
REFERENCES
- 1.Adams I. P., Dack S., Dickinson F. M., Ratledge C. 2002. The distinctiveness of ATP:citrate lyase from Aspergillus nidulans. Biochim. Biophys. Acta 1597:36–41 [DOI] [PubMed] [Google Scholar]
- 2.Apirion D. 1965. The two-way selection of mutants and revertants in respect of acetate utilization and resistance to fluoro-acetate in Aspergillus nidulans. Genet. Res. 6:317–329 [DOI] [PubMed] [Google Scholar]
- 3.Armitt S., McCullough W., Roberts C. F. 1976. Analysis of acetate non-utilizing (acu) mutants in Aspergillus nidulans. J. Gen. Microbiol. 92:263–282 [DOI] [PubMed] [Google Scholar]
- 4.Boulton C. A., Ratledge C. 1981. Correlation of lipid accumulation in yeasts with possession of ATP:citrate lyase. J. Gen. Microbiol. 127:169–176 [Google Scholar]
- 5.Brock M., Buckel W. 2004. On the mechanism of action of the antifungal agent propionate. Propionyl-CoA inhibits glucose metabolism in Aspergillus nidulans. Eur. J. Biochem. 271:3227–3241 [DOI] [PubMed] [Google Scholar]
- 6.Carman A. J., Vylkova S., Lorenz M. C. 2008. Role of acetyl coenzyme A synthesis and breakdown in alternative carbon source utilization in Candida albicans. Eukaryot. Cell 7:1733–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connerton I. F., Fincham J. R. S., Sandeman R. A., Hynes M. J. 1990. Comparison and cross-species expression of the acetyl-CoA synthetase genes of the ascomycete fungi, Aspergillus nidulans and Neurospora crassa. Mol. Microbiol. 4:451–460 [DOI] [PubMed] [Google Scholar]
- 8.David H., Hofmann G., Oliveira A. P., Jarmer H., Nielsen J. 2006. Metabolic network driven analysis of genome-wide transcription data from Aspergillus nidulans. Genome Biol. 7:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lucas J. R., Dominguez A. I., Valenciano S., Turner G., Laborda F. 1999. The acuH gene of Aspergillus nidulans, required for growth on acetate and long-chain fatty acids, encodes a putative homologue of the mammalian carnitine/acylcarnitine carrier. Arch. Microbiol. 171:386–396 [DOI] [PubMed] [Google Scholar]
- 10.De Lucas R. J., Martínez O., Pérez P., López I. M., Valenciano S., Laborda F. 2001. The Aspergillus nidulans carnitine carrier encoded by the acuH gene is exclusively located in the mitochondria. FEMS Microbiol. Lett. 201:193–198 [DOI] [PubMed] [Google Scholar]
- 11.De Virgilio C., Burckert G., Barth J.-M., Neuhaus N., Boller T., Wiemken A. 1992. Cloning and disruption of a gene required for growth on acetate but not on ethanol: the acetyl-coenzyme A synthetase gene of Saccharomyces cerevisiae Yeast 8:1043–1051 [DOI] [PubMed] [Google Scholar]
- 12.Eisendle M., Oberegger H., Zadra I., Haas H. 2003. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding L-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 49:359–375 [DOI] [PubMed] [Google Scholar]
- 13.Eisendle M., Schrettl M., Kragl C., Muller D., Illmer P., Haas H. 2006. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot. Cell 5:1596–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatland B. L., Ke J., Anderson M. D., Mentzen W. I., Cui L. W., Allred C. C., Johnston J. L., Nikolau B. J., Wurtele E. S. 2002. Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol. 130:740–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flikweert M. T., Van Der Zanden L., Janssen W. M., Steensma H. Y., Van Dijken J. P., Pronk J. T. 1996. Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast 12:247–257 [DOI] [PubMed] [Google Scholar]
- 16.Flipphi M., Mathieu M., Cirpus I., Panozzo C., Felenbok B. 2001. Regulation of the aldehyde dehydrogenase gene (aldA) and its role in the control of the coinducer level necessary for induction of the ethanol utilization pathway in Aspergillus nidulans. J. Biol. Chem. 276:6950–6958 [DOI] [PubMed] [Google Scholar]
- 17.Han K.-H., Lee D.-B., Kim J.-H., Kim M.-S., Han K.-Y., Kim W.-S., Park Y.-S., Kim H.-B., Han D.-M. 2003. Environmental factors affecting development of Aspergillus nidulans. J. Microbiol. 41:34–40 [Google Scholar]
- 18.Hynes M. J. 1977. Induction of the acetamidase of Aspergillus nidulans by acetate metabolism. J. Bacteriol. 131:770–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes M. J., Murray S. L., Duncan A., Khew G. S., Davis M. A. 2006. Regulatory genes controlling fatty acid catabolism and peroxisomal functions in the filamentous fungus, Aspergillus nidulans. Eukaryot. Cell 5:794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes M. J., Murray S. L., Khew G. S., Davis M. A. 2008. Genetic analysis of the role of peroxisomes in the utilization of acetate and fatty acids in Aspergillus nidulans. Genetics 178:1355–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz M. E., Hynes M. J. 1989. Isolation and analysis of the acetate regulatory gene, facB, from Aspergillus nidulans. Mol. Cell. Biol. 9:5696–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly J. M., Drysdale M. R., Sealy-Lewis H. M., Jones I. G., Lockington R. A. 1990. Alcohol dehydrogenase III in Aspergillus nidulans is anaerobically induced and post-transcriptionally regulated. Mol. Gen. Genet. 222:323–328 [DOI] [PubMed] [Google Scholar]
- 23.Kensche P. R., Oti M., Dutilh B. E., Huynen M. A. 2008. Conservation of divergent transcription in fungi. Trends Genet. 24:207–211 [DOI] [PubMed] [Google Scholar]
- 24.Kratzer S., Schuller H.-J. 1995. Carbon source-dependent regulation of the acetyl-CoA synthetase-encoding gene ACS1 from Saccharomyces cerevisiae. Gene 161:75–79 [DOI] [PubMed] [Google Scholar]
- 25.Kuswandi, Roberts C. F. 1992. Genetic control of the protocatechuic acid pathway in Aspergillus nidulans. J. Gen. Microbiol. 138:817–823 [Google Scholar]
- 26.Lockington R. A., Borlace G. N., Kelly J. M. 1997. Pyruvate decarboxylase and anaerobic survival in Aspergillus nidulans. Gene 191:61–67 [DOI] [PubMed] [Google Scholar]
- 27.Mathieu M., Nikolaev I., Scazzocchio C., Felenbok B. 2005. Patterns of nucleosomal organization in the alc regulon of Aspergillus nidulans: roles of the AlcR transcriptional activator and the CreA global repressor. Mol. Microbiol. 56:535–548 [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka M., Ueda Y., Aiba S. 1980. Role and control of isocitrate lyase in Candida lipolytica. J. Bacteriol. 144:692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijer S., de Jongh W. A., Olsson L., Nielsen J. 2009. Physiological characterisation of an acuB deletion in Aspergillus niger. Appl. Microbiol. Biotechnol. 84:157–167 [DOI] [PubMed] [Google Scholar]
- 30.Morrice J., MacKenzie D. A., Parr A. J., Archer D. B. 1998. Isolation and characterisation of the acetyl-CoA carboxylase gene from Aspergillus nidulans. Curr. Genet. 34:379–385 [DOI] [PubMed] [Google Scholar]
- 31.Nayak T., Szewczyk E., Oakley C. E., Osmani A., Ukil L., Murray S. L., Hynes M. J., Osmani S. A., Oakley B. R. 2006. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172:1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogales J., Macchi R., Franchi F., Barzaghi D., Fernandez C., Garcia J. L., Bertoni G., Diaz E. 2007. Characterization of the last step of the aerobic phenylacetic acid degradation pathway. Microbiology 153:357–365 [DOI] [PubMed] [Google Scholar]
- 33.Nowrousian M. 2009. A novel polyketide gene cluster is involved in fruiting body morphogenesis in the filamentous fungi Sordaria macrospora and Neurospora crassa. Curr. Genet. 55:185–198 [DOI] [PubMed] [Google Scholar]
- 34.Nowrousian M., Kuck U., Loser K., Weltring K.-M. 2000. The fungal acl1 and acl2 genes encode two polypeptides with homology to the N- and C-terminal parts of the animal ATP citrate lyase polypeptide. Curr. Genet. 37:189–193 [DOI] [PubMed] [Google Scholar]
- 35.Nowrousian M., Ringelberg C., Dunlap J. C., Loros J. J., Kück U. 2005. Cross-species microarray hybridization to identify developmentally regulated genes in the filamentous fungus Sordaria macrospora. Mol. Genet. Genomics 273:137–149 [DOI] [PubMed] [Google Scholar]
- 36.Nowrousian M., Masloff S., Poggeler S., Kück U. 1999. Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity. Mol. Cell. Biol. 19:450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osmani S. A., Scrutton M. C. 1983. The sub-cellular localization of pyruvate carboxylase and of some other enzymes in Aspergillus nidulans. Eur. J. Biochem. 133:551–560 [DOI] [PubMed] [Google Scholar]
- 38.Pfitzner A., Kubicek C. P., Röhr M. 1987. Presence and regulation of ATP:citrate lyase from the citric acid producing fungus Aspergillus niger. Arch. Microbiol. 147:88–91 [DOI] [PubMed] [Google Scholar]
- 39.Pronk J. T., Steensma H. Y., Van Dijken J. P. 1996. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 12:1607–1633 [DOI] [PubMed] [Google Scholar]
- 40.Saint-Prix F., Bonquist L., Dequin S. 2004. Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: the NADP+-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiology 150:2209–2220 [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed.Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 42.Sandeman R. A., Hynes M. J. 1989. Isolation of the facA (acetyl-coenzyme A synthetase) and acuE (malate synthase) genes of Aspergillus nidulans. Mol. Gen. Genet. 218:87–92 [DOI] [PubMed] [Google Scholar]
- 43.Schuller H.-J. 2003. Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr. Genet. 43:139–160 [DOI] [PubMed] [Google Scholar]
- 44.Shashi K., Bachhawat A. K., Joseph R. 1990. ATP:citrate lyase of Rhodotorula gracilis: purification and properties. Biochim. Biophys. Acta 1033:23–30 [DOI] [PubMed] [Google Scholar]
- 45.Starai V. J., Escalante-Semerena J. C. 2004. Acetyl-coenzyme A synthetase (AMP forming). Cell Mol. Life Sci. 61:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stemple C. J., Davis M. A., Hynes M. J. 1998. The facC gene of Aspergillus nidulans encodes an acetate-inducible carnitine acetyltransferase. J. Bacteriol. 180:6242–6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi H., McCaffery J. M., Irizarry R. A., Boeke J. D. 2006. Nucleocytosolic acetyl-coenzyme A synthetase is required for histone acetylation and global transcription. Mol. Cell 23:207–217 [DOI] [PubMed] [Google Scholar]
- 48.Todd R. B., Andrianopoulos A., Davis M. A., Hynes M. J. 1998. FacB, the Aspergillus nidulans activator of acetate utilization genes, binds dissimilar DNA sequences. EMBO J. 17:2042–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todd R. B., Murphy R. L., Martin H. M., Sharp J. A., Davis M. A., Katz M. E., Hynes M. J. 1997. The acetate regulatory gene facB of Aspergillus nidulans encodes a Zn(II) Cys6 transcriptional activator. Mol. Gen. Genet. 254:495–504 [DOI] [PubMed] [Google Scholar]
- 50.Todd R. B., Davis M. A., Hynes M. J. 2007. Genetic manipulation of Aspergillus nidulans: meiotic progeny for genetic analysis and strain construction. Nat. Protoc. 2:811–821 [DOI] [PubMed] [Google Scholar]
- 51.Tsitsigiannis D. I., Keller N. P. 2007. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 15:109–118 [DOI] [PubMed] [Google Scholar]
- 52.van den Berg M. A., de Jong-Gubbels P., Kortland C. J., van Dijken J. P., Pronk J. T., Yde Steensma H. 1996. The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae. differ with respect to kinetic properties and transcriptional regulation. J. Biol. Chem. 271:28953–28959 [DOI] [PubMed] [Google Scholar]
- 53.van Roermund C. W. T., Elgersma Y., Singh N., Wanders R. J. A., Tabak H. 1995. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 14:3480–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallner A., Blatzer M., Schrettl M., Sarg B., Lindner H., Haas H. 2009. Ferricrocin, a siderophore involved in intra- and transcellular iron distribution in Aspergillus fumigatus. Appl. Environ. Microbiol. 75:4194–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q., Zhang Y., Yang C., Xiong H., Lin Y., Yao J., Li H., Xie L., Zhao W., Yao Y., Ning Z.-B., Zeng R., Xiong Y., Guan K.-L., Zhao S., Zhao G.-P. 2010. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327:1004–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wellen K. E., Hatzivassiliou G., Sachdeva U. M., Bui T. V., Cross J. R., Thompson C. B. 2009. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wynn J. P., Hamid A. A., Midgley M., Ratledge C. 1998. Widespread occurrence of ATP:citrate lyase and carnitine acetyltransferase in filamentous fungi. World J. Microbiol. Biotechnol. 14:145–147 [Google Scholar]
- 58.Xie X., Wilkinson H. H., Correa A., Lewis Z. A., Bell-Pedersen D., Ebbole D. J. 2004. Transcriptional response to glucose starvation and functional analysis of a glucose transporter of Neurospora crassa. Fungal Genet. Biol. 41:1104–1119 [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y.-Q., Keller N. P. 2004. Blockage of methylcitrate cycle inhibits polyketide production in Aspergillus nidulans. Mol. Microbiol. 52:541–550 [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y., Brock M., Keller N. P. 2004. Connection of propionyl-CoA metabolism to polyketide biosynthesis in Aspergillus nidulans. Genetics 168:785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S., Xu W., Jiang W., Yu W., Lin Y., Zhang T., Yao J., Zhou L., Zeng Y., Li H., Li Y., Shi J., An W., Hancock S. M., He F., Qin L., Chin J., Yang P., Chen X., Lei Q., Xiong Y., Guan K.-L. 2010. Regulation of cellular metabolism by protein lysine acetylation. Science 327:1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]