Abstract
Fungi contain several hexokinases, which are involved either in sugar phosphorylation or in carbon source sensing. Glucose and fructose phosphorylations appear to rely exclusively on glucokinase and hexokinase. Here, we characterized the catalytic glucokinase and hexokinase from the opportunistic human pathogen Aspergillus fumigatus and showed that both enzymes display different biochemical properties and play different roles during growth and development. Glucokinase efficiently activates glucose and mannose but activates fructose only to a minor extent. Hexokinase showed a high efficiency for fructose activation but also activated glucose and mannose. Transcript and activity determinations revealed high levels of glucokinase in resting conidia, whereas hexokinase was associated mainly with the mycelium. Consequentially, a glucokinase mutant showed delayed germination at low glucose concentrations, whereas colony growth was not overly affected. The deletion of hexokinase had only a minor impact on germination but reduced colony growth, especially on sugar-containing media. Transcript determinations from infected mouse lungs revealed the expression of both genes, indicating a contribution to virulence. Interestingly, a double-deletion mutant showed impaired growth not only on sugars but also on nonfermentable nutrients, and growth on gluconeogenic carbon sources was strongly suppressed in the presence of glucose. Furthermore, the glkA hxkA deletion affected cell wall integrity, implying that both enzymes contribute to the cell wall composition. Additionally, the absence of either enzyme deregulated carbon catabolite repression since mutants displayed an induction of isocitrate lyase activity during growth on glucose-ethanol medium. Therefore, both enzymes seem to be required for balancing carbon flux in A. fumigatus and are indispensable for growth under all nutritional conditions.
Aspergillus fumigatus is an opportunistic human pathogen and is able to cause life-threatening invasive aspergillosis mainly in immunocompromised patients (34). Only a limited number of antifungals are currently available to combat fungal infections. Nutrition assimilation is a prerequisite for infection, and a better understanding of the metabolic processes during infection may help to identify new antifungal drug targets. However, since infection is a dynamic process, high metabolic versatility is assumed to favor adaptation to rapidly changing environmental conditions within a host (4).
Glucose is highly abundant in some sites within the human body, and the concentration in the bloodstream ranges between 6 and 8 mM (12). Additionally, the brain of vertebrates contains high glucose and low protein levels, and investigations of a diploid Saccharomyces cerevisiae hexokinase 2 mutant (hex2Δ/hex2Δ) showed that Hex2p is essential for successful survival within murine brains (29). Although the glucose concentration within tissues may be much lower, a role for glucose metabolism in pathogenesis is conceivable. The utilization of glucose could be beneficial for pathogenic fungi, because it allows an easy acquisition of building block precursors for biomass formation, reducing equivalents and energy. The initial step in sugar utilization is its uptake and subsequent activation to a sugar phosphate. Bacteria frequently use a coupled phosphoenolpyruvate-dependent carbohydrate:phosphotransferase system for sensing, uptake, and activation of sugars (30). In contrast, fungi seem to possess specific hexose transporters and activate sugars by soluble hexokinases via the consumption of ATP (6).
The activation of hexoses, especially of glucose and fructose, in relatives of A. fumigatus, that is, Aspergillus nidulans and Aspergillus niger, has previously been studied. Previous investigations revealed that both fungi contain at least two catalytically active hexose kinases called glucokinase and hexokinase (19, 35, 36, 41). Several other enzymes with high sequence similarity to hexose kinases have been detected by genome analyses, but these enzymes seem to possess regulatory functions in carbon sensing rather than contributing directly to the activation of hexoses to sugar phosphates (3, 18, 28).
Glucokinase and hexokinase from A. niger have been purified and biochemically characterized by homologous overproduction and subsequent purification of the enzymes (35, 36). Comparison of the catalytic properties of both enzymes showed that glucokinase possesses a very high specificity for glucose, with a specific activity of 233 U/mg and a Km value of 63 μM. The activation of fructose was not assumed to occur naturally, because the Km for fructose was approximated to be 120 mM. Additionally, it was shown previously that the activity of the glucokinase was not significantly inhibited by the addition of the hexokinase inhibitor trehalose-6-phosphate (T6P) (35). In contrast, purified A. niger hexokinase showed a specific activity of 220 U/mg for fructose and a Km of 2 mM but was also significantly active with glucose (specific activity = ∼20 U/mg; Km = 0.35 mM). Fructose phosphorylation activity was inhibited by trehalose-6-phosphate in a concentration-dependent manner, which allowed the discrimination of glucokinase and hexokinase activities in cell extracts (36). Those investigations implied that glucokinase might be mainly responsible for glucose metabolism whereas the main function of hexokinase is the activation of fructose. However, none of the respective genes had been deleted in A. niger, and the in vivo contribution of each enzyme to sugar metabolism remained speculative.
Although a detailed biochemical characterization of these two enzymes in the model organism A. nidulans has not been performed, mutants with defective hexokinase (frA1) (38) and glucokinase (glkA4) as well as a double mutant (19) have been constructed and phenotypically analyzed. Those authors investigated the contribution of both enzymes to sugar metabolism and their impact on carbon catabolite repression. That analysis revealed no visible growth defect for the glkA mutant, which implied that the function was completely compensated for by the hexokinase. In contrast, the hexokinase mutant was no longer able to grow on fructose as the sole carbon source, confirming that glucokinase is indeed unable to perform fructose phosphorylation in vivo. However, on glucose as the sole carbon source, no phenotype was observed for the hexokinase mutant. As expected, the double mutant was unable to grow on glucose or fructose. In addition, this double mutant displayed deregulated carbon catabolite repression, which was not observed for the single mutants (19). However, the mutants investigated in that study were generated by UV mutagenesis, and it might be possible, although unlikely, that one enzyme still possessed low catalytic activity or some regulatory function. In contrast to investigations of A. nidulans, the deletion of the hexokinase in the fruit-pathogenic fungus Botrytis cinerea revealed a pleiotropic growth defect on various carbon sources, whereas a glucokinase mutant, in agreement with data for A. nidulans, displayed no altered phenotype. Furthermore, for B. cinerea, attempts to generate a double-deletion strain were unsuccessful, implying the synthetic essentiality of the two genes (40).
Due to these discrepancies between A. nidulans and B. cinerea, we aimed to elucidate the impact of glucokinase and hexokinase on the growth and development of A. fumigatus. Of special interest was the investigation of the physiological role of glucokinase, since the deletion of this enzyme in both A. nidulans and B. cinerea did not alter their phenotypes. For this purpose, we performed recombinant protein productions with Escherichia coli and recorded the biochemical parameters of both enzymes in vitro. These data were used to predict the role of both enzymes in vivo. Additionally, we generated deletion mutants and studied their phenotypes at different developmental stages and during growth on various carbon sources. Our investigations revealed that glucokinase is associated mainly with resting conidia and seems to be required for glucose activation from storage sugars such as trehalose. Additionally, glucokinase might contribute to glucose phosphorylation under conditions of low glucose concentrations in the environment. In contrast, hexokinase is associated mainly with the mycelium and plays a major role in sugar utilization during vegetative growth. Additionally, severe phenotypes of a double-deletion mutant on glycolytic and gluconeogenic carbon sources demonstrated that at least one enzyme must be present to allow normal growth, conidiation, and cell wall integrity.
MATERIALS AND METHODS
Media, growth conditions, and preparation of cell extracts.
To obtain conidial suspensions, all strains were inoculated on malt extract agar slants (Fluka, Taufkirchen, Germany) and incubated for 5 to 7 days at room temperature. Strains with delayed growth and conidiation were incubated at 30°C. Media were overlaid with phosphate-buffered saline (PBS) plus 0.1% Tween 20 (PBS-Tween), and conidia were scraped from the mycelium. Conidial suspensions were filtered through 40-μm cell strainers (BD Biosciences, CA) to remove clumps and mycelial fragments. Conidial suspensions were stored for a maximum of 7 days at 4°C without a significant loss of viability. For testing of growth phenotypes on different carbon sources, Aspergillus minimal media were prepared as described by the Fungal Genetic Stock Center (http://www.fgsc.net/Aspergillus/protocols/MediaForAspergillus.pdf), with the pH adjusted to 6.5. For solid media, 2% agar was added prior to sterilization. Carbon sources were either malt extract (Fluka), potato dextrose broth (Sigma), Sabouraud medium (Sigma), peptone (1%), Casamino Acids (1%), bovine serum albumin (1%), starch (1%), lecithin from egg yolk (1%; Fluka), glucose (50 mM, if not indicated otherwise), ribose (50 mM), mannose (50 mM), galactose (50 mM), trehalose (25 mM), lactose (25 mM), saccharose (25 mM), fructose (50 mM), sorbose (50 mM), glucosamine (50 mM), acetate (100 mM), or ethanol or glycerol (each 100, 50, or 10 mM). Incubations were performed at 37°C, and liquid cultures were agitated at 210 rpm on a rotary shaker. For the preparation of cell extracts from mycelia, liquid cultures were filtered through Miracloth filter gauze (Merck, Darmstadt, Germany). The retained mycelium was washed once with water and pressed dry. Cells were disrupted under liquid nitrogen in a mortar, and the powdered mycelium was suspended in an appropriate buffer for subsequent enzyme activity determinations. For the preparation of cell extracts from conidia, fresh conidial suspensions were washed once with an appropriate buffer, resuspended as a thick paste, and mixed in 0.5-ml screw-cap vials with zirconia beads (diameter, 0.5 mm; Carl Roth GmbH, Karlsruhe, Germany). Conidia (ca. 1 × 109 conidia in 400 μl) were disrupted in a Speed Mill (Analytik Jena, Jena, Germany) three times for 2 min, with 2 min on ice after each cycle. All crude extracts were centrifuged at 15,000 × g for 5 min, and the cell-free supernatants were collected. Protein concentrations were determined by using Bradford protein assay concentrate as described by the manufacturer (Bio-Rad, Munich, Germany).
Germination analysis.
To determine the germination rates of different A. fumigatus isolates, freshly harvested conidia were labeled with fluorescein isothiocyanate (FITC) as described previously (26). After three washes of labeled conidia with PBS-Tween, the number of conidia in suspension was counted with a hemocytometer, and 100,000 conidia were used to inoculate 1 ml of the respective medium added to wells of four-well LabTek chamber slides (Nalgene Nunc, New York, NY). Slides were incubated at 37°C in a humid incubator in the presence of 5% CO2 for the times indicated in Results. Germination was terminated by removing the medium and fixing the cells with 1% paraformaldehyde (PFA) for 9 min at room temperature. PFA was removed, and slides were washed with 50 mM (NH4)2SO4 and subsequently incubated for 9 min in the presence of 50 mM (NH4)2SO4. After a washing step with PBS the chambers were removed, and cells were embedded in a DAPI (4′,6-diamidino-2-phenylindole)-containing mounting solution (ProLong Gold Antifade reagent with DAPI; Invitrogen). Slides were sealed and incubated for at least 12 to 24 h at 4°C prior to fluorescence microscopy with an AxioImager fluorescence microscope (Zeiss, Jena, Germany). Bright-field, FITC fluorescence, and DAPI fluorescence pictures were recorded at a ×40 magnification, and pictures were overlaid by using the MetaMorph software package (version 6.1; Molecular Devices, CA). Overlays were magnified with PowerPoint (Microsoft Office; Microsoft Corporation, WA), and hard-copy printouts were used to evaluate each single cell. For each condition, at least six independent pictures were evaluated, resulting in more than 300 individual cells evaluated for each strain and culturing condition. Germination was classified by a numbering system (score of 1 to 5), as explained in more detail in Results. In parallel, the viability of conidia was tested by incubating conidia for 7 to 8 h in Sabouraud complete medium. Due to the poor growth of the ΔglkA ΔhxkA double mutant on Sabouraud medium, 1% peptone-containing medium was used for viability control. Evaluation of at least 1,000 conidia was performed directly with a fluorescence microscope using FITC labeling of conidia as a readout system. Resting conidia appeared as small but brightly fluorescent spheres, whereas swollen conidia were three to four times larger, and the fluorescence of conidia was interrupted at the site of germ tube formation. For microscopic evaluation of germination and colony formation of the double-deletion strain, glass slides were coated with minimal medium containing 1.5% agarose. Between 200 and 1,000 conidia were plated onto the slides and incubated in a humid chamber for various time points. For microscopic analysis the medium was overlaid with 20 μl of PBS, and a coverslip was applied. Cells were observed in bright field using a ×10 to ×40 magnification.
Recombinant production and purification of GlkA, HxkA, and HxkB.
Sequences of all oligonucleotides used in this study are listed in Table SA1 in the supplemental material. For the amplification of coding regions of glucokinase and hexokinase, cDNA was used as a template. The RNA for cDNA synthesis was derived from A. fumigatus cultures grown for 20 h at 37°C on liquid medium containing 50 mM glucose and 0.5% (wt/vol) peptone. RNA extraction and reverse transcription were performed as described previously (5). The open reading frame of glkA was amplified from cDNA with the sequence-specific oligonucleotides cDNAglkAAfBam_up and cDNAglkAAfHind_d, hxkA was amplified with oligonucleotides HxkAfNco_up and HxkAfHind_down, and hxkB was amplified by using oligonucleotides HxkBcDNABgl_f and HxkBcDNAHind_r. All amplifications were performed with Phusion proofreading polymerase (New England Biolabs, Frankfurt am Main, Germany). PCR fragments were cloned into the pJet1.2/blunt vector (MBI Fermentas, St. Leon-Rot, Germany) and transferred into E. coli DH5α cells (Invitrogen, Karlsruhe, Germany). Plasmid DNA was isolated with the NucleoSpin kit (Machery-Nagel, Düren, Germany). Three plasmids each, containing either the glkA, the hxkA, or the hxkB gene, were selected for sequencing to confirm the predicted intron positions and sequences of the genes. The glkA gene was excised by BamHI and HindIII restriction, the hxkB gene was excised by BglII and HindIII restriction, and the hxkA gene was excised by NcoI and HindIII restriction. All fragments were subsequently cloned into a modified pET43.1a expression vector containing an N-terminal His tag (22). After the transformation of E. coli DH5α cells, plasmid DNA was isolated, and E. coli BL21(DE3) Rosetta 2 cells (Merck/Novagen) were transformed. Positive clones were transferred into 10 ml of Overnight Express Instant TB medium (Merck/Novagen) and incubated for 22 h at 30°C. For HxkB purification, incubation at 18°C for 48 h was required to avoid the formation of inclusion bodies. Cells were harvested by centrifugation, resuspended in 4 ml HEPES buffer (50 mM [pH 7.5]) (buffer A), and disrupted by sonication (Sonoplus; Bandelin, Berlin, Germany). After the removal of cell debris by centrifugation, the clear supernatant was loaded onto a Ni-nitrilotriacetic acid (NTA) agarose column (1-ml bed volume) previously equilibrated by gravity flow with buffer A. The column was rinsed with 8 ml buffer A containing 30 mM imidazole and 100 mM NaCl. Enzymes were eluted with buffer A containing 200 mM imidazole and 150 mM NaCl. Protein concentrations were determined by the Bio-Rad protein assay (Bio-Rad) with bovine serum albumin as a standard. The purity of fractions was determined by sodium dodecyl sulfate (SDS)-PAGE analysis on NuPage Bis-Tris 4 to 12% gradient gels using an MES (morpholineethanesulfonic acid)-buffered running system (Invitrogen). Pure fractions were combined and desalted against buffer A by using centrifugal filter devices with a molecular cutoff of 30 kDa (Millipore, Schwalbach, Germany). Glycerol was added to a final concentration of 50%, and enzymes were stored at −20°C. Under these conditions all enzyme preparations remained stable for at least 6 months.
Deletion of glkA and hxkA from A. fumigatus.
For the deletion of the coding regions of glkA and hxkA, upstream and downstream fragments containing an overlapping NotI restriction site were amplified, which allowed the assembly of both fragments by subsequent PCR as described previously (17). The glkA upstream fragment was amplified with oligonucleotides Bam-215glkAAf_up (P1) and NotGlkAAfup_r (P2), and the downstream fragment was amplified with NotGlkAAfdown_f (P3) and BamGlkAAfdown_r (P4). For the hxkA deletion, the upstream fragment was amplified with oligonucleotides BamHxkAAFup_f (P1) and NotHxkAAFup_r (P2), and the downstream fragment was amplified with NotHxkAAFdown_f (P3) and BamHxkAAFdown_r (P4). All fragments were gel purified, and aliquots of the respective up- and downstream fragments were mixed, denatured, annealed, and amplified by PCR for five cycles prior to the addition of oligonucleotides P1 and P4 and further amplification for 30 cycles. The assembled up- and downstream fragments were gel purified and subcloned into the pJet1.2/blunt vector. Fragments were excised by BamHI restriction and subcloned into a BamHI-restricted and -dephosphorylated pUC19 vector (Fermentas). The resulting plasmids, ΔglkAAf_pUC and ΔhxkAAf_pUC, were restricted with NotI and dephosphorylated. The pyrithiamine resistance cassette (ptrA) was excised with NotI from plasmid ptrA-pJET1, and the hygromycin resistance cassette (hph) was excised from plasmid hph-pCRIV. ptrA was cloned into ΔhxkAAf_pUC, yielding ΔhxkAAf_pUC/ptrA, and hph was cloned into ΔglkAAf_pUC, yielding ΔglkAAf_pUC/hph. For the transformation of the A. fumigatus ΔakuB pyrG+ strain (7) containing a deletion of the akuB gene, which is required for the nonhomologous end-joining repair mechanism, the deletion cassettes were excised by BamHI restriction. Fungal transformation was carried out according to standard procedures (48) by using 0.1 μg pyrithiamine/ml as selection marker for the hxkA deletion and 240 μg hygromycin B/ml as a selection marker for the glkA deletion. Transformants were purified by the repeated streaking of conidia onto selective medium, and genomic DNA was isolated from glucose-grown cultures by a yeast DNA purification kit (Biozym, Hess. Oldendorf, Germany). Probes for Southern blot analysis were labeled by PCR with digoxigenin-11-dUTPs (Roche Diagnostics, Mannheim, Germany). The probe for glkA deletion strains was directed against the downstream region, and the 900-bp probe was amplified with oligonucleotides NotGlkAAf_down_f and BamGlkAAf_down_r. For hxkA deletion strains the 800-bp probe was directed against the upstream region and amplified with oligonucleotides BamHxkAAFup_f and NotHxkAAFup_r. From each deletion approach, eight independent transformants were randomly selected for genomic DNA isolation. The DNA for glkA deletion strains was restricted with HindIII, and a shift of the wild-type fragment from 4.9 kb to 7.7 kb was expected. For hxkA the genomic DNAs were restricted with BamHI, and a shift of the wild-type fragment from 3.1 kb to 6.0 kb was expected. Bands were visualized by CDPStar luminescence after hybridization with α-digoxigenin Fab fragments conjugated with alkaline phosphatase according to the manufacturer's protocol (Roche Diagnostics).
Generation of glkA hxkA double-deletion mutants.
To investigate the phenotype of a mutant strain devoid of any catalytically active hexose kinase, the hexokinase was deleted in the glucokinase deletion background. For this purpose, one of the glucokinase deletion strains was selected and transformed with the construct ΔhxkAAf_pUC/ptrA for the hxkA deletion. Transformants were selected on medium containing glycerol as the sole carbon source and pyrithiamine as a selectable marker. Small colonies appeared approximately 7 days after transformation, and these colonies were streaked repeatedly onto glycerol-pyrithiamine medium. Simultaneously, the strains were checked for their resistance to hygromycin, which is indicative of the ΔglkA background. Genomic DNA of the wild type, 10 transformants, the ΔglkA strain, and an ΔhxkA strain were restricted with EcoRI, and Southern blotting was performed with probes against the glkA downstream region and the hxkA upstream region. For the glkA deletion, a shift of the hybridizing fragment from 10.9 to 4.4 kb was expected, whereas for hxkA, the wild-type fragment was expected to shift from 4.9 to 3.2 kb.
Complementation of glkA and hxkA deletion mutants.
For the complementation of glkA and hxkA deletion mutants, one representative clone each was selected. Since the glkA deletion did not result in a severe growth phenotype, we used the pyrithiamine resistance cassette to screen for complemented strains. Due to the fact that the hxkA deletion resulted in an inability to use fructose as a substrate, no additional selection marker was required for complementation, and transformants were selected on fructose-containing regeneration plates. However, to distinguish complemented mutants from wild-type contaminations, we introduced an artificial HindIII restriction site into the downstream noncoding region of hxkA. For both complementation approaches, we expected a homologous integration of the construct at the original genomic locus. For glkA complementation a 250-bp upstream fragment together with the whole coding sequence and a 190-bp downstream region were amplified with oligonucleotides Hind250glkAAf_up and GlkAAfNotdo_r. An additional downstream fragment of approximately 700 bp, overlapping with its NotI restriction site to the downstream fragment of the former PCR product, was amplified with oligonucleotides GlkAAfNotdo_f and HindglkAdo_r2. As described above, PCR fragments were gel purified, mixed, and fused by PCR using oligonucleotides Hind250glkAAf_up and HindglkAdo_r2. The fragment was first cloned into the pJet1.2 cloning vector, excised by HindIII restriction, and subcloned into pUC19. The vector was linearized with NotI, and the ptrA resistance cassette was inserted. The complete complementation cassette was excised by HindIII restriction and used for the transformation of the A. fumigatus ΔglkA mutant. Clones were selected by pyrithiamine resistance and subsequently by the loss of hygromycin resistance, which was indicative of recombination at the original locus. Transformants were checked by Southern analysis using a 720-bp probe directed against the downstream region of glkA, which was amplified with oligonucleotides GlkAAfNotdo_f and HindGlkAdo_r. Genomic DNA was restricted with BamHI, leading to the expected fragment size of 8.8 kb for the wild type and 7.7 and 6.3 kb, respectively, for the complemented strains, depending on the orientation of the ptrA cassette in the two independent plasmids used for transformation.
For the complementation of the hxkA mutant, an 870-bp upstream fragment together with the coding region and a 180-bp downstream region were amplified with oligonucleotides CompHxkAAfKpnF and HindCompHxkAAfR. Additionally, a 560-bp downstream fragment was amplified with oligonucleotides HindCompHxkAAfF and CompHxkAAfKpnR, whereby the forward primer containing a HindIII restriction site was complementary to HindCompHxkAAfR. As described above, both fragments were fused by PCR, amplified with oligonucleotides CompHxkAAfKpnF and CompHxkAAfKpnR, and subcloned into the pJet1.2 vector. The complementation cassette was removed by KpnI restriction and used directly for the transformation of the ΔhxkA mutant. After the selection of transformants on fructose medium, strains were checked for the loss of pyrithiamine resistance, which was indicative of homologous integration at the hxkA locus. Genomic DNA of transformants was restricted with HindIII, and the probe directed against the upstream region of hxkA was used for Southern analysis. A shift of the wild-type fragment from 7.2 to 4.6 kb was expected.
qRT-PCR for determination of glkA and hxkA expression.
To isolate RNA from conidia and mycelium, the respective cell type was either disrupted by using a Speed Mill or by grinding under liquid nitrogen as described above. For RNA extraction the RNeasy plant minikit (Qiagen, Hilden, Germany) with a DNase Zero treatment (Biozym) was applied. The absence of contaminating DNA was checked by PCR on the isolated total RNA with primers against the citrate synthase gene as described previously (24). An aliquot of the RNA was reverse transcribed by using SuperScript III reverse transcriptase and an anchored oligo(dT)20 primer (both from Invitrogen) as described previously (24). Quantitative real-time PCR (qRT-PCR) was performed with a Rotor-Gene 6000 system (Qiagen) with the DyNAmo Flash SYBR green quantitative PCR kit (New England Biolabs). Each set of oligonucleotides was tested for its efficiency to amplify the respective partial gene. Final data analysis was performed by using the efficiency values incorporated in the 2−ΔΔCT method (31, 37). Actin (primer set ACT1-for and ACT1-rev) and β-tubulin (primer set RT_Tub_f and RT_Tub_r) served as housekeeping genes for data normalization. Transcript levels for hxkA were determined with the primer set qRT_hxkAAf_for and qRT_ hxkAAf_rev, whereas glkA transcript levels were determined by using the oligonucleotides qRT_glkAAf_for and qRT_glkAAf_rev. Comparative analysis between actin and β-tubulin showed that the latter was not constitutively expressed in resting conidia, because transcript levels for this gene showed extremely high sample-to-sample variation. Therefore, only the data from act1 expression were used for the normalization of glkA and hxkA transcript levels.
Semiquantitative determination of glkA and hxkA transcript levels from infected mouse lungs.
Lungs of mice were obtained from an independent study to investigate the time-dependent fungal burden under different immunosuppression regimens (25). In brief, 6- to 8-week-old male BALB/c-J mice were immunosuppressed either with the cytostatic drug cyclophosphamide (200 mg/kg body weight at day −4 and day −1) or with the corticosteroid cortisone acetate (1 mg/g body weight at day −3 and at the day of infection). Mice were infected intranasally with suspensions containing 2 × 106 conidia of A. fumigatus wild-type strain CBS144.89. This strain is the parental strain of the ΔakuB strain that was used to create the glkA and hxkA deletion mutants. Two mice from each immunosuppression regimen were sacrificed 24 h after infection, and two additional mice were sacrificed 72 h after infection. Lungs were removed, frozen in liquid nitrogen, and ground to a fine powder. RNA was isolated and transcribed into cDNA as described elsewhere previously (24). Due to unspecific amplification and very low transcript levels of glkA and hxkA, a quantitative evaluation by qRT-PCR approaches was not possible. Therefore, a semiquantitative approach was chosen, in which different amounts of cDNA served as templates for amplification by standard PCR with a SpeedCycler (Analytik Jena AG, Jena, Germany) using GoTaq polymerase and the same primers as those described above. After 35 amplification cycles the PCR products were loaded on an ethidium bromide-stained 2% agarose gel. Bands were visualized by UV illumination, and the intensities of the bands specific for glkA and hxkA were quantified by using the TraceQuantity method of the QuantityOne software package (Bio-Rad Laboratories GmbH, Munich, Germany). From these quantifications, the ratio between glkA and hxkA transcript levels in each individual sample was calculated.
Enzyme assays and biochemical characterization of hexose kinases.
The ATP-dependent phosphorylation of sugars was determined at pH 7.5, as described elsewhere previously (36), by utilizing a coupled assay system with pyruvate kinase and lactate dehydrogenase to monitor ADP formation during the reaction. Glucose phosphorylation was independently determined via glucose-6-phosphate dehydrogenase (35) but yielded the same specific activities and was therefore not monitored further. Glucose, fructose, mannose, galactose, glucosamine, 2-deoxyglucose (2-DG), ribose, lactose, trehalose, and sorbose were tested as substrates. Km values were determined by varying the concentration of the sugars while keeping all other components constant. The Km values were calculated by double-reciprocal plotting of the activity versus the substrate concentration. By adding different amounts of trehalose-6-phosphate to the standard assay mixture, the inhibition of phosphorylating activity was tested. At least three activity determinations were performed under each condition tested, and data represent mean values with standard deviations.
Isocitrate lyase activity was determined, as previously described (11), by using a phenylhydrazine-based system to measure the release of glyoxylate from isocitrate.
Sensitivity of glkA and hxkA deletion strains to cell wall-stressing compounds.
Several different cell wall-stressing agents were used to investigate the cell wall integrity of A. fumigatus mutant strains in comparison to the wild type (45). All strains except the double-deletion strain were tested for sensitivity on agar plates containing either 50 mM glucose or 100 mM glycerol minimal medium. Plates were supplemented with one of the following compounds: calcofluor white (150 and 300 μg/ml), sodium dodecyl sulfate (0.01 and 0.02%), caffeine (5 and 10 mM), or Congo red (20 and 40 μg/ml). For inclusion of the ΔglkA ΔhxkA double-deletion mutant, 1%-peptone-containing agar plates were supplemented with 100 μg calcofluor white, 0.01% sodium dodecyl sulfate, or 40 μg/ml Congo red. Serial 10-fold dilutions (range of between 1 × 105 and 1 × 101 conidia in 5 μl) were prepared and spotted onto the plates. All plates were incubated at 37°C for up to 144 h.
RESULTS
Identification, purification, and determination of initial activity of GlkA, HxkA, and HxkB.
For the identification of glucokinase and hexokinase coding sequences, we used the respective A. niger protein sequences in a BLAST search against the genome of sequenced A. fumigatus strain A1163. The A. fumigatus glucokinase GlkA was identified by using A. niger GlkA (GenBank accession number Q92407 for the protein) as a template, and the resulting A. fumigatus enzyme (AFUB_096090; accession number EDP47757) showed 82% identity over the whole sequence. The A. fumigatus hexokinase HxkA was identified by using the A. niger hexokinase HxkA (accession number CAA08922) as a template, and the resulting A. fumigatus protein (AFUB_022950; accession number EDP54242) showed 88% sequence identity. A third enzyme, named HxkB (AFUB_094300; accession number EDP47586), was also selected since it showed 48% and 33% sequence identities to A. fumigatus HxkA and GlkA, respectively. Two additional putative hexose kinases (AFUB_089570 [accession number EDP48243] and AFUB_031350 [accession number EDP55074]) were annotated previously (18), but their sequence identity to GlkA and HxkA is below 25% (see Table SA2 in the supplemental material). These enzyme most likely belong to the regulatory hexose kinases, and we therefore did not focus on these additional enzymes.
To determine the biochemical properties of all three selected A. fumigatus hexose kinases, we recombinantly produced the enzymes in E. coli. The open reading frames of the respective hexose kinases were amplified from total cDNA, sequenced, and subcloned into expression vectors with an N-terminal His tag. In all cases, cDNA sequencing confirmed the open reading frames predicted for glkA, hxkA, and hxkB. The overproduction of all enzymes was performed with BL21(DE3) Rosetta 2 cells using OvernightExpress Instant TB medium, whereby GlkA and HxkA were produced at 30°C and HxkB was produced at 18°C. At higher incubation temperatures, the overproduction of HxkB led to the formation of inclusion bodies, whereas at 18°C, most of the protein was soluble. All proteins were purified to near homogeneity by chromatography over Ni-NTA Sepharose (Fig. 1). Purified HxkB displayed no activity with glucose, 2-deoxyglucose, fructose, mannose, galactose, ribose, or trehalose, implying that this hexokinase-like protein either is not catalytically active and may act as a regulatory hexokinase or was not produced in an active form. Therefore, we were not able to provide further details on the biochemical characteristics of HxkB. In contrast, purified GlkA and HxkA showed catalytic activities and were analyzed in more detail.
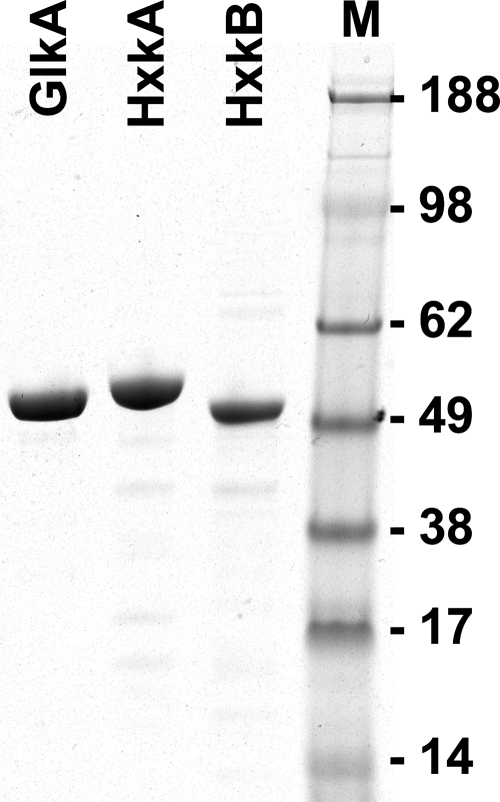
Fig. 1.
SDS-PAGE analysis of purified recombinant GlkA, HxkA, and HxkB. Between 2 μg (HxkB) and 3 μg (GlkA and HxkA) of protein was loaded, and the gel was stained with Coomassie blue. Lane M, molecular mass marker with masses in kDa indicated at the right. Expected masses with the N-terminal His tag were 55.8 kDa for GlkA, 55.6 kDa for HxkA, and 54.0 kDa for HxkB. Allowing for some variation in mobility within the gel, all purified proteins met their expected molecular masses.
Substrate specificity of GlkA and HxkA and inhibition by trehalose-6-phosphate.
We first determined the catalytic activities and substrate affinities of GlkA and HxkA with glucose and fructose. With glucose, GlkA showed a specific activity of 52 U/mg (substrate turnover number = 47.2 s−1) and a Km value of 25 μM, leading to a catalytic efficiency of 1.89 × 106 M−1 s−1. Although GlkA still showed a specific activity of 38 U/mg with fructose (substrate turnover number = 34.5 s−1), the Km value for fructose was 31.2 mM, leading to a catalytic efficiency of 1.11 × 103 M−1 s−1. Therefore, the phosphorylation of glucose by GlkA is approximately 1,700 times more efficient than that of fructose, and we concluded that GlkA does not support fructose phosphorylation under in vivo conditions.
HxkA displayed a specific activity of 7.8 U/mg (substrate turnover number = 7.04 s−1) and a Km value of 0.23 mM with glucose, leading to a catalytic efficiency of 3.06 × 104 M−1 s−1, which is 62 times less efficient than the efficiency determined for GlkA. However, with fructose, HxkA displayed a specific activity of 21.6 U/mg (substrate turnover number = 19.5 s−1) and a Km value of 2.1 mM, leading to a catalytic efficiency of 9.29 × 103 M−1 s−1. Although this efficiency with fructose is only 8.4 times higher than the efficiency for fructose activation by GlkA, the 15-times-lower Km value is likely sufficient for catalyzing fructose activation under in vivo conditions. These determinations additionally revealed that HxkA also displayed a higher efficiency for glucose phosphorylation than for fructose activation (factor of 3.3), in agreement with a previously reported biochemical characterization of hexokinase from A. niger (36).
We then determined activities with other sugars by using different substrate concentrations (1 mM, 10 mM, and 100 mM) in the test assay mixture. The resulting activities were compared with the activity of GlkA with glucose or the activity of HxkA with fructose, and the respective activities were set as 100% (Table 1). Besides the activities with glucose and fructose, both enzymes were also able to activate mannose and 2-DG, whereby GlkA showed a higher affinity for both substrates than did HxkA. Glucosamine, although a substrate for both enzymes, inhibited its own phosphorylation when applied at concentrations above 10 mM. Neither enzyme phosphorylated the other tested sugars.
Table 1.
Substrate spectrum and activity with respect to the substrate concentration of recombinant GlkA and HxkA from A. fumigatus
| Substrate | % activity |
|||||
|---|---|---|---|---|---|---|
| GlkA |
HxkA |
|||||
| 100 mM | 10 mM | 1 mM | 100 mM | 10 mM | 1 mM | |
| Glucose | 100 | 100 | 96 | 79 | 66 | 57 |
| Fructose | 15 | 2 | 0 | 100 | 95 | 26 |
| Mannose | 58 | 54 | 54 | 15 | 13 | 13 |
| 2-Deoxyglucose | 70 | 68 | 60 | 70 | 68 | 27 |
| Glucosamine | 3 | 18 | 17 | 35 | 43 | 19 |
| Lactose | 0 | 0 | 0 | 0 | 0 | 0 |
| Galactose | 0 | 0 | 0 | 0 | 0 | 0 |
| Ribose | 0 | 0 | 0 | 0 | 0 | 0 |
| Trehalose | 0 | 0 | 0 | 0 | 0 | 0 |
| Sorbose | 0 | 0 | 0 | 0 | 0 | 0 |
Trehalose-6-phosphate (T6P) has been described to be an inhibitor of hexokinase but not glucokinase activity (19, 35, 36). To confirm this finding for the A. fumigatus enzymes, we tested the inhibition of glucose and fructose activation by GlkA and HxkA in the presence of different inhibitor concentrations. GlkA was not affected in sugar phosphorylation, confirming the results for A. niger and A. nidulans. In contrast, HxkA activity was inhibited by T6P, depending on the substrate and inhibitor concentration. Glucose phosphorylation was inhibited only when 1 mM, but not 10 mM, glucose was applied to the assay mixture, and an equal concentration of 1 mM glucose and 1 mM T6P inhibited HxkA by 96%. Fructose phosphorylation by HxkA was always reduced. At 1 mM fructose, the addition of 0.1 mM T6P inhibited the activity by 93%, and the addition of 1 mM T6P inhibited the enzyme by 98%. In the presence of 10 mM fructose, 0.1 mM T6P inhibited the activity by 35%, and 1 mM T6P reduced the activity by 90%. These results show that the mode of inhibition seems competitive with respect to the affinity for the natural substrate. It furthermore shows that T6P inhibition can be used to discriminate between GlkA- and HxkA-derived activities in cell extracts.
Single and double deletions of glkA and hxkA from A. fumigatus.
To study the impact of GlkA and HxkA at different developmental stages and on different carbon sources, we aimed to delete the coding sequence from A. fumigatus by utilizing a parental strain (ΔakuB) with a defective nonhomologous end-joining repair mechanism, which significantly increases the rate of homologous recombination (7). The deletion of glkA proved problematic. First, the sequence of the 5′-untranslated region of AFUB_096090 from strain A1163, resembling the same A. fumigatus subspecies used in this study, contained a large sequence gap. Therefore, we utilized the 5′-untranslated region annotated for strain Af293 (locus tag AFUA_6G02230) for primer design. Generally, we use an 800- to 1,000-nucleotide flanking region for homologous recombination. However, it turned out that although we obtained specific PCR products, these fragments were not amplified in E. coli. We therefore reduced the size of the upstream fragment to 215 bp, which was usable for the construction of the glkA deletion construct. Despite this small upstream region we obtained several transformants and selected eight of them for Southern blot analysis. All eight transformants showed a single homologous integration into the glkA locus (see Fig. SA1A in the supplemental material).
The generation of the hxkA deletion cassette was performed by standard procedures, and no problems arose in the cloning of the upstream and downstream fragments. Interestingly, transformants showed a rather delayed conidiation on glucose minimal medium. Eight transformants were selected for Southern analysis, and all clones showed a single homologous integration into the hxkA locus (see Fig. SA2A in the supplemental material). One representative glkA mutant and one hxkA mutant were selected for complementation. Initial growth tests revealed no altered phenotype for glkA on agar plates, whereas the hxkA mutant grew poorly and did not sporulate on fructose. Therefore, the complementation of glkA required the use of the pyrithiamine resistance cassette as a selectable marker during transformation, whereas hxkA was complemented by selecting transformants for their abilities to grow on fructose (Fig. SA1B and SA2B).
We next attempted to generate a glkA hxkA double mutant. Initial attempts in which either the ΔglkA or the ΔhxkA strain served as an acceptor and different media were used for regeneration were unsuccessful. However, the transformation of the ΔglkA strain with the ΔhxkA deletion construct, with transformant regeneration in the presence of glycerol, yielded several small colonies 7 days after transformation. All transformants continued to grow poorly following subculture on fresh glycerol medium, but all transformants were resistant to hygromycin and pyrithiamine, as expected for a double-deletion strain. Southern blot analysis with probes visualizing the glucokinase and the hexokinase deletion confirmed that all 10 selected transformants showed the expected shifts of hybridizing fragments compared to the single-deletion strains (see Fig. SA3 in the supplemental material). Therefore, it can be concluded that viable strains devoid of both catalytic hexose kinases can be generated, but their growth phenotypes are more severe than that described previously for a respective double-mutant from A. nidulans, which was described only to fail to grow on glucose and fructose (19).
Analysis of growth of mutants on different carbon sources.
A previous investigation of glucokinase and hexokinase mutants from A. nidulans indicated that only the mutation of hexokinase, but not glucokinase, leads to a defect in sugar utilization, whereby the defect of the hexokinase mutant was restricted to an inability to grow on fructose (19). Interestingly, the respective mutants of Botrytis cinerea showed a similar unaltered phenotype for a glucokinase mutant, but a hexokinase mutant was severely affected on various sugars and also some gluconeogenic carbon sources such as acetate and glycerol (40). Since the mutation in the A. nidulans glkA4 mutant was not analyzed by sequencing, it remains unclear whether the mutated enzyme still retained some residual activity. To test whether the A. fumigatus orthologues had similar functions, we inoculated solid medium containing different sugars or gluconeogenic carbon sources with conidia of the wild type; the glkA, the hxkA, and the glkA hxkA deletion strains; and the complemented strains (Fig. 2).
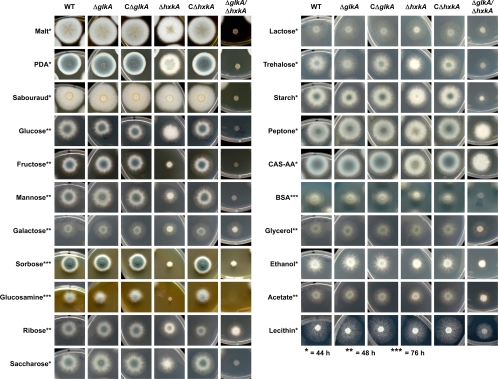
Fig. 2.
Growth analysis of glkA and hxkA mutants. All plates were spot inoculated with 1 × 103 conidia and incubated for 44, 48, or 76 h, as indicated by the number of asterisks. WT, wild type; ΔglkA, glkA deletion mutant; CΔglkA, complemented glkA deletion mutant; ΔhxkA, hxkA deletion mutant; CΔhxkA, complemented hxkA deletion mutant; ΔglkA ΔhxkA, double-deletion mutant. The complete media malt extract agar (Malt), Sabouraud medium, and potato dextrose agar (PDA) were prepared as recommended by the manufacturers. All monosaccharides were used at a final concentration of 50 mM, and disaccharides were used at a concentration of 25 mM. Starch, peptone, Casamino Acids (CAS-AA), bovine serum albumin (BSA), and lecithin were added to a final concentration of 1% (wt/vol). Glycerol, ethanol, and acetate were used at a concentration of 100 mM. For a detailed explanation of growth phenotypes, refer to the text.
As described for the other fungi, the glkA deletion did not overtly affect the phenotype on the different carbon sources. The only minor differences in comparison to the wild type and the complemented strain were observed with trehalose, in which the glkA mutant colonies always appeared to be somewhat delayed in biomass formation. However, with complete medium as well as all other sugars and nonfermentable carbon sources, no difference in colony formation was observed, implying that GlkA function was completely complemented by HxkA. Therefore, the glkA phenotype was in agreement with previously reported observations of A. nidulans and B. cinerea (19, 40). In contrast, the hxkA deletion strain showed pleiotropic growth phenotypes similar to those of B. cinerea but not A. nidulans (19, 40). In general, conidium formation of the ΔhxkA mutant was delayed on nearly all carbon sources tested, which was visualized by the delayed appearance of the gray-greenish color on top of the colonies. Additionally, and as expected, this mutant was strongly inhibited in growth on fructose. This finding confirmed the results from activity determinations, in which HxkA showed a much lower Km for fructose than did GlkA. However, the mutant was also unable to grow on glucosamine and exhibited an extreme delay in growth on sorbose. The former was unexpected, because both GlkA and HxkA were able to activate glucosamine, whereby HxkA was significantly more active with this substrate than GlkA. The strong effect on sorbose was unexpected, because neither enzyme used this sugar as a substrate. An additional strong delay was observed with the disaccharide saccharose, which can be explained by its composition of glucose and fructose. Also, on mannose, which was an excellent substrate for the glucokinase, delayed growth and conidiation were observed. Interestingly, reduced growth was also observed for some gluconeogenic carbon sources, especially ethanol and acetate but also glycerol. This indicates that hexokinase may also be involved in the activation of glucose derived from gluconeogenesis.
The most severe phenotypes were observed for the double-deletion strain. This mutant failed to grow on glucose, fructose, mannose, glucosamine, sorbose, and saccharose. Some growth on galactose and lactose (galactose containing) was observed, which may be due mainly to the Leloir pathway, which generates glucose-6-phosphate from galactose-1-phosphate in several steps (44). Some very minor growth on starch was also observed. The degradation of starch yields not only free glucose units but also some glucose-1-phosphate by the glycogen/starch phosphorylase, which may be encoded by AFUA_1G12920 in A. fumigatus. Interestingly, growth was also strongly retarded with complete medium containing glucose and amino acids/peptides, although growth on peptone and Casamino Acids was only slightly affected. Despite the relatively strong growth on peptone, this mutant failed to produce substantial quantities of conidia under these conditions (even after 2 weeks of incubation [not shown]). Interestingly, on bovine serum albumin, which requires protease secretion for growth, the colony formation of the mutant was also strongly retarded. Some biomass formation on ribose, lecithin, ethanol, acetate, and glycerol was also observed, but growth was strongly delayed, and significant conidiation was not observed.
This phenotypic analysis indicates that (i) HxkA and GlkA are the main catalytic hexose kinases; (ii) both HxkA and GlkA are required for normal conidiation, but HxkA plays a major role; (iii) even on gluconeogenic carbon sources, at least one of the hexose kinases is required for normal biomass formation; (iv) both enzymes seem to be present in mycelium, but HxkA has a greater impact on sugar metabolism; and (v) GlkA does not significantly contribute to the metabolism of fructose, galactose, glucosamine, and sorbose, since the ΔhxkA strain and the double mutant exhibited very similar phenotypes under these conditions.
Cell wall integrity of hexose kinase mutants.
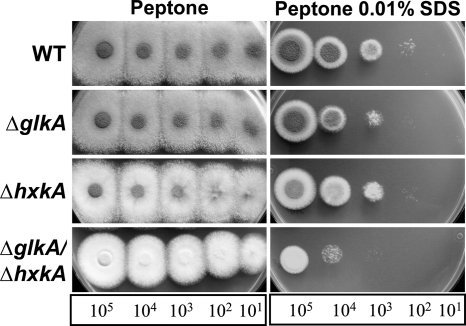
We additionally tested the hexose kinase mutants for their sensitivities to the cell wall stress-inducing compounds calcofluor white, sodium dodecyl sulfate, caffeine, and Congo red. In a first screen on glucose- and glycerol-containing media, the double-deletion strain was omitted due to its severe growth defect on these media. This analysis revealed that both single mutants displayed no or only a very minor increase in the sensitivity to cell wall-stressing agents compared to the parental wild-type strain (data not shown). However, since both mutants seemed, in principle, to be able to phosphorylate various sugars required for the synthesis of cell wall components and glycoproteins, we included the double-deletion strain. Here, we used peptone medium as a nutrient source, because it was shown to contribute best to the biomass formation of the ΔglkA ΔhxkA mutant (Fig. 2). With this medium the effects of calcofluor white, Congo red, and sodium dodecyl sulfate were tested. As shown in Fig. 3, the double-deletion strain showed colony formation, although slightly delayed in growth and without conidiation, at all dilutions when spotted onto peptone medium. However, the addition of each cell wall stress-inducing agent severely affected biomass formation, and sensitivity was strongly increased compared to those of the wild type and the single-deletion mutants (shown only for 0.01% SDS). Therefore, the presence of at least one catalytically active hexose kinase is essential for the full integrity of the fungal cell wall.
Fig. 3.
Effect of cell wall stress-inducing agents on growth of hexose kinase mutants. WT, wild type; ΔglkA, glkA deletion mutant; ΔhxkA, hxkA deletion mutant; ΔglkA ΔhxkA, double-deletion mutant. Media contained 1% (wt/vol) peptone as a nutrient source. Only the effect of 0.01% SDS is shown. Plates were photographed after 60 h of incubation at 37°C.
Impact of GlkA and HxkA on conidium germination.
Our growth analyses implied that GlkA is functionally redundant in the presence of intact HxkA. Therefore, we asked why this enzyme, which seems to be conserved in nearly all fungal species but has not resulted in a severe phenotype when deleted, was maintained during evolution. Furthermore, although GlkA showed a very high affinity for glucose and had the highest specific activity for this substrate, it only partially complemented the phenotype of an hxkA mutant. The minor growth defect on trehalose, however, provided a clue to the in vivo function of GlkA.
Trehalose is one of the main storage compounds within conidia of different fungal species. This sugar protects conidia from oxidative and heat stress (16) and is degraded for energy metabolism in the germinating conidium. Studies of A. nidulans have shown that trehalose breakdown in conidia is dependent mainly on TreB, a neutral trehalase. The deletion of the treB gene resulted in no visible germination defect in the presence of high glucose concentrations, but germination was significantly delayed when only trace concentrations of glucose were present in the medium (9). Since glucose released from trehalose needs to be activated to glucose-6-phosphate and, furthermore, the initial glucose concentration may be far below the Km of the hexokinase, we assumed that GlkA might be of major importance during early conidial germination. To test this hypothesis, we performed germination experiments with liquid media containing either different concentrations of glucose (0.1 to 10 mM) or the gluconeogenic carbon source ethanol or glycerol at a fixed concentration of 10 mM. Using glucose, germination was stopped after 7 to 8 h, whereas this time was prolonged to approximately 11 h with glycerol and to 13 h with ethanol. Since germination on minimal medium is not synchronized, we evaluated at least six microscopic fields (≥300 individual cells) under each condition and classified them in a numbering system between 1 and 5 (for a detailed classification, see the legend of Fig. 4A). The sum of the numbers of all cells was calculated and divided by the number of cells counted, leading to a minimal value of 1.0 (all cells resting) or a maximum of 5.0 (all cells germinated, branched, and containing multiple nuclei). The incubation of conidia of all strains except the double-deletion strain for 7 to 8 h in complete Sabouraud medium and evaluation of approximately 1,000 individual cells independently were performed to test the viability of the conidial preparations. All conidial preparations displayed a viability of between 93% and 98%.
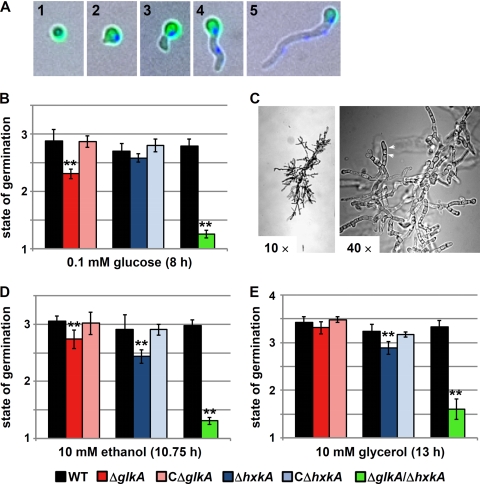
Fig. 4.
Analysis of conidium germination in the presence of different carbon sources. (A) Classification of the germination state. The pictures show overlays of bright-field images with fluorescent photographs to visualize FITC-labeled conidia (green) and DAPI-stained nuclei (blue). These pictures were used to classify each single cell from germination assays. Classification was as follows: 1, resting conidium with bright FITC fluorescence and nuclei not or barely visible; 2, swollen conidium and germ tube sometimes visible, with a brightly stained nucleus; 3, germinated conidium with a single nucleus, sometimes migrating toward the germ tube; 4, germinated conidium with at least two nuclei but without branching hyphae; 5, germinated conidium with three or more nuclei, with hyphae sometimes starting to branch or a germ tube at a second site. (B) Analysis of germination in the presence of 0.1 mM glucose. (C) Microcolony formation of a ΔglkA ΔhxkA double-deletion mutant grown for 163 h on a glass slide covered with 50 mM glucose minimal medium. Magnifications are indicated. At a ×40 magnification the short septated hyphae (arrowheads) and the hyperbranching phenotype are visible. (D) Analysis of germination in the presence of 10 mM ethanol. (E) Analysis of germination in the presence of 10 mM glycerol. For each analysis, the wild-type, deletion mutant, and complemented strains were incubated on the same chamber slide. The variations observed for wild-type germination between ΔglkA, ΔhxkA, and ΔglkA ΔhxkA analyses are due to slight variations in the incubation times but did not influence the overall results (as determined from time response studies). Incubation times were 7 to 8 h for 0.1 mM glucose, ca. 11 h for 10 mM glycerol, and ca. 13 h for 10 mM ethanol. To obtain representative data, at least six microscopic fields from each strain and each condition with 50 or more cells each were evaluated, and data show the mean values with standard deviations. Significance was calculated by a Student's t test, and two stars indicate P values of ≤0.01.
Evaluation of the different growth conditions confirmed our hypothesis. At 0.1 mM glucose, the wild-type and complemented strains showed no significant difference in germination speed, whereas the germination of the glkA mutant was significantly delayed (Fig. 4B). Increasing the concentration of glucose to 2 or 10 mM generally increased the germination speed but also reduced the germination delay between the glkA mutant and the wild type (not shown). In contrast, the deletion of hxkA resulted in no significant delay in germination at either glucose concentration, implying that GlkA but not HxkA is the major glucose-activating enzyme in germinating conidia. However, due to the minor germination delay of the glkA mutant at higher glucose concentrations, this enzyme appears to be especially important at extremely low glucose concentrations. Germination analysis of the double-deletion mutant was problematic. Using peptone as a viability control we found that more than 60% of conidia remained in the resting state after 8 and 13 h of incubation. This may indicate that the double-deletion strain either has a strongly reduced viability of conidia or germinates extremely asynchronously. Nevertheless, germination analyses were performed with glucose, glycerol, and ethanol. As expected from the viability control, most conidia remained in the resting state on glucose, with only a small proportion of conidia, which showed some swelling within the 8-h observation period (Fig. 4B). Therefore, germination on glucose requires at least one active hexose kinase. To confirm this assumption, we microscopically determined the germination and colony formation of the double mutant from glass slides covered with glucose minimal medium. After 20 h some swollen but no germinating conidia were detected (not shown). However, 163 h after inoculation, some microcolonies were observed, which showed a hyperbranching phenotype and did not develop further (Fig. 4C). Interestingly, on the gluconeogenic carbon sources glycerol and ethanol, GlkA and HxkA had similar impacts on germination (Fig. 4D and E). Both deletions caused a retardation of germination on ethanol but had little effect on germination on glycerol. The deletion of both hexose kinases led to an additional delay in germination, confirming the contribution of both enzymes to germination and growth on gluconeogenic carbon sources.
Due to the more pronounced effect of GlkA on germination in the presence of low glucose concentrations, this enzyme could be responsible for the activation of glucose from stored trehalose. The degradation of stored trehalose reserves is predicted to release only small amounts of free glucose, which requires an enzyme with a high glucose affinity. In contrast, HxkA appears to be involved mainly in sugar activation during vegetative growth regardless of the carbon source applied to the growth medium.
Growth inhibition by 2-DG.
To test whether GlkA is generally required in the presence of low glucose concentrations, we performed growth analyses in the presence of 2-deoxyglucose (2-DG). The catalytic parameters of GlkA and HxkA indicated that both enzymes activated 2-DG in a manner similar to that for the activation of glucose. GlkA activated 2-DG at nearly maximum rates at 1 mM, whereas HxkA was significantly less effective at low 2-DG concentrations (Table 1). The in vivo activation of 2-DG leads to 2-DG-6-phosphate, which cannot be further metabolized and thereby inhibits growth (6). Solid media that contained glycerol as the major carbon source were prepared and were supplemented with 2-DG at concentrations ranging from 25 μg/ml (0.15 mM) to 100 μg/ml (0.6 mM). All strains were affected in growth and sporulation by the addition of small amounts of 2-DG (see Fig. SA4 in the supplemental material). However, the glkA deletion strain was more resistant to the addition of 2-DG, whereas the hxkA mutant was most strongly affected. Therefore, it can be concluded that the high-affinity enzyme GlkA is mainly involved in the activation of 2-DG when present at low concentrations.
Presence of GlkA and HxkA activities in conidia and mycelium.
For GlkA to phosphorylate trehalose-derived glucose early during germination, this enzyme must be present in resting conidia. Furthermore, assuming that HxkA is the major sugar-activating enzyme during vegetative growth, the presence of a significant proportion of this enzyme during hyphal growth was expected. To discriminate the different activities, we utilized the above-described inhibition of HxkA by T6P, whereby this inhibitor did not affect GlkA activity. To determine activities for glucose and fructose phosphorylation from both enzymes, we always utilized the respective substrate at 10 mM and added 1 mM T6P for the inhibition of HxkA activity when fructose was the substrate. This inhibitor concentration was shown to strongly decrease the fructose-activating activity of HxkA.
We first determined activities from resting conidia of the wild type and the two mutant strains (Table 2). As expected, high levels of glucose- and fructose-activating activities were found for the wild-type extract. However, since activity with fructose made up only one-third of the glucose activity, a significant proportion of the activity was assumed to be derived from GlkA. In agreement with this assumption, a glkA mutant showed low glucose-activating activity (reduced by a factor of 7 in comparison to the wild type) and relatively high fructose-activating activity, with the latter being sensitive to T6P inhibition. In contrast, an hxkA mutant displayed high levels of glucose-phosphorylating activity (reduced by a factor of 1.3 in comparison to the wild type), and the low, but significant, activity with fructose was insensitive to inhibition. Therefore, we conclude that, indeed, high concentrations of GlkA and only a minor proportion of HxkA are present within conidia, supporting the idea of the proposed role of GlkA in activating trehalose-derived glucose. It furthermore implies that GlkA and HxkA seem to be the only major glucose- and fructose-activating enzymes, since the sum of the activities derived from the mutants approximately met the levels determined for the wild type. Due to difficulties in obtaining sufficient amounts of conidia of the double-deletion strain for the preparation of crude extracts, the hexose-phosphorylating activity was not determined. However, the fact that only a minor proportion of conidia from the double mutant germinated within 24 h in the presence of glucose additionally supports the assumption that no other enzyme with significant hexose-phosphorylating activity is present in conidia.
Table 2.
Hexokinase and glucokinase activities determined from cell extracts of different A. fumigatus strainsa
| Source of extracts | Mean Aspec (mU/mg) ± SD (% inhibition) |
||
|---|---|---|---|
| Glucose | Fructose | Fructose + T6P | |
| Conidia | |||
| WT | 435.8 ± 41 | 141.3 ± 15.4 | 24.5 ± 12.6 (82.7) |
| ΔglkA | 60.8 ± 10 | 130.1 ± 14.2 | 37.0 ± 14.5 (71.6) |
| ΔhxkA | 330.2 ± 34 | 62.0 ± 13.4 | 59.4 ± 9.3 (4.2) |
| Mycelium (glucose grown) | |||
| WT | 297.8 ± 41 | 305.9 ± 41 | 63.6 ± 14 (79.2) |
| ΔglkA | 204.7 ± 26 | 304.2 ± 31 | 76.7 ± 7 (74.8) |
| ΔhxkA | 149.5 ± 9 | 44.7 ± 9 | 40.6 ± 3 (9.2) |
| Mycelium (ethanol grown) | |||
| WT | 189.1 ± 48 | 183.2 ± 33 | 59.0 ± 5 (67.8) |
| ΔglkA | 184.4 ± 39 | 182.9 ± 47 | 82.3 ± 24 (55.0) |
| ΔhxkA | 188.8 ± 23 | 61.8 ± 9 | 61.0 ± 10 (1.3) |
| ΔglkA ΔhxkA | <10 | <10 | ND |
Substrates were used at a final concentration of 10 mM. For inhibition, 1 mM T6P was added. Aspec, specific activity. WT, wild type; ND, not determined.
We then tried to confirm that HxkA contributes mainly to sugar activation in vegetative mycelium (Table 2). In glucose-grown wild-type mycelia, the level of fructose-phosphorylating activity exceeded that of the glucose-phosphorylating activity and was highly sensitive to T6P inhibition, indicating high levels of HxkA. In the glkA mutant, the level of activity for glucose phosphorylation was reduced, but activity for fructose stayed at high levels and was sensitive to T6P inhibition. In contrast, the hxkA mutant displayed only low levels of fructose-phosphorylating activity, and this activity was only slightly affected by T6P. Using ethanol as a carbon source, the levels of hexose-phosphorylating activities of the wild type were always lower than those with glucose, but, as observed with glucose, the activities for glucose and fructose phosphorylation were in a similar range, and fructose phosphorylation was highly sensitive to T6P inhibition. Unexpectedly, investigations of the two mutants implied that for each mutant, the level of the respective residual enzyme (HxkA in the glkA mutant and GlkA in the hxkA mutant) increased to some extent, since the sum of the activities from both mutants exceeded that determined for the wild type.
Determinations of hexose kinase activity from the double-deletion strain grown on ethanol revealed values below the detection limit of 10 mU/mg in crude cell-free preparations. This finding indicates that no other major hexose-phosphorylating enzyme is induced on gluconeogenic carbon sources. Therefore, our results show that both enzymes are present in conidia and in vegetative mycelia regardless of the growth condition applied. However, HxkA is the major sugar-activating enzyme during vegetative growth, whereas GlkA seems to be the dominant enzyme in resting conidia.
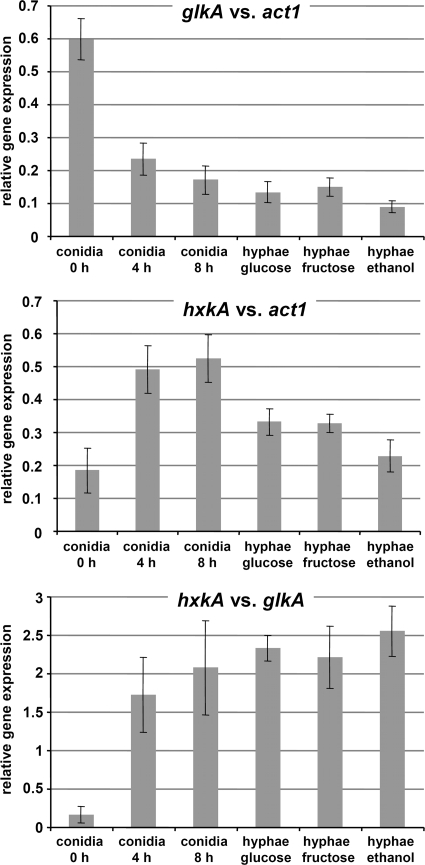
Determination of glkA and hxkA transcript levels by qRT-PCR.
Our activity determinations revealed that GlkA, but not HxkA, is highly abundant in resting conidia. In contrast, HxkA seems to constitute the major enzyme during vegetative growth. Due to the very early presence of GlkA activity, we next tested whether this was reflected by the mRNA level. Therefore, we isolated mRNA from resting and swollen conidia (0 and 4 h), early germ tubes (8 h), and glucose-grown mycelia (15 h). In addition, we analyzed fructose- and ethanol-grown mycelia. For the standardization of glkA and hxkA transcript levels, data were normalized against the transcript levels of the actin gene act1. Our analysis showed that glkA transcripts were extremely abundant in resting conidia but that levels steadily declined during the germination process (Fig. 5A). In contrast, hxkA transcripts were less abundant in resting conidia, but expression was strongly induced during early germination (4 and 8 h) and stayed at a rather high level in the mycelium (Fig. 5B) regardless of the carbon source present in the medium.
Fig. 5.
Transcript analysis of glkA and hxkA from different developmental states by quantitative real-time PCR. Data shown represent mean values of data from two biological replicates, each measured in three technical replications, and error bars give standard deviations. Conidia 0 h, resting conidia; conidia 4 h and conidia 8 h, incubation for 4 h and 8 h, respectively, in 50 mM glucose minimal medium; hyphae glucose, hyphae fructose, and hyphae ethanol, mycelium grown for 18 to 21 h on the respective carbon sources. Glucose and fructose concentrations were 50 mM, and the ethanol concentration was 100 mM. (A) Transcript levels of glkA normalized against actin. High transcript levels were obtained from resting conidia, which steadily declined during germination. In vegetative mycelium, a constantly low level of expression was observed. (B) Transcript levels of hxkA normalized against actin. The expression of hxkA is strongly induced during germination and constantly expressed in vegetative mycelium. (C) Direct comparison of the ratio of hxkA transcript levels to glkA transcript levels. Levels of glkA were used for normalization. In resting conidia, levels of glkA transcripts exceeded those of hxkA. This ratio inverts during early germination and remains in favor of hxkA in vegetative mycelia.
We additionally determined the ratio between levels of glkA and hxkA transcripts to visualize the switch of glkA and hxkA gene expression patterns. Here, the glkA transcript levels were used for normalization. This analysis confirmed that glkA transcripts were approximately six times more abundant in resting conidia (Fig. 5C), whereas this ratio was inverted early during germination. Mycelia contained hxkA transcript levels that were approximately two times higher than glkA transcript levels. Interestingly, this ratio in the mycelium was not affected by the carbon source, since the same ratios were obtained from glucose-, fructose-, and ethanol-grown mycelia. Therefore, qRT-PCR independently confirmed our activity determinations showing that GlkA is the major conidium-associated sugar-phosphorylating enzyme, whereas HxkA is mainly mycelium associated.
Expression of glkA and hxkA in lungs of infected mice.
Phenotypic analysis of the double-deletion strain implied that glkA and hxkA may be expressed and may play a role during lung infection and the manifestation of invasive aspergillosis. Therefore, we investigated the expressions of both genes from the lungs of mice, which were immunosuppressed with either cortisone acetate or the cytostatic drug cyclophosphamide, which renders mice neutropenic. In addition, lungs of mice sacrificed 1 or 3 days after infection were investigated.
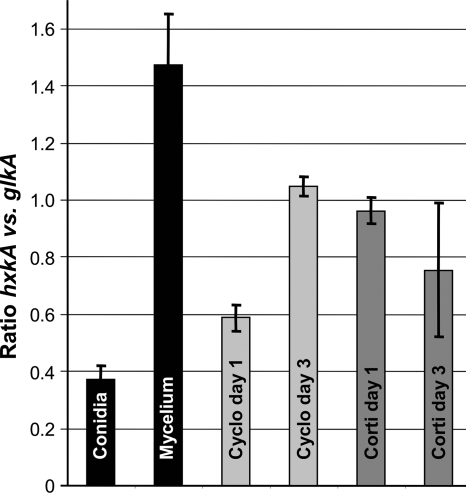
Previous studies of the growth behavior of A. fumigatus with both immunosuppression regimens have shown that conidia germinate within 1 day when mice were treated with corticosteroids (5, 25), but at later time points, neutrophils were recruited and attacked outgrowing hyphae, reducing the number of living fungal cells at the cost of severe tissue destruction. In contrast, the germination of conidia is delayed in neutropenic animals, but, once germinated, the mycelium can grow unrestricted through the lung parenchyma (25). In a first approach we attempted to quantify the expression levels of glkA and hxkA by qRT-PCR using SYBR green as a fluorescent marker for gene amplification. However, we noticed that the small proportion of fungal cDNA among the large amount of cDNA derived from the lung tissue precluded exact data evaluation. A melting-curve analysis revealed that significant amounts of unspecific products were amplified, which prohibited a quantification of the fluorescence signals. Therefore, we turned to a semiquantitative analysis in which amplifications were performed by PCR and products were separated by agarose gel electrophoresis. Using cDNA from resting conidia and mycelium as controls, we identified the bands corresponding to glkA and hxkA, which were subsequently quantified. However, data analysis was hampered, especially for mice sacrificed at day 3 under corticosteroid treatment, a condition which coincides with a low number of living fungal cells (25). Figure 6 shows the mean values of the ratios between glkA and hxkA transcripts, in which the results from two mice from each time point and immunosuppression regimen, including two independent evaluations, were combined. Although this data evaluation is less exact than that with qRT-PCR for fungal cultures and does not consider primer efficiency during amplification, it reflects the tendency of gene transcription. As expected, resting conidia show a much higher glkA than hxkA transcript level, whereas the level of hxkA was higher in the mycelium. Under cyclophosphamide treatment at 1 day postinfection, the level of hxkA only slightly increased, and the ratio was still in favor of glkA. This coincides with the observation that under this regimen and at this time point, conidia started to swell but did not undergo substantial germination (25). At day 3 postinfection the level of hxkA was similar to that of glkA, and histopathological analyses have repeatedly shown that at this time point, a massive parenchyma infiltration with fungal mycelium takes place (25). Contrarily, early after infection of corticosteroid-treated mice, the level of hxkA had already increased. This coincides with the observation of rapid conidial germination under this regimen (5, 25). Three days after infection, the determination of transcript levels was very difficult due to extremely weak signals leading to a high variation of transcript ratios, as reflected by the large standard deviations.
Fig. 6.
Determination of the ratio of hxkA transcript levels to glkA transcript levels by semiquantitative analysis of PCR products. After separation on a 2% agarose gel, band intensities were determined by using the TraceQuantity method from the QuantityOne software package (Bio-Rad). Black bars show the transcript ratios from resting conidia (Conidia) and glucose-grown mycelium (Mycelium). Light gray bars show transcript levels determined from lungs of infected mice immunosuppressed with cyclophosphamide (Cyclo) and sacrificed 1 or 3 days after infection. Dark gray bars show the same analysis as the light gray bars, but mice were immunosuppressed with cortisone acetate (Corti). For each analysis the mean values from two independent biological probes measured in replicates were used, and standard deviations are indicated.
Although this is only a preliminary analysis, it shows that the germination of conidia coincides with an increase in hxkA transcript levels under infectious conditions. However, glkA transcripts remained rather abundant, and the ratio between hxkA and glkA levels did not reach that determined with cultured mycelia. This may reflect the continuous supply of very low glucose concentrations in the infected tissues, which might require a high-affinity sugar kinase, such as GlkA, for efficient glucose metabolism.
Influence of GlkA and HxkA on carbon catabolite repression.
In fungi the utilization of alternative carbon sources in the presence of glucose is generally downregulated by a carbon catabolite repression system. In aspergilli this repression system is governed mainly by the DNA-binding protein CreA (15, 33), whereas in Saccharomyces cerevisiae Mig1p plays a major role in carbon catabolite repression (21). Studies of S. cerevisiae revealed that the hexokinase Hxk2p additionally participates in the regulation of alternative carbon utilization (23) and fermentative growth in the presence of glucose (39). This regulation appears to be different from that of A. nidulans, in which only the mutation of both glucokinase and hexokinase but not of a single hexose kinase led to derepressed carbon catabolite repression (19). However, since our analyses showed that A. fumigatus glkA and hxkA mutants behaved somewhat different from that from A. nidulans, we investigated the release of carbon catabolite repression in our mutants and analyzed the activity of the glyoxylate cycle enzyme isocitrate lyase.
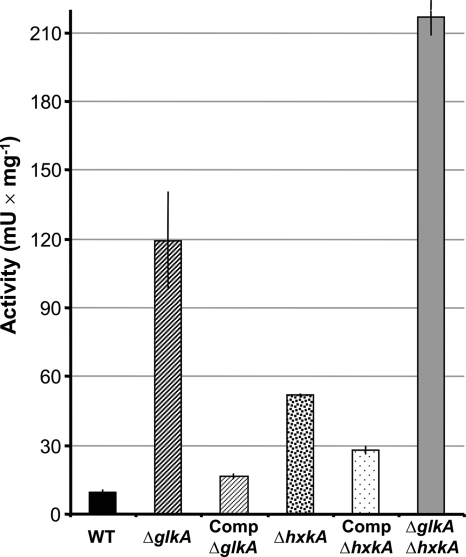
Previous investigations have shown that A. fumigatus does not produce isocitrate lyase in the presence of glucose or glycerol but produced large amounts of enzyme when acetate or ethanol was given as the sole carbon and energy source (11). Wild-type, mutant, and complemented strains were grown for 19 h on either glucose, glucose-ethanol, or ethanol (30 h), and isocitrate lyase activity was determined from cell extracts. The double-deletion mutant was harvested only from glucose-ethanol (72 h) and ethanol (48 h), since the biomass obtained from glucose liquid medium (168 h) was not sufficient for the preparation of cell extracts. For all glucose-containing media we confirmed that glucose was not consumed at the time of harvest. Activity determinations showed that all tested strains did not exhibit detectable isocitrate lyase activity when grown solely on glucose (<2 mU/mg) (details not shown). In contrast, when ethanol was used as the sole carbon source, the level of isocitrate lyase activity increased in all strains to ≥400 mU/mg (details not shown). However, when the mixture of glucose and ethanol was investigated, only the wild-type and complemented strains displayed low levels of isocitrate lyase activity, as expected for a functional carbon catabolite repression system. In contrast, both the glkA and the hxkA mutants showed a significant increase in isocitrate lyase activity, whereby the activity increased stronger in the ΔglkA strain, implying that GlkA may have a stronger impact on the regulation of carbon catabolite repression than HxkA (Fig. 7). The strongest increase in isocitrate lyase activity was observed for the double-deletion strain, indicating a cumulative effect of glkA and hxkA deletions. Therefore, in A. fumigatus both hexose kinases play a role in carbon catabolite repression, with a clearly stronger contribution being made by GlkA. This is in contrast to the results with A. nidulans, in which carbon catabolite repression was relieved only when both enzymes were disrupted simultaneously (19).
Fig. 7.
Determination of isocitrate lyase activity from mycelia grown in the presence of glucose and ethanol. A mixture of 50 mM glucose and 100 mM ethanol was used as the carbon source. Activity was determined in two independent biological replicates, each measured in three technical replications. Mean values with standard deviations are shown. WT, wild type; ΔglkA, glkA deletion mutant; CΔglkA, complemented glkA deletion mutant; ΔhxkA, hxkA deletion mutant; CΔhxkA, complemented hxkA deletion mutant; ΔglkA ΔhxkA, double-deletion mutant. In comparison to the wild type, the activities increased in the ΔglkA strain by a factor of 12, in the ΔhxkA strain by a factor of 5, and in the double-deletion strain by a factor of 22.
DISCUSSION
In this study we investigated the impact of the glucokinase GlkA and the hexokinase HxkA on the physiology of A. fumigatus. Together, these enzymes represent the only active hexose kinases, although other related enzymes, such as HxkB, are present in the genome of A. fumigatus. However, recombinantly produced HxkB showed no sugar-phosphorylating activity, and the sum of the sugar kinase activities from the glkA and the hxkA mutants, as determined from conidia or from glucose-grown mycelia, met the activity values determined for the wild type. Additionally, the ΔglkA ΔhxkA double mutant was unable to grow on several sugars, displayed severe growth phenotypes on all nutrient sources tested, and showed no detectable hexose-phosphorylating activity on ethanol.
The main focus of this study was the elucidation of the contribution of each enzyme during germination, growth, and conidiation on various carbon sources. In particular, the role of GlkA was elusive, since the mutation of this gene in several other fungi, such as A. nidulans (19) or B. cinerea (40), revealed no visible phenotype. In terms of colony morphology on various carbon sources, our investigations confirmed these observations. However, a more detailed analysis implied that glucokinase is a high-affinity glucose-phosphorylating enzyme required for the activation of intracellular trace amounts of glucose. The growth of a glkA deletion strain was less inhibited in the presence of small amounts of 2-deoxyglucose and showed a slightly reduced growth rate when trehalose was given as the sole carbon source. Trehalose degradation in the mycelium requires the action of an acid trehalase, which releases free glucose molecules. Therefore, the concentration of available glucose is directly dependent on the activity of the trehalase. This glucose must be phosphorylated by a sugar kinase before entering glycolysis (10). Aside from the utilization of trehalose from the surrounding medium, trehalose is one of the major storage compounds in conidia, which becomes mobilized by the neutral trehalase during germination. Therefore, an A. nidulans treB deletion mutant showed a reduced germination speed when only trace amounts of glucose were present in the surrounding growth medium. However, at high glucose concentrations, such a mutant showed no altered germination phenotype (9), implying a reduced importance for feeding from trehalose when glucose can be easily acquired from the medium.
These observations led us to investigate the conidial germination of glkA and hxkA mutants in the presence of low and high glucose concentrations. Interestingly, the phenotype of the glkA mutant resembled the phenotype of the A. nidulans treB mutant exactly (9). Due to the strongly reduced germination speed of the glkA mutant but not of the hxkA mutant at a very low glucose concentration, a major impact of GlkA on the activation of glucose from trehalose breakdown is likely. In agreement, both activity determinations and transcript analyses showed that GlkA is the major sugar kinase in resting conidia. In addition, since higher glucose concentrations relieved the phenotype of delayed germination, it can be concluded that trehalose breakdown is of less importance when large amounts of glucose can be obtained from the medium. In this situation the increasing levels of HxkA during germination seem to be sufficient for glucose phosphorylation. The importance of at least one functional hexose kinase for germination in the presence of glucose was also confirmed by investigations of the glkA hxkA double-deletion strain. Conidia of this mutant displayed strongly reduced viability, since, regardless of the carbon source used, at least 50 to 60% of conidia did not start to swell within 14 h of incubation. In addition, swelling and germination of the residual conidia were strongly delayed, independent of the presence of 0.1 or 10 mM glucose. Once germinated on glucose, conidia were able to form only microcolonies with very short septated hyphae and a hyperbranching phenotype. The size of these microcolonies remained constant during further incubation, which implies that colony formation did not involve the utilization of exogenous sugars but might be due to the consumption of internal lipid reserves, which are present in conidia (11).
Besides the major association of GlkA with conidia and its importance for rapid germination in the presence of low glucose concentrations, our activity and transcript level determinations showed that HxkA is the major sugar kinase during vegetative growth. Contrary to previously reported observations of A. nidulans (19, 38), the A. fumigatus hxkA deletion strain showed a growth defect not only in the presence of fructose, as expected from the high catalytic efficiency for this substrate, but also on several other sugars and was strongly delayed in conidiation. The major impact of hexokinase and, to a lesser extent, of glucokinase on conidiation was also confirmed by observations with the double-deletion strain. Colonies of this mutant did not develop the same characteristic gray-greenish color, which indicates the production of melanized conidia (Fig. 2). Additionally, the amounts of conidia harvested from several plates containing either glycerol, peptone, or ribose medium were not sufficient for the preparation of cell extracts. Several reasons may account for the reduced production and viability of conidia. As mentioned above, conidia contain several storage compounds, such as trehalose, mannitol, and lipids (9, 11), and the synthesis of these compounds may require high levels of glucose-phosphorylating activity. In addition, reduced conidiation and abnormal cellular morphology were also observed for an A. fumigatus phosphomannose isomerase (pmi1) mutant, which is unable to catalyze the interconversion of mannose-6-phosphate (Man-6-P) and fructose-6-phosphate (13). Mannose-6-phosphate is essential for cell wall integrity and is derived from fructose-6-phosphate via Pmi1 or from the direct activation of mannose by GlkA or HxkA. Therefore, the reduced viability and conidiation rate might be directly due to a shortage of mannose-6-phosphate. In agreement, the single-deletion strains did not show an increased sensitivity to cell wall-stressing agents, whereas the double-deletion strain was severely affected. This finding implies that the double-deletion strain is unable to produce sufficient mannose-6-phosphate from fructose-6-phosphate and may additionally contain reduced glucan and chitin contents in the vegetative hyphae.
Although HxkA is the major hexose kinase during vegetative growth, GlkA can at least partially complement the deletion phenotype on various sugars, which coincides with the similar substrate spectra of both enzymes. The strong phenotype of the ΔhxkA mutant on fructose was expected, since the apparent Km of GlkA for this sugar was extremely high (31.2 mM). However, the strong phenotype of the ΔhxkA mutant on mannose, glucosamine, and sorbose was unexpected. Both enzymes showed a very high affinity for mannose, which was assumed to lead to complementation by GlkA similar to that observed with glucose. Also, glucosamine was shown to provide a substrate for both enzymes, but under in vivo conditions GlkA seems rather ineffective in the phosphorylation of this sugar amine. Most interestingly, neither GlkA nor HxkA displayed any activity with sorbose, but HxkA seems to be essential for its metabolism. This is in agreement with observations of A. nidulans that showed that sorbose-phosphorylating activity is absent in a hexokinase mutant (14). Therefore, sorbose either becomes converted to fructose prior to phosphorylation or is activated by an enzyme, which is directly dependent on the presence of active HxkA.
As expected, the double-deletion strain was strongly retarded or unable to grow on most sugars. Exceptions were ribose, galactose, and lactose, on which conidiation was strongly reduced but biomass formation occurred. Ribose may rely on a special ribokinase for activation, whereas galactose and lactose may rely on a galactokinase for activation, leading to glucose-6-phosphate via the Leloir pathway (44). However, we were surprised by the negative effect of glucose in the presence of alternative gluconeogenic carbon sources. On Casamino Acids and peptone, the growth of the double mutant was pronounced, but with the complete media malt extract, Sabouraud medium, and potato dextrose broth, growth was strongly delayed. Additionally, the supplementation of ethanol medium with glucose led to a prolonged growth rate (not shown). It can be assumed that glucose is efficiently imported, which may cause different negative effects. Glucose may activate carbon catabolite repression directly via CreA, although our analyses showed that carbon catabolite repression is much less pronounced in the double mutant. Nevertheless, the presence of high intracellular glucose concentrations may trigger glycolysis. Due to the fact that glucose cannot become phosphorylated, cells will enter starvation. Another scenario is a disturbance of the osmotic equilibrium, because cells are unable to convert glucose into trehalose or glycogen with the consequence of strong water influx and high internal osmotic pressure.
Interestingly, the deletion of both hexose-phosphorylating enzymes also retarded germination and growth on the gluconeogenic carbon sources glycerol, acetate, ethanol, and lecithin, indicating that some proportion of glucose-6-phosphate from gluconeogenesis is used for the production of storage reservoirs such as glucose, glycogen, trehalose, or other sugars to provide glucose-6-phosphate independently from gluconeogenesis. The deletion of both hexose kinases traps these storage compounds in a nonmetabolizable state, wasting the energy of glucose synthesis without the ability to earn this energy back. Both GlkA and, especially, HxkA were shown to be present in ethanol-grown mycelia, supporting the idea of sugar phosphorylation from storage compounds. In addition, aside from the importance in the production of mannose-6-phosphate for mannoproteins (13), large amounts of glucose are also required for chitin production. Therefore, it can be assumed that gluconeogenesis not only provides glucose-6-phosphate, which is directly consumed for biosynthetic purposes, but also produces glucose, which can be used when needed.
Another interesting observation was the above-described deregulated carbon catabolite repression with respect to the increase in levels of isocitrate lyase activity in the presence of glucose and ethanol. In wild-type cells this increase is prohibited by a functional repression system, which is assumed to be based mainly on the negative transcriptional regulator CreA (1, 15, 42, 43). However, it has been shown that CreA-dependent regulation alone cannot completely explain the effect of glucose on the repression of alternative carbon source utilization. However, the energy and redox status of the cells may also contribute to this regulation (33). Our observations for the glkA mutant, the hxkA mutant, and the double mutant are in agreement with this assumption. In the absence of the inducing carbon source ethanol, no isocitrate lyase activity was detected, confirming glucose repression and the requirement for an inducing signal. With ethanol as the sole carbon source, a high level of isocitrate lyase activity was detected, and values were indistinguishable among the different strains, indicating a maximal rate of expression in the absence of glucose. However, on a mixed carbon source consisting of glucose and ethanol, both single mutants showed an increase in isocitrate lyase activity, which was even more pronounced in the double-deletion strain. Interestingly, despite the fact that HxkA is the major hexose kinase in vegetative mycelia, the increase in isocitrate lyase activity showed a higher dependency on the presence of GlkA. Therefore, we conclude that the deletion of either enzyme unbalances the equilibrium of glucose uptake and utilization. Carbon catabolite repression may therefore depend not only on the presence of free glucose but also on the accumulation of intermediates from its degradation, as previously assumed (8, 33).
Since A. fumigatus is an opportunistic human pathogen that is able to cause severe invasive aspergillosis in immunocompromised patients (2), we were also interested in the expression of glkA and hxkA under infectious conditions. It is likely that glucose provides a substrate during pathogenesis (4), which would require hexose kinase activity for degradation. However, our phenotypic analyses of the double-deletion mutant implied that either HxkA or GlkA is required to support growth even in the absence of glucose. Although it was problematic to determine the exact expression ratios of both genes, we obtained the unexpected result that hxkA levels did not significantly increase over the level of glkA and might even decrease at later time points in corticosteroid-treated animals. In the presence of low glucose concentrations, glkA expression might be favored, since the Km for glucose (25 μM for GlkA) was nine times lower than that determined for HxkA (0.23 mM). Therefore, although the hxkA deletion mutant displayed a more severe phenotype during vegetative growth, GlkA might significantly contribute to virulence during lung infection. The specific contribution of each enzyme and the possible attenuation in virulence will be tested in future experiments. However, it can be assumed that at least the double deletion will display strongly reduced virulence due to its general growth defects on most nutrient sources.
In conclusion, investigations of the double-deletion strain showed the important contribution of hexose kinases throughout the life cycle of A. fumigatus, independent of the carbon sources present in the environment. No other major hexose kinase that can replace the function of GlkA and HxkA is present. Fifteen independent transformation attempts were required to obtain the double-deletion strain, indicating the importance of the correct transformation conditions for recovering mutants with such severe phenotypes. This also may explain why the deletion of glucokinase and hexokinase in B. cinerea was reported previously to be lethal (40). However, we cannot explain the rather weak phenotype of A. nidulans mutants defective in both genes (19), but this may be the result of gene mutations rather than complete gene deletions. Contrary to previous investigations speculating that GlkA might be a redundant enzyme and dispensable for proper cellular function, we show that both the GlkA and HxkA enzymes have perfectly adapted to their specific developmental niche. GlkA is highly efficient in glucose activation and not inhibited by trehalose-6-phosphate, which makes the enzyme exquisitely suited for the activation of glucose from trehalose degradation during conidial germination. HxkA, although inhibited by trehalose-6-phosphate and possessing a reduced affinity for glucose, shows a broader substrate spectrum and is expressed mainly in growing hyphae. Under these conditions, high-affinity sugar transport systems (6, 20, 27, 32, 41, 47) can increase the intracellular sugar concentration, which makes the high glucose affinity of GlkA less important.
Due to the 36% sequence identity of both enzymes, it seems likely that a gene duplication event led to the evolution of GlkA and HxkA (18). Although both enzymes retained the same biochemical function, i.e., the activation of hexoses to hexose-6-phosphates, their temporal regulation diverged, supporting the idea of the evolutionary principles of gene duplications in fungi in which duplicated metabolic genes tend to retain their biochemical function but become integrated into new regulatory networks (46).
Supplementary Material
ACKNOWLEDGMENTS
We thank G. Steinmetzer for technical assistance during cloning and purification of recombinant enzymes and for preparation of media for growth analyses. We are additionally grateful to M. Poetsch for matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) analyses of purified recombinant enzymes and D. Wilson for critical reading of the manuscript.
This work was supported by funds from the Hans Knoell Institute.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 7 May 2010.
REFERENCES
- 1.Agger T., Petersen J. B., O'Connor S. M., Murphy R. L., Kelly J. M., Nielsen J. 2002. Physiological characterisation of recombinant Aspergillus nidulans strains with different creA genotypes expressing A. oryzae alpha-amylase. J. Biotechnol. 92:279–285 [DOI] [PubMed] [Google Scholar]
- 2.Balloy V., Chignard M. 2009. The innate immune response to Aspergillus fumigatus. Microbes Infect. 11:919–927 [DOI] [PubMed] [Google Scholar]
- 3.Bernardo S. M., Gray K. A., Todd R. B., Cheetham B. F., Katz M. E. 2007. Characterization of regulatory non-catalytic hexokinases in Aspergillus nidulans. Mol. Genet. Genomics 277:519–532 [DOI] [PubMed] [Google Scholar]
- 4.Brock M. 2009. Back to the roots. Microb. Biotechnol. 2:132–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brock M., Jouvion G., Droin-Bergere S., Dussurget O., Nicola M. A., Ibrahim-Granet O. 2008. Bioluminescent Aspergillus fumigatus, a new tool for drug efficiency testing and in vivo monitoring of invasive aspergillosis. Appl. Environ. Microbiol. 74:7023–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown C. E., Romano A. H. 1969. Evidence against necessary phosphorylation during hexose transport in Aspergillus nidulans. J. Bacteriol. 100:1198–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva Ferreira M. E., Heinekamp T., Hartl A., Brakhage A. A., Semighini C. P., Harris S. D., Savoldi M., de Gouvea P. F., de Souza Goldman M. H., Goldman G. H. 2007. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 44:219–230 [DOI] [PubMed] [Google Scholar]
- 8.David H., Krogh A. M., Roca C., Akesson M., Nielsen J. 2005. CreA influences the metabolic fluxes of Aspergillus nidulans during growth on glucose and xylose. Microbiology 151:2209–2221 [DOI] [PubMed] [Google Scholar]
- 9.d'Enfert C., Bonini B. M., Zapella P. D., Fontaine T., da Silva A. M., Terenzi H. F. 1999. Neutral trehalases catalyse intracellular trehalose breakdown in the filamentous fungi Aspergillus nidulans and Neurospora crassa. Mol. Microbiol. 32:471–483 [DOI] [PubMed] [Google Scholar]
- 10.d'Enfert C., Fontaine T. 1997. Molecular characterization of the Aspergillus nidulans treA gene encoding an acid trehalase required for growth on trehalose. Mol. Microbiol. 24:203–216 [DOI] [PubMed] [Google Scholar]
- 11.Ebel F., Schwienbacher M., Beyer J., Heesemann J., Brakhage A. A., Brock M. 2006. Analysis of the regulation, expression, and localisation of the isocitrate lyase from Aspergillus fumigatus, a potential target for antifungal drug development. Fungal Genet. Biol. 43:476–489 [DOI] [PubMed] [Google Scholar]
- 12.Egi M., Bellomo R., Stachowski E., French C. J., Hart G. K., Hegarty C., Bailey M. 2008. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit. Care Med. 36:2249–2255 [DOI] [PubMed] [Google Scholar]
- 13.Fang W., Yu X., Wang B., Zhou H., Ouyang H., Ming J., Jin C. 2009. Characterization of the Aspergillus fumigatus phosphomannose isomerase Pmi1 and its impact on cell wall synthesis and morphogenesis. Microbiology 155:3281–3293 [DOI] [PubMed] [Google Scholar]
- 14.Fekete E., Karaffa L., Sandor E., Banyai I., Seiboth B., Gyemant G., Sepsi A., Szentirmai A., Kubicek C. P. 2004. The alternative D-galactose degrading pathway of Aspergillus nidulans proceeds via L-sorbose. Arch. Microbiol. 181:35–44 [DOI] [PubMed] [Google Scholar]
- 15.Felenbok B., Flipphi M., Nikolaev I. 2001. Ethanol catabolism in Aspergillus nidulans: a model system for studying gene regulation. Prog. Nucleic Acid Res. Mol. Biol. 69:149–204 [DOI] [PubMed] [Google Scholar]
- 16.Fillinger S., Chaveroche M. K., van Dijck P., de Vries R., Ruijter G., Thevelein J., d'Enfert C. 2001. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 147:1851–1862 [DOI] [PubMed] [Google Scholar]
- 17.Fleck C. B., Brock M. 2008. Characterization of an acyl-CoA:carboxylate CoA-transferase from Aspergillus nidulans involved in propionyl-CoA detoxification. Mol. Microbiol. 68:642–656 [DOI] [PubMed] [Google Scholar]
- 18.Flipphi M., Sun J., Robellet X., Karaffa L., Fekete E., Zeng A. P., Kubiecek C. P. 2009. Biodiversity and evolution of primary carbon metabolism in Aspergillus nidulans and other Aspergillus spp. Fungal Genet. Biol. 46(Suppl. 1):S19–S44 [DOI] [PubMed] [Google Scholar]
- 19.Flipphi M., van de Vondervoort P. J., Ruijter G. J., Visser J., Arst H. N., Jr., Felenbok B. 2003. Onset of carbon catabolite repression in Aspergillus nidulans. Parallel involvement of hexokinase and glucokinase in sugar signaling. J. Biol. Chem. 278:11849–11857 [DOI] [PubMed] [Google Scholar]
- 20.Forment J. V., Flipphi M., Ramon D., Ventura L., Maccabe A. P. 2006. Identification of the mstE gene encoding a glucose-inducible, low affinity glucose transporter in Aspergillus nidulans. J. Biol. Chem. 281:8339–8346 [DOI] [PubMed] [Google Scholar]
- 21.Gancedo J. M. 1998. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62:334–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hortschansky P., Eisendle M., Al-Abdallah Q., Schmidt A. D., Bergmann S., Thon M., Kniemeyer O., Abt B., Seeber B., Werner E. R., Kato M., Brakhage A. A., Haas H. 2007. Interaction of HapX with the CCAAT-binding complex—a novel mechanism of gene regulation by iron. EMBO J. 26:3157–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Z., Yue Y., Jiang H., Zhang B., Sherwood P. W., Michels C. A. 2000. Analysis of the mechanism by which glucose inhibits maltose induction of MAL gene expression in Saccharomyces. Genetics 154:121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim-Granet O., Dubourdeau M., Latge J. P., Ave P., Huerre M., Brakhage A. A., Brock M. 2008. Methylcitrate synthase from Aspergillus fumigatus is essential for manifestation of invasive aspergillosis. Cell. Microbiol. 10:134–148 [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim-Granet O., Jouvion G., Hohl T., Droin-Bergere S., Philippart F., Kim O. Y., Adib-Conquy M., Schwendener R., Cavaillon J.-M., Brock M. 2010. In vivo bioluminescence imaging and histopathopathologic analysis reveal distinct roles for resident and recruited immune effector cells in defense against invasive aspergillosis. BMC Microbiol. 10:105 doi:10.1186/1471-2180-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim-Granet O., Philippe B., Boleti H., Boisvieux-Ulrich E., Grenet D., Stern M., Latge J. P. 2003. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 71:891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jørgensen T. R., vanKuyk P. A., Poulsen B. R., Ruijter G. J., Visser J., Iversen J. J. 2007. Glucose uptake and growth of glucose-limited chemostat cultures of Aspergillus niger and a disruptant lacking MstA, a high-affinity glucose transporter. Microbiology 153:1963–1973 [DOI] [PubMed] [Google Scholar]
- 28.Katz M. E., Masoumi A., Burrows S. R., Shirtliff C. G., Cheetham B. F. 2000. The Aspergillus nidulans xprF gene encodes a hexokinase-like protein involved in the regulation of extracellular proteases. Genetics 156:1559–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kingsbury J. M., Goldstein A. L., McCusker J. H. 2006. Role of nitrogen and carbon transport, regulation, and metabolism genes for Saccharomyces cerevisiae survival in vivo. Eukaryot. Cell 5:816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lengeler J. W., Jahreis K. 2009. Bacterial PEP-dependent carbohydrate:phosphotransferase systems couple sensing and global control mechanisms. Contrib. Microbiol. 16:65–87 [DOI] [PubMed] [Google Scholar]
- 31.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 32.MacCabe A. P., Miro P., Ventura L., Ramon D. 2003. Glucose uptake in germinating Aspergillus nidulans conidia: involvement of the creA and sorA genes. Microbiology 149:2129–2136 [DOI] [PubMed] [Google Scholar]
- 33.Mogensen J., Nielsen H. B., Hofmann G., Nielsen J. 2006. Transcription analysis using high-density micro-arrays of Aspergillus nidulans wild-type and creA mutant during growth on glucose or ethanol. Fungal Genet. Biol. 43:593–603 [DOI] [PubMed] [Google Scholar]
- 34.Nucci M., Anaissie E. 2009. Fungal infections in hematopoietic stem cell transplantation and solid-organ transplantation—focus on aspergillosis. Clin. Chest Med. 30:295–306 [DOI] [PubMed] [Google Scholar]
- 35.Panneman H., Ruijter G. J., van den Broeck H. C., Driever E. T., Visser J. 1996. Cloning and biochemical characterisation of an Aspergillus niger glucokinase. Evidence for the presence of separate glucokinase and hexokinase enzymes. Eur. J. Biochem. 240:518–525 [DOI] [PubMed] [Google Scholar]
- 36.Panneman H., Ruijter G. J., van den Broeck H. C., Visser J. 1998. Cloning and biochemical characterisation of Aspergillus niger hexokinase—the enzyme is strongly inhibited by physiological concentrations of trehalose 6-phosphate. Eur. J. Biochem. 258:223–232 [DOI] [PubMed] [Google Scholar]
- 37.Pfaffl M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts C. F. 1963. The genetic analysis of carbohydrate utilization in Aspergillus nidulans. J. Gen. Microbiol. 31:45–58 [DOI] [PubMed] [Google Scholar]
- 39.Rossell S., Lindenbergh A., van der Weijden C. C., Kruckeberg A. L., van Eunen K., Westerhoff H. V., Bakker B. M. 2008. Mixed and diverse metabolic and gene-expression regulation of the glycolytic and fermentative pathways in response to a HXK2 deletion in Saccharomyces cerevisiae. FEMS Yeast Res. 8:155–164 [DOI] [PubMed] [Google Scholar]
- 40.Rui O., Hahn M. 2007. The Botrytis cinerea hexokinase, Hxk1, but not the glucokinase, Glk1, is required for normal growth and sugar metabolism, and for pathogenicity on fruits. Microbiology 153:2791–2802 [DOI] [PubMed] [Google Scholar]
- 41.Ruijter G. J., Panneman H., van den Broeck H. C., Bennett J. M., Visser J. 1996. Characterisation of the Aspergillus nidulans frA1 mutant: hexose phosphorylation and apparent lack of involvement of hexokinase in glucose repression. FEMS Microbiol. Lett. 139:223–228 [DOI] [PubMed] [Google Scholar]
- 42.Ruijter G. J., Visser J. 1997. Carbon repression in aspergilli. FEMS Microbiol. Lett. 151:103–114 [DOI] [PubMed] [Google Scholar]
- 43.Scazzocchio C. 1992. Control of gene expression in the catabolic pathways of Aspergillus nidulans: a personal and biased account. Biotechnology 23:43–68 [PubMed] [Google Scholar]
- 44.Sellick C. A., Campbell R. N., Reece R. J. 2008. Galactose metabolism in yeast—structure and regulation of the Leloir pathway enzymes and the genes encoding them. Int. Rev. Cell Mol. Biol. 269:111–150 [DOI] [PubMed] [Google Scholar]
- 45.Valiante V., Heinekamp T., Jain R., Härtl A., Brakhage A. A. 2008. The mitogen-activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet. Biol. 45:618–627 [DOI] [PubMed] [Google Scholar]
- 46.Wapinski I., Pfeffer A., Friedman N., Regev A. 2007. Natural history and evolutionary principles of gene duplication in fungi. Nature 449:54–61 [DOI] [PubMed] [Google Scholar]
- 47.Wei H., Vienken K., Weber R., Bunting S., Requena N., Fischer R. 2004. A putative high affinity hexose transporter, hxtA, of Aspergillus nidulans is induced in vegetative hyphae upon starvation and in ascogenous hyphae during cleistothecium formation. Fungal Genet. Biol. 41:148–156 [DOI] [PubMed] [Google Scholar]
- 48.Weidner G., d'Enfert C., Koch A., Mol P. C., Brakhage A. A. 1998. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5′-monophosphate decarboxylase. Curr. Genet. 33:378–385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.