Abstract
Streptococcus mutans is a key contributor to the formation of the extracellular polysaccharide (EPS) matrix in dental biofilms. The exopolysaccharides, which are mostly glucans synthesized by streptococcal glucosyltransferases (Gtfs), provide binding sites that promote accumulation of microorganisms on the tooth surface and further establishment of pathogenic biofilms. This study explored (i) the role of S. mutans Gtfs in the development of the EPS matrix and microcolonies in biofilms, (ii) the influence of exopolysaccharides on formation of microcolonies, and (iii) establishment of S. mutans in a multispecies biofilm in vitro using a novel fluorescence labeling technique. Our data show that the ability of S. mutans strains defective in the gtfB gene or the gtfB and gtfC genes to form microcolonies on saliva-coated hydroxyapatite surfaces was markedly disrupted. However, deletion of both gtfB (associated with insoluble glucan synthesis) and gtfC (associated with insoluble and soluble glucan synthesis) is required for the maximum reduction in EPS matrix and biofilm formation. S. mutans grown with sucrose in the presence of Streptococcus oralis and Actinomyces naeslundii steadily formed exopolysaccharides, which allowed the initial clustering of bacterial cells and further development into highly structured microcolonies. Concomitantly, S. mutans became the major species in the mature biofilm. Neither the EPS matrix nor microcolonies were formed in the presence of glucose in the multispecies biofilm. Our data show that GtfB and GtfC are essential for establishment of the EPS matrix, but GtfB appears to be responsible for formation of microcolonies by S. mutans; these Gtf-mediated processes may enhance the competitiveness of S. mutans in the multispecies environment in biofilms on tooth surfaces.
Oral diseases related to dental biofilms afflict the majority of the world's population, and dental caries is still the single most prevalent and costly oral infectious disease (12, 32). Dental caries results from the interaction of specific bacteria with constituents of the diet within a biofilm formed on the tooth surface known as plaque (5, 36). Streptococcus mutans is a key contributor to the formation of biofilms associated with dental caries disease, although other microorganisms may also be involved (3); S. mutans (i) effectively utilizes dietary sucrose (and possibly starch) to rapidly synthesize exopolysaccharides (EPS) using glucosyltransferases and a fructosyltransferase that adsorb to surfaces, (ii) adheres tenaciously to glucan-coated surfaces, and (iii) is acidogenic and acid tolerant (5, 30).
In general, biofilms develop after initial attachment of microbes to a surface, followed by formation of highly structured cell clusters (or microcolonies) and further development and stabilization of the microcolonies, which are in a complex extracellular matrix (6, 49). The majority of biofilm matrices contain exopolysaccharides, and dental biofilms are no exception; up to 40% of the dry weight of dental plaque is composed of polysaccharides (depending on the type of carbohydrate consumption and the time of plaque collection), which are mostly glucans synthesized by microbial glucosyltransferases (Gtfs) (for a review, see reference 36). S. mutans plays a major role in the development and establishment of the EPS matrix in dental biofilms. This bacterium produces at least three Gtfs, which are products of the gtfB, gtfC, and gtfD genes; GtfB synthesizes mostly insoluble glucans containing elevated amounts of α-1,3-linked glucose, GtfC synthesizes a mixture of insoluble and soluble glucans (rich in α-1,6-linked glucose), and GtfD synthesizes predominantly soluble glucans (for reviews, see references 30 and 36). The Gtfs secreted by S. mutans bind avidly to the pellicle formed on the tooth surface and to bacterial surfaces and are enzymatically active; when they are exposed to sucrose, glucans are formed in situ within minutes (17, 33, 38, 40, 46). It is noteworthy that most nonstreptococcal oral bacteria (e.g., Actinomyces and Veillonella spp.) do not produce glucans unless Gtfs are adsorbed on their surfaces (33, 46). The glucans synthesized in situ provide binding sites for colonization and accumulation of S. mutans on the apatitic surface and for binding to each other through interactions with several membrane-associated glucan-binding proteins and surface glucans (8, 39, 47). The exopolymers also contribute to the bulk and physical integrity and stability of the biofilm matrix (for a review, see reference 36). The glucan-mediated processes promote tight adherence and coherence of bacterial cells bound to each other and to the apatitic surface, which leads to the formation of microcolonies by S. mutans and thereby modulates the initial steps of cariogenic biofilm development.
When dietary sucrose is consumed frequently, S. mutans, as a member of the oral biofilm community, continues to synthesize polysaccharides and metabolize this sugar to form organic acids. The elevated amounts of EPS, which may involve upregulation of gtf genes in response to pH and carbohydrate availability (29), increase the virulence of the biofilms (42, 51). In addition, the ability of S. mutans to utilize some extra- and intracellular polysaccharides as short-term storage compounds provides an additional ecological benefit and simultaneously increases the amount of acid produced and the extent of acidification within the biofilm (5, 7). The persistence of this aciduric environment leads to selection and dominance of highly acid-tolerant (and acidogenic) organisms, such as S. mutans (32, 37); the low-pH environment in the biofilm matrix results in dissolution of enamel, thus initiating the pathogenesis of dental caries (32, 36).
Recently, we have shown that EPS produced by S. mutans Gtfs modulate the initial formation, sequence of assembly, and structural organization of microcolonies by this bacterium on apatitic surfaces (50). However, it was unclear which of the Gtf enzymes were associated with these processes. Furthermore, the polysaccharides may also modulate the formation of microcolonies by complex ecological interactions in a multispecies system. In this study, we investigated (i) the role of each of the S. mutans gtf genes in EPS matrix and microcolony development on a saliva-coated hydroxyapatite (sHA) surface and (ii) the influence of exopolysaccharides on establishment of microcolonies at distinct developmental phases during formation of biofilms by S. mutans in the presence of Streptococcus oralis and Actinomyces naeslundii.
(This study was presented at 5th ASM Conference on Biofilms, Cancun, Mexico, 15 to 19 November 2009.)
MATERIALS AND METHODS
Bacterial strains.
S. mutans UA159 (ATCC 700610; serotype c), a proven virulent cariogenic dental pathogen selected for genomic sequencing (1), and gtf mutants of this strain were used for single-species biofilm assays. The mutant strains (gtfB, gtfC, gtfD, and gtfB gtfC mutants) were generated by a standard allelic replacement method and were verified by DNA sequence analysis (S.-J. Ahn, S.-J. Ahn and R. A. Burne, unpublished data); the gtf mutant strains were kindly provided by Robert A. Burne (Department of Oral Biology, University of Florida, Gainesville, FL). S. mutans UA159, A. naeslundii ATCC 12104, and S. oralis ATCC 35037 were used for the mixed-species biofilm investigation. The cultures were stored at −80°C in tryptic soy broth containing 20% glycerol.
S. mutans biofilm assay using gtf mutants.
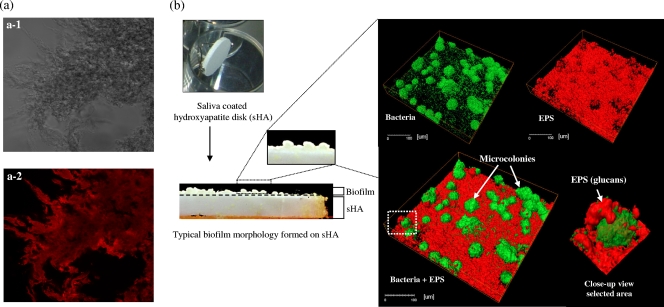
Hydroxyapatite disks (diameter, 1.25 cm; Clarkson Chromatography Products, Inc., South Williamsport, PA) were coated with filter-sterilized clarified human whole saliva (11, 23). Cells of S. mutans UA159 or one of the gtf mutant strains were grown in ultrafiltered (10-kDa-cutoff membrane; Prep/Scale; Millipore, MA) buffered tryptone-yeast extract broth (UFTYE) containing 2.5% tryptone and 1.5% yeast extract (pH 7.0) with 1% (wt/vol) glucose to mid-exponential phase (optical density at 600 nm [OD600], 0.5). All of the gtf mutants except the gtfD mutant had similar growth rates, as determined by OD600 using an automated turbidimetric system (Bioscreen C lab system; Oy Growth Curves Ab Ltd., Helsinki, Finland). Single-species biofilms were formed on sHA disks placed vertically using a disk holder in a 24-well plate (Fig. 1 b) containing batch (static) cultures (11, 23). The bacterial cells were grown in 2.8 ml (per well) of UFTYE containing 1% (wt/vol) sucrose and an sHA disk at 37°C in the presence of 5% CO2 for 24 h. At the end of the experimental period (24-h-old biofilms), the biofilms were dip washed three times and then gently swirled in physiological saline to remove loosely adherent material. The biofilms were then subjected to confocal imaging analysis (50).
FIG. 1.
(a) Water-insoluble glucans synthesized by S. mutans glucosyltransferase B (labeled with Alexa Fluor 647; maximum absorbance wavelength, 647 nm; maximum fluorescence emission wavelength, 668 nm). (a-1) Phase-contrast image of glucans before excitation with a laser at 633 nm (×20 oil objective; numerical aperture, 0.70). (a-2) Fluorescence image of glucans (adapted from reference 22). (b) Saliva-coated hydroxyapatite (sHA) biofilm model: simultaneous visualization of EPS (red) and bacteria and microcolonies (green) in a three-dimensional image of an S. mutans biofilm formed on the surface of an sHA disk.
Multispecies biofilm assay.
The multispecies biofilm method used was based on a batch culture approach using saliva-coated hydroxyapatite (sHA) disks (16, 23) and was designed to mimic the formation of biofilms according to the “ecological plaque-biofilm” concept (32). We used S. oralis ATCC 35037 and A. naeslundii ATCC 12104, in addition to S. mutans UA159, to generate multispecies biofilms in the presence of sucrose or glucose; these three organisms are found in supragingival plaque in humans (32). S. oralis is one of the most commonly detected early colonizers of the tooth surface (3, 14); strain ATCC 35037 produces soluble glucans and can be highly acid tolerant (21). A. naeslundii is also present at the early stages of plaque formation and may be also associated with development of root caries (32); strain ATCC 12104 is acidogenic and produces EPS, including fructans (2, 4).
S. mutans UA159, A. naeslundii ATCC 12104, and S. oralis ATCC 35037 cells were grown in UFTYE with 1% glucose at 37°C in the presence of 5% CO2 to mid-exponential phase (OD600 for streptococci, 0.5; OD600 for A. naeslundii, 0.75). The bacterial suspensions were mixed to obtain an inoculum containing a defined microbial population consisting of S. mutans (102 CFU/ml), A. naeslundii (106 CFU/ml), and S. oralis (107 CFU/ml). Biofilms were formed on sHA disks mounted vertically (like those used in the S. mutans biofilm assay) in batch cultures that were incubated for 115 h. The mixed population containing S. mutans, A. naeslundii, and S. oralis was inoculated into 2.8 ml of UFTYE with 0.1% sucrose and incubated at 37°C in the presence of 5% CO2. During the first 19 h, the organisms were grown without disturbance to allow initial biofilm formation. Then, after 19 h, the culture medium was replaced, and the biofilms were grown until 29 h to establish a mixed-species community. After 29 h of biofilm growth, the biofilms were transferred to UFTYE containing 1% sucrose or 1% glucose to induce environmental changes to simulate a cariogenic challenge. The culture medium was then changed twice daily (8 a.m. and 6 p.m.) until the end of the experimental period (115 h). The biofilms were analyzed using confocal imaging and biochemical assays at 29 h, 67 h, 91 h, and 115 h.
Laser scanning confocal fluorescence microscopy imaging of biofilms.
The architecture and structural organization of the biofilms were examined by simultaneous in situ labeling of extracellular polysaccharides (EPS) and bacterial cells as described by Klein et al. (22). Briefly, 1 μM Alexa Fluor 647-labeled dextran conjugate (molecular weight, 10,000; maximum absorbance wavelength, 647 nm; maximum fluorescence emission wavelength, 668 nm; Molecular Probes Inc., Eugene, OR) was added to the culture medium during formation and development of the biofilms. The fluorescently labeled dextran was used as an acceptor and was incorporated into newly formed glucan by Gtfs (such as GtfB) (Fig. 1a) during synthesis of the extracellular polysaccharide matrix during biofilm development, but it did not stain the bacterial cells at concentrations used in this study (22). The bacterial cells in biofilms were labeled with 2.5 μM SYTO 9 green fluorescent nucleic acid stain (480/500 nm; Molecular Probes Inc., Eugene, OR). For S. mutans UA159 and mutant strain single-species biofilms, confocal imaging was performed using a Leica TCS SP1 microscope (Leica Lasertechnik GmbH, Heidelberg, Germany) equipped with argon ion and helium-neon lasers set at 488 and 633 nm, respectively (22, 50). Triple dichroic filters (488, 543, and 633 nm) and emission filters (Chroma Technology Corp., Rockingham, VT) were used for detection of Alexa Fluor 647 and SYTO 9. Confocal images were acquired using a ×40, 0.8-numerical-aperture water immersion objective lens, which provided an optical section thickness of approximately 1 μm. For multispecies biofilms, imaging was performed using an Olympus FV 1000 two-photon laser scanning microscope (Olympus, Tokyo, Japan) equipped with a ×10, 0.45-numerical-aperture water immersion objective lens. Two-photon imaging provides enhanced visualization of multispecies biofilms, which are thicker and have larger microcolonies than single-species biofilms, especially at later stages of biofilm development. The excitation wavelength was 810 nm, and the emission wavelength filter for SYTO 9 was a 495/540 OlyMPFC1 filter, while the filter for Alexa Fluor 647 was an HQ655/40M-2P filter. A laser wavelength of ∼800 nm provides the maximum power and can excite a wide range of fluorophores (from blue through red) in the two-photon mode (10); 810 nm was determined to be the optimal wavelength for Alexa Fluor 647 excitation when the wavelengths available from our laser source (700 to 1,020 nm) were tested. The emission spectrum of Alexa Fluor 647 excited at 810 nm was the same as the emission spectrum for excitation at 633 nm used in single-photon confocal imaging. Each biofilm was scanned at five randomly selected positions, and z series were generated by optical sectioning at each of these positions.
Image analyses using COMSTAT.
Three independent biofilm experiments were performed, and 10 image stacks (512- by 512-pixel tagged image file format) were collected for each experiment. The confocal image stacks were analyzed by the COMSTAT image-processing software (18) as described by Klein et al. (22). This software was written as a script in Matlab 5.1 (The MathWorks, Natick, MA) equipped with the Image Processing Toolbox, which generated several measurements for quantifying and characterizing the three-dimensional structure of biofilms (18). In this study, the biomass of EPS and bacterial cells and the number of microcolonies were calculated using COMSTAT to determine the structural differences among the different biofilms. The three-dimensional architecture of the biofilms was visualized using Amira 5.0.2 (Mercury Computer Systems Inc., Chelmsford, MS). The confocal fluorescence data were imported into the software, and three-dimensional images of each of the components in the biofilms were created using voltex and iso-surface rendering (22, 50).
Biochemical and microbiological analyses.
The multispecies biofilms were removed and homogenized by sonication in a sterile 0.89% (wt/vol) NaCl solution (30-s pulse at an output of 7 W; Branson Sonifier 150; Branson Ultrasonics, Danbury, CT). An homogenized suspension was used to determine the dry weight, the number of viable cells (by plating on blood agar using an automated spiral plater and determining the total number of CFU per biofilm), and the polysaccharide content. The three species were differentiated by observation of colonial morphology in conjunction with microscopic examination of cells from selected colonies (16). The amounts of soluble and insoluble extracellular polysaccharides were determined by the phenol-sulfuric acid method (using glucose as the standard) as described by Koo et al. (24) and Duarte et al. (11).
Statistical analysis.
The COMSTAT, biochemical, and microbiological data were analyzed using analysis of variance (ANOVA), and the F test was used to examine differences among the test groups (different S. mutans strains and distinct multispecies growth conditions). When significant differences were detected, pairwise comparisons were made between all of the groups using Tukey's method to adjust for multiple comparisons. The statistical software JMP (version 3.1; SAS Institute, Cary, NC) was used to perform the analyses. The level of significance used was 5%.
RESULTS AND DISCUSSION
The attachment of bacterial cells on surfaces and the formation of highly structured cell clusters (or microcolonies) may be linked to the ability of organisms to survive and persist within biofilms (microbial fitness) and to express virulence (19, 28). At the same time, the extracellular matrix provides mechanical stability to maintain a spatial arrangement for microconsortia over a prolonged period and could also affect the diffusion properties of the biofilms (6, 13).
S. mutans cells can attach initially to saliva-coated surfaces (albeit in low numbers) through sucrose-independent mechanisms mediated primarily by lectin-like interactions between specific pellicle proteins (e.g., agglutinins) and adhesins (e.g., P1) present on the bacterial cell surface (15). In contrast, this bacterium binds avidly to glucan-coated surfaces, particularly those synthesized in situ by GtfB and GtfC, in larger numbers and with greater adhesion strength than it binds to uncoated or saliva-coated apatitic surfaces (8, 27, 39). Moreover, S. mutans, alone or mixed with other species, can develop into microcolonies only when sucrose is available (26, 50), suggesting a potential role of Gtfs and glucans in the formation and establishment of structured microcolonies.
Role of S. mutans Gtfs in EPS matrix and microcolony development in biofilms.
We used mutant strains defective of each of the gtf genes and our confocal fluorescence imaging approach (22) (Fig. 1) to investigate which Gtfs from S. mutans modulate EPS matrix and microcolony formation.
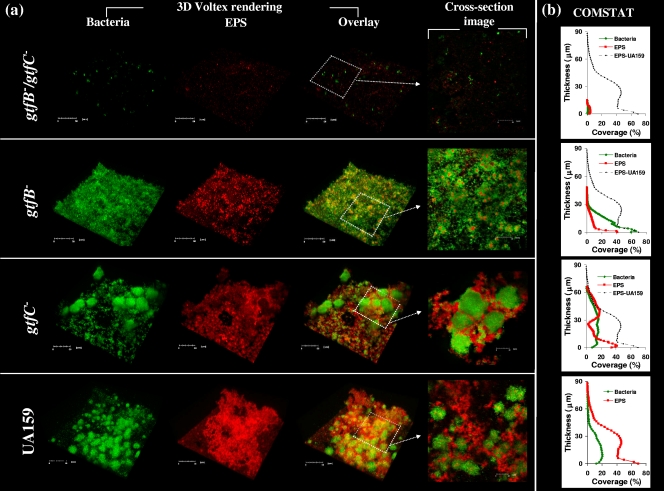
Figure 2 a shows a representative three-dimensional image of bacteria (green) and glucans (red) in biofilms formed by the parental strain (UA159) and gtf mutants. Furthermore, the vertical distribution of glucans and bacteria from the disk surface to the fluid phase interface was calculated using the confocal imaging data sets and COMSTAT (Fig. 2b). Our data show that the mutations in the strain with defects in both the gtfB (associated with insoluble glucan synthesis) and gtfC (associated with soluble and insoluble glucan synthesis) genes markedly disrupted the ability of this strain to synthesize the EPS matrix and form microcolonies on a saliva-coated hydroxyapatite (sHA) surface; only single cell chains and occasional small clusters of cells of the gtfB gtfC mutant strain were observed on the surface (Fig. 2a). Consequently, deletion of gtfB and gtfC resulted in poor biofilm formation and minimal accumulation of bacterial cells and polysaccharides on the sHA surface as determined by COMSTAT (Fig. 2b). For the mutant strain defective in either gtfB or gtfC there was also significant (albeit not complete) loss of the ability to synthesize the EPS matrix (compared to parental strain UA159) (Fig. 2a and 2b). However, deletion of each of these genes resulted in a distinct pattern of bacterial accumulation on the apatitic surfaces. The gtfB mutant uniformly attached to and accumulated on the surface, but it did not form microcolonies according to the COMSTAT analysis. In contrast, deletion of gtfC did not decrease the ability of the resulting organism to form microcolonies, although there were fewer microcolonies of the gtfC mutant than of parental strain UA159 (17 ± 4 microcolonies for the gtfC mutant compared with 33 ± 9 microcolonies for UA159). Cross-sectional images of the biofilms formed by the parental strain and the gtfC mutant showed that there were polysaccharides surrounding and enmeshing the microcolonies and filling the spaces between the microcolonies. These observations indicate that the presence of a functional gtfB gene is critical for clustering of bacterial cells and for further stabilization and development into microcolonies (Fig. 2a).
FIG. 2.
(a) Representative three-dimensional images of biofilms formed by S. mutans UA159 and gtf mutant strains in the presence of 1% (wt/vol) sucrose. (b) COMSTAT analysis of the distribution of bacteria and EPS from the disk surface to the fluid phase interface.
The simultaneous synthesis of glucans, particularly by GtfB and GtfC, appears to be essential for establishment of the extracellular matrix, but GtfB may be responsible for modulating the formation of microcolonies by S. mutans cells. The role of GtfD could not be assessed precisely in our experiments due to a significant reduction in the bacterial growth rate as a result of deletion of gtfD (an effect not observed with other gtf deletions). GtfD may also contribute to this process because water-soluble glucans produced by GtfD act as primers for synthesis of insoluble glucan by GtfB, indicating that there is positive cooperativity in establishment of the exopolysaccharide matrix (47). The specific roles of GtfB and GtfC in S. mutans biofilm formation could be associated with their specific localization, spatial distribution, and type of glucan product. Although all three enzymes secreted by S. mutans bind to an experimental salivary pellicle formed on hydroxyapatite (sHA), GtfC has the greatest affinity for the sHA surface, followed by GtfB and then GtfD (46). The presence of small hydrophobic domains in the C terminus of GtfC may be associated with the pellicle-binding activity of this enzyme, possibly through interactions with specific salivary macromolecules present in the pellicle, such as lysozyme and α-amylase (45, 46). The glucans formed in the pellicle by surface-adsorbed GtfB and GtfC (but not GtfD) have been shown to increase the adherence of S. mutans GS-5 and Streptococcus sobrinus 6715 to an apatitic surface (39, 47). The presence of α-1,6 linkages in conjunction with 1,3 linkages on the GtfB- and GtfC-derived glucan is critical for mediating bacterial adhesion, possibly by conferring a specific structure or conformation that serves as a bacterial binding site (47). Thus, it appears that bacterial cells were still able to attach to and accumulate on an sHA surface even after deletion of gtfB (or gtfC) due to the presence of a functional gtfC (or gtfB) gene (Fig. 2a); previous studies have shown that a single gtfC deletion did not decrease the ability of the S. mutans mutant to form biofilms, either alone or with other organisms (43).
The polysaccharides formed by GtfB appear to be critical for microcolony development, which may be associated with the type of glucans and the localization of the enzyme. GtfB binds with greater avidity to the S. mutans cell surface than the other enzymes and, more importantly, in an enzymatically active form (46); the carboxyl-terminal repeating units of GtfB have a major role in the specific binding of the enzyme to the cell surface of oral streptococci (20). The presence of surface-bound GtfB glucans enhances the binding of bacteria to each other, promoting the initial clustering of cells and formation of microcolonies. Furthermore, GtfB synthesizes more glucans with elevated amounts of α-1,3-linkages with a higher percentage of 3-linked branch points than other Gtfs, which produce a highly insoluble and structurally rigid polymer (25), allowing vertical growth of the microcolonies and increasing the thickness of the biofilms.
Thus, with their specific localization and spatial distribution, highly adherent and structurally rigid insoluble glucans serve as (i) a matrix that holds bacterial cells together and mediates the initial assembly on the surface of sHA (which is mediated primarily by GtfC) and (ii) a supporting frame for further development into microcolonies, which may provide mechanical stability by tightly and stably binding the bacterial cells together and to the surface (which is mediated mostly by GtfB). These polymers, in addition to their interactions with specific glucan-binding proteins expressed on the S. mutans cell surface (31), are essential for maintaining the three-dimensional structure of microcolonies over time. This observation could explain why deletion of both gtfB and gtfC resulted in a nearly complete loss of the ability of the organisms to form biofilms.
Furthermore, GtfB binds to other oral microorganisms, including nonmutans streptococci, Lactobacillus species, and Actinomyces species, in an active form which could provide additional binding sites for S. mutans (5, 46). Considering that bacterial fitness and expression of virulence in biofilms may be linked to microcolony formation (19, 28), this specific glucan-dependent mechanism may modulate the establishment and survival of S. mutans within complex biofilms in a context not previously considered.
Development of an S. mutans biofilm in a mixed-species system.
We investigated whether the presence of other relevant oral species influences the formation of the EPS matrix and microcolonies by S. mutans. Analyses of the microbial species composition in dental biofilms have shown that the majority (47 to 90%) of cultivable bacteria are nonmutans streptococci, such as S. oralis, and that one-third of the remaining bacteria are Actinomyces species, such as A. naeslundii (32, 34, 35). The bacterial composition of a human dental biofilm is relatively stable when the biofilm is exposed to minor environmental changes (32). The formation and establishment of biofilms related to dental caries involve a change in a key environmental factor, e.g., persistent availability of sucrose (32, 36). Here, we hypothesized that S. mutans Gtfs trigger the formation of structured microcolonies after introduction of sucrose, which contributes to establishment of this pathogen in a mixed-species environment and simultaneously increases the biomass and exopolysaccharide content of the biofilms.
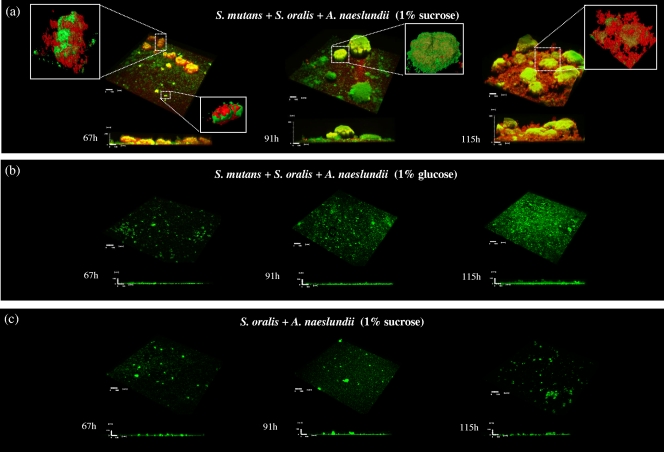
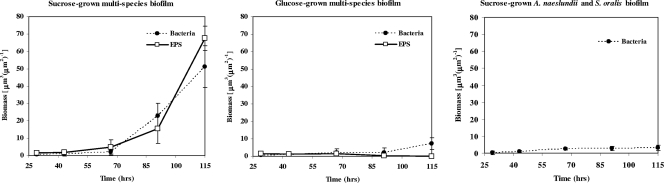
As shown in Fig. 3, both an EPS matrix and microcolonies were detected only in biofilms formed in the presence of S. mutans and sucrose, which resulted in thicker biofilms containing significantly more bacterial and EPS biomass than other biofilms, as measured by COMSTAT (P < 0.05) (Fig. 4). In contrast, in biofilms grown in the presence of glucose or sucrose but without S. mutans there was minimal accumulation of bacterial cells (or microcolony formation) and glucans, although some cell clusters were present on the apatitic surface (Fig. 3 and 4). The residual EPS detected in the glucose biofilms (Fig. 4) was likely due to polysaccharides synthesized during the first 29 h, when cultures were grown in the presence of 0.1% (wt/vol) sucrose to allow initial establishment of a biofilm. In addition, it is possible that our labeling technique did not detect soluble exopolymers synthesized by S. oralis and A. naeslundii in the presence of sucrose (such as fructans). Figure 3 also shows selected areas of a sucrose-grown multispecies biofilm and the structural relationship between bacteria and EPS at the microscale. The images show that exopolysaccharides were closely associated with bacterial cells throughout biofilm development and surrounded the cells and bound them together and to the sHA surface. The ability of GtfB to bind on the surface of Actinomyces spp. and oral streptococci (46) increases the amount of insoluble glucans synthesized in situ, promoting S. mutans binding to other organisms and concomitantly providing additional structural support for microcolony development. This effect could explain the development of the highly organized, large microcolonies enmeshed in an intricate web-like exopolysaccharide matrix observed at the late stage of the biofilm formation process (115 h) (Fig. 3). Results of previous studies have shown that sucrose induces the expression of gtfB and gtfC by S. mutans in single-species biofilms (22, 29, 41), directly influencing the biomass, EPS content, and stability of S. mutans biofilms (22, 50). Recently, we observed that S. mutans exhibits a higher level of gtfB expression (compared with the expression of other gtf genes) as multispecies biofilms transition from an early stage (43 h) to later stages of development (91 to 115 h) (H. Koo, M. J. Klein, and J. Xiao, unpublished results).
FIG. 3.
Representative three-dimensional images of multispecies biofilms grown in the presence of sucrose (a) or glucose (b) and a two-species biofilm grown in the presence of sucrose (c). The images are three-dimensional images of bacteria (green) and EPS (red) and selected enlarged areas for visualization of the structural relationship between bacterial cells and glucans.
FIG. 4.
Biomasses of EPS and bacterial cells in multispecies biofilms formed in the presence of 1% (wt/vol) sucrose or 1% (wt/vol) glucose and in sucrose-grown two-species (A. naeslundii and S. oralis) biofilms, as determined by COMSTAT analysis. The data are means ± standard deviations (n = 30) from three independent experiments. The values for the EPS and bacterial biomasses for the sucrose-grown multispecies biofilm at 67 h, 91 h, and 115 h are significantly different from the values for the glucose-grown multispecies and sucrose-grown two-species biofilms (P < 0.05, as determined by ANOVA for all pairs using the Tukey-Kramer honestly significant difference test).
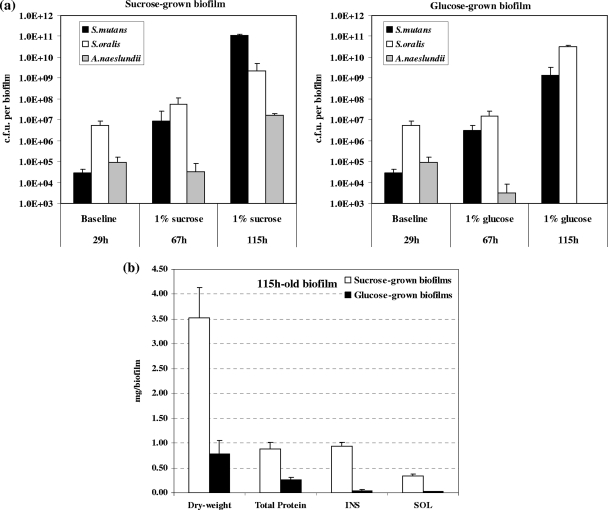
In parallel, the composition and proportion of the bacterial species and the amounts of both insoluble and soluble EPS in the biofilm matrix were profoundly altered by the presence of sucrose. Figure 5 a shows the changes in the microbial populations in the biofilms over time. At 29 h (baseline), the biofilms were comprised of mostly S. oralis, followed by A. naeslundii and S. mutans. After introduction of 1% sucrose, S. mutans became the major species in the biofilms (at 115 h), and concomitantly, the level of insoluble exopolysaccharides (mostly insoluble glucans) increased from an undetectable level at 29 h (data not shown) to approximately 30% of the biofilm dry weight at 115 h (Fig. 5b). In contrast, S. oralis ATCC 35307 (an acid-tolerant strain), rather than S. mutans UA159, was the dominant species in biofilms formed after introduction of 1% glucose. The glucose-grown biofilms contained approximately 10-fold fewer viable (total) bacterial cells and 4.5-fold less biomass than the biofilms that formed in the presence of sucrose. It is noteworthy that the biochemical and microbiological data agree well with the confocal imaging (Fig. 3) and COMSTAT (Fig. 4) analysis results. However, biomass measurements obtained with COMSTAT may be underestimated due to limitation of the laser penetration in the inner layers of the large (and thicker) microcolonies found in mature biofilms (at 115 h).
FIG. 5.
Biochemical analyses of multispecies biofilms formed in the presence of 1% (wt/vol) sucrose or 1% (wt/vol) glucose. (a) Average number of CFU recovered per biofilm. (b) Dry weight and biochemical composition of the biofilms. The data are means ± standard deviations (n = 9) from three independent experiments. The dry weight and the total amounts of protein and insoluble (INS) and soluble (SOL) polysaccharides in the sucrose-grown multispecies biofilm at 115 h are significantly different from the values for the glucose-grown multispecies biofilm (P < 0.05, as determined by ANOVA for all pairs using the Tukey-Kramer honestly significant difference test).
Furthermore, the presence of EPS within and surrounding microcolonies throughout the biofilm may create chemical gradients due to the differential diffusion (more cross-linked glucans may limit diffusion) of nutrients and metabolic products throughout the matrix, which could affect the microenvironment pH in the biofilms (9, 28, 44, 48). This structural organization could protect the bacteria from inimical influences of antimicrobials and other environmental assaults (26, 50). Fructans and soluble glucans synthesized by S. oralis and A. naeslundii may also contribute to the overall polysaccharide synthesis in the extracellular matrix (7), although their role in the structural stability of biofilms remains to be explored.
Collectively, the results described here emphasize the importance of S. mutans Gtfs and the EPS matrix in the development of highly stable and structured microcolonies and their influence on the biomass, architecture, and pathogenicity of the multispecies biofilms. The production of glucans by GtfB and GtfC acting in concert at different sites may enhance the ability of S. mutans to compete with other oral species and allow this bacterium to persist at high levels on the apatitic surface for prolonged periods of time. Our data further support and expand the observations made in previous in vitro and in vivo studies which indicated that both the gtfB and gtfC genes are required for sucrose-dependent colonization of hard surfaces by S. mutans and in the pathogenesis of dental caries in vivo (42, 51). The reactions of glucosyltransferases in pellicles and within biofilms are diverse and complex, and further elucidation of how the exopolymers synthesized by these enzymes influence the biophysical and diffusion properties of the matrix would increase our understanding of the formation and metabolic activity of microcolonies and potentially identify novel therapeutic targets that can be used to effectively disrupt the development of pathogenic biofilms.
Acknowledgments
We thank Sang-Joon Ahn and Sug-Joon Ahn (of the laboratory of Robert A. Burne at University of Florida, Gainesville, FL) for providing the gtf mutant strains. We are also grateful to Bruno B. Silva for technical assistance with confocal imaging.
This research was supported in part by Public Health Service research grant 1R01 DE018023 from the National Institute of Dental and Craniofacial Research.
Footnotes
Published ahead of print on 16 March 2010.
REFERENCES
- 1.Ajdić, D., W. M. McShan, R. E. McLaughlin, G. Savić, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, P. Z., and W. H. Bowen. 1990. Immunochemical studies on levans from several strains of Actinomyces viscosus. Arch. Oral Biol. 35:55-62. [DOI] [PubMed] [Google Scholar]
- 3.Beighton, D. 2005. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent. Oral Epidemiol. 33:248-255. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron, L. J., E. Morou-Bermudez, and R. A. Burne. 2000. Characterization of the fructosyltransferase gene of Actinomyces naeslundii WVU45. J. Bacteriol. 182:3649-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen, W. H. 2002. Do we need to be concerned about dental caries in the coming millennium? J. Am. Dent. Assoc. 133:1405-1407. [DOI] [PubMed] [Google Scholar]
- 6.Branda, S. S., S. Vik, L. Friedman, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. [DOI] [PubMed] [Google Scholar]
- 7.Burne, R. A., Y. Y. Chen, D. L. Wexler, H. Kuramitsu, and W. H. Bowen. 1996. Cariogenicity of Streptococcus mutans strains with defects in fructan metabolism assessed in a program-fed specific-pathogen-free rat model. J. Dent. Res. 75:1572-1577. [DOI] [PubMed] [Google Scholar]
- 8.Cross, S. E., J. Kreth, L. Zhu, R. Sullivan, W. Shi, F. Qi, and J. K. Gimzewski. 2007. Nanomechanical properties of glucans and associated cell-surface adhesion of Streptococcus mutans probed by atomic force microscopy under in situ conditions. Microbiology 153:3124-3132. [DOI] [PubMed] [Google Scholar]
- 9.Dibdin, G. H., and R. P. Shellis. 1988. Physical and biochemical studies of Streptococcus mutans sediments suggest new factors linking the cariogenicity of plaque with its extracellular polysaccharide content. J. Dent. Res. 67:890-895, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Drobizhev, M., S. Tillo, N. S. Makarov, T. E. Hughes, and A. Rebane. 2009. Absolute two-photon absorption spectra and two-photon brightness of orange and red fluorescent proteins. J. Phys. Chem. B 113:855-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte, S., M. I. Klein, C. P. Aires, J. A. Cury, W. H. Bowen, and H. Koo. 2008. Influences of starch and sucrose on Streptococcus mutans biofilms. Oral Microbiol. Immunol. 23:206-212. [DOI] [PubMed] [Google Scholar]
- 12.Dye, B. A., S. Tan, V. Smith, B. G. Lewis, L. K. Barker, G. Thornton-Evans, P. I. Eke, E. D. Beltrán-Aguilar, A. M. Horowitz, and C. H. Li. 2007. Trends in oral health status: United States, 1988-1994 and 1999-2004. Vital Health Stat. 11:1-92. [PubMed] [Google Scholar]
- 13.Flemming, H. C., T. R. Neu, and D. J. Wozniak. 2007. The EPS matrix: the “house of biofilm cells.” J. Bacteriol. 189:7945-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara, T., T. Hoshino, T. Ooshima, S. Sobue, and S. Hamada. 2000. Purification, characterization, and molecular analysis of the gene encoding glucosyltransferase from Streptococcus oralis. Infect. Immun. 68:2475-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbons, R. J. 1996. Role of adhesion in microbial colonization of host tissues: a contribution of oral microbiology. J. Dent. Res. 75:866-870. [DOI] [PubMed] [Google Scholar]
- 16.Guggenheim, B., E. Giertsen, P. Schüpbach, and S. Shapiro. 2001. Validation of an in vitro biofilm model of supragingival plaque. J. Dent. Res. 80:363-370. [DOI] [PubMed] [Google Scholar]
- 17.Hannig, C., A. Ruggeri, B. Al-Khayer, P. Schmitz, B. Spitzmüller, D. Deimling, K. Huber, W. Hoth-Hannig, W. H. Bowen, and M. Hannig. 2008. Electron microscopic detection and activity of glucosyltransferase B, C, and D in the in situ formed pellicle. Arch. Oral. Biol. 53:1003-1010. [DOI] [PubMed] [Google Scholar]
- 18.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersbøll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, L. R. 2008. Microcolony and biofilm formation as a survival strategy for bacteria. J. Theor. Biol. 251:24-34. [DOI] [PubMed] [Google Scholar]
- 20.Kato, C., and H. K. Kuramitsu. 1991. Molecular basis for the association of glucosyltransferases with the cell surface of oral streptococci. FEMS Microbiol. Lett. 63:153-157. [DOI] [PubMed] [Google Scholar]
- 21.Kirchherr, J. L., G. H. Bowden, M. F. Cole, Y. Kawamura, D. A. Richmond, M. J. Sheridan, and K. A. Wirth. 2007. Physiological and serological variation in Streptococcus mitis biovar 1 from the human oral cavity during the first year of life. Arch. Oral Biol. 52:90-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein, M. I., S. Duarte, J. Xiao, S. Mitra, T. H. Foster, and H. Koo. 2009. Structural and molecular basis of the role of starch and sucrose in Streptococcus mutans biofilm development. Appl. Environ. Microbiol. 75:837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo, H., B. D. Schobel, K. Scott-Anne, G. Watson, W. H. Bowen, J. A. Cury, P. L. Rosalen, and Y. K. Park. 2005. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 84:1016-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo, H., M. F. Hayacibara, B. D. Schobel, J. A. Cury, P. L. Rosalen, Y. K. Park, A. M. Vacca-Smith, and W. H. Bowen. 2003. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 52:782-789. [DOI] [PubMed] [Google Scholar]
- 25.Kopec, L. K., A. M. Vacca-Smith, and W. H. Bowen. 1997. Structural aspects of glucans formed in solution and on the surface of hydroxyapatite. Glycobiology 7:929-934. [DOI] [PubMed] [Google Scholar]
- 26.Kreth, J., L. Zhu, J. Merritt, W. Shi, and F. Qi. 2008. Role of sucrose in the fitness of Streptococcus mutans. Oral Microbiol. Immunol. 23:213-219. [DOI] [PubMed] [Google Scholar]
- 27.Kuramitsu, H. K. 1974. Adherence of Streptococcus mutans to dextran synthesized in the presence of extracellular dextransucrase. Infect. Immun. 9:764-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence, J. R., G. D. Swerhone, U. Kuhlicke, and T. R. Neu. 2007. In situ evidence for microdomains in the polymer matrix of bacterial microcolonies. Can. J. Microbiol. 53:450-458. [DOI] [PubMed] [Google Scholar]
- 29.Li, Y., and R. A. Burne. 2001. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiology 147:2841-2848. [DOI] [PubMed] [Google Scholar]
- 30.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch, D. J., T. L. Fountain, J. E. Mazurkiewicz, and J. A. Banas. 2007. Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol. Lett. 268:158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh, P. D. 2003. Are dental diseases examples of ecological catastrophes? Microbiology 149:279-294. [DOI] [PubMed] [Google Scholar]
- 33.McCabe, R. M., and J. A. Donkersloot. 1977. Adherence of Veillonella species mediated by extracellular glucosyltransferase from Streptococcus salivarius. Infect. Immun. 18:726-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyvad, B., and M. Kilian. 1987. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand. J. Dent. Res. 95:369-380. [DOI] [PubMed] [Google Scholar]
- 35.Nyvad, B., and M. Kilian. 1990. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 24:267-272. [DOI] [PubMed] [Google Scholar]
- 36.Paes Leme, A. F., H. Koo, C. M. Bellato, G. Bedi, and J. A. Cury. 2006. The role of sucrose in cariogenic dental biofilm formation—new insight. J. Dent. Res. 85:878-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quivey, R. G., Jr., W. L. Kuhnert, and K. Hahn. 2000. Adaptation of oral streptococci to low pH. Adv. Microb. Physiol. 42:239-274. [DOI] [PubMed] [Google Scholar]
- 38.Rölla, G., J. E. Ciardi, K. Eggen, W. H. Bowen, and J. Afseth. 1983. Free glucosyl- and fructosyltransferase in human saliva and adsorption of these enzymes to teeth in vivo, p. 21-30. In R. J. Doyle and J. E. Ciardi (ed.), Glucosyltransferases, glucans, sucrose, and dental caries. IRL Press, Washington, DC.
- 39.Schilling, K. M., and W. H. Bowen. 1992. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect. Immun. 60:284-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilling, K. M., and W. H. Bowen. 1988. The activity of glucosyltransferase adsorbed onto saliva-coated hydroxyapatite. J. Dent. Res. 67:2-8. [DOI] [PubMed] [Google Scholar]
- 41.Shemesh, M., A. Tam, and D. Steinberg. 2007. Differential gene expression profiling of Streptococcus mutans cultured under biofilm and planktonic conditions. Microbiology 153:1307-1317. [DOI] [PubMed] [Google Scholar]
- 42.Tanzer, J. M., M. L. Freedman, and R. J. Fitzgerald. 1985. Virulence of mutants defective in glucosyltransferase, dextran-mediated aggregation, or dextranase activity, p. 204-211. In S. E. Mergenhagen and B. Rosan (ed.), Molecular basis of oral microbial adhesion. American Society for Microbiology, Washington, DC.
- 43.Thurnheer, T., J. R. van der Ploeg, E. Giertsen, and B. Guggenheim. 2006. Effects of Streptococcus mutans gtfC deficiency on mixed oral biofilms in vitro. Caries Res. 40:163-171. [DOI] [PubMed] [Google Scholar]
- 44.Thurnheer, T., R. Gmur, S. Shapiro, and B. Guggenheim. 2003. Mass transport of macromolecules within an in vitro model of supragingival plaque. Appl. Environ. Microbiol. 69:1702-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vacca-Smith, A. M., A. R. Venkitaraman, R. G. Quivey, Jr., and W. H. Bowen. 1996. Interactions of streptococcal glucosyltransferases with alpha-amylase and starch on the surface of saliva-coated hydroxyapatite. Arch. Oral Biol. 41:291-298. [DOI] [PubMed] [Google Scholar]
- 46.Vacca-Smith, A. M., and W. H. Bowen. 1998. Binding properties of streptococcal glucosyltransferases for hydroxyapatite, saliva-coated hydroxyapatite, and bacterial surfaces. Arch. Oral Biol. 3:103-110. [DOI] [PubMed] [Google Scholar]
- 47.Venkitaraman, A. R., A. M. Vacca-Smith, L. K. Kopec, and W. H. Bowen. 1995. Characterization of glucosyltransferase B, GtfC, and GtfD in solution and on the surface of hydroxyapatite. J. Dent. Res. 74:1695-1701. [DOI] [PubMed] [Google Scholar]
- 48.Vroom, J. M., K. J. De Grauw, H. C. Gerritsen, D. J. Bradshaw, P. D. Marsh, G. K. Watson, J. J. Birmingham, and C. Allison. 1999. Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy. Appl. Environ. Microbiol. 65:3502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao, J., and H. Koo. 4 November 2009, posting date. Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J. Appl. Microbiol. [Epub ahead of print.] doi: 10.1111/j.1365-2672.2009.04616.x. [DOI] [PubMed]
- 51.Yamashita, Y., W. H. Bowen, R. A. Burne, and H. K. Kuramitsu. 1993. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 61:3811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]