Abstract
Several bacterial strains isolated from granitic rock material in front of the Damma glacier (Central Swiss Alps) were shown (i) to grow in the presence of granite powder and a glucose-NH4Cl minimal medium without additional macro- or micronutrients and (ii) to produce weathering-associated agents. In particular, four bacterial isolates (one isolate each of Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp.) were weathering associated. In comparison to what was observed in abiotic experiments, the presence of these strains caused a significant increase of granite dissolution (as measured by the release of Fe, Ca, K, Mg, and Mn). These most promising weathering-associated bacterial species exhibited four main features rendering them more efficient in mineral dissolution than the other investigated isolates: (i) a major part of their bacterial cells was attached to the granite surfaces and not suspended in solution, (ii) they secreted the largest amounts of oxalic acid, (iii) they lowered the pH of the solution, and (iv) they formed significant amounts of HCN. As far as we know, this is the first report showing that the combined action of oxalic acid and HCN appears to be associated with enhanced elemental release from granite, in particular of Fe. This suggests that extensive microbial colonization of the granite surfaces could play a crucial role in the initial soil formation in previously glaciated mountain areas.
Glaciers in alpine regions are highly sensitive to changes in climatic conditions (29). Increasing global atmospheric temperatures over the last decades have resulted in the recession of alpine glaciers (18). Forefields of temperate alpine glaciers provide unique opportunities to study initial soil formation as well as microbial and plant succession along the chronosequences (12, 26, 34, 36). The forefields close to the glacier terminus are initially vegetation free and consist mainly of rock material with high fractions of silt-sized grains with low C and N content and small amounts of available nutrients (14). Mineral weathering is a key process in the formation of soils (1, 26), and the crucial importance of microbially promoted mineral weathering for nutrient acquisition is increasingly recognized (2, 4, 39, 46). Recently exposed rock surfaces can be considered primary ecosystems where only a few microbes that are adapted due to their mineral-weathering abilities can grow (17). Some cations of rock-forming minerals are essential for proper cell functions. However, our understanding of geochemically significant microbes in forefields of temperate alpine glacier is still very limited but is crucial for increasing our knowledge of nutrient mobilization and the buildup of organic matter that is essential for the development of macroorganisms.
The area of the Damma glacier in Central Switzerland is characterized by a relatively homogenous granitic rock basement and is used as field site of the interdisciplinary research project “Biosphere-Geosphere interactions: Linking climate change, weathering, soil formation and ecosystem evolution (BigLink)” (5). In the frame of this research project, we studied the functional roles of granite-colonizing microbes as biotic weathering agents in previously glaciated areas. So far, relatively little is known about microbe-granite interactions, especially regarding the release of trace elements. Several studies have examined the dissolution of specific granite-forming minerals in the presence of actively metabolizing bacteria or compounds that simulate metabolic activity (24, 30, 31, 37, 38, 44). There is a general agreement that microbially produced organic acids, siderophores, and extracellular polysaccharides can all promote dissolution of minerals. Previous dissolution experiments have mainly been performed with (i) commercially obtained minerals (23, 45), (ii) model microorganisms that were commercially obtained from culture collections (3, 35, 45), or (iii) laboratory strains, such as those of Bacillus subtilis (23) and Burkholderia fungorum (47). Most studies have focused on individual mineral specimens rather than on the mixture of minerals that are present in granite rock (47). Few studies observed mineral weathering of collected rock and bacteria isolated from volcanic areas covered with vegetation (30, 31). Moreover, there are no studies on microbial weathering for such immediately deglaciated environments combining functional and taxonomic investigations, probably due to the difficulties in obtaining heterotrophic bacterial isolates from granitic glacier forefields. In spite of this, a comprehensive culture collection containing approximately 500 bacterial strains, which were isolated from the glacier tongue of the Damma glacier, was established. Full-length 16S rRNA gene sequences of 120 isolates revealed that many isolates obtained from oligotrophic media were closely related to readily cultivable heterotrophic bacteria (e.g., Arthrobacter sp., Collimonas sp., Paenibacillus sp., and Pseudomonas sp.). These bacteria have been found to enhance mineral dissolution (39).
Our aim was to characterize the impact of microorganisms on granite weathering. We performed laboratory dissolution experiments with sterile crushed granite rock material, 12 bacterial strains, and 1 algal strain. To investigate the potential weathering abilities of these isolates, granite dissolution experiments were performed abiotically with model agents, such as HCl for proton-promoted weathering or oxalate and citrate and KCN for ligand-promoted weathering.
MATERIALS AND METHODS
Characterization and preparation of granite samples.
For the dissolution experiments, we used granite samples from the slope of the Winterstock mountain (46°38′ N, 08°28′ E), near the Damma glacier, which is located in the Central Alps of Switzerland (5). Unweathered fist-sized stone samples were collected with a hammer. The rocks were first crushed to a grain size of <0.6 mm with a hammer and a jaw breaker (Retsch BB51; Germany). From this material, a representative amount was separated and milled within ethanol with a McCrone micronizing mill (McCrone Scientific, United Kingdom), using agate grinding elements, to a grain size of <20 μm. The mineralogical composition (Table 1) of this material was determined with X-ray powder diffraction (XRD). The quantitative determination was carried out with Rietveld analysis of the XRD pattern using the program AutoQuan (Seifert GE). The total specific surface area of the <63-μm fraction was determined with 7-point N2-BET (Brunauer, Emmett, and Teller) in the relative pressure range characterized by a p/p0 value of 0.01 to 0.30 (Autosorb-1 MP; Quantachrome, Germany). The total specific surface area of the granite powder resulted in a value of 2.5 m2 g−1. The chemical composition (Table 2) of the rock was measured with X-ray fluorescence spectroscopy (XRF; Spectro X Lab 2000 spectrometer). For that reason, 4 g of the <0.6-mm fraction was milled in a tungsten carbide rotary disc mill, mixed with 0.9 g of Licowax C Micropowder PM (Fluxona), and pressed at 1.5 tons cm−2 to form the pellets.
TABLE 1.
Mineralogical composition of granite from the Damma area
| Mineral | Composition | % (wt/wt)a |
|---|---|---|
| Albite | NaAlSi3O8 | 35.0 ± 0.9 |
| Quartz | SiO2 | 25.9 ± 0.5 |
| Microcline | KAlSi3O8 | 14.1 ± 0.8 |
| Muscovite | K(Al)2(AlSi3)O10(OH,F)2 | 8.8 ± 0.7 |
| Biotite | K[(Mg,FeII)]3(AlSi3)O10(OH,F)2 | 5.8 ± 0.5 |
| Epidote | Ca2[Fe(III),Al]Al2(SiO4)(Si2O7)O(OH) | 6.0 ± 0.5 |
| Chlorite | (Mg,Fe)6(Si,Al)4O10(OH)8 | 2.7 ± 0.6 |
| Magnetite | Fe(III)2Fe(II)O4 | 0.6 ± 0.2 |
Values are means ± standard error.
TABLE 2.
Elemental composition of granite from the Damma area (XRF analysis)
| Element | % | ppm |
|---|---|---|
| Si | 28.1 | |
| Al | 6.3 | |
| K | 3.1 | |
| Fe | 2.5 | |
| Ti | 0.3 | |
| Na | 1.5 | |
| Ca | 1.3 | |
| Ba | 1.2 | |
| Cr | 0.6 | |
| Mg | 0.5 | |
| W | 0.4 | |
| Sr | 0.3 | |
| Zr | 0.3 | |
| Rb | 0.1 | |
| Ce | 86 | |
| Cl | 75 | |
| P | 69 | |
| Mn | 481 | |
| Zn | 47 | |
| V | 45 | |
| Co | 43 | |
| La | 42 | |
| Nd | 41 | |
| Y | 31 | |
| Sm | 22 | |
| Ga | 20 | |
| S | <20 | |
| Pb | 15 | |
| Th | 13 | |
| Pr | 7 | |
| Ni | 7 | |
| Cu | 5 | |
| Hf | 4 | |
| Sn | 3 | |
| Tl | 2 | |
| Br | 1 | |
| Ge | 1 | |
| Mo | 1 |
Microorganisms.
From the fine granitic sand at the glacier front of the Damma glacier, approximately 500 microbes were obtained on oligotrophic medium. We used 1/100 Ravan medium (43) and 1/160 nutrient agar both solidified with washed agar (20) supporting growth of oligotrophic bacteria. Pure cultures were prepared from isolated colonies after repeated streaking and preserved at −80°C as glycerol stock cultures. Genomic DNA was extracted from these isolates and processed for PCR amplification and 16S rRNA gene sequencing. For amplification of 16S rRNA genes from isolates, primers 27f and 1495r, described by Bianciotto et al. (6), were used. The cycle sequencing reactions were carried out with purified PCR products, and the excess dye terminator was removed using a Montage SEQ96 sequencing reaction cleanup kit (Millipore). DNA sequencing was performed using an ABI 3730 sequencer (Applied Biosystems). The resulting nucleotide sequences were blasted using the National Centre of Biotechnology Information (NCBI) database to obtain the closest species match. Twelve heterotrophic bacterial species were selected (Table 3) based on the abundance in the culture collection and literature describing them to produce bioweathering agents (39). Green algae were isolated and purified on basal bold medium (BBM) (8) and were assigned to Chlorella sp. based on 18S rRNA gene sequences.
TABLE 3.
Bacteria isolated from the Damma glacier tongue used in granite dissolution experimentsa
| Isolate | Closest GenBank relative | Sequence identity (%) | GenBank accession no. | Class(es) |
|---|---|---|---|---|
| A | Arthrobacter sp. | 99 | GU213306 | Actinobacteria |
| B | Frigoribacter sp. | 100 | GU213358 | Alphaproteobacteria |
| C | Paenibacillus sp. | 98 | GU213302 | Bacilli |
| D | Janthinobacterium sp. | 99 | GU213411 | Betaproteobacteria |
| E | Leifsonia sp. | 99 | GU213305 | Actinobacteria |
| F | Oxalobacter sp. | 99 | GU213389 | Betaproteobacteria |
| G | Paucibacter sp. | 98 | GU213383 | Betaproteobacteria |
| H | Pedobacter steynii | 99 | GU213382 | Bacteroidetes, Sphingobacteria |
| I | Polaromonas sp. | 99 | GU213399 | Betaproteobacteria |
| J | Pseudomonas sp. | 100 | GU213349 | Gammaproteobacteria |
| K | Rhodococcus erythropolis | 100 | GU213360 | Actinobacteria |
| L | Variovorax sp. | 99 | GU213355 | Betaproteobacteria |
The isolated bacteria were stored at −80°C and characterized by 16S rRNA gene sequencing. The GenBank database at the NCBI website (http://www.ncbi.nlm.nih.gov/) was used for phylogenetic affiliation (>1,300 bp) of the isolates.
Granite dissolution experiments.
Mineral dissolution experiments were run with microcosms. Three grams of crushed granite (<20-μm fraction) was placed into acid-washed 100 ml glass Erlenmeyer culture flasks and autoclaved. Each microcosm received 90 ml of filter-sterilized (pore size of 0.2 μm) minimal growth medium (adjusted to a pH of about 6.9 with 1 M NaOH). The base constituents of the medium were glucose (0.6 g liter−1; 3.3 mM; purity grade, 99.5%) and NH4Cl (0.1 g liter−1; 1.9 mM; purity grade, 99.8%) without any other macro- or micronutrients. To prepare the inoculum, bacteria were grown in a sterilized 2% LB (Lennox) medium for 3 to 4 days and then harvested by centrifugation at 3,000 × g. Cell pellets were then resuspended and rinsed three times in 0.8% (wt/vol) NaCl solution prepared in Millipore water to remove culture medium before the dissolution experiments were started. Each microcosm received 500 μl of the different bacterial isolates (optical density at 600 nm [OD600] of 0.4; approximately 5 × 108 cells ml−1).
The algal isolate was grown in liquid BBM and then treated similarly to the bacterial isolates before inoculation. The flasks were gently shaken at 100 rpm in a climate chamber under light/dark conditions (14:10 h) with a light intensity of 60 μmol photons m−2 s−1 at 25°C throughout the course of the experiment. There were three replicate microcosms for each isolate.
Abiotic experiments with crushed granite under proton-promoted and ligand-promoted dissolution regimes were performed in triplicates with growth medium (glucose-NH4Cl) and (i) HCl (1 mM), (ii) oxalic acid (10 mM), (iii) citric acid (3 mM), or (iv) KCN (1 mM). Hydrochloric acid was used as a model acid. Oxalic acid and citric acid, which are commonly found in the Damma glacier forefield (14), were used as model ligands. The supplies of the organic acids were adjusted to the carbon concentrations of the growth medium. Cyanide produced by cyanogenic bacterial strains is known to solubilize metals from electronic waste material (15). Cyanide was added as KCN. All abiotic experiments were performed as previously described for the biotic experiments.
From each microcosm, aliquots were sampled at regular time intervals for analysis of pH, glucose, organic acids, cyanide, siderophores, and dissolved elements. Aliquots (4 ml after 1, 2, and 4 days and 16 ml after 8 and 16 days) were collected with sterile syringes and filter sterilized through 0.2-μm cellulose acetate filters. One fraction of the aliquots was immediately used for the measurements of pH and organic acids. Another fraction of the aliquots was acidified with 10 μl nitric acid (>65% purity) for elemental analysis, and the remaining fraction was stored in acid-cleaned glass vials either at 4°C (for the analyses of iron, cyanide, and siderophores) or −20°C (for the analyses of glucose) for further analyses.
Chemical analyses.
Total dissolved macro- and micronutrients were measured with inductively coupled plasma mass spectrometry (ICP-MS) (Sciex Elan 6000; Perkin-Elmer, Boston, MA). In addition to ICP-MS measurements, iron concentrations were also quantified with the ferrozine method according to Buss et al. (11). The pH was determined using a modified bromocresol green assay, as previously described (38), by replacing bromocresol with bromthymol blue in our assays. The absorbance at 595 nm was compared with a calibration curve of known pH. Glucose concentration was analyzed by using an Amplex red glucose kit (Invitrogen; Carlsbad, CA) and a Tecan Infinite200 (Tecan AG, Hombrechtikon, Switzerland) microplate fluorescence-luminescence reader. The organic acid concentrations were measured by ion chromatography (ICS-2000; Dionex AG, Switzerland). The concentration of siderophores in the filtrate was measured by the liquid chrome azurol S (CAS) assay (33). In addition, we also used a solid CAS assay to determine the ability of isolates to produce siderophores. Slots of CAS agar were inoculated with 100 μl glucose-NH4Cl medium containing cells of one of the isolates (OD600 = 0.4). The changes of color in the agar were monitored. The concentration of dissolved CN− in the solution was measured with the methemoglobin test (32). The test is based on the changes in the spectrum of the methemoglobin as a result of interactions with CN−. Methemoglobin and CN− form stable complexes exhibiting maximum absorption at 422 nm, in comparison to 407 nm, the maximum absorption for methemoglobin alone.
Cell density.
Cell counts occurring before and after the incubations were measured with flow cytometry. The cells attached to the granite surface were removed using sonication (Branson Digital Sonifier; 3 times at 20 s each; power, 40 W) according to the method of Lindahl and Bakken (25). Almost all cells were separated from granite (25) and were located in the supernatants. The solutions in the flasks were decanted, and separated granite samples were poured into 50-ml Falcon tubes and mixed with a phosphate-buffered saline (PBS) solution (1× PBS, pH 7.4). For cell counts, 1 ml of PBS solution containing granite samples was poured into Eppendorf tubes containing 0.5 ml Histodenz (1.3 g ml−1; Sigma catalog no. D2158). The samples were centrifuged at 17,000 × g and 4°C for 90 min, and the supernatants (containing cells) were poured into new tubes. The cells were stained with Sybr green (Invitrogen, Carlsbad, CA) and counted with a CyFlow Space flow cytometer (Partec GmbH, Münster, Germany) as described by Hammes and Egli (19).
Statistical analysis.
All statistical calculations were performed with the program STATISTICA 8.0 (StatSoft, Tulsa, OK). Independent t tests comparing the results from the experimental settings (isolates) and the control medium (glucose) were conducted. The dependence of each elemental concentration in solution on sampling date or isolate was analyzed by repeated analyses of variance (ANOVA).
RESULTS
Glucose consumption and cell counts.
Within the 16-day incubation period, the glucose concentrations decreased to nearly 20 percent relative to the initial concentrations, indicating microbial respiration with no significant (P < 0.05) differences between isolates (data not shown). In the control incubations without bacterial cells, the glucose concentrations remained unchanged.
Simultaneously with the decrease in glucose concentrations, the cell densities increased from 2.5 × 108 initially to a maximum of 5.0 × 109 cells, indicating that the isolates were able to grow in the glucose-NH4Cl medium in the presence of granite (Fig. 1). Flow cytometer analyses revealed significantly less growth (4.0 × 108 cells) in the absence of granite. There were considerable differences in cell densities between isolates. The Frigoribacter sp. showed the lowest cell densities (3.0 × 108 cells), similar to what was found for the controls without granite. Interestingly, a major part of the bacterial cells (except in the cases of Pedobacter sp. and Pseudomonas sp.) was attached at the granite surfaces since there were approximately six times more cells adhering to the granite surface than suspended in the solution, indicating an intimate interaction between bacterial cells and granite. The highest cell counts were exhibited by Arthrobacter sp., Paenibacillus sp., Janthinobacterium sp., and Variovorax sp., with approximately 3.0 × 109 cells attached on the granite surfaces and about of 2.5 × 108 planktonic cells (Fig. 1).
FIG. 1.
Total cell counts (mean ± standard error; n = 3) of sessile (filled bars) and planktonic (empty bars) cells after 16 days of dissolution experiments. Batch reactors contained 3 g of granite and 90 ml of growth medium. The dashed line shows the initial cell numbers (at time zero). The investigated isolates are represented as follows: A, Arthrobacter sp.; B, Frigoribacter sp.; C, Paenibacillus sp.; D, Janthinobacterium sp.; E, Leifsonia sp.; F, Oxalobacter sp.; G, Paucibacter sp.; H, Pedobacter steynii; I, Polaromonas sp.; J, Pseudomonas sp.; K, Rhodococcus erythropolis; L, Variovorax sp.; and GA, Chlorella sp.
Changes in pH values.
The pH values of the unbuffered cultures were monitored over the entire experimental period since pH changes could be significant with respect to granite dissolution. The initial pH values were about 6.9 in all biotic experiments and the growth medium (glucose-NH4Cl) alone (Fig. 2). In the abiotic experiments with oxalic acid, citric acid, HCl, and KCN, the initial pH values were 4.7, 4.7, 5.7, and 7.5, respectively. In biotic experiments, the pH values of the isolates ranged between 5.5 and 7.2 during incubation (Fig. 2). The pH values of five bacterial isolates (one isolate each of Arthrobacter sp., Frigoribacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp.) rapidly decreased during the exponential phase of cell growth. The pH dropped to 5.5 to 6.3 within 2 days, and after this time, the pH increased again (to pH 6.5 to 7.0). Experiments with Arthrobacter sp. showed the greatest pH decline (to 5.5), whereas the microalgae (Chlorella sp.) did not change the pH in the medium (Fig. 2). Control incubations without cells (glucose-NH4Cl only) did not show a change in pH over the course of the incubations. In abiotic experiments with oxalic acid, citric acid, and HCl, the pH values increased after 1 day to 6.1, 5.7, and 6.7, respectively, and thereafter remained constant. In contrast, in experiments with KCN, the pH values did not significantly change during the incubation.
FIG. 2.
Changes of pH (mean; n = 3) in solution over time (0, 1, 2, 4, 8, and 16 days). Results are shown for biotic experiments with granite containing bacteria (A to L) or green algae (GA) and for abiotic controls with granite containing 3.3 mM glucose (Me+). The investigated isolates are represented as follows: A, Arthrobacter sp; B, Frigoribacter sp.; C, Paenibacillus sp.; D, Janthinobacterium sp.; E, Leifsonia sp.; F, Oxalobacter sp.; G, Paucibacter sp.; H, Pedobacter steynii; I, Polaromonas sp.; J, Pseudomonas sp.; K, Rhodococcus erythropolis; L, Variovorax sp.; and GA, Chlorella sp.
Dissolved elements.
Dissolution experiments were conducted in order to better understand the mechanisms of granite dissolution and the release of trace metal nutrients. In these experiments, dissolved iron was used as an overall indicator of mineral dissolution. The results obtained with the ferrozine method and the ICP-MS measurements of dissolved iron correlated well (coefficient of variation, 15%). Therefore, the ferrozine method was used for iron measurements in addition to ICP-MS in order to yield iron data with greater time resolution than the other parameters. The impacts of bacteria on dissolution of Fe differed with isolates (Fig. 3 a). Experiments with 4 isolates (1 isolate each of Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp.) out of 13 isolates showed significantly (P < 0.01) higher Fe concentrations than those in the solutions for the other experiments. The highest Fe concentrations were measured after 4 or 8 days of incubation, ranging from 93 μM Fe (Leifsonia sp.) to 140 μM Fe (Arthrobacter sp.) (Fig. 3a). These Fe concentrations were 13 to 20 times higher than those in the experiments containing the culture medium (glucose-NH4Cl) without bacterial cells. Five isolates (one isolate each of Frigoribacter sp., Paenibacillus sp., Oxalobacter sp., Variovorax sp., and Chlorella sp.) did not increase the release of Fe from granite, since they showed Fe concentrations in solution similar to those observed in the experiments with growth medium alone (7 μM Fe). In contrast, the Paucibacter sp., Pedobacter sp., Pseudomonas sp., and Rhodococcus sp. isolates showed slightly increased Fe concentrations in solution (up to 28 μM Fe) compared to those observed in the experiments with the growth medium alone. Abiotic experiments with oxalic acid, citric acid, and hydrochloric acid released large amounts of Fe from granite (Fig. 3b). In experiments with oxalic acid and HCl, the Fe concentrations in solution exceeded 2.5 mM. In contrast, citric acid released considerably less Fe in solution (1 mM Fe). In comparison to the level for the applied acids, the salt KCN (1 mM) released small amounts of Fe into solution, similar to the level for the glucose medium (Fig. 3b).
FIG. 3.
Iron concentration (μM; mean ± standard error; n = 3) in solution over time, quantified with the ferrozine method. (a) Biotic experiments with granite containing bacteria (A to L) or green algae (GA) and growth medium (glucose-ammonium chloride) alone. (b) Abiotic controls with granite containing 3.3 mM glucose (Me+), 10 mM oxalic acid (Ox), 3 mM citric acid (Cit), 1 mM HCl (HCl), or 1 mm KCN (CN−). The investigated isolates are represented as follows: A, Arthrobacter sp.; B, Frigoribacter sp.; C, Paenibacillus sp.; D, Janthinobacterium sp.; E, Leifsonia sp.; F, Oxalobacter sp.; G, Paucibacter sp.; H, Pedobacter steynii; I, Polaromonas sp.; J, Pseudomonas sp.; K, Rhodococcus erythropolis; L, Variovorax sp.; and GA, Chlorella sp.
Dissolution rates were calculated according to the method of Liermann et al. (24). Four isolates (one isolate each of Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp.) showed highest Fe dissolution rates, exceeding 1.4 × 10−12 mol m−2 s−1 (Table 4). The impact of bacteria on the release of Ca and Mg was also significant (Table 4). In five isolates (one isolate each of Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., Paucibacter sp., and Polaromonas sp.), the dissolution rates ranged from 0.44 × 10−12 to 0.46 × 10−12 mol Ca m−2 s−1 and from 0.50 × 10−12 to 0.62 × 10−12 mol Mg m−2 s−1, respectively. These rates were significantly (P < 0.05) higher than rates in uninoculated culture media (for Ca, 0.28 × 10−12 mol m−2 s−1; for Mg, 0.38 × 10−12 mol m−2 s−1). In addition, the isolate also had a strong impact on the rate of release of K (Table 4). Except for the Paenibacillus sp., Rhodococcus sp., and Variovorax sp. isolates, all isolates showed significantly (P < 0.05) high release rates for K. Again, the highest rates were measured in experiments with Arthrobacter sp., Leifsonia sp., Janthinobacterium sp., and Polaromonas sp., exceeding 3.6 × 10−12 mol K m−2 s−1 (Table 4). The bacterial isolate had a weak impact on the rate of dissolution of P. In particular, Pedobacter sp. and Oxalobacter sp. released more P into solution than controls without cells. The rates of dissolution of P for these two isolates averaged 0.03 × 10−12 mol m−2 s−1 (Table 4). Experiments with the bacterial isolates of Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., Paucibacter sp., and Polaromonas sp. released up to six times more Mn into solution than the experiments without bacterial cells (Table 4). Experiments with Frigoribacter sp., Paenibacillus sp., Oxalobacter sp., Rhodococcus sp., and Variovorax sp. had the lowest Mn dissolution rates, which were even lower than those in the abiotic glucose experiments. The dissolution rates for trace nutrients, such as Co, Cu, Mo, Ni, V, and Zn, were low (<0.01 × 10−12 mol m−2 s−1) and are not reported in Table 4.
TABLE 4.
Mobilization of specific elements in the first 8 days of the experimenta
| Isolateb | Dissolution rate (10−12 mol m−2 s−1) |
Significance of test isolate concn vs medium (Me+) concnc |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Mg | Ca | Al | Fe | Mn | P | K | Mg | Ca | Al | Fe | Mn | P | |
| A | 3.64 ± 0.04 | 0.62 ± 0.00 | 0.46 ± 0.01 | 0.34 ± 0.06 | 1.34 ± 0.10 | 0.05 ± 0.00 | 0.00 ± 0.00 | ++d | ++ | ++ | + | ++ | ++ | |
| B | 3.26 ± 0.02 | 0.29 ± 0.02 | 0.17 ± 0.01 | 0.12 ± 0.02 | 0.03 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | + | − | −− | − | |||
| C | 3.11 ± 0.04 | 0.25 ± 0.01 | 0.14 ± 0.01 | 0.06 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | −− | −− | −− | −− | −− | ||
| D | 3.82 ± 0.01 | 0.61 ± 0.01 | 0.46 ± 0.02 | 0.29 ± 0.06 | 1.43 ± 0.06 | 0.05 ± 0.00 | 0.00 ± 0.00 | ++ | ++ | ++ | + | ++ | ++ | |
| E | 3.83 ± 0.05 | 0.52 ± 0.03 | 0.45 ± 0.03 | 0.19 ± 0.05 | 0.80 ± 0.16 | 0.04 ± 0.00 | 0.00 ± 0.00 | ++ | ++ | ++ | ++ | ++ | ||
| F | 3.31 ± 0.03 | 0.21 ± 0.02 | 0.18 ± 0.03 | 0.07 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.00 | ++ | −− | − | −− | −− | −− | ++ |
| G | 3.51 ± 0.03 | 0.50 ± 0.01 | 0.44 ± 0.01 | 0.09 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.00 ± 0.00 | ++ | ++ | ++ | −− | ++ | ||
| H | 3.56 ± 0.05 | 0.38 ± 0.01 | 0.34 ± 0.04 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 | ++ | −− | − | ++ | ++ | ||
| I | 3.79 ± 0.04 | 0.55 ± 0.02 | 0.46 ± 0.01 | 0.26 ± 0.01 | 0.96 ± 0.07 | 0.04 ± 0.00 | 0.00 ± 0.00 | ++ | ++ | ++ | ++ | ++ | ++ | |
| J | 3.38 ± 0.09 | 0.41 ± 0.00 | 0.32 ± 0.01 | 0.04 ± 0.00 | 0.06 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.01 | + | −− | + | ++ | |||
| K | 3.12 ± 0.02 | 0.28 ± 0.01 | 0.21 ± 0.02 | 0.06 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | − | − | −− | −− | − | ||
| L | 2.83 ± 0.04 | 0.29 ± 0.02 | 0.22 ± 0.02 | 0.06 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | −− | − | − | −− | −− | − | |
| GA | 3.38 ± 0.06 | 0.40 ± 0.01 | 0.32 ± 0.00 | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | + | −− | −− | + | |||
| Me+ | 3.14 ± 0.03 | 0.38 ± 0.02 | 0.28 ± 0.02 | 0.14 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | |||||||
The concentration parameter values for calculating the dissolution rates (mean ± standard error; n = 3) were obtained by the ICP-MS measurements. Rates are not listed when the values for all experiments were <0.01 × 10−12 mol m−2 s−1. Concentrations observed after 8 days were used for statistical analysis.
A, Arthrobacter sp.; B, Frigoribacter sp.; C, Paenibacillus sp.; D, Janthinobacterium sp.; E, Leifsonia sp.; F, Oxalobacter sp.; G, Paucibacter sp.; H, Pedobacter steynii; I, Polaromonas sp.; J, Pseudomonas sp.; K, Rhodococcus erythropolis; L, Variovorax sp.; GA, Chlorella sp.
++, highly significant (higher elemental concentration in biotic than in abiotic experiments; P < 0.01); +, significant (higher elemental concentration in biotic than in abiotic experiments; P < 0.05); −−, highly significant (lower elemental concentration in biotic than in abiotic experiments; P < 0.01); −, significant (lower elemental concentration in biotic than in abiotic experiments; P < 0.05).
Independent t tests comparing results from isolate and control experiments were performed.
Formation of metabolites.
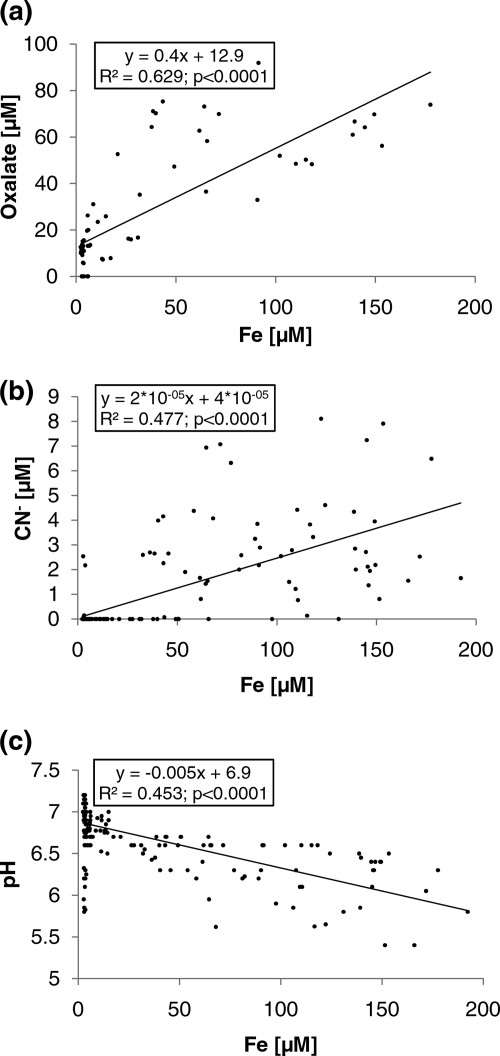
In order to determine which organic acids were responsible for the observed enhancement of granite dissolution rates, ion chromatography (IC) analyses were conducted. Oxalic acid was the only organic acid that was present significantly above the detection limit with all isolates (except for the Chlorella sp. isolate) and which could be unambiguously identified. The Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp. isolates produced the largest amounts of oxalate, as measured at both time points (8 and 16 days) (Fig. 4 a). The oxalate concentrations were highly significant (P < 0.01) between isolates but not (P > 0.05) between sampling times. Surprisingly, in the glucose-NH4Cl medium with granite and without bacterial cells, small amounts of oxalate were observed after 8 days (but not in the media without granite) (Fig. 4a). We suggest that this organic acid was probably formed abiotically from glucose in the presence of the granite powder. Thus, an exudation of oxalate most likely occurred only when the concentrations were higher than those in the controls. The four isolates with the highest oxalate formation also showed the highest levels of elemental release, particularly for Fe, Ca, K, Mg, and Mn. A clear relationship was found between oxalate formation and Fe dissolution (R2 = 0.63) (Fig. 5).
FIG. 4.
Formation of metabolites during the dissolution experiments with granite. (a) Concentrations of oxalic acid (μM; mean ± standard error; n = 3) in solution after 8 and 16 days of incubation. (b) Concentrations of CN− (μM; mean ± standard error) over time. The investigated isolates are represented as follows: A, Arthrobacter sp.; B, Frigoribacter sp.; C, Paenibacillus sp.; D, Janthinobacterium sp.; E, Leifsonia sp.; F, Oxalobacter sp.; G, Paucibacter sp.; H, Pedobacter steynii; I, Polaromonas sp.; J, Pseudomonas sp.; K, Rhodococcus erythropolis; L, Variovorax sp.; and GA, Chlorella sp. Results are shown for abiotic controls containing 3.3 mM glucose with (Me+) or without (Me−) granite.
FIG. 5.
Relationships between oxalic acid (μM) and iron concentrations (μM) in solution during the experiment, n = 78 (a), between CN− (μM) and Fe (μM) concentrations (n = 195) (b), and between pH and iron concentration (μM; n = 195) (c).
With respect to cyanide formation, the same isolates were exceptionally active as in the case of oxalate (Fig. 4b). The cyanide concentrations were highly significant (P < 0.01) between the isolates. Four out of the 13 isolates were significant HCN producers (cyanogens). Two of the isolates (one isolate each of Arthrobacter sp. and Leifsonia sp.) belong to the Actinobacteria, and two (one isolate each of Janthinobacterium sp. and Polaromonas sp.) belong to the Betaproteobacteria. Variovorax sp., within the Betaproteobacteria, also secreted small amounts of HCN. In contrast, the other tested Actinobacteria (Rhodococcus sp.), Betaproteobacteria (Oxalobacter sp. and Paucibacter sp.), and bacteria from all other classes did not produce HCN, nor did the only tested eukaryotic organism (Chlorella sp.). The release of cyanide is a fast reaction since significant amounts were already detected 1 day after incubation (Fig. 4b). The four HCN producers (Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp.) showed the highest level of formation of oxalate and the strongest releases of Fe, Ca, K, Mg, and Mn from granite powder. Clear relationships between CN− concentration and Fe mobilization (R2 = 0.48) and between CN− and oxalate concentrations (R2 = 0.56) were found (Fig. 5).
The formation of siderophores was also examined in our experiment. However, with both approaches (liquid CAS and solid CAS agar), we were not able to determine siderophores successfully. In the liquid CAS assay, all measured siderophore concentrations were zero or negative, indicating that the dissolved Fe in solution could have negatively affected the colorimetric measurements. The blue CAS agar test did not show changes of colors within the 2-week incubation period, indicating that there were no amounts or only small amounts of siderophores produced by the tested isolates.
DISCUSSION
Bacteria, which were isolated from fine granitic sand in the recently deglaciated area of the glacier forefield, proved to be very efficient in the promotion of granite weathering. Under laboratory conditions, 12 out of 13 microorganisms grew readily in the presence of granite powder as a trace nutrient source. Our dissolution data revealed that four isolates in particular (one isolate each of Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp.) accelerated elemental release from granite. The Fe release from granite mainly occurred in the first 4 days of incubation, which is in agreement with other studies (24, 47). The measured elemental concentrations in solution were most probably lower than the effectively released elemental quantities, due to the uptake of elements by the growing of bacterial cultures, due to the uptake of elements by adsorption of elements on the walls of the incubation flasks, and due to the formation of secondary precipitates. As a result, the elemental concentrations in solution decreased again after 4 or 8 days (shown for Fe in Fig. 3a).
Our bacterial isolates of Arthrobacter sp. and Leifsonia sp. belong to the Actinobacteria, whereas Janthinobacterium sp. and Polaromonas sp. belong to the Betaproteobacteria. Other Betaproteobacteria isolated from soils (Burkholderia sp. and Collimonas sp.) were recently shown to enhance the release of Fe from biotite (38, 40) and granite (47). Janthinobacterium agaricidamnosum DSM9628 commercially obtained from a culture collection was also able to weather biotite, but with a very low efficiency compared to the level for Collimonas strains (40). Kalinowski et al. (21) reported on an Arthrobacter species, which enhanced the release of Fe from hornblende. However, the organism was later assigned to Bacillus sp. on the basis of partial 16S rRNA gene sequencing (10). Vuorinen et al. (41) observed elevated concentrations of K, Ca, Fe, Na, and Mg in the culture solutions of Pseudomonas aeruginosa after incubation in the presence of Rapakivi granite. So far, no mineral weathering experiments with green algae, which are often present on rock surfaces (17), were performed. Our microalgae isolate of Chlorella sp. was found to be very common at the Damma glacier tongue, as shown by sequence analysis of an 18S rRNA gene clone library. Chlorella sp. was not a very weathering-efficient microorganism, although the releases of K and Mn were slightly enhanced compared to the levels for the bacteria-free experiments.
Bacterial strains under nutrient-limited conditions were found to produce several organic acids, such as acetate, gluconic acid, citrate, and oxalate, and thereby to increase mineral dissolution rates in laboratory experiments (37, 45). In particular, four isolates (one isolate each of Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp.) showed very distinct formation of oxalic acid. The same isolates accelerated elemental release from granite the most. Among the organic acids, oxalate formed by bacteria has been shown to be very effective in the dissolution of silicates, mainly plagioclase feldspars (44). A conspicuous feature of our isolates is that the four distinct bacterial species were able to produce cyanide but that no other isolate showed HCN production. Hydrogen cyanide is a highly toxic compound usually formed in an early stage of bacterial growth (22). So far, no meaningful role is known for HCN in primary bacterial metabolism, and HCN is generally considered a secondary metabolite (7). Cyanide-producing bacteria can act as biocontrol plant growth-promoting bacteria and help plants in their defense against fungal pathogens by inhibiting cytochrome c oxidase and several other metalloenzymes. This property was predominantly described for Pseudomonas strains (7). The impact of HCN on mineral weathering was examined in just a few studies. Several HCN-forming strains from the genus Pseudomonas were shown to dissolve minerals (15). However, our Pseudomonas isolate from the forefield of the Damma glacier did not form HCN under our experimental conditions.
Our four most efficient bioweathering isolates also attained the highest cell densities at the granite surface. However, in many bacterial dissolution experiments, the population of attached bacteria is not determined, and the relative importance of their activity is not reported in an explicit manner (35, 46, 47). Classical techniques, useful for the direct enumeration of free bacteria in solution, are seldom adaptable to the determination of bacteria adhering to solid surfaces. Sonication and Histodenz density gradient centrifugation together with flow cytometry analyses offered a useful method for determining cell densities at the granite surface which are responsible for the direct attack on the granite surface. Microbe-surface interactions play a significant role in weathering processes (42). Attachment to mineral surfaces mainly by production of extracellular polysaccharides creates microenvironments (biofilms) that protect bacteria against environmental stress. In these microenvironments, bacteria extract inorganic nutrients directly from the mineral matrix (42). Therefore, any dissolution-enhancing metabolites are most concentrated at or near the mineral surface where dissolution occurs.
Microorganisms are known to influence the dissolution of minerals mainly by acidification, by complexation by ligands, and by redox reactions. Oxalate can act as a ligand which directly affects mineral dissolution by complexing metal ions at the mineral surfaces and thereby facilitating the release of metals to solution through ligand-promoted dissolution (16, 27, 46). Cyanide is also able to form water-soluble metal complexes, thus increasing mineral solubility (13).
In addition, particularly oxalic acid (pKa values of 1.2 and 4.2) may decrease the solution pH and thus accelerate mineral dissolution via proton-promoted dissolution. The pH values in experiments with Arthrobacter sp., Janthinobacterium sp., Leifsonia sp., and Polaromonas sp. and with the noncyanogen Frigoribacter sp. decreased during the first 4 to 8 days. As a consequence, a negative correlation between pH and Fe concentration in solution (R2 = 0.45) was observed. Research results obtained by other authors suggest that the pH decrease during the initial phase of mineral dissolution was due to the production of low-molecular-weight organic acids or due to the presence of siderophores (9, 24, 30). In some microbial experiments on dissolution of apatite and biotite, the production of organic acids decreased solution pH to between 3 and 5 (46). Protons might bind to bridging oxide ions at the mineral surface and thus weaken the bondings between the oxygen and the metal atoms, resulting in the detachment of the metal species (16).
In our batch experiments with microorganisms, it was difficult to distinguish between the effects of protons and organic ligands on the kinetics of mineral dissolution. However, we assume that the main effect arises from oxalate, primarily through ligand-promoted dissolution and secondarily through pH decrease and therefore increase of proton-promoted dissolution. In addition to the kinetic effects, the thermodynamic solubility of the minerals increases as a consequence of the formation of dissolved metal complexes with oxalate and cyanide.
Both ligands, oxalate and cyanide, appear to be associated with the enhanced release of elements, in particular of Fe. We assume that the main weathering-associated agent in our experiments was oxalic acid but that cyanogens had advantages compared to noncyanogens, probably due to trace nutrient mobilization in the early stages of bacterial growth. Our HCN-producing bacteria of Arthrobacter sp., Leifsonia sp., Janthinobacterium sp., and Polaromonas sp. may exert a special advantage in their natural habitat by producing HCN, which may help in mobilizing nutrients and trace elements and inhibit the growth of competing microorganisms.
Siderophores produced by microorganisms were documented to be an alternative mechanism for increasing the release of iron from minerals (24). The siderophore production is critically dependent on scarce iron availability (28). With the liquid CAS assay, we were not able to measure siderophores in solution (functional groups of siderophores may be occupied with released Fe), and therefore, the influence of siderophores on mineral weathering could not be examined.
Acknowledgments
Financial support for this study was provided by the “Biosphere-Geosphere interactions: Linking climate change, weathering, soil formation and ecosystem evolution (BigLink)” project of the Competence Center Environment and Sustainability (CCES) of the ETH Domain. This research was also supported by the Genetic Diversity Centre (GDC) of ETH Zurich.
We thank the BigLink consortium, in particular Stefano Bernasconi and Rienk Smittenberg (ETH), for scientific support. We also thank Thomas Egli and Aline Frossard (EAWAG) for their help in performing flow cytometry. Daniela Steiner (WSL) is acknowledged for valuable technical support in the laboratory. We also thank Ursula Graf and Alessandro Schlumpf of the WSL Central Laboratory for ICP-MS and IC measurements.
Footnotes
Published ahead of print on 4 June 2010.
REFERENCES
- 1.Anderson, S. P., J. I. Drever, C. D. Frost, and P. Holden. 2000. Chemical weathering in the foreland of a retreating glacier. Geochim. Cosmochim. Acta 64:1173-1189. [Google Scholar]
- 2.Banfield, J. F., W. W. Barker, S. A. Welch, and A. Taunton. 1999. Biological impact on mineral dissolution: application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl. Acad. Sci. U. S. A. 96:3404-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker, W. W., S. A. Welch, S. Chu, and J. F. Banfield. 1998. Experimental observations of the effects of bacteria on aluminosilicate weathering. Am. Miner. 83:1551-1563. [Google Scholar]
- 4.Bennett, P. C., J. R. Rogers, and W. J. Choi. 2001. Silicates, silicate weathering and, microbial ecology. Geomicrobiol. J. 18:3-19. [Google Scholar]
- 5.Bernasconi, S., and BigLink Project Members. 2008. Weathering, soil formation and initial ecosystem evolution on a glacier forefield: a case study from the Damma Glacier, Switzerland. Miner. Mag. 72:19-22. [Google Scholar]
- 6.Bianciotto, V., C. Bandi, D. Minerdi, M. Sironi, H. V. Tichy, and P. Bonfante. 1996. An obligately endosymbiotic mycorrhizal fungus itself harbors obligately intracellular bacteria. Appl. Environ. Microbiol. 62:3005-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumer, C., and D. Haas. 2000. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol. 173:170-177. [DOI] [PubMed] [Google Scholar]
- 8.Bold, H. C. 1949. The morphology of Chlamydomonas chlamydogama sp. nov. Bull. Torrey Bot. Club 76:101-108. [Google Scholar]
- 9.Brantley, S. L., L. J. Liermann, M. Bau, and S. Wu. 2001. Uptake of trace metals and rare earth elements from hornblende by a soil bacterium. Geomicrobiol. J. 18:37-61. [Google Scholar]
- 10.Brantley, S. L., R. L. Guynn, L. J. Liermann, A. Anbar, J. Barling, and G. Icopini. 2004. Fe isotopic fractionation during mineral dissolution with and without bacteria. Geochim. Cosmochim. Acta 68:3189-3204. [Google Scholar]
- 11.Buss, H. L., M. A. Bruns, M. J. Schultz, J. Moore, C. F. Mathur, and S. L. Brantley. 2005. The coupling of biological iron cycling and mineral weathering during saprolite formation, Luquillo Mountains, Puerto Rico. Geobiology 3:247-260. [Google Scholar]
- 12.Caccianiga, M., and C. Andreis. 2004. Pioneer herbaceous vegetation on glacier forelands in the Italian Alps. Phytocoenologia 34:55-89. [Google Scholar]
- 13.Campbell, S. C., G. J. Olson, T. R. Clark, and G. McFeters. 2001. Biogenic production of cyanide and its application to gold recovery. J. Ind. Microbiol. Biotechnol. 26:134-139. [DOI] [PubMed] [Google Scholar]
- 14.Edwards, I. P., H. Bürgmann, C. Miniaci, and J. Zeyer. 2006. Variation in microbial community composition and culturability in the rhizosphere of Leucanthemopsis alpina (L.) Heywood and adjacent bare soil along an alpine chronosequence. Microb. Ecol. 52:679-692. [DOI] [PubMed] [Google Scholar]
- 15.Faramarzi, A. M., and H. Brandl. 2006. Formation of water-solube metal cyanide complexes from solid minerals by Pseudomonas plecoglossicida. FEMS Microbiol. Lett. 259:47-52. [DOI] [PubMed] [Google Scholar]
- 16.Furrer, G., and W. Stumm. 1986. The coordination chemistry of weathering: I. Dissolution kinetics of δ-Al2O3 and BeO. Geochim. Cosmochim. Acta 50:1847-1860. [Google Scholar]
- 17.Gorbushina, A. A., and W. J. Broughton. 2009. Microbiology of the atmosphere-rock interface: how biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu. Rev. Microbiol. 63:431-450. [DOI] [PubMed] [Google Scholar]
- 18.Haeberli, W., M. Hoelzle, F. Paul, and M. Zemp. 2007. Integrated monitoring of mountain glaciers as key indicators of global climate change: the European Alps. Ann. Glaciol. 46:150-160. [Google Scholar]
- 19.Hammes, F. A., and T. Egli. 2005. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 39:3289-3294. [DOI] [PubMed] [Google Scholar]
- 20.Janssen, P., P. Yates, B. Grinton, P. Taylor, and M. Sait. 2002. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl. Environ. Microbiol. 68:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalinowski, B. E., L. J. Liermann, S. L. Brantley, A. Barnes, and C. G. Pantano. 2000. X-ray photoelectron evidence for bacteria enhanced dissolution of hornblende. Geochim. Cosmochim. Acta 64:1331-1343. [Google Scholar]
- 22.Knowles, C. J., and A. W. Bunch. 1986. Microbial cyanide metabolism. Adv. Microb. Physiol. 27:73-111. [DOI] [PubMed] [Google Scholar]
- 23.Lee, J. U., and J. B. Fein. 2000. Experimental study of the effects of Bacillus subtilis on gibbsite dissolution rates under near-neutral pH and nutrient poor conditions. Chem. Geol. 166:193-202. [Google Scholar]
- 24.Liermann, J., B. E. Kalinowski, S. L. Brantley, and J. G. Ferry. 2000. Role of bacterial siderophores in dissolution of hornblende. Geochem. Cosmochim. Acta 64:587-602. [Google Scholar]
- 25.Lindahl, V., and L. R. Bakken. 1995. Evaluation of methods for extraction of bacteria from soil. FEMS Microbiol. Ecol. 16:135-142. [Google Scholar]
- 26.Mavris, C., M. Egli, M. Plötze, J. D. Blum, A. Mirabella, D. Giaccai, and W. Haeberli. 2010. Initial stages of weathering and soil formation in the Morteratsch proglacial area (Upper Engadine, Switzerland). Geoderma 155:359-371. [Google Scholar]
- 27.Neaman, A., J. Chorover, and S. Brantley. 2006. Effects of organic ligands on granite dissolution in batch experiments at pH 6. Am. J. Sci. 306:451-473. [Google Scholar]
- 28.Neilands, J. B. 1995. Siderophores. Structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed] [Google Scholar]
- 29.Oerlemans, J. 2005. Extracting a climate signal from 169 glacier records. Science 308:675-677. [DOI] [PubMed] [Google Scholar]
- 30.Puente, M. E., Y. Bashan, C. Y. Li, and V. K. Lebsky. 2004. Microbial populations and activities in the rhizoplane of rock-weathering desert plants. 1. Root colonization and weathering of igneous rocks. Plant Biol. 6:629-642. [DOI] [PubMed] [Google Scholar]
- 31.Puente, M. E., C. Y. Li, and Y. Bashan. 2009. Rock-degrading endophytic bacteria in cacti. Environ. Exp. Bot. 66:389-401. [Google Scholar]
- 32.Rudolf von Rohr, M., G. Furrer, and H. Brandl. 2009. Effect of iron and phosphate on bacterial cyanide formation determined by methemoglobin in two-dimensional gradient microcultivations. J. Microbiol. Methods 79:71-75. [DOI] [PubMed] [Google Scholar]
- 33.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 34.Sigler, W., and J. Zeyer. 2002. Microbial diversity and activity along the forefields of two receding glaciers. Microb. Ecol. 43:397-407. [DOI] [PubMed] [Google Scholar]
- 35.Song, W., N. Ogawa, C. T. Oguchi, T. Hatta, and Y. Matsukura. 2008. Effect of Bacillus subtilis on granite weathering: a laboratory experiment. Catena 70:275-281. [Google Scholar]
- 36.Tscherko, D., J. Rustemeier, A. Richter, W. Wanek, and E. Kandeler. 2003. Functional diversity of the soil microflora in primary succession across two glacier forelands in the Central Alps. Eur. J. Soil Sci. 54:685-696. [Google Scholar]
- 37.Ullman, W. J., D. L. Kirchman, S. A. Welch, and P. Vandevivere. 1996. Laboratory evidence for microbially mediated silicate mineral dissolution in nature. Chem. Geol. 132:11-17. [Google Scholar]
- 38.Uroz, S., C. Calvaruso, M. P. Turpault, J. C. Pierrot, C. Mustin, and P. Frey-Klett. 2007. Effect of the mycorrhizosphere on the genotypic and metabolic diversity of the bacterial communities involved in mineral weathering in a forest soil. Appl. Environ. Microbiol. 73:3019-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uroz, S., C. Calvaruso, M. P. Turpault, and P. Frey-Klett. 2009. Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol. 17:378-387. [DOI] [PubMed] [Google Scholar]
- 40.Uroz, S., C. Calvaruso, M. P. Turpault, J. C. Pierrot, C. Mustin, and P. Frey-Klett. 2009b. Efficient mineral weathering is a distinctive functional trait of the bacterial genus Collimonas. Soil Biol. Biochem. 41:2178-2186. [Google Scholar]
- 41.Vuorinen, A., S. Mantere-Alhonen, R. Uusinoka, and P. Alhonen. 1981. Bacterial weathering of rapakivi granite. Geomicrobiol. J. 2:317-325. [Google Scholar]
- 42.Warren, L. A. 2005. Biofilms and metal geochemistry: the relevance of micro-organism-induced geochemical transformations, p. 11-34. In G. M. Gadd, K. T. Semple, and H. M. Lappin-Scott (ed.), Microorganisms and earth systems—advances in geomicrobiology. Cambridge University Press, Cambridge, United Kingdom.
- 43.Watve, M., V. Shejval, C. Sonawane, M. Rahalkar, A. Matapurkar, Y. Shouche, M. Patole, N. Phadnis, A. Champhenkar, K. Damle, S. Karandikar, V. Kshirsagar, and M. Jog. 2000. The K′ selected oligophilic bacteria: a key to uncultured diversity? Curr. Sci. 78:1535-1542. [Google Scholar]
- 44.Welch, S. A., W. W. Barker, and J. F. Banfield. 1999. Microbial extracellular polysaccharides and plagioclase dissolution. Geochim. Cosmochim. Acta 63:1405-1419. [Google Scholar]
- 45.Welch, S. A., and W. J. Ullman. 1999. The effect of microbial glucose metabolism on bytownite feldspar dissolution rates between 5° C and 35° C. Geochim. Cosmochim. Acta 63:3247-3259. [Google Scholar]
- 46.Welch, S. A., A. E. Taunton, and J. F. Banfield. 2002. Effect of microorganisms and microbial metabolites on apatite dissolution. Geomicrobiol. J. 19:343-367. [Google Scholar]
- 47.Wu, L., A. D. Jacobson, and M. Hausner. 2008. Characterization of elemental release during microbe-granite interactions at T = 28° C. Geochim. Cosmochim. Acta 72:1076-1095. [Google Scholar]