Abstract
This study investigated the therapeutic potential of bacterial polysaccharides by employing a model system based on enteroxigenic Escherichia coli (ETEC)-induced hemagglutination of erythrocytes. Exopolysaccharides produced by strains of Lactobacillus reuteri inhibited ETEC-induced hemagglutination of porcine erythrocytes. No effect was observed for dextran produced from Weissella cibaria and commercially available oligo- and polysaccharides.
Lactic acid bacteria (LAB) are important in food production due to their positive contribution to flavor and preservation of the final product. Some of these food-grade microorganisms are also valuable for their ability to synthesize exopolysaccharides (EPSs). EPSs are high-molecular-weight sugar polymers, which remain attached to the microorganism as capsular EPS or become excreted into the environment in the form of slime or ropy EPS (26, 30). LAB utilize two distinct biosynthetic pathways to produce EPSs. Heteropolysaccharides (HePSs) comprised of two to eight repeating units of monosaccharides are assembled by cell wall-bound glycosyltransferases in low quantities from intracellular sugar nucleotide precursors (4). Extracellular glycansucrases (glucan- or fructansucrases) synthesize homopolysaccharides (HoPSs) consisting of either glucose or fructose from sucrose, and their yield can be as high as 40 g liter−1 (13, 19). EPS formation by glycansucrases has been reported for lactobacilli of the species Lactobacillus reuteri, L. pontis, L. panis, L. acidophilus, and L. frumenti (28, 29). HoPS synthesis and the corresponding genes have been especially well characterized in L. reuteri (27). All EPSs used in this study were HoPSs formed in the presence of sucrose. In addition to HoPS synthesis, glycansucrases are capable of producing oligosaccharides (OSs). Several OSs are found to have prebiotic properties as they can benefit host health by acting as nondigestible food ingredients and by enhancing the growth of desired microbial members of the gastrointestinal microbiota (7). Emerging research efforts have investigated potential applications of OSs as antiadhesive agents in preventing pathogen colonization. Shoaf et al. (24) suggested that commercial oligo- and polysaccharides reduce the adherence of enteropathogenic Escherichia coli (EPEC) to cell lines. Martín-Sosa et al. (16) reported antiadhesive properties of human milk oligosaccharides against human strains of enterotoxigenic Escherichia coli (ETEC) and uropathogenic E. coli. Similarly, Martín et al. (15) confirmed the binding of milk oligosaccharides to ETEC isolated from calves. In contrast, adhesion studies with EPS are limited.
The inhibition of ETEC is of special interest to the swine industry because ETEC is the primary cause for diarrhea in neo- and postnatal piglets and results in substantial economic losses. We therefore aimed to test for antiadhesive properties of EPSs synthesized by LAB and commercially available prebiotics against porcine ETEC strains. Hemagglutination assays were used; these assays are generally accepted as an effective model system for testing ETEC adherence as hemagglutination resembles the attachment of K88-positive bacteria to the gut wall (10).
Hemagglutination assays.
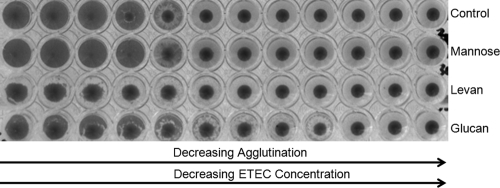
Four porcine ETEC strains positive for the K88 (F4) antigen were obtained from the Escherichia coli Laboratory (EcL) at the University of Montréal. The strains used were E. coli ECL13086 (O149, virotype STa:STb:LT:EAST1:F4), ECL13795 (O149, virotype STb:LT:EAST1:F4), ECL13998 (O149, virotype STa:STb:LT:EAST1:F4:Paa), and ECL14048 (O149, virotype STb:LT:EAST1:F4:Paa). Overnight cultures were recovered from Minca agar (9) with 1 ml of phosphate-buffered saline (PBS; 150 mM, pH 7.2). For preparation of erythrocytes, porcine whole blood (Innovative Research, Novi, Michigan) was washed three times in PBS and resuspended in PBS at 5%. Hemagglutination tests were conducted based on the protocol used by Martín et al. (15). Briefly, ETEC suspensions, which contained between 2.5 × 1011 and 2.5 × 1012 CFU of bacterial cells in 25 μl, were diluted 2-fold in V-bottomed 96-well polystyrene microtiter plates (Corning, Corning, NY). Twenty-five microliters of PBS or PBS containing EPS was added and incubated for 5 min. Finally, 25 μl of 5% erythrocyte suspension in PBS was applied to the wells and mixed gently. Microtiter plates were inspected visually after 2 h of incubation at 4°C. All four porcine ETEC strains agglutinated porcine erythrocytes and were resistant to mannose at 10 mg ml−1 (Fig. 1).
FIG. 1.
Hemagglutination of porcine erythrocytes in the presence of mannose, levan (Lactobacillus reuteri LTH5794), and glucan (Lactobacillus reuteri FUA3048) at 10 mg ml−1, using enterotoxogenic E. coli ECL14048, which was serially diluted 2-fold. PBS (150 mM at pH 7.2) was used as a negative control.
Preparation of EPS.
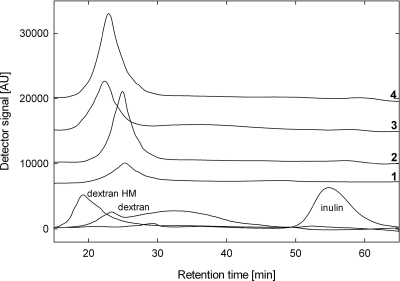
EPS was obtained from L. reuteri strains TMW1.656, LTH5794, and FUA3048 and Weissella cibaria strains 10M and W58. L. reuteri TWM1.656, LTH5794, and FUA3048 formed reuteran, levan, and an uncharacterized glucan, respectively (Table 1). W. cibaria 10M and W58 formed a dextran, and the corresponding dextransucrase genes were amplified by PCR using primers GTFWcFor (5′-GCATCTTTCAATACTTGAGG-3′) and GTFWcRev (5′-CATGACTTGTTGGCATAGC). The amplified 993- and 981-bp sequences of W. cibaria 10M and W58, respectively, were 97% homologous to the dextransucrase dsrWc gene of Weissella cibaria CMU (ACK38203) (12, 21). Strains were grown in modified MRS medium containing 100 g liter−1 sucrose as the sole carbon source (25). EPS was harvested via ethanol precipitation (29), dialyzed, and further purified by hot phenol extraction (32). Mono-, di-, and oligosaccharides were removed by dialysis using Spectra/Por 2 molecular porous membrane tubing (molecular weight cutoff [MWCO], 12,000 to 14,000) (Spectrum Laboratories Inc., Rancho Dominguez, CA) at 4°C with frequent changes of ultrapure water. UltraPure buffer-saturated phenol (Invitrogen, Burlington, Canada) was added to each sample at an equal volume. The samples were incubated in a water bath at 70°C for 70 min, cooled on ice for 30 min, and centrifuged at 3,000 × g for 20 min at 4°C. The aqueous layer was collected and dialyzed to remove phenol for 4 days. Samples were freeze-dried. SDS-PAGE was used to confirm removal of proteins. Commercially available dextrans (dextran, 1 × 105 to 2 × 105 Da; dextran HM, 5 × 106 to 4 × 107 Da; Sigma, Oakville, Canada), isomalto-oligosaccharides (IMO) (VitaSugar; BioNeutra Inc., Edmonton, Alberta, Canada), cellobiose (Sigma), inulin from chicory (Sigma), raffinose (Difco, Mississauga, Canada), and Raftiline ST (Orafti, Tienen, Belgium) were included for comparison. Sizes of bacterial EPSs were determined by size-exclusion chromatography using a Superdex 200 column (GE Healthcare, Baie d'Urfe, Canada). Water was used as a solvent at a flow rate of 0.4 ml min−1. EPS was detected with a refractive index (RI) detector. EPSs produced by L. reuteri LTH5794 and FUA3048 were slightly larger than EPSs synthesized by L. reuteri TMW1.656 and W. cibaria W58 and 10M (Fig. 2 and data not shown).
TABLE 1.
Strains used for EPS production
| Strain used for EPS production | Origin | EPS formation visible on modified MRS | Glycosyltransferase gene(s) (PCR) | HoPS monosaccharide component | EPS | Reference(s) |
|---|---|---|---|---|---|---|
| Lactobacillus reuteri | ||||||
| TMW1.656 | Sourdough | + | gtfA, inu | Glucose | Reuteran | 11, 22 |
| LTH5794 | Human intestine | + | gtfB | Fructose | Levanb | 5, 22 |
| FUA3048 | Mouse intestine | + | NDc | Glucose | Glucan | This study |
| Weissella cibaria | ||||||
| 10 M | Sourdough | + | dexWca | Glucose | Dextran | This study; 12, 21 |
| W58 | Sourdough | + | dexWc | Glucose | Dextran | This study; 12, 21 |
Amplification of partial sequence using primers GTFWcFor (5′-GCATCTTTCAATACTTGAGG-3′) and GTFWcRev (5′-CATGACTTGTTGGCATAGC-3′) obtained from accession no. ACK28203. dexWc is the dex gene from W. cibaria.
D. Bundle, personal communication.
ND, not determined.
FIG. 2.
Size-exclusion chromatography of EPSs produced by W. cibaria 58W (line 1) and L. reuteri TMW1.656 (line 2), LTH5794 (line 3), and FUA3048 (line 4) in comparison to commercially available dextran, dextran HM, and inulin.
Purified bacterial EPSs and commercial oligo- and polysaccharides were tested at concentrations of 2.5, 5, and 10 mg ml−1 in hemagglutination assays. Glucan, levan, and reuteran consistently inhibited hemagglutination at 10 mg ml−1 for all ETEC strains (Table 2; Fig. 1). Antihemagglutination activities for these EPSs were also observed at 5 mg ml−1 in a few strain-dependent cases but were less pronounced. In contrast, antihemagglutination activities were not observed at 10 mg ml−1 using commercially available oligo- and polysaccharides; tests with lower sugar concentrations were not conducted. Overall triplicate tests were conducted with three different EPS preparations, fresh ETEC cultures, and blood.
TABLE 2.
Inhibition erythrocyte agglutination of four porcine ETEC strains tested against bacterial EPSs and commercially available prebiotics
| EPS source and oligosaccharide/polysaccharide | Agglutination of ETEC at concn (mg ml−1)a: |
||
|---|---|---|---|
| 10 | 5 | 2.5 | |
| Bacterial | |||
| Reuteran (Lactobacillus reuteri TMW1.656) | + | +/− | − |
| Levan (Lactobacillus reuteri LTH5794) | + | +/− | − |
| Glucan (Lactobacillus reuteri FUA3048) | + | +/− | − |
| Dextran (Weissella cibaria 10M) | − | ND | ND |
| Dextran (Weissella cibaria 58W) | − | ND | ND |
| Dextran (Sigma) | − | ND | ND |
| Dextran HM (Sigma) | − | ND | ND |
| Commercial | |||
| IMO (VitaSugar; BioNeutra Inc.) | − | ND | ND |
| Cellobiose (Sigma) | − | ND | ND |
| Inulin from chicory (Sigma) | − | ND | ND |
| Raffinose (Difco) | − | ND | ND |
| Raftiline ST (Orafti) | − | ND | ND |
ND, not determined; +, agglutination observed; −, agglutination not observed; +/−, strain-dependent agglutination.
Our results indicate that LAB EPSs can interfere with ETEC adhesion and therefore have the potential to benefit the swine industry. Rapid proliferation of ETEC is attributed to fimbria-triggered attachment to specific receptors on intestinal enterocytes and subsequent secretion of heat-labile and heat-stable toxins (8). In particular, K88 fimbriae were found to be associated with the colonization of the small intestines of neonatal and postnatal piglets by interacting with glycoproteins in ileal mucus and receptors on porcine intestinal epithelial cells (3, 10, 18, 23, 33). K88 fimbriae are composed of a single adhesin major protein subunit and minor subunits that are suspected to regulate fimbrial expression (1, 31). K88 fimbriae bind to cell surface receptors that contain carbohydrate structures, with β-d-galactose being an essential component (2, 6, 23). Galactose-containing glycoprotein in pig gastric mucin, glucosamine, and chondrosine inhibited hemagglutination in ETEC K88 strains (17, 20). Inhibition of adherence was suspected to be nonspecific as the presence of large hydrophobic glucoside molecules likely disrupted hydrophobic interactions between fimbriae and the receptor (20). Bacterial EPSs successfully decreased the adherence of ETEC strains. The presence of aggregatory compounds, receptor analogues, or competitive exclusion might contribute to binding inhibition (14). Interestingly, our results indicated that among glucans, the α-(1-4)-, α-(1-6)-linked reuteran and a glucan of undetermined linkage type had antihemagglutination activity but the predominately α-(1-6)-linked dextran did not (30). In the case of fructans, the β-(2-6)-linked levan was found to have antiadherence properties but the β-(2-6)-linked inulins (Sigma and Orafti) did not (30). Although the glucans and fructans tested were identical in composition, they differ greatly in molecule weight and structure. Yet, whereas no correlation between molecule weight and antiagglutination activity could be observed, our findings suggest that antiadherence requires a certain degree of structural specificity. Further studies on the structure/function relationships among EPSs, ETEC adhesins, and host cell receptors are needed to gain a deeper understanding of the antiadhesive properties of bacterial EPSs and to determine the mode of interaction of ETEC and EPSs.
Currently, antibiotics are widely used in pig production at subtherapeutic and therapeutic levels. However, increasing public health concerns have led to efforts to reduce antibiotic use. The application of bacterial EPSs might be an alternative to prevent and treat diarrhea caused by ETEC. EPSs can be produced in cereal fermentations and can be easily incorporated into feed in liquid and dry form (11, 21, 27).
Acknowledgments
ACIDF, AVAC, Christian Hansen A/S, and BioNeutra Inc. are thanked for financial support.
Footnotes
Published ahead of print on 14 May 2010.
REFERENCES
- 1.Bakker, D., P. T. J. Willemsen, L. H. Simmons, F. G. van Zijderveld, and F. K. de Graaf. 1992. Characterisation of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol. Microbiol. 6:247-255. [DOI] [PubMed] [Google Scholar]
- 2.Bijlsma, S. G., and J. F. Frik. 1987. Hemagglutination patterns of the different variants of Escherichia coli K88 antigen with porcine, bovine, guinea pig, chicken, ovine, and equine erythrocytes. Res. Vet. Sci. 43:122-123. [PubMed] [Google Scholar]
- 3.Conway, P. L., A. Welin, and P. S. Cohen. 1990. Presence of K88 specific receptors in porcine ileal mucus is age dependent. Infect. Immun. 58:3178-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vuyst, L., F. de Vin, F. Vanigelgem, and B. Degeest. 2001. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 11:687-707. [Google Scholar]
- 5.Gänzle, M. G., and C. Schwab. 2009. Ecology of exopolysaccharide formation by lactic acid bacteria: sucrose utilization, stress tolerance, and biofilm formation, p. 263-278. In M. Ullrich (ed.), Bacterial polysaccharides: current innovations and future trends. Academic Press, London, United Kingdom.
- 6.Gibbons, R. A., G. W. Jones, and R. Shellwood. 1975. An attempt to identify the intestinal receptor for the K88 adhesin by means of a haemagglutination inhibition test using glycoproteins and fractions from sow colostrums. J. Gen. Microbiol. 86:228-240. [DOI] [PubMed] [Google Scholar]
- 7.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiotica: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 8.Gross, R. J., S. M. Scotland, and B. Rowe. 1976. Enterotoxin testing of Escherichia coli causing epidemic enteritis in the United Kingdom. Lancet i:629-630. [DOI] [PubMed]
- 9.Guinée, P. A. M., J. Veldkamp, and W. H. Jansen. 1977. Improved Minca medium for the detection of K99 antigen in calf enterotoxigenic strains of Escherichia coli. Infect. Immun. 15:676-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, G. W., and J. M. Rutter. 1974. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J. Gen. Microbiol. 84:135-144. [DOI] [PubMed] [Google Scholar]
- 11.Kaditzky, S., J. Behr, A. Stocker, P. Kaden, M. G. Gänzle, and R. F. Vogel. 2008. Influence of pH on the formation of glucan by Lactobacillus reuteri TMW 1.106 exerting a protective function against extreme pH values. Food Biotechnol. 22:398-418. [Google Scholar]
- 12.Kang, H. K., J. S. Oh, and D. Kim. 2009. Molecular characterization and expression analysis of the glucansucrase DSRWC from Weissella cibaria synthesizing a α (1→6) glucan. FEMS Microbiol. Lett. 292:33-41. [DOI] [PubMed] [Google Scholar]
- 13.Korakli, M., M. Pavlovic, M. G. Gänzle, and R. F. Vogel. 2003. Exopolysaccharide and kestose production by Lactobacillus sanfranciscensis LTH2590. Appl. Environ. Microbiol. 69:2073-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korakli, M., and R. F. Vogel. 2006. Structure/function relationship of homopolysaccharides producing glycansucrase and therapeutic potential of their synthesised glycans. Appl. Microbiol. Biotechnol. 71:790-803. [DOI] [PubMed] [Google Scholar]
- 15.Martín, M. J., S. Martín-Sosa, and P. Hueso. 2002. Binding of milk oligosaccharides by several enterotoxigenic Escherichia coli strains isolated from calves. Glyconj. J. 19:5-11. [DOI] [PubMed] [Google Scholar]
- 16.Martín-Sosa, S., M. Martín, and P. Hueso. 2002. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J. Nutr. 132:3067-3072. [DOI] [PubMed] [Google Scholar]
- 17.Meng, Q., M. S. Kerley, T. J. Russel, and G. L. Allee. 1998. Lectin like activity of Escherichia coli K88, Salmonella choleraesuis, and Bifidobacteria pseudolongum of porcine gastrointestinal origin. J. Anim. Sci. 76:551-556. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe, J. W., K. A. Krogfelt, H. C. Krivan, S. C. Cohen, and D. C. Laux. 1991. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect. Immun. 59:91-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monsan, P., S. Bozonnet, C. Albenne, G. Joucla, R.-M. Willemot, and M. Remaud-Siméon. 2001. Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 11:675-685. [Google Scholar]
- 20.Payne, D. 1994. A study of K88-mediated haemagglutination by enterotoxigenic Escherichia coli (ETEC). New Microbiol. 17:99-110. [PubMed] [Google Scholar]
- 21.Schwab, C., M. Mastrangelo, A. Corsetti, and M. G. Gänzle. 2008. Formation of oligosaccharides and polysaccharides by Lactobacillus reuteri LTH5448 and Weissella cibaria 10M in sorghum sourdoughs. Cereal Chem. 85:679-684. [Google Scholar]
- 22.Schwab, C., and M. G. Gänzle. 2005. Exopolysaccharide production by intestinal lactobacilli, p. 83-96. In G. W. Tannock (ed.), Probiotics and prebiotics: scientific aspects. Caister Academic Press, Norwich, United Kingdom.
- 23.Sellwood, R. 1980. The interaction of the K88 antigen with porcine intestinal epithelial cell brush borders. Biochim. Biophys. Acta 632:326-335. [DOI] [PubMed] [Google Scholar]
- 24.Shoaf, K., G. L. Mulvey, G. D. Armstrong, and R. W. Hutkins. 2006. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 74:6920-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolz, P., G. Böcker, W. P. Hammes, and R. F. Vogel. 1995. Utilization of electron acceptors by lactobacilli isolated from sourdough. I. Lactobacillus sanfrancisco. Z. Lebensm. Unters. Forsch. 201:91-96. [Google Scholar]
- 26.Sutherland, I. W. 1972. Bacterial exopolysaccharides. Adv. Microb. Physiol. 8:143-213. [DOI] [PubMed] [Google Scholar]
- 27.Tieking, M., and M. G. Gänzle. 2005. Exopolysaccharides from cereal-associated lactobacilli. Trends Food Sci. Technol. 16:79-84. [Google Scholar]
- 28.Tieking, M., S. Kaditzky, M. G. Gänzle, and R. F. Vogel. 2003. Biodiversity and potential for baking applications of glycosyltransferases in lactobacilli for use in sourdough fermentation, p. 58-59. In L. de Vuyst (ed.), Sourdough, from fundamentals to applications. Vrije Universiteit Brussel, Brussels, Belgium.
- 29.Tieking, M., M. Korakli, M. A. Ehrmann, M. G. Gänzle, and R. F. Vogel. 2003. In situ production of exopolysaccharides during sourdough fermentation by cereal and intestinal isolates of lactic acid bacteria. Appl. Environ. Microbiol. 69:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Hijum, S., S. Kralj, L. K. Ozimek, L. Dijkhuizen, and I. G. H. van Geel-Schutten. 2006. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 70:157-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Zijderveld, F. G. 1990. Epitope analysis of the F4 (K88) fimbrial antigen complex of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect. Immun. 58:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 33.Wilson, R. A., and D. H. Francis. 1986. Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am. J. Vet. Res. 47:213-217. [PubMed] [Google Scholar]