Abstract
With the aim of explaining the variations in microcystin (MC) concentrations during cyanobacterial blooms, we studied several Microcystis aeruginosa populations blooming in different freshwater ecosystems located in the same geographical area. As assessed by real-time PCR, it appeared that the potentially MC-producing cells (mcyB+) were predominant (70 to 100%) in all of these M. aeruginosa populations, with the exception of one population in which non-MC-producing cells always dominated. Apart from the population in the Grangent Reservoir, we found that the proportions of potentially MC-producing and non-MC-producing cells varied little over time, which was consistent with the fact that according to a previous study of the same populations, the intergenic transcribed spacer (ITS) genotype composition did not change (38). In the Grangent Reservoir, the MC-RR variant was the dominant microcystin variant throughout the bloom season, despite changes in the ITS composition and in the proportions of mcyB+ cells. Finally, the variations in total MC concentrations (0.3 to 15 μg liter−1) and in the MC cellular quotas (0.01 to 3.4 pg cell−1) were high both between and within sites, and no correlation was found between the MC concentrations and the proportion of mcyB+ cells. All of these findings demonstrate that very different results can be found for the proportions of potentially MC-producing and non-MC-producing cells and MC concentrations, even in M. aeruginosa populations living in more or less connected ecosystems, demonstrating the importance of the effect of very local environmental conditions on these parameters and also the difficulty of predicting the potential toxicity of Microcystis blooms.
Microcystins (MCs) are the most common cyanotoxins and have been involved in several animal and human poisoning episodes (8). These hepatotoxic cyclic heptapeptides are synthesized by a multifunctional enzyme complex (10, 40), and the discovery of the gene cluster encoding this complex has made it possible in recent years to develop molecular tools for studying the relative proportions of MC-producing and non-MC-producing cells in natural cyanobacterial populations. Potentially MC-producing and non-MC-producing cells can coexist in these populations, but the factors and processes governing the dynamics of these subpopulations remain unclear.
Some recent papers on the Microcystis genus have shown that the proportions of potentially MC-producing cells can differ considerably from lake to lake. For example, in Lake Wannsee, Germany, this proportion was between 0 and 40% (28), as it was in Lake Oneida, United States (18), and in Lake Mikata, Japan (48). In contrast, large variations over time (6 to 93%) of potentially MC-producing cells were found in the Grangent Reservoir, France (4). Major variations (30 to 80%) were also found in a natural French population of Planktothrix agardhii (3), and the variations in the proportions of potentially MC-producing cells reflected those of the MC concentrations. However, only 54% of the variation in MC concentrations could be explained by changes in the proportion of MC-producing cells, suggesting that a considerable part of the MC concentrations was also due to variations in MC cell quotas. These findings suggest that the toxic risks during cyanobacterial proliferations are due to variations in both the proportion of MC-producing cells and the production of MC by the toxic cells.
Numerous papers have already investigated the impact of various biotic and abiotic environmental factors on microcystin production by toxic cells. These studies demonstrate that MC production can be influenced by temperature (35), light (46), nutrients such as nitrogen and phosphorus (12, 32), pH (39), iron (42), xenobiotics (17, 34, 45), and predators (22, 23, 47). Despite inconsistent results, the production of microcystins by the cells does seems to be linked to their growth rate (11, 31, 33), which is itself affected by environmental conditions. On the other hand, several studies of variations in the proportions of MC-producing cells have demonstrated the potential influence of nutrient concentrations (9, 48) and light and temperature (5), and two papers (3, 5) have suggested that there is a negative correlation between the proportions of MC-producing cells and the abundance of cyanobacterial cells. These findings are consistent with the data of Kardinaal and Visser (26), showing that in Dutch lakes there is a negative relationship between the densities of cyanobacterial cells and the mean MC concentration in the cells.
In an overall attempt to explain the variations of toxicity during cyanobacterial blooms, we studied the spatiotemporal variations in MC concentrations and in the proportions of MC-producing and non-MC-producing cells in several Microcystis aeruginosa populations blooming in different freshwater ecosystems located in the same geographical area. The point of this study was to analyze these variations in terms of the characteristics of these ecosystems and the population dynamics of the M. aeruginosa populations. In addition, these data were compared to the variations in the intergenic transcribed spacer (ITS) composition of the same populations recently reported by Sabart et al. (38). The proportion of potentially MC-producing cells was estimated by a real-time quantitative PCR approach, the change in threshold cycle (ΔCT) method recently developed by Briand et al. for Planktothrix (3) and Microcystis (4) and targeting the mcyB (mcyA for Planktothrix) and phycocyanin (PC) genes.
MATERIALS AND METHODS
Study sites, sampling, and counting of cyanobacterial abundances.
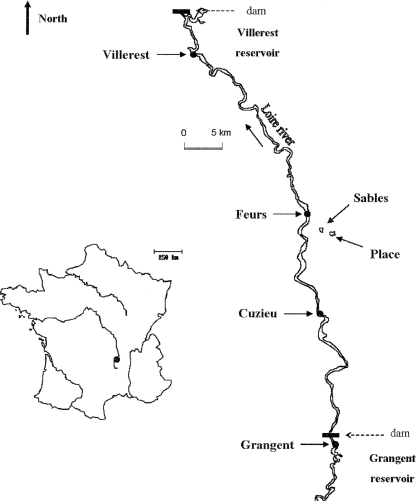
The study was conducted in 2006 at six sampling stations located in the catchment area of the river Loire in central France (Fig. 1). Four of the stations were located in the river Loire itself: one in the Grangent Reservoir (45°27N, 4°15′E, area of 3.65 km2, length of 21 km, maximum depth of 50 m, and volume of 57.4 × 106 m3), one in the Villerest Reservoir (45°55′N, 4°02′E, area of 7.12 km2, length of 34 km, maximum depth of 45 m, and volume of 130 × 106 m3) located 70 km downstream of Grangent, and another two located in the fluvial section between these two reservoirs (Cuzieu, 45°36′N, 4°14′E; and Feurs, 45°44′N, 4°12′E). In addition, two small ponds (Place Pond, 45°43′N, 4°14′E, area of 0.08 km2, maximum depth of 2.5 m, and volume of 0.13 × 106 m3; and Sables Pond, 45°43′N, 4°14′E, area of 0.1 km2, maximum depth of 1.4 m, and volume of 0.145 × 106 m3) dedicated to fish production and located close to the river (3 km) were also studied. Microcystis was the only MC-producing genus that was present at our study sites during our sampling period. Further biotic and abiotic parameters for these sites are given in Table 1.
FIG. 1.
Geographical location in France of the study area and of the sampling sites.
TABLE 1.
Characteristics of the six sampling sites
| Characteristic | Result from: |
|||||
|---|---|---|---|---|---|---|
| Grangent Reservoir | Villerest Reservoir | Cuzieu (Loire River) | Feurs (Loire River) | Sables Pond | Place Pond | |
| Minimum Secchi disk depth (m) | 1.0 | 0.7 | NDa | ND | 0 | 0.2 |
| Chlorophyll a concn (mg m−3) | ||||||
| Annual mean | 11.0 | 20.5 | 4.8 | 16.0 | 73.2 | 52.4 |
| Maximum | 47.6 | 62.9 | 9.8 | 35.2 | 149.6 | 87.8 |
| Maximum temp (°C) | 25.2 | 27 | 28.2 | 28.3 | 26.6 | 27.7 |
| Total phosphorus (mean annual concn of inflow [mg liter−1]) | 0.04 | 0.08 | 0.09 | 0.09 | 2.37 | 1.45 |
| Proportion (%) of M. aeruginosa in: | ||||||
| Phytoplankton | 86 | ND | ND | ND | 51 | 42 |
| Cyanobacteria | 94 | ND | ND | ND | 81 | 81 |
| Trophic statusb | Eutrophic | Eutrophic | Hypereutrophic | Hypereutrophic | ||
ND, not determined.
From OCDE (31a).
The study sites were sampled twice a month between June and September 2006. Water samples (20 liters) were collected using an electric pump equipped with a 20-μm-pore mesh (29), from a depth of 0.5 m below the surface at all stations. For the Grangent Reservoir, the 0- to 10-m surface water layer was sampled in order to obtain a greater concentration of cyanobacteria for subsequent analysis. The Microcystis colonies obtained were then resuspended in 1 ml of distilled water and kept at −20°C until analyzed.
The abundance of Microcystis cells in the samples was estimated by microscopic cell counts. One milliliter of each sample was fixed with 1% glutaraldehyde, and then the colonial structure of Microcystis was rapidly disrupted (<1 min) by ultrasonication (at 20 kHz) (36). Microcystis cells were counted in triplicate using a hematimeter (Thoma hematimeter).
Microcystin analysis.
The intracellular microcystins were extracted twice by sonication in 75% methanol (MeOH). The lysate was centrifuged to remove cell debris, and then the supernatant was filtered through a 0.2-μm-pore syringe filter to remove any remaining smaller particulates. The samples were evaporated using a concentrator (Concentrator 5301; Eppendorf, Hamburg Germany), and the extracts obtained were dissolved in 100 μl 20% MeOH.
Microcystin concentrations were determined by the protein phosphatase 2A (PP2A) inhibition bioassay developed by Rivasseau et al. (37). This assay is based on the degradation of paranitrophenylphosphate (pNPP; colorless) into paranitrophenol (pNP; yellow) by the PP2A. This reaction is inhibited by microcystins. The activity of the PP2A was determined by measuring the color produced as a result of the formation of pNP from the substrate pNPP. The assay was carried out in triplicate in a 96-well microtitration plate. Controls (in which sample was replaced by MeOH), blanks (in which there was no sample), and sample blanks (in which all components were present except the enzyme) were also included in the assays. Methanolic samples of MC were diluted in 100 μl buffer B (40 mM Tris-HCl, 34 mM MgCl2, 4 mM EDTA, 4 mM dithiothreitol, pH 8.3). 50 μl of buffer B containing 27 mU/ml PP2A, and 0.5 mg ml−1 bovine serum albumin, and 50 μl of buffer B containing 28 mM pNPP was added to initiate the enzymatic reaction. After incubation for 60 min at 37°C, the absorbance was measured at 405 nm on a MultiSkan Ascent microplate-reader (Thermo, Vantaa, Finland). The toxin concentrations were calculated from a standard MC-LR calibration curve. The cellular quotas were expressed in picogram equivalents of MC-LR per cell, and the microcystin concentrations were expressed in microgram equivalents of MC-LR per liter of lake water.
In order to obtain further insight into the proportions of the specific congeners of microcystin present, selected samples were also analyzed by high-pressure liquid chromatography (HPLC)-mass spectrometry (MS). Liquid chromatography (LC)-MS measurements were performed using a micrOTOF electrospray ionization-time-of-flight (ESI-TOF) mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) coupled to an HPLC system (Agilent Technologies, Waghaeusel-Wiesental, Germany) controlled by HyStar chromatography software (Bruker Daltonik GmbH). A mobile-phase system consisting of water (A) and acetonitrile (B) (each containing 0.1% formic acid) was used, and separation was performed with an RP-18 column (100 by 2.1 mm, 3.5-μm particle size; Agilent Zorbax Eclipse Plus) and the following gradient: 0 to 2 min, 98% A; 2 to 12 min, 98% to 2% A (linear); and 12 to 13 min, 2% to 98% A.
Data sets were acquired in positive electrospray (ESI) mode in a scan range from 50 to 1,500 m/z at a sampling rate of 2 Hz. Sodium formate solution was used for the calibration and injected at the beginning of each chromatographic run by infusion (Harvard Apparatus; flow rate, 220 μl/h). Quality control samples (MC-LR) and blank runs were interspersed between the samples under investigation.
The LC-MS data were smoothed before automatic peak integration on extracted ion chromatograms (EICs) created at a 10-mDa mass accuracy, using internal routines constructed using DataAnalysis software (Bruker Daltonik GmbH). Chromatographic trace definitions were based on the measured masses of MC-LR, MC-RR, MC-YR, and MC-LY.
The microcystin concentrations in the samples were quantified on the basis of standard curves. The linearity of the TOF detector was suitable for the range of toxin concentrations in samples. The relative proportion of each of the MC congeners was calculated as a ratio of the MC pool (defined as the sum of the four congeners measured).
Chemicals.
All reagents were of HPLC-MS grade (>99.9%) or analytical grade. Methanol was purchased from Sigma-Aldrich (Saint Quentin Fallavier, France), and acetonitrile and water were obtained from Rhiedel de Hahn (Chromasolv LC-MS grade; Sigma-Aldrich, Saint Quentin Fallavier, France). pNPP, dithiothreitol, and bovine serum albumin were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). The four microcystin standards MC-LR, MC-RR, MC-YR, and MC-LY were purchased from Alexis Biochemicals (Enzo Life Sciences, Lausanne, Switzerland). PP2A purified from human red blood cells was obtained from Upstate (Millipore Corporation, Billerica, MA).
DNA extraction and multiplex real-time PCR.
The proportion of potentially MC-producing cells was determined by real-time PCR analysis. DNA was extracted using the DNeasy plant minikit (Qiagen), as described previously by Sabart et al. (38). Two target genes were used: the intergenic spacer region within the phycocyanin (PC) operon, and the mcyB region. The primers and probes used for the PC operon and mcyB genes (listed in Table 2) have been described previously (4, 28).
TABLE 2.
Real-time PCR primers and probes used in this study
| Gene region and primer or probe | Sequence (5′→3′)a |
|---|---|
| mcyB region | |
| Primers | |
| 30F | CCTACCGAGCGCTTGGG |
| 108R | GAAAATCCCCTAAAGATTCCTGAGT |
| Probe | FAM-CACCAAAGAAACACCCGAATCTGAGAGG-TAMRA |
| PC gene region | |
| Primers | |
| 188F | GCTACTTCGACCGCGCC |
| 254R | TCCTACGGTTTAATTGAGACTAGCC |
| Probe | Cy5-CCGCTGCTGTCGCCTAGTCCCTG-BHQ-2 |
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine; Cy5, 5-indocarboxycyanine; BHQ-2, black hole quencher 2.
Amplification by real-time PCR was carried out using an Mx3005P thermal cycler (Stratagene, Netherlands). All of the reactions were performed in 20-μl volumes in 96-well plates. The multiplex reaction mixture contained 10 μl of 2× Quantitec probe PCR kit mix (Qiagen), a 300 nM (300 fmol μl−1) concentration of each primer, a 100 nM concentration of the TaqMan probe for PC gene amplification, and a 900 nM concentration of each primer, a 250 nM concentration of the TaqMan probe for mcyB, and 2 μl of template containing various amounts of genomic DNA. The mixture was then filled to 20 μl with sterile Millipore water. Each sample was prepared in triplicate. Negative controls without DNA were included for each PCR run. For each run of samples, serial dilutions containing 1.1 × 101, 1.1, 1.1 × 10−1, 1.1 × 10−2, 1.1 × 10−3, 1.1 × 10−4, and 1.1 × 10−5 ng of the genomic DNA μl−1 were prepared from the DNA extract of an MC-producing M. aeruginosa strain to serve as external standards for the real-time PCR. The temperature cycle consisted of an initial preheating step of 15 min at 95°C, followed by 40 cycles each consisting of 30 s at 95°C (denaturing), 1 min at 60°C (annealing), and 30 s at 72°C (extension). For data analysis, the fluorescence threshold of all of the samples was set manually to 132 (relative fluorescence) for PC gene amplification and to 665 for mcyB amplification, in order to obtain the best PCR efficiency using linear-log calibration curves. The sizes of the amplicons were 66 and 78 bp for the PC and mcyB genes, respectively.
We used a novel method to estimate the relative proportion of the mcyB+ subpopulation in the Microcystis population: the change in threshold cycle (ΔCT). This method was originally developed by Briand et al. (3) for Planktothrix and then used for Microcystis (4). Briefly, standard curves were established by relating the known quantity of DNA (in picograms) to the threshold cycle (CT) number (the cycle number at which the fluorescence first exceeds the threshold) for several axenic strains from the Pasteur Culture Collection (PCC). The resulting ΔCT between the two genes (ΔCT = CT for the PC gene − CT for mcyB) was deduced from the regression equations. An equation was established from the ΔCT values theoretically calculated for proportions of mcyB+ genotype strains of 100, 50, 25, and 12.5% of the population: y = 3.32 × log (x) − 8.78, where y is the ΔCT and x is the initial percentage of the mcyB+ genotype strains (4).
Data analysis.
Pearson's and Spearman's correlations and analysis of variance (ANOVA) were performed using the XLSTAT-Pro 7.5 software (Addinsoft).
RESULTS
Population dynamics, microcystin concentrations, and proportions of MC-producing cells in the M. aeruginosa population of the Grangent Reservoir.
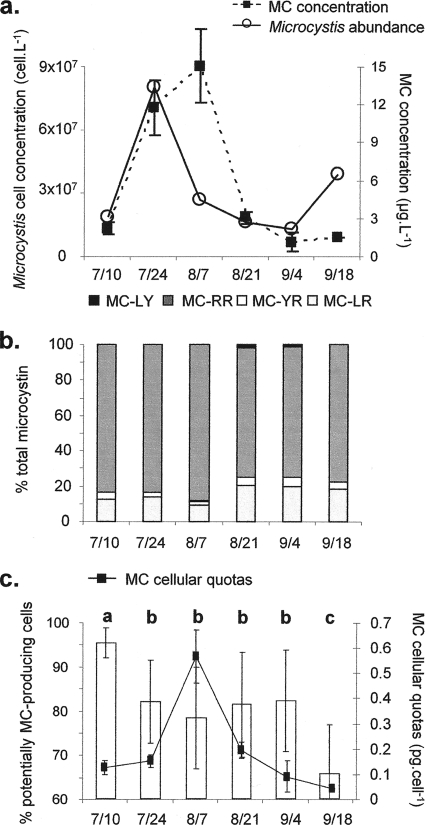
As previously reported in the paper by Sabart et al. (38), in 2006 the peak of M. aeruginosa cells in the Grangent Reservoir was reached on 24 July (8.05 × 107 cells liter−1). After that, a marked decrease was found from 7 August to 4 September, reaching values of about 1.9 × 107 cells liter−1. Finally, the last sampling date (18 September) was marked by an increase in the cell concentration (about 3.9 × 107 cells liter−1) (Fig. 2a).
FIG. 2.
Temporal dynamics of Microcystis population, the potentially MC-producing cells, and microcystin in the Grangent Reservoir in 2006. (a) Microcystis cell and microcystin concentrations; (b) distribution of microcystin variants expressed as a percentage of total microcystin; (c) proportion of potentially MC-producing cells (histograms) and MC cellular quotas. The error bars indicate standard deviations. Different letters indicate significant differences in the proportion of potentially MC-producing cells.
The pattern of MC concentration (estimated after extracting the MC from the cells) was similar overall to that of the concentration of Microcystis cells (Fig. 2a). The only exception was observed on 7 August, when there was a decrease in the cell abundance of M. aeruginosa but an increase in the MC concentration. There was no significant change over time in the relative proportions of each MC variant (Fig. 2b). MC-RR was always the dominant variant (73 to 88%), the second most abundant being MC-LR, which accounted for 9 to 20% of the total MC. The other two variants (MC-YR and MC-LY) were always detected in low concentrations and accounted together for less than 10% of the total MC.
The proportions of MC-producing cells assessed by real-time PCR ranged from 95% to 66% during the study period (Fig. 2c). The highest value was found at the first sampling date, just before the abundance of M. aeruginosa cells peaked. After an initial dip, this proportion subsequently remained stable at around 80% until the beginning of September, just before falling to the lowest proportion, which was recorded on 18 September.
Finally, the MC cellular quotas (Fig. 2c) appeared to be very stable throughout the study, except on 7 August, when the cellular quota reached a value six times higher than that at other times (repeated-measures ANOVA, Tukey's post hoc test; P < 0.0001). With regard to the relative proportion of MC-producing cells, we also calculated the cellular quotas taking only toxic cells into account. These quotas were multiplied by 1.26 (± 0.15)-fold on average compared to the cellular quotas calculated for all Microcystis cells present. These MC cellular quotas were significantly correlated with the changes in MC concentrations (Spearman's r2 = 0.78; P = 0.033), but no correlation with Microcystis cells densities or with the proportion of MC-producing cells was detected.
Population dynamics, microcystin concentrations, and proportions of MC-producing cells in the M. aeruginosa population located downstream of Grangent.
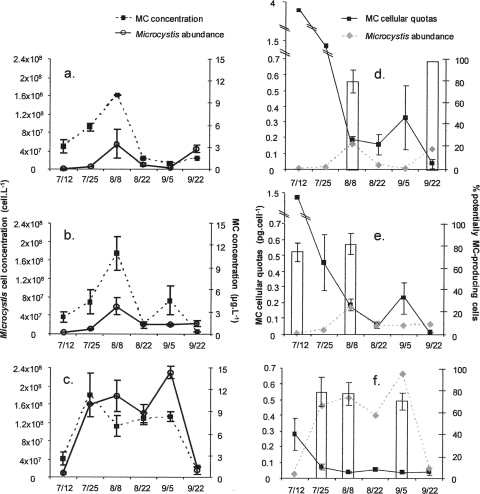
The two fluvial M. aeruginosa populations contained lower cell concentrations than that in the Grangent Reservoir (Fig. 3 a and b), with no significant difference being found between them (Spearman's r2 = 0.89; P = 0.017). The maximum cell densities (about 5.7 × 107 cells liter−1) were found on 8 August. The cell concentrations of the Microcystis population in the Villerest Reservoir were higher than those in the Grangent Reservoir (comprising between 9 × 106 and 2.3 × 108 cells liter−1), and the pattern of change was very different, with cell concentrations in the Villerest population increasing throughout the summer to peak on 4 September (Fig. 3c).
FIG. 3.
Temporal dynamics of Microcystis population, the potentially MC-producing cells, and microcystin in the Loire River in 2006. Shown are the Microcystis cell and microcystin concentrations in Cuzieu (a), Feurs (b), and Villerest (c), as well as the proportion of potentially MC-producing cells (histograms) and MC cellular quotas in Cuzieu (d), Feurs (e), and Villerest (f). The error bars indicate standard deviations.
In the Loire River (Cuzieu and Feurs), the MC concentrations displayed a similar pattern (Pearson's r2 = 0.74; P = 0.027), with maximum values of 10.1 and 10.8 μg liter−1 recorded for Cuzieu and Feurs, respectively (Fig. 3a and b). Moreover, the changes in these MC concentrations were synchronous with those of the Microcystis cell concentrations, as previously found in the Grangent Reservoir, but MC concentrations in the river were up to four times higher than those in the Grangent Reservoir. In the Villerest Reservoir (Fig. 3c), the MC concentrations were in the same range as those in the Loire River. As at Cuzieu and Feurs, there was a positive correlation (Pearson's r2 = 0.7; P = 0.039) between the temporal variations in MC concentrations and Microcystis cell concentrations.
The proportions of potentially MC-producing cells in the M. aeruginosa samples from the Loire River and from the Villerest Reservoir ranged from 70 to 100% and did not display any significant fluctuation over the sampling period (Fig. 3d to f).
Finally, the MC cellular quotas appeared to be much higher in samples from the Loire River than in those from the Villerest Reservoir (Fig. 3d to f) or Grangent Reservoir (Fig. 2c). The variations in these MC cellular quotas were similar at the two river sampling sites at Cuzieu and Feurs (Spearman's r2 = 1; P = 0.03), with the highest values recorded at the first sampling date and a subsequent progressive decline until the last sampling date (Fig. 3d and e). In the Villerest Reservoir, there was no significant change in the MC cellular quotas over the sampling period (Fig. 3f). Where possible (i.e., when the relative proportion of MC-producing genotypes was known), we also calculated the MC cellular quotas, taking into account only the toxic cells. As previously observed for the Grangent Reservoir, these quotas were multiplied by 1.25 (± 0.13)-fold on average compared to the cellular quotas calculated for all of the Microcystis cells.
Population dynamics, microcystin concentrations, and proportions of MC-producing cells in the M. aeruginosa population of the Sable and Place Ponds.
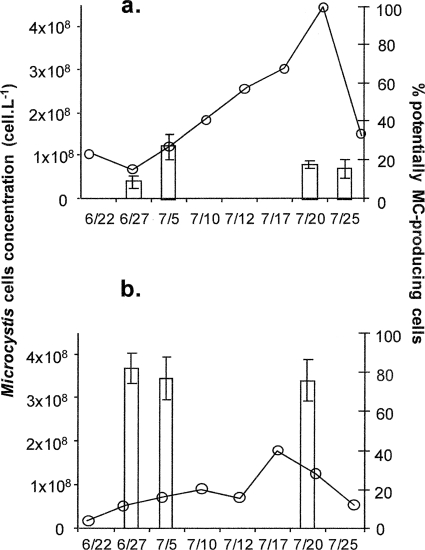
M. aeruginosa cell abundances in these ponds (Fig. 4 a and b) were in the same range as those of the Villerest Reservoir (1.1 × 107 to 4.3 × 108 cells liter−1). These two ponds displayed two very different patterns with regard to the proportion of MC-producing cells present. In the Sable Pond, the M. aeruginosa population was dominated by non MC-producing cells throughout the study (9.2 to 27.6%, with a mean value of 17.8%) (Fig. 4a). On the other hand, the proportion of potentially MC-producing cells was very high in the Place Pond and was characterized by little temporal variation (75.5 to 82%) (Fig. 4b).
FIG. 4.
Temporal dynamics of Microcystis population and the potentially MC-producing cells in the ponds in 2006. Shown are the Microcystis cell concentrations and proportions of potentially MC-producing cells (histograms) in Sables Pond (a) and Place Pond (b). The error bars indicate standard deviations.
DISCUSSION
This study is the first one to estimate changes in both MC concentrations and the proportions of MC-producing cells during a bloom season, on the basis of measurements of several M. aeruginosa populations located in the same geographical area but living in separate but connected freshwater ecosystems. Our results show that large variations can be found in both the MC concentrations and the proportions of potentially MC-producing cells, depending on the ecosystem and also depending on time.
Comparison of the changes in potentially MC-producing cells in the different ecosystems.
It is interesting to look at the variations in the proportions of potentially MC-producing cells in the M. aeruginosa population of the Grangent Reservoir alongside the dynamics of the M. aeruginosa population and the temporal variations in the ITS composition of this population, which were investigated in the same samples by Sabart and colleagues (38). In the present study, the development of the proliferation (in July) was associated with a decrease in the proportion of MC-producing cells and in ITS genotypic diversity, with the dominance of just a few genotypes (38). The same concomitant evolution was found in the Grangent Reservoir in 2007 by Briand et al. (4). In their study, there was a more marked decline in the proportion of MC-producing cells (from 93% to 14%), while the ITS genotypic composition was dominated by three genotypes when the M. aeruginosa population reached its peak. The magnitude of the blooms and the pattern of their development over the course of the summer were quite different in these 2 years (with lower cellular abundances in 2007), as were the environmental conditions (a hot and dry summer in 2006 versus a wet summer in 2007). Despite these differences in the overall annual patterns of the ecosystem, the patterns of change in the genotypic composition of the M. aeruginosa population were similar, which suggests that potential toxicity confers a competitive advantage in the early stages of a bloom.
The postbloom study that we carried out in 2006 allowed us to analyze the evolution of an M. aeruginosa population under changing environmental conditions (cooler weather, destratification of the water column, and changes in the hydrological regime [38]). Indeed, dramatic changes in the genotypic composition occurred 2 weeks after the decrease in biomass (21 August), whereas the change in the proportion of toxic cells occurred only in mid-September and was concomitant with a significant drop in water level that caused disruption of the ecosystem. The results obtained during this interesting period showed that changes in the ITS composition of the population were not always associated with changes in the proportions of potentially MC-producing cells, suggesting that characteristics other than the ability to produce MC are selected for during bloom events. From this study and that of Briand et al. (4), it looks as though toxic cells tend to dominate in M. aeruginosa populations when cyanobacteria do not dominate the phytoplankton community (e.g., at the onset of the bloom, as illustrated by the results from July 2006 and May 2007, and also when the bloom resumes, as illustrated by those from August 2006 just before the cyanobacteria peaked).
At the sampling points located downstream of the Grangent Reservoir (Cuzieu, Feurs, and Villerest), we did not find any significant changes in the proportion of MC-producing cells, which was high throughout the study. This is an interesting finding given that the same dominant ITS genotype was also found throughout the study at these sites (38). This dominant genotype selected under fluvial conditions was probably an MC-producing genotype. Finally, the two ponds, which are connected in winter, displayed very different proportions of MC-producing cells (18 versus 78%), which is consistent with the contrasting ITS genotype compositions of these two M. aeruginosa populations (38). No major variations in the proportions of MC-producing cells were found in the M. aeruginosa populations in these two ecosystems, in agreement with the fact that no dramatic change was found in their ITS genotypic compositions (38). All of these findings demonstrate that it is local environmental conditions rather than global processes, such as climate, that seem to have a major impact on the potential toxicity of M. aeruginosa populations. Indeed, despite the fact that all of the populations studied here were exposed to the same overall climatic conditions due to their being located in the same area, variable proportions of toxic cells were found in these populations associated with contrasting patterns in the evolution of these proportions. Moreover, when our results are compared with all of the data available on the proportions of toxic cells in cyanobacterial populations (Table 3), it emerged that it was not possible to establish any link between the different descriptors of the ecosystems (e.g., trophic status, surface, etc.) and the proportions of MC-producing cells in the cyanobacterial populations, which confirms the importance of local conditions and probably the complexity of interactions occurring between toxic and nontoxic cells in the populations. These findings seem to contradict the hypothesis of Davis et al. (9), who propose that global changes, and climate warming in particular, could be leading to the dominance of toxic cells during cyanobacterial blooms.
TABLE 3.
Overview of the proportion of potentially microcystin-producing cells in cyanobacterial populations from the literature and characteristics of the study sites
| Cyanobacterial genus or species | Site | Type of ecosystem | Latitude and longitude | Trophic status | Surface area (km2) | Maximal depth (m) | Depth of sampling (m) | Targeted genes | % of toxic cells | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Microcystis sp. | Lake Wannsee, Germany | 52.25°N, 13.10°E | Hypereutrophic | 2.82 | 8.5 | Integrated from the water column | mcyB, PC gene | 0.9-38.3 | 27 | |
| Microcystis sp. | Lake Champlain, Untied States | Natural freshwater lake | 44.62°N, 73.37°W | Hypereutrophic | 1.13 | 122 | Surface water | mcyD, 16S rRNA gene | 6 | 9 |
| Microcystis sp. | Lake Agawam, United States | Natural freshwater lake (glacial) | 40.88°N, 72.39°W | Eutrophic | 0.5 | 4 | Surface water | mcyD, 16S rRNA gene | 0.01-1.56 | 9 |
| Microcystis sp. | Mill Pond, United States | 40.91°N, 72.36°W | Hypereutrophic | 8 | Surface water | mcyD, 16S rRNA gene | 9 | |||
| Microcystis sp. | Lake Ronkonkoma, United States | Natural freshwater lake (glacial) | 40.83°N, 73.12°W | 1.5 | 27 | Surface water | mcyD, 16S rRNA gene | 12-100 | 9 | |
| Planktothrix agardhii | Lake Viry-Châtillon, France | Artificial freshwater lake | 48.40°N, 2.23°E | 0.98 | 2.8 | 0.5 | mcyA, PC gene | 31-93 | 3, 5 | |
| Microcystis sp. | Lake Erie, United States | Natural lake (glacial) | 42.04°N, 81.19°W | Eutrophic | 25.77 | 64 | 1 | mcyD, 16S rRNA gene | 0-59.5 | 36a |
| Microcystis sp. | Lake Oneida, United States | Natural freshwater lake | 43.18°N, 75.92°W | 207 | 6.4 | 1 | mcyD, 16S rRNA gene | 0-37 | 18 | |
| Microcystis sp. | Hirosawa-no-ike Pond, Japan | Artificial pond used for carp fishery | 35.01° N, 135.41°E | Hypereutrophic | 0.14 | 1.8 | mcyA, 16S rRNA gene | 80 | 17a | |
| Microcystis sp. | Lake Mikata, Japan | Natural freshwater lake | 35.33°N, 135.53°E | 3.6 | 2 | 0.5 | mcyA, PC gene | 0.5-35 | 48 | |
| Microcystis aeruginosa | Lake Grangent, France | Artificial freshwater lake (dam on the Loire River) | 45.27°N, 4.15°E | Eutrophic | 3.65 | 50 | 0.5 | mcyB, PC gene | 6-93 | 4 |
| Integrated (0-10) | mcyB, PC gene | 65.6-94.7 | This study | |||||||
| Microcystis aeruginosa | Lake Villerest, France | Artificial freshwater lake (dam on the Loire River) | 45.55°N, 4.02°E | Eutrophic | 7.12 | 45 | 0.5 | mcyB, PC gene | 70.3-76.8 | This study |
| Microcystis aeruginosa | Sables Pond, France | Pond | 45.43°N, 4.14°E | Hypereutrophic | 0.1 | 1.4 | 0.5 | mcyB, PC gene | 9.2-27.6 | This study |
| Microcystis aeruginosa | Place Pond, France | Pond | 45.43°N, 4.14°E | Hypereutrophic | 0.08 | 2.5 | 0.5 | mcyB, PC gene | 75.5-82 | This study |
| Microcystis aeruginosa | Cuzieu, France | River Loire | 45.36°N, 4.14°E | 0.5 | mcyB, PC gene | 81.9-100 | This study | |||
| Microcystis aeruginosa | Feurs, France | River Loire | 45.44°N, 4.12°E | 0.5 | mcyB, PC gene | 75-82.2 | This study |
Relationship between variations in MC production, proportions of mcyB+ cells, and cell abundances of Microcystis.
In the Grangent Reservoir, the proportions of the MC variants barely changed over time, as previously reported in other lakes (15, 16), and the major MC variant was always MC-RR, whereas MC-LR has been reported to be the dominant MC variant in most Microcystis blooms (2, 13, 43, 44). Previous studies have demonstrated the influence of various environmental factors, such as light, water temperature (1, 35), and also amino acid availability (41), on the MC composition of cyanobacterial populations. However, considering the lack of change in the MC variant composition in the Grangent Reservoir during this study and the fact that the M. aeruginosa population was exposed to major variations in the water temperature or availability of light during the study period (38), it seems to be unlikely that these factors have any impact on MC composition in the Grangent Reservoir. Fastner et al. (16) suggested that the lack of change in MC variant composition during cyanobacterial blooms could result from the absence of any change in the genotype composition of the cyanobacterial population, but our findings do not corroborate this hypothesis, because we detected major changes in the ITS composition of the Grangent population of M. aeruginosa during the summer of 2006 (38), suggesting that environmental determinism was involved in the MC variant composition.
The concentrations of MCs were in a range of the same order as those reported in the literature for Microcystis blooms (3, 6, 13, 18, 19, 26), and they were frequently above the WHO advisory level for drinking water of 1 μg liter−1 equivalent of MC-LR. Overall, in both the reservoirs and the fluvial sites, the MC concentrations in the water parallel the changes in Microcystis abundance, but not those in the proportion of potentially MC-producing cells. Our findings are inconsistent with previous results (20, 27), which found that changes in MC concentrations were predominantly a result of changes in the proportion of MC-producing cells. In contrast to these studies, our results indicate that measurement of the mcy gene with real-time PCR is not an appropriate way to predict MC concentrations in freshwater ecosystems. The lack of correlation between MC concentrations in the water and the proportion of the mcyB+ cells could be due to the presence of an incomplete mcy operon, leading to an overestimation of the proportion of MC-producing genotypes, as previously suggested by Hotto et al. (18).
Our MC cellular quotas were generally higher than those found in experimental studies performed using isolated strains (21, 33, 46), but they were usually in the same range as those found in cyanobacterial populations in other lakes (3, 7, 14). These high values could be due to an overestimation generated by the fact that non-colony-forming M. aeruginosa cells may have been taken into account in the MC analysis but not in the cell count (48). Despite this possible overestimation, the highest MC cellular quotas were found at the beginning of the sampling period at the three sampling sites located downstream of Grangent and generally when Microcystis cell abundance was low (for example, after the biomass peak in Grangent, when the bloom was starting in Villerest or at the two fluvial sites). In the same way, high MC cellular quotas preceding blooms of Planktothrix and Microcystis have been reported in Dutch lakes (24, 25), suggesting that high concentrations of MC in the early stages of a bloom could enhance growth efficiency or could act as a grazing deterrent. We can also assume that high cellular quotas during the early stages of the bloom are due to the recruitment of highly toxic cells, since high MC contents have been found in the benthic colonies in the Grangent Reservoir (30).
In conclusion, we found major variations in space and time in the proportion of potentially MC-producing cells in different M. aeruginosa populations living in interconnected freshwater ecosystems located in the same geographical area. We also show that the relative selection of the MC-producing and non-MC-producing genotypes occurs at the scale of each ecosystem and probably depends on many local environmental factors and processes. The variations in the proportion of potentially microcystin-producing genotypes did not explain those of the MC concentrations. Finally, the fact that the highest MC cellular quotas were observed at low Microcystis cell abundances at each site suggests that toxic cells have an advantage under suboptimal conditions for growth. These findings are of particular interest with regard to the ecological role of microcystin, which could be implicated in sustaining Microcystis under adverse conditions or fluctuating environments.
Acknowledgments
This work was conducted in the framework of a research program with Electricité de France Research & Development (EDF R&D). This work was also funded in part by the ANR research program SEST 2007 (project MATRICS).
We thank Pierre Souvignet and Thierry Vasquez for field sampling and Monika Ghosh for improving the English text.
Footnotes
Published ahead of print on 28 May 2010.
REFERENCES
- 1.Amé, M. V., and D. A. Wunderlin. 2005. Effects of iron, ammonium and temperature on microcystin content by a natural concentrated Microcystis aeruginosa population. Water Air Soil Pollut. 168:235-248. [Google Scholar]
- 2.Baldia, S. F., M. C. G. Conaco, T. Nishijima, S. Imanishi, and K. I. Harada. 2003. Microcystin production during algal bloom occurrence in Laguna de Bay, the Philippines. Fish. Sci. 69:110-116. [Google Scholar]
- 3.Briand, E., M. Gugger, J. C. Francois, C. Bernard, J. F. Humbert, and C. Quiblier. 2008. Temporal variations in the dynamics of potentially microcystin-producing strains in a bloom-forming Planktothrix agardhii (cyanobacterium) population. Appl. Environ. Microbiol. 74:3839-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briand, E., N. Escoffier, C. Straub, M. Sabart, C. Quiblier, and J. F. Humbert. 2009. Spatiotemporal changes in the genetic diversity of a bloom-forming Microcystis aeruginosa (Cyanobacteria) population. ISME J. 3:419-429. [DOI] [PubMed] [Google Scholar]
- 5.Briand, E., C. Yepremian, J. F. Humbert, and C. Quiblier. 2008. Competition between microcystin- and non-microcystin-producing Planktothrix agardhii (Cyanobacteria) strains under different environmental conditions. Environ. Microbiol. 10:3337-3348. [DOI] [PubMed] [Google Scholar]
- 6.Briand, J. F., S. Jacquet, C. Flinois, C. Avois-Jacquet, C. Maisonnette, B. Leberre, and J. F. Humbert. 2005. Variations in the microcystin production of Planktothrix rubescens (Cyanobacteria) assessed from a four-year survey of Lac du Bourget (France) and from laboratory experiments. Microb. Ecol. 50:418-428. [DOI] [PubMed] [Google Scholar]
- 7.Briand, J. F., C. Robillot, C. Quiblier-Lloberas, and C. Bernard. 2002. A perennial bloom of Planktothrix agardhii (Cyanobacteria) in a shallow eutrophic French lake: limnological and microcystin production studies. Arch. Hydrobiol. 153:605-622. [Google Scholar]
- 8.Carmichael, W. W. 1992. Cyanobacteria secondary metabolites—the cyanotoxins. J. Appl. Bacteriol. 72:445-459. [DOI] [PubMed] [Google Scholar]
- 9.Davis, T. W., D. L. Berry, G. L. Boyer, and C. J. Gobler. 2009. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8:715-725. [Google Scholar]
- 10.Dittmann, E., B. A. Neilan, M. Erhard, H. von Döhren, and T. Börner. 1997. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 26:779-787. [DOI] [PubMed] [Google Scholar]
- 11.Downing, T. G., C. Meyer, M. M. Gehringer, and M. van de Venter. 2005. Microcystin content of Microcystis aeruginosa is modulated by nitrogen uptake rate relative to specific growth rate or carbon fixation rate. Environ. Toxicol. 20:257-262. [DOI] [PubMed] [Google Scholar]
- 12.Downing, T. G., C. S. Sember, M. M. Gehringer, and W. Leukes. 2005. Medium N:P ratios and specific growth rate comodulate microcystin and protein content in Microcystis aeruginosa PCC7806 and M. aeruginosa UV027. Microb. Ecol. 49:468-473. [DOI] [PubMed] [Google Scholar]
- 13.Dyble, J., G. L. Fahnenstiel, R. W. Litaker, D. F. Millie, and P. A. Tester. 2008. Microcystin concentrations and genetic diversity of Microcystis in the lower Great Lakes. Environ. Toxicol. 23:507-516. [DOI] [PubMed] [Google Scholar]
- 14.Fahnenstiel, G. L., D. F. Millie, J. Dyble, R. W. Litaker, P. A. Tester, M. J. McCormick, R. Rediske, and D. Klarer. 2008. Microcystin concentrations and cell quotas in Saginaw Bay, Lake Huron. Aquat. Ecosyst. Health Manag. 11:190-195. [Google Scholar]
- 15.Fastner, J., M. Erhard, W. W. Carmichael, F. Sun, K. L. Rinehart, H. Rönicke, and I. Chorus. 1999. Characterization and diversity of microcystins in natural blooms and strains of the genera Microcystis and Planktothrix from German freshwaters. Arch. Hydrobiol. 145:147-163. [Google Scholar]
- 16.Fastner, J., M. Erhard, and U. Neumann. 2001. Microcystin variants in Microcystis and Planktothrix dominated field samples, p. 148-152. In I. Chorus (ed.), Cyanotoxins: occurrence, causes, consequences. Springer, Berlin, Germany.
- 17.Gong, Y., L. R. Song, X. Q. Wu, B. D. Xiao, T. Fang, and J. T. Liu. 2009. Effects of arsenate on microcystin content and leakage of Microcystis strain PCC7806 under various phosphate regimes. Environ. Toxicol. 24:87-94. [DOI] [PubMed] [Google Scholar]
- 17a.Ha, J. H., T. Hidaka, and T. Hiroshi. 2009. Quantification of toxic Microcystis and evaluation of its dominance ratio in blooms using real-time PCR. Environ. Sci. Technol. 43:812-818. [DOI] [PubMed] [Google Scholar]
- 18.Hotto, A. M., M. F. Satchwell, D. L. Berry, C. J. Gobler, and G. L. Boyer. 2008. Spatial and temporal diversity of microcystins and microcystin-producing genotypes in Oneida Lake, NY. Harmful Algae 7:671-681. [Google Scholar]
- 19.Izydorczyk, K., T. Jurczak, A. Wojtal-Frankiewicz, A. Skowron, J. Mankiewicz-Boczek, and M. Tarczynska. 2008. Influence of abiotic and biotic factors on microcystin content in Microcystis aeruginosa cells in a eutrophic temperate reservoir. J. Plankton Res. 30:393-400. [Google Scholar]
- 20.Jacoby, J. M., D. C. Collier, E. B. Welch, F. J. Hardy, and M. Crayton. 2000. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can. J. Fish. Aquat. Sci. 57:231-240. [Google Scholar]
- 21.Jähnichen, S., T. Ihle, T. Petzoldt, and J. Benndorf. 2007. Impact of inorganic carbon availability on microcystin production by Microcystis aeruginosa PCC 7806. Appl. Environ. Microbiol. 73:6994-7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang, M. H., K. Ha, M. C. Lucas, G. J. Joo, and N. Takamura. 2004. Changes in microcystin production by Microcystis aeruginosa exposed to phytoplanktivorous and omnivorous fish. Aquat. Toxicol. 68:51-59. [DOI] [PubMed] [Google Scholar]
- 23.Jang, M. H., K. Ha, and N. Takamura. 2008. Microcystin production by Microcystis aeruginosa exposed to different stages of herbivorous zooplankton. Toxicon 51:882-889. [DOI] [PubMed] [Google Scholar]
- 24.Janse, I., W. E. A. Kardinaal, M. Kamst-van Agterveld, M. Meima, P. M. Visser, and G. Zwart. 2005. Contrasting microcystin production and cyanobacterial population dynamics in two Planktothrix-dominated freshwater lakes. Environ. Microbiol. 7:1514-1524. [DOI] [PubMed] [Google Scholar]
- 25.Janse, I., W. E. A. Kardinaal, M. Meima, J. Fastner, P. M. Visser, and G. Zwart. 2004. Toxic and nontoxic Microcystis colonies in natural populations can be differentiated on the basis of rRNA gene internal transcribed spacer diversity. Appl. Environ. Microbiol. 70:3979-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kardinaal, W. E. A., I. Janse, M. Kamst-van Agterveld, M. Meima, J. Snoek, L. R. Mur, J. Huisman, G. Zwart, and P. M. Visser. 2007. Microcystis genotype succession in relation to microcystin concentrations in freshwater lakes. Aquat. Microb. Ecol. 48:1-12. [Google Scholar]
- 27.Kurmayer, R., G. Christiansen, and I. Chorus. 2003. The abundance of microcystin-producing genotypes correlates positively with colony size in Microcystis sp. and determines its microcystin net production in Lake Wannsee. Appl. Environ. Microbiol. 69:787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurmayer, R., and T. Kutzenberger. 2003. Application of real-time PCR for quantification of microcystin genotypes in a population of the toxic cyanobacterium Microcystis sp. Appl. Environ. Microbiol. 69:6723-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latour, D., H. Giraudet, and M. J. Salençon. 2004. Sampling method of colonial cyanobacteria in lake. The case study of Microcystis aeruginosa on the reservoir of Grangent (Loire, France). C. R. Biol. 327:105-113. [DOI] [PubMed] [Google Scholar]
- 30.Latour, D., M. J. Salençon, J. L. Reyss, and H. Giraudet. 2007. Sedimentary imprint of Microcystis aeruginosa (Cyanobacteria) blooms in Grangent reservoir (Loire, France). J. Phycol. 43:417-425. [Google Scholar]
- 31.Long, B. M., G. J. Jones, and P. T. Orr. 2001. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 67:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.OECD. 1982. Eutrophication of waters. Monitoring, assessment and control. Final report. OECD Cooperative Programme on Monitoring of Inland Waters (Eutrophication Control), Environment Directorate, Organization for Economic Cooperation and Development, Paris, France.
- 32.Oh, H. M., S. J. Lee, M. H. Jang, and B. D. Yoon. 2000. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat. Appl. Environ. Microbiol. 66:176-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orr, P. T., and G. J. Jones. 1998. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 43:1604-1614. [Google Scholar]
- 34.Pan, X. J., F. Y. Chang, L. J. Kang, Y. D. Liu, G. B. Li, and D. H. Li. 2008. Effects of gibberellin A3 on growth and microcystin production in Microcystis aeruginosa (Cyanophyta). J. Plant Physiol. 165:1691-1697. [DOI] [PubMed] [Google Scholar]
- 35.Rapala, J., K. Sivonen, C. Lyra, and S. I. Niemelä. 1997. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds, C. S., and G. H. M. Jaworski. 1978. Enumeration of natural Microcystis populations. Br. Phycol. J. 13:269-277. [Google Scholar]
- 36a.Rinta-Kanto, J. M., E. A. Konopko, J. M. DeBruyn, R. A. Bourbonniere, G. L. Boyer, and S. W. Wilhelm. 2009. Lake Erie Microcystis: relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae 8:665-673. [Google Scholar]
- 37.Rivasseau, C., P. Racaud, A. Deguin, and M. C. Hennion. 1999. Development of a bioanalytical phosphatase inhibition test for the monitoring of microcystins in environmental water samples. Anal. Chim. Acta 394:243-257. [Google Scholar]
- 38.Sabart, M., D. Pobel, D. Latour, J. Robin, M. J. Salençon, and J. F. Humbert. 2009. Spatiotemporal changes in the genetic diversity in French bloom-forming populations of the toxic cyanobacterium, Microcystis aeruginosa. Environ. Microbiol. Rep. 1:263-272. [DOI] [PubMed] [Google Scholar]
- 39.Song, L. R., T. Sano, R. H. Li, M. Watanabe, Y. D. Liu, and K. Kaya. 1998. Microcystin production of Microcystis viridis (cyanobacteria) under different culture conditions. Phycol. Res. 46:19-23. [Google Scholar]
- 40.Tillett, D., E. Dittmann, M. Erhard, H. von Döhren, T. Börner, and B. A. Neilan. 2000. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem. Biol. 7:753-764. [DOI] [PubMed] [Google Scholar]
- 41.Tonk, L., D. B. van de Waal, P. Slot, J. Huisman, H. C. P. Matthijs, and P. M. Visser. 2008. Amino acid availability determines the ratio of microcystin variants in the cyanobacterium Planktothrix agardhii. FEMS Microbiol. Ecol. 65:383-390. [DOI] [PubMed] [Google Scholar]
- 42.Utkilen, H., and N. Gjølme. 1995. Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Environ. Microbiol. 61:797-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasconcelos, V. M., K. Sivonen, W. R. Evans, W. W. Carmichael, and M. Namikoshi. 1996. Hepatotoxic microcystin diversity in cyanobacterial blooms collected in Portuguese freshwaters. Water Res. 30:2377-2384. [Google Scholar]
- 44.Vezie, C., L. Brient, K. Sivonen, G. Bertru, J. C. Lefeuvre, and M. Salkinoja Salonen. 1997. Occurrence of microcystin-containing cyanobacterial blooms in freshwaters of Brittany (France). Arch. Hydrobiol. 139:401-413. [Google Scholar]
- 45.Wang, J. X., P. Xie, and N. Guo. 2007. Effects of nonylphenol on the growth and microcystin production of Microcystis strains. Environ. Res. 103:70-78. [DOI] [PubMed] [Google Scholar]
- 46.Wiedner, C., P. M. Visser, J. Fastner, J. S. Metcalf, G. A. Codd, and L. R. Mur. 2003. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 69:1475-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, H., P. Xie, Z. X. Ke, Y. Q. Liu, S. K. Wu, J. Xu, and L. G. Guo. 2006. The impact of planktivorous fishes on microcystin concentrations in Meiliang Bay, Lake Taihu, China. J. Freshw. Ecol. 21:721-723. [Google Scholar]
- 48.Yoshida, M., T. Yoshida, Y. Takashima, N. Hosoda, and S. Hiroishi. 2007. Dynamics of microcystin-producing and non-microcystin-producing Microcystis populations is correlated with nitrate concentration in a Japanese lake. FEMS Microbiol. Lett. 266:49-53. [DOI] [PubMed] [Google Scholar]