Abstract
The effect of dietary feeding of rice bran and phytic acid on the glucose metabolism in high fat-fed C57BL/6N mice fed was investigated. The mice were given with either a high fat diet only (HF group) or a high fat diet supplemented with rice bran (HF-RB group) or phytic acid (HF-PA group) for 7 weeks. The control mice (NC group) received a normal diet. At the end of the experimental period, the HF group exhibited substantially higher blood glucose level than the NC group. However, the HF-RB and HF-PA groups showed a marked decrease in the blood glucose level relative to HF mice. Furthermore, significantly higher glucokinase (GK) activity and lower phosphoenolpyruvate carboxykinase (PEPCK) activity were observed in HF-RB and HF-PA mice compared with that of the NC and HF ones. It was also found that the glucose-6-phosphatase (G6pase) activity and hepatic glycogen concentration were considerably higher in HF-RB and HF-PA groups, respectively, than that of the HF mice. These findings demonstrate that both rice bran and phytic acid could reduce the risk of high fat diet-induced hyperglycemia via regulation of hepatic glucose-regulating enzyme activities.
Keywords: rice bran, phytic acid, glucose metabolism, diabetes, high fat-fed mice
Introduction
Diabetes mellitus, a metabolic disease characterized by hyperglycemia and often associated with obesity, is one of the leading causes of death in most developed countries [1]. Its incidence has rapidly increased in epidemic proportions due to poor eating habit and sedentary lifestyle. Scientific studies have shown that chronic consumption of a high fat diet results in increased body weight and poor glucose regulation [2–4]. Individuals with higher intake of fat are more prone to develop glucose metabolism disorder, type 2 diabetes, or impaired glucose tolerance than those with lower fat intake [5]. A wide range of oral medicines are currently being used for treating diabetes, however, various side effects and high rates of secondary failures have been associated with the available anti-diabetic medicine [6]. Thus, finding natural drugs with hypoglycemic activity has become stronger and more urgent.
Rice bran, a by-product of rice milling industry and commonly used as animal feed, has been the subject of many researches for the past years due to its health-promoting phytochemicals that have strong antioxidant activities [7–9] and hypocholesterolemic effects [10, 11]. It also contains high amount of phytic acid (9.5–14%) [12], a dietary fiber component found in most grains and legumes which has been shown to have antioxidant and anticancer properties [13–15]. Recent researches also revealed that phytic acid has hypoglycemic and antihyperlipidemic effects in diabetic mice [16, 17].
The high-fat diet-fed C57BL/6 mouse model is widely used by researchers in investigating the pathophysiology of impaired glucose tolerance and type 2 diabetes for the development of new treatments [18–20]. While the in vitro antioxidant potential of rice bran and phtyic acid has been well documented, reports on their physiological functions in relation to glucose metabolism in animal models have been limited. Since oxidative stress is considered to be a key factor in the development of diabetes and its associated health disorders, the strong antioxidant activity of rice bran and phytic acid may be useful in preventing the development of diabetic hyperglycemia under a high fat diet condition. Hence, this study was conducted to investigate the effects of dietary feeding of rice bran and phytic acid on the glucose metabolism in high fat-fed C57BL/6N mice.
Materials and Methods
Animals and diet
Thirty-two male C57BL/6N mice of 4 weeks of age, weighing 12 g, were obtained from Orient Inc. (Seoul, Korea). They were individually housed in stainless steel cages in a room maintained at 25°C with 50% relative humidity and 12/12 h light/dark cycle and fed with a pelletized chow diet for 2 weeks after arrival. The mice were then randomly divided into 4 dietary groups (n = 8). The first and second groups were fed with a normal and high fat (17%, w/w) diets, respectively, while the other two groups were fed with high fat diet supplemented with either rice bran (RPC, Gimcheon, Korea) or phytic acid (Tsuno, Osaka, Japan). The composition of the experimental diet (Table 1) was based on the AIN-76 semisynthetic diet. The mice were fed for 7 weeks and allowed free access to food and water during the experimental period. The daily food intake of mice was constant (3 g/day) throughout the study. At the end of the experimental period, the mice were anaesthetized and sacrificed. Blood samples were collected and centrifuged at 1,000 × g for 15 min at 4°C to obtain the plasma. The livers were removed, rinsed with physiological saline and stored at −70°C until analysis.
Table 1.
Composition of the experimental diets (%)
| Component | Dietary group1 |
|||
|---|---|---|---|---|
| NC | HF | HF-RB | HF-PA | |
| Casein | 20 | 20 | 16.7 | 20 |
| DL-Methionine | 0.3 | 0.3 | 0.3 | 0.3 |
| Sucrose | 50 | 50 | 36.46 | 49.5 |
| Corn starch | 15 | |||
| Cellulose | 5 | 5 | 5 | |
| Corn oil | 5 | 3 | 3 | |
| Cholinbitartrate | 0.2 | 0.2 | 0.2 | 0.2 |
| Mineral mixture2 | 3.5 | 3.5 | 1.34 | 3.5 |
| Vitamin mixture3 | 1 | 1 | 1 | 1 |
| Lard | 17 | 14 | 17 | |
| Rice bran | 30 | |||
| Phytic acid | 0.5 | |||
| Total (%) | 100 | 100 | 100 | 100 |
1 NC, normal diet; HF, high fat diet; HF-RB, high fat diet + rice bran; HF-PA, high fat diet + phytic acid. 2 AIN-76 mineral mixture. 3 AIN-76 vitamin mixture.
Measurement of blood glucose level
The blood glucose level in mice was measured using Accu-Chek Active Blood Glucose Test Strips (Roche Diagnostics GmbH, Mannheim, Germany). Blood samples were drawn from the tail vein of the mice before and after 3 and 7 weeks of feeding the animals with experimental diets.
Determination of insulin and glycogen levels
The insulin content was measured using radioimmunoassay kits (TMB Mouse Insulin ELISA kit, Sibayagi, Gunma, Japan). The glycogen concentration in liver was determined using the method described by Seifter et al. [21]. Fresh liver (100 mg) was mixed with 30% KOH and heated at 100°C for 30 min. The mixture was then added with 1.5 mL ethanol (95%, v/v) and kept overnight at 4°C. The pellet was mixed with 4 ml distilled water. A 500 µL of the mixture was added with 0.2% anthrone (in 95% H2SO4) and the absorbance of the sample solution was measured at 620 nm. The results were calculated on the basis of a standard calibration curve of glucose.
Measurement of hepatic glucose-regulating enzyme activities
The hepatic enzyme source was prepared according to the method developed by Hulcher and Oleson [22]. Briefly, the liver tissue (0.3 g) was homogenized with 6 mL Tris buffer and the homogenates were then centrifuged at 600 × g for 10 min at 4°C. The supernatant was ultracentrifuged at 100,000 × g for 1 h at 4°C to isolate the cytosolic fraction for glucokinase (GK) and phosphoenolpyruvate carboxykinase (PEPCK) assay. The residue was mixed with 1 mL Tris buffer and re-ultracentrifuged at 100,000 × g for 1 h at 4°C. The supernatant was discarded and the residue was collected for the G6pase assay. The GK activity was determined based from the method of Davidson and Arion [23] with slight modification. A 0.98 mL of the reaction mixture containing 50 mM Hepes-NaOH (pH 7.4), 100 mM KCl, 7.5 mM MgCl2, 2.5 mM dithioerythritol, 10 mg/mL albumin, 10 mM glucose, 4 units of glucose-6-phosphate dehydrogenase, 50 mM NAD+ and 10 µL cytosol was pre-incubated at 37°C for 10 min. The reaction was initiated with the addition of 10 µL of 5 mM ATP and the mixture was incubated at 37°C for 10 min. The change in absorbance at 340 nm was recorded. The glucose-6-phosphatase (G6pase) activity was measured using the method described by Alegre et al. [24]. The reaction mixture contained 765 µL of 131.58 mM Hepes-NaOH (pH 6.5), 100 µL of 18 mM EDTA (pH 6.5), 100 µL of 265 mM glucose-6-phosphate, 10 µL of 0.2 M NADP+, 0.6 IU/mL mutarotase and 0.6 IU/mL glucose dehydrogenase. After pre-incubation at 37°C for 3 min, the mixture was added with 5 µL microsome and incubated at 37°C for 4 min. The change in absorbance at 340 nm was measured. The PEPCK activity was determined based from the method developed by Bentle and Lardy [25]. The reaction mixture consisted of 72.92 mM sodium Hepes (pH 7.0), 10 mM dithiothreitol, 500 mM NaHCO3, 10 mM MnCl2, 25 mM NADH, 100 mM IDP, 200 mM PEP, 7.2 unit of malic dehydrogenase and 10 µL cytosol. The enzyme activity was determined based from the decrease in the absorbance of the mixture at 350 nm at 25°C.
Statistical analysis
All data are presented as the mean ± SE. The data was evaluated by one-way ANOVA using a Statistical Package for Social Sciences software program (SPSS Inc., Chicago, IL) and the differences between the means were assessed using Duncan’s multiple range test. Statistical significance was considered at p<0.05.
Results
Body weight gain
Prior to feeding the mice with the experimental diets, all the animals exhibited similar body weights. However, at the end of the experimental period, a substantial increase in the body weight of HF mice was observed relative to that of the control group (data not shown). The HF-RB and HF-PA groups, on the other hand, showed considerably lower body weights than the HF group, suggesting that both rice bran and phytic acid could control the increase in body weight of mice under high fat diet condition.
Blood glucose levels
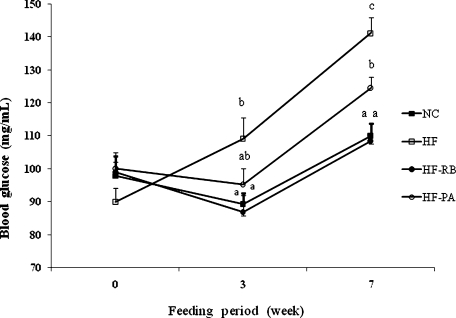
The initial blood glucose levels in mice prior to feeding with experimental diets did not significantly differ among the groups (Fig. 1). However, high-fat feeding resulted in a marked increase in the glucose level of mice after 3 and 7 weeks. On the final week, the HF-RB and HF-PA mice exhibited substantially lower glucose level compared with the HF group.
Fig. 1.
Effect of rice bran and phytic acid supplementation on the blood glucose level in high fat fed-mice. Means not sharing a common superscript are significantly different at p<0.05 (n = 8). NC, normal diet; HF, high fat diet; HF-RB, high fat diet + rice bran; HF-PA, high fat diet + phytic acid.
Insulin and glycogen levels
There was no significant difference in the insulin level among the mice groups (Table 2). Supplementation with rice bran in the diet did not significantly change the glycogen content in mice. On the other hand, the hepatic glycogen content was considerably higher in HF-PA group than that of the control and HF mice.
Table 2.
Insulin and glycogen concentrations1 in mice fed with high fat diet supplemented with rice bran and phytic acid
| Dietary group2 | Insulin (ng/mL) | Glycogen (mg/g liver) |
|---|---|---|
| NC | 1.58 ± 0.01a | 2.55 ± 0.16a |
| HF | 1.45 ± 0.01a | 3.14 ± 0.21a |
| HF-RB | 1.87 ± 0.02a | 3.91 ± 1.33ab |
| HF-PA | 2.41 ± 0.04a | 5.77 ± 0.92b |
1 Values are means ± SE (n = 8). Means in the same column not sharing a common superscript are significantly different at p<0.05, which pertains to the “a and b”, in the table. 2 NC, normal diet; HF, high fat diet; HF-RB, high fat diet + rice bran; HF-PA, high fat diet + phytic acid.
Hepatic glucose-regulating enzyme activities
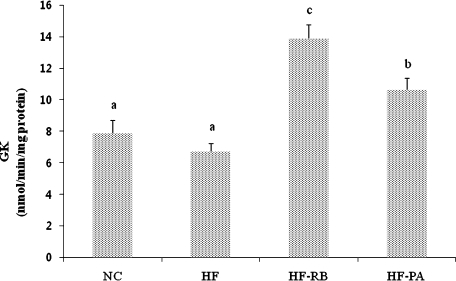
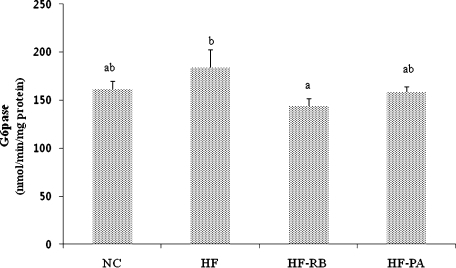
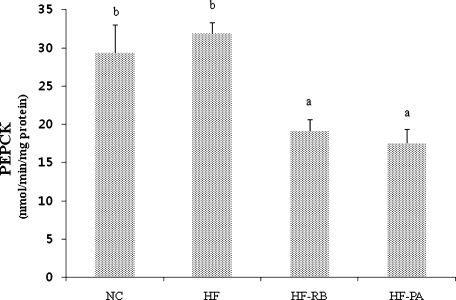
The hepatic GK enzyme activity was significantly higher in mice fed with rice bran and phytic acid than that of the control and HF-fed ones (Fig. 2). While high fat feeding did not significantly affect the activities of G6pase and PEPCK enzymes (Figs. 3 and 4), dietary feeding of rice bran suppressed the elevation of G6pase activity (Fig. 3). Moreover, both HF-RB and HF-PA groups exhibited significantly lower PEPCK activity than that of the NC and HF ones (Fig. 4).
Fig. 2.
Effect of rice bran and phytic acid supplementation on the hepatic GK enzyme activity in high fat fed-mice. Bars not sharing a common superscript are significantly different at p<0.05 (n = 8). NC, normal diet; HF, high fat diet; HF-RB, high fat diet + rice bran; HF-PA, high fat diet + phytic acid.
Fig. 3.
Effect of rice bran and phytic acid supplementation on the hepatic G6pase enzyme activity in high fat fed-mice. Bars not sharing a common superscript are significantly different at p<0.05 (n = 8). NC, normal diet; HF, high fat diet; HF-RB, high fat diet + rice bran; HF-PA, high fat diet + phytic acid.
Fig. 4.
Effect of rice bran and phytic acid supplementation on the hepatic PEPCK enzyme activity in high fat fed-mice. Bars not sharing a common superscript are significantly different at p<0.05 (n = 8). NC, normal diet; HF, high fat diet; HF-RB, high fat diet + rice bran; HF-PA, high fat diet + phytic acid.
Discussion
In vitro studies on the antioxidant capacity of rice bran and phytic acid revealed that these plant components have strong antioxidant activity [13, 26]. Since diabetes is a free radical-mediated disease, the effect of rice bran and phytic acid on the glucose metabolism in high fat-fed mice was investigated. In the present study, a high fat-diet resulted in a marked increase in the blood glucose level in mice. However, both rice bran and phytic acid were able to suppress the increase in glucose concentration. Previous investigations also showed that rice bran powder and its water soluble fraction could decrease serum glucose level in human subjects with diabetes [27, 28]. It was also found that the fiber components of rice bran isolated from watery extract exhibited hypoglycemic effect in mice with normal serum glucose [29]. Likewise, Lee et al. [17] reported that dietary feeding of phytic acid resulted in a significant reduction in the blood glucose levels in diabetic mice.
The antihyperglycemic action of rice bran and phytic acid is probably associated with the marked enhancement of GK activity and inhibition of G6pase and PEPCK in the liver. Hepatic GK enzyme plays a major role in the regulation of glucose homeostasis [30]. An increase in the expression of hepatic GK could cause an increase in the utilization of blood glucose for energy production or glycogen storage in the liver [31], thereby resulting in reduced blood glucose level. G6pase and PEPCK enzymes, on the other hand, regulate gluconeogenesis and glucose output in the liver [30, 32]. Hence, a decrease in G6pase and PEPCK activities signifies a decrease in hepatic glucose production. Although no significant difference was observed in the insulin levels among the groups, the results suggest that mice fed with rice bran and phytic acid had a tendency towards higher insulin levels than animals fed with high fat diet alone. An enhanced rate of glycogenesis was observed in HF-PA mice, as manifested by a significant increase in the hepatic glycogen concentration. The HF-RB group also showed a relatively higher glycogen content than HF and NC mice, although the difference is not statistically significant. The activities of hepatic glucose-regulating enzymes are reported to be partly regulated by insulin. High levels of insulin were shown to inhibit hepatic glucose production via stimulation of GK gene transcription and glycogen synthesis and inhibition of gluconeogenesis [31, 32].
A high fat diet negatively affects glucose metabolism and the regulation of blood glucose is essential in preventing the development of diabetes. Hyperglycemia promotes the formation of reactive oxygen species (ROS), which cause cellular damage [33]. The liver undergoes free radical-mediated injury in diabetes, and an increased ROS is related to the damage of hepatic glucose-regulating enzymes [34]. Antioxidants have long been recognized as a means of treating diabetes as they were shown to reduce blood glucose concentrations by protecting the cells against the toxic effects of ROS under hyperglycemic condition [35, 36]. Although the antioxidant potential of rice bran and phytic acid has been well documented, further studies are still needed to elucidate the underlying mechanism of the antihyperglycemic action of rice bran and phytic acid in order to have a better understanding of their therapeutic potential.
Conclusion
Results of this study demonstrate that rice bran and phytic acid could improve the blood glucose metabolism in high fat-fed C57BL/6N mice. The enhanced glucose metabolism may be partly due to the activation of GK, and inhibition of G6pase and PEPCK enzymes in the liver. The hypoglycemic effect of rice bran and phytic acid illustrates that these plant components may be beneficial for the treatment of diabetic hyperglycemia.
References
- 1.McGill M., Felton A.M. New global recommendations: a multidisciplinary approach to improving outcomes in diabetes. Prim. Care Diabetes. 2007;1:49–55. doi: 10.1016/j.pcd.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Alsaif M.A., Duwaihy M.M.S. Influence of dietary fat quantity and composition on glucose tolerance and insulin sensitivity in rats. Nutr. Res. 2004;24:417–425. [Google Scholar]
- 3.Messier C., Whately K., Liang J., Du L., Puissant D. The effects of high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in C57BL/6 mice. Behav. Brain Res. 2007;178:139–145. doi: 10.1016/j.bbr.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Petro A.E., Cotter J., Cooper D.A., Peters J.C., Surwit S.J., Surwit R.S. Fat carbohydrate and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53:454–457. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein A.H., Schwab U.S. Relationship of dietary fat to glucose metabolism. Atherosclerosis. 2000;150:227–243. doi: 10.1016/s0021-9150(99)00504-3. [DOI] [PubMed] [Google Scholar]
- 6.Inzucchi S.E. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. J. Am. Med. Assoc. 2002;287:360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 7.Chotimarkorn C., Benjakul S., Silalai N. Antioxidant components and properties of five long-grained rice bran extracts from commercial available cultivars in Thailand. Food Chem. 2008;111:636–641. [Google Scholar]
- 8.Devi R.R., Jayalekshmy A., Arumughan C. Antioxidant efficacy of phytochemical extracts from defatted rice bran in the bulk oil system. Food Chem. 2007;104:658–664. doi: 10.1016/j.fct.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal S., Bhanger M.I., Anwar F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005;93:265–272. [Google Scholar]
- 10.Kahlon T.S., Chow F.I., Sayre R.N. Cholesterol lowering properties of rice bran. Cereal Food World. 1994;39:99–103. [Google Scholar]
- 11.Revilla E., Santa Maria C., Miramontes E., Bautista J., Garcia-Martinez A., Cremades O., Cert R., Parrado J. Nutraceutical composition, antioxidant activity and hypocholesterolemic effect of water-soluble enzymatic extract from rice bran. Food Res. Int. 2009;42:387–393. [Google Scholar]
- 12.Martin-Tereso J., Gonzalez A., Laar H.V., Burbano C., Pedrosa M.M., Mulder K., den Hartog L.A., Verstegen M.W.A. In situ ruminal degradation of phytic acid in formaldehyde-treated rice bran. Animal Feed Sci. Technol. 2009;152:286–297. [Google Scholar]
- 13.Graf E., Eaton J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990;8:61–69. doi: 10.1016/0891-5849(90)90146-a. [DOI] [PubMed] [Google Scholar]
- 14.Shamsuddin A.M. Anti-cancer function of phytic acid. Int. J. Food Sci. Technol. 2002;37:769–782. [Google Scholar]
- 15.Shamsuddin A.M., Vucenik I. IP6 and inositol in cancer prevention and therapy. Curr. Cancer Ther. Rev. 2005;1:259–269. [Google Scholar]
- 16.Lee S.H., Park H.J., Cho S.Y., Jung H.J., Cho S.M., Cho Y.S., Lillehoj H.S. Effects of dietary phytic acid on serum and hepatic lipid levels in diabetic KK mice. Nutr. Res. 2005;25:869–876. [Google Scholar]
- 17.Lee S.H., Park H.J., Chun H.K., Cho S.Y., Cho S.M., Lillehoj H.S. Effects of dietary phytic acid on the blood glucose level in diabetic KK mice. Nutr. Res. 2006;26:474–479. [Google Scholar]
- 18.Schreyer S.A., Wilson D.L., LeBoeuf R.C. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis. 1998;136:17–24. doi: 10.1016/s0021-9150(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 19.Surwit R.S., Kuhn C.M., Cochrane C., McCubbin J.A., Feinglos M.N. Diet induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 20.Winzell M.S., Ahren B. The high fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53:S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 21.Seifter S., Dayton S., Navic B., Muntwyler E. The estimation of glycogen with the anthrone reagent. Arch. Biochem. 1950;25:191–200. [PubMed] [Google Scholar]
- 22.Hulcher F.H., Oleson W.H. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J. Lipid Res. 1973;14:625–631. [PubMed] [Google Scholar]
- 23.Davidson A.L., Arion W.J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch. Biochem. Biophys. 1987;253:156–167. doi: 10.1016/0003-9861(87)90648-5. [DOI] [PubMed] [Google Scholar]
- 24.Alegre M., Ciudad C.J., Fillat C., Guinovart J.J. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal. Biochem. 1988;173:185–189. doi: 10.1016/0003-2697(88)90176-5. [DOI] [PubMed] [Google Scholar]
- 25.Bentle L.A., Lardy H.A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J. Biol. Chem. 1976;251:2916–2921. [PubMed] [Google Scholar]
- 26.Lai P., Li K.Y., Lu S., Chen H.H. Phytochemicals and antioxidant properties of solvent extracts from Japonica rice bran. Food Chem. 2009;117:538–544. [Google Scholar]
- 27.Qureshi A.A., Sami S.A., Khan F.A. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. J. Nutr. Biochem. 2002;13:175–187. doi: 10.1016/s0955-2863(01)00211-x. [DOI] [PubMed] [Google Scholar]
- 28.Tazakori Z., Dehghan M.H., Iranparvar M., Zare M., Foladi N., Mohmmadi R. Effect of rice bran powder on blood glucose levels and serum lipid parameters in diabetes patient II. Res. J. Biol. Sci. 2007;2:252–255. [Google Scholar]
- 29.Hikino H., Takahashi M., Oshima Y., Konno C. Isolation and hypoglycemic activity of orizabrans A, B, C and D glycans of Oryza sativa bran. Planta Medica. 1988;54:1–3. doi: 10.1055/s-2006-962316. [DOI] [PubMed] [Google Scholar]
- 30.Akiyama S., Katsumata S., Suzuki K., Ishimi Y., Wu J., Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010;46:87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iynedjian P.B., Gjinovci A., Renold A.E. Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats. J. Biol. Chem. 1988;263:740–744. [PubMed] [Google Scholar]
- 32.Friedman J.E., Sun Y., Ishizuka T., Farrell C.J., McCormack S.E., Herron L.M., Hakimi P., Lechner P., Yun J.S. Phosphoenolpyruvate carboxykinase (GTP) gene transcription and hyperglycemia are regulated by glucocorticoids in genetically obese db/db transgenic mice. J. Biol. Chem. 1997;272:31475–31481. doi: 10.1074/jbc.272.50.31475. [DOI] [PubMed] [Google Scholar]
- 33.Hong J.H., Cha Y.S., Rhee S.J. Effects of the cellcultured Acanthopanax senticosus extract on antioxidative defense system and membrane fluidity in the liver of type 2 diabetes mouse. J. Clin. Biochem. Nutr. 2009;45:101–109. doi: 10.3164/jcbn.08-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lelli S.M., San L.C., Viale M.D., Mazzetti M.B. Response of glucose metabolism enzymes in an acute porphyria model role of reactive oxygen species. Toxicology. 2005;216:49–58. doi: 10.1016/j.tox.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Kaneto H., Kajimoto Y., Miyagawa J., Matsuoka T., Fujitani Y., Umayahara Y., Hanafusa T., Matsuzawa Y., Yamasaki Y., Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 36.Modak M., Dixit P., Londhe J., Ghaskadbi S., Devasagayam T.P.A. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007;40:163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]