Abstract
This study was performed to examine the effect of different fat sources, lard, sunflower oil (SO), and fish oil (FO) in high-fat and low-fat diet on reactive oxygen species generation by blood phagocytes, glutathione redox status in erythrocytes, and total plasma antioxidant ability in rats. Whole blood chemiluminescence (CL) did not differ between three low-fat fed groups. However, baseline and phorbol myristate acetate (PMA)-stimulated CL in blood of high-lard fed rats were lower than in low-lard and high-SO fed animals. Phagocyte-stimulated oxidative burst was higher in rats fed high-SO diet than in those fed low-SO and high-FO diets. The highest level of oxidize glutathione (GSSH), the lowest reduce glutathione (GSH)/GSSG ratio in erythrocytes, and the highest plasma activity to reduce ferric ions were observed in rats fed both diets contaning linoleic acid-rich sunflower oil compared to animals fed the corresponding energy from other fats. 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of plasma was lower in high-lard and high-FO fed rats compared to the corresponding low-fat diets, and the lowest in low-FO fed rats among low-fat fed animals. We presume from our results that linoleic acid may have dual effect, prooxidative in blood cells but maintaining total antioxidant plasma ability.
Keywords: high-fat diet, blood chemiluminescence, erythrocyte GSH/GSSG, FRAP, DPPH
Introduction
Besides fuel, dietary fats in mammals provide essential fatty acids for the membrane synthesis, protein and carbohydrate modifications, and signalling compounds [1]. However, a high-fat diet depends on its fatty acids composition which could cause various health problems. The effects of a high-fat diet on oxidative stress in response to different dietary fats have been studied extensively [2–4], and comprise of a wide range of the oxidative stress parameters. It has been reported that high dietary polyunsaturated fatty acids series 3 (n-3 PUFA) increased erythrocyte membrane susceptibility to peroxidation [4, 5] and lipid peroxide products in rat liver and kidney [6]. Further research has demonstrated that dietary supplementation with n-3 PUFA has no effect on erythrocyte membrane peroxidation [7], or has a positive effect on the glutathione level and antioxidant enzyme activity in blood [8]. The question remains whether there is increased susceptibility of the membranes to peroxidation, or an increased amount of the lipid peroxidation products resulted from enhanced reactive oxygen species (ROS) generated by blood phagocytes, or dysfunction of the antioxidant systems in blood, or both. Health concequences of a high saturated fatty acid dieting are investigated most frequently in relation to the lipid metabolism and mechanisms of hyperlipidemia which play a crucial role in arteriosclerosis, hypertension, and ultimately cardiovascular disease [9, 10]. The studies on the effect of a high saturated fatty acid diet on oxidative stress in particular tissues have shown different results. Zhang and his co-workes [11] have reported that a high lard diet increases NADPH-dependent ROS production in the rat cerebral cortex, and Ronis et al. [12] have demonstrated increased liver membrane resistance to oxidative stress in rats fed high-fat diet. However, tissue differences in response to particular dietary fats are also likely [13]. In this study we investigated effect of saturated fatty acid rich lard on oxidative-antioxidative status in peripheral blood cells and plasma in comparition to PUFA-rich diets.
Highly specialized erythrocytes (RBC), despite lacking mitochondria, are subject to free radical exposure due to the auto-oxidation of hemoglobin under high oxygen pressure in the arterial blood and abundant heme iron content. An increased intraerythrocytic ROS concentration results in erythrocytes’ membrane lipid peroxidation, acceleration of their senescence, and can cause damage to other intracellular protein [14]. Maintenance of the prooxidant-antioxidant balance in RBC is also important to other tissues since RBCs are the mobile detoxifying elements in the circulation [15], or when ROS diffuse out of them may be a reason of tissue microinjury [16]. Cellular glutathione (GSH) is non-protein thiol which together with associated enzymes forms the powerful antioxidant and detoxifying system. GSH is readily non-enzymatically oxidized to gluthatione disulfide (GSSG) in the presence of ROS, and the GSH/GSSG ratio is often used as an indicator of the cellular redox state [17]. Because dietary fats greatly modify erythrocyte membrane composition affecting their susceptibility to oxidation [4, 18], the GSH redox status in erythrocytes is an important parameter of oxidative stress. In this study we investigated the effects of different fats in low-fat and high-fat diets on ROS generation by blood phagocytes, the GSH/GSSG ratio in erythrocytes, and total plasma antioxidant capacity in rats.
Materials and Methods
Chemicals
Ethylenediaminetetraacetic acid (EDTA), phorbol 12-myristate 13-acetate (PMA), luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), 5-sulphosalicilic acid, 5,5'-dithiobis-2-nitrobenzoic acid (DTNB), glutathionee reductase (E.C.1.6.4.2.), β-nicotinamide adenine dinucleotide phosphate reduced form (NADPH), reduced glutathione (GSH), 2-vinylpiridyne, 2,4,6-tripyridyl-s-triazyne (TPTZ), 1,1-diphenyl-2-picrylhydrazyl (DPPH), acetonitryl were purchased from Sigma-Aldrich Chemical (Poznan, Poland). All chemicals were of the analytical grade purity.
Animals and diets
Male Wistar rats (150–160 g) were housed in plastic boxes in the controlled animal facility on a 12 h light/dark cycle. The protocol of the experiment was approved by the Government Ethical Committee for Animal Care. After acclimatization, the rats were randomized into six dietary groups. Three control groups were fed low-fat diets (10% energy from fat) prepared with lard (composed mostly with monosaturated and saturated fatty acid), sunflower oil (SO), and fish oil (FO), and three groups were fed high-fat diets (40% energy from fat) compose of the same fats. The fish oil diets were supplemented with soybean oil to 10% of total fat content in the diet, to maintain adequate intake of essential n-6 PUFA. The fatty-acid composition of fats used to prepare the diets was analyzed by gas-liquid chromatography or given by a supplier, and summarised in Table 1. Purified diets were prepared once a week and kept in daily rations in sealed bags at −20°C. Their composition, caloric density and compound suppliers are presented in Table 2. Diets and tap water were provided ad libitum daily at 15.00 h. Food intake was recorded daily and corrected for spillage (±0.1 g). After 6 weeks of feeding followed by overnight fasting, the rats were anesthetized with ketamine and xylazine (i.p. 20 and 10 mg/kg, respectively), blood was withdrawn by way of heart puncture, and then the animals were decapitated.
Table 1.
Fatty acid composition in dietary fats
| Fatty acids | Lard† |
Sunflower oil‡ |
Fish oil‡ |
|---|---|---|---|
| % by weight | |||
| Saturated fatty acids | 42.6 | 9.2 | 29.0 |
| Monounsaturated fatty acids | 50.3 | 30.5 | 26.0 |
| Polyunsaturated fatty acids | 6.5 | 60.1 | 33.3 |
| Linoleic acid (18:2n-6) | 6.3 | 59.5 | 1.3 |
| Arachidonic acid (20:4n-6) | — | — | 0.7 |
| α-Linolenic acid (18:3n-3) | 0.2 | 0.6 | 1.3 |
| EPA (20:5n-3) | — | — | 12.2 |
| DHA (22:5n-3) | — | — | 10.8 |
| Total n-6 PUFA | 6.3 | 59.5 | 2.6 |
| Total n-3 PUFA | 0.2 | 0.6 | 30.7 |
PUFA, polyunsturated fatty acids; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. † determined by gas liquid chromatography at the National Food and Nutrition Institute (Warsaw); ‡ given by supplier listed under Table 2.
Table 2.
Composition and energy density of the purified diets
| Low-fat diet |
High-fat diet |
|
|---|---|---|
| g/kg | ||
| Casein | 194.4 | 228.6 |
| Sucrose | 291.5 | 201.1 |
| Corn starch1 | 364.4 | 252.6 |
| Fat2 | 52.5 | 203.4 |
| Cellulose3 | 48.6 | 57.1 |
| AIN-93 Mineral Mix4 | 34.0 | 40.0 |
| AIN-93 Vitamin Mix4 | 9.7 | 11.4 |
| Choline bitartrate5 | 1.9 | 2.3 |
| L-Cystine5 | 2.9 | 3.4 |
| Energy density (kcal/g) | 3.92 | 4.62 |
1 Stobimyl XMH 042 (Stockmeier Food, Germany); 2 Lard, (Pamso Com. Inc, Pabianice, Poland), Menhaden Fish Oil (Omega Protein, Inc. Hammond, LA), Sunflower Oil (Fat Processing Com. Inc, Warsaw; Poland); 3 Arbocel® (J. Rettenmaier & Söhne Gmbh + Co Faserstoff-Werke, Rosenberg, Germany); 4 Research Diets, Inc (New Brunswick, NJ); 5 Sigma-Aldrich Ltd (Poznan, Poland).
Chemiluminescence assay
Luminol-dependent whole blood chemiluminescence (CL) was measured according to Kukovetz et al. [19] with some modifications [20]. Tubes with three microlitres of fresh blood, and 947 µl of the mixture solution were placed into the thermostatically controlled 1251 luminometer (Bio-Orbit®, Turku, Finland) and incubated at 37°C. After 30 min of incubation the resting CL (Baseline) was recorded for 1 min. Then 50 µl of phorbol-12-myristate-13-acetate (PMA) in sterile 0.9% NaCl was added by an automatic dispenser to a final concentration of 10−5 M and CL was measured continuously for 20 min. CL peak intensity (Peak) and total CL in response to PMA (Total) measured as the area under the CL intensity curve until returning to baseline were calculated by MultiUse v. 2.01 software. CL signal in all samples returned to a baseline between 6th to 9th minute of recording. The mixture solution containing 0.025% w/v luminol and 5% w/v glucose in deionized pyrogen-free water was freshly prepared. The experiment was run in triplicate. The baseline CL and CL Peak were expressed in arbitrary units corresponding to milivoltage potential (aU) per 104 leukocytes in the blood sample (aU/104 WBC). The Total CL was expressed in arbitrary unit × seconds per 104 WBC (aU × s/104 WBC).
Erythrocyte hemolysis
The hemoglobin concentration and hematocrit were measured using hematological apparatus (ABX Micros, Montpellier, France) calibrated for the rat blood. Packed red blood cells were washed three times with buffered saline solution, pH 7.4, hemolysed with 5 volumes of ice-cold distillated water, deproteinized with 10% 5-sulphosalicilic acid, and centrifuged at 4°C.
GSH and GSSG measurement
The Tietze’s GSH recycling method based on the sequential GSH oxidation by 5,5'-dithiobis-2-nitrobenzoic acid (DTNB), and reduction by the glutathione reductase in the presence of NADPH was applied [21]. The formation of 2-nitro-5-thiobenzoic acid was measured spectrophotometrically at 412 nm for 5 min. The total glutathione concentration was calculated on the basis of a calibration curve obtained with authentic GSH in range 1.5–50 µM. Briefly, the samples were added to 115 mM phosphate buffer, pH 7.4, containing EDTA (5 mM), NADPH (0.2 mM), and DTNB (0.6 mM). The reaction was initiated by addition of glutathione reductase (0.8 U/L). Oxidized glutathione was determined by the same method after derivatization of native GSH with 2-vinylpiridyne (185 mM) [22]. The lower detection limit for GSSG concentration was 0.1 µM. All measurements were performed in duplicate.
Ferric reducing ability of plasma (FRAP)
This method is based upon the reduction of ferric tripyridyltriazine Fe (III)-TPTZ to coloured ferrous tripyridyltriazine Fe (II)-TPTZ at low pH resulting in an increase in absorbance at 593 nm. This assay was performed according to Benzie and Strain [23]. The FRAP solution consisted of 300 mM acetate buffer, pH 3.6, 10 mM TPTZ in 40 mM HCl, and 20 mM FeCL3 in the ratio 10:1:1 was prepared freshly. The FRAP solution (900 µl) was pre-warmed to 37°C, next 50 µl of plasma and 50 µl of deionized water were added, and all reagents were incubated at 37°C for 20 min. Then, the absorbance was measured in spectrophotometer (Ultrospec III, Pharmacia LKB, Cambridge, England) in duplicate. The results were expressed in Fe (II) ions using the calibration curve prepared with the FRAP reagent containing Fe2SO4 in range 0.5–50 mM.
Plasma radical scavenging activity
DPPH test based on decomposition and discolouring of red stable 1,1-diphenyl-2-picrylhydrazyl radicals by a variety of plasma antioxidants acting as electron donors was assayed as Takao et al. [24]. Briefly, 970 µl of 50% methanol, 5 µl of 10 mM in methanol were combined and the control absorbance was measured at 520 nm (A520). The samples were incubated at 20°C for 3 min, then 25 µl of deproteinized with acetonitryl plasma was added, shaken, and decrease in the A520 related to DPPH decomposition over a 30-min incubation period was recorded. The DPPH radical-scavenging activity of the sample was measured in triplicate, and calculated according the formula: % radical scavenging activity = (the control absorption – the sample absorption/the control absorption) × 100.
Statistical analysis
Data are shown as means ± SEM. The ANOVA test followed by the Turkey post-hock test was used to compare the effect of high-fat diets. The Scheffe post-hock test was applied to compare the effect of the fat type and the t test was applied to compare the effect of the high-fat diet with the corresponding low-fat diet. Statistical significance was accepted at the p<0.05.
Results
Body mass gain and energy intake
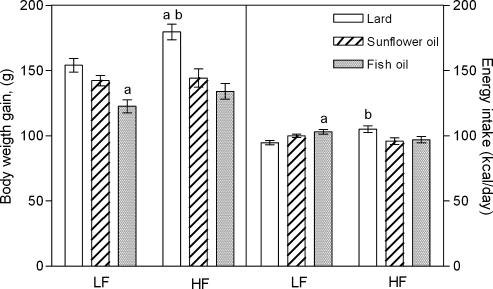
Fig. 1 presents the weight gain of rats fed low-fat and high-fat diets prepared with lard, sunflower oil (SO), and fish oil (FO), and their mean caloric intake during a six-week feeding period. Rats fed high-lard gained most weight compared to animals fed high-SO and high-FO (p<0.05 and p<0.01, respectively), and low-lard (p<0.05). They also consumed more energy from food than animals fed the other fats in high-fat diet (p<0.01). The weight gain of rats fed low-FO was significantly less at the end of the feeding period compared to rats fed the other low-fat diets (p<0.05), although they ate significantly more calories compared to low-lard fed rats (p<0.05). The final weight gain and mean energy intake of high-SO and low-SO fed rats were not significantly different from each other.
Fig. 1.
The body weight gain and mean daily caloric intake of rats fed for 6 weeks with low-fat (LF) and high-fat (HF) diets containing lard, sunflower oil, or fish oil. Values are expressed as mean ± SEM (n = 10 per dietary group). Letters depict significant difference p<0.05 as: a vs low-lard diet; b vs other HF diets.
Blood phagocyte chemiluminescence
Table 3 presents the luminol-dependent whole blood baseline CL, Peak CL intensity, and Total CL after PMA stimulation of blood phagocyte in rats fed the low-fat and high-fat diets containing different fats. The CL parameters did not differ significantly between rats fed the low-fat diets, although a tendency to increase baseline and PMA-evoked phagocyte oxidative burst in low-FO fed animals was observed. The baseline and PMA-stimulated phagocyte chemiluminescence were significantly lower in high-lard fed animals compared to low-lard (p<0.05), high-SO (p<0.01), and high-FO (p<0.05) fed rats. The Peak and Total CL of high-SO fed rats were 1.33- and 1.25-fold higher, respectively, than of animals fed low-SO (p<0.05), although there was no significant difference in the baseline blood CL between low-SO and high SO fed rats. Among rats fed the high-fat diets, the CL Peak intensity of blood phagocytes after PMA stimulation was significantly lower in high-FO than in high-SO fed animals (p<0.01).
Table 3.
The effect of 6-week feeding low-fat and high-fat diets containing lard, sunflower oil, or fish oil as a fat source on reactive oxygen species (ROS) generation by circulating blood phagocytes
| Whole blood chemiluminescence | Low-fat diet |
High-fat diet |
||||
|---|---|---|---|---|---|---|
| Lard | Sunflower oil | Fish oil | Lard | Sunflower oil | Fish oil | |
| Baseline [aU/104 WBC] | 1.81 ± 0.14 | 1.83 ± 0.17 | 2.08 ± 0.34 | 1.16 ± 0.08† | 2.12 ± 0.21‡ | 1.70 ± 0.19 |
| Peak [aU/104 WBC] | 3.21 ± 0.24 | 3.05 ± 0.19 | 4.23 ± 0.46 | 2.44 ± 0.09† | 4.26 ± 0.27†‡ | 3.52 ± 0.21‡§ |
| Total [aU × s/104 WBC] | 2267 ± 226 | 2139 ± 88 | 3034 ± 358 | 1706 ± 68† | 2838 ± 263†‡ | 2491 ± 132‡ |
Peak-chemiluminescence intensity (peak height in mV) after phorbol myristate acetate (PMA) addition; Total – area under the chemiluminescence intensity curve until returning to baseline after PMA addition (integrated value), aU – arbitrary units, WBC – white blood cells. Values are means of 5–7 samples; † p<0.05 vs the corresponding low-fat diet; ‡ p<0.01 vs high lard; § p<0.05 vs high sunflower oil.
Glutathione redox status in erythrocytes
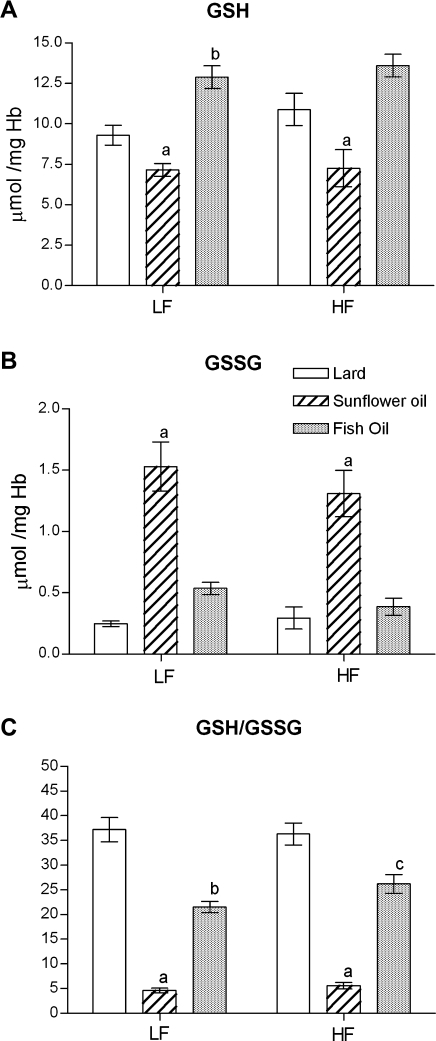
There were no significant differences in the erythrocyte GSH and GSSH levels between rats fed low-fat and high-fat diet prepared with the same fats, however there were significant differences in their concentrations between animals fed various fats in the same energy quantity in the diet (Fig. 2). The lowest GSH and the highest GSSH concentrations were observed in RBC of rats fed both low and high SO diets (p<0.001 vs low and high FO; p<0.01 vs low and high lard). Consequently, the GSH/GSSG ratio was the lowest in the erythrocytes of animals fed low and high SO (Fig. 2C). Although the reduced GSH level in the erythrocytes of low-FO fed rats was significantly higher than of low-lard fed animals (p<0.01; Fig. 2A), but the antioxidant status measured as the GSH/GSSG ratio was significantly lower in these rats compared to low- lard fed animals (p<0.01; Fig. 2C). The GSH/GSSG ratio was significantly higher in low and high-lard fed groups compared to animals fed the same energy with SO and FO in the diet (Fig. 2C).
Fig. 2.
The effect of the low-fat (LF) and high-fat (HF) diets containing lard, sunflower oil, or fish oil on the concentrations of (A) glutathione (GSH), (B) oxidized glutathione (GSSG), and (C) GSH/GSSG ratio in rat erythrocytes. Values are expressed as mean ± SEM (n = 5–8). Significant differences are as follows: a p<0.01 vs other LF or HF diets; b p<0.01 vs low-lard diet; c p<0.01 vs high-lard diet.
Total plasma antioxidant ability
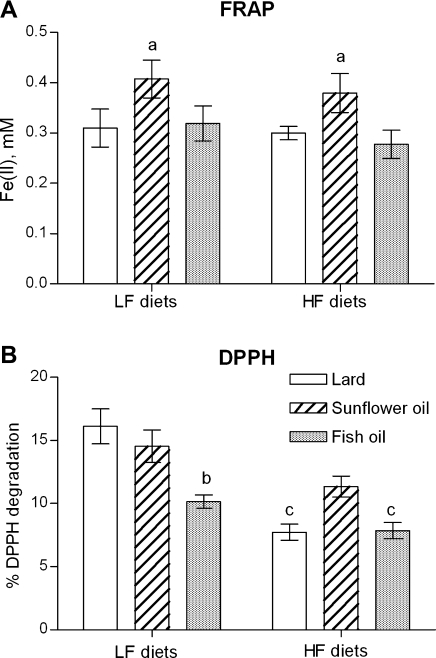
The total antioxidant plasma capacity was evaluated by two methods which gave slightly different results. Plasma potency to reduce Fe (III) in the FRAP test did no show any differences between animals fed high-fat and low-fat containing the same fat type. However, plasma of rats fed both low and high SO had the highest antioxidant capacity among groups fed the same energy with lard and FO (p<0.05; Fig. 3A). DPPH radical scavenging activity of plasma was respectively 2.08- and 1.29-times lower in rats fed high-lard and high-FO than in rats fed the corresponding low-fat diet (p<0.001 and p<0.05, respectively; Fig. 3B). In addition, deproteinized antioxidant plasma activity in the DPPH test was significantly lower in rats fed low-FO than in animals fed low-lard and low-SO (p<0.05; Fig. 3B). Only a tendency to increase plasma anti-DPPH activity between rats fed high-SO and those fed the other high-fat diets was noted.
Fig. 3.
The effect of the low-fat (LF), and high-fat (HF) diets containing lard, sunflower oil, or fish oil on (A) plasma ability to reduce Fe (III) in tripyridyltriazine complex (FRAP test), and (B) plasma capacity to scavenging 1,1-diphenyl-2-picrylhydrazyl radicals (DPPH test). Values are expressed as mean ± SEM (n = 5–10). A significant differences are as follows: a p<0.05 vs other LF or HF diets; b p<0.01 vs other LF diets; c p<0.05 vs the corresponding LF diet.
Discussion
Luminol-dependent CL is a sensitive tool for phagocyte oxidative burst determination [25]. In our study, the baseline and PMA-stimulated reactive species production by blood phagocytes, mainly due to the oxidant-generating enzyme NADPH oxidase, a leakage of ROS from the mitochondrial chain [19, 26], and to some extent by the xanthine-xanthine oxidase system [27] were significantly different between high-fat and low-fat fed rats depending on the fat source in the diet. The different effect of FO diet rich in n-3 PUFA and SO diet rich in n-6 PUFA on reactive species production by blood neutrophils in the present in vivo study is in accordance with the findings of in vitro studies. It has been found that n-3 PUFA suppressed the stimulated production of superoxide radicals by human neutrophils [28], and inhibited the NADPH oxidase-dependent superoxide radical production in the whole and disrupted neutrophils comparing to the effect of arachidonic acid [29]. Moreover, two-week pretreatment of rats with eicosapentaenoic fatty acid (C20:5n-3) resulted in attenuation of the pulmonary macrophage respiratory burst [30]. In contrast, n-6 PUFAs are able to directly promote NADPH oxidase assembly and activation [31, 32]. The main component of sunflower oil, linoleic acid (C18:2n-6), may also indirectly activate NADPH oxidise by protein kinase C stimulation [33, 34]. Serezani et al. [35] have demonstrated that linoleic acid stimulates neutrophil H2O2 release in a dose-dependent and NADPH oxidase-dependent manner. Further, they reported that linoleic acid-induced H2O2 generation depends on 5-lipooxigenase activity and leukotriene B4 synthesis. An augmentation of leukotriene B4 in rat leukocytes in response to the addition of sunflower oil to a basic fish oil diet has been reported also in the earlier study [36]. Our results confirmed in vivo that a high-SO diet causes greater blood phagocyte oxidative burst than an ingestion of high FO. It may suggest that a high quantity of linoleic acid in a diet can augment the risk of tissues microinjury by circulating neutrophils [37].
The mechanism of the oxidant-generated enzyme inhibition by saturated fatty acids is less easily recognized and understood. To our knowledge there are no studies which demonstrate a direct effect of saturated fatty acids on NADPH oxidase activity. However, it has been found that coconut oil-enriched diet in rats significantly reduced the generation of O2- in neutrophils, partially due to the reduction of glucose-6-phosphate dehydrogenase activity [38]. Furthermore, the carnitine palmitoyltransferase and cytochrome oxidase activities were found to be lower in brown adipose tissue of rats fed lard than of those fed high-PUFA [39]. It is also likely, that ingestion of high saturated fat decreased the saturated fatty acid to PUFA ratio in neutrophil membranes resulted in a decrease in oxidative species-generated enzyme activity in blood phagocytes.
The current determination of the erythrocyte GSH/GSSG ratio and total antioxidant capacity of plasma showed that an augmented phagocyte oxidative burst in high-SO fed rats did not cause parallel pro-oxidative changes in RBC and blood plasma. The lowest GSH/GSSG ratio showing up the intracellular thiol redox potential [17] was found in both low-SO and high-SO fed rats. However, plasma capability to reduce ferric ions was greater in these rats compared to plasma of rats fed lard and FO in the same quantity in the diet. The prominent PUFA component in cellular phospholipids is linoleic acid and its derivate arachidonic acid [40]. A high ingestion of sunflower oil causes an incorporation of linoleic acid into erythrocyte phosphatidylocholine in the highest amount [41]. Linoleic acid and arachidonic acid are well known activators of NADPH oxidase [42, 43], and may impair GSH/glutathione peroxidase and catalase-mediated catabolism of H2O2 in erythrocytes [14]. Moreover, Yuan and co-workers [44] reported fat type-dependent changes of antioxidant enzymes activity in RBC of Wistar Kyoto rats. Other tissues are also affected by a diet containing SO as a fat source. Ghosh et al. [45] demonstrated a very low level of GSH in the myocardium associated with myocardial necrosis in rats fed high-SO diet. All these findings suggest that a high ingestion of linoleic acid has profound pro-oxidative effects in different tissues and its incorporation into membrane phosphatidylocholine may be a good biomarker of a short-term antioxidant dietary intervention [41].
Our results suggest that high-fat diet containing sunflower oil causes oxidative stress in erythrocytes. This is consistent with recent study showing enhancement of systemic lipid peroxidation in rabbits fed with high amount of sunflower oil [46]. In addition, high DNA adducts level as markers for DNA-damage derived from lipid peroxidation products were found in the liver of sunflower oil fed rats [47]. Taking these into consideration it is difficult to explain why sunflower oil rich diet increased plasma antioxidant capacity. Total antioxidant plasma capacity was measured by two complementary methods, and evaluated various pools of antioxidants in plasma [48]. One of powerful plasma antioxidant, uric acid, did not reach the highest level in plasma of SO fed rats (data not shown). Perhaps, this diet could facilitate ingestion of some low molecular weight antioxidants e.g. vitamins, thus plasma antioxidant activity was raised. On the other hand, it cannot be excluded that elevated plasma antioxidant activity is an unspecific secondary effect to systemic oxidative stress phenomenon. However, the precise explanation of sunflower oil induced rise of plasma antioxidant ability requires further studies.
In summary, peripheral blood phagocytes produced less ROS in rats fed high-lard and plasma of these animals had lower ability to decompose DPPH radicals than rats fed high-SO. The erythrocyte glutathione redox status was the highest in rats fed the diet containing lard, and the lowest in rats fed SO. These findings indicate that a diet high in linoleic acid-rich sunflower oil causes a greater phagocyte oxidative burst than ingestion the same energy from saturated fatty acids and marine origin n-3 PUFA, augments oxidative stress in erythrocytes, and can lead to tissue microinjury. However, total antioxidant plasma ability in rats fed sunflower oil was higher than in rats fed equal energy from other fats.
Acknowledgment
We thank M. Kopytek for biochemical determinations, A. Sarniak A. Kliszko, Z. Sedzinska for technical assistance, and J. Ciechowicz for statistical analysis. This work was supported by the State Committee for Scientific Research, Poland (grant No. 214/P05/2002/23).
Abbreviations
- aU
arbitrary units of chemiluminescence
- CL
chemiluminescence
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- DTNB
5,5'-dithiobis-2-nitrobenzoic acid
- EDTA
ethylenediaminetetraacetic acid
- FRAP
ferric reducing ability of plasma
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- FO
fish oil
- NADPH
β-nicotinamide adenine dinucleotide phosphate reduced form
- PMA
phorbol 12-myristate 13-acetate
- PUFA
polyunsaturated fatty acids
- RBC
red blood cells
- ROS
reactive oxygen species
- SO
sunflower oil
- TPTZ
2,4,6-tripyridyl-s-triazyne
- WBC
white blood cells
References
- 1.Spector A.A. Essentiality of fatty acids. Lipids. 1999;34:S1–3. doi: 10.1007/BF02562220. [DOI] [PubMed] [Google Scholar]
- 2.Beltowski J., Wójcicka G., Górny D., Marciniak A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J. Physiol. Pharmacol. 2000;51:883–896. [PubMed] [Google Scholar]
- 3.Folmer V., Soares J.C.M., Gabriel D., Rocha J.B.T. A high fat diet inhibits δ-aminolevulinatedehydratase and increase lipid peroxidation in mice (Mus musculus) J. Nutr. 2003;133:2168–2170. doi: 10.1093/jn/133.7.2165. [DOI] [PubMed] [Google Scholar]
- 4.Palozza P., Sgarlata E., Luberto C.H., Piccioni E., Anti M., Marra G., Armeloo F., Franceschelli P., Bartoli G.M. n-3 Fatty acids induce oxidative modifications in human erythrocytes depending on dose and duration of dietary supplementation. Am. J. Clin. Nutr. 1996;64:297–304. doi: 10.1093/ajcn/64.3.297. [DOI] [PubMed] [Google Scholar]
- 5.van den Berg J.J., de Fouw N.J., Kuypers F.A., Roelofsen B., Houtsmuller U.M., Op den Kamp J.A. Increased n-3 polyunsaturated fatty acid content of red blood cells from fish oil-fed rabbits increases in vitro lipid peroxidation, but decreases hemolysis. Free Radic. Biol. Med. 1991;11:393–399. doi: 10.1016/0891-5849(91)90156-w. [DOI] [PubMed] [Google Scholar]
- 6.Sekine S., Kubo K., Tadokoro T., Saito M. Effect of docosahexaenoic acid ingestion on temporal change in urinary excretion of mercapturic acid in ODS rats. J. Clin. Biochem. Nutr. 2007;41:184–190. doi: 10.3164/jcbn.2007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ando K., Nagata K., Beppu M., Kikugawa K., Kawabata T., Hasegawa K., Suzuki M. Effect of n-3 fatty acid supplementation on lipid peroxidation and protein aggregation in rat erythrocyte membranes. Lipids. 1998;33:505–511. doi: 10.1007/s11745-998-0234-6. [DOI] [PubMed] [Google Scholar]
- 8.Hsu H.C., Lee Y.T., Chen M.F. Effects of fish oil and vitamin E on the antioxidant defense system in diet-induced hypercholesterolemic rabbits. Prostaglandins Other Lipid Mediat. 2001;66:99–108. doi: 10.1016/s0090-6980(01)00146-0. [DOI] [PubMed] [Google Scholar]
- 9.Yang R.L., Shi Y.H., Li W., Le G.W. Increasing oxidative stress with progressive hyperlipidemia in human: relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008;43:154–158. doi: 10.3164/jcbn.2008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popovic T., Ranic M., Bulajic P., Milicevic M., Arsic A., Vucic V., Glibetic M. Effects of n-3 fatty acids supplementation on plasma phospholipids fatty acid composition in patients with obstructive jaundice—a pilot study. J. Clin. Biochem. Nutr. 2009;45:370–375. doi: 10.3164/jcbn.09-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Dong F., Ren J., Driscoll M.J., Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp. Neurol. 2004;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Ronis M.J.J., Korourian S., Zipperman M., Hakkak R., Badger T.M. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J. Nutr. 2004;134:904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 13.Lin M.H., Lu S.C., Huang P.C., Liu Y.C., Liu S.Y. A high-cholesterol, n-3 polyunsaturated fatty acid diet causes different response in rats and hamsters. Ann. Nutr. Metab. 2005;49:386–391. doi: 10.1159/000088891. [DOI] [PubMed] [Google Scholar]
- 14.Cimen M.Y. Free radical metabolism in human erythrocytes. Clin. Chim. Acta. 2007;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Brown J.M., Grosso M.A., Terada L.S., Beehler C.J., Toth K.M., Whitman G.J., Harken A.H., Repine J.E. Erythrocytes decrease myocardial hydrogen peroxide levels and reperfusion injury. Am. J. Physiol. 1989;256:H584–588. doi: 10.1152/ajpheart.1989.256.2.H584. [DOI] [PubMed] [Google Scholar]
- 16.Johnson R.M., Goyette G. Jr., Ravindranath Y., Ho Y.S. Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic. Biol. Med. 2005;39:1407–1417. doi: 10.1016/j.freeradbiomed.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 18.Oarada M., Furukawa H., Majima T., Miyazawa T. Fish oil diet affects on oxidative senescence of red blood cells linked to degeneration of spleen cells in mice. Biochem. Biophys. Acta. 2000;1487:1–14. doi: 10.1016/s1388-1981(00)00076-7. [DOI] [PubMed] [Google Scholar]
- 19.Kukovetz E.M., Bratschitsch G., Hofer H.P., Egger G., Schaur R.J. Influence of age on the release of reactive oxygen species by phagocytes as measured by a whole blood chemiluminescence assay. Free Radic. Biol. Med. 1997;22:433–438. doi: 10.1016/s0891-5849(96)00334-6. [DOI] [PubMed] [Google Scholar]
- 20.Szkudlarek U., Luczynska M., Kasielski M., Kaucka S., Nowak D. Exhaled hydrogen peroxide correlates with the release of reactive oxygen species by blood phagocytes in healthy subjects. Respir. Med. 2003;97:718–725. doi: 10.1053/rmed.2003.1506. [DOI] [PubMed] [Google Scholar]
- 21.Tietze F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione, applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 22.Griffith O.W. Determination of glutathionee and glutathionee disulfide using glutathione reductase and 2-vinylopyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 23.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 24.Takao T., Kitatani F., Watanabe N., Yagi A., Sakata K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci. Biotechnol. Biochem. 1994;58:1780–1783. [Google Scholar]
- 25.Lojek A., Ciz M., Marnila P., Duskova M., Lilius E.M. Measurement of whole blood phagocyte chemiluminescence in the Wistar rat. Biolumin. Chemilumin. 1997;12:225–231. doi: 10.1002/(SICI)1099-1271(199709/10)12:5<225::AID-BIO448>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 26.Miesel R., Murphy M.P., Kroger H. Enhanced mitochondrial radical production in patients which rheumatoid arthritis correlates with elevated levels of tumor necrosis factor alpha in plasma. Free Radic. Res. 1996;25:161–169. doi: 10.3109/10715769609149921. [DOI] [PubMed] [Google Scholar]
- 27.Nunes F.A., Kumar C., Chance B., Brass C.A. Chemiluminescent measurement of increased free radical formation after ischemia/reperfusion Mechanisms of free radical formation in the liver. Dig. Dis. Sci. 1995;40:1045–1053. doi: 10.1007/BF02064197. [DOI] [PubMed] [Google Scholar]
- 28.Heine J., Scheinichen D., Jaeger K., Andre M., Leuwer M. In vitro influence of parenteral lipid emulsions on the respiratory burst of neutrophils. Nutrition. 1999;15:540–545. doi: 10.1016/s0899-9007(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 29.Schneider S.M., Fung V.S., Palmblad J., Babior B.M. Activity of the leukocyte NADPH oxidase in whole neutrophils and cell-free neutrophil preparations stimulated with long-chain polyunsaturated fatty acids. Inflammation. 2001;25:17–23. doi: 10.1023/a:1007019510569. [DOI] [PubMed] [Google Scholar]
- 30.Sharif S., Broman M., Babcock T., Ong E., Jho D., Rudnicki M., Helton W.S., Espat N.J. A priori dietary omega-3 lipid supplementation results in local pancreatic macrophage and pulmonary inflammatory response attenuation in a model of experimental acute edematous pancreatitis. J. Parenter. Entera. Nutr. 2006;30:271–276. doi: 10.1177/0148607106030004271. [DOI] [PubMed] [Google Scholar]
- 31.Rubinek T., Levy R. Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes. Biochim. Biophys. Acta. 1993;1176:51–58. doi: 10.1016/0167-4889(93)90176-p. [DOI] [PubMed] [Google Scholar]
- 32.Sellmayer A., Obermeier H., Danesch U., Aepfelbacher M., Weber P.C. Arachidonic acid increases activation of NADPH oxidase in monocytic U937 cells by accelerated translocation of p47-phox and costimulationof protein kinase C. Cell Signal. 1996;8:397–402. doi: 10.1016/0898-6568(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 33.Holian O., Nelson R. Action of long-chain fatty acids on protein kinase C activity, comparison of omega-6 and omega-3 fatty acids. Anticancer Res. 1992;12:975–980. [PubMed] [Google Scholar]
- 34.Nixon J.B., McPhail L.C. Protein kinase C (PKC) isoforms translocate to Triton-insoluble fractions in stimulated human neutrophils, correlation of conventional PKC with activation of NADPH oxidase. J. Immunol. 1999;16:4574–4582. [PubMed] [Google Scholar]
- 35.Serezani C.H., Aronoff D.M., Jancar S., Peters-Golden M. Leukotriene B4 mediates p47phox phosphorylation and membrane translocation in polyunsaturated fatty acid-stimulated neutrophils. J. Leukoc. Biol. 2005;78:976–984. doi: 10.1189/jlb.1004587. [DOI] [PubMed] [Google Scholar]
- 36.James M.J., Cleland L.G., Gibson R.A., Hawkes J.S. Interaction between fish and vegetable oils in relation to rat leukocyte leukotriene production. J. Nutr. 1991;121:631–637. doi: 10.1093/jn/121.5.631. [DOI] [PubMed] [Google Scholar]
- 37.Murphy P.G., Myers D.S., Webster N.R., Jones J.G., Davis M.J. Direct detection of free radical generation in an in vivo model of acute lung injury. Free Radic. Res. Commun. 1991;15:167–176. doi: 10.3109/10715769109049137. [DOI] [PubMed] [Google Scholar]
- 38.Lopes L.R., Laurindo F.R., Mancini-Filho J., Curi R., Sannomiya P. NADPH-oxidase activity and lipid peroxidation in neutrophils from rats fed fat-rich diets. Cell Biochem. Funct. 1999;17:57–64. doi: 10.1002/(SICI)1099-0844(199903)17:1<57::AID-CBF811>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi H., Matsuo T., Tokuyama K., Suzuki M. Effect of dietary fat type on beta-oxidation of brown adipose tissue and Na+ channel density of brain nerve membrane in rats. J. Nutr. Sci. Vitaminol. 1996;42:161–166. doi: 10.3177/jnsv.42.161. [DOI] [PubMed] [Google Scholar]
- 40.Salem N., Litman B., Kim H.Y., Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 41.Skeaff C.M., Hodson L., McKenzie J.E. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J. Nutr. 2006;136:565–569. doi: 10.1093/jn/136.3.565. [DOI] [PubMed] [Google Scholar]
- 42.Hartfield P.J., Robinson J.M. Arachidonic acid activates NADPH oxidase by a direct, calmodulin-regulated mechanism. Prostaglandins Other Lipid Mediat. 1998;56:1–6. doi: 10.1016/s0090-6980(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 43.Mollapour E., Linch D.C., Roberts P.J. Activation and priming of neutrophil nicotinamide adenine dinucleotide phosphate oxidase and phospholipase A(2) are dissociated by inhibitors of the kinases p42(ERK2) and p38(SAPK) and by methyl arachidonyl fluorophosphonate, the dual inhibitor of cytosolic and calcium-independent phospholipase A(2) Blood. 2001;97:2469–2477. doi: 10.1182/blood.v97.8.2469. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Y.V., Kitts D.D., Godin D.V. Variations in dietary fat and cholesterol intakes modify antioxidant status of SHR and WKY rats. J. Nutr. 1998;128:16020–16030. doi: 10.1093/jn/128.10.1620. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh S., Kewalramani G., Yuen G., Pulinilkunnil T., An D., Innis S.M., Allard M.F., Wambolt R.B., Qi D., Abrahani A., Rodrigues B. Induction of mitochondrial nitrative damage and cardiac dysfunction by chronic provision of dietary omega-6 polyunsaturated fatty acids. Free Radic. Biol. Med. 2006;41:1413–1424. doi: 10.1016/j.freeradbiomed.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Sabitha P., Vasudevan D.M., Kamath P. Effect of high fat diet without cholesterol supplementation on oxidative stress and lipid peroxidation in New Zealand white rabbits. J. Atheroscler. Thromb. 2010;17:213–218. doi: 10.5551/jat.2667. [DOI] [PubMed] [Google Scholar]
- 47.Eder E., Wacker M., Lutz U., Nair J., Fang X., Bartsch H., Beland F.A., Schlatter J., Lutz W.K. Oxidative stress related DNA adducts in the liver of female rats fed with sunflower-, rapeseed-, olive- or coconut oil supplemented diets. Chem. Biol. Interact. 2006;159:81–98. doi: 10.1016/j.cbi.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Janaszewska A., Bartosz G. Assay of total antioxidant capacity, comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Invest. 2007;62:231–236. doi: 10.1080/003655102317475498. [DOI] [PubMed] [Google Scholar]