Abstract
We investigated the energy expenditure in hospitalized patients with severe or moderate ulcerative colitis (UC), and compared them to healthy controls. Thirteen patients (5 women and 8 men; mean age 31.8 years; mean BMI 19.0 kg/m2) and 10 healthy volunteers were enrolled in this study. The resting energy expenditure (mREE) levels were determined by indirect calorimetry. The mREEs of the UC patients were significantly higher than those of healthy controls (26.4 ± 3.6 vs 21.8 ± 1.7 kcal/kg/day), although the mREEs of the UC patients were almost the same as the predicted REEs (pREEs) calculated by the Harris-Benedict equation (26.4 ± 2.4 kcal/kg/day vs 26.5 ± 2.6 kcal/kg/day). The mREE/pREE ratio, which reflects stress, was 1.0 ± 0.15. In the UC patients, a significant correlation was observed between the mREEs and the clinical activity index. In conclusion, UC patients showed a hyper-metabolic status as evaluated by their mREE/body weight. Energy expenditure was significantly correlated with disease activity. From our observations, we recommend that nutritional management with more than 30–35 kcal/ideal body weight/day (calculated by the mREE × activity factor) may be optimal for active severe or moderate ulcerative colitis.
Keywords: ulcerative colitis, resting energy expenditure, indirect calorimetry
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the digestive tract of unknown etiology [1, 2]. Typical symptoms include abdominal pain, bloody diarrhea, weight loss and fever. Patients with UC have various nutrition deficiencies or metabolic disturbances, although the prevalence of nutritional deficiencies or metabolic disturbances is lower than in patients with Crohn’s disease (CD) [3, 4].
Nutritional support is essential for severe or moderate UC patients, as well as for CD patients. Corticosteroids and immunosuppressive drugs are effective in UC patients, but nutritional therapy such as total parenteral nutrition (TPN) is integral to the management of UC patients. The aims of nutritional support in inflammatory bowel disease (IBD) are to treat or to prevent nutritional deficits, to reduce disease activity and the need for surgery, and to maintain remission. An evaluation of the energy expenditure of UC patients is critical in planning optimal nutritional therapy [5]. Typically, the total energy of parenteral nutrition or enteral nutrition is determined by using the predicted resting expenditure (pREE) calculated by the Harris-Benedict equation [6], and the total energy requirement is calculated by pREE × activity factor × stress factor [7]. On the other hand, the mREE can be determined by indirect calorimetry.
There have been several studies reporting that the energy expenditure of CD patients changes to a hyper-metabolic status [8–12]. Recently, we have also reported the energy expenditure of Japanese CD patients [13]. However, there are a few reports on energy metabolism in adult UC patients [4, 14, 15]. In this study, we evaluated the energy metabolism of hospitalized UC patients with moderate to severe clinical activity, and determined the optimal energy requirements for nutritional management.
Subjects and Methods
Patients
Thirteen UC patients with moderate to severe clinical activity (5 women and 8 men, median age 31.8 years old) and 10 healthy volunteers were enrolled in this study. The patients were admitted to the Gastroenterology Unit of Shiga University of Medical Science Hospital. The ethics committee of the Shiga University of Medical Science approved this study. All patients had their diagnosis of UC established by endoscopic, histologic, and clinical criteria. All patients were defined as the total colitis type, and their clinical activity index [16] was is 9.8 ± 3.5. Nine patients were receiving total parenteral nutrition (TPN), and four patients were receiving peripheral parenteral nutrition (PPN). Prednisone (30–80 mg/day) was taken by all patients. Cyclosporine therapy [16, 17] had just been started in 6 patients, and leukocytapheresis therapy [18] had been started in one patient.
Indirect calorimetry
The mREEs and respiratory quotients (RQ) were measured by computed open-circuit indirect calorimetry (AE-300S; Minato Medical Science Co., Osaka, Japan) [13]. Indirect calorimetry (IC) was performed in the hospital room on the morning after a 10-h overnight fast in healthy controls. However, the infusion of parenteral nutrition was maintained in the UC patients. Period flow and gas calibration were performed prior to all measurements. After resting for a minimum of 30 min, the patients were assessed in a supine position with a facemask. A pump drew ambient air through a facemask at a constant rate. After equilibrium was reached for 10 min, respiratory exchange was performed continuously over 30 min. The mREE and RQ data were obtained every minute.
The mREE was calculated from the oxygen consumption (VO2) and carbon dioxide production (VCO2) by the Weir equation [19]:
| mREE = (3.94 × VO2 + 1.11 × VCO2) × 1.44 |
Measurement of the non protein RQ was calculated as RQ = VCO2/VO2. The measured mREE was then compared with the pREE (predicted resting energy expenditure] calculated by the Harris and Benedict equation.:
| Men: pREE = 66.47 + 13.75 × W [weight (kg)] + 5.0 × H [height (cm)] − 6.75 × A [age (yrs)]. |
| Women: pREE = 665.09 + 9.56 × W [weight (kg)] + 1.84 × H [height (cm)] − 4.67 × A [age (yrs)]. |
Statistical analyses
Differences between the groups were analyzed with Kruskal-Wallis tests. A p value <0.05 was considered to be statistically significant. Correlations were investigated with Spearman rank correlation tests.
Results
The patients did not differ significantly from the controls in their age or height, but had significantly lower body weights (p<0.01) and lower BMIs (p<0.001) (Table 1).
Table 1.
Background of the UC patients and healthy controls
| UC patients | controls | p value | |
|---|---|---|---|
| Patients number | 13 | 10 | |
| Female/male | 5/8 | 3/7 | |
| Age (y) | 31.8 ± 11.7 | 41.2 ± 18.8 | 0.155 |
| Height (cm) | 168.4 ± 6.6 | 165.5 ± 8.3 | 0.360 |
| Body weight (kg) | 53.5 ± 7.6 | 64.5 ± 11.4 | 0.009 |
| BMI (kg/mm2) | 18.8 ± 2.4 | 23.5 ± 2.3 | <0.001 |
BMI, body mass index.
The average hemoglobin, red blood cell count, white blood cell count and platelet counts in the UC patients were 10.0 ± 2.1 g/dl, 371.0 ± 81.3 × 104/mm3, 9980 ± 4453/mm2, 33.8 ± 19.5 × 104/mm3 respectively. The serum albumin and total-cholesterol levels were 2.97 ± 0.47 g/dl and 139.0 ± 51.6 mg/dl, respectively.
The mREE in the UC patients determined by indirect calorimetry was 1412.5 ± 272.0 kcal/day, and the mREE in the healthy controls was 1402.7 ± 242.0 kcal/day, respectively. Although the mREE in the UC patients was almost the same as in the controls (Table 2), the mREE/body weight of the UC patients (26.4 ± 3.6 kcal/day) was significantly higher than that of the controls (21.8 ± 1.7 kcal/day) (Table 2).
Table 2.
pREE, mREE, and RQ of UC patients and healthy controls
| UC patients | controls | p value | |
|---|---|---|---|
| pREE (kcal/day) | 1407.0 ± 154.1 | 1484.7 ± 233.0 | 0.347 |
| mREE (kcal/day) | 1412.5 ± 272.0 | 1402.7 ± 242.0 | 0.930 |
| mREE/pREE (%) | 100.2 ± 14.8 | 94.8 ± 10.2 | 0.328 |
| pREE/body weight (kcal/kg/day) | 26.5 ± 2.6 | 23.1 ± 2.4 | 0.003 |
| mREE/body weight (kcal/kg/day) | 26.4 ± 3.6 | 21.8 ± 1.7 | 0.001 |
| RQ | 0.92 ± 0.12 | 0.83 ± 0.07 | 0.049 |
pREE, predicted resting energy expenditure; mREE, resting energy expenditure measured by indirect calorimetry; RQ, respiratory quotient.
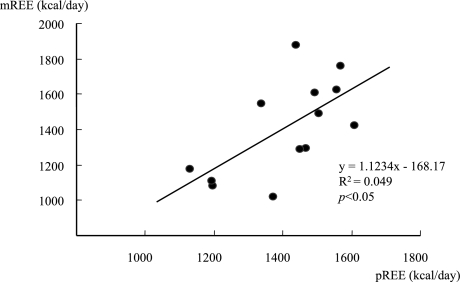
In UC patients, the pREE calculated by the Harris-Benedict equation was 1407.0 ± 154.0 kcal/day, and there was a positive correlation between the pREE and mREE (p<0.05) (Fig. 1). Similar observations were noted in the controls (p<0.01). The mREE/pREE ratio in the UC patients (1.00 ± 0.15) was higher than in the controls (0.94 ± 0.10) (Table 2), but not significant.
Fig. 1.
Correlation between the measured resting energy expenditure (mREE) and the predicted resting energy expenditure (pREE). The mREE was measured by indirect calorimetry, and the pREE was calculated by the Harris-Benedict equation. There was a positive correlation between the mREE and pREE in UC patients.
The RQ of these patients measured by indirect calorimetry was 0.92 ± 0.12, and this was significantly higher than the controls. There was no significant correlation between the RQ and mREE. There were no significant correlations between the mREE and BMI, or between the RQ and BMI in the UC patients.
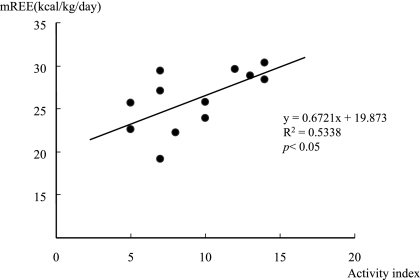
As shown in Fig. 2, there was a significant positive correlation between the clinical activity index and the mREE/body weight ratio. However, there was no significant correlation between the CRP and mREE/body weight ratio.
Fig. 2.
Correlation between the mREE and disease activity index in UC patients (n = 13). The mREE in UC patients exhibited a positive correlation with the disease activity index.
Discussion
This is the first report on the resting energy expenditure in Japanese patients with UC. This study showed that the mREE measured by IC in hospitalized UC patients was significantly higher than in healthy controls, indicating a hyper-metabolic status of the active UC patients. Recently, we examined the energy metabolism in Japanese CD patients by indirect calorimetry, and showed that the mREE of CD patients was 24.4 ± 2.4 kcal/body weight/day [13]. These combined observations suggest that energy metabolism in UC patients was increased to a much greater extent than in CD patients.
The resting energy expenditure (pREE), calculated by the Harris-Benedict equation, has been widely used to evaluate the energy status of patients [5]. The total energy requirement is calculated by pREE × activity factor × stress factor [6]. Theoretically, the pREE is expected to be equal to the mREE in healthy humans, and the mREE/pREE ratio is a marker for a hyper-metabolic status [12]. From our results, the mREE/pREE in the healthy controls and UC patients were 94.8 ± 10.2% and 100.2 ± 14.8%, respectively. The stress factor in patients with IBD is reported to be 1.1–1.3, similar to patients with cancer or chronic obstructive pulmonary disease. This adjustment may result in an actual energy requirement increase of approximately 45 kcal/kg/day. Energy requirements are often lower than the predicted requirements if the patients are not very lean with a low fat mass [4]. Our study suggests that 30–35 kcal/ideal body weight/day may be optimal for severe or moderate hospitalized UC patients, and suggests that the calculation by pREE × active factor × 1.1–1.3 as a stress factor may be over the energy requirement for active UC patients. High-energy intakes during TPN therapies also have the risk of overfeeding.
In this study, we showed that disease activity affected the energy expenditure in adult UC patients. Proinflammatory cytokines such as interferon-γ or tumor necrosis factor-α have been reported to have significant effects on energy metabolism in patients with systemic inflammatory diseases, including IBD [20–24]. There was no significant correlation between the mREE and CRP in our study. Previously, Wiskin et al. reported that there was no significant correlation between mREE and CRP in children with inflammatory bowel disease [25], and our present result confirms their report. The disease activity score and CRP sometimes do not move in parallel in active UC patients [26, 27]. Osada et al. reported that clinical symptoms reflected the activity of distal colon, whereas CRP reflected the activity of proximal colon [28]. In this study, there was no significant relationship between activity index and CRP in severe or moderate UC patients.
Previously, it has been reported that the RQ is lowered in active IBD patients [12, 15]. A reduced RQ means that fat, but not carbohydrate, is mainly utilized as the fuel substrate, which resembles a starvation pattern. However, the RQ in active UC was 0.92 ± 0.12, and was significantly higher than that in the healthy controls. This unexpected result can be explained by continuing parenteral nutrition during the measurements by indirect calorimetry, whereas the healthy controls were examined after overnight fasting. To determine the nutritional substrate metabolism in active UC patients, further studies are necessary in subjects who had not started parenteral nutrition or steroid therapy.
In conclusion, moderate to severe UC patients have a hyper-metabolic status, and there is a significant positive relationship between their resting energy expenditure and disease activity. From our results, the daily energy requirements for Japanese patients with moderate to severe UC are recommended as 30–35 kcal/ideal body weight.
References
- 1.Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart D.C., Carding S.R. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 3.Han P.D., Burke A., Baldassano R.N., Rombeau J.L., Lichtenstein G.R. Nutrition and inflammatory bowel disease. Gatroenterol. Clin. North Am. 1999;28:423–443. doi: 10.1016/s0889-8553(05)70063-7. [DOI] [PubMed] [Google Scholar]
- 4.Hrabovský V., Zadák Z., Bláha V., Hyspler R., Karlík T., Martínek A., Mendlová A. Cholesterol metabolism in active Crohn’s disease. Wien. Klin. Wochenschr. 2009;121:270–275. doi: 10.1007/s00508-009-1150-6. [DOI] [PubMed] [Google Scholar]
- 5.Barot L.R., Rombeau J.L., Steinberg J.J., Crosby L.O., Feurer I.D., Mullen J.L. Energy expenditure in patients with inflammatory bowel disease. Ann. Surg. 1981;116:460–462. doi: 10.1001/archsurg.1981.01380160070014. [DOI] [PubMed] [Google Scholar]
- 6.Harris J.A., Benedict F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. U.S.A. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long C.L., Schaffel N., Geiger J.W., Schiller W.R., Blakemore W.S. Metabolic response to injury and illness: Estimation of energy and protein needs from indirect calorimetry and nitrogen balance. J. Parenteral. Entr. Nutr. 1979;3:452–456. doi: 10.1177/014860717900300609. [DOI] [PubMed] [Google Scholar]
- 8.Chan A.T., Fleming C.R., O’Fallon W.M., Huizenga K.A. Estimated versus measured basal energy requirement in patients with Crohn’s disease. Gastroenterology. 1986;91:75–78. doi: 10.1016/0016-5085(86)90441-5. [DOI] [PubMed] [Google Scholar]
- 9.Kushner R.F., Schoeller D.A. Resting and total energy expenditure in patients with inflammatory bowel disease. Am. J. Clin. Nutr. 1991;53:161–165. doi: 10.1093/ajcn/53.1.161. [DOI] [PubMed] [Google Scholar]
- 10.Rigaud D., Cerf M., Angel Alberto L., Sobhani I., Mignon M. Increase of resting energy expenditure during flare-ups in Crohn’s disease. Gatroenterol. Clin. Biol. 1993;17:932–937. [PubMed] [Google Scholar]
- 11.AI-Janouni R., Hebuterne X., Pouget I., Rampal P. Energy metabolism and substrate oxidation in patients with Crohn’s disease. Nutrition. 2000;16:173–178. doi: 10.1016/s0899-9007(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 12.Schneeweiss B., Lochs H., Zauner C., Fischer M., Wyatt J., Maier-Dobersberger T., Schneider B. Energy and substrate metabolism in patients with active Crohn’s disease. J. Nutr. 1999;129:844–848. doi: 10.1093/jn/129.4.844. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki M., Johtatsu T., Kurihara M., Iwakawa H., Tanaka T., Tsujikawa T., Bamba S., Andoh A., Fujiyama Y. Energy metabolism in Japanese patients with Crohn’s disease. J. Clin. Biochem. Nutr. 2010;46:68–72. doi: 10.3164/jcbn.09-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barot L.R., Rombeau J.L., Feurer I.D., Mullen J.L. Caloric requirement in patients with inflammatory bowel disease. Ann. Surg. 1982;195:214–218. doi: 10.1097/00000658-198202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capristo E., Gaetano A.D., Mingrone G., Addolorato G., Greco A.V., Castagneto M., Gasbarrini G. Multivaliate identification of metabolic features in inflammatory bowel disease. Metabolism. 1999;48:952–956. doi: 10.1016/s0026-0495(99)90188-9. [DOI] [PubMed] [Google Scholar]
- 16.Lichitiger S., Present D.H., Kornbluth A., Gelernt I., Bauer J., Galler G., Michelassi F., Hanauer S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994;330:1841–1845. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 17.Durai D., Hawthorne A.B. Review article: how and when to use ciclosporin in ulcerative colitis. Aliment. Pharmacol. Ther. 2005;22:907–916. doi: 10.1111/j.1365-2036.2005.02680.x. [DOI] [PubMed] [Google Scholar]
- 18.Sawada K., Kusugami K., Suzuki Y., Bamba T., Munakata A., Hibi T., Shimoyama T. Leukocytapheresis in ulcerative colitis: results of a multicenter double-blind prospective case-control study with sham apheresis as placebo treatment. Am. J. Gastroenterol. 2005;100:1362–1369. doi: 10.1111/j.1572-0241.2005.41089.x. [DOI] [PubMed] [Google Scholar]
- 19.Weir J.B.V. New methods for calculating metabolic rate with special reference to protein matabolism. J. Physiol. 1949;109:254–259. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Xun K., Chen L., Wang Y. TNF-alpha. A potent lipid metabolism regulator. Cell Biohem. 2009;27:407–416. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]
- 21.McNamara M.J., Alexander H.R., Norton J.A. Cytokines and their role in the pathophysiology of cancer cachaxia. JPEN J. Parenter. Enteral. Nutr. 1992;16:50S–55S. doi: 10.1177/014860719201600603. [DOI] [PubMed] [Google Scholar]
- 22.Tisdale M.J. Cancer cachexia: metabolic alterations and clinical manifestations. Nutrition. 1997;13:1–7. doi: 10.1016/s0899-9007(96)00313-9. [DOI] [PubMed] [Google Scholar]
- 23.Bonville D.A., Parker T.S., Levine D.M., Gordon B.R., Hydo L.J., Eachempati S.R., Borie P.S. The relationship of hypocholesterolemia to cytokine concentrations and mortality in critically ill patients with sustemic inflammatory response syndrome. Surg. Infect. 2004;5:39–49. doi: 10.1089/109629604773860291. [DOI] [PubMed] [Google Scholar]
- 24.McLean S.J., Rombeau J.L. Cytokines and inflammatory bowel disease: a review. J. Parenter. Enteral. Nutr. 1998;23:20–24. doi: 10.1177/014860719902300506. [DOI] [PubMed] [Google Scholar]
- 25.Wiskin A.E., Wootton S.A., Culliford D.J., Afzal N.A., Jackson A.A., Beattie R.M. Impact of disease activity on resting energy expenditure in children with inflammatory bowel disease. Clin. Nutr. 2009;28:652–656. doi: 10.1016/j.clnu.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Schoepfer A.M., Beglinger C., Straumann A., Trummler M., Renzulli P., Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm. Bowel. Dis. 2009;15:1851–1858. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 27.Moscandrew M.E., Loftus E.V. Diagnostic advances in inflammatory bowel disease (imaging and laboratory) Curr. Gastroenterol. Rep. 2009;11:488–495. doi: 10.1007/s11894-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 28.Osada T., Ohkusa T., Okayasu I., Yoshida T., Hirai S., Beppu K., Shibuya T., Sakamoto N., Kobayashi O., Nagahara A., Terai T., Watanabe S. Correlations among total colonoscopic findings, clinical symptoms, and laboratory markers in ulcerative colitis. J. Gastroenterol. Hepatol. 2008;23 Suppl 2:S262–267. doi: 10.1111/j.1440-1746.2008.05413.x. [DOI] [PubMed] [Google Scholar]