Abstract
There is limited data about the mucosal lesions of portal hypertensive enteropathy (PHE) detected by capsule endoscopy, and there is no scoring system to evaluate their severity. Our aim is to create a reliable scoring system for PHE, and to explore the possible usefulness of using transient elastograhy (TE) in that field. We compared the medical records of 31 patients with liver cirrhosis and portal hypertension with 29 control patients. We found that the mucosal lesions compatible with PHE were significantly more common in cirrhotic patients than in control patients (67.7% vs 6.9%, p<0.001). Cirrhotic patients with high TE score (p = 0.018), high Child-Pugh grade, large esophageal varices (EV), portal hypertensive gastropathy, and history of endoscopic variceal injection sclerotherapy or ligation (EIS/EVL) were significantly associated with PHE. Using our scoring system, we found that patients with higher TE score (p = 0.004), high Child-Pugh score (p = 0.011), larger EV (p = 0.006), and prior EIS/EVL (p = 0.006) were significantly associated with higher PHE score. We concluded that using our scoring system might be helpful in grading PHE severity, and TE might be a new non-invasive method for detecting the presence and severity of PHE in cirrhotic patients.
Keywords: capsule endoscopy, portal hypertensive enteropathy, scoring system, transient elastography
Introduction
Patients with liver cirrhosis exhibit portal hypertension (PHT), which causes various pathological changes in the entire gastrointestinal tract (from esophagus to anus) [1]. In the recent past, the term portal hypertensive intestinal vasculopathy has been used to explain the effects of PHT on the entire bowel and includes portal hypertensive gastropathy (PHG), enteropathy, and colonopathy [2]. Among these pathological lesions, esophageal varices (EV), PHG, and portal hypertensive colonopathy represent common sources of bleeding, and they are usually diagnosed and treated by esophagogastroduodenoscopy (EGD) and colonoscopy [2, 3].
Portal hypertensive gastropathy is a well-established cause of bleeding in cirrhotic patients, present in up to 98% of patients with PHT [4], and accounts for 25.8% of acute bleeding episodes [5]. Similarly, portal hypertensive colonopathy has been known and described [3, 6, 7]. Although it is found in up to 70% of patients with PHT and is more frequent in patients with evidence of esophageal varices or PHG [8], it seems to be a rare cause of bleeding [6, 7].
The recognized existence of PHG and portal hypertensive colonopathy suggests that the small intestine might also show mucosal changes related to PHT, which is called portal hypertensive enteropathy (PHE). In fact, in 1989, Thiruvengadam and Gostout reported three patients with blood loss, who had diffuse erythema and scattered petechia not only in the stomach but also in the duodenum and jejunum [8].
The small intestine constitutes the largest part of the gut. However, little is known about the endoscopic features of the small bowel in different diseases, especially liver cirrhosis [9]. Our limited knowledge about small intestine is attributed to the limitations of conventional endoscopy because only small parts of it can be examined by EGD or colonoscopy [10]. Nowadays, completely the small intestine is easily explored with the advent of new endoscopic methods, such as capsule endoscopy (CE) [6, 11]. Capsule endoscopy discovered also many diseases in small intestine [12, 13].
FibroScan® apparatus
Transient elastography is a novel, ultrasound based technology that involves acquisition of pulse-echo ultrasound signals to measure liver stiffness using FibroScan apparatus [14]. In brief, the tip of an ultrasound transducer probe is placed between ribs over the right lobe of the liver. The probe transmits a low-amplitude (vibration and frequency) signal to the liver, which in turn induces an elastic shear wave that propagates through liver tissue. The pulse-echo ultrasound allows measurement of wave velocity, expressed in kilopascals, a measure of liver stiffness. Normal liver stiffness is reported to be in the range of 4–6 kilopascals (kPa) [15]. Transient elastography is associated with attractive features beyond the fact that it is noninvasive. Most important, it assesses a relatively large sample-across an area of 1–2 cm of the liver, estimated to be some 100 times greater than a liver biopsy specimen is. Additionally, transient elastography allows multiple readings to be taken (from slightly different areas, thereby providing data on an even larger sample). This is critical because sampling error associated with liver biopsy [16] is likely due to the small portion of the liver sampled [17].
Measurement of the hepatic venous pressure gradient (HVPG) is currently considered the golden standard for the evaluation of PHT [18]. Nevertheless, HVPG measurement is invasive, relatively expensive, difficult, time consuming, and available only in major centers. Many recent studies proposed the use of liver stiffness measurement (LSM) using TE for the detection of liver cirrhosis and the prediction of its related complications, including the presence of EV and variceal bleeding [19, 20]. Vizzutte et al. proposed that liver stiffness measurement by using TE may represent a non-invasive, rapid, cheap, and easy method for the prediction of clinically significant (HVPG ≥10 mm Hg) and severe portal hypertension (HVPG ≥12 mm Hg) with a cut-off value of LSM ≥13.6 kPa and ≥17.6 kPa, respectively [21].
To our knowledge, there are limited prospective studies in which CE was used to assess the frequency, features, and scoring system of the mucosal lesions of PHE. In addition, there are no prospective trials that have studied the association between the TE score, and the presence/severity of mucosal lesions of PHE detected by CE to declare whether or not TE has a clinical impact in that field.
The purpose of this study was to create a reliable scoring system for the small bowel mucosal findings of PHE in order to evaluate its severity, and to explore the clinical impact of using transient elastography in that field.
Materials and Methods
Patients
This is a non-randomised, controlled, prospective study of a cohort of 31 patients with documented liver cirrhosis and PHT. They were admitted to our hospital from June 2008 to October 2009. Written informed consent was obtained from all the patients after a precise explanation of the nature and purpose of our study. The ethics committee of our hospital approved the study.
Inclusion criteria were evidence of liver cirrhosis and PHT. Patients with a prior history of endoscopic variceal injection sclerotherapy (EIS) or ligation (EVL) were also included. Exclusion criteria were the following: recent history or current intake of medications which affect the degree of PHT (such as diuretics and beta-blockers), and the intestinal mucosa (such as non-steroidal anti-inflammatory drugs), presence of renal or cardiac impairment, and patients with enteritis from another cause, such as Crohn’s disease.
The control arm consisted of 29 patients who were selected from our hospital’s patients with obscure gastrointestinal bleeding that were diagnosed as; bleeding duodenal ulcer (two patients), colonic angioectasia (one patient), colonic ulcer (one patient), and colonic diverticulosis (25 patients). The indication of CE was to exclude any other bleeding points in the small intestine. All control patients had no clinical, laboratory, and radiological evidence of liver cirrhosis and/or PHT.
Liver cirrhosis was diagnosed by the history of chronic liver disease, clinical features, laboratory and typical radiological and/or histological data of liver cirrhosis. Portal hypertension was diagnosed by the endoscopic evidence of esophageal and/or gastric varices and/or TE score ≥13.6 kPa [21].
Severity of liver cirrhosis was graded according to Child-Pugh score [22]. A high Child Pugh score was considered if it was more than grade 6.
Transient elastography
Transient elastography was performed using the FibroScan® apparatus (Echosens, Paris, France). The operator was a staff physician who had previously performed at least 100 determinations in patients with chronic liver disease. The median value of 10 successful acquisitions was kept as representative of TE score of the patients [23].
Endoscopic findings
Esophageal and gastric varices were graded by using the standard criteria proposed by the Japanese Society for Portal Hypertension [24]. The form (F) of varices was classified as follows: straight small-calibred varices (F1); moderately enlarged, beady varices (F2); or markedly enlarged, nodular, or tumor-shaped varices (F3). Esophageal varices (F2) with signs of impending bleeding, such as the red-color sign or telangiectasia, and/ or those with form F3 were considered large EV.
Portal hypertensive gastropathy was diagnosed by the recognition of elementary lesions, such as a mosaic-like pattern, red point lesions, cherry-red spots, or black-brown spots [25].
Capsule endoscopy findings and scoring system
Capsule endoscopy was performed within one month after performing EGD. A Pill Cam SB (Given Imaging, Yoqneam, Israel) and RAPID system software version 5 were used. The CE digital image stream was reviewed and interpreted by two staff endoscopists blinded to the clinical and endoscopic information. Esophageal varices PHG (as detected by CE) were not considered in this study.
Our scoring system of PHE depends on the classification of the mucosal lesions of small bowel (SB) into four main types; 1—red spots, 2—angioectasias, 3—SB varices, and 4—inflammatory-like lesions. The first three types comprised the vascular lesions of PHE. Each of these four lesions was worth two points if it was multiple (more than two lesions) and only one point if it was not. We calculated the positive points for every patient to make a final PHE score out of a maximum of eight points.
Study Design
Data collected on each patient included age, sex, underlying hepatic pathology, liver function tests, blood count, Child-Pugh class and grade, TE score, prior history of EIS and/or EVL, and prior history of variceal bleeding.
We compared the prevalence and characteristics of SB mucosal lesions between the cirrhotic and control patients.
Cirrhotic patients with and those without PHE were compared to determine whether the PHE findings were significantly associated with TE score and the other clinical characteristics.
We explored the relationship between our scoring system and the clinical outcome of the patients by studying the statistical relationship between the PHE score and all the clinical and endoscopic data of patients, including the TE score.
Statistical analysis
The data is shown as mean ± SD. Comparisons were performed using the student t test and the chi-square test. Correlations between continuous data were done using Pearson’s correlation. Differences were considered statistically significant when the p value was equal to or less than 0.05. Statistical analysis was performed using statistical software (SPSS, version 16; SPSS Inc, Chicago, IL).
Results
During the study period, 31 cirrhotic patients with PHT (19 females, and 12 males, mean age of all patients was 70.8 ± 8 years), and 29 control patients (9 females, and 20 males, mean age of all patients was 68.9 ± 11 years) were enrolled in the study and were evaluated by CE. All patients completed the CE procedure uneventfully. No complications occurred, and the exit of the capsule was confirmed in all the cases. The clinical, and endoscopic characteristics of cirrhotic and control patients are listed in Table 1.
Table 1.
Clinical, and endoscopic characteristics of cirrhotic and control patients
| Characteristic | Cirrhotic Patients (n = 31) | Control Patients (n = 29) | p value |
|---|---|---|---|
| Prior variceal bleeding, n (%) | 5 (16.1%) | NA | |
| Prior EIS/EVL, n (%) | 8 (25.8%) | NA | |
| Underlying hepatic pathology, n (%): | |||
| HCV | 8 (25.8%) | NA | |
| HCC | 20 (64.5%) | ||
| Others | 3 (9.7%) | ||
| Child-Pugh class, n (%): | |||
| A | 20 (64.5%) | NA | |
| B | 10 (32.3%) | ||
| C | 1 (3.2%) | ||
| TE score, mean ± SD | 26 ± 12.4 | 6.8 ± 1.4 | <0.001* |
| EGD findings: | |||
| Esophageal varices, n (%) | |||
| Presence | 26 (83.9%) | NA | |
| Large EV | 8 (25.8%) | ||
| Gastric varices, n (%) | 16 (51.6%) | ||
| PHG, n (%) | 20 (64.5%) | ||
| Capsule endoscopic findings: | |||
| Prevalence, n (%) | 21 (67.7%) | 2 (6.9%) | <0.001* |
| SB mucosal lesions; n (%) | |||
| 1—Red spots | 17 (54.8%) | 1 (3.4%) | <0.001* |
| 2—Angioectasias | 16 (51.6%) | 0 | <0.001* |
| 3—Inflammatory like lesions | 13 (41.9%) | 1 (3.4%) | <0.001* |
| 4—Varices | 5 (16.1%) | 0 | 0.024* |
* p<0.05 was considered statistically significant. EIS, endoscopic injection sclerotherapthy; EVL, endoscopic variceal ligation; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; TE, transient elastography; EGD, esophagogastroduodenoscopy; EV, esophageal varices; PHG, portal hypertensive gastropathy; SB, small bowel; NA, non applicable.
Transient elastography score was significantly different between cirrhotic patients and control patients (p<0.001).
Capsule endoscopic findings
The mucosal findings detected by the CE in both cirrhotic and control patients are listed in Table 1 (Figs. 1, 2). Twenty-one of the cirrhotic patients (67.7%) were found to have CE signs of PHE; nineteen patients (61.3%) of them had diffuse PHE, and twenty patients (64.5%) had more than one lesion. Active bleeding was seen during endoscopic examination in only one case (3.2%) and was submitted to double balloon endoscopy. On the other side, the mucosal findings were present only in two of the control patients (67.7% vs 6.9%, p<0.001). None of these patients revealed diffuse lesions (61.3% vs 0, p<0.001), and none of them had more than one lesion (64.5% vs 0, p<0.001).
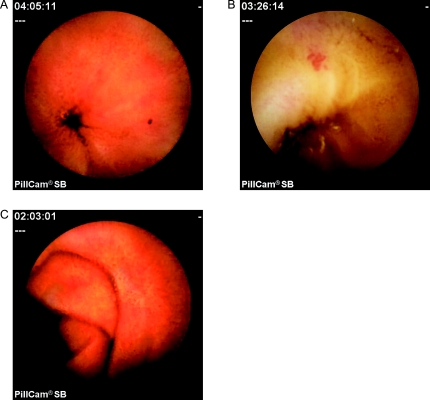
Fig. 1.
Capsule endoscopic views of the vascular lesions of portal hypertensive enteropathy. A: Red spot. B: Angioectasia. C: Serpiginous small bowel varix.
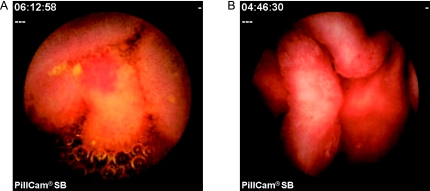
Fig. 2.
Capsule endoscopic views of the inflammatory lesions of portal hypertensive enteropathy. A: Erythema. B: Edematous villi.
The association between PHE and clinical characteristics of the patients
A comparison of cirrhotic patients with and those without PHE is shown in Table 2. Higher TE score (p = 0.018), high Child-Pugh score (p = 0.041), larger EV (p = 0.023), PHG (p = 0.049), and prior EIS/EVL (p = 0.023) were all significantly associated with PHE. However, there was no difference between these two groups of patients with regard to hemoglobin level, hematocrit value, and prior history of variceal bleeding.
Table 2.
Comparison of cirrhotic patients with and those without portal hypertensive enteropathy
| Variable | with PHE (n = 21) | without PHE (n = 10) | p value |
|---|---|---|---|
| Laboratory findings; mean ± SD | |||
| Haemoglobin, g/dl | 11.2 ± 2.1 | 11.1 ± 1.1 | 0.913 |
| Haematocrit value, % | 32.6 ± 5.3 | 29.4 ± 10.6 | 0.27 |
| High Child-Pugh score, n (%) | 10 (47.6%) | 1 (10%) | 0.041* |
| TE score, kPa; mean ± SD | 29 ± 12.6 | 18 ± 7.8 | 0.018* |
| Endoscopic findings; | |||
| Large EV, n (%) | 8 (38.1%) | 0 (0%) | 0.023* |
| Gastric varices, n (%) | 11 (52.4%) | 5 (50%) | 0.901 |
| PHG, n (%) | 16 (76.2%) | 4 (40%) | 0.049* |
| Prior variceal bleeding, n (%) | 5 (23.8%) | 0 | 0.092 |
| Prior EVL /EIS, n (%) | 8 (38.1%) | 0 | 0.023* |
* p<0.05 was considered statistically significant. PHE, portal hypertensive enteropathy; TE, transient elastography; kPa, kilopascal; EV, esophageal varices; PHG, portal hypertensive gastropathy; EIS, endoscopic injection sclerotherapthy; EVL, endoscopic variceal ligation.
The association between PHE score and clinical characteristics of the patients
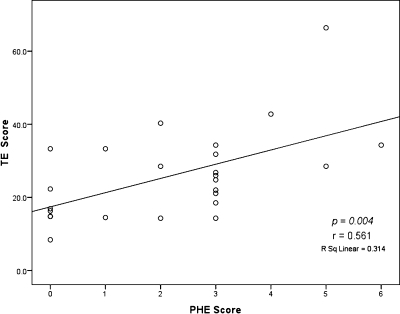
Using our scoring system, we sought to evaluate the relationship between the score of PHE findings and the clinical parameters in the cirrhotic patients to determine whether the PHE score was associated with liver disease severity (Child-Pugh score), and the endoscopic findings of EGD (Table 3). As a result, we found that cirrhotic patients with a high Child-Pugh score (p = 0.011), large EV (p = 0.006), and prior EIS/EVL (p = 0.006) were significantly associated with a higher PHE score (severe PHE). Moreover, patients with a higher TE score were significantly related with the PHE score (r = 0.561, p = 0.004) (Fig. 3).
Table 3.
The relation between PHE score and patients’ clinical variables
| Variable | PHE score mean ± SD | p value | |
|---|---|---|---|
| High Child Pugh score | Yes† | 3.4 ± 1.9 | 0.011* |
| No† | 1.8 ± 1.6 | ||
| Large EV | Yes | 3.7 ± 1.4 | 0.006* |
| No | 1.7 ± 1.8 | ||
| Gastric Varices | Yes | 2.1 ± 1.7 | 0.631 |
| No | 2.4 ± 2.1 | ||
| PHG | Yes | 2.8 ± 1.6 | 0.289 |
| No | 1.7 ± 2.1 | ||
| Prior EIS/EVL | Yes | 3.8 ± 1.4 | 0.006* |
| No | 1.9 ± 1.8 | ||
| Prior variceal bleeding | Yes | 3.4 ± 1.7 | 0.136 |
| No | 2 ± 1.9 |
* p<0.05 was considered statistically significant. †Yes, presence of the variable; †No, absence of the variable. PHE, portal hypertensive enteropathy; EV, esophageal varices; PHG, portal hypertensive gastropathy; EIS, endoscopic injection sclerotherapthy; EVL, endoscopic variceal ligation.
Fig. 3.
Linear distribution of TE scores according the PHE score.
Discussion
Liver cirrhosis is a progressive chronic liver disease, and its clinical features are dependent on the disease duration and the nature of the etiological factors [26]. Increased resistance to portal blood flow due to liver cirrhosis leads to PHT [27]. In fact, PHT results in the development of gastroesophageal and/or ectopic (colonic, enteric) varices as well as other mucosal lesions in the stomach, small intestine, and colon [2, 28].
While portal hypertensive gastropathy and colonopathy are considered as sources of non-variceal bleeding in patients with liver cirrhosis and PHT, data on PHE is limited [27]. The true prevalence and the clinical significance of PHE are unknown. Likewise, the features of this condition are not completely described because, in most instances, the endoscopic diagnosis of PHE is impossible beyond the limit of insertion of an enteroscope or the distal ileum (retrograde ileoscopy).
Because CE examines the entire small bowel (SB), we believed that it might reveal the mucosal changes and the true extent of involvement of the small bowel in patients with liver cirrhosis and PHT. This is important in view of the enormous surface area of the SB and its inaccessibility during routine gastrointestinal endoscopy and because the study of the SB mucosa by enteroscopy is time consuming and invasive [10].
In this study, the prevalence of SB mucosal lesions compatible with PHE was significantly higher in cirrhotic patients than in non-cirrhotic patients (67.7% vs 6.9%, p<0.001). This prevalence of PHE among the cirrhotic patient group was in accordance with that observed by three previous similar studies [10, 27, 29]. Nevertheless, presence of such lesions in non-cirrhotic patients (control group) was a matter of conflict. Figueiredo et al. [29], and Goulas et al. [27] revealed similar results to ours, but De Palma et al. [10] revealed an absence of such lesions among the non-cirrhotic patients.
The mucosal lesions of PHE included red spots with highest percentage of presence (54.8%), followed by angioectasias (51.6%), inflammatory-like lesions (41.9%), and lastly SB varices (16.1%).
In our present study, a comparison of cirrhotic patients with and without PHE revealed that cirrhotic patients with a high TE score, worsening Child-Pugh class, larger EV, PHG, and a prior history of EIS/EVL were significantly associated with PHE. However, there was no difference between these two groups of cirrhotic patients with regard to hemoglobin level, haematocrit value, and prior history of variceal bleeding. Our data was almost in accordance with those of De Palma et al. [10], but it was not in accordance with those of Figueiredo et al. [29] and Goulas et al. [27].
Currently, there is no scoring system for grading the severity of mucosal abnormalities of PHE in cirrhotic patients with PHT, making comparisons between studies difficult. De Palma et al. [10] supposed that PHE lesions detected by CE might be classified into two categories: inflammatory-like lesions (edema, erythema, granularity, friability) and vascular lesions (red spots, angioectasias, and varices). Kodama et al. [30], in a study using double balloon endoscopy, proposed that PHE lesions might be classified into two categories: villous abnormalities and vascular lesions. Then, Kodama et al. subclassified each category into three further subcategories and gave one point for every positive finding of these six subcategories. As a result, a scoring system with a maximum of six points was created. Nevertheless, his scoring system was not significantly associated with any of the clinical variables of patients, except for presence of ascites.
We proposed to make a scientifically reliable scoring system using great modifications in that previous system, and we statistically studied it with all variables of the patients, especially TE score.
Our scoring system depends on the classification of the mucosal lesions into main four lesions, as shown in Table 1: inflammatory-like lesions and three vascular lesions, which are red spots, angioectasisas, and SB varices. Each type of these four lesions is worth two points if it was multiple (more than two lesions) and one point if it was not. As a result, the total score was eight points maximally. In our scoring system, we aimed to give greater attention to the vascular lesions more than inflammatory ones, as they seem to be the most important lesions clinically, which might be sources for overt or occult bleeding. Moreover, bleeding vascular lesions can be treated endoscopically with injection sclerotherapy for varices and argon plasma coagulation for angioectasisas or red spots.
Cirrhotic patients with a high Child-Pugh score (p = 0.011), large EV (p = 0.006), and prior EIS/EVL (p = 0.006) were significantly associated with a higher PHE score. Moreover, we found that patients with a higher TE score were significantly related with the PHE score (p = 0.004). As a result, we thought that our scoring system might have a prognostic value, because it was significantly associated with parameters of liver disease severity (Child-Pugh score), and PHT (EV, TE score, and prior EIS/EVL).
Capsule endoscopy has a useful role in detection of mucosal lesions of PHE, and in assessment of its severity (PHE score). Nevertheless, CE is an expensive investigation, which could not be used daily for cirrhotic patients. We proposed that TE had an important clinical impact in such field, because using TE as a non-invasive and cheap maneuver could be helpful to suspect the presence and severity of PHE, especially if these patients have bleeding and conventional endoscopy was negative.
In conclusion, the mucosal changes compatible with PHE are significantly more common in cirrhotic patients than in non-cirrhotic patients. Higher PHE score was related to the degree of liver disease, and portal hypertension. Transient elastography might be a new non-invasive method that can be used in detection of mucosal lesions of PHE. The exact clinical significance of PHE remains unknown. In the future, further large-scale studies using our scoring system are required to declare the significance of PHE, and to determine a LSM cutoff value using transient elastography for best prediction of PHE.
Abbreviations
- PHT
portal hypertensive enteropathy
- PHG
portal hypertensive gastropathy
- EV
esophageal varices
- EGD
esophagogastroduodenoscopy
- CE
capsule endoscopy
- HVPG
hepatic venous pressure gradient
- TE
transient elastography
- kPa
kilopascal
- LSM
liver stiffness measurement
- EIS
endoscopic injection sclerotherapy
- EVL
endoscopic variceal ligation
- SB
small bowel
References
- 1.Sarfeh I.J., Tarnawaski A. Gastric mucosal vasculopathy in portal hypertension. Gastroenterology. 1987;93:1129–1131. doi: 10.1016/0016-5085(87)90579-8. [DOI] [PubMed] [Google Scholar]
- 2.Viggiano T.R., Gostout C.J. Portal hypertensive intestinal vasculopathy: a review of the clinical, endoscopic, and histopathologic features. Am. J. Gastroenterol. 1992;87:944–954. [PubMed] [Google Scholar]
- 3.Kozarek R.A., Botoman V.A., Bredfeldt J.E., Roach J.M., Patteson D.J., Ball T.J. Portal colopathy: prospective study of colonoscopy in patients with portal hypertension. Gastroenterology. 1991;101:1192–1197. doi: 10.1016/0016-5085(91)90067-u. [DOI] [PubMed] [Google Scholar]
- 4.Burak K.W., Beck P.L. Diagnosis of portal hypertensive gastropathy. Curr. Opin. Gastroenterol. 2003;19:477–482. doi: 10.1097/00001574-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Primignani M., Carpinelli L., Preatoni P., Battaglia G., Carta A., Prada A., Cestari R., Angeli P., Galla A., Rossi A., Spinzi G., De Franchis R. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC) Gastroenterology. 2000;119:181–187. doi: 10.1053/gast.2000.8555. [DOI] [PubMed] [Google Scholar]
- 6.Bresci G., Parisi G., Capria A. Clinical relevance of colonic lesions in cirrhotic patients with portal hypertension. Endoscopy. 2006;38:830–835. doi: 10.1055/s-2006-944629. [DOI] [PubMed] [Google Scholar]
- 7.Misra S.P., Dwivedi M., Misra V., Dharmani S., Kunwar B.K., Arora J.S. Colonic changes in patients with cirrhosis and in patients with extrahepatic portal vein obstruction. Endoscopy. 2005;37:454–459. doi: 10.1055/s-2005-861252. [DOI] [PubMed] [Google Scholar]
- 8.Thiruvengadam R., Gostout C.J. Congestive gastroenteropathy—an extension of nonvariceal upper gastrointestinal bleeding in portal hypertension. Gastrointest. Endosc. 1989;35:504–507. doi: 10.1016/s0016-5107(89)72898-4. [DOI] [PubMed] [Google Scholar]
- 9.Misra S.P., Dwivedi M., Misra V., Gupta M. Ilial varices and portal hypertensive ileopathy in patients with cirrhosis and portal hypertension. Gastrointest. Endosc. 2004;60:778–783. doi: 10.1016/s0016-5107(04)02049-8. [DOI] [PubMed] [Google Scholar]
- 10.De Palma G.D., Rega M., Masone S., Persico F., Siciliano S., Patrone F., Matantuono L., Persico G. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest. Endosc. 2005;62:529–534. doi: 10.1016/s0016-5107(05)01588-9. [DOI] [PubMed] [Google Scholar]
- 11.Iddan G., Meron G., Glukhovsky A., Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi K., Umegaki E., Watanabe T., Yoda Y., Morita E., Murano M., Tokioka S., Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. J. Gastroenterol. 2009;44:879–888. doi: 10.1007/s00535-009-0102-2. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi K., Yoda Y., Amagase K., Kato S., Tokioka S., Murano M., Takeuchi A., Umegaki E. Prevention of NSAID-induced small intestinal mucosal injury: prophylactic potential of lansoprazole. J. Clin. Biochem. Nutr. 2009;45:125–130. doi: 10.3164/jcbn.SR09-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandrin L., Fourquet B., Hasquenoph J.M., Yon S., Fournier C., Mal F., Christidis C., Ziol M., Poulet B., Kazemi F., Beaugrand M., Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound. Med. Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Fraquelli M., Rigamonti C., Casazza G., Conte D., Donato M.F., Ronchi G., Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E., Grimaldi A., Capron F., Poynard T., LIDO Study Group Sampling variability of liver biopsy in non-alcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 17.Bedossa P., Dargere D., Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Burroughs A.K., Groszmann R., Bosch J., Grace N., Garcia-Tsao G., Patch D., Garcia-Pagan J.C., Dagher L. Assessment of therapeutic benefit of antiviral therapy in chronic hepatitis C: is hepatic venous pressure gradient a better end point? Gut. 2002;50:425–427. doi: 10.1136/gut.50.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziol M., Handra-Luca A., Kettaneh A., Christidis C., Mal F., Kazemi F., de Lédinghen V., Marcellin P., Dhumeaux D., Trinchet J.C., Beaugrand M. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 20.Castera L., Vergniol J., Foucher J., Le Bail B., Chanteloup E., Haaser M., Darriet M., Couzigou P., De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI and liver biopsy for the assessment of liver fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Vizzutti F., Arena U., Romanelli R.G., Rega L., Foschi M., Colagrande S., Petrarca A., Moscarella S., Belli G., Zignego A.L., Marra F., Laffi G., Pinzani M. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 22.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transaction of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 23.Carrion J.A., Navasa M., Bosch J., Bruguera M., Gilabert R., Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl. 2006;12:1791–1798. doi: 10.1002/lt.20857. [DOI] [PubMed] [Google Scholar]
- 24.Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J. Surg. 1995;19:420–422. doi: 10.1007/BF00299178. [DOI] [PubMed] [Google Scholar]
- 25.Rondonotti E., Villa F., Signorelli C., de Franchis R. Portal hypertensive enteropathy. Gastrointest. Endosc. Clin. N. Am. 2006;16:277–286. doi: 10.1016/j.giec.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Higaki N., Matsui H., Imaoka H., Ikeda Y., Murakami H., Hiasa Y., Matsuura B., Onji M. Characteristic endoscopic features of portal hypertensive enteropathy. J. Gastroenterol. 2008;43:327–331. doi: 10.1007/s00535-008-2166-9. [DOI] [PubMed] [Google Scholar]
- 27.Goulas S., Triantafyllidou K., Karagiannis S., Nicolaou P., Galanis P., Vafiadis I., Tzivras M., Mavrogiannis C. Capsule endoscopy in the investigation of patients with portal hypertension and anemia. Can. J. Gastroenterol. 2008;22:469–474. doi: 10.1155/2008/534871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrero R.J., Barkin J.S. Wireless capsule endoscopy and portal hypertensive intestinal vasculopathy. Gastrointest. Endosc. 2005;62:535–537. doi: 10.1016/j.gie.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 29.Figueiredo P., Almeida N., Lérias C., Lopes S., Gouveia H., Leitão M.C., Freitas D. Effect of portal hypertension in the small bowel: an endoscopic approach. Dig. Dis. Sci. 2008;53:2144–2150. doi: 10.1007/s10620-007-0111-z. [DOI] [PubMed] [Google Scholar]
- 30.Kodama M., Uto H., Numata M., Hori T., Murayama T., Sasaki F., Tsubouchi N., Ido A., Shimoda K., Tsubouchi H. Endoscopic characterization of the small bowel in patients with portal hypertension evaluated by double balloon endoscopy. J. Gastroenterol. 2008;43:589–596. doi: 10.1007/s00535-008-2198-1. [DOI] [PubMed] [Google Scholar]