Abstract
A triple therapy based on a proton pump inhibitor (PPI), amoxicillin (AMPC), and clarithromycin (CAM) is recommended as a first-line therapy for Helicobacter pylori (H. pylori) eradication and is widely used in Japan. However, a decline in eradication rate associated with an increase in prevalence of CAM resistance is viewed as a problem. We investigated CAM resistance and eradication rates over time retrospectively in 750 patients who had undergone the triple therapy as first-line eradication therapy at Nagoya City University Hospital from 1995 to 2008, divided into four terms (Term 1: 1997–2000, Term 2: 2001–2003, Term 3: 2004–2006, Term 4: 2007–2008). Primary resistance to CAM rose significantly over time from 8.7% to 23.5%, 26.7% and 34.5% while the eradication rate decreased significantly from 90.6% to 80.2%, 76.0% and 74.8%. Based on the PPI type, significant declines in eradication rates were observed with omeprazole or lansoprazole, but not with rabeprazole. A decrease in the H. pylori eradication rate after triple therapy using a PPI + AMPC + CAM has been acknowledged, and an increase in CAM resistance is considered to be a factor. From now on, a first-line eradication regimen that results in a higher eradication rate ought to be investigated.
Keywords: amoxicillin, clarithromycin resistance, Helicobacter pylori, proton pump inhibitor, triple therapy
Introduction
The greatest association of Helicobacter pylori (H. pylori) infection is with peptic ulcer disease. Recently, the association between H. pylori and the development of gastric cancer is more clearly established than before, and by the announcement of the Japanese Society for Helicobacter Research, H. pylori eradication should be undertaken in all subjects who are infected [1, 2]. The treatment to eradicate H. pylori by a triple therapy using a proton pump inhibitor (PPI), amoxicillin (AMPC) and clarithromycin (CAM) was approved and covered by insurance in Japan in November, 2000. In February 2003, the eradication therapy went into broad use as a result of the eradication guidelines of the Japanese Society for Helicobacter Research. The initial eradication rate was good at about 90% [3], but a decline in eradication rate attributable to an increase in CAM resistance was pointed out. Consequently, the Japanese Society for Helicobacter Research conducted sensitivity surveillance from 2002 through 2006 in order to determine the prevalence of H. pylori drug resistance in Japan. The results revealed that the CAM resistance rate from 2002 to 2003 was 18.9%, followed by 21.1% in 2003–2004, 27.7% in 2004–2005 [4]. The reports also demonstrated that the CAM resistance rate in Japan since 2003 exceeded 20%, the condition the Maastricht III Consensus Report recommends the triple therapy using PPI, AMPC and CAM [5]. In such a situation (CAM resistance rate >20%), it is recommended that drug sensitivity tests be carried out prior to eradication [5], but in Japan sensitivity testing is not common because eradication therapies other than the PPI, AMPC, and CAM triple therapy are not approved for the first-line eradication therapy. In this study, we conducted a retrospective analysis of the status of eradication rate by PPI, AMPC, and CAM in our hospital in Japan, where the rate of CAM resistance is high.
Materials and Methods
We retrospectively investigated the H. pylori eradication rate over time in 750 patients, who had been diagnosed as H. pylori-infected by at least one positive result from culture test, microscopy or 13C-urea breath test (UBT). The 750 patients had received the triple therapy based on a PPI (omeprazole (OPZ) 20 mg or lansoprazole (LPZ) 30 mg or rabeprazole (RPZ) 10 mg twice as day), AMPC (750 mg twice as day), and CAM (200 mg or 400 mg twice a day) at Nagoya City University Hospital from January, 1997 until December, 2008. Successful eradication was determined by performing a UBT following the first month after eradication, and Δ<2.5‰ was defined as successful eradication. We also investigated the relationship between the eradication rate and the H. pylori CAM primary resistance (from the flat dilution method, MIC >8 µg/ml) in four terms detected at Nagoya City University Hospital from January 1997 until December 2008. Terms were divided as follows; Term 1: 1997–2000, before eradication therapy was approved and covered by insurance in Japan; Term 2: 2001–2003, the first half of period when only omeprazole and lansoprazole were approved, Term 3: 2004–2006, the latter half of period when only omeprazole and lansoprazole were approved; Term 4: 2007–2008, after rabeprazole was approved. The eradication rate by type of PPI was evaluated as well.
The Kruskal-Wallis test and the χ2 test were used for the statistical analysis. p<0.05 was considered statistically significant.
Results
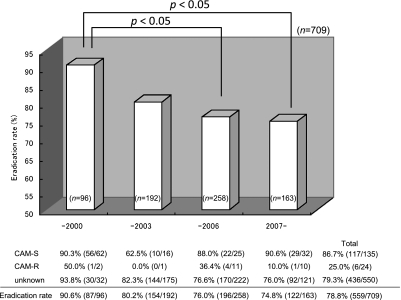
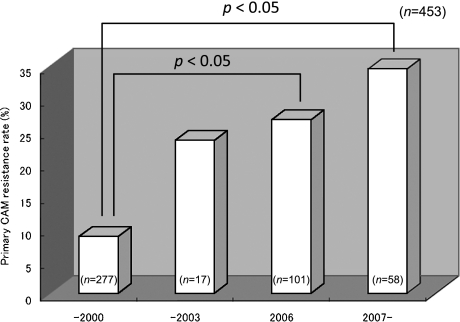
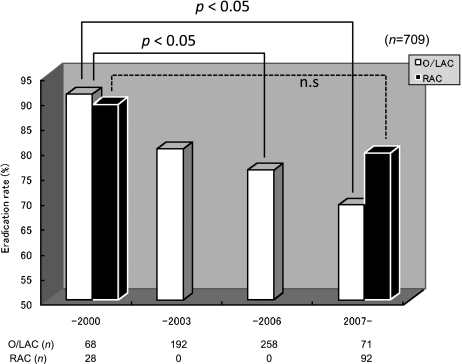
Table 1 presents the patients’ background in this study. 491 men and 259 women with the average age of 56.1 ± 14.2 years were enrolled in the study. The classification of disorders were gastric ulcers (258/750, 34.4%), duodenal ulcers (188/750 25.1%), gastroduodenal ulcers (57/750, 7.6%), other gastrointestinal disorders (247/750, 32.9%). The patients with gastrointestinal disorders mainly comprised patients with atrophic gastritis or patients having undergone endoscopic treatment for a gastric tumor. 41 patients did not visit for the evaluation of H. pylori eradication. 709 patients were tested by UBT to determine whether the H. pylori was eradicated, and successful eradication was assessed in 559 of these patients. We divided the study into the four terms of 1997 to 2000 (Term 1), before H. pylori eradication therapy was indicated and covered by insurance in Japan; the first half of the period from 2001 to 2006, when eradication therapy was based on OPZ and LPZ (Term 2, from 2001 to 2003); and the second half of that (2004–2006, Term 3); and 2007 and later years (Term 4), when treatment with RPZ was approved for insurance coverage. There were no differences based on gender or type of disorder from Term 1 to Term 4, but the ages were significantly higher in Terms 3 and 4 compared it in Term 1. Eradication rates significantly declined over time from 90.6% to 80.2%, 76.0% and 74.8% between Term 1 and Term 4 (Fig. 1). On the other hand, primary CAM resistance rose significantly over time between 1997 and 2008 from 8.7% in Term 1, prior to 2000; 23.5% in Term 2; 26.7% in Term 3; and 34.5% in Term 4 (Fig. 2). In 159 patients who were tested for CAM susceptibility, the eradication rate in those with CAM susceptibility was 86.7% while the eradication rate in those with CAM-resistant bacteria was 25.0%, which resulted in a significant difference. Therefore, the rise in primary CAM resistance is considered to be a major factor leading the decline in the first-line eradication rate based on triple therapy with the PPI, AMPC, and CAM. We also investigated the differences over time in the eradication rate for the different types of PPIs. RPZ was not used for eradication treatment from 2000, when eradication therapy was approved in Japan, until 2007, when RPZ was approved for insurance coverage. Therefore, we compared the eradication rates by RPZ for Term 1 with Term 4. For OPZ and LPZ, eradication rates were compared in all four terms. A significant decline over time in eradication rates by OPZ/LPZ from Terms 1 to Term 4 was observed; 91.2%, 80.2%, 76.0% and 69.0%. On the other hand, no significant difference was found in the RPZ eradication rates; 89.2% in Term 1 and 79.4% in Term 4 (Fig. 3).
Table 1.
Patients’ background
| Total | –2000 | –2003 | –2006 | 2007– | |
|---|---|---|---|---|---|
| Number of Patients | 750 | 103 | 197 | 267 | 183 |
| Age | 56.6 ± 14.2 | 53.0 ± 13.4 | 55.2 ± 14.2 | 58.1 ± 13.4* | 57.8 ± 15.4* |
| Sex (M/F) | 491/259 | 63/40 | 134/63 | 182/85 | 112/71 |
| Disorder | |||||
| Gastric ulcer | 258 (34.4%) | 21 (20.4%) | 78 (39.6%) | 81 (30.3%) | 78 (42.6%) |
| Duodenal ulcer | 188 (25.1%) | 28 (27.2%) | 53 (26.9%) | 58 (21.7%) | 49 (26.8%) |
| Gastroduodenal ulcer | 57 (7.6%) | 11 (10.7%) | 14 (7.1%) | 21 (7.9%) | 11 (6.0%) |
| Other | 247 (32.9%) | 43 (41.7%) | 52 (26.4%) | 107 (40.1%) | 45 (24.6%) |
*p<0.05 significantly different compared with –2000 group.
Fig. 1.
Eradication rates significantly declined from 90.6% (1997–2000) to 80.2% (2001–2003), 76.0% (2004–2006) and 74.8% (2007–2008). The eradication rate of CAM-resistant bacteria (25.0%) was significant lower than that of CAM susceptibility (86.7%). CAM-S, clarithromycin susceptibility; CAM-R, clarithromycin resistance.
Fig. 2.
Primary CAM resistance rose significantly from 8.7% (1997–2000) to 23.5% (2001–2003), 26.7% (2004–2006), and 34.4% (2007–2008).
Fig. 3.
Eradication rates by OPZ/LPZ significantly decline from 91.2% (1995–2000) to 80.2% (2001–2003), 76.0% (2004–2006) and 69.0% (2007–2008). On the other hand, no significant difference was found in the RPZ eradication rates between the term of before 2000 and after 2007. O/LAC, omeprazole or lansoprazole + amoxicillin + clarithromycin regimen. RAC, rabeprazole + amoxicillin + clarithromycin regimen.
Discussion
The H. pylori eradication rate by triple therapy based on a PPI, AMPC and CAM is thought to be affected by age, smoking habits, drug compliance, polymorphisms of the CYP2C19 gene, and drug susceptibility, and CAM resistance, in particular, is viewed to have a large effect on the eradication rate [6–10]. That arises from the big deterioration in eradication rate from about 80.6%–98.3% for CAM-susceptible bacteria to 0%–33.3% for CAM-resistant bacteria [11, 12]. In the 2005 Maastricht III Consensus Report, triple therapy based on a PPI, AMPC, and CAM were recommended as the primary eradication drugs in areas where CAM resistance is <20% [5]. In a report on the eradication rate in the United Kingdom over the 11 years from 1991 to 2001, a good eradication rate of 89.9% was reported for triple therapy using a PPI, AMPC, and CAM when the CAM resistance ranged 3.8–10.3% [13]. In the present study period, the CAM resistance had been low at 8.7% with a good eradication rate at 90.6% for the triple therapy before 2000, when eradication therapy was approved in Japan. However, after that, the CAM resistance rate rose over 20%, the surveillance by the Japanese Society for Helicobacter Research revealed [6]. In our facility, as well, CAM resistance had exceeded 20% in 2000 and reached >30% over the past two years, which resulted in the remarkable decline in eradication rate along, falling to the 70% level. Triple therapy with a PPI, AMPC and CAM needs to be re-examined in Japan. Triple therapy using a PPI, AMPC, and metronidazole (MNZ) is used in second-line eradication, and a high eradication rate over 90% has been reported in Japan [14–16]. In addition, new approaches to improve the eradication rate by concomitant with a mucoprotective drug or lactobacillus are attempted [17, 18]. Triple therapy using MNZ as first line eradication and new regimen using quinolone like levofloxacin, gatifloxacin, sitafloxacin or garenoxacin need to be developed [19, 20].
Mean age of patients taking significantly rose in Term 3 and 4 compared to Term 1 in the present study as seen in table 1. However, Broutet et al. [21] reported higher failure rate among young patients, therefore, the significant higher age in Term 3 and 4 was considered not to have influenced the lowering eradication rate in the present study.
On the other hand, the eradication rate for triple therapies is reported to depend on the degree of acid secretion suppression, in addition to CAM resistance [22], and the importance of acid secretion suppression has also drawn attention. The effect of suppressing acid secretion by a PPI was reported to rely on a polymorphism of the CYP2C19 gene [23]. That the eradication rates for triple therapy based on a PPI, AMPC and CAM differ due to polymorphisms of the CYP2C19 gene is also reported in a meta-analysis [24]. In patients with CAM-resistant bacteria, in particular, the eradication rate is reported to be greatly affected by polymorphisms of the CYP2C19 gene [25]. It was shown that the eradication rate for a double eradication therapy of PPI and AMPC is affected by the extent to which acid secretion can be suppressed [26], and that is thought to be because the PPI's ability to suppress acid secretion affects the eradication rate more for CAM-resistant bacteria. In Japan, OPZ, LPZ and RPZ are used in the eradication treatment, but RPZ is reported to be less likely affected by CYP2C19 than the other two PPIs [27, 28]. In fact, a meta-analysis reported the less influence of CYP2C19 polymorphism on triple therapy using RPZ unlike the case of OPZ or LPZ [24]. In Japan, there is a great deal of variety in polymorphisms of the CYP2C19 gene [29], and that is why we investigated eradication rates in triple therapy groups and in the group using RPZ and the other groups using the OPZ or LPZ. While the eradication rates in OPZ and LPZ groups decreased significantly over time, no apparent decline in eradication rate was seen for the RPZ group. Less influence of CYP2C19 polymorphisms on RPZ might explain the reason. Also, RPZ itself is indicated to possess antibiotic effects of against H. pylori, and it might have helped as well [30]. However, we did not conduct detailed investigations because it was a retrospective study, nor did we measure CYP2C19 polymorphisms, and H. pylori drug susceptibility tests were carried out only for some cases.
In the current situation where prevalence of primary resistance to CAM increased, it was difficult to ascertain the reasons why differences arose in the effectiveness of H. pylori eradication by acid secretion-suppressing agents and triple therapy based on a PPI, AMPC, and CAM. However, the eradication rate for large-scale triple therapy using RPZ is reported to be good and to be ≥90%, in spite of increase in the CAM resistance rate in recent years [31]. In addition, a prospective study reported a higher eradication rate of 91% with RPZ than it of 81% with LPZ prior to our report [12]. The selection of a PPI should also be important in first line eradication today, and we need to make it clearer hereafter.
The present study demonstrated the evident decline in eradication rates for the triple therapy using a PPI, AMPC and CAM in Japan, and the increase in CAM resistance rate is considered to be associated with the lower eradication rate. The first line eradication with new regimens must be developed and used. At the same time, PPI from which a higher eradication rate can be obtained should be selected from current regimen as well.
Acknowledgment
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Abbreviations
- CAM
clarithromycin
- PPI
proton pump inhibitor
- AMPC
amoxicillin
- H. pylori
Helicobacter pylori
- UBT
urea breath test
- OPZ
omeprazole
- LPZ
lansoprazole
- RPZ
rabeprazole
- MNZ
metronidazole
References
- 1.Suzuki H., Hibi T., Marshall B.J. Helicobacter pylori: present status and future prospects in Japan. J. Gastroenterol. 2007;42:1–15. doi: 10.1007/s00535-006-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki H., Iwasaki E., Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79–87. doi: 10.1007/s10120-009-0507-x. [DOI] [PubMed] [Google Scholar]
- 3.Asaka M., Sugiyama T., Kato M., Satoh K., Kuwayama H., Fukuda Y., Fujioka T., Takemoto T., Kimura K., Shimoyama T., Shimizu K., Kobayashi S. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001;6:254–261. doi: 10.1046/j.1523-5378.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi I., Murakami K., Kato M., Kato S., Azuma T., Takahashi S., Uemura N., Katsuyama T., Fukuda Y., Haruma K., Nasu M., Fujioka T. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J. Clin. Microbiol. 2007;45:4006–4010. doi: 10.1128/JCM.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfertheiner P., Megraud F., O’Morain C., Bazzoli F., El-Omar E., Graham D., Hunt R., Rokkas T., Vakil N., Kuipers E.J. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perri F., Villani M.R., Festa V., Quitadamo M., Andriulli A. Predictors of failure of Helicobacter pylori eradication with the standard ‘Maastricht triple therapy’. Aliment. Pharmacol. Ther. 2001;15:1023–1029. doi: 10.1046/j.1365-2036.2001.01006.x. [DOI] [PubMed] [Google Scholar]
- 7.Furuta T., Shirai N., Takashima M., Xiao F., Hanai H., Nakagawa K., Sugimura H., Ohashi K., Ishizaki T. Effects of genotypic differences in CYP2C19 status on cure rates for Helicobacter pylori infection by dual therapy with rabeprazole plus amoxicillin. Pharmacogenetics. 2001;11:341–348. doi: 10.1097/00008571-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Miwa H., Misawa H., Yamada T., Nagahara A., Ohtaka K., Sato N. Clarithromycin resistance, but not CYP2C-19 polymorphism, has a major impact on treatment success in 7-day treatment regimen for cure of H. pylori infection: a multiple logistic regression analysis. Dig. Dis. Sci. 2001;46:2445–2450. doi: 10.1023/a:1012371702918. [DOI] [PubMed] [Google Scholar]
- 9.Poon S.K., Chang C.S., Su J., Lai C.H., Yang C.C., Chen G.H., Wang W.C. Primary resistance to antibiotics and its clinical impact on the efficacy of Helicobacter pylori lansoprazole-based triple therapies. Aliment. Pharmacol. Ther. 2002;16:291–296. doi: 10.1046/j.1365-2036.2002.01184.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T., Kawakami K., Kataoka M., Takei K., Taira S., Itoi T., Moriyasu F., Takagi Y., Aoki T., Matsubayasiu J., Mukai K., Rimbara E., Noguchi N., Sasatsu M. The effectiveness of packaged medicine in eradication therapy of Helicobacter pylori in Japan. J. Clin. Biochem. Nutr. 2006;38:73–76. [Google Scholar]
- 11.Hoshiya S., Watanabe K., Tokunaga K., Tanaka A., Ninomiya H., Shingaki M., Itoh T., Saito S., Ishida H., Takahashi S. Relationship between eradication therapy and clarithromycin-resistant Helicobacter pylori in Japan. J. Gastroenterol. 2000;35:10–14. [PubMed] [Google Scholar]
- 12.Murakami K., Sato R., Okimoto T., Nasu M., Fujioka T., Kodama M., Kagawa J., Sato S., Abe H., Arita T. Eradication rates of clarithromycin-resistant Helicobacter pylori using either rabeprazole or lansoprazole plus amoxicillin and clarithromycin. Aliment. Pharmacol. Ther. 2002;16:1933–1938. doi: 10.1046/j.1365-2036.2002.01368.x. [DOI] [PubMed] [Google Scholar]
- 13.Cameron E.A., Powell K.U., Baldwin L., Jones P., Bell G.D., Williams S.G. Helicobacter pylori: antibiotic resistance and eradication rates in Suffolk, UK, 1991–2001. J. Med. Microbiol. 2004;53:535–538. doi: 10.1099/jmm.0.05499-0. [DOI] [PubMed] [Google Scholar]
- 14.Murakami K., Okimoto T., Kodama M., Sato R., Watanabe K., Fujioka T. Evaluation of three different proton pump inhibitors with amoxicillin and metronidazole in retreatment for Helicobacter pylori infection. J. Clin. Gastroenterol. 2008;42:139–142. doi: 10.1097/MCG.0b013e31802cbc1a. [DOI] [PubMed] [Google Scholar]
- 15.Shirai N., Sugimoto M., Kodaira C., Nishino M., Ikuma M., Kajimura M., Ohashi K., Ishizaki T., Hishida A., Furuta T. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur. J. Clin. Pharmacol. 2007;63:743–749. doi: 10.1007/s00228-007-0302-8. [DOI] [PubMed] [Google Scholar]
- 16.Matsuhisa T., Kawai T., Masaoka T., Suzuki H., Ito M., Kawamura Y., Tokunaga K., Suzuki M., Mine T., Takahashi S., Sakaki N. Efficacy of metronidazole as second-line drug for the treatment of Helicobacter pylori Infection in the Japanese population: a multicenter study in the Tokyo Metropolitan Area. Helicobacter. 2006;11:152–158. doi: 10.1111/j.1523-5378.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.W., Kim G.H., Cheong J.Y., Yang U.S., Park S.K., Song C.S., Kang D.H., Song G.A. H. pylori eradication: a randomized prospective study of triple therapy with or without ecabet sodium. World J. Gastroenterol. 2008;14:908–912. doi: 10.3748/wjg.14.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bortoli N., Leonardi G., Ciancia E., Merlo A., Bellini M., Costa F., Mumolo MG., Ricchiuti A., Cristiani F., Santi S., Rossi M., Marchi S. Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am. J. Gastroenterol. 2007;102:951–956. doi: 10.1111/j.1572-0241.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 19.Nishizawa T., Suzuki H., Hibi T. Quinolone-Based Third-Line Therapy for Helicobacter pylori Eradication. J. Clin. Biochem. Nutr. 2009;44:119–124. doi: 10.3164/jcbn.08-220R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki H., Nishizawa T., Muraoka H., Hibi T. Sitafloxacin and garenoxacin may overcome the antibiotic resistance of Helicobacter pylori with gyrA mutation. Antimicrob. Agents Chemother. 2009;53:1720–1721. doi: 10.1128/AAC.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broutet N., Tchamgoué S., Pereira E., Lamouliatte H., Salamon R., Mégraud F. Risk factors for failure of Helicobacter pylori therapy—results of an individual data analysis of 2751 patients. Aliment. Pharmacol. Ther. 2003;17:99–109. doi: 10.1046/j.1365-2036.2003.01396.x. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto M., Furuta T., Shirai N., Kodaira C., Nishino M., Ikuma M., Ishizaki T., Hishida A. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12:317–323. doi: 10.1111/j.1523-5378.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 23.Furuta T., Sugimoto M., Shirai N., Ishizaki T. CYP2C19 pharmacogenomics associated with therapy of Helicobacter pylori infection and gastro-esophageal reflux diseases with a proton pump inhibitor. Pharmacogenomics. 2007;8:1199–1210. doi: 10.2217/14622416.8.9.1199. [DOI] [PubMed] [Google Scholar]
- 24.Zhao F., Wang J., Yang Y., Wang X., Shi R., Xu Z., Huang Z., Zhang G. Effect of CYP2C19 genetic polymorphisms on the efficacy of proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: a meta-analysis. Helicobacter. 2008;13:532–541. doi: 10.1111/j.1523-5378.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- 25.Furuta T., Shirai N., Takashima M., Xiao F., Hanai H., Sugimura H., Ohashi K., Ishizaki T., Kaneko E. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin. Pharmacol. Ther. 2001;69:158–168. doi: 10.1067/mcp.2001.113959. [DOI] [PubMed] [Google Scholar]
- 26.Furuta T., Ohashi K., Kamata T., Takashima M., Kosuge K., Kawasaki T., Hanai H., Kubota T., Ishizaki T., Kaneko E. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann. Intern. Med. 1998;129:1027–1030. doi: 10.7326/0003-4819-129-12-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Shirai N., Furuta T., Moriyama Y., Okochi H., Kobayashi K., Takashima M., Xiao F., Kosuge K., Nakagawa K., Hanai H., Chiba K., Ohashi K., Ishizaki T. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment. Pharmacol. Ther. 2001;15:1929–1937. doi: 10.1046/j.1365-2036.2001.01108.x. [DOI] [PubMed] [Google Scholar]
- 28.Adachi K., Katsube T., Kawamura A., Takashima T., Yuki M., Amano K., Ishihara S., Fukuda R., Watanabe M., Kinoshita Y. CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment. Pharmacol. Ther. 2000;14:1259–1266. doi: 10.1046/j.1365-2036.2000.00840.x. [DOI] [PubMed] [Google Scholar]
- 29.Kubota T., Chiba K., Ishizaki T. Genotyping of S-mephenytoin 4'-hydroxylation in an extended Japanese population. Clin. Pharmacol. Ther. 1996;60:661–666. doi: 10.1016/S0009-9236(96)90214-3. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H., Miyazawa M., Nagahashi S., Sato M., Bessho M., Nagata H., Miura S., Ishii H. Rabeprazole treatment attenuated Helicobacter pylori-associated gastric mucosal lesion formation in Mongolian gerbils. J. Gastroenterol. Hepatol. 2003;18:787–795. doi: 10.1046/j.1440-1746.2003.03038.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuwayama H., Asaka M., Sugiyama T., Fukuda Y., Aoyama N., Hirai Y., Fujioka T., Japan Rabeprazole Study Group Rabeprazole-based eradication therapy for Helicobacter pylori: a large-scale study in Japan. Aliment. Pharmacol. Ther. 2007;25:1105–1113. doi: 10.1111/j.1365-2036.2007.03298.x. [DOI] [PubMed] [Google Scholar]