Abstract
Purpose
To date there remains no effective therapy for metastatic melanoma and at the molecular level disease progression remains poorly understood. Recent work by our group led to the development of a novel phenotype switching model for melanoma progression, wherein cells transition back-and-forth between states of proliferation and invasion to drive disease progression. To explore the model’s clinical relevance we interrogated phenotype-specific expression patterns in human melanoma patient material.
Experimental Design
A matched primary/metastasis pair from a human melanoma patient was obtained and immunohistochemically stained for proliferative and invasive phenotype markers. These were also stained for hypoxia and blood vessel markers.
Results
Proliferative phenotype markers Melan-A and Mitf showed consistent anti-correlation with invasive phenotype marker Wnt5A and hypoxia marker Glut-1. These also correlated with observed intra-tumoural vascularisation patterns. Similar pattern distributions were present in both primary and metastasis samples. Strikingly, we observed that late phase metastatic melanoma cells adopt morphologies and behaviours identical to very early phase cells.
Conclusions
The expression patterns observed closely matched expectations derived from previous in vitro and xenografting experiments. These results highlight the likelihood that disease progression involves melanoma cells retaining the capacity to regulate the expression of metastatic potential critical factors according to changing microenvironmental conditions.
Keywords: Melanoma, phenotype switching, immunohistochemistry
Introduction
Malignant melanoma is a cancer of the skin derived from melanocytes for which, despite decades of extensive clinical and basic research, there remains no effective therapy for metastatic forms of the disease. To improve patient outlook it is critical we understand the changes in cellular behaviour and molecular biology which take place during metastatic progression. Where melanoma analysis has the most relevant outcome for patients is the point at which lesions undergo immunohistopathological examination. Here there is repeated opportunity to re-evaluate what we know about melanoma biology in the human context. The structures observed and the molecules identified as being present (or absent) inform our interpretation of what is going on in situ. Presuppositions about how melanoma biology works that we bring with us to the microscope make a critical contribution to this interpretation.
In recent years our group conceived the phenotype switching model for melanoma progression (Figure 1A). This model evokes a biphasic mechanism wherein melanoma cells, in response to microenvironmental signals, switch back-and-forth between states of proliferation and invasion to drive metastatic disease [1,2]. However, while in vitro experiments on human cells and mouse xenografts yielded critical information for deriving the model, it is important that these observations are examined in the context of a human patient to provide clinical relevance.
Figure 1. Phenotype switching model for melanoma progression.
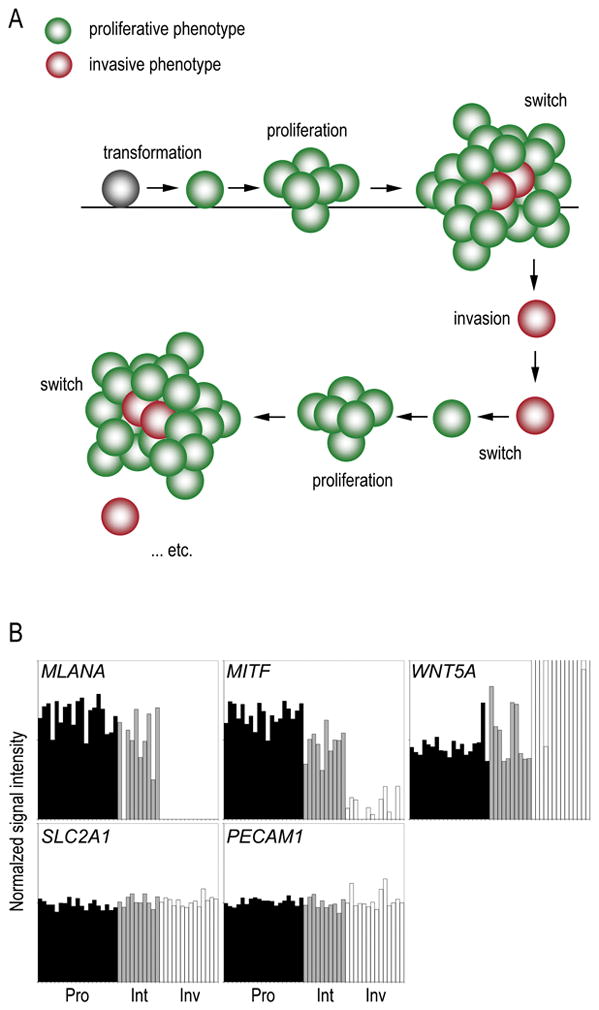
(A) After transformation melanoma cells are initially of the proliferative phenotype which promotes tumorigenesis. Changing microenvironmental conditions (such as increasing hypoxia) signals the switch of some cells to an invasive phenotype which escape the primary lesion. These cells lodge elsewhere in the body and switch back to the proliferative phenotype to seed a metastatic lesion, which allows the cycle to be repeated. (B) Transcription profiles for genes expressing the six marker factors chosen for this study are compared in the context of the phenotype switching hypothesis. Factors chosen include Melan-A (MLANA), Mitf (MITF), Wnt5A (WNT5A), Glut-1 (SLC2A1) and CD31 (PECAM1). Black bars represent expression in proliferative phenotype cells (Pro), grey bars represent intermediate phenotype cells (Int), and white bars represent expression in invasive phenotype cells (Inv). Only MLANA, MITF and WNT5A show significant (p < 0.001) differences in gene expression between proliferative and invasive phenotype cells. Data was extracted from GEO using accession GSE4843 and sample phenotype assignments are as previously established [1].
We present here an immunohistochemically-based study of two lesions removed from a 46 year-old female patient. This patient reported to our department with abdominal pain which subsequent CT scan analysis revealed to be caused by a polyploidy lesion of the gallbladder. A cholecystectomy was performed and histological examination identified this lesion to be a metastasis of melanoma. Additional examination of the patient revealed a primary melanocytic lesion on the back of the neck. The excised neck lesion was diagnosed as an ulcerated nodular melanoma with Breslow index of 3.5 mm. Nine months later routine PET-CT staging showed evidence of pulmonary metastases, the patient was staged as pT3bN0M1c. This patient-matched primary/metastasis pair is here interpreted immunohistochemically in the context of the phenotype switching model.
Methods and Materials
Gene expression data retrieval
Previously published experiments had phenotyped a library of 45 melanoma cell lines from the University Hospital of Mannheim collection according to whole genome gene expression profiling [1]. DNA microarray expression data for this collection was retrieved from NCBI’s Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) using accession number GSE4843 (Mannheim dataset). Data was normalized by setting all probe set values below 0.01 to 0.01 and dividing by the median probe set value within each sample, then by the median of their values across the samples. For selected genes normalized probe set data was extracted for samples previously identified as proliferative or invasive phenotype [1]. Normalized expression data for individual probe sets was interrogated for the significance of the difference in their expression between sample classes (proliferative phenotype versus invasive phenotype) using a two-tailed Student’s t-test.
Immunohistochemistry
From our sample archives blocks of paraffin-embedded formalin-fixed tissues corresponding to a matched primary/metastatic lesion pair from a 46 year old female patient were sourced for sectioning onto glass slides. For antigen retrieval, slides were heated with cell conditioner 1 (Ventana Medical Systems, Tucson, AZ). For each sample we assessed immunostaining for the following antigens; Melan-A (clone A103, 1:60 - Novacastra, Newcastle, UK), Mitf (clone 5+D5, 1:50 - Abcam, Cambridge, UK), biotinylated-Wnt5A (BAF645, 1:50 - R&D Systems, Minneapolis, MN), Glut-1 (AB1341, 1:1000 - Chemicon, Temecula, CA) and CD31 (JC70A, 1:40 - Dako). Staining was performed using kits supplied by Ventana or Dako. Endogenous biotin was blocked with the appropriate kit. Antigen-specific antibodies were applied and revealed with either the iVIEW DAB detection kit (Ventana) or the ChemMate detection kit (Dako). Slides were counterstained with haematoxylin.
Image acquisition and analysis
Stained slides were imaged at 0.25 μm per pixel resolution using a ScanScope XT (Aperio, Vista, CA, USA). Full-slide scans were captured as high-resolution (0.21 microns/pixel) two-dimensional vector graphic files and selected regions were extracted using ImageScope software (Aperio). Analyses were performed using both slides and acquired images by trained histopathologists (DM, RD).
Results
Target gene expression patterns
We extracted DNA microarray data for genes known to have phenotype-specific expression patterns as well as other genes believed to have roles in metastatic progression (Figure 1B). Melan-A, encoded by the MLANA gene, is a protein of unknown function found in both melanosomal and Golgi-network structures of melanocytic cells [3]. Melan-A protein, also known as MART1 (Melanoma Antigen Recognized by T-cells), is processed by melanoma cells into a peptide that is presented at their surface, allowing them to be targeted by tumor infiltrating lymphocytes. MLANA mRNA expression in vitro is highest in proliferative phenotype melanoma cells and near background levels in invasive phenotype cells (p < 0.001). Microphthalmia-associated transcription factor (Mitf) is critical for the regulation of melanocyte development and survival [4]. It is responsible for regulating the expression of many genes including Melan-A [5,6] and is thought to be central to the regulation of cell phenotype during melanoma progression [2,7]. MITF expression in vitro is closely related to that of MLANA, with significantly increased expression in proliferative phenotype cells (p < 0.001). Wingless-type MMTV integration site family 5A (Wnt5A) is a secreted signalling protein of the non-canonical Wnt pathway involved in regulation of cell fate and embryogenic patterning [8]. In melanoma Wnt5A is reported to antagonize the expression of both Melan-A and Mitf, making cells less susceptible to T-cell cytolysis [9]. Correspondingly, we find the in vitro expression of its gene (WNT5A) is significantly higher in invasive phenotype cells (p < 0.001) and anticorrelates with MLANA/MITF expression [1,9,10]. Glucose transporter type 1 (Glut-1), encoded by the SLC2A1 gene, is an important glucose transporter in neuronal tissues and in cancer is associated with intra-lesional hypoxia [11]. As hypoxia is considered to be a critical factor in effecting phenotype switching in melanoma cells, we used Glut-1 expression as a proxy for identifying intra-lesional regions of hypoxia. In vitro, where oxygen levels are normal, SLC2A1 mRNA expression does not change across melanoma phenotypes. The CD31 antigen (also known as platelet-endothelial cell adhesion molecule 1, PECAM1) is a transmembrane protein involved in adhesive interactions between endothelial cells which is widely used as a marker in IHC examinations of vascular endothelia [12,13]. We find that in vitro PECAM1 expression is unchanged across melanoma cell phenotypes.
Primary melanoma lesion immunohistochemistry
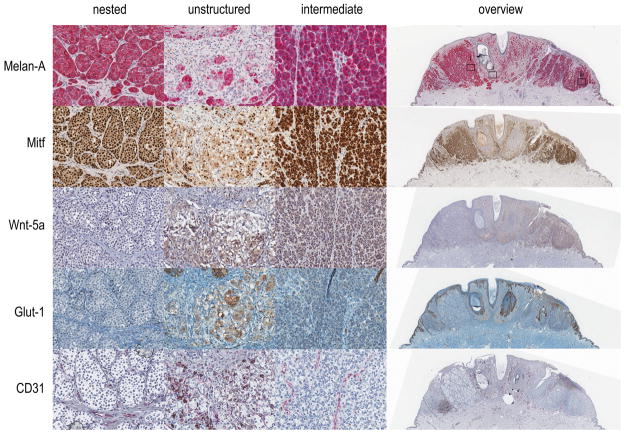
The primary melanoma lesion was observed to have three morphologically distinct regions. The first (which we refer to as nested) is an area of monomorphic groups of cells with round nuclei separated by a canalicular network of elongated cells with flattened nuclei. The second region (unstructured) is an area of cells in which the nested structures are broken up and intermixed. The third (intermediate) resembles the nested region except that the cell nests are irregular with fewer and smaller cells that are more tightly packed together and have less well-defined nuclei. This intermediate region also has a canalicular network but it is significantly less coherently arranged than in the nested region. The primary tumour was stained for Melan-A, Mitf, Wnt5A, Glut-1 and CD31, and each of the three identified regions were assessed in detail (Figure 2).
Figure 2. Immunostaining a primary melanoma lesion.
A 3 mm thick primary melanoma lesion fixed in formalin and embedded in paraffin was sectioned and immunostained for Melan-A, Mitf, Wnt5A, Glut-1 and CD31. Examples of three separate regions including (i) nested, (ii) unstructured, and (iii) intermediate are shown in detail, as well as an overview of the complete section, for each marker.
The nested region of the primary lesion showed that the cell nests consistently stained for Melan-A while the canalicular network remained unstained. Mitf staining was clearly apparent in cell nest nuclei and absent from the canalicular network. For Wnt5A we observed no staining in either cell nests or network cells. There was a little Glut-1 staining among network cells (marking possible erythrocytes) and none in cell nests. Interestingly, there was significant CD31 staining among network cells, particularly where vessel lumens were apparent.
In unstructured regions, staining for both Melan-A and Mitf was lighter and heterogeneous. Conversely, staining patterns for Glut-1 and Wnt5A were significantly increased. CD31 staining was also present, but revealed fewer well-defined vesicular structures than was seen in nested regions. All staining patterns appeared heterogeneous and were not related to particular cell morphologies. Interestingly, it is apparent that there are signs of cytoplasmic pigmentation in cells unstained by Melan-A. One possibility is that these cells had previously expressed melanocytic factors, or alternatively they may simply represent melanophages.
Melan-A and Mitf staining patterns in the intermediate region was consistent with nested staining patterns, although the intensity of staining was heterogeneous with some nest cells being unstained and cytoplasmic Mitf staining was heavier. Wnt5A and Glut-1 staining patterns, however, appeared to include nest cells as well as network cells. CD31 staining in the intermediate region was strikingly aligned with subgroups of network cells, but there were fewer clear vessel structures than could be discerned in nested regions.
Gallbladder melanoma lesion immunohistochemistry
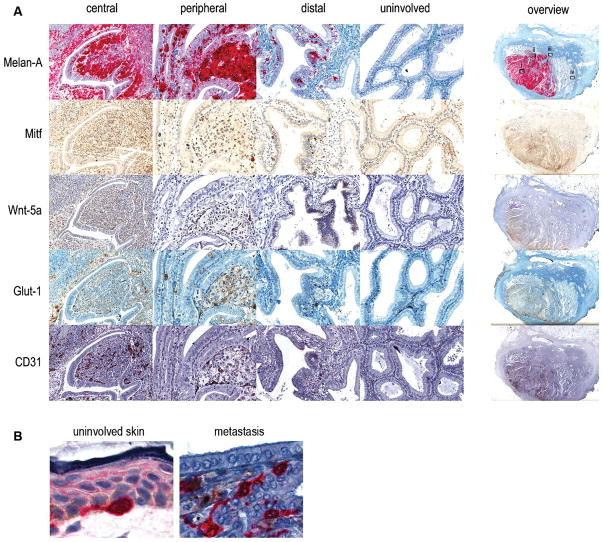
A matching melanoma metastasis to the gallbladder from the same patient was available. We identified four distinct regions of this tumour to compare antigen-detection patterns. The first (central) is deep in the main metastasis adjacent to remnant columnar epithelia (not associated with lumina characteristic of healthy tissue). The second (peripheral) is on the metastasis periphery adjacent to largely intact columnar epithelial tissues within the gallbladder. The third region (distal) is clearly separated from the main metastatic structure and involves columnar epithelia enclosing a large lumen. A fourth region (uninvolved) was selected from an area rich in columnar epithelia unaffected by the metastasis. As with the primary lesion this metastasis was immunoassayed for Melan-A, Mitf, Wnt5A, Glut-1 and CD31 (Figure 3).
Figure 3. Immunostaining a gallbladder melanoma lesion.
(A) A gallbladder melanoma metastasis fixed in formalin and embedded in paraffin was sectioned and immunostained for Melan-A, Mitf, Wnt5A, Glut-1 and CD31. Examples of four separate regions including (i) central, (ii) peripheral, (iii) distal, and (iv) uninvolved are shown in detail, as well as an overview of the complete section, for each marker. (B) Detail of a Melan-A positive melanocyte in uninvolved skin (left) and the peripheral area of the gall bladder epithelium showing invading Melan-A positive tumor cells (right).
Most cells within central regions stained for Melan-A and Mitf, many of these cells appeared to be arranged in nests not dissimilar from those identified in the nested regions of the primary lesion. On the other hand, some cells adjacent to remnant columnar epithelia stained less strongly for these markers. We found also that a number of Melan-A/Mitf positive cells had infiltrated and dispersed within the remnant columnar epithelia. By comparison, staining both for Glut-1, CD31 and Wnt5A was limited to cells adjacent to columnar remnants. In the middle of the tumour, it is likely that CD31 staining is limited to macrophages.
Melan-A staining of peripheral regions typically highlighted cells much as they appear in central tissues but there were many cells dispersed within intact columnar epithelia (Fig. 3B). Mitf staining was weaker but corresponded closely to Melan-A staining. Interestingly, staining for Wnt5A and Glut-1 largely corresponded with Melan-A/Mitf expression, although staining was less consistent in the columnar epithelia. CD31 was clearly apparent in the stroma separating columnar epithelia.
In the distal region Melan-A staining revealed melanoma cells dispersed among columnar epithelia and not in the stroma. Most Melan-A stained cells aligned on the basement membrane and, as seen in similarly structured peripheral regions, tended to disperse along it. Mitf staining, although weaker, closely followed the dispersed basal membrane-aligned pattern. Wnt5A staining was somewhat different, in which nearly all cells of the columnar epithelia were positive for this marker, while stromal regions were not. Glut-1 staining, on the other hand, revealed a small number of marker-positive cells dispersed throughout the stroma which are most likely erythrocytes within stromal vasculature. CD31 staining was apparent in both the columnar epithelia (particularly near the apical membrane within these cells) as well as the stroma (similar to the pattern observed in this tissue type in peripheral regions).
While Melan-A staining was absent within uninvolved regions there was occasional staining for Mitf, although far less frequently than in any of the other regions examined, and it was restricted to the stromal interstices. While Wnt5A staining was absent, Glut-1 staining was identical to that found in the distal area with occasional labelling of erythrocytes. In comparison, uninvolved stromal regions were consistently stained for CD31 indicating the presence of vesicular structures.
Closer examination of the columnar epithelium in the peripheral and distal regions of the gall bladder metastasis highlighted Melan-A positive cells invading these structure (Fig. 3A). Interestingly, in comparison with a melanocyte of uninvolved skin there is no morphological difference and also signs of pigment are visible around Melan-A positive tumor cells in the columnar epithelium of the gall bladder (Fig. 3B).
Discussion
In previous studies our group derived a model for melanoma progression in which melanoma cells switch back-and-forth between phenotypes of proliferation and invasion to drive disease progression [1,2]. To distinguish between proliferative and invasive cells in vivo we used phenotype-specific markers Melan-A and Mitf (expressed by proliferative phenotype cells) and Wnt5A (expressed by invasive phenotype cells). Importantly, we show here that the phenotypes are readily identifiable in each of a matched primary/metastasis pair, as predicted by the phenotype switching model. We also show cells which express markers from both phenotypes confirming the presence of so-called intermediate phenotype melanoma cells which were predicted by DNA microarray studies [1].
In nested regions of the primary lesion nests of cells staining strongly for both Melan-A and Mitf did not stain for Wnt5A, indicating that these were proliferative phenotype melanoma cells. This differed in unstructured regions of the same lesion where Melan-A and Mitf staining was greatly reduced in cells with significant expression of Wnt5A, showing that these are invasive phenotype melanoma cells. These inverse expression patterns could also be seen in central regions of the gallbladder metastasis. Clonal evolution models attribute heterogeneity of staining patterns that persist between primary and metastatic lesions to stochastic processes [14]. This infers that since nearly all lesions retain cells which express melanocytic markers the cells responsible for metastatic spread retain these characteristics, while other melanoma cells have, due to accumulated mutation, presumably lost both their capacity to express melanocytic markers and metastatic potential [15]. Following the stochastic model this would have to be true because melanoma metastases almost always present with melanocytic characteristics. However, we have shown in vitro that while melanoma cells expressing melanocytic markers are proliferative they are weakly invasive [1]. Furthermore, we have demonstrated that cells which do not express melanocytic markers still give rise to xenograft lesions containing melanocytic cells [2]. This along with findings made by others [16] demonstrates that melanoma cells may switch on or off gene sets according to microenvironmental signalling. We therefore argue that cells expressing Wnt5A but not melanocyte markers are the cell type which escaped the primary lesion to seed the gallbladder metastasis, and there reverted to a proliferative (and melanocytic) phenotype. Indeed, many Melan-A expressing cells which have evolved from the cells which invaded columnar epithelial structures have taken on a dispersal pattern and morphology which is nearly indistinguishable from normal epithelial melanocytes (Fig 3B). This phenomenon highlights the plasticity of melanoma cells and further supports the phenotype switching hypothesis of melanoma progression. The basement membrane of the gallbladder is structured similarly to that of the skin [17], and it is likely that melanoma cells encountering this microenvironment are induced to morphologies which recall those of melanocytes at the epidermal/dermal interface.
Interestingly, in both primary lesion intermediate tissues and metastatic peripheral regions melanoma cells are stained by markers from both phenotypes. In our prior studies we have reserved discussion to melanoma cells which represented opposite ends of the phenotype spectrum of proliferation and invasion and argued that cells must switch between these to drive metastatic disease. We previously acknowledged there were cell line examples which appeared to be intermediates populating the continuum between phenotype extremes, and their expression profiles included genes from both proliferative and invasive signatures [2]. We conclude, based on co-expression of markers from both phenotypes, that the cells in the intermediate region of the primary lesion and the peripheral region of the metastasis are indeed intermediate phenotype melanoma cells.
We were also interested in examining the staining patterns of CD31, a specific marker for blood vasculature, in human melanoma tissues. Therefore, the expression pattern of blood vessel marker was compared against those of known phenotype-specific factors (Mitf/Wnt5A). In nested regions of the primary we observed extensive CD31 staining of network cells, particularly of cells enclosing lumens. In conjunction with Glut-1 expression (a hypoxia marker) being absent in cell nests this strongly hints at the presence of a functional microcirculatory system. Such a distribution of structures and markers has bearing on the phenotype switching model for melanoma progression. It has been previously proposed by us that hypoxia provides a mechanism for driving the switch from a proliferative to an invasive melanoma cell phenotype [2]. It would explain why in nested regions, well serviced by oxygen-supplying blood vessels, there is no evidence of Wnt5A-expressing invasive phenotype cells. This is in stark contrast to the unstructured regions of the primary where the arrangement is a disorganized mass of differently shaped cells. Here there is, despite frequent CD31 staining, very little indication of the presence of a functioning microcirculatory system and increased Glut-1 staining suggests that hypoxia levels are high. Accordingly, Melan-A/Mitf levels are reduced and Wnt5A staining is strong, suggesting that these cells are invasive phenotype melanoma cells.
There were apparent off-target stainings in the gallbladder metastasis. Among the most interesting were the few Mitf-positive nuclei identified in uninvolved regions. These cells are distinct from Mitf-positive cells found in other regions because they do not express Melan-A and they are located in the stroma. This suggests that these cells are instead mast cells, which express Mitf and were previously reported to be present in the connective tissues of gallbladder [18,19]. Furthermore, gallbladder is a derivative of the endoderm and normal melanocytic cells have yet to be described in organs of endodermal origin [20]. Wnt5A expression in distal but not uninvolved region epithelia is difficult to explain. It may be unrelated to the melanoma metastasis as this factor is known to be involved in apicobasal cell polarization in developing gut epithelia [21,22].
In conclusion we show here, in a matched primary/metastasis pair from a human melanoma patient, expression patterns of factors associated with proliferation, invasion and vascularisation which correlate with what is expected from the phenotype switching model of melanoma progression. This parallels earlier studies performed under culturing conditions and in xenografted mouse experiments. Furthermore, as pathologists we focus on cells whose morphology has significantly deviated from non-neoplastic progenitor cells (e.g. large size, pleomorphic nuclei) to identify the malignant drivers of metastatic disease. However, the example we present here shows that melanoma cells in a distal metastasis can present in a form identical to normal epidermal melanocytes. This chameleon-like behaviour, in which cellular shape and molecular complement remain highly plastic throughout disease progression, highlights the difficulty faced by pathologists as they seek to adequately interpret what the microscope reveals.
Acknowledgments
This work was supported by grants from Krebsliga-Oncosuisse (KSH), the Julia Bangerter Rhyner Stiftung (RD) and the Intramural Research Program of the National Institute on Aging, Baltimore, MD (ATW).
References
- 1.Hoek KS, Schlegel NC, Brafford P, Sucker A, Ugurel S, Kumar R, et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 2006;19:290–302. doi: 10.1111/j.1600-0749.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoek KS, Eichhoff OM, Schlegel NC, Doebbeling U, Schaerer L, Hemmi S, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 3.De Maziere AM, Muehlethaler K, van Donselaar E, Salvi S, Davoust J, Cerottini JC, et al. The melanocytic protein Melan-A/MART-1 has a subcellular localization distinct from typical melanosomal proteins. Traffic. 2002;3:678–693. doi: 10.1034/j.1600-0854.2002.30909.x. [DOI] [PubMed] [Google Scholar]
- 4.Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the Microphthalmia Transcription Factor Network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- 5.Du J, Miller AJ, Widlund HR, Horstmann MA, Ramaswamy S, Fisher DE. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol. 2003;163:333–343. doi: 10.1016/S0002-9440(10)63657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoek KS, Schlegel NC, Eichhoff OM, Widmer DS, Praetorius C, Einarsson SO, et al. Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell Melanoma Res. 2008;21:665–676. doi: 10.1111/j.1755-148X.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- 7.Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS, et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006;20:3426–3439. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 9.Dissanayake SK, Olkhanud PB, O’Connell MP, Carter A, French AD, Camilli TC, et al. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68:10205–10214. doi: 10.1158/0008-5472.CAN-08-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 11.Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy. 2007;53:233–256. doi: 10.1159/000104457. [DOI] [PubMed] [Google Scholar]
- 12.Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990;43:752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzu I, Bicknell R, Harris AL, Jones M, Gatter KC, Mason DY. Heterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumours. J Clin Pathol. 1992;45:143–148. doi: 10.1136/jcp.45.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 15.de Wit NJ, van Muijen GN, Ruiter DJ. Immunohistochemistry in melanocytic proliferative lesions. Histopathology. 2004;44:517–541. doi: 10.1111/j.1365-2559.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- 16.Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 17.Juvonen T, Kairaluoma MI, Malinen H, Niemela O. Extracellular matrix proteins in bile and serum of patients with gallstone disease. Connect Tissue Res. 1993;29:171–180. doi: 10.3109/03008209309016824. [DOI] [PubMed] [Google Scholar]
- 18.Nechushtan H, Zhang Z, Razin E. Microphthalmia (mi) in murine mast cells: regulation of its stimuli-mediated expression on the translational level. Blood. 1997;89:2999–3008. [PubMed] [Google Scholar]
- 19.Toledo OM, Morales CR, Pereyra LA, Jordao T, Montes GS. Migrating mast cells in the gallbladder epithelium of cattle and sheep. A comparative morphologic and histochemical study. Histochemistry. 1981;72:433–442. doi: 10.1007/BF00501785. [DOI] [PubMed] [Google Scholar]
- 20.Safioleas M, Agapitos E, Kontzoglou K, Stamatakos M, Safioleas P, Mouzopoulos G, et al. Primary melanoma of the gallbladder: does it exist? Report of a case and review of the literature. World J Gastroenterol. 2006;12:4259–4261. doi: 10.3748/wjg.v12.i26.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuyama M, Aizawa S, Shimono A. Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS genetics. 2009;5:e1000427. doi: 10.1371/journal.pgen.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Dev Biol. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]