Abstract
The epidermal growth factor receptor (EGFR), a member of the ErbB family of receptor tyrosine kinases, plays an important role in the control of cell growth and differentiation. Disruption of its signaling leads to neoplastic cell proliferation, migration, stromal invasion, resistance to apoptosis, and angiogenesis.
EGFR is overexpressed in a variety of solid tumors, including colorectal cancer (CRC), and its overexpression is associated with poorer prognosis. One class of agents that is currently used to target EGFR in the treatment of metastatic CRC (mCRC) is the monoclonal antibodies. While the monoclonal antibody EGFR inhibitors lack many of the severe side effects commonly observed with cytotoxic chemotherapy, they are associated with a set of unique dermatological toxicities. This paper reviews the safety profile of the anti-EGFR monoclonal antibodies cetuximab and panitumumab in the treatment of mCRC.
Keywords: epidermal growth factor receptor, skin toxicity, cetuximab, panitumumab
1. INTRODUCTION
The epidermal growth factor receptor (EGFR), a member of the ErbB family of receptor tyrosine kinases1,2,3, is a transmembrane glycoprotein composed of an extracellular ligand-binding domain, a transmembrane lipophilic segment, and an intracellular protein kinase domain4.
EGFR is activated by EGF-like ligands, including EGF, transforming growth factor alpha (TGF-α), amphiregulin, heparin-binding EGF-like growth factor, betacellulin, and epiregulin5. Binding of these ligands results in EGFR dimerization, which leads to high-affinity ligand binding, activation of the intrinsic protein tyrosine kinase (TK) activity, and tyrosine autophosphorylation. Activation of the intracellular protein TK leads to recruitment and phosphorylation of several intracellular substrates, triggering a variety of cellular responses including cell division, survival, motility, invasion, adhesion, and cellular repair6,7. EGFR therefore plays an important role in the control of cell growth and differentiation8, and disruption of its signaling leads to neoplastic cell proliferation, migration, stromal invasion, resistance to apoptosis, and angiogenesis9.
EGFR is overexpressed in a variety of solid tumors, including colorectal cancer (CRC), squamous cell cancer of the head and neck, and non-small-cell lung cancer. EGFR overexpression is associated with poorer prognosis in these malignancies10–14 and, in CRC, may be associated with an advanced disease stage15–18.
One class of agents that is currently used to target EGFR in the treatment of metastatic CRC (mCRC) is the monoclonal antibodies (MoAbs), which compete with endogenous ligands, including EGF and TGF-α to block ligand-dependant activation of EGFR, and induce receptor internalization and consequent downregulation19. This paper reviews the safety profile of the anti-EGFR MoAbs cetuximab and panitumumab in the treatment of mCRC.
2. DERMATOLOGICAL TOXICITIES
While the MoAb EGFR inhibitors lack many of the severe side effects commonly observed with cytotoxic chemotherapy, they are associated with a set of unique dermatological toxicities. The majority of patients treated with a MoAb EGFR inhibitor will experience dermatological side effects, most notably the papulopustular skin rash, which can impact quality of life and affect adherence to therapy20.
EGFR is expressed in the basal layer of the epidermis and contributes to the stimulation of epidermal growth, inhibition of differentiation, and acceleration of wound healing. Inhibition of EGFR results in impaired growth and migration of keratinocytes, and inflammatory chemokine expression by these cells. The resulting inflammatory cell recruitment and subsequent cutaneous injury account for the majority of dermatological symptoms associated with anti-EGFR therapy including papulopustular eruption, hair growth disorders, periungual and nail plate abnormalities, xerosis, telangiectasias, and pruritus13,21–29. Disruptions to this barrier may also promote bacterial overgrowth30, further exacerbating injury to the cutaneous tissue.
Skin toxicities have been reported in 80–95% of patients with mCRC treated with cetuximab and panitumumab monotherapy (Tables 1, 2)31, with a similar frequency seen in trials of cetuximab in combination with chemotherapy (Table 3). The most severe skin toxicities (grades 3–4) have been seen in trials combining bevacizumab with the EGFR inhibitors32–34 In the CAIRO2 trial, the addition of cetuximab to capecitabine, oxaliplatin and bevacizumab in the first-line treatment of mCRC did not result in excessive toxicity33. However, an overall increase in grade 3–4 toxicity was seen in the cetuximab arm, which was fully attributed to cetuximab-related skin toxicity. In the BOND trial, grade 3–4 skin toxicity occurred at a rate of 13% in the group treated with cetuximab and irinotecan13. In BOND2, the grade 3 skin rash was observed in 21% of patients treated with a combination of cetuximab, irinotecan, and bevacizumab35, suggesting that there is considerable worsening of skin toxicity with the addition of bevacizumab to an anti-EGRF treatment regimen.
Table I.
AEs in cetuximab monotherapy trials31
| Saltz et al. (2004) | Cunningham et al. (2004) | Lenz et al. (2006) | Jonker et al. (2007) | Wierzbicki et al. (2008) | |
|---|---|---|---|---|---|
| n of patients | 57 | 115 | 346 | 287 | 85 |
| Any AE, n (%) | NR | 50 (43.5) | NR | 226 (78.5) | 81 (95.3) |
| Any skin toxicity (%) | 88 | 80 | 82.9 | 88.6 | NR |
| Phase | II | II/III | II | III | II |
| Grade 3/4 AEs, % | Acne, 16; asthenia, 4; atrial fibrillation, 2; hypokalemia, 2; rash, 2; vomiting, 2; confusion, 2; diarrhea, 2; headache, 2 | Dyspnea, 13.0; asthenia, 10.4; acne-like rash, 5.2; abdominal pain, 5.2; nausea/vomiting, 4.3; anemia, 2.6; diarrhea, 1.7; thrombocytopenia, 0.9; stomatitis, 0.9 | Acne, 4.9; asthenia, 2.0; headache, 1.2; diarrhea, 1.2; nausea, 0.6; dry skin, 0.6; fever, 0.3 | Fatigue, 33.0; dyspnea, 16.3; abdominal pain, 13.2; pain–other, 14.9; infection without neutropenia, 12.8: rash or desquamation, 11.8; hypomagnesemia, 5.8; edema, 5.2; anorexia, 8.3; constipation, 3.5; nausea, 5.6; vomiting, 5.6; confusion, 5.6 | Dermatitis, 4.7; hypomagnesemia, 4.7; dyspnea 2.4; headache, 1.2 |
| Onset of skin toxicity | 1–3 wks | 1–3 wks | 8–19 days | NR | NR |
| Infusion reactions, type, n (%) n of patients | Allergic reactions, 3 (5) | Hypersensitivity reaction, 4 (3.5) | Hypersensitivity reaction, 26 (7.5) | Hypersensitivity reaction, 13 (4.5) | Infusion reaction grade ≥3, (3.5) |
Table II.
AEs in panitumumab monotherapy trials31
| Van Cutsem et al. (2007) | Van Cutsem et al. (2008) | Berlin et al. (2006) | Hecht et al. (2008) | Hecht et al. (2007) | |
|---|---|---|---|---|---|
| 229 | 176 | 93 | 203 | 148 | |
| Any AE, n (%) | 79 (35) | 32 (18) | 23 (25) | 88 (42) | 18 (12) |
| Any skin toxicity (%) | 90% | NR | 96 | NR | 95 |
| Phase | III | II | II | II | II |
| Grade 3/4 AE, % | Acneiform rash, 7.4; abdominal pain, 7.4; erythema, 5.2; dyspnea, 4.8; fatigue, 4.4; anorexia, 3.5; asthenia, 3.1; constipation, 2.6; pruritus, 2.2; skin exfoliation, 2.2; vomiting, 2.2; hypomagnesemia, 3.0; back pain, 1.7; paronychia, 1.3; diarrhea, 1.3; nausea, 0.9; rash, 0.9; skin fissures, 0.9; edema, 0.9; cough 0.4 | Acne, 6.2; erythema, 5.1; rash, 4.5; other skin manifestations, 2.3; paronychia, 1.7; pruritus, 1.1; skin exfoliation, 0.6; diarrhea, 0.6; conjunctivitis, 0.6 | Acneiform rash, 9.9; erythema, 6.6; rash, 3.3; pruritus, 2.2; paronychia, 2.2; hypokalemia, 2.2; exfoliation, 1.1; skin fissures, 1.1; vomiting, 1.1; anorexia, 1.1; hypomagnesemia, 1.1 | Acneiform rash, 6; erythema, 5; pruritus, 3; rash, 3; exfoliation, 3; nausea/vomiting, 2; fatigue/asthenia, 2; diarrhea, 2; dyspnea, 1; infections, 6 | Rash, 3; fatigue, 3; vomiting, 1; pruritus, 1; nausea, 1; diarrhea, 1; dyspnea, 1 |
| Onset of skin toxicity | 12–15 days | NR | 6–13 days | NR | 9–14 days |
| Infusion reactions, type, n (%) | Infusion reaction, 0 (0); only one grade 2 reaction | Moderate hypersensitivity, 1 (0.6) | Infusion reaction, 1 (1) | Infusion reaction, grade 3 or 4, 7 (3) | Hypersensitivity reaction, 1 (0.7) |
Table III.
Adverse events of trials with combination cetuximab therapy in the treatment of metastatic colorectal cancer31
| BOND (Cunningham 2004) N=212 | CRYSTAL (Raoul 2009) N=87 | OPUS (Bokemeyer ECCO 2007) N=337 | BOND2 (Saltz 2007) N=83 | CAIRO2 (Tol Ann Oncol 2008) n=192 | ||
|---|---|---|---|---|---|---|
| Treatment regimen | CET + IRI | CET + IRI +5-FU | CET plus 5-FU/FA/oxaliplatin (FOLFOX-4) | CET + BEV + IRI n=43 | CET + BEV N=40 | Capecitabine + oxaliplatin + BEV + CET |
| Any AE | 65.1 | NR | NR | NR | NR | NR |
| Skin toxicity | 80 | 52% (rash) | NR | 83 | 85 | 92 |
| Grade 3/4 AE, % | Anemia 4.7 Neutropenia 9.4 Thrombocytopenia 0.5 Diarrhea 21.2 Asthenia 13.7 Acne-like rash 9.4 Nausea and vomiting 7.1 Abdominal pain 3.3 Stomatitis 2.4 Dyspnea 1.4 Fever 2.4 |
Leukopenia 21.2 Diarrhea 12 Vomiting 12 Rash 12 Acne 10 Asthenia 10 Intestinal obstruction 10 Abdominal pain 6 Mucous membrane disorder 6 Dyspnea 8 Atrial fibrillation 4 Deep thrombophelbitis 4 Gamma glutamyl transpeptidase increased 4 Hypokalaemia 4 Liver function test abnormal 4 Skin disorder 4 Thrombosis 4 Urinary tract infection 4 Weight loss 4 |
Neutropenia 27.6 Diarrhea 7.1 Neurotoxicity 3.5 Leukopenia 7.1 Fatigue 3.5 Skin reactions 14.1 Infusion-related reactions 4.1 |
Skin rash 21 Paronychial cracking 7 |
Skin rash 20 Paronychial cracking 5 Headache 5 |
Acneiform skin rash 26 Nail changes 6 Dry skin 0.5 Hand–foot syndrome 16 Diarrhoea 23 Nausea 6 Vomiting 6 Hypertension 4 Proteinuria 0.5 Cardiac ischaemia 2 Cerebrovascular ischaemia 0.5 Sensory neuropathy 6 Thromboembolic event 8 Infection 5 Febrile neutropenia 0 Allergic reaction 7 Bleeding 1 Gastrointestinal perforation 1 Hypomagnesaemia 2 |
The PACCE (Panitumumab Advanced Colorectal Cancer Evaluation) study evaluated the efficacy and safety of adding panitumumab to combination chemotherapy with bevacizumab for the first-line treatment of mCRC34. PACCE was stopped early when a planned interim analysis revealed an increased incidence of toxicity with no improvement of increased efficacy in the panitumumab arm. Grade 3–4 skin toxicity was observed in 36% of patients treated with panitumumab.
2.1. Papulopustular rash
The most common adverse event among mCRC patients treated with the anti-EGFR MoAbs is the papulopustular rash, characterized by erythematous inter- and intrafollicular papulopustules and commonly affecting sun-exposed areas of the body36 such as the face, neck, shoulders, upper body, and scalp37,38. In clinical trials of cetuximab and panitumumab monotherapy, papulopustular rash occurred in the majority of patients, but in most cases were mild-to-moderate in severity (Tables 1, 2).
The onset of rash is early, generally developing over a period of six weeks after starting treatment39. The first week is characterized by sensory disturbance, erythema, and edema, followed by papulopustular eruption during the second week. Crusting appears by Week 4. If treated successfully, the papulopustular eruptions disappear and make way for erythema and dry skin by Week 6. In some cases, the rash may improve spontaneously40, but may also persist26. Phase I escalation studies have shown the rash to be dose-dependant40–42.
Although the rash may resemble acne vulgaris, it is clinically and histologically different. Therefore, terms that imply a similarity with acne, such as “acneiform rash”, “acneiform follicular rash”, “acne-like rash”, “maculopapular rash”, or “monomorphic pustular lesions”, should not be used to describe the papulopustular rash associated with anti-EGFR therapy36,37,43,44.
2.1.1. Correlation between the efficacy of EGFR inhibitors and the occurrence of rash
Data from the clinical trials of cetuximab and panitumumab in the treatment of mCRC suggest a positive correlation between the presence and severity of rash and survival13,32,45–49. In the first phase II open-label trial of cetuximab monotherapy for the treatment of refractory mCRC, longer survival times were observed in patients who experienced a rash of any grade compared with patients who did not have a rash (p = 0.02)32. In the pivotal BOND study comparing cetuximab in combination with irinotecan with cetuximab alone for the treatment of mCRC, patients with skin reactions had higher response rates than patients without skin reaction (25.8% vs. 6.3% in the combination group; 13.0% vs. 0% in the monotherapy group; p = 0.005)13.
Similar results have been observed in phase II and III studies of panitumumab. In a phase II study of 148 patients with EGFR-positive mCRC, grades 2–4 skin toxicity was associated with longer PFS (HR 0.67; 95% CI 0.50 to 0.90) and OS (HR 0.72; 95% CI 0.54 to 0.97) compared with grades 0–1 skin toxicity49. In the pivotal phase III, open-label trial comparing panitumumab monotherapy with best supportive care for the treatment of mCRC, exploratory analysis revealed a trend toward longer progression-free survival (HR 0.62; 95% CI 0.44–0.88) and overall survival (HR 0.59, 95% CI 0.42–0.85) in patients with grade 2–4 skin toxicity compared with patients with grade 1 skin toxicity50.
The correlation between rash and response to the anti-EGFR treatment suggests that treatment response might be optimized by increasing the dose until the appearance of rash. The phase I/II EVEREST (Evaluation of Various Erbitux Regimens by Means of Skin and Tumor Biopsies) trial randomly assigned patients with no rash or grade I rash to treatment with standard-dose cetuximab (250 mg/m2/week) plus irinotecan or an increasing dose of cetuximab (50 mg/m2 every two weeks until grade 2 or higher toxicity, tumor response, to a maximum dose of 500 mg/m2)51. Skin toxicity and response rates both increased with dose escalation. Mean PFS was 4.8 months in the dose-escalation group compared with 3.9 months in those who received standard-dose cetuximab51. As KRAS mutation status has been shown to be a predictor of tumor response to anti-EGFR treatment, the EVEREST trial sought to determine whether dose escalation would also be able to induce a response in patients with KRAS mutations. KRAS and skin toxicity were found to be independent predictors of outcomes. Among patients with wild-type KRAS tumors and grade 0–1 rash, dose escalation improved response rates compared with the standard-dose group (46.4% vs. 21.1%). However, none of the patients with KRAS mutations achieved a response, regardless of the dose51.
These results suggest that the characteristic rash associated with EGFR inhibitors may have potential as a surrogate marker of efficacy in patients with KRAS wild type tumors.
2.1.2. Management of skin rash associated with cetuximab and panitumumab
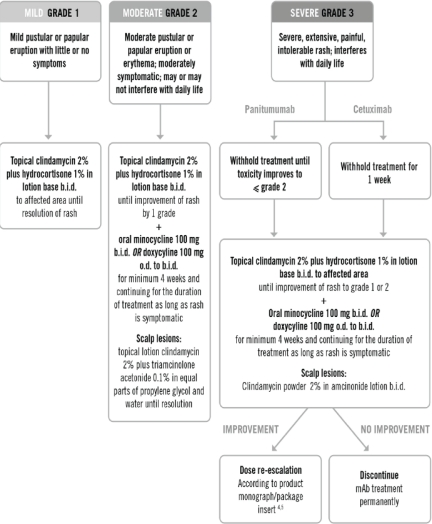
Canadian guidelines have recently been developed for the prevention and management of dermatological toxicities associated with anti-EGFR MoAb treatment52. General principles include practicing sun-protective measures and avoidance of activities and products that are likely to dry the skin (e.g. long, hot showers; alcohol-based/perfumed products; over-the-counter acne medications). Oatmeal baths and creams may provide symptomatic relief. Management should be individualized according to the type, severity, and location of the rash. Specific treatment recommendations for mild, moderate, and severe rash are outlined in the British Columbia Cancer Agency’s rash protocol for EGFR inhibitors (Fig. 1). Twice daily application of topical clindamycin 2% plus hydrocortisone 1% in a lotion base is recommended for the treatment of mild rash. Moderate and severe rash may require the addition of oral minocycline or doxycycline.
Figure 1.
Treatment recommendations for rash mediated by monoclonal antibody targeting of epidermal growth factor receptor, by severity (Adapted from the BC Cancer Agency’s EGFR inhibitors rash protocol.)
Researchers at the Memorial Sloan-Kettering Cancer Center evaluated the ability of topical tazarotene, with or without oral minocycline, to reduce or prevent papulopustular rash when administered in conjunction with cetuximab therapy53. Forty-eight patients with mCRC who were about to start therapy with cetuximab were randomly assigned to receive daily treatment with oral minocycline (n = 24) or placebo (n = 24), and topical tazarotene on either the left or right side of their face. Both therapies were administered for eight weeks. During the first four weeks, minocycline treatment was associated with significantly fewer facial lesions and lower rates of severe itch compared with placebo (20% vs 50%, p = 0.05). By Week 8, these differences were no longer significant. Tazarotene application did not produce any clinical benefit and was, in fact, associated with significant irritation, resulting in its discontinuation in one-third of patients.
The largest randomized prospective study to demonstrate the efficacy of prophylactic intervention in reducing the risk of skin toxicity with an anti-EGFR therapy was the STEPP (Skin Toxicity Evaluation Protocol with Panitumumab) trial. The STEPP trial evaluated the differences between pre-emptive and reactive treatments for skin toxicities associated with EGFR inhibition by panitumumab in 58 patients receiving panitumumab plus FOLFIRI or irinotecan-only chemotherapy for second-line treatment of mCRC54. Patients with mCRC who had previously failed treatment with oxaliplatin-based chemotherapy were randomly assigned to biweekly treatment with FOLFIRI–based chemotherapy plus panitumumab or treatment with irinotecan-based chemotherapy plus panitumumab every three weeks. Within each treatment group, patients were randomly assigned to receive skin toxicity treatment 24 hours before the first panitumumab dose and then daily through Week 6 (preemptive) or after skin toxicity developed (reactive)54. Skin toxicity treatment included the use of skin moisturizers, sunscreen, 1% hydrocortisone cream, and doxycycline 100 mg bid. Preemptive treatment reduced the incidence of grade 2 or greater skin toxicities by more than 50% compared with reactive treatment, without additional side effects. Time to severe skin toxicity and time to first occurrence of a grade 2 or greater skin toxicity were also significantly delayed by preemptive treatment. Prophylactic management of skin toxicity was not associated with any reduction in efficacy when compared to the reactive skin toxicity arm.
In addition to treatment with topical and oral antibiotics, specific dose reductions and treatment delays are recommended for patients who develop severe rash on cetuximab (Table 4)55.
Table IV.
Grades 3 and 4 events in the STEPP trial20
| Pmab + FOLFIRI Q2W | Pmab + Iri Q3W | Pmab + FOLFIRI Q2W | Pmab + Iri Q3W KRAS | |||||
|---|---|---|---|---|---|---|---|---|
| P (n=28) | R (n=27) | P (n=20) | R (n=20) | WT KRAS (n=32) | Mut KRAS (n=19) | WT KRAS (n=17) | Mut KRAS (n=19) | |
| Dermatitis acneiform - n (%) | 2 (7) | 7 (26) | 0 (0) | 3 (15) | 7 (22) | 2 (11) | 1 (6) | 2 (11) |
| Diarrhea - n (%) | 6 (21) | 9 (33) | 1 (5) | 6 (30) | 10 (31) | 4 (21) | 2 (12) | 4 (21) |
| Dehydration - n (%) | 3 (11) | 7 (26) | 0 (0) | 6 (30) | 5 (16) | 4 (21) | 1 (6) | 4 (21) |
| Neutropenia - n (%) | 3 (11) | 7 (26) | 1 (5) | 5 (25) | 5 (16) | 3 (16) | 3 (18) | 3 (16) |
| Deep vein thrombosis – n (%) | 0 (0) | 1 (4) | 0 (0) | 1 (5) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
2.2. Xerosis
Xerosis, or excessive dryness of the skin, is generally characterized by diffuse, fine scaling. Xerosis occurs in up to 35% of patients treated with an EGFR inhibitor56, and is more common among older patients or those with a history of atopic eczema37. Xerosis may be complicated by chronic asteototic eczema, or “winter eczema”, which is characterized by pruritic, dry, cracked, and polygonally fissured skin with irregular scaling.
Xerosis is associated with a significant decrease in free fatty acids in the stratum corneum. Cutaneous loss of these fatty acids increases transepidermal water loss, causing the cells to shrink and reducing the skin’s elasticity. This disruption of the epidermal layer can lead to inflammation and an increased risk of infection by Staphylococcus aureus or, less commonly, herpes simplex virus type I23,24. Systemic and/or topical antibiotics may be required57. Pruritus may be alleviated with antihistamines39, and anecdotal reports suggest that pregabalin may control pruritus associated with cetuximab therapy58.
Painful fissures may appear on the palms, fingertips, soles of the feet and toes, and on the lips20,21,24,59,60. Fissures should be treated with emollients, and sealed with cyanoacrylate or flurandernolide tape that delivers high-potency steroids and protects against mechanical trauma61.
2.3. Paronychia
Paronychia is an infection that occurs where the nail and skin meet at the side or the base of a fingernail or toenail. Occurring in 10% to 15% of patients treated with cetuximab and gefitinib24,26,29,62, this side effect generally presents four to eight weeks from the start of treatment with an EGFR inhibitor MoAb. Paronychia associated with EGFR blockade is characterized by an erythematous and painful inflammation of the nail fold, which may swell and form granulation tissue37. The nails may become brittle and slower growing. In severe cases, paronychia perungual abscess and pyogenic granuloma of the nail fold may develop62. Secondary bacterial (S. aureus) or fungal (Candida albicans) infection is common in paronychia associated with EGFR inhibition29,63,64.
Minocycline or doxycycline 100 mg bid and high-potency topical steroids may be effective in the treatment of paronychia61. Extreme cases may require nail local steroid injections or nail fulguration.
2.4. Hair changes
Some patients who undergo treatment with an EGFR inhibitor may experience changes to their hair, most notably an increased growth of the eyelashes (trichomegaly)37,65,66. However, the patient’s scalp hair may become finer, brittle, and curly, and hypertrichosis of the face may develop67, suggesting that the mechanism regulating hair growth may differ in different parts of the body66. In three clinical studies investigating the safety and efficacy of panitumumab monotherapy in mCRC in a single institution, hirsutism was reported in half of women who received panitumumab for more than six weeks68.
Hair changes usually appear later during the course of treatment – two to five months after beginning treatment – and generally resolve within a month of discontinuing treatment37.
2.5. Telangiectasias and hyperpigmentation
Telangiectasias are small dilated blood vessels that develop in a small proportion of patients taking EGFR inhibitors, generally appearing on the face, chest, back, and limbs. Hyperpigmentation may result from fading telangiectasias24,59,60 or as a consequence of inflammation. Because telangiectasias and hyperpigmentation usually occur as a result of photosensitivity, patients being treated with an EGFR inhibitor should be counseled to practice sun protection. The Canadian Dermatology Association has outlined sun protection for the general population, which includes wearing an SPF 30 broad-spectrum sunscreen, scheduling outdoor activities before 11 am and after 4 pm, and wearing appropriate clothing to cover the skin, including hat. Darker-skinned individuals, in particular, are susceptible to hyperpigmentation24,43,59,60,68,69.
2.6. Radiation dermatitis
With the increasing use of EGFR inhibitors with or following radiotherapy, recent reports have indicated a potential for cetuximab to enhance the severity of radiation dermatitis70–72. The radiation oncology department of the University of Dusseldorf, Germany, observed two cases of unusually severe radiation dermatitis among a small group of five patients with head and neck cancer (HNC) treated with irradiation and concurrent cetuximab71. The appearance of these cases prompted the researchers to conduct a survey of members of the EORTC Head and Neck Radiation Oncology Group. Among 71 HNC patients from 15 institutions who had been treated with cetuximab and concurrent radiotherapy and for whom information on dermatological reactions was available, 15 and 20 patients developed grade 3 and grade 4 radiation dermatitis (49%), respectively73. In another recent report of 13 consecutive patients with HNC treated with concurrent cetuximab and radiotherapy, 10 (77%) experienced severe skin reactions (grade 3–4), which were associated with low treatment compliance and delays in completing RT74.
These results are in contradiction to results of a large, multinational, randomized study, where the concurrent addition of cetuximab to radiation treatment did not increase the rate of grade 3–4 radiation dermatitis (11% cetuximab plus radiotherapy vs 8.5% radiotherapy alone; p = 0.27)75. A potential reason for this discrepancy in the German study is selection bias, as institutions that had observed cases of severe radiation dermatitis may have been more likely to respond to the survey than those who had not. As well, the total number of patients might have been underestimated due to the lack of formal registry of cetuximab patients in most institutions. Another potential reason for the increase in radiation dermatitis observed in the more recent studies is the removal of radiation dermatitis as a dose-limiting toxicity of radiotherapy, since the introduction of megavoltage radiotherapy. As a result, severe radiation dermatitis may have been underreported in the earlier trial71,73.
3. OCULAR TOXICITIES
Ocular toxicities such as conjunctivitis and blepharitis with increased lacrimation have been reported for both cetuximab and panitumumab47,76.
Blepharitis, or inflammation of the lid margin, results from inflammation of the meibomian glands, which contain EGFR-expressing cells. Symptoms include itching and watering of the eyes and lids, and crusting of the lashes. In general, treatment of blepharitis includes warm compresses, eyelid scrubs, and topical antibiotic. Eyelid cultures should be obtained if the condition fails to improve with these measures77. While there are no clear guidelines for dose-modifications with EGFR inhibitors, in the case of cetuximab, Dranko et al. recommend following the dose-modification regimen recommended for papulomacular rash (Table 5)77,78.
Table V.
Dose Modifications Guidelines for severe acneiform rash in patients taking cetuximab78
| Severe Acneiform Rash | Cetuximab | Outcome | Cetuximab Dose Modification | |
|---|---|---|---|---|
| 1st | occurrence | Delay infusion 1 to 2 weeks | Improvement No improvement |
Continue at 250 mg/m2 Discontinue cetuximab |
| 2nd | occurrence | Delay infusion 1 to 2 weeks | Improvement No improvement |
Reduce dose to 200 mg/m2 Discontinue cetuximab |
| 3rd | occurrence | Delay infusion 1 to 2 weeks | Improvement No improvement | Reduce dose to 150 mg/m2 Discontinue cetuximab |
| 4th | occurrence | Discontinue ERBITUX | ||
4. HYPOMAGNESEMIA
Hypomagnesemia has emerged as a relatively common side effect of cetuximab and panitumumab therapy48,79–84. In the early trials of anti-EGFR therapies, the incidence of hypomagnesemia was underestimated80,81, likely because these trials focused on patients with overt hypomagnesemia and who therefore had time to develop hypomagnesemia during the relatively short treatment intervals85–87. However, postmarketing experience with the anti-EGFR MoAbs began to reveal reports of severe hypomagnesemia88, and the incidence was found to increase with increasing duration of treatment87. In a retrospective review of 114 mCRC patients treated with cetuximab at the Roswell Park Cancer Center in Buffalo, grade 3–4 hypomagnesemia was observed in 5%, 23%, and 47% of patients who were treated with cetuximab for less than three months, for three to six months, and for more than six months, respectively87.
Panitumumab is associated with a similar risk of hypomagnesemia. Among patients with mCRC who were treated with panitumumab, with or without best supportive care, magnesium concentrations were reduced in 36% of the 231 patients treated with panitumumab (3% grade 3–4) compared with 1% of those receiving best supportive care alone50. A lower frequency of hypomagnesemia was observed in the PACCE trial, where the combination of panitumumab, fluorouracil, oxaliplatin, and leucovorin in the first-line treatment of mCRC was associated with only a 4% incidence of grade 3–4 hypomagnesemia34. This low frequency of hypomagnesemia has been attributed to a lack of stringent guidelines for magnesium monitoring84.
The timing of the onset of hypomagnesemia during treatment with anti-EGFR MoAbs can be inferred from the rate of magnesium loss and the duration of treatment. Among 98 consecutive patients with mCRC treated with anti-EGFR MoAbs in a Belgium study, 97% experienced a progressive decrease in serum magnesium concentrations after initiation of treatment, with a median time to hypomagnesemia of 99 days (range 12–639 days)79. Serum magnesium concentrations returned to normal shortly after discontinuation of the EGFR inhibitor79. The slope of the change in serum magnesium concentrations from baseline was calculated with three early time points and was found to correlate well with the slope of the entire dataset, suggesting that early characterization of magnesium wasting might be possible in clinical practice.
The mechanisms responsible for hypomagnesemia in association with anti-EGFR MoAbs have not been well defined. Increased EGFR expression in the ascending loop of Henle, where 70% of filtered magnesium is reabsorbed, may result in damage to the renal tubule and interfere with magnesium transport85,88.
Symptoms of hypomagnesemia can be cardiovascular, neuromuscular, or behavioral89. Cardiovascular symptoms include ventricular ectopic beats, hypertension, enhancement of digoxin-induced dysrhythmia, and cardiomyopathies and, in more severe cases, ventricular tachycardia, ventricular fibrillation, atrial fibrillation, and multifocal atrial tachycardia. Neuromuscular and behavioral symptoms include weakness, confusion, tetany, agitation, tremors and depression and, in more severe cases, convulsions, psychosis, ataxia, spasticity, and delirium. Hypocalcemia has been reported in association with hypomagnesemia87 and can contribute to neuromuscular symptoms. This hypomagnesimic hypocalcemia can only be corrected by replacing magnesium levels. The pathophysiology of hypocalcemia in this setting is related to hypomagnesemiainduced PTH resistance.
The impact of hypomagnesemia in patients undergoing anti-EGFR treatment for mCRC has been underestimated, most likely because magnesium levels are rarely measured during routine screening84. Patients’ electrolytes should be periodically monitored during – and for eight weeks after – the completion of anti-EGFR therapy. Hypomagnesemia should be suspected in mCRC patients being treated with cetuximab or panitumumab who present with chronic diarrhea, hypocalcemia, refractory hypokalemia, and ventricular arrhythmia84.
Hypokalemia has similarly been associated with anti-EGFR therapy, although to a lesser extent than hypomagnesemia. The exact mechanism has not been elucidated and it has been observed in the absence of diarrhea. Hypokalemia typically responds well to oral potassium supplementation.
4.1. Management of hypomagnesemia
Management of hypomagnesemia is dependent on the grade of severity outlined in Table 6. Patients with grade I hypomagnesemia are generally asymptomatic and do not require replacement therapy90. In patients with grade 2 hypomagnesemia, oral supplementation is generally ineffective and poorly tolerated due to diarrhea79,87. Weekly intravenous treatment with magnesium sulfate 4 g has been shown to be effective for patients with magnesium levels of 0.9 to 1.0 mg/dL (0.37–0.41 mmol/L)90. For patients with grade 2 hypomagnesemia who are asymptomatic and without cardiac risk factors, weekly monitoring without magnesium supplementation may be considered.
Table VI.
Grades of Severity of Hypomagnesemia: National Cancer Institute–Common Toxicity Criteria Version 3
| Grade 0 | Within normal limits |
| Grade 1 | < LLN–1.2 mg/dL or < LLN–0.5 mmol/L |
| Grade 2 | < 1.2–0.9 mg/dL or < 0.5–0.4 mmol/L |
| Grade 3 | < 0.9–0.7 mg/dL or < 0.4–0.3 mmol/L |
| Grade 4 | < 0.7 mg/dL or < 0.3 mmol/L |
Abbreviation: LLN = lower limit of normal
Grade 3–4 hypomagnesemia is associated with symptoms of fatigue, cramps, and somnolence79, which are often attributed to cytotoxic chemotherapy and, therefore, go unreported. However, the patient’s energy level and performance status may be improved by normalizing magnesium levels of those with grade 3–4 hypomagnesemia90. Replacement therapy is particularly important for these patients, as grade 3–4 hypomagnesemia puts the patient at increased risk for cardiac arrhythmia, which may lead to sudden death78.
Management of grade 3–4 hypomagnesemia is challenging, requiring intravenous treatment with magnesium sulfate 6 to 10 g a minimum of two times per week. In severe cases, daily supplementation may be necessary, which can be extremely limiting and inconvenient for the patient90. In such cases, a four-to-eight-week break from EGFR inhibition may be considered, as magnesium concentrations return to normal approximately four to eight weeks after discontinuation. The patient may then be rechallenged with the EGFR inhibitor following reversal of the hypomagnesemia90.
5. DIARRHEA
Grade 3–4 diarrhea occurred in up to 2% of patients in the EGFR inhibitor monotherapy trials (Tables 1, 2). The incidence and severity of diarrhea is increased when cetuximab or panitumumab is given in combination with chemotherapy (Table 3), but is generally in the range expected for irinotecan therapy.
Grade 3–4 diarrhea occurred in up to 28% of patients in trials combining an EGFR inhibitor with chemotherapy (Table 3). In the CRYSTAL trial, the rate of grade 3–4 diarrhea was increased by the addition of cetuximab to FOLFIRI, compared with FOLFIRI alone (15.2% vs. 10.5%)48. In CAIRO233, cetuximab increased the rate of grade 1–2 diarrhea, but not grade 3–4 diarrhea, when added to a regimen of capecitabine, oxaliplatin and bevacizumab.
The combination of panitumumab and IFL (5-fluorouracil [5-FU], leucovorin [LV], and irinotecan) is not recommended due to the high incidence of diarrhea seen in clinical trials. A phase II trial assessed the incidence of grade 3–4 diarrhea in mCRC patients treated with panitumumab in combination with first-line irinotecan-containing regimens91. Patients were initially treated with panitumumab (2.5 mg/kg weekly via a 1-hour infusion) in combination with IFL. However, the protocol was later amended to substitute folinic acid, 5-FU, and irinotecan (FOLFIRI) for IFL due to toxicity with IFL. In all, 19 patients were treated with IFL and 24 with FOLFIRI in combination with panitumumab. Grade 3–4 diarrhea was observed in 58% of patients in the IFL group and 25% of patients in the FOLFIRI group. In the PACCE trial34, the addition of panitumumab to either bevacizumab/irinotecan-based chemotherapy or to bevacizumab/oxaliplatin-based chemotherapy resulted in an increased incidence of grade 3–4 diarrhea, compared with the chemotherapy regimens alone.
Management of diarrhea should be aggressive, with treatment including loperamide or diphenoxylate92. General management may include bowel rest, hydration, and replacement of electrolytes. Hospitalization is required for patients with dehydration, fever, neutropenia, or nausea and vomiting that prevents adequate oral hydration93.
6. INFUSION REACTIONS
Severe infusion reactions have been reported in approximately 3.5–7.5% of mCRC patients treated with cetuximab (Table 1). In randomized trials of panitumumab monotherapy in mCRC, infusion-related reactions of all grade occurred in 0.6–3% of patients (Table 2).
A higher incidence of infusion reactions associated with cetuximab treatment was recently reported in a study in Tennessee and North Carolina, which included data for 88 patients in clinical trials and 55 patients outside of trials94. Among those in clinical trials, the grade 3–4 IRs occurred at a rate of 22%. However, rates of hypersensitivity reactions were much lower (< 1%) in most centers in the Northeast.
Most severe (grade 4) infusion reactions with cetuximab occur within a few minutes of taking the first dose. However, Needle et al. reported that 33% of grade 3–4 infusion reactions occurred after the second dose; less severe reactions may appear with subsequent treatments, suggesting differences in the underlying mechanisms responsible for mild and severe infusion reactions95.
Severe hypersensitivity reactions to cetuximab are thought to be largely due to IgE-mediated anaphylaxis, associated with the preexistence of IgE antibodies prior to treatment with cetuximab96. Following previous exposure to an antigen, IgE reaginic antibodies are released into the circulation by plasma cells derived from B lymphocytes under the influence of helper T-cells. These antibodies bind to receptors on tissue mast cells or blood-borne basophils, thereby sensitizing them. Subsequent reexposure to the antigen cross-links the Fab portions of two surface-bound IgE molecules, activating the cell and triggering the release of chemical mediators. Following reports of increased hypersensitivity reactions to cetuximab in the southeastern United States, IgE antibodies against cetuximab were detected in pretreatment blood samples of 68% of patients who had a hypersensitivity reaction to the drug. In contrast, IgE antibodies were detected in only 2% of those without a reaction (p < 0.001). The IgE antibody was discovered to be specific for the sugar galactose-α-1,3-galactose, expressed on the Fab portion of the cetuximab heavy chain. While the reason for the regional distribution of IgE antibodies to galactose-α-1,3-galactose in the United States is unclear, tick bites have been proposed as a potential etiology96.
The mechanism of panitumumab hypersensitivity reactions is not clearly understood. In a phase III trial of panitumumab monotherapy in patients with mCRC, 1.4% of patients tested positive for neutralizing antibodies47. However, a correlation between neutralizing antibodies and infusion reactions has yet to be demonstrated.
Patients may be able to continue treatment with an EGFR inhibitor following mild to moderate infusion reactions. Nielsen et al. described two patients with grade 2 infusion reactions to cetuximab, who were successfully rechallenged with cetuximab under controlled conditions97. Prednisone and antihistamines were administered prior to treatment, and the cetuximab infusion was started on a low rate, with gradual titration. The patients showed no evidence of an acute reaction during or after the cetuximab infusion and were able to continue treatment under the same treatment protocol. More recently, the addition of corticosteroids to antihistamines prior to treatment with cetuximab has been shown to reduce infusion-related reactions, without altering anti-tumor efficacy98.
There are also limited data demonstrating successful treatment with an alternative anti-EGFR MoAb following a severe infusion reaction with one anti-EGFR MoAb. Saif et al. described two mCRC patients with severe infusion reactions to panitumumab who were successfully challenged with cetuximab99, and three patients with severe hypersensitivity reactions to cetuximab who were successfully challenged with panitumumab100. The two patients who were switched to cetuximab received premedication with prednisone and antihistamines, and were treated with cetuximab according to a prolonged infusion time and gradual dose escalation99. None of the three patients successfully switched to panitumumab received pretreatment100.
7. CONCLUSIONS
The majority of patients treated with an MoAb EGFR inhibitor for mCRC will experience dermatological side effects, the most common of which is the papulopustular skin rash, which occurs early during the course of treatment and can impact the patient’s quality of life. The severity of the rash is dose-dependant and is also correlated with efficacy of treatment. Therefore, every effort should be made to ensure adherence to therapy. Most cases are mild-to-moderate in nature and will respond to treatment with topical antibiotics. More severe cases may require the addition of oral antibiotics, along with dose reduction or treatment delays. Preemptive treatment has been shown to delay the time to skin toxicity. Based on two randomized studies, the prophylactic use of systemic oral antibiotics, namely doxycycline or minocycline, reduces the risk of grade 2 and higher skin toxicity. While this practice may eliminate the reliability of skin toxicity as a predictive factor of response, it does not reduce anti-EGFR efficacy. Given the inconvenience associated with severe skin toxicities, many practices have moved to prophylactic oral antibiotic administration.
Other dermatological side effects of both cetuximab and panitumumab include xerosis, fissures, hyperpigmentation, and changes to the hair and nails. Recent reports have also indicated a potential for cetuximab to enhance the severity of radiation dermatitis.
Less common but important side effects include diarrhea and infusion reactions. Hypomagnesemia has emerged more recently as a side effect of EGFR inhibitors and should be considered in patients who develop fatigue and muscle weakness on therapy. Serum magnesium levels should be monitored routinely in patients undergoing treatment with cetuximab or panitumumab for mCRC.
8. ACKNOWLEDGEMENTS
The authors acknowledge the support of Bristol-Myers Squibb Inc. in providing funding for third-party medical writing. The authors also acknowledge the medical writing contributions of Maryka Hladki of Science & Medicine Canada.
9. REFERENCES
- 1.Di Marco E, Albanese E, Benso S, et al. Expression of epidermal growth factor receptor and transforming growth factor alpha in human larynx carcinoma. Cancer Lett. 1992;65(3):189–99. doi: 10.1016/0304-3835(92)90231-j. [DOI] [PubMed] [Google Scholar]
- 2.Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19(3):183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen PJ, Berger JE, Meneses J, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376(6538):337–41. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 4.Klapper LN, Kirschbaum MH, Sela M, et al. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- 5.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410(1):83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 6.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 7.Yarden Y. The EGFR family and its ligands in human cancer. Signaling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 8.Castillo L, Etienne-Grimaldi MC, Fischel JL, et al. Pharmacological background of EGFR targeting. Ann Oncol. 2004;15(7):1007–12. doi: 10.1093/annonc/mdh257. [DOI] [PubMed] [Google Scholar]
- 9.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7(10):2958–70. [PubMed] [Google Scholar]
- 10.Mayer A, Takimoto M, Fritz E, et al. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71(8):2454–60. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 12.Ciardiello F. An update of new targets for cancer treatment: receptor-mediated signals. Ann Oncol. 2002;13(Suppl 4):29–38. doi: 10.1093/annonc/mdf635. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecanrefractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 14.Zlobec I, Vuong T, Hayashi S, et al. A simple and reproducible scoring system for EGFR in colorectal cancer: application to prognosis and prediction of response to preoperative brachytherapy. Br J Cancer. 2007;96(5):793–800. doi: 10.1038/sj.bjc.6603619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross ME, Zorbas MA, Danels YJ, et al. Cellular growth response to epidermal growth factor in colon carcinoma cells with an amplified epidermal growth factor receptor derived from a familial adenomatous polyposis patient. Cancer Res. 1991;51(5):1452–59. [PubMed] [Google Scholar]
- 16.Radinsky R. Modulation of tumour cell gene expression and phenotype by the organ-specific metastatic environment. Cancer Metastasis Rev. 1995;14(4):323–28. doi: 10.1007/BF00690601. [DOI] [PubMed] [Google Scholar]
- 17.Radinsky R, Risin S, Fan D, et al. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1(1):19–31. [PubMed] [Google Scholar]
- 18.Prewett MC, Hooper AT, Bassi R, et al. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8(5):994–1003. [PubMed] [Google Scholar]
- 19.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21(14):2787–99. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell EP, Pérez–Soler R, Van Cutsem E, et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park) 2007;21(11 Suppl 5):4–9. [PubMed] [Google Scholar]
- 21.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nature Rev Cancer. 2005;6(10):803–12. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 22.Lacouture ME, Melosky BL. Cutaneous reactions to anti-cancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007;12(6):1–5. [PubMed] [Google Scholar]
- 23.Segaert S, Tabernero J, Chosidow O, et al. The management of skin reactions in cancer patients receiving epidermal growth factor receptor targeted therapies. J Dtsch Dermatol Ges. 2005;3(8):599–606. doi: 10.1111/j.1610-0387.2005.05058.x. [DOI] [PubMed] [Google Scholar]
- 24.Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(9):1425–33. doi: 10.1093/annonc/mdi279. [DOI] [PubMed] [Google Scholar]
- 25.Folprecht G, Lutz MP, Schöffski P, et al. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006;17(3):450–56. doi: 10.1093/annonc/mdj084. [DOI] [PubMed] [Google Scholar]
- 26.Busam KJ, Capodieci P, Motzer R, et al. Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001;144(6):1169–76. doi: 10.1046/j.1365-2133.2001.04226.x. [DOI] [PubMed] [Google Scholar]
- 27.Molinari E, De Quatrebarbes J, André T, et al. Cetuximab-induced acne. Dermatology. 2005;211(4):330–33. doi: 10.1159/000088502. [DOI] [PubMed] [Google Scholar]
- 28.Bouché O, Brixi-Benmansour H, Bertin A, et al. Trichomegaly of the eyelashes following treatment with cetuximab. Ann Oncol. 2005;16(10):1711–12. doi: 10.1093/annonc/mdi300. [DOI] [PubMed] [Google Scholar]
- 29.Boucher KW, Davidson K, Mirakhur B, et al. Paronychia induced by cetuximab, an antiepidermal growth factor receptor antibody. J Am Acad Dermatol. 2002;47(4):632–33. doi: 10.1067/mjd.2002.124621. [DOI] [PubMed] [Google Scholar]
- 30.Grenader T, Gipps M, Goldberg A. Staphylococcus aureus bacteremia secondary to severe erlotinib skin toxicity. Clin Lung Cancer. 2008;9(1):59–60. doi: 10.3816/CLC.2008.n.010. [DOI] [PubMed] [Google Scholar]
- 31.Peeters M, Price T, Van Laethern JL. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: where are we today? Oncologist. 2009;14(1):29–39. doi: 10.1634/theoncologist.2008-0167. [DOI] [PubMed] [Google Scholar]
- 32.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–08. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 33.Tol J, Koopman M, Rodenburg CJ, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol. 2008;19(4):734–38. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 34.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–80. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 35.Saltz LB, Lenz H-J, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared With cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. Clin Oncol. 2007;25(29):4557–61. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 36.Jacot W, Bessis D, Jorda E, et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol. 2004;151(1):238–41. doi: 10.1111/j.1365-2133.2004.06026.x. [DOI] [PubMed] [Google Scholar]
- 37.Bianchini D, Jayanth A, Chua YJ, et al. Epidermal growth factor receptor inhibitor-related skin toxicity: mechanisms, treatment, and its potential role as a predictive marker. Clin Colorectal Cancer. 2008;7(1):33–43. doi: 10.3816/CCC.2008.n.005. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell EP, LaCouture M, Shearer H, et al. Updated results of STEPP, a phase 2, open-label study of pre-emptive versus reactive skin toxicity treatment in metastatic colorectal cancer (mCRC) patients receiving panitumumab+FOLFIRI or irinotecan-only chemotherapy as second-line treatment European Society for Medical Oncology (ESMO) International Symposium. 10th World Congress on Gastrointestinal CancerJune 25–28, 2008Barcelona, Spain (Abstract O-021) Available online at: www.worldgicancer.com/WCGI/WGIC08/WGIC08_pressinfo_combined.pdf; cited December 15, 2008. [Google Scholar]
- 39.Lacouture ME. Insights into the pathophysiology and management of dermatologic toxicities to EGFR-targeted therapies in colorectal cancer. Cancer Nurs. 2007;30(4 Suppl 1):S17–26. doi: 10.1097/01.NCC.0000281758.85704.9b. [DOI] [PubMed] [Google Scholar]
- 40.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19(13):3267–79. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 41.Foon KA, Yang XD, Weiner LM, et al. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys. 2004;58(3):984–90. doi: 10.1016/j.ijrobp.2003.09.098. [DOI] [PubMed] [Google Scholar]
- 42.Rowinsky EK, Schwartz GH, Gollob JA, et al. Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol. 2004;22(16):3003–15. doi: 10.1200/JCO.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 43.Kimyai-Asadi A, Jih MH. Follicular toxic effects of chimeric anti-epidermal growth factor receptor antibody cetuximab used to treat human solid tumors. Arch Dermatol. 2002;138(1):129–31. doi: 10.1001/archderm.138.1.129. [DOI] [PubMed] [Google Scholar]
- 44.Walon L, Gilbeau C, Lachapelle JM. [Acneiform eruptions induced by cetuximab] Ann Dermatol Venereol. 2003;130(4):443–46. [PubMed] [Google Scholar]
- 45.Lenz HJ, Van Cutsem E, Khambata-Ford S, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24(30):4914–21. doi: 10.1200/JCO.2006.06.7595. [DOI] [PubMed] [Google Scholar]
- 46.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–48. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 47.Van Cutsem E, Siena S, Humblet Y, et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol. 2008;19(1):92–98. doi: 10.1093/annonc/mdm399. [DOI] [PubMed] [Google Scholar]
- 48.Van Cutsem E, Nowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial. J Clin Oncol. 2007;25(18 Suppl):4000. [Google Scholar]
- 49.Hecht JR, Patnaik A, Berlin J, et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer. 2007;110(5):980–88. doi: 10.1002/cncr.22915. [DOI] [PubMed] [Google Scholar]
- 50.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 51.Tejpar S, Peeters M, Humblet Y, et al. Phase I/II study of cetuximab dose-escalation in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST): Pharmacokinetic (PK), Pharmacodynamic (PD) and efficacy data. J Clin Oncol. 2007;25(18 Suppl):4037. [Google Scholar]
- 52.Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16(1):16–26. doi: 10.3747/co.v16i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25(34):5390–96. doi: 10.1200/JCO.2007.12.6987. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell EP, LaCouture ME, Shearer H, et al. A phase II, open-label trial of skin toxicity (ST) evaluation (STEPP) in metastatic colorectal cancer (mCRC) patients (pts) receiving panitumumab (pmab) + FOLFIRI or irinotecan-only chemotherapy (CT) as 2nd-line treatment (tx): Interim analysis. J Clin Oncol. 2008;26(15S):15007. [Google Scholar]
- 55.Grothey A. Recognizing and managing toxicities of molecular targeted therapies for colorectal cancer. Oncology (Williston Park) 2006;20(14 Suppl 10):21–28. [PubMed] [Google Scholar]
- 56.Agero AL, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55(4):657–70. doi: 10.1016/j.jaad.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Soler R. Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer. 2006;8(Suppl 1):S7–14. doi: 10.3816/clc.2006.s.008. [DOI] [PubMed] [Google Scholar]
- 58.Porzio G, Aielli F, Verna L, et al. Efficacy of pregabalin in the management of cetuximab-related itch. J Pain Symptom Manage. 2006;32(5):397–98. doi: 10.1016/j.jpainsymman.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Lynch TJ, Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12(5):610–21. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 60.Dick FE, Crawford GH. Managing cutaneous side effects of epidermal growth factor receptor (HER1/EGFR) inhibitors. Commun Oncol. 2005;2:492–96. [Google Scholar]
- 61.Roé E, García Muret MP, Marcuello E, et al. Description and management of cutaneous side effects during cetuximab or erlotinib treatments: a prospective study of 30 patients. J Am Acad Dermatol. 2006;55(3):429–37. doi: 10.1016/j.jaad.2006.04.062. [DOI] [PubMed] [Google Scholar]
- 62.Chang GC, Yang TY, Chen KC, et al. Complications of therapy in cancer patients: Case 1. Paronychia and skin hyperpigmentation induced by gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22(22):4646–48. doi: 10.1200/JCO.2004.02.168. [DOI] [PubMed] [Google Scholar]
- 63.Shah NT, Kris MG, Pao W, et al. Practical management of patients with non-small-cell lung cancer treated with gefitinib. J Clin Oncol. 2005;23(1):165–74. doi: 10.1200/JCO.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 64.Dainichi T, Tanaka M, Tsuruta N, et al. Development of multiple paronychia and periungual granulation in patients treated with gefitinib, an inhibitor of epidermal growth factor receptor. Dermatology. 2003;207(3):324–25. doi: 10.1159/000073100. [DOI] [PubMed] [Google Scholar]
- 65.Nakano J, Nakamura M. Paronychia induced by gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Dermatol. 2003;30(3):261–62. doi: 10.1111/j.1346-8138.2003.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 66.Dueland S, Sauer T, Lund-Johansen F, et al. Epidermal growth factor receptor inhibition induces trichomegaly. Acta Oncol. 2003;42(4):345–46. doi: 10.1080/02841860310006038. [DOI] [PubMed] [Google Scholar]
- 67.Pascual JC, Bañuls J, Belinchon I, et al. Trichomegaly following treatment with gefitinib (ZD1839) Br J Dermatol. 2004;151(5):1111–12. doi: 10.1111/j.1365-2133.2004.06265.x. [DOI] [PubMed] [Google Scholar]
- 68.Drelich DA, Rose L, Ramirez M, et al. Dermatological toxicities of panitumumab in the treatment of patients with metastatic colorectal cancer (mCRC) from three clinical studies. J Clin Oncol. 2007;25(18 Suppl):14551. [Google Scholar]
- 69.Lacouture ME, Basti S, Patel J, et al. The SERIES clinic: an interdisciplinary approach to the management of toxicities of EGFR inhibitors. J Support Oncol. 2006;4(5):236–38. [PubMed] [Google Scholar]
- 70.Berger B, Belka C. Severe skin reaction secondary to concomitant radiotherapy plus cetuximab. Radiat Oncol. 2008;3:5. doi: 10.1186/1748-717X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bölke E, Gerber PA, Lammering G, et al. Development and management of severe cutaneous side effects in head-and-neck cancer patients during concurrent radiotherapy and cetuximab. Strahlenther Onkol. 2008;184(2):105–10. doi: 10.1007/s00066-008-1829-z. [DOI] [PubMed] [Google Scholar]
- 72.Azad A. Severe cutaneous toxicity following treatment with radiotherapy and cetuximab: a case report. Cases J. 2009;2(1):25. doi: 10.1186/1757-1626-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giro C, Berger B, Bölke E, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol. 2009;90(2):166–71. doi: 10.1016/j.radonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Pryor DI, Porceddu SV, Burmeister BH, et al. Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother Oncol. 2009;90(2):172–76. doi: 10.1016/j.radonc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 76.Tonini G, Vincenzi B, Santini D, et al. Ocular toxicity related to cetuximab monotherapy in an advanced colorectal cancer patient. J Natl Cancer Inst. 2005;97(8):606–07. doi: 10.1093/jnci/dji104. [DOI] [PubMed] [Google Scholar]
- 77.Dranko S, Kinney C, Ramanathan RK. Ocular toxicity related to cetuximab monotherapy in patients with colorectal cancer. Clin Colorectal Cancer. 2006;6(3):224–25. doi: 10.3816/CCC.2006.n.040. [DOI] [PubMed] [Google Scholar]
- 78.Erbitux (cetuximab) Product Monograph. Montreal, Quebec: Bristol-Myers Squibb Canada; 2009. [Google Scholar]
- 79.Tejpar S, Piessevaux H, Claes K, et al. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol. 2007;8(5):387–94. doi: 10.1016/S1470-2045(07)70108-0. [DOI] [PubMed] [Google Scholar]
- 80.Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(14):2311–19. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 81.Jonker DJ, Karapetis CS, Moore M, et al. Randomized phase III trial of cetuximab monotherapy plus best supportive care (BSC) versus BSC alone in patients with pretreated metastatic epidermal growth factor receptor (EGFR)-positive colorectal carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) and the Australasian Gastro-Intestinal Trials Group (AGITG) [abstract]; Program and proceedings of the 2007 annual meeting of the American Association for Cancer Research; Presented at the American Association for Cancer Research Annual Meeting; April 14–18, 2007; Los Angeles, CA. Philadelphia, PA: American Association for Cancer Research; 2007. [Google Scholar]
- 82.Giusti RM, Shastri KA, Cohen MH, et al. FDA drug approval summary: panitumumab (Vectibix) Oncologist. 2007;12(5):577–83. doi: 10.1634/theoncologist.12-5-577. [DOI] [PubMed] [Google Scholar]
- 83.Saif MW, Cohenuram M. Role of panitumumab in the management of metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6(2):118–24. doi: 10.3816/CCC.2006.n.028. [DOI] [PubMed] [Google Scholar]
- 84.Saif MW. Management of hypomagnesemia in cancer patients receiving chemotherapy. J Support Oncol. 2008;6(5):243–48. [PubMed] [Google Scholar]
- 85.Schrag D, Chung KY, Flombaum C, et al. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. 2005;97(16):1221–24. doi: 10.1093/jnci/dji242. [DOI] [PubMed] [Google Scholar]
- 86.Van Cutsem E, Peeters M, Siena S, et al. Panitumumab significantly improves progression-free survival in patients with metastatic colorectal cancer. Ann Oncol. 2006;17:vi19–vi27. [Google Scholar]
- 87.Fakih MG, Wilding G, Lombardo J. Cetuximab-induced hypomagnesemia in patients with colorectal cancer. Clin Colorectal Cancer. 2006;6(2):152–56. doi: 10.3816/CCC.2006.n.033. [DOI] [PubMed] [Google Scholar]
- 88.Kuo T, Cho CD, Halsey J, et al. Phase II study of gefitinib, fluorouracil, leucovorin, and oxaliplatin therapy in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2005;23(24):5613–19. doi: 10.1200/JCO.2005.08.359. [DOI] [PubMed] [Google Scholar]
- 89.Iannello S, Belfiore F. Hypomagnesemia. A review of pathophysiological, clinical and therapeutical aspects. Panminerva Med. 2001;43(1):177–209. [PubMed] [Google Scholar]
- 90.Fakih M. Management of anti-EGFR-targeting monoclonal antibody-induced hypomagnesemia. Oncology (Williston Park) 2008;22(1):74–76. [PubMed] [Google Scholar]
- 91.Berlin J, Posey J, Tchekmedyian S, et al. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin Colorectal Cancer. 2007;6(6):427–32. doi: 10.3816/CCC.2007.n.011. [DOI] [PubMed] [Google Scholar]
- 92.Held-Warmkessel J. Diarrhea. In: Camp-Sorrell D, Hawkins RA, editors. Clinical manual for the oncology advanced practice nurse. 2nd ed. Pittsburgh, PA: Oncology Nursing Society; 2006. pp. 425–33. [Google Scholar]
- 93.Maroun JA, Anthony LB, Blais N, et al. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Curr Oncol. 2007;14(1):13–20. doi: 10.3747/co.2007.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644–48. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 95.Needle MN. Safety experience with IMC-C225, an anti-epidermal growth factor receptor antibody. Semin Oncol. 2002;29(5 Suppl 14):55–60. doi: 10.1053/sonc.2002.35648. [DOI] [PubMed] [Google Scholar]
- 96.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nielsen DL, Pfeiffer P, Jensen BV. Re-treatment with cetuximab in patients with severe hypersensitivity reactions to cetuximab. Two case reports. Acta Oncol. 2006;45(8):1137–38. doi: 10.1080/02841860600871764. [DOI] [PubMed] [Google Scholar]
- 98.Siena S, Glynne-Jones R, Thaler J, et al. Infusion-related reactions (IRR) associated with cetuximab plus irinotecan treatment in patients with irinotecan-resistant metastatic colorectal cancer (mCRC): Findings from the MABEL study. J Clin Oncol. 2007;25(18S):4137. [Google Scholar]
- 99.Saif MW, Syrigos KI, Hotchkiss S, et al. Successful desensitization with cetuximab after an infusion reaction to panitumumab in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2009;65(1):107–12. doi: 10.1007/s00280-009-1009-6. [DOI] [PubMed] [Google Scholar]
- 100.Saif MW, Peccerillo J, Potter V. Successful re-challenge with panitumumab in patients who developed hypersensitivity reactions to cetuximab: report of three cases and review of literature. Cancer Chemother Pharmacol. 2009;63(6):1017–22. doi: 10.1007/s00280-008-0831-6. [DOI] [PubMed] [Google Scholar]