Abstract
Objective
Patients with schizophrenia demonstrate significant impairments of early visual processing, potentially implicating dysfunction of the magnocellular visual pathway. The present study evaluates transient visual evoked potential (tVEP) responses to stimuli biased toward the magnocellular (M) or parvocellular (P) systems in patients with schizophrenia vs. normal volunteers first to evaluate relative contributions of M and P systems to specific tVEP components in schizophrenia and, second, to evaluate integrity of early M and P processing in schizophrenia.
Methods
Seventy-four patients with schizophrenia and schizoaffective disorder were compared with 59 control subjects using separate stimuli to assess the tVEP response to M, P and mixed M/P conditions. Stimuli were biased toward M vs. P processing by manipulation of chromatic and achromatic contrast. C1, P1, N1 and P2 components were compared between patients and controls. All subjects showed 20/32 vision or better.
Results
Waveforms were obtained to low contrast (M), chromatic contrast (P) and high contrast (mixed M/P) stimuli in both patients and controls. C1 was present to P and mixed M/P stimuli. Patients showed a significant reduction in amplitude and an increase in latency of the C1 component. P1 was elicited primarily by M and mixed M/P stimuli, whereas N1 was elicited primarily by P and mixed M/P stimuli. Patients showed reductions in both P1 and N1 amplitudes across conditions. However, only reductions in P1 amplitude survived covariation for between group differences in visual acuity. Further, P1 amplitude reductions in the M condition correlated with a proxy measure of global outcome.
Conclusions
M- and P-selective stimuli elicit differential components of the tVEP. Patients with schizophrenia show significant reductions in response even to simple visual stimuli. Deficits, particularly within the M system, may correlate significantly with global outcome and level of community functioning.
Significance
Whereas deficits in high-order cognitive processing have been extensively documented in schizophrenia, integrity of early-stage sensory processing has been studied to a lesser degree. The present findings suggest that deficits in early-stage visual processing are significantly related to overall clinical outcome in schizophrenia. Further, between-group differences in visual acuity may influence VEP results, even for subjects with ‘normal’ vision (20/32 or better).
Keywords: Transient visual evoked potentials, VEP, ERP, Schizophrenia, Visual processing, P1, Outcome
1. Introduction
Patients with schizophrenia demonstrate severe neurocognitive deficits that affect not only higher-order cognitive function (Goldberg and Gold, 1995; Goldman-Rakic, 1994; Green and Nuechterlein, 1999b; Jaeger et al., 2003; Saykin et al., 1994) but also early levels of perceptual processing (Braff et al., 1991; Javitt et al., 1999; Turetsky et al., 2003). In the visual system, deficits have been shown to include increased visual thresholds (Cadenhead et al., 1997; Schechter et al., 2003), greater sensitivity to backward masking (Braff et al., 1991; Butler et al., 1996; Green and Nuechterlein, 1999a; Schechter et al., 2003), and decreased contrast sensitivity (Butler et al., 2005; Keri et al., 2002; Slaghuis and Curran, 1999), as well as motion perception (Chen et al., 1999; Li, 2002; Schwartz et al., 1999) and eye tracking deficits (Holzman et al., 1974; Levy et al., 1993; Trillenberg et al., 2004). Further, it has been suggested that these impairments may contribute to ‘upstream’ cognitive and social impairments (Brenner et al., 2002; Bruder et al., 1998; Kee et al., 1998; Ohno et al., 2000; Perry and Braff, 1994; Sergi and Green, 2003) and overall functional outcome of patients (Green et al., 2000). The exact nature of the deficits, while having been described behaviorally, have not been well characterized in relation to their neurophysiological substrate.
Traditional models of visual processing subdivide the visual system into distinct ‘transient’ vs. ‘sustained’ psychophysical channels, with apparent hyperactivity being noted particularly in visual backward masking paradigms (Green et al., 1994; Schechter et al., 2003). In contrast, more recent models subdivide into distinct magnocellular (M) vs. parvocellular (P) pathways defined based on neuroanatomy and response properties of underlying neurons with schizophrenia associated with M system underactivity on the basis of contrast sensitivity (Butler et al., 2005; Keri et al., 2002,1998; Slaghuis, 1998) and steady state VEPs (Butler et al., 2001, 2005). Properties of M neurons correspond closely to those of the ‘transient’ pathway, whereas properties of the P neurons correspond to those of the sustained pathway. M and P neurons respond differentially to specific stimulus types. The present study takes advantage of the differential response properties of M vs. P neurons to investigate functioning of these discrete systems, along with contributions of these systems to overall clinical outcome in schizophrenia.
Both the M and P systems begin in the retina and project via the lateral geniculate nucleus (LGN) to primary visual cortex (striate cortex, V1). The systems, however, are distinguished by response properties to simple visual stimuli, as well as by functional role. M cells are particularly sensitive to low luminance contrast stimuli, and respond to stimuli with luminance contrast levels as low as 1%, whereas cortical neurons that receive P input do not respond below ∼8% contrast (Derrington and Lennie, 1984; Kaplan et al., 1988; Merigan and Maunsell, 1993; Tootell et al., 1988). In addition, M cells are relatively insensitive to chromatic contrast in the absence of luminance contrast, whereas the P cells are highly responsive to color contrast alone(Logothetis et al., 1990). Differences in responsivity can be used to bias processing towards the M vs. P pathways. Both M and P neurons within LGN project initially to striate cortex (V1), which is located in the vicinity of the calcarine fissure and generates activity in the midline occipital region relative to frontocentral electrodes. Integrity of the early visual pathways can thus be investigated using M-vs. P-selective stimuli, together with recordings sensitive to V1 activation.
Transient visual stimuli elicit a sequence of visual evoked potentials referred to as the transient VEP (tVEP). The earliest readily detectable cortical response to visual stimuli occurs at ∼80 ms. This component has alternatively been termed C1 (Jeffreys, 1971), N1 or N80 (Regan, 1989). Consistent with contemporary nomenclature, the term C1 is used in the present study. C1 inverts for upper vs. lower-field stimuli, consistent with a primary generator in V1 (Di Russo et al., 2001; Vanni et al., 2004). Both P-(Ellemberg et al., 2001; Previc, 1988) and M- (Di Russo et al., 2001; Ellemberg et al., 2001; Maier et al., 1987; Schroeder et al., 1991) system contributions to C1 subcomponent generation have been described. P1, a positive component that generally peaks about 100 ms following stimulus onset, also reflects early activation of visual cortex (Di Russo et al., 2001; Ellemberg et al., 2001; Schroeder et al., 1991), particularly via the M system (Ellemberg et al., 2001; Previc, 1988). Multiple P1 generators have been identified, with the largest activity observed over dorsal stream sites, with extrastriate (V3, V3a and middle occipital gyrus) (Di Russo et al., 2001; Vanni et al., 2004) as well as striate contributions (Aine et al., 1995; Maier et al., 1987; Vanni et al., 2004). P1 is followed by N1, a negative deflection peaking at approximately 150 ms with multiple generators that may represent P-mediated activation of ventral stream structures such as lateral occipital complex (Bentin et al., 1999; Doniger et al., 2000, 2001, 2002).
Although behavioral (Butler et al., 2005; Keri et al., 2002, 2004; O'Donnell et al., 1996, 2002; Slaghuis, 1998, 2004) and steady state VEP (Butler et al., 2001, 2005) studies suggest deficits in early-stage visual processing in schizophrenia particularly involving underactivity of the M system, previous studies of transient VEP (tVEP) in schizophrenia have been conflicting. Initial, as well as current, studies of the tVEP (Shagass et al., 1977; Straumanis et al., 1982; van der Stelt et al., 2004) have found no impairment in the early components of the waveform, reporting a deficit only at 200 ms (Shagass, 1980) or later (van der Stelt et al., 2004). In other studies, however, significant P1 deficits have been observed (Basinska, 1998; Doniger et al., 2002; Foxe et al., 2001; Matsuoka et al., 1996; Romani et al., 1986; Spencer et al., 2003). Stimulus types, recording characteristics (e.g., filter frequencies) and patient types have varied considerably across studies, leaving the basis for the divergence in results unresolved.
The present study takes advantage of the differential properties of M vs. P systems to analyze mechanisms underlying impaired early visual processing in schizophrenia. tVEPs were biased towards M vs. P processing through manipulation of luminance and color contrast. This method of segregating pathways has been effective in previous electrophysiological (Butler et al., 2001; Ellemberg et al., 2001; Greenstein et al., 1998) and psychophysical studies (Livingstone and Hubel, 1987, 1988; Schechter et al., 2003). Three stimulus conditions were utilized. Stimuli were shown at either high contrast (100% achromatic contrast), low contrast (4% achromatic contrast) or chromatic contrast (100% chromatic contrast at isoluminance). The high contrast is a mixed condition, eliciting both an M and P pathway response. The low contrast condition biases processing towards the M pathway, since the P pathway does not respond below ∼8% luminance contrast (Kaplan et al., 1988; Merigan and Maunsell, 1993; Tootell et al., 1988). The chromatic condition biases processing towards the color-sensitive P pathway because the M pathway does not respond to chromatic contrast where there is no difference in luminance between stimulus elements (i.e. presentation at isoluminance) (Logothetis et al., 1990).
We hypothesized that these different stimulus conditions would differentially affect generation of the discrete C1, P1 and N1 components, to the extent that these components depend differentially on M vs. P pathway input. Furthermore, we expected that components dependent upon M pathway input would be preferentially affected in schizophrenia. In particular, we hypothesized that P1 would be reduced in amplitude in schizophrenia, in accord with our earlier findings (Butler et al., 2001; Doniger et al., 2002; Foxe et al., 2001), and that the reduction would be most pronounced in the M-biased (low luminance contrast) condition.
This study also examined the relationship between early perceptual deficits and clinical features of schizophrenia, including symptoms and functional outcome. Symptoms were measured by the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962) and Scale for the Assessment of Negative Symptoms (SANS)(Andreasen, 1984). Functional outcome was measured by the problem-solving factor subscale of the Independent Living Scales (ILS-PS) (Loeb, 1996). The ILS-PS predicts more strongly the capacity of individuals with schizophrenia or other neuropsychiatric disorders to function independently in the community than measures of verbal memory or Global Assessment of Functioning (GAF) scores (Revheim and Medalia, 2004a,b) and is therefore used as a proxy measure for functional outcome. A large sample of patients and controls was utilized to more thoroughly investigate the tVEP deficits and associated impairments in schizophrenia.
2. Methods
2.1. Subjects
Seventy-four patients (58 male, 16 females) meeting the criteria for DSM-IV diagnosis of schizophrenia (n=58) or schizoaffective disorder (n=16) provided written informed consent and participated in the study. Patients were recruited from the Clinical Research and Evaluation Facility (CREF) at the Nathan Kline Institute (NKI), an inpatient and outpatient state psychiatric facility. Diagnosis was obtained by means of chart review, consultation with physicians and the Structured Clinical Interview for DSM-IV (SCID; (First et al., 1997)). Patients were excluded if they had any neurological or ophthalmologic disorders that may affect performance or met criteria for alcohol or substance dependence within the last six months or abuse within the last month. Seventy-four patients were taking atypical antipsychotic medication at the time of testing. Two were also taking typical antipsychotics and one was taking aripiprizol, a new medication, which does not yet have an established chlorpromazine equivalence. Mean±SEM chlorpromazine equivalence was 1185.7±65.15 mg/day (range: 334–3417 mg/day).
Fifty-nine healthy volunteers (33 men, 26 women) also participated in the study. Controls with a history of SCID-defined psychiatric disorder, neurological or ophthalmologic disorders, or subjects who met criteria for alcohol or substance dependence within the last six months or abuse within the last month were excluded.
Patients and controls did not differ significantly in age (patients: 38.4±1.2; controls: 35.9±1.3 years), though they differed in gender composition [χ2=18.66, df=1, P<.001] and socioeconomic status as measured by the four-factor Hollingshead scale (patients: 22.8±1.0; controls: 51.6±1.6; [t= −15.23, df=124, P<.001]). In initial analysis patients with schizophrenia and schizoaffective disorder were compared using t-tests and showed no significant difference in response on any of the tVEP measures, thus allowing the groups to be combined. Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962) total score (mean±SEM) was 36.0±1.3 and scale for the assessment of negative symptoms (SANS)(Andreasen, 1984) total score (mean±SEM; including global scores) was 30.4±1.4.
Patients were rated using the ILS-PS (Loeb, 1996). The ILS-PS is a semi-structured interview designed to assess the likelihood of successful independent community living by utilizing effective strategies, abstract reasoning and judgment (Revheim and Medalia, 2004a,b). Questions on the ILS-PS include items such as ‘tell me two reasons why it is important to pay your bills’ and ‘what might you do if both your lights and your TV went off at the same time.’ Raw scores on the ILS-PS are converted to a standard score. Mean±SEM ILS-PS scaled score was 32.8±1.4. Cut-off scores were determined based on published norms of a normative sample (Loeb, 1996). Two categories of patients, low and high functioning, were subsequently differentiated (low function ≤ 39 and high functioning ≧40). Mean±SEM for the low functioning and high functioning groups were 26.7±.1.0 (n=51) and 48.3±1.2 (n=20), respectively.
2.2 Procedure
2.2.1. Apparatus
Stimuli were presented using a VENUS system (Neuroscientific Corp., Farmingdale, NY) and RGB monitor with a frame rate of ∼119 Hz (noninterlaced). Use of the VENUS system allows precise control over luminance and chromatic contrast. Viewing distance was 114 cm and the stimulus field subtended 8°×8° of visual angle. Luminance of the monitor was set at ∼100 cd/m2.
VEPs were recorded at Oz relative to a frontal reference. The EEG was amplified 20,000x (bandpass, 0.1 to 100 Hz) and averaged online using the VENUS system. Averaged transient waveform was then filtered through a lowpass filter with a corner frequency of 50 Hz and all odd harmonics were extracted.
2.2.2. Stimuli
Three conditions were presented; a 100% achromatic contrast condition, a 4% achromatic contrast condition and a 100% chromatic contrast condition. All stimuli were simple checkerboards consisting of 16×16 checks (check size=30 minarc) and were presented with 1 Hz square-wave modulation to evoke a transient response (60 s duration with two reversals per cycle=120 pattern reversals). In the achromatic conditions (100% and 4%), contrast was defined by Michelson's contrast: contrast= (Lmax−Lmin)/(Lmax+Lmin), where Lmax is maximal luminance (i.e. bright checks) and Lmin is minimal luminance (i.e. dark checks).
The chromatic condition was presented at isoluminance to null the response of the M system. Isoluminance refers to the condition when there is no difference in luminance across a spatial pattern that is defined in terms of chromatic contrast. To determine the isoluminant point for each participant, the electrophysiological technique of Zemon et al. (1991) was used, and isoluminance was estimated through manipulation of the ratio of red and green guns of the RGB display monitor. Signals from the two guns were modulated in counterphase (i.e. 180° out of phase with respect to one another). The red–green ratio at which a minimum of the amplitude and an inflection of the phase of the fundamental frequency component were seen was used as the isoluminant point (for detailed methodology see Greenstein et al., 1998). Ten repetitions of each stimuli were used to confirm the isoluminant point.
Each individual's isoluminant point was used in presenting chromatic contrast stimuli. Presentation of chromatic contrast stimuli involved a similar counterphase modulation of the red and green guns with the blue gun set to zero. Chromatic contrast was defined by the depth of modulation of the red gun. The green gun was always modulated in counterphase with the red gun at a depth of modulation used to yield isoluminance for each participant.
2.2.3. General procedure
Prior to VEP testing, visual acuity was assessed for all participants using the ETDRS charts. Only participants with vision of 20/32 or better were included in the study, consistent with ICD-9-CM criteria for ‘normal’ vision (International Classification of Diseases, 9th Revision, Clinical Modification). A small but significant between-group difference in visual acuity was found between patients (acuity mean±SEM: 0.88±.03) and controls (acuity mean±SEM: 1.07±.03) [t=−5.125, df=105, P<.001], however, acuity did not correlate with component amplitude or latencies in either group other than N1 amplitude [r=−.36, P=.007, n=57], P2 amplitude [r=.31, P=.02, n=57] and P1 latency [r=−.31, P=.02, n=57] in the high contrast (mixed M/P) condition for patients alone. Nevertheless, acuity was used as a covariate for all conditions. While all participants had acuities of 20/32or better (i.e. were able to read 20/32 line on the ETDRS chart), numerical acuity values were recorded for only 57 patients and 49 controls.
All participants sat in a dimly lit room and light adapted to the mean luminance of the display for several minutes. Participants were than instructed to fixate on the center of the display during each run. The experimenters (A.S. and M.J.) monitored the gaze of each participant during each run to ensure steady fixation. Any runs in which gaze was unsteady (the participant looked away from center or blinked excessively) were rejected and repeated. In addition, if the EEG trace contained sizable deflections from baseline or other noise activity, the run was rejected and repeated. Brief rest periods were provided between runs while data were stored. Testing was performed in one session, with the 100% achromatic condition presented first and the two other conditions then presented in counter-balanced order. All VEP testing was performed binocularly.
2.3. Statistical analysis
EEG signals were amplified and recorded. Successive responses to 60 presentations (cycles of contrast-reversal) were averaged online and the subsequent waveform was stored for analysis. The averaged transient waveform was then filtered in the frequency domain by means of a discrete Fourier transform with a corner frequency of the lowpass filter set at 50 Hz and all odd harmonics removed. A running t-test was used across the range of time to compare the two groups (control and schizophrenia). To avoid artifacts due to autocorrelation, a range of points were described as significantly different only when at least 11 contiguous time points were significant at the P<.05 level (Guthrie and Buchwald, 1991).
The waveform was then analyzed for amplitude and latency of its constituent components. Amplitude was derived as the peak voltage within a 24 ms window around the group's average peak amplitude. Latency was defined as the timepoint of the individual's peak. Amplitude and latencies were calculated for C1 (∼80 ms), P1 (∼100 ms), N1 (∼150 ms) and P2 (∼200 ms) components, where applicable. Amplitudes and latencies of each condition were averaged and then compared by ANOVA, each with two between-group factors (group and gender). Gender differences in waveform components were analyzed with follow-up t-tests between patients and controls and gender when a significant group by gender effect was found. Given the significant correlation between visual acuity and several of the VEP components in patients (P1 latency, N1 amplitude and P2 amplitude of the high contrast condition), acuity was included as a covariate in the analysis of all components.
Pearson product–moment correlation coefficients were used to examine relationships between significantly different waveform components between groups and clinical variables (BPRS and SANS scores), ILS-PS rating scores, and medication. ANOVA with follow-up t-tests for significant components were used to discern differences between low and high-functioning patients (as defined above) and controls where waveform components significantly correlated with ILS-PS.
All participants received the high contrast (mixed M/P) condition (74 patients and 59 controls). 60 patients and 49 controls received the low contrast (M) condition and 30 patients and 18 controls received the chromatic contrast (P) condition.
3. Results
Figs. 1–3 show the averaged waveform, running t-test results and component amplitude of the three conditions for patients and controls. As expected, the morphology of the waveforms differs as a function of stimulus type.
Fig. 1.
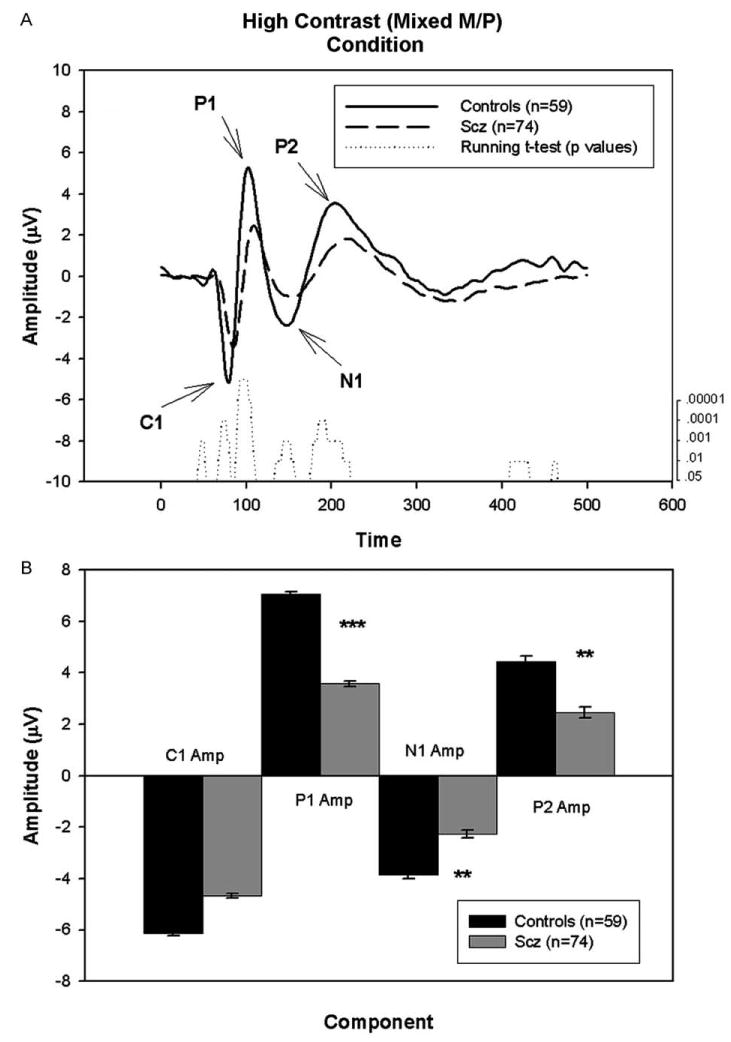
A. Graph showing the group average waveforms for the high contrast (mixed M/P) condition over time for controls and patients with running t-test. B. Bar graph showing group average C1, P1, N1 and P2 component amplitudes with SEM for patients and controls. **P<.005, ***P<.001
Fig. 3.
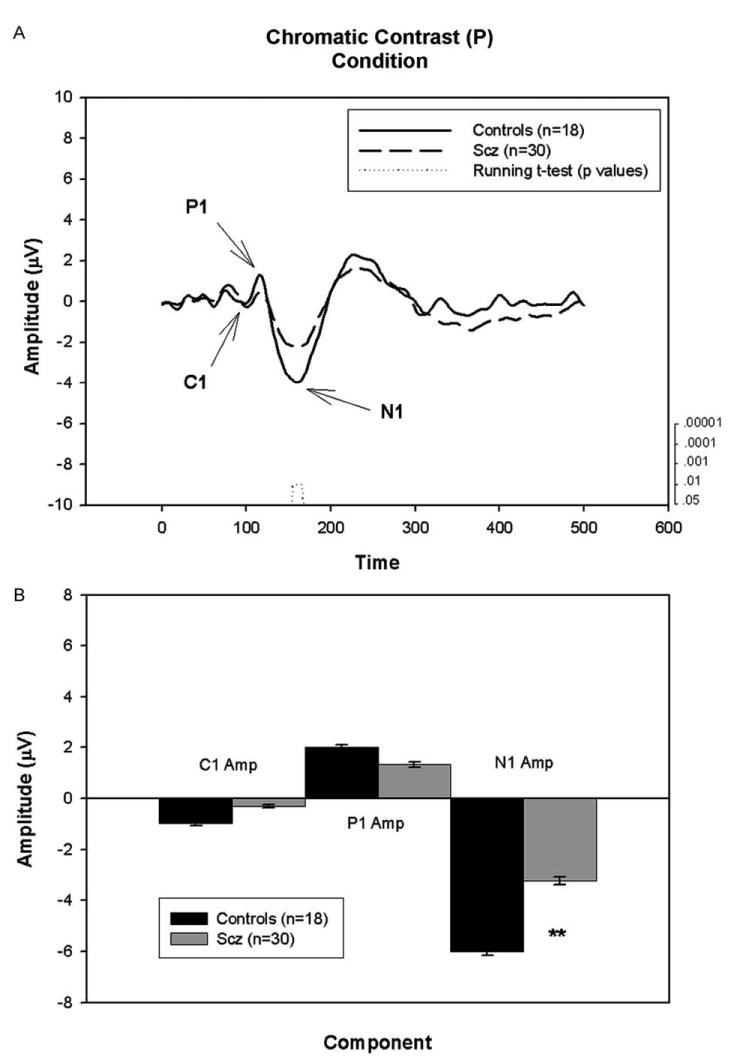
A. Graph showing the group average waveforms for the chromatic contrast (P) condition over time for controls and patients with running t-test. B. Bar graph showing group average C1, P1, and N1 component amplitudes with SEM for patients and controls. **P<.005.
3.1. Analysis of tVEP waveforms and component analysis
3.1.1. C1 Component
The earliest negative deflection, C1 occurred at ∼80 ms in the high contrast (mixed M/P) condition (Fig. 1). A slight deflection occurred at this latency as well in the chromatic contrast (P) condition (Fig. 3). No discernable component was present in the low contrast (M) condition (Fig. 2).
Fig. 2.
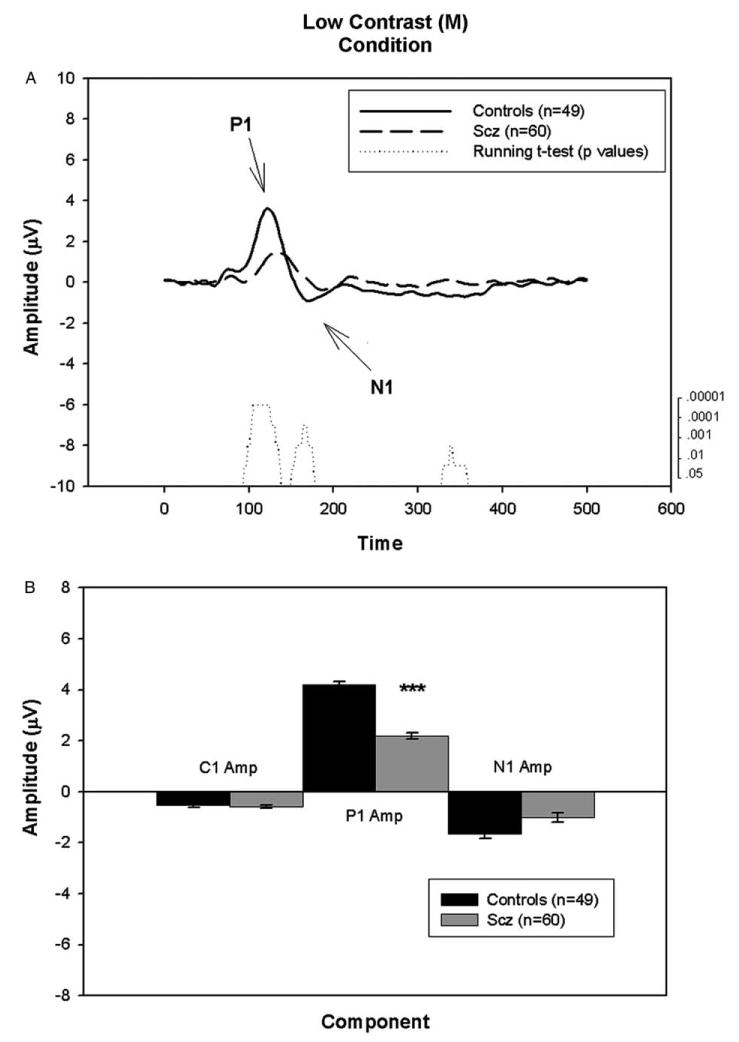
A. Graph showing the group average waveforms for the low contrast (M) condition over time for controls and patients with running t-test. B. Bar graph showing group average C1, P1, and N1 component amplitudes with SEM for patients and controls. ***P<.001
A significant between-group difference was observed in C1 amplitude in the high contrast (mixed M/P) condition [F=4.17, df=1/129, P=.04] but not in the chromatic contrast (P) conditions [F=2.23, df=1/44, P=.14]. While the running t-test revealed several consecutive timepoints with P<.05 between patients and controls in the high contrast (mixed M/P) condition, the number of consecutive points did not meet predetermined criteria for significance (11 consecutive points).
The between-group differences in C1 amplitude in the high contrast (mixed M/P) condition did not remain statistically significant following covariation for acuity [F=2.42, df=1/105, P=.12].
A significant group by gender interaction [F=4.06, df= 1/129, P=.05], but no gender effect [F=.70, df=1/129, P=.40] was observed for the C1 latency component in the high contrast (mixed M/P) condition. Follow-up t-tests demonstrated that while the C1 amplitude of male patients and controls were virtually the same (control males= −4.95±.59 μV; patient male= −4.93±.96 μV; t=−.02, df=1/89, P=.98), amplitudes for the female patients (3. 81±.63 μV) were significantly lower than for the female controls (−7.67±1.2 μV; t=2.41, df=1/36.1, P<.007). Upon covariation for acuity, however, the group by gender interaction was no longer significant [F=.71, df=1/105, P=.40]. No significant interaction or main effects for gender were observed for the other conditions.
Significant increases in C1 latency (P<.01) were observed for patients relative to controls in both the high contrast and chromatic contrast conditions (Table 1). No significant group by gender interaction or main effect for gender was observed for any of the conditions. The between-group difference in C1 latency remained significant following covariation for visual acuity in both high contrast (mixed M/P) [F=11.93, df=1/105, P=.001], and chromatic contrast (P) [F=4.25, df=1/35, P=.05] conditions.
Table 1.
Group means and ANOVA (group effect) for latency components
| Component | Patients (M±SEM) | Controls (M±SEM) | ANOVA (F, P value) |
|---|---|---|---|
| High contrast (mixed M/P) | |||
| C1 Latency | 85.4±.72 | 79.4±.66 | 29.51, <.001 |
| P1 Latency | 109.9±.87 | 104.0±.85 | 24.25, <.001 |
| N1 Latency | 147.6±1.3 | 142.9±1.4 | 9.05, .003 |
| P2 Latency | 210.0±1.2 | 204.5±1.3 | 10.58, .001 |
| Low contrast (M) | |||
| P1 Latency | 152.5±18.8 | 113.2±10.7 | 1.14, .29 |
| N1 Latency | 179.9±1.2 | 175.4±1.4 | 1.82, .18 |
| Chromatic contrast (P) | |||
| C1 Latency | 59.6±1.2 | 68.3±2.3 | 11.20, .002 |
| P1 Latency | 107.4±2.0 | 112.0±2.6 | 1.52, .22 |
| N1 Latency | 160.2±1.8 | 157.5±2.8 | .13, .72 |
All df for high contrast (mixed M/P) condition: 1/129; all df for low contrast (M) condition: 1/105; all df for chromatic contrast (P) condition: 1/44.
3.1.2. P1 amplitude
A large positive peak, P1, was present at ∼120 ms in both the low contrast (M) and high contrast (mixed M/P) conditions (Figs. 2 and 1, respectively). In contrast, only a minor peak at ∼100 ms was present in the chromatic contrast (P) condition (Fig. 3). P1 amplitude was reduced by nearly 50% compared with controls in both the low [F=16.80, df= 1/105, P<.001] and high [F=14.25, df=1/129, P<.001] contrast conditions. No significant between-group amplitude differences were found in the chromatic contrast (P) condition. Consistent with these results, the running t-test reveals a significant difference between the groups (21 points with P<.05; range of p values: P=10−6 to P=.03) from 94.5 ms to 136.5 ms in the low contrast (M) condition and a significant difference between the groups (11 points with P<.05; range of p values: P=10−6-P=.013) from 88 ms to 109.2 ms in the high contrast (mixed M/P) condition.
The between-group differences in P1 amplitude remained significant for both the low [F=10.14, df= 1/84, P=.002] and high [F=5.17, df=1/105, P=.03] contrast conditions following covariation for acuity.
A significant increase in latency of the P1 component was obtained for patients relative to controls of (Table 1) in the high contrast (mixed M/P) condition. This latency difference remained significant following covariation for visual acuity [F=8.54, df=1/106, P=.004]. Latencies in the other conditions were not significantly different between patients and controls (Table 1). No significant group by gender interaction or main effect for gender was observed for any of the conditions.
3.1.3. N1 Amplitude
A large negative peak, N1, was observed at ∼150 ms in both the high contrast (mixed M/P) condition and chromatic contrast (P) conditions (Figs. 1 and 3, respectively). In contrast, only a minor deflection at ∼180 ms was observed in the low contrast (M) condition (Fig. 2). Between-group ANOVAs demonstrated a significant decrease of N1 amplitude in patients relative to controls in both the high contrast (mixed M/P) [F=9.73, df=1/129, P=.002] and chromatic contrast (P) [F=8.54, df=1/44, P=.005] conditions. No significant difference between groups was found in the low contrast (M) condition.
Running t-tests revealed several consecutive timepoints with P<.05 between patients and controls in both high contrast (mixed M/P) and chromatic contrast (P) conditions. However, these were fewer than the predetermined criteria for statistical significance in both conditions, suggesting absent or non-reliable between-group difference. Further, between-group differences in N1 amplitude did not remain statistically significant following covariation for acuity in either the high contrast (mixed M/P) [F=2.93, df=1/105, P=.09] or chromatic (P) [F=3.47, df=1/35, P=.07] conditions. In the high contrast (mixed M/P) condition a significant correlations between N1 amplitude and acuity was observed for patients only (r=−.36, P=.007, n=57).
A significant increase of latency of the N1 component was obtained for patients relative to controls in the high contrast (mixed M/P) condition, but not in any other condition (Table 1). When acuity was used as a covariate for the latency of the N1 component in the high contrast (mixed M/P) condition, however, no significant between group difference was observed [F=3.46, df=1/106, P=.07].
A significant group by gender interaction [F=9.40, df= 1/129, P=.003], but no gender effect [F=1.91, df=1/129, P=.17] was observed for the N1 latency component in the high contrast (mixed M/P) condition. Similar findings were obtained even after using acuity as a covariate (significant group by gender interaction [F=9.65, df=1/106, P=.002] and no gender effect [F=.74, df=1/106, P=.39]). Follow-up t-tests demonstrated that while the latencies of male patients and controls were virtually the same (control males=146.9±1.8 ms; patient male=146.8±1.5 ms; t=−.51, df=1/89, P=.96), latencies for the female patients (150.3±2.7 ms) were significantly longer than for the female controls (137.8±2.0; t=5.20, df=1/40, P<.001). No significant interaction or main effects for gender were observed for the other conditions.
3.1.4. P2 Amplitude
A large positive peak, P2, was present at ∼200 ms in the high contrast (mixed M/P) condition only (Fig. 1). No similar peak was found in either the low contrast (M) condition or chromatic contrast (P) condition (Figs. 2 and 3, respectively). Between-group ANOVAs demonstrate a significant nearly 50% reduction in P2 amplitude in patients relative to controls [F=9.22, df=1/129, P=.003]. Consistent with the group differences, the running t-test demonstrated a significant difference between the waveforms of patients and controls in the high contrast (mixed M/P) condition (27 points with P<.05; range of p values: P=.0008−.05) from 176–231 ms.
While there was a significant between-group effect for the P2 amplitude in the high contrast (mixed M/P), when acuity was used as a covariate, no significant between group-difference in amplitude was observed [F=1.39, df= 1/105, P=.24].
No significant latency differences were found between patients and controls (Table 1). No significant group by gender interaction or main effect for gender was observed for any of the conditions.
3.2. Correlations of VEP components with clinical measures, ILS-PS and medication
No significant correlations between BPRS or SANS scores and any components of any of the tVEP were found. However, a significant correlation was found between ILS-PS scores and the P1 amplitude of the low contrast (M) condition [r=.33, n=59, P=.01]. Given this significant correlation, an ANOVA was performed between controls and ILS-PS groups (high and low functioning patients) for the P1 amplitude component of the low contrast (M) condition, and a significant difference between the groups was found [F=7.66, df=2/104, P=.001]. Post hoc tests revealed that while low functioning patients (mean±SEM: 1.90 μV±0.26) had a significant decrease of the P1 amplitude relative to controls (mean±SEM: 4.11 μV± 0.51) [Tukey HSD: P<.001], the high functioning patients (mean±SEM: 3.14 μV±0.57) did not differ significantly from controls or low functioning patients [Tukey HSD: P=.4, P=.3, respectively]. No other significant correlations were found between waveform components for any transient conditions and ILS-PS scores.
No significant correlations were found between medication (chlorpromazine equivalence) and components of any of the tVEP conditions.
4. Discussion
The aim of the current study was to examine the tVEP profile of patients with schizophrenia relative to controls in context of M vs. P pathway function as well as the possible relationship to level of community functioning. We have demonstrated that patients with schizophrenia exhibit significant deficits in select components of the early tVEP in low contrast (M), chromatic contrast (P) and high contrast (mixed M/P) conditions relative to controls. These findings are significant in several ways. First, our findings confirm reported P1 deficits in patients compared to controls (Basinska, 1998; Doniger et al., 2002; Foxe et al., 2001; Matsuoka et al., 1996; Romani et al., 1986; Spencer et al., 2003). Furthermore, the fact that the P1 component was particularly prominent in the low contrast (M) and high contrast (mixed M/P) conditions but not in the chromatic contrast (P) condition is further indication of the M genesis of this component (Di Russo et al., 2001; Ellemberg et al., 2001; Previc, 1988). The decreased P1 in patients in those conditions thus suggests a preferential M impairment.
In addition to P1 deficits, an N1 deficit in patients compared to controls was found. Our findings of a large N1 component only in the high contrast (mixed M/P) and chromatic contrast (P) conditions are consistent with the N1 emerging from ventral stream based upon P system input (Allison et al., 1999; Bentin et al., 1999; Di Russo et al., 2001; Doniger et al., 2000, 2001). The present findings, however, also provide a potential cautionary note regarding the study of N1 deficits in schizophrenia. Although all subjects were required to have visual acuity of 20/32 or better, nonetheless in the patient group N1 amplitude and latency in the high contrast (mixed M/P) condition and N1 amplitude in the chromatic (P) condition correlated significantly with acuity. Further, when acuity was used as a covariate, no significant group difference remained for these measures. Deficits in C1 generation also did not survive covariation for visual acuity. N1 sensitivity to acuity is consistent with the contribution of the parvocellular stream in N1 generation and with the sensitivity of the parvocellular system to small stimulus elements. In contrast, P1 deficits in the low contrast (M) and high contrast (mixed M/P) conditions persisted despite covariation.
A limitation of the present study is the use of only a single electrode located at Oz and referenced to the central midline. Since ERP components are dissociated in space, as well as time, the present findings must be considered preliminary, and follow-up with high-density recordings to isolate C1, P1 and N1 generators is required. Nevertheless, these findings strongly support the existence of early visual processing deficits in schizophrenia, occurring potentially within both the M and P visual pathways.
In addition to showing deficits in early visual processing in schizophrenia, the present study also shows direct correlation between early visual measures and global outcome as measured by the ILS-PS. The ILS-PS is a problem solving measure that differentiates individuals requiring continued inpatient hospitalization vs. those who have the capability to function in supervised outpatient settings (Revheim and Medalia, 2004a). In the present study, despite amplitude reductions in multiple components, only P1 amplitude in the M-selective (4% achromatic) condition correlated with ILS-PS score. Furthermore, high functioning patients did not differ significantly from controls in this measure, potentially accounting for a portion of the heterogeneity across studies.
At present, the mechanism by which impaired visual processing contributes to poor outcome is unknown, although the M pathway in particular contributes to processes that may be critical for real world function, such as object recognition (Doniger et al., 2002), motion processing (Chen et al., 1999), visual attention (Steinman et al., 1998; Vidyasagar, 1999) or reading (Cornelissen et al., 1998; Demb et al., 1998; Greatrex and Drasdo, 1995; Livingstone et al., 1991; Romani et al., 2001; Stein and Walsh, 1997). Alternatively, VEP components may simply serve as phenotypic markers for poor outcome forms of the disorder. Future studies involving prodromal and first-episode patients, as well as unaffected family members of schizophrenia probands, may be needed to disentangle state vs. trait features of visual processing impairments in schizophrenia.
Another important finding of this study is the apparent lack of a medication effect on the VEP in schizophrenia. Our finding of no correlation between cpz equivalence and VEP is consistent with our earlier report of ssVEP in schizophrenia (Butler et al., 2001, 2002), as well as reports of the absence of medication effects on visual backward masking (Butler et al., 2003; Cadenhead et al., 1997; Green et al., 1999) and no effect of acute haloperidol application on the VEP (Jibiki et al., 1993). The dissociation between medication and the VEP further highlights the utility of the VEP as a measure of the underlying pathological process of schizophrenia rather than the interfering medication effects.
Finally, an interesting observation of this study is the absence of the expected better VEP performance for women compared to their male counterparts in the schizophrenia group. Among the normal population, women's VEP responses are consistently larger and faster than men's (Celesia et al., 1987; Halliday et al., 1982; Mitchell et al., 1987; Stockard et al., 1979). In the present study, this advantage was lost and female patients with schizophrenia showed significantly increased latencies of the N1 component at high contrast (mixed M/P) that were greater than those of their male counterparts. A similar but less robust pattern was observed for the C1 amplitude at high contrast (mixed M/P) as well. The group X gender differences persist, even after using visual acuity as a covariate. Given the role of the N1 component in object recognition (Doniger et al., 2001), this finding is consistent with an earlier findings of significantly greater critical stimulus duration required for female vs. male patients in letter identification (Cadenhead et al., 1997). Similarly, this may relate to lower premorbid cognitive functioning of female compared to male adolescents who will develop schizophrenia early in life (Weiser et al., 2000) and other reports of greater neuropsychological impairments in women who develop schizophrenia relative to men (Lewine et al., 1996).
In summary, sensory processing deficits continue to be relatively understudied in schizophrenia. In contrast to some recent reports, the present study shows significant dysfunction even of early visual processes in schizophrenia. Stimulus manipulations, particularly luminance and chromatic contrast variations, successfully biased activations toward M vs. P pathways in the present study. Deficits in M pathway processing, in particular, correlated significantly with global outcome. These deficits, which may be related to both structural and functional abnormalities within the early visual pathway in schizophrenia (Butler et al., 2005), are deserving of further study.
Acknowledgments
This study was supported in part by a Lieber Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (PDB), USPHS grants RO1 MH66374 (PDB), RO1 MH49334 and K02 MH01439 (DCJ), and a Burroughs Wellcome Translational Scientist Award (DCJ).
References
- Aine CJ, Supek S, George JS. Temporal dynamics of visual-evoked neuromagnetic sources: effects of stimulus parameters and selective attention. Int J Neurosci. 1995;80:79–104. doi: 10.3109/00207459508986095. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, Spencer D, McCarthy G. Electrophysiological studies of human face perception I: potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–30. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Basinska A. Altered electrophysiological pattern of target detection in schizophrenia in the continuous attention test. Acta Neurobiol Exp. 1998;58:207–20. doi: 10.55782/ane-1998-1275. [DOI] [PubMed] [Google Scholar]
- Bentin S, Mouchetant-Rostaing Y, Giard MH, Echallier JF, Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: time course and scalp distribution. J Cognit Neurosci. 1999;11:235–60. doi: 10.1162/089892999563373. [DOI] [PubMed] [Google Scholar]
- Braff DL, Saccuzzo DP, Geyer MA. Information processing dysfunctions in schizophrenia: studies of visual backward masking, sensorimotor gating, and habituation. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of schizophrenia. Vol. 5. New York, NY: Elsevier; 1991. pp. 303–34. [Google Scholar]
- Brenner CA, Lysaker PH, Wilt MA, O’Donnell BF. Visual processing and neuropsychological function in schizophrenia and schizoaffective disorder. Psychiatry Res. 2002;111:125–36. doi: 10.1016/s0165-1781(02)00139-7. [DOI] [PubMed] [Google Scholar]
- Bruder G, Kayser J, Tenke C, Rabinowicz E, Friedman M, Amador X, Sharif Z, Gorman J. The time course of visuospatial processing deficits in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 1998;107:399–411. doi: 10.1037//0021-843x.107.3.399. [DOI] [PubMed] [Google Scholar]
- Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40:295–8. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–33. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Early-stage vision and schizophrenia. Am J Psychiatry. 2002;159:678–9. [Google Scholar]
- Butler PD, DeSanti LA, Maddox J, Harkavy-Friedman JM, Amador XF, Goetz RR, Javitt DC, Gorman JM. Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res. 2003;59:199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead KS, Geyer MA, Butler RW, Perry W, Sprock J, Braff DL. Information processing deficits of schizophrenia patients: relationship to clinical ratings, gender and medication status. Schizophr Res. 1997;28:51–62. doi: 10.1016/s0920-9964(97)00085-6. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Kaufman D, Cone S. Effects of age and sex on pattern electroretinograms and visual evoked potentials. Electroencephalogr Clin Neurophysiol. 1987;68:161–71. doi: 10.1016/0168-5597(87)90023-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–54. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Hansen PC, Hutton JL, Evangelinou V, Stein JF. Magnocellular visual function and children’s single word reading. Vis Res. 1998;38:471–82. doi: 10.1016/s0042-6989(97)00199-5. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Functional magnetic resonance imaging of early visual pathways in dyslexia. J Neurosci. 1998;18:6939–51. doi: 10.1523/JNEUROSCI.18-17-06939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–40. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp. 2001;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Snodgrass JG, Schroeder CE, Javitt DC. Activation timecourse of ventral visual stream object-recognition areas: high density electrical mapping of perceptual closure processes. J Cogn Neurosci. 2000;12:615–21. doi: 10.1162/089892900562372. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Schroeder CE, Murray MM, Higgins BA, Javitt DC. Visual perceptual learning in human object recognition areas: a repetition priming study using high-density electrical mapping. Neuroimage. 2001;13:305–13. doi: 10.1006/nimg.2000.0684. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–20. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Hammarrenger B, Lepore F, Roy MS, Guillemot JP. Contrast dependency of VEPs as a function of spatial frequency: the parvocellular and magnocellular contributions to human VEPs. Spat Vis. 2001;15:99–111. doi: 10.1163/15685680152692042. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders-patient edition. New York, NY: New York State Psychiatric Institute; 1997. [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired PI generation revealed by high-density electrical mapping. Neuro Report. 2001;12 doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Gold JM. Neurocognitive functioning in patients with schizophrenia: an overview. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology, the fourth generation of progress. New York, NY: Raven Press Ltd; 1995. pp. 1245–57. [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–57. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Greatrex JC, Drasdo N. The magnocellular deficit hypothesis in dyslexia: a review of reported evidence. Ophthalmic Physiol Opt. 1995;15:501–6. [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Backward masking performance as an indicator of vulnerability to schizophrenia. Acta Psychiatr Scand Suppl. 1999a;395:34–40. doi: 10.1111/j.1600-0447.1999.tb05981.x. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophr Bull. 1999b;25:309–19. doi: 10.1093/oxfordjournals.schbul.a033380. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51:945–51. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. Am J Psychiatry. 1999;156:1367–73. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the right stuff? Schizophr Bull. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Greenstein VC, Seliger S, Zemon V, Ritch R. Visual evoked potential assessment of the effects of glaucoma on visual subsystems. Vis Res. 1998;38:1901–11. doi: 10.1016/s0042-6989(97)00348-9. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–4. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Halliday AM, Barrett G, Carroll WM, Kriss A. Problems in defining the normal limits of the visual evoked potential. Adv Neurol. 1982;32:1–9. [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW. Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry. 1974;31:143–51. doi: 10.1001/archpsyc.1974.01760140005001. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Czobor P, Berns SM. Basic neuropsychological dimensions in schizophrenia. Schizophr Res. 2003;65:105–16. doi: 10.1016/s0920-9964(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Liederman E, Cienfuegos A, Shelley AM. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophr Bull. 1999;25:763–75. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA. Source locations of pattern-related visual evoked potentials (VEP) components. Electroencephalogr Clin Neurophysiol. 1971;30:367. [PubMed] [Google Scholar]
- Jibiki I, Kurokawa K, Fukushima T, Yamaguchi N. Acutely administered haloperidol has little effect on steady-state visual evoked potentials from pattern-reversal stimulations in treated schizophrenics. Jpn J Psychiatry Neurol. 1993;47:51–5. doi: 10.1111/j.1440-1819.1993.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM, Purpura K. Color and luminance contrast as tools for probing the primate retina. Neurosci Res. 1988 8:S151–S65. doi: 10.1016/0921-8696(88)90014-x. [DOI] [PubMed] [Google Scholar]
- Kee KS, Kern RS, Green MF. Perception of emotion and neurocognitive functioning in schizophrenia: what's the link? Psychiatry Res. 1998;81:57–65. doi: 10.1016/s0165-1781(98)00083-3. [DOI] [PubMed] [Google Scholar]
- Keri S, Antal A, Szekeres G, Szendi I, Kovacs Z, Janka Z, Benedek G. Testing basic visual functions in the evaluation of extrapyramidal side effects of antipsychotic agents. Orv Hetil. 1998;139:235–8. [PubMed] [Google Scholar]
- Keri S, Antal A, Szekeres G, Benedek G, Janka Z. Spatiotemporal visual processing in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:190–6. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18:537–42. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull. 1993;19:461–536. doi: 10.1093/schbul/19.3.461. [DOI] [PubMed] [Google Scholar]
- Lewine RR, Walker EF, Shurett R, Caudle J, Haden C. Sex differences in neuropsychological functioning among schizophrenic patients. Am J Psychiatry. 1996;153:1178–84. doi: 10.1176/ajp.153.9.1178. [DOI] [PubMed] [Google Scholar]
- Li CS. Impaired detection of visual motion in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:929–34. doi: 10.1016/s0278-5846(02)00207-5. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Psychophyscial evidence for seperate channels for the perception of form, color, movement, and depth. J Neurosci. 1987;7:3416–68. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–9. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc Natl Acad Sci USA. 1991;88:7943–7. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb PA. ILS: independent living scales manual. San Antonio, TX: The Psychological Corporation, Harcourt Race Jovanovich, Inc.; 1996. [Google Scholar]
- Logothetis NK, Schiller PH, Charles ER, Hurlbert AC. Perceptual deficits and the activity of the color-opponent and broad-band pathways at isoluminance. Science. 1990;247:214–7. doi: 10.1126/science.2294602. [DOI] [PubMed] [Google Scholar]
- Maier J, Dagnelie G, Spekreijse H, van Dijk BW. Principal components analysis for source localization of VEPs in man. Vis Res. 1987;27:165–77. doi: 10.1016/0042-6989(87)90179-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Saito H, Ueno T, Sato M. Altered endogenous negativities of the visual event-related potential in remitted schizophrenia. Electroencephalogr Clin Neurophysiol. 1996;100:18–24. doi: 10.1016/0168-5597(95)00212-x. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? In: Cowan WM, Shooter EM, Stevens CF, Thompson RF, editors. Ann rev neuroscience. Palo Alto, CA: Annual Reviews, Inc.; 1993. pp. 369–402. [DOI] [PubMed] [Google Scholar]
- Mitchell KW, Howe JW, Spencer SR. Visual evoked potentials in the older population: age and gender effects. Clin Phys Physiol Meas. 1987;8:317–24. doi: 10.1088/0143-0815/8/4/004. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–92. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Potts GF, Nestor PG, Stylianopoulos KC, Shenton ME, McCarley RW. Spatial frequency discrimination in schizophrenia. J Abnorm Psychol. 2002;111:620–5. doi: 10.1037//0021-843x.111.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T, Ikebuchi E, Henomatsu K, Kasai K, Nakagome K, Iwanami A, Hiramatsu K, Hata A, Fukuda M, Honda M, Miyauchi M. Psychophysiological correlates of social skills deficits in persons with schizophrenia. Psychiatry Res. 2000;100:155–67. doi: 10.1016/s0925-4927(00)00077-9. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psych Rep. 1962;10:799–812. [Google Scholar]
- Perry W, Braff DL. Information-processing deficits and thought disorder in schizophrenia. Am J Psychiatry. 1994;151:363–7. doi: 10.1176/ajp.151.3.363. [DOI] [PubMed] [Google Scholar]
- Previc F. The neurophysiological significance of the N1 and P1components of the visual evoked potential. Clin Vis Sci. 1988;3:195–202. [Google Scholar]
- Regan D. Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. New York, NY: Elsevier; 1989. [Google Scholar]
- Revheim N, Medalia A. The independent living scales (ILS) as a measure of functional outcome for schizophrenia. Psychiatric Services. 2004 doi: 10.1176/appi.ps.55.9.1052. [DOI] [PubMed] [Google Scholar]
- Revheim N, Medalia A. Verbal memory, problem-solving skills and community status in schizophrenia. Schizophr Res. 2004b;68:149–58. doi: 10.1016/j.schres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Romani A, Zerbi F, Mariotti G, Callieco R, Cosi V. Computed tomography and pattern reversal visual evoked potentials in chronic schizophrenic patients. Acta Psychiatr Scand. 1986;73:566–73. doi: 10.1111/j.1600-0447.1986.tb02726.x. [DOI] [PubMed] [Google Scholar]
- Romani A, Conte S, Callieco R, Bergamaschi R, Versino M, Lanzi G, Zambrino CA, Cosi V. Visual evoked potential abnormalities in dyslexic children. Funct Neurol. 2001;16:219–29. [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–31. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Tenke CE, Givre SJ, Arezzo JC, Vaughan HG., Jr Striate cortical contribution to the surface-recorded pattern-reversal VEP in the alert monkey. Vis Res. 1991;31:1143–57. doi: 10.1016/0042-6989(91)90040-c. [DOI] [PubMed] [Google Scholar]
- Schwartz BD, Maron BA, Evans WJ, Winstead DK. High velocity transient visual processing deficits diminish ability of patients with schizophrenia to recognize objects. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:170–7. [PubMed] [Google Scholar]
- Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2003;59:233–41. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- Shagass C. Brain potential studies of psychopathology. Comp Psychiatry. 1980;21:483–91. doi: 10.1016/0010-440x(80)90051-6. [DOI] [PubMed] [Google Scholar]
- Shagass C, Straumanis JJ, Jr, Roemer RA, Amadeo M. Evoked potentials of schizophrenics in several sensory modalities. Biol Psychiatry. 1977;12:221–35. [PubMed] [Google Scholar]
- Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Exp Brain Res. 2004;156:196–211. doi: 10.1007/s00221-003-1771-3. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Curran CE. Spatial frequency masking in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1999;108:42–50. doi: 10.1037//0021-843x.108.1.42. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–11. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–52. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Steinman SB, Steinman BA, Garzia RP. Vision and attention. II: Is visual attention a mechanism through which a deficient magnocellular pathway might cause reading disability? Optom Vis Sci. 1998;75:674–81. doi: 10.1097/00006324-199809000-00023. [DOI] [PubMed] [Google Scholar]
- Stockard JE, Stockard JJ, Westmoreland BF, Corfits JL. Brainstem auditory-evoked responses. Normal variation as a function of stimulus and subject characteristics. Arch Neurol. 1979;36:823–31. doi: 10.1001/archneur.1979.00500490037006. [DOI] [PubMed] [Google Scholar]
- Straumanis JJ, Shagass C, Roemer RA. Influence of antipsychotic and antidepressant drugs on evoked potential correlates of psychosis. Biol Psychiatry. 1982;17:1101–22. [PubMed] [Google Scholar]
- Tootell RB, Hamilton SL, Switkes E. Functional anatomy of macaque striate cortex. IV. Contrast and magno-parvo streams. J Neurosci. 1988;8:1594–609. doi: 10.1523/JNEUROSCI.08-05-01594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillenberg P, Lencer R, Heide W. Eye movements and psychiatric disease. Curr Opin Neurol. 2004;17:43–7. doi: 10.1097/00019052-200402000-00008. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Owzar K, Johnson SC, Doty RL, Gur RE. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry. 2003;53:403–11. doi: 10.1016/s0006-3223(02)01865-6. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61:237–48. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- Vanni S, Warnking J, Dojat M, Delon-Martin C, Bullier J. Sequence of pattern onset responses in the human visual areas: an fMRI constrained VEP source analysis. Neuroimage. 2004;21:801–17. doi: 10.1016/j.neuroimage.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR. A neuronal model of attentional spotlight: parietal guiding the temporal. Brain Res Brain Res Rev. 1999;30:66–76. doi: 10.1016/s0165-0173(99)00005-3. [DOI] [PubMed] [Google Scholar]
- Weiser M, Reichenberg A, Rabinowitz J, Weiser M, Reichenberg A, Rabinowitz J, Kaplan Z, Mark M, Nahon D, Davidson M. Gender differences in premorbid cognitive performance in a national cohort of schizophrenic patients. Schizophr Res. 2000;45:185–90. doi: 10.1016/s0920-9964(99)00190-5. [DOI] [PubMed] [Google Scholar]
- Zemon V, Siegfried J, Gordon J. Magno and parvo pathways in humans studied using VEPs to luminance and chromatic contrast (abstract) Invest Opthalmol Vis Sci. 1991 32:1033. [Google Scholar]


