Abstract
Individuals with schizophrenia show magnocellular visual pathway abnormalities similar to those described in dyslexia, predicting that reading disturbance should be a common concomitant of schizophrenia. To date, however, reading deficits have not been well established, and, in fact, reading is often thought to be normal in schizophrenia based upon results of tests such as the WRAT, which evaluate single word reading. This study evaluated “real world” reading ability in schizophrenia, relative to functioning of the magnocellular visual pathway. Standardized psychoeducational reading tests and contrast sensitivity measures were administered to 19 patients and 10 controls. Analyses of between group differences were further refined by classification of participants into reading vs. non-reading impaired groups using a priori and derived theoretical models. Patients with schizophrenia, as a group, showed highly significant impairments in reading (p<0.04–p<0.001), with particular deficits on tests of rate, comprehension and phonological awareness. Between 21% and 63% of patients met criteria for dyslexia depending upon diagnostic model vs. 0–20% of the controls. The degree of deficit correlated significantly with independent measures of magnocellular dysfunction. Reading impairment in schizophrenia reaches the level of dyslexia and is associated with compromised magnocellular processing as hypothesized. Findings related to symptoms, functioning and recommendations for reading ability assessment are discussed.
Keywords: Reading disorders, Dyslexia, Visual deficits, Magnocellular processing, Contrast sensitivity, Schizophrenia
1. Introduction
Mastery of the three R's – reading, writing, and arithmetic – is critical for professional success (Caspi et al., 1998). Despite extensive research on cognition in schizophrenia, relatively little work has focused on these basic academic skills (Schirmer et al., 2005). Furthermore, when reading has been studied, tests such as the Wide Range Achievement Test (WRAT) reading subtest have primarily been used. Rather than measuring all aspects of reading ability, this test assesses only single word recognition which, as a measure of premorbid IQ, is largely unaffected in schizophrenia (Dalby and Williams, 1986; Harvey et al., 2000; Kremen et al., 1996). Further, recent sensory studies have demonstrated substantial impairments in functioning of the magnocellular visual system in schizophrenia (Butler et al., 2005). Dysfunction of the magnocellular system, in turn, is implicated in the clinical phenomenon of dyslexia, a condition where reading is impaired relative to overall cognitive function (Demb et al., 1998). Based upon these two observations, a high rate of reading impairment in schizophrenia would be expected.
In its broadest sense, dyslexia is defined as a specific deficit in reading relative to other aspects of cognitive function (Manzo and Manzo, 1993). Initial criteria for dyslexia excluded individuals with low IQ or education, poor nutrition, poverty, or presence of co-morbid other mental disabilities. Currently, severe reading impairment (dyslexia) is differentiated from poor reading in general. Specifically, dyslexia is defined as a developmental disorder characterized by major difficulties in learning to decode printed material (Vellutino and Fletcher, 2005), and, in particular, to convert printed material into appropriate phonological representations (Hoover and Gough, 1990). Current models emphasize core deficits in word recognition at the orthographic (awareness of spelling patterns or letter combinations) and/or phonological (awareness of sound structure) levels (Vellutino et al., 2004; Vellutino and Fletcher, 2005) rather than placing emphasis on language impairment alone (Tallal, 2000).
Reading deficits are predicted strongly by recent research demonstrating impaired functioning of the magnocellular visual pathway in schizophrenia. The magnocellular (M) pathway is one of two primary low-level visual pathways in the human brain, and is primarily responsible for processing low spatial frequency and motion information, and for organizing visual space. Magnocellular processing deficits have been extensively linked to dyslexia (Demb et al., 1998; Talcott et al., 1998; Romani et al., 2001; Ridder et al., 1997). Although evidence for M-pathway involvement in dyslexia varies somewhat across studies, it has nevertheless been proposed that up to 75% of individuals with dyslexia have visual processing deficits attributable to M-pathway dysfunction (Talcott et al., 1998; Ridder et al., 1997). Furthermore, deficits have been detected in reading-impaired children and adults using an assortment of psychophysical measures including critical flicker fusion and coherent motion detection (Talcott et al., 1998), contrast sensitivity (Lovegrove, 1993), and visual evoked potentials (Romani et al., 2001). Respectively, disabled readers have elevated detection thresholds, lower magnocellular-related contrast sensitivity, and smaller electrophysiological responses than controls. Many of the same M-system deficits, including reduced ability to detect low spatial frequency (Butler et al., 2001, 2005) and motion (Kim et al., 2005) stimuli, and reductions in visual evoked potentials (Schechter et al., 2003) have been found in schizophrenia. However, the relationship of M-system dysfunction to reading impairment has not been previously investigated in schizophrenia.
Along with visual deficits, dyslexia is also frequently associated with deficits in phonological processing, resulting potentially from auditory-level disturbances in phonemic sequencing. Patients with schizophrenia, like those with dyslexia, show deficits in early auditory processing including, for example, deficits in tone matching (Javitt et al., 2000), mismatch negativity generation (Javitt et al., 1995b) and ability to detect phonetic boundaries (Cienfuegos et al., 1999). Thus, dyslexia-like deficits would be expected in schizophrenia based upon consideration of auditory, as well as visual, processing dysfunction.
Although most studies in schizophrenia have evaluated only single word reading, scattered studies have obtained findings suggestive of fundamental disturbances in reading ability. For example, Fuller et al. (2002) found that patients who developed schizophrenia showed relatively intact reading on the Iowa Tests of Basic Skills while in 4th and 8th grades, but reduced reading ability while in 11th grade. The Iowa emphasizes passage comprehension, rather than single word reading, and thus may be more indicative of “real world” reading skills. Similarly, Hayes and O'Grady (2003) demonstrated reduced passage comprehension relative to single word reading in schizophrenia, but did not examine potential sensory antecedents. To our knowledge, this is the first study to utilize multiple, standardized, psychoeducationally-based reading batteries in schizophrenia. Statistical comparisons are made relative to both local non-psychiatric comparison subjects and published norms of specific test batteries.
2. Methods
2.1. Participants
Twenty-nine individuals signed written informed consent to participate after procedures had been fully explained: 19 patients with schizophrenia or schizoaffective disorder diagnosed by SCID interview and 10 community-dwelling adults. All participants met inclusion criteria (18 to 55 years old; visual acuity corrected to 20/30 for near and far distances, IQ>85, native English speakers). Individuals with a history of neurological impairment, mental retardation, color vision deficits, or current alcohol or drug abuse (<1 month) or substance dependence (<6 months) were excluded.
Groups did not differ significantly in age, Quick IQ score (Ammons and Ammons, 1962), parental Hollings-head socioeconomic status (SES), Edinburgh score for handedness, gender, or ethnicity (Table 1). Groups differed significantly on years of education (t=−4.78, df=25.2, p<0.01) and SES (t=−3.96, df=26, p<0.01).
Table 1.
Demographic data: sample characteristics for patients and controls [mean (S.D.)]
| Patients (n=19) | Controls (n=10) | |
|---|---|---|
| Age | 38.3 (9.6) | 28.7 (9.0) |
| Education** | 12.4 (2.3) | 15.2 (0.85) |
| Quick IQ | 103.1 (7.1) | 104.6 (8.2) |
| Subject SES** | 26.9 (9.4)a | 43.1 (12.0) |
| Parental SES | 43.2 (22.2)b | 47.0 (11.2) |
| Edinburgh score | 18.0 (2.5) | 13.9 (6.5) |
| Gender | ||
| Male | 18 (95%) | 6 (60%) |
| Female | 1 (5%) | 4 (40%) |
| Ethnicity | ||
| White, Non-Hispanic | 7 (37%) | 7 (70%) |
| Black, Non-Hispanic | 7 (37%) | 2 (20%) |
| Hispanic | 2 (10%) | 0 (0%) |
| Other | 3 (16%) | 1 (10%) |
n=18.
n=16.
p<0.01.
The patients had on average been ill for 16.8±8.6 years (mean±S.D.) prior to testing and were all receiving antipsychotic medication (1077.7±574 chlorpromazine equivalents). Scores for positive, negative, cognitive, depression and excitement factors on the Positive and Negative Syndrome Scale (PANSS) (Lindenmayer et al., 1994) were 13.5±4.1, 13.9±5.3, 12.1±3.5, 13.8±5.0, and 8.7±3.2, respectively. Global level of function as measured by the Independent Living Scales (ILS-PB) (Revheim and Medalia, 2004) was 40.1±12.2, with some individuals requiring maximum supervision (37% score < 40) while others were capable of independent living (26% score 50+). Limited neuropsychological testing was obtained using the WAIS-3 (Wechsler, 1997) Working Memory Index (WMI: 81.2±15.6, n=12) and Processing Speed Index (PSI: 80.5±8.3, n=18). These measures were obtained for patients only, but were significantly reduced vs. population norms.
2.2. Instruments
Instruments assessed both single word and passage reading, and incorporated tests sensitive to both global comprehension, as well as to specific orthographic and phonological processes. Specific measures included the following.
The Wide Range Achievement Test (WRAT3), reading subtest, (Wilkinson, 1993) measures basic word-reading skill without demands on comprehension. Raw scores are converted into standard scores using age-related norms with a mean of 100 and a S.D. of 15.
The Gray Oral Reading Test (GORT-4) (Wiederholt and Bryant, 2001) measures oral reading skills and offers norms through age 18 years 11 months. Individuals read aloud up to 14 stories and answer five multiple-choice questions that follow. The GORT-4 provides five separate scores: Rate (amount of time taken to read the story); Accuracy (ability to pronounce each word correctly); Fluency (Rate+Accuracy); and Comprehension (correctness of answers to multiple-choice questions) reported as standard scores with a mean of 10 and a S.D. of 3. An overall reading ability, the Oral Reading Quotient (ORQ), with a mean of 100 and S.D. of 15, combines fluency and comprehension scores.
The Comprehensive Test of Phonological Processing (CTOPP) (Wagner et al., 1999) measures three components of phonological processing: phonological awareness, phonological memory, and rapid naming; using 12 subtests. Norms are provided for ages 7 through 24 years old. Five composite scores are obtained based on subtest scores: Phonological Awareness (PA), Phonological Memory (PM), Rapid Naming (RN), Alternate Phonological Awareness (APA), and Alternate Rapid Naming (ARN), and are reported as a standard score with a mean of 100 and a S.D. of 15. PA and APA measure the awareness and ability to grasp the phonological structure of oral language, using real words or nonwords, respectively. PM measures the ability to code information phonologically and to store it in short-term or working memory. RN or ARN incorporates the retrieval of phonological information from memory as an individual executes a series of operations (line reading) with speed and repetition.
The Woodcock–Johnson III Tests of Achievement (WJ-III) (Woodcock et al., 2001) measures academic strengths and weaknesses. Seven specific reading tests were used to obtain cluster scores: Broad Reading (BR), Basic Reading Skills (BRS), Reading Comprehension (RC) and Phoneme/Grapheme Knowledge (PGK), reported as a standard score with a mean of 100 and a S.D. of 15. BR measures reading decoding, reading speed and comprehension of paragraphs. BRS measures sight vocabulary, phonics and structural analysis. RC measures overall comprehension, vocabulary and reasoning. PGK measures proficiency in phonic and orthographic processes used during decoding and encoding.
The Nelson–Denny Reading Test (NDRT) (Brown et al., 1993) measures vocabulary, reading comprehension and reading rate for individuals at the high school and college level. The timed multiple-choice vocabulary (NDRT-V) and passage comprehension (NDRT-C) sections are performed silently and combine to form the total score (NDRT-T). All scores are reported as grade equivalent levels or percentile scores.
2.3. Procedures
Reading measures were administered according to specified directions over 1–2 sessions, for a total of 3–4 h of testing. Testing occurred in a well-lit/quiet room, with opportunities for rest periods. Scoring was performed by hand or computer software (e.g. WJ-III) and age-norms were used or approximated (e.g. oldest age groups for GORT-4 and CTOPP).
2.3.1. Contrast sensitivity functions
Integrity of magnocellular functioning was evaluated using contrast sensitivity, as previously described (Butler et al., 2005). Briefly, participants were tested binocularly following light adaptation to background luminance. Horizontal sine-wave gratings were then presented at 0.5, 7, and 21 c/deg on one half (either right or left side) of a visual display, with the other side remaining blank. Subjects had to state which side of the display contained the grating. Each grating was presented for 500 ms. Contrast was varied across trials using an up-and-down transformed response method to determine contrast sensitivity (the reciprocal of threshold) associated with 70.7% correct responses for each spatial frequency. The mean of 10 reversals was used to obtain thresholds.
2.4. Statistical analyses
Demographic characteristics between groups were analyzed with t-tests and Mann–Whitney U-tests. Group differences on reading measures were analyzed with t-tests and followed up with MANCOVA using education or SES as covariates. For patients, symptom profiles and limited neuropsychological test scores were correlated with reading measures to explore potential confounds.
In order to overcome limitations imposed by nomothetic analyses, idiographic reading profiles were explored on a case-by-case basis using several models for diagnosing reading impairment, including “dyslexia” (described below). Participants were then correctly classified into sub-groups (impaired vs. non-impaired) as per the pre-determined criterion. Individuals assigned to the “reading impaired” group were those individuals classified as having reading deficiencies in at least two of the five models.
Group analyses for contrast sensitivity measures were performed using one-way ANOVA for the three subgroups of participants: patients with or without reading impairment and controls. Further validation of sub-group analyses was executed by using one-way ANOVA to determine group differences on an alternate reading measure not used in the primary classification schemas.
3. Results
3.1. Reading impairment
Because of the relative lack of information regarding reading dysfunction in schizophrenia and lack of consensus regarding “ideal” reading tests, four separate test batteries were used (GORT-4, CTOPP, WJ-III, and NDRT). An omnibus MANOVA demonstrated first that patients showed significant impairments in reading relative to controls across respective test batteries (Table 2). In order to identify specific subtests that were particularly sensitive to reading dysfunction, individual subtests were compared between patients and controls using Bonferroni-corrected t-tests. Significant between-group differences were found on the following measures: GORT-4-Rate, GORT-4-Fluency, GORT-4-ORQ, CTOPP-APA, WJ-III-BR and NDRT-C (Table 2). Scores on GORT-4-Rate, Comprehension and ORQ; CTOPP-PA, CTOPP-RN, CTOPP-APA, CTOPP-ARN, WJ-III-BR and NDRT-C were also significantly below adult population norms (p<0.01), as well as CTOPP-PM and NDRT-T (p<0.05). Significant correlations with NDRT-C were found for GORT-4-Rate and CTOPP-APA, respectively (r=0.82, n=29, p=0.001; r=0.63, n=29, p=0.001).
Table 2.
Means and standard deviations for reading measures for patients (n=19) and controls (n=10)
| Patients
|
Controls
|
t | Adj. df | pa | |||
|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | ||||
| WRAT3 (SS) | 98.9 | 13.8 | 109.1 | 5.5 | −2.2 | 27 | (0.04) |
| GORT-4 (SS) (F=12.08, df=1,27, p=0.002) | |||||||
| Rate** | 7.8 | 3.3 | 11.0 | 0.9 | −3.9 | 22.8 | 0.001 |
| Accuracy | 9.5 | 4.6 | 13.1 | 1.4 | −3.2 | 23.3 | (0.004) |
| Fluency | 7.9 | 5.8 | 13.7 | 1.5 | −4.1 | 22.1 | 0.001 |
| Comprehension** | 6.3 | 3.5 | 9.6 | 2.1 | −2.7 | 27 | (0.01) |
| ORQ** | 82.6 | 23.1 | 109.9 | 9.5 | −3.5 | 27 | 0.001 |
| CTOPP-Composite Scores (SS) (F=10.07, df=1,27, p=0.004) | |||||||
| CTOPP-PA** | 80.7 | 23.9 | 94.6 | 9.2 | −2.2 | 25.6 | (0.03) |
| CTOPP-PM* | 92.4 | 13.7 | 105.1 | 10.9 | −2.5 | 27 | (0.02) |
| CTOPP-RN** | 89.6 | 14.7 | 99.4 | 15.3 | −1.7 | 27 | (0.10) |
| CTOPP-APA** | 74.3 | 21.2 | 97.3 | 12.6 | −3.7 | 26.4 | 0.001 |
| CTOPP-ARN** | 80.6 | 13.9 | 96.7 | 15.4 | −2.9 | 27 | (0.008) |
| WJ-III (Cluster Scores) (SS) (F=4.81, df=1,27, p=0.04) | |||||||
| WJ-III-BR** | 91.4 | 11.9 | 105.0 | 7.5 | −3.3 | 27 | 0.003 |
| WJ-III-BRS | 96.5 | 14.6 | 102.1 | 8.2 | −1.1 | 27 | (0.28) |
| WJ-III-C | 96.4 | 13.4 | 106.4 | 11.3 | −2.0 | 27 | (0.05) |
| WJ-III-PGK | 96.0 | 12.3 | 102.7 | 9.2 | −1.5 | 27 | (0.15) |
| Nelson–Denny Reading Test (GE) (F=9.63, df=1,27, p=0.004) | |||||||
| NDRT-T* | 10.0 | 5.3 | 15.6 | 3.1 | −3.1 | 27 | (0.005) |
| NDRT-V | 11.4 | 5.1 | 15.8 | 3.3 | −2.5 | 27 | (0.02) |
| NDRT-C** | 8.9 | 5.0 | 15.2 | 3.4 | −3.5 | 27 | 0.002 |
Critical level based upon Bonferroni correction for 19 comparisons=p<0.003.
p<.05 vs. adult norms.
p<.01 vs. adult norms.
The NDRT-C assesses reading performance relative to grade appropriate norms. Grade equivalent reading scores were significantly reduced for patients (8.9±5.0) relative to years of education completed (12.4±2.3), with the mean difference being 3.4±3.8 years (paired t=3.9, df=18, n=19, p=0.001). In contrast, controls showed no significant difference between education level completed (15.2±0.85) and reading ability (15.2±3.4), leading to a significant group × diagnosis interaction (F=5.51, df=1,27, n=29, p=0.03).
As opposed to the significant deficits in specific reading functions, patients showed only a marginal deficit in WRAT3 reading scores vs. control (p<0.04) that did not survive Bonferroni correction. Further, patient WRAT3 scores were not significantly different from population norms (t=−0.35, p=0.73), consistent with prior literature.
3.2. Models of diagnostic classification
Several theoretical models for diagnosing reading impairment were used in order to classify participants on an individual basis. This idiographic approach was proposed to further elucidate differences between individuals distinguished as reading impaired or non-impaired.
Based upon available literature, five models were selected (Fletcher et al., 1992; Aaron, 1995; Boder, 1970; Wolf and Bowers, 1999; Ramus et al., 2003) and participants were classified according to the criteria. Significantly more patients were identified as having a specific reading disability across models than controls (21–63% vs. 0–20%). Since multiple models were employed, it was decided a priori that individuals assigned to the “reading impaired” group would be classified as having reading deficiencies in at least 2 of the 5 models, which led to identification of nine patients as reading impaired.
As a final step, we defined an experimental diagnostic model using two tests, GORT-4-Rate and CTOPP-APA, which showed the greatest between-group differences. These tests tap orthographic and phonological awareness, respectively. Using deficient performance on these two tests, we identified 9 patients and no controls who met criteria for reading disturbance (Fisher exact test p=0.011). This group subsumed the individuals identified by other models, while nevertheless maintaining between-group specificity.
In order to validate group reading performance differences for our experimental model, we looked at group differences for percentile scores for NDRT-reading rate. Overall significant differences were found (F=4.1, df=2,28, n=29, p=0.03); with significant Bonferroni post hoc comparisons between impaired patients (12.7±16.3, n=9) and non-impaired patients (48.6±39.7, n=10, p=0.05), but not between impaired or non-impaired patients and non-impaired controls (45.1±28.0, n=10).
3.3. Reading impairment and visual processing
Within the patient group as a whole, GORT-4-Rate and Fluency deficits were significantly related to contrast sensitivity for low (0.5 c/deg) (r=0.56, n=18, p=0.01; r=0.64, n=18, p=0.004 respectively) but not medium (7 c/deg) (r=0.34, n=18, p=0.16; r=0.33, n=18, p=0.18), or high (21 c/deg) (r=0.31, n=18, p=0.21; r=0.27, n=18, p=0.27) spatial frequency stimuli.
In categorical analyses, mean contrast sensitivity measures at 0.5 c/deg were significantly reduced in the group of patients with reading impairment (43.7±18.4) compared to patients without (94.5±28.9) (F=11.48, n=28, p=0.001) (Fig. 1). By comparison, the patient groups showed no significant contrast sensitivity differences at 7 or 21 c/deg (97.8±31.2 vs. 118.6±50.1 and 4.0±2.1 vs. 5.0±5.0 respectively).
Fig. 1.
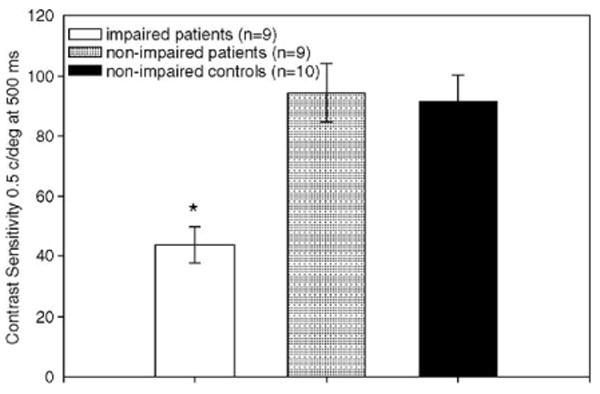
Contrast sensitivity by group.
3.4. Relationship to symptoms, neurocognitive and functional outcome measures
GORT-4-Comprehension correlated significantly and inversely with the PANSS Cognitive-factor score (r=−0.68, n=18, p=0.002). In contrast, neither the WAIS-3 Working Memory Index (r=0.12, n=12, p=0.71) nor the WAIS-3 Processing Speed Index (r=0.16, n=18, p=0.52) significantly predicted GORT-4 performance. No other significant relationships were found for reading tests with symptoms or neurocognitive tests.
GORT-4-Comprehension (r=0.64, n=19, p=0.003); GORT-4-ORQ (r=0.61, n=19, p=0.006); NDRT-T (r=0.63, n=19, p=0.004); and NDRT-C (r=0.63, n=19, p=0.004) were all also significantly correlated with ILS-PB scores, with higher levels of reading comprehension being associated with less need for community supervision.
Since significant differences were found between patient and comparison groups on education and SES, the analyses for between group differences were repeated using education or SES as covariates. Using MANCOVA, no significant between-group differences survived, suggesting that impairments in reading may have significantly influenced the reduced educational achievements and SES levels of the subjects since education and SES were significantly interrelated (r=0.74, p=<0.01). However, significant between group differences remained for five of six measures (GORT-Rate, GORT-Fluency, GORT-ORQ, CTOPP-APA, and NDRT-C) following covariation for parental SES.
4. Discussion
4.1. Summary of findings
Although reading is a critical life skill, it has been consistently understudied in schizophrenia. The major finding of this study is that patients show substantial impairments in reading ability vs. both normal comparison subjects (p<0.003) and age-appropriate norms (p<0.01). As a group, patients' reading level was 3.4 years below their achieved educational levels. Between 21% and 63% of patients met criteria for dyslexia depending upon diagnostic model. Further, deficits correlated both with independent measures of visual magnocellular functioning and with global achievement as measured by the SES, suggesting that failure to achieve appropriate reading levels may significantly reflect underlying perceptual level disturbances and may significantly affect occupational and role functioning. This finding is congruent with previous results regarding limitations in attainment and compromised performance commensurate with education in secondary school for individuals with schizophrenia (Fuller et al., 2002).
In contrast to substantial deficits in specific reading functions, patients showed no substantial deficit in single word reading, as measured by the WRAT3, nor in premorbid IQ, consistent with prior research. Reading deficits were significantly impaired not only on measures of comprehension (e.g., GORT-4-Comprehension), but also on tests that specifically evaluated orthographic (e.g., GORT-Rate/Fluency) and phonological (e.g., CTOPP-APA) processing. Among batteries tested, the deficits were most pronounced on the GORT and CTOPP. Least deficits were observed on WJ-III tests where life experience and language comprehension skills may permit the use of compensatory strategies (e.g., guessing based on context: e.g., WJ-III-C) to overcome mechanistic deficits. Impaired scores on some tests, such as the GORT-4-Comprehension, were significantly related to more global cognitive functioning, as reflected by PANSS cognitive factor score, suggesting a contribution of higher order dysfunction. In contrast, impaired performance on GORT-4-Rate and CTOPP-APA were unrelated to generalized cognitive symptomatology, suggesting that symptoms do not interfere with visual and auditory aspects of reading. Further, deficits in GORT-4-Rate and CTOPP-APA also showed correlations with Comprehension, suggesting both “bottom up” and “top down” influences on impaired reading comprehension in schizophrenia. Our finding of low GORT-4-Comprehension and NDRT-C scores is consistent with prior reports of low comprehension during silent reading in schizophrenia (Hayes and O'Grady, 2003).
4.2. Reading and visual processing deficits
To answer our research questions of whether a distinct pattern of reading deficits for individuals with schizophrenia emerged and whether these deficits are associated with magnocellular processing deficits, we used a range of models for diagnosing specific reading disability and classified our sample accordingly. The prevalence of reading impairment in our patient sample was higher than in our comparison group (0–20%) or the general population (2–20%) (Spafford and Grosser, 2005), with almost half the patients and no controls meeting criteria for our proposed Model 6. Together with the discrepancy between reading level and education, this is highly suggestive that individuals with schizophrenia are prone to learning disabilities particularly in the years immediately preceding illness onset. Reading skills normally improve during high school years. If the poor reading scores of our patients predated onset of their illness, our results may help to explain the limited number of patients who participated in post-high school education. Most diagnostic models appeared approximately equivalently successful in identifying reading dysfunction in schizophrenia. Overall, patients were classified as having generalized, rather than specific orthographic or phonological patterns, consistent with documented deficiencies in both early auditory (Javitt et al., 2000; Javitt et al., 1995a; Cienfuegos et al., 1999) and visual (Butler et al., 2005, 2001; Kim et al., 2005; Schechter et al., 2003) processing in schizophrenia.
As predicted, we found a strong relationship between reading impairment and magnocellular processing deficits as demonstrated by impaired contrast sensitivity to low-, but not high-, spatial frequency stimuli. Patients with reading impairment had lower contrast sensitivity (higher thresholds) than either non-impaired patients or controls. Performance by patients without reading impairment did not differ significantly on contrast sensitivity measures compared to controls.
4.3. Study limitations and implications of reading impairment for future studies
Study limitations include a small sample size and constricted variability of ethnicity and socioeconomic status in our patient sample, which limit generalizability of the results. In addition, further evaluation with a broad array of neurocognitive measures would be beneficial for investigating additional potential contributions to reading comprehension (e.g. verbal learning measures).
We believe that reading, as a complex and integrative functional behavior, is an important area for future research in sensory processing deficits in schizophrenia. We suggest that researchers view single word reading (WRAT3) performance with caution because it may have limited value as a true estimate of reading ability. We also recommend consideration of a brief reading battery inclusive of the GORT-4, as a test of global reading ability that can focus on rate, fluency and comprehension as separate components, as well as CTOPP phonological processing (e.g. CTOPP-APA), for adults with schizophrenia for potential diagnosis of specific reading disorder. Deficits in reading ability should also be considered in distribution of written materials (e.g., legal notifications, consent forms, psychoeducational materials).
In summary, while patients do indeed show relatively intact single word reading, as per previous reports, they nevertheless show highly impaired reading ability when confronted with “real world” materials. The deficits are manifest not only as reduced comprehension, but also as deficits in decoding the orthographic and phonological information necessary for parsing written text and converting written information into the “inner speech” necessary for semantic decoding. Since reading fluency depends on lower level processes of phonological processing and orthographic processing being intact (Wolf and Katzir-Cohen, 2001), our data suggest that impaired comprehension in our patients is related to these earlier processes as expected. It has been said that the act of reading requires the reader to simultaneously read the lines (decode for comprehension), read between the lines (make inferences about meaning) and read beyond the lines (transfer the knowledge) (Manzo and Manzo, 1993). While individuals with schizophrenia are known to have difficulty in inferential and abstract thinking and transferring information from one situation to another, our findings highlight the implicit difficulties in the first step of just “reading the lines”.
Although the present study did not employ specific measures of occupational or role performance, reading ability was found to correlate with global outcome as measured by the ILS-PB. Because none of the subjects in the present study had a childhood history of schizophrenia and had not previously been diagnosed with dyslexia during childhood, it is quite possible that the deficits we observed did not manifest until relatively late in childhood or during teen-age years. Indeed, such a supposition is consistent with a retrospective analysis of reading scores among individuals who subsequently developed schizophrenia. The finding that reading deficits in schizophrenia show both phonological and orthographic antecedents suggest that reading remediation approaches designed for treating developmental dyslexia may be effective also in schizophrenia remediation.
Acknowledgments
This study was made possible with grant support from NIMH, R01-MH049334, awarded to DCJ and NIMH, R01-MH66374 awarded to PDB.
The authors would like to acknowledge Dolores Perin, Ph.D., Teachers College, Columbia University, for her expertise and consultation regarding reading theory and assessment.
References
- Aaron PG. Differential diagnosis of reading disabilities. School Psychology Review. 1995;24:345–360. [Google Scholar]
- Ammons RB, Ammons CH. The Quick Test (QT): provisional manual. Psychological Reports. 1962;11:111–161. [Google Scholar]
- Boder E. Developmental dyslexia: a new diagnostic approach based on the identification of three subtypes. The Journal of School Health. 1970 June;:289–290. [PubMed] [Google Scholar]
- Brown JI, Fishco VV, Hanna G. Nelson–Denny Reading Test: Manual for Scoring and Interpretation. The Riverside Publishing Company; Itasca, IL: 1993. [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Archives of General Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Wright BR, Moffitt TE, Silva PA. Early failure in the labor market: childhood and adolescent predictors of unemployment in the transition to adulthood. American Sociological Review. 1998;63:424–451. [Google Scholar]
- Cienfuegos A, March L, Shelley AM, Javitt DC. Impaired categorical perception of synthetic speech sounds in schizophrenia. Biological Psychiatry. 1999;45:82–88. doi: 10.1016/s0006-3223(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Dalby JT, Williams R. Preserved reading and spelling ability in psychotic disorders. Psychological Medicine. 1986;16:171–175. doi: 10.1017/s0033291700002609. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Best M, Heeger DJ. Psychophysical evidence for a magnocellular pathway deficit in dyslexia. Vision Research. 1998;38:1555–1559. doi: 10.1016/s0042-6989(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Francis DJ, Rourke BP, Shaywitz SE. The validity of discrepancy-based definitions of reading disabilities. Journal of Learning Disabilities. 1992;25:555–561. doi: 10.1177/002221949202500903. [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O'Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. American Journal of Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Moriarty PJ, Friedman JI, White L, Parrella M, Mohs RC, Davis KL. Differential preservation of cognitive functions in geriatric patients with lifelong chronic schizophrenia: less impairment in reading compared with other skill areas. Biological Psychiatry. 2000;47:962–968. doi: 10.1016/s0006-3223(00)00245-6. [DOI] [PubMed] [Google Scholar]
- Hayes RL, O'Grady BM. Do people with schizophrenia comprehend what they read? Schizophrenia Bulletin. 2003;29:499–507. doi: 10.1093/oxfordjournals.schbul.a007022. [DOI] [PubMed] [Google Scholar]
- Hoover WA, Gough PB. The simple view of reading. Reading and Writing. 1990;2:127–160. [Google Scholar]
- Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Archives of General Psychiatry. 1995a;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Archives of General Psychiatry. 1995b;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia. Archives of General Psychiatry. 2000;57:1131–1135. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophrenia Research. 2005;76:55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Pepple JR, Lyons MJ, Tsuang MT. The “3 Rs” and neuropsychological function in schizophrenia: an empirical test of the matching fallacy. Neuropsychology. 1996;10:22–31. doi: 10.1016/0165-1781(94)02652-1. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Bernstein-Hyman R, Grochowski S. Five-factor model of schizophrenia. Journal of Nervous and Mental Disease. 1994;182:631–638. doi: 10.1097/00005053-199411000-00006. [DOI] [PubMed] [Google Scholar]
- Lovegrove W. Weakness in the transient visual system: a causal factor in dyslexia? In: Tallal P, Galaburda AM, Llinas RR, von Euler C, editors. Temporal Information Processing in the Nervous System: Special Reference to Dyslexia and Dysphasia. The New York Academy of Sciences; New York: 1993. pp. 57–69. [DOI] [PubMed] [Google Scholar]
- Manzo AV, Manzo UC. Literacy Disorders: Holistic Diagnosis and Remediation. Harcourt Brace Jovanovich College Publishers; Fort Worth: 1993. [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126:841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Revheim N, Medalia A. The Independent Living Scales as a measure of functional outcome for schizophrenia. Psychiatric Services. 2004;55:1052–1054. doi: 10.1176/appi.ps.55.9.1052. [DOI] [PubMed] [Google Scholar]
- Ridder WH, Borsting E, Cooper M, McNeel B, Huang E. Not all dyslexics are created equal. Optometry and Vision Science. 1997;74:99–104. doi: 10.1097/00006324-199702000-00021. [DOI] [PubMed] [Google Scholar]
- Romani A, Conte S, Callieco R, Bergamaschi R, Versino M, Lanzi G, Ambrino CA, Cosi V. Visual evoked potential abnormalities in dyslexic children. Functional Neurology. 2001;16:219–229. [PubMed] [Google Scholar]
- Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophrenia Research. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schirmer TN, Meyer KA, Samarasinghe R. Teaching literacy and mathematics skills to adult psychiatric inpatients: an evaluation of the adult literacy program at Hawaii State Hospital. Psychiatric Rehabilitation Journal. 2005;28:251–259. doi: 10.2975/28.2005.251.259. [DOI] [PubMed] [Google Scholar]
- Spafford CS, Grosser GS. Dyslexia and Reading Difficulties: Research and Resource Guide for Working with All Struggling Readers. Second. Pearson Education, Inc; Boston: 2005. [Google Scholar]
- Talcott JB, Hansen PC, Willis-Owen C, McKinnell IW, Richardson AJ, Stein JF. Visual magnocellular impairment in adult developmental dyslexics. Neuro-ophthalmology. 1998;20:187–201. [Google Scholar]
- Tallal P. The science of literacy: from the laboratory to the classroom. PNAS. 2000;97:2402–2404. doi: 10.1073/pnas.97.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM. Developmental dyslexia. In: Snowling MJ, Hulme C, editors. The Science of Reading: A Handbook. Blackwell Publishing; Malden, MA: 2005. pp. 362–378. [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? Journal of Child Psychology and Psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. CTOPP: Comprehensive Test of Phonological Processing (Examiner's Manual) Pro-Ed; Austin, TX: 1999. [Google Scholar]
- Wechsler D. The Wechsler Adult Intelligence Scale. Third. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Wiederholt JL, Bryant BR. Examiner's Manual. Fourth. Pro-Ed; Austin, TX: 2001. Gray Oral Reading Tests (GORT-4) [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test 3 Administrative Manual. Wide Range, Inc; Wilmington, DE: 1993. [Google Scholar]
- Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology. 1999;91:415–438. [Google Scholar]
- Wolf M, Katzir-Cohen T. Reading fluency and its intervention. Scientific Studies of Reading. 2001;5:211–239. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock–Johnson III Tests of Achievement. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]


